Published online Sep 26, 2015. doi: 10.4330/wjc.v7.i9.562
Peer-review started: May 21, 2015
First decision: June 3, 2015
Revised: July 7, 2015
Accepted: August 16, 2015
Article in press: August 17, 2015
Published online: September 26, 2015
Processing time: 125 Days and 22.5 Hours
AIM: To present our initial clinical experience using this innovative software solution for guidance of percutaneous structural heart disease interventions.
METHODS: Left atrial appendage, atrial septal defect and paravalvular leak closure, transaortic valve repair and MitraClip® procedures were performed in the catheter laboratory under fluoroscopic and echocardiographic guidance. The two-dimensional and three-dimensional images generated by the transesophageal echocardiography probe were interfaced with the fluoroscopic images in real-time using the EchoNavigator®-system.
RESULTS: The application of the novel image fusion technology was safe and led to a better appreciation of multimodality imaging guidance due to improved visualization of the complex relationship between catheter devices and anatomical structures.
CONCLUSION: The EchoNavigator®-system is a feasible and safe tool for guidance of interventional procedures in structural heart disease. This innovative technology may improve confidence of interventional cardiologists in targeting and positioning interventional devices in order to increase safety, accuracy, and efficacy of percutaneous interventions in the catheter laboratory.
Core tip: Interventions in structural heart disease require adequate echocardiographic and fluoroscopic imaging for safe accomplishment of the procedure. Recently, a novel fusion imaging technology has been introduced, allowing for the first time to merge echocardiographic and fluoroscopic images in the catheter laboratory in real time. As one of the first centers worldwide, we used this innovative technology for guidance of interventions in structural heart disease, demonstrating its potential benefits for guiding complex interventions in structural heart disease.
- Citation: Balzer J, Zeus T, Hellhammer K, Veulemans V, Eschenhagen S, Kehmeier E, Meyer C, Rassaf T, Kelm M. Initial clinical experience using the EchoNavigator®-system during structural heart disease interventions. World J Cardiol 2015; 7(9): 562-570
- URL: https://www.wjgnet.com/1949-8462/full/v7/i9/562.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i9.562
Adequate peri-procedural image guidance is indispensable for safe accomplishment of cardiovascular interventions[1]. In contrast to coronary interventions where fluoroscopy remains the dominant imaging modality, the evaluation and treatment of structural heart disease requires continuous soft-tissue information that cannot be provided by fluoroscopy alone. Therefore, percutaneous interventions in the catheter laboratory are usually monitored additionally by two-dimensional (2D) and especially three-dimensional (3D) transesophageal echocardiography (TEE)[2]. Both techniques are presented side by side on different screens, necessitating the interventionalist to mentally reconstruct and fuse the presented information. Recently, the EchoNavigator®-system (Philips Healthcare, Best, The Netherlands) has been introduced as a novel software solution, allowing to merge echocardiographic and fluoroscopic images on the same display in real time[3,4]. In this study we aim to present our initial clinical experience with this innovative technology and describe its potential benefits during percutaneous interventions in structural heart disease.
From January 2014 until July 2014 we used the EchoNavigator® software for guidance of 127 interventions in structural heart disease [3 paravalvular leaks, 11 atrial septal defects (ASDs), 31 transapical transcatheter aortic valve repair (TAVR) procedures, 35 left atrial appendage (LAA) occlusions, and 47 MitraClip® procedures]. Conscious sedation with continuous hemodynamic monitoring was applied in all cases, with the exception of the 31 transapical TAVR procedures, where general anesthesia was applied. After insertion of the procedure specific sheath, patients were sedated, and the TEE probe was inserted by an experienced echocardiographer before initiating the procedure by the interventionalist. For the additional application of this fusion imaging technology no ethical approval was necessary. All patients gave written informed consent for the performance of each interventional procedure.
