Published online Nov 26, 2015. doi: 10.4330/wjc.v7.i11.719
Peer-review started: May 8, 2015
First decision: June 3, 2015
Revised: July 23, 2015
Accepted: September 16, 2015
Article in press: September 18, 2015
Published online: November 26, 2015
Processing time: 208 Days and 10.2 Hours
The endothelium exerts multiple actions involving regulation of vascular permeability and tone, coagulation and fibrinolysis, inflammatory and immunological reactions and cell growth. Alterations of one or more such actions may cause vascular endothelial dysfunction. Different risk factors such as hypercholesterolemia, homocystinemia, hyperglycemia, hypertension, smoking, inflammation, and aging contribute to the development of endothelial dysfunction. Mechanisms underlying endothelial dysfunction are multiple, including impaired endothelium-derived vasodilators, enhanced endothelium-derived vasoconstrictors, over production of reactive oxygen species and reactive nitrogen species, activation of inflammatory and immune reactions, and imbalance of coagulation and fibrinolysis. Endothelial dysfunction occurs in many cardiovascular diseases, which involves different mechanisms, depending on specific risk factors affecting the disease. Among these mechanisms, a reduction in nitric oxide (NO) bioavailability plays a central role in the development of endothelial dysfunction because NO exerts diverse physiological actions, including vasodilation, anti-inflammation, antiplatelet, antiproliferation and antimigration. Experimental and clinical studies have demonstrated that a variety of currently used or investigational drugs, such as angiotensin-converting enzyme inhibitors, angiotensin AT1 receptors blockers, angiotensin-(1-7), antioxidants, beta-blockers, calcium channel blockers, endothelial NO synthase enhancers, phosphodiesterase 5 inhibitors, sphingosine-1-phosphate and statins, exert endothelial protective effects. Due to the difference in mechanisms of action, these drugs need to be used according to specific mechanisms underlying endothelial dysfunction of the disease.
Core tip: The endothelium is involved in the regulation of vascular tone and permeability, coagulation and fibrinolysis, inflammatory and immunological reactions and cell growth. Cardiovascular risk factors cause vascular endothelial dysfunction through impairing endothelium-derived vasodilators, enhancing endothelium-derived vasoconstrictors, producing reactive oxygen species and reactive nitrogen species, activating inflammatory and immune reactions and promoting thrombosis. Among these mechanisms, a reduction in nitric oxide bioavailability plays a central role in the development and progression of endothelial dysfunction. A variety of currently used or investigational drugs exert endothelial protective effects according to specific mechanisms underlying endothelial dysfunction of the disease.
- Citation: Su JB. Vascular endothelial dysfunction and pharmacological treatment. World J Cardiol 2015; 7(11): 719-741
- URL: https://www.wjgnet.com/1949-8462/full/v7/i11/719.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i11.719
The endothelium is formed by a monolayer of endothelial cells. It constitutes a physical barrier between blood and tissues and regulates the exchange of molecules between blood and tissues. In addition, endothelial cells metabolize, synthesize and release a variety of substances, including vasoactive substances regulating vascular tone, blood pressure and local blood flow, the substances participating in coagulation, fibrinolysis and inflammatory and immunological reactions, reactive oxygen species (ROS) and reactive nitrogen species (RNS) involved in oxidation and nitrosylation of proteins and lipids, and growth factors promoting cell growth (Table 1). Any perturbation affecting the capacity and equilibrium of the endothelium as a physical barrier and to metabolize, synthesize and release these substances will cause endothelial dysfunction, which contributes to the development and progression of cardiovascular diseases. After summarizing the role of a number of endothelium-derived vasoactive substances and risk factors of endothelial dysfunction, this review focus on several categories of pharmacological substances that may be used for improving endothelial function.
| Vasoactive substances |
| Endothelium-derived vasodilators |
| Adrenomedullin |
| Endothelium-derived hyperpolarizing factors |
| Kinins |
| Nitric oxide |
| Prostacyclin |
| Endothelium-derived vasoconstrictors |
| Angiotensin II |
| Endothelin-1 |
| Vasoconstrictor prostanoids |
| Coagulation and fibrinolysis |
| Coagulation |
| Factor V |
| Heparan sulfate |
| Protein C |
| Protein S |
| Thrombomodulin |
| Tissue factor |
| von Willebrand factor |
| Fibrinolysis |
| Plasminogen activator inhibitor |
| Tissue plasminogen activator |
| Urokinase |
| Growth factors |
| Basic fibroblast growth factor |
| Insulin-like growth factor |
| Platelet-derived growth factor |
| Transforming growth factor |
| Inflammatory and immunological mediators |
| Cytokines |
| Interleukins |
| Monocyte chemoattractant protein 1 |
| Transforming growth factor |
| Tumor necrosis factor-α |
| Adhesion molecules |
| Intercellular adhesion molecules |
| Platelet-endothelial cell adhesion molecules |
| Selectins |
| Vascular cell adhesion molecules |
| Reactive oxygen species and reactive nitrogen species |
| Reactive oxygen species |
| Hydrogen peroxide (H2O2) |
| Hydroperoxyl (HO2) |
| Superoxide (O2-) |
| Reactive nitrogen species |
| Nitrite (NO2-) |
| Nitrogen dioxide (NO2) |
| Peroxynitrite (ONOO-) |
| Nitryl chloride (NO2Cl) |
The endothelium releases a variety of vasoactive substances, including different vasodilators such as nitric oxide (NO), prostacyclin, kinins, and endothelium-derived hyperpolarizing factors (EDHF), vasoconstrictors such as endothelin-1 and PGH2, and ROS. Among endothelium-derived vasodilators, NO occupies a central position because changes in the release of endothelial NO play a crucial role in the perturbation of vascular homeostasis and in the development of endothelial dysfunction associated with various cardiovascular disorders.
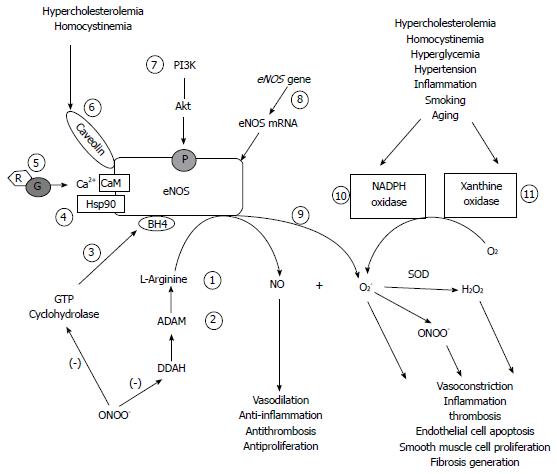
NO is synthesized from the amino acid L-arginine by a family of enzymes, the NO synthase (NOS), through the L-arginine-NO pathway. Three isoforms of NOS have been identified. Neuronal NOS (nNOS), initially found in the nervous system, is also constitutively expressed in skeletal and cardiac muscles, vessels and many other tissues. Endothelial NOS (eNOS) is constitutively expressed mainly in endothelial cells, whereas the expression of inducible NOS (iNOS) can be stimulated by diverse factors such as cytokines and endotoxin in different circumstances. The endothelium-derived NO release is primarily ensured by eNOS and complemented by nNOS expressed in vascular endothelial cells. Therefore, eNOS is primordial in the regulation of NO production by endothelial cells. The activity of eNOS depends on several factors, including eNOS mRNA and protein expression, the abundance of asymmetric dimethylarginine (ADMA, an endogenous eNOS inhibitor that competes with L-arginine for binding to eNOS)[1,2], the quantity and quality of cofactors such as tetrahydrobiopterin (BH4) and NADPH that are necessary for eNOS catalyzing NO production from L-arginine[3,4], its interaction with caveolin and heat shock protein 90 (hsp90)[5,6], and its translational modifications such as phosphorylation at different sites by multiple kinases or phosphatases [for example, phosphorylation at ser-1179 by phosphotidylinositol-3-kinase (PI3K)/protein kinase B (Akt) activates eNOS to initiate NO synthesis][7,8] and S-nitrosylation at cysteine residues[9]. In addition, excessive superoxide (O2-) and hydrogen peroxide (H2O2) production due to increased NAD(P)H oxidase[10,11] and eNOS uncoupling induced by changes in oxidized low density lipoprotein (OxLDL), caveolin-1, BH4, a switch from S-nitrosylation to S-glutathionylation and oxidation of the zinc-thiolate complex by peroxynitrite (ONOO-) also affects effective eNOS activity[12-15].