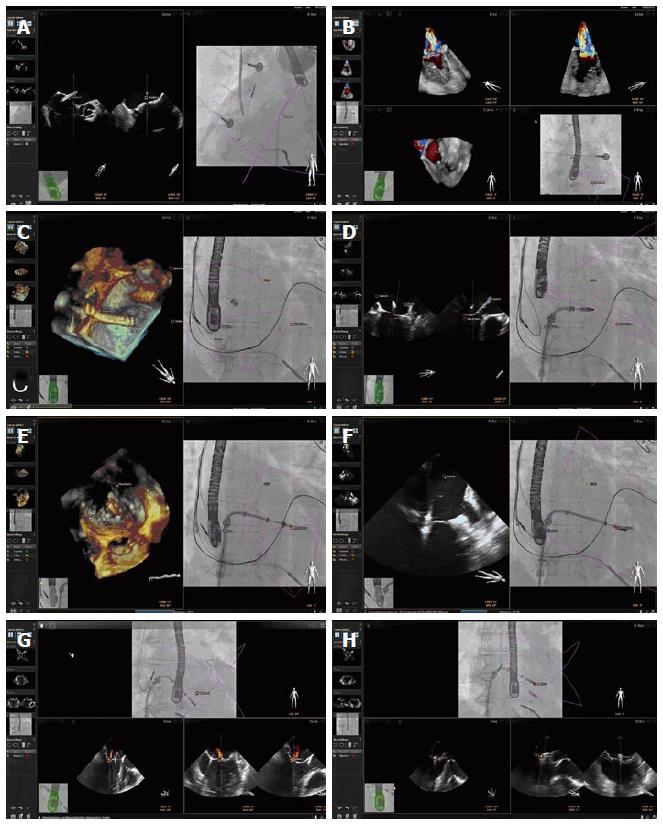
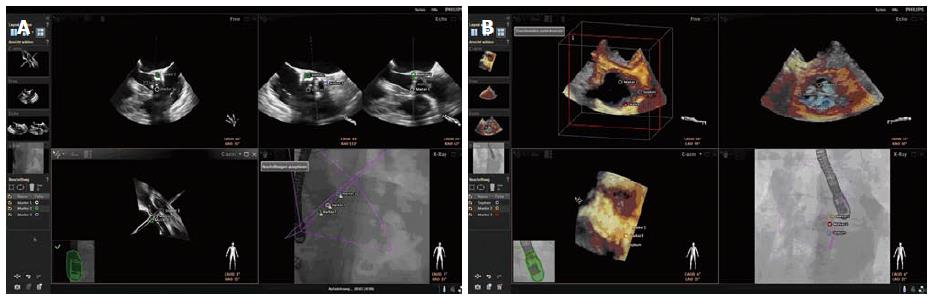
The technology of the EchoNavigator®-system relies on a real-time co-registration and visualization of 2D/3D TEE and fluoroscopy. The method consists of an image-based TEE probe localization and calibration algorithm. This algorithm automatically finds and tracks the position and the direction of the TEE probe within the fluoroscopic image[5]. After synchronization of TEE and fluoroscopy images, the system automatically tracks and follows the rotation of the C-Arm, based on the angulation of the gantry[6]. The results of this co-registration process are visualized to the interventional cardiologist on a large specific display that can be divided and arranged in up to 4 sections at discretion of the interventionalist. The 4 sections are assigned to different functions, depicting different views and are labeled as follows: (1) Echo: The Echo-view demonstrates online the images from the echo machine that can only be manipulated by the echocardiographer; (2) X-ray: The X-ray-view displays the actual fluoroscopic view depending on the angulation of the gantry. For a precise co-registration of the TEE probe, the probe has to be central in this view, the correctness of the co-registration being illustrated by a green edging of the probe. In case that the co-registration is not correct, e.g., after movement of the TEE probe, the edging of the probe will turn into red; (3) C-Arm: The beam flow of the matrix array transducer is marked as a purple 3D sector in the X-ray-view, presenting the 3D Echo information of this sector in the C-Arm-view. Changes in angulation, rotation or position of the TEE probe are immediately registered and updated on the fluoroscopic image; and (4) Free: The Free-view also displays 2D and 3D TEE information that can be manipulated by the interventionalist with a sterile covered mouse on the catheter table. The interventionalist can rotate and crop into 3D data sets in any direction.
Specific points of interest can be marked in the ultrasound image by the interventionalist that will automatically appear on the fluoroscopic image (Figure 1). There is also the opportunity to switch between different TEE modalities (2D, 3D, 2D and 3D Color Doppler), and different view settings (2D, X-Plane, 3D Zoom and 3D Full Volume).
All 127 procedures were performed using the EchoNavigator®-system for peri-interventional guidance. The application led to safe accomplishment of all performed procedures without any complications related to the peri-procedural imaging guidance.
Percutaneous edge to edge repair of mitral regurgitation using the MitraClip® system: The unique visualization of the mitral valve apparatus using 3D TEE for planning and performing the procedure allows improved understanding of the morphological and functional changes induced by the MitraClip® system. In this context, a recent study demonstrated that the procedural effects of the MitraClip® system upon the mitral valve apparatus can best be detected using 3D TEE with various offline reconstruction techniques[7]. The peri-interventional evaluation of the mitral valve, including the leaflets, the annulus, and the subvalvular apparatus using 3D TEE is therefore of major importance. On the other hand, the orientation of the guiding system and the dedicated structures of the Clip with its grippers can be much better delineated using fluoroscopy. Therefore, a multimodality approach for guidance of MitraClip® implantations using 2D/3D TEE and fluoroscopy is of essential importance. Our results demonstrate that the translation of specific echocardiographic markers into the X-ray-view can improve the visualization of the complex relationship between catheter devices and anatomical structures during MitraClip® procedures (Figure 2). In this context, the EchoNavigator®-system was especially usefull for the transseptal puncture, that can be performed using fluoroscopy and 2D TEE imaging alone. In special situations, the 3D images improve the visualization of the transseptal puncture side for better definition of the correct height above the mitral valve, necessary for sufficient movement of the delivery guide and the device[8]. The fact, that the designated point of puncture can be marked in either the 2D or the 3D echocardiographic view, is very helpful for placement of the needle in the fluoroscopic image. Severe complications can arise when the Clip perforates the thin wall of the atrial septum, leading to cardiac tamponade[9]. The designation of three echocardiographic orientation points (interatrial septum at puncture site, crista terminalis between pulmonary vein and the LAA, and the center of the mitral valve) into the fuoroscopic image can prevent injury of the left atrium, even when the Clip movements are monitored with X-ray within this virtual triangle. When more than one Clip needs to be implanted, the exact relation of the Clips to each other can be misjudged due to blooming artefacts of the echocardiographic image[10]. In this situation, fluoroscopy is very helpful to illustrate the spatial relation of both Clips. The EchoNavigator®-system enables the translation of the residual jet from the echocardiographic image into the X-ray image for exact implantation of the second Clip.
LAA occlusion: In non-valvular atrial fibrillation the interventional closure of the LAA has shown to be a successful alternative to oral anticoagulation[11]. The main steps of the procedure are the transseptal crossing of the guiding catheter into the left atrium and the placement of the occluder into the LAA. Basically, the intervention can be performed using fluoroscopy alone, but the advantages of 3D TEE for peri-interventional guidance during the procedure could be clearly demonstrated in recent studies[12]. 2D TEE allows the interventionalist to measure the orifice of the appendage in different 2D cut planes, but 3D TEE additionally allows 3D measurement of the perimeter for exact definition of the landing zone and correct device selection[13]. The EchoNavigator®-system revealed to be very useful for the performance of the transseptal puncture (Figure 3). Furthermore, the exact delineation of the landing zone derived from the echocardiographic images allowed for secure implantation of the device, therewith preventing mismatch and dislocation of the occluder.
ASD occlusion: Interventional techniques for the closure of interatrial communications can be performed using echocardiography as the only imaging modality. In a recent study the feasibility of interventional closure of ASDs without fluoroscopy was demonstrated[14]. TEE provides an imaging technique to guide ASD closures, providing fast and complete information about the underlying pathomorphology, improving spatial orientation, and additionally monitoring online the appropriate position of the device without loss of image quality. Furthermore, the additional information supplied by the 3D images helps to better understand the anatomy and the pathomorphology of the defect during guidance of the intervention, leading to shorter procedure times and less radiation exposure to the patient[15]. Our initial experience with the EchoNavigator®-system in such procedures indicates safe implantation of devices in ASDs. After placement of the echocardiographic marker delineating the ASD into the X-ray-view, the guide wire passage orientated towards this point enormously facilitated the procedure (Figure 4).
TAVR: For safe and precise performance of TAVI procedures, knowledge about the exact anatomy of the aortic root an its surrounding structures obtained by different imaging modalities is indispensable[16]. The use of TEE for peri-interventional guidance is limited, as it often requires general anesthesia and the probe may also partially obstruct the optimal fluoroscopic view. Particularly in patients treated over the transapical approach where general anesthesia is needed anyway, TEE has its place during and after valve implantation. For secure valve implantation, the exact knowledge about the alignment of the hinge points of the three leaflets is crucial[17]. With fluoroscopic imaging alone it is difficult to place the gantry in a position, where the hinge points are in the exact same cut plane. 3D TEE has proven to be very useful for aortic annular sizing and exact delineation of the hinge points during valve sizing and implantation[18]. The EchoNavigator®-system allows transfer of specific 2D and 3D echocardiographic markers into the fluoroscopic image (Figure 5). This allows the interventionalist to correct the position of the gantry to the point where all three hinge point markers derived from echocardiography create one orthogonal plane.