NO released by eNOS participates in the regulation of vascular tone. NO activates soluble guanylate cyclase by binding to its ferrous heme, leading to the conversion of guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP) that causes vascular smooth muscle relaxation. Moreover, NO exerts antiinflammatory, antiplatelet, antiproliferative and antimigration actions that contribute to the maintenance of an adequate environment for the endothelium. Lacking eNOS gene in mice induces insulin resistance, hyperlipidemia and hypertension[16]. NO released by nNOS also participates in the regulation of vascular tone, especially for the regulation of vascular tone in skeletal muscles. Altered nNOS activity and protein levels contribute to muscular damage due to sustained vasoconstriction in patients with Duchenne muscular dystrophy[17,18] and endothelial dysfunction in dogs with Duchenne muscular dystrophy[19].
Prostacyclin (also called PGI2) is generated from arachidonic acid by cyclooxygenase (COX) in endothelial cells. Activation of IP receptors by PGI2 activates adenylate cyclase to synthesize cyclic adenosine monophosphate (cAMP) from adenosine triphosphate, causing vascular smooth muscle relaxation. However, PGI2 synthesis can be inactivated by increased cytokines[20] and under certain conditions, PGI2 exerts vasoconstrictor action and contributes to endothelial dysfunction[21].
The term “EDHF” describes a set of substances causing vascular myocyte hyperpolarization and spreading endothelial hyperpolarization to vascular myocytes, resulting in vascular myocyte relaxation, which is not affected by blocking NO or prostacyclin production[22]. Interestingly, in eNOS/COX-1 double-knockout mice, EDHF-mediated vasodilation plays a compensatory role for the absence of endothelial NO[23]. EDHF hyperpolarize myocyte membranes by opening calcium-activated potassium channels named as BKca, IKca, and SKca according to their conductance (big, intermediate, and small conductance), leading to K+ efflux[24-26]. EDHF-mediated vasodilation also involves epoxyeicosatrienoic acids (EETs), gap junction, reactive oxygen species and hydrogen peroxide, depending on the vascular beds and vessel types[24,25,27]. Cytochrome P450 epoxygenase catalyzes the production of EETs from arachidonic acid in different vessels and EETs participate in, at least partly, the hyperpolarization and relaxation of myocytes in these vessels[28,29]. It is worth noting that EDHF-mediated vasodilation is a complex phenomenon, which involves multiple signaling pathways that may be not exclusive in response to different stimuli[24-26]. Altered EDHF signalings may account for endothelial dysfunction in some cardiovascular disorders as suggested by studies in animals. For example, a defect in connexins that compose gap junctions is partly responsible for impaired vasodilator responses in hypertensive rats[30] and in diabetic rats[31]. However, the role of EDHF in endothelial dysfunction in human cardiovascular diseases remains elusive. This may be related to the difficulty that the function of EDHF can only be deciphered after impairment of NO and prostacyclin-mediated responses.
Kinins such as bradykinin can be generated in vessel walls, especially in endothelial cells that contain the components such as kinin precursors and kinin-generating enzymes, necessary for the production of kinins[32]. The biological effects of kinins are mediated by stimulation of constitutively expressed B2 receptors and inducible B1 receptors. In endothelial cells, activation of B2 receptors by bradykinin releases NO, prostacyclin, EDHF and tissue plasminogen activator, which exert diverse physiological and pathological actions on cardiovascular system, including regulation of coronary vascular tone and local blood flow of organs, coagulation, fibrinolysis, and water-electrolyte[33,34]. Stimulation of B1 receptors by its agonists also induce NO-mediated vasodilation[35]. Due to very short half-life in the blood, bradykinin essentially plays an autocrine/paracrine role. Experimental studies have demonstrated a protective role of bradykinin B2 receptors on cardiovascular function. It is, at least in part, due to opposing effects of bradykinin B2 receptor activation on angiotensin II AT1 receptor activation because of multiple cross-talks between the kallikrein-kinin system and the renin-angiotensin system[36]. This explains the contribution of kinins to the cardiovascular protective effects of angiotensin-converting (ACE) inhibitors and angiotensin AT1 receptor blockers. Deletion of both B1 and B2 receptors in diabetic mice exacerbates nephropathy as indicated by increased oxidative stress, mitochondrial DNA deletions and renal expression of fibrogenic genes, suggesting a protective role of the kallikrein-kinin system on diabetic nephropathy[37]. However, these mice exhibit neither accelerated cardiac dysfunction nor ROS production, challenging the protective role of kinins in this setting[38]. Otherwise, kinins can increase endothelial permeability[39] and are involved in inflammatory responses by activating phospholipase A2 to release arachidonic acid that is used for the production of vasoconstrictor prostanoids, which may be harmful for endothelial function.