Percutaneous closure of prosthetic paravalvular leaks: Different multimodality imaging techniques have been proposed for peri-interventional guidance of these procedures, including a combination of fluoroscopy and TEE[19]. The advantage of 3D TEE is the improved spatial resolution of the defect, especially during placement of the guide wire through the defect after transseptal puncture[20]. The interaction between the prosthetic leaflets and the occluder system leading to possible obstruction and malfunction of the prosthetic valve can better be illustrated by 3D TEE. The application of the EchoNavigator®-system is very helpful for percutaneous closure of paravalvalvular leaks (Figure 6). It allows the interventionalist to focus more upon the fluoroscopic image as it better delineates the catheter and the devices. Especially the precise steering of the guide wire through the defect is facilitated using this innovative technology.
The major findings of the present study are: (1) the application of the EchoNavigator®-system is feasible and safe during interventions in structural heart disease; (2) the EchoNavigator®-system allows merged display of echocardiographic (2D, 3D, Color-Doppler, X-Plane Imaging) and fluoroscopic images in real-time, allowing the interventionalist to interact with both imaging modalities simultaneously.
The benefits of fusion imaging technologies have already been described in the recent past, especially for the combination of CT-angiography and fluoroscopy images during transapical TAVR procedures for exact assignment of the correct site for the transapical access[21]. The integration of echocardiographic images into the X-ray image in real-time during percutaneous interventions were hampered so far by the complex nature of the echocardiographic data. The EchoNavigator®-system is the first integrative solution to merge the two major important imaging modalities echocardiography and fluoroscopy in real time during interventions in structural heart disease. This technology facilitates the procedures, as echocardiographic soft tissue information is copied into the fluoroscopic image in real-time without time-consuming offline reconstruction. Traditionally, the interventional cardiologist is more familiar with the standard fluoroscopy projections compared with the classical TEE orientations. This new technology therewith fulfills the needs of the traditional interventionalist, who is not always used to the different 2D and 3D image orientation from TEE, by delineating the information from echocardiography into the more familiar fluoroscopic image. Only few studies have described the application of the EchoNavigator® during structural heart disease interventions so far. Sündermann et al[4] were the first to document an influence of radiation dose and procedure time under guidance of MitraClip procedures using the EchoNavigator®. They discovered a reduction in radiation and procedure time especially in complex procedures were more than one clip was implanted. This underlines the strength of this technology, simplifying the exact detection of the target point for the implantation of more than one clip next to the already implanted clip. The more complex the intervention becomes during the procedure, the more beneficial is the gain of the additional information given by the supporting software. Recently, González Gómez et al[22] described the advantage of the technique for transseptal punctures, according to our own results. Especially in MitraClip procedures the exact height of the puncture is essential for the success of the intervention. Using the EchoNavigator® the puncture site can even be defined before pullback of the needle from the superior caval vein into the right atrium. The precision of this fusion imaging technology is remarkable. Our own experience indicates that the applied markers depicting the target structures correlate very well with the definite location of the catheter devices. Moreover, in cases where the matching of echocardiographic and fluoroscopic data was unsatisfactory, we switched back to the conventional image guidance without the overlay. This approach always warrants an exit strategy in cases where the confidence into the fusion technology is somehow affected, anticipating possible misguidance by the EchoNavigator®-system. Next to the advantages of the EchoNavigator®, there also are some limitations. The technology described in this paper is based on a co-registration process of the TEE probe into the fluoroscopic image, simultaneously transferring the data into the virtual coordinate system of the X-ray display. The specific points of interest that can be marked with the software do not follow the acute movements of the echocardiographic speckles, making the combined image with the marked reference points quite static. The newest release of the EchoNavigator® allows a translation of the entire echocardiographic dataset into the X-ray-image, making the fusion of both modalities even more impressive, as recently published by our group[23].