Adrenomedullin (AM), a vasodilator peptide initially identified from human pheochromocytoma, can be secreted by vascular cells, especially by endothelial cells[40-42]. AM exerts its action in the cardiovascular system through receptor complexes composed of the calcitonin receptor-like receptor and receptor activity-modifying proteins. In vessels, the receptors for AM are expressed in both endothelial and smooth muscle cells[43,44]. AM-induces endothelium-dependent and - independent vasodilation, depending upon species and vascular beds[42]. AM-induced endothelium-dependent vasodilation is mediated by PI3K/Art/NO/cGMP pathway, the activation of cGMP-stimulated protein kinase G and/or the production of a vasodilator prostanoid (likely prostacyclin)[42,45,46], whereas AM-induced endothelium-independent vasodilation involves the opening of K+ channels (calcium-activated K+ channels or ATP sensitive K+ channels, probably depending upon vascular beds) and the activation of cAMP-dependent protein kinase A[42,47]. In addition to vasodilator effect, AM was shown to inhibit angiotensin II-induced ROS generation by NAPDH[48], and AM -deficient mice developed insulin resistance due to increased ROS[49]. AM protects bone marrow-derived mononuclear cell and endothelial progenitor cells from apoptosis and exerts a vascular protective role[50,51]. AM also exerts an protective effect on endothelial barrier function and reduces endothelial permeability in response to inflammation and endotoxin[52-56]. Otherwise, AM posses angiogenesis property and participates in vascular remodeling [50,57,58]. Plasma AM levels were shown to be higher in many pathological situations such as arteriosclerosis, sepsis, essential or pulmonary hypertension and heart failure[52,59-63], whereas intracoronary AM levels were lower in patients with stable coronary disease[64] and in infants with brain damage after surgery under cardiopulmonary bypass[65]. Increased AM levels were interpreted as a compensatory mechanism to protect cardiac and vascular function[61,66]. Expression of receptors for AM was shown to be increased in rats with heart failure though its signification remains elusive[67,68]. Despite its protective role in the cardiovascular system, AM was shown to be involved in the growth of different tumors such as prostate, colorectal and bladder tumors, and AM and its receptors are potential targets for the treatment of these tumors[69-71].
Endothelial cells express ACE and angiotensin AT1 and AT2 receptors. Once released, angiotensin II immediately binds to these receptors and those expressed on smooth muscle cells. Although angiotensin II causes both vasoconstriction via AT1 receptors and vasorelaxation by stimulating AT2 receptors, angiotensin II-induced vasoconstriction is predominant in many circumstances. Moreover, angiotensin II exerts multiple actions affecting endothelial function. Angiotensin II upregulates endothelial receptors for OxLDL, stimulates OxLDL uptake, and enhances OxLDL-mediated ROS generation and endothelial cell apoptosis[72]. Angiotensin II increases receptors for vascular endothelial growth factors and matrix metalloproteinases (MMPs), which may account for increases in endothelial permeability and vascular remodeling[73-75]. Angiotensin II increases the expression of plasminogen activator inhibitor type 1 (a natural inhibitor of tissue-type plasminogen activator and urokinase-type plasminogen activator) in endothelial cells[76], thereby favoring thrombosis. Angiotensin II favors inflammation by inducing COX-2 expression[74] and increasing cytokine tumor necrosis factor-alpha (TNF-α)[75]. Although angiotensin II can upregulate eNOS and inducible NO synthase (iNOS) expression, it reduces eNOS-derived NO by promoting eNOS uncoupling through monocyte-dependent S-glutathionylation[77]. In addition, the activation of AT2 receptors also contributes to the angiotensin II-induced vascular remodeling[78]. These actions of angiotensin II may contribute to endothelial dysfunction as the renin-angiotensin system is activated, as is the case in atherosclerosis[79] and heart failure.
Although endothelin-1 can upregulate eNOS expression by enhancing eNOS mRNA stability via protein tyrosine kinases and protein kinase C-dependent pathways[80,81], endothelin