Our manuscript is supposed to give “tips and tricks” while working with the EchoNavigator® technology according to our initial experience in 127 patients. Our study lacks information about the benefit of the technology in the context of reducing the procedure length or the radiation dose. Data demonstrating these effects are currently only available for MitraClip procedures[4]. Prospective randomized multi-center studies with a larger sample size are necessary to demonstrate potential benefits of this promising technology for the patient. At this time, the main benefit of the EchoNavigator®-system is the facilitation of procedures by online fusion of two important imaging modalities, leading to better confidence of the interventionalist into the procedure and producing a better communication between the echocardiographer and the operator.
In conclusion, the EchoNavigator®-system is a feasible and safe tool for guidance of interventional procedures in structural heart disease. This innovative technology may accelerate the learning curve of interventional cardiologists in order to increase safety, accuracy, and efficacy of percutaneous interventions in the catheter laboratory. Further research is necessary to evaluate the clinical value of this promising new tool, but it is likely that such a visualization technology might have a significant impact on the success and the safety of cardiovascular procedures in the catheter laboratory.
The number of percutaneous interventional procedures for the treatment of structural heart disease in patients that are ineligible for conventional open heart surgery is increasing permanently. Less invasive techniques allow for save accomplishment of these highly complex interventions in the catheter laboratory. Still, complications can arise during the procedure due to inadequate imaging of the target structures, often with fatal outcome for the patient.
Side by side imaging of cardiac soft tissue anatomy and catheter devices using echocardiography and fluoroscopy is essential for safe performance of interventional procedures in structural heart disease, though assuming mental fusion and reconstruction of each imaging modality by the interventionalist. Online fusion imaging technologies can determine a faster and better understanding of the complex relationship between anatomical landmarks and catheter devices and have the potential to facilitate the procedure.
In the current study the authors demonstrate for the first time the application of a novel image fusion technology (EchoNavigator®) to guide different types of complex interventions in structural heart disease. To our knowledge, no similar studies presenting such a broad applicability of this hybrid imaging technique have been published so far.
The study results of the present study suggest that online fusion of echocardiographic soft tissue anatomy and fluoroscopic catheter devices is a breakthrough for precise monitoring of interventions in structural heart disease. The impressive images in this article implicate the value of this innovative technology for upcoming interventional procedures that afford even more an exact delineation of cardiac anatomy for safe performance of complex procedures. Furthermore, the technique does not exclude the standard operating procedure using solely echocardiography and fluoroscopy side-by-side. The generated overlay images rather must be considered accessory with in fact tremendous additional value.
The name of the new software “EchoNavigator®” implicates the way it is used for monitoring of interventions in the catheter laboratory. 2D and 3D echo information can be translated into the fluoroscopic image for best navigation of the procedure using a combination of both imaging modalities.
This is an excellent manuscript about the clinical experience using the EchoNavigator®-system. The authors have suggested that the EchoNavigator®-system is a feasible and safe tool for guidance of interventional procedures, such as left atrial appendage, atrial septal defect and paravalvular leak closure, transaortic valve repair and MitraClip® in structural heart disease. This manuscript is nicely structured and very well written.
P- Reviewer: Amiya E, Nunez-Gil IJ, Ueda H S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | van der Hoeven BL, Schalij MJ, Delgado V. Multimodality imaging in interventional cardiology. Nat Rev Cardiol. 2012;9:333-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 2. | Faletra FF, Pedrazzini G, Pasotti E, Muzzarelli S, Dequarti MC, Murzilli R, Schlossbauer SA, Slater IP, Moccetti T. 3D TEE during catheter-based interventions. JACC Cardiovasc Imaging. 2014;7:292-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Corti R, Biaggi P, Gaemperli O, Bühler I, Felix C, Bettex D, Kretschmar O, Falk V, Grünenfelder J. Integrated x-ray and echocardiography imaging for structural heart interventions. EuroIntervention. 2013;9:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Sündermann SH, Biaggi P, Grünenfelder J, Gessat M, Felix C, Bettex D, Falk V, Corti R. Safety and feasibility of novel technology fusing echocardiography and fluoroscopy images during MitraClip interventions. EuroIntervention. 2014;9:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Gao G, Penney G, Ma Y, Gogin N, Cathier P, Arujuna A, Morton G, Caulfield D, Gill J, Aldo Rinaldi C. Registration of 3D trans-esophageal echocardiography to X-ray fluoroscopy using image-based probe tracking. Med Image Anal. 2012;16:38-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Housden RJ, Ma Y, Arujuna A, Nijhof N, Cathier P, Gijsbers G, Bullens R, Gill J, Rinaldi CA, Parish V. Extended-field-of-view three-dimensional transesophageal echocardiography using image-based X-ray probe tracking. Ultrasound Med Biol. 2013;39:993-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Altiok E, Hamada S, Brehmer K, Kuhr K, Reith S, Becker M, Schröder J, Almalla M, Lehmacher W, Marx N. Analysis of procedural effects of percutaneous edge-to-edge mitral valve repair by 2D and 3D echocardiography. Circ Cardiovasc Imaging. 2012;5:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Swaans MJ, Post MC, Van den Branden BJ, Van der Heyden JA. A complicated transseptal puncture during Mitraclip procedure: saved by 3D-TEE. Eur J Echocardiogr. 2011;12:E45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Tamburino C, Ussia GP, Maisano F, Capodanno D, La Canna G, Scandura S, Colombo A, Giacomini A, Michev I, Mangiafico S. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur Heart J. 2010;31:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Kische S, Nienaber C, Ince H. Use of four MitraClip devices in a patient with ischemic cardiomyopathy and mitral regurgitation: “zipping by clipping”. Catheter Cardiovasc Interv. 2012;80:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, Halperin JL, Holmes D. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127:720-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 513] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 12. | Nucifora G, Faletra FF, Regoli F, Pasotti E, Pedrazzini G, Moccetti T, Auricchio A. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: implications for catheter-based left atrial appendage closure. Circ Cardiovasc Imaging. 2011;4:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Perk G, Biner S, Kronzon I, Saric M, Chinitz L, Thompson K, Shiota T, Hussani A, Lang R, Siegel R. Catheter-based left atrial appendage occlusion procedure: role of echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Schubert S, Kainz S, Peters B, Berger F, Ewert P. Interventional closure of atrial septal defects without fluoroscopy in adult and pediatric patients. Clin Res Cardiol. 2012;101:691-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Balzer J, van Hall S, Rassaf T, Böring YC, Franke A, Lang RM, Kelm M, Kühl HP. Feasibility, safety, and efficacy of real-time three-dimensional transoesophageal echocardiography for guiding device closure of interatrial communications: initial clinical experience and impact on radiation exposure. Eur J Echocardiogr. 2010;11:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Bloomfield GS, Gillam LD, Hahn RT, Kapadia S, Leipsic J, Lerakis S, Tuzcu M, Douglas PS. A practical guide to multimodality imaging of transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2012;5:441-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Kasel AM, Cassese S, Bleiziffer S, Amaki M, Hahn RT, Kastrati A, Sengupta PP. Standardized imaging for aortic annular sizing: implications for transcatheter valve selection. JACC Cardiovasc Imaging. 2013;6:249-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 18. | Jilaihawi H, Doctor N, Kashif M, Chakravarty T, Rafique A, Makar M, Furugen A, Nakamura M, Mirocha J, Gheorghiu M. Aortic annular sizing for transcatheter aortic valve replacement using cross-sectional 3-dimensional transesophageal echocardiography. J Am Coll Cardiol. 2013;61:908-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Krishnaswamy A, Kapadia SR, Tuzcu EM. Percutaneous paravalvular leak closure- imaging, techniques and outcomes. Circ J. 2013;77:19-27. [PubMed] |
| 20. | Kliger C, Eiros R, Isasti G, Einhorn B, Jelnin V, Cohen H, Kronzon I, Perk G, Fontana GP, Ruiz CE. Review of surgical prosthetic paravalvular leaks: diagnosis and catheter-based closure. Eur Heart J. 2013;34:638-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Kliger C, Jelnin V, Sharma S, Panagopoulos G, Einhorn BN, Kumar R, Cuesta F, Maranan L, Kronzon I, Carelsen B. CT angiography-fluoroscopy fusion imaging for percutaneous transapical access. JACC Cardiovasc Imaging. 2014;7:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | González Gómez A, Hernández-Antolín R, Zamorano JL. Eco-X Ray Fusion for Transseptal Puncture. Rev Esp Cardiol (Engl Ed). 2015;68:714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Balzer J, Zeus T, Blehm A, Westenfeld R, Lichtenberg A, Kelm M, Rassaf T. Intraprocedural online fusion of echocardiography and fluoroscopy during transapical mitral valve-in-valve implantation. Can J Cardiol. 2015;31:364.e9-364.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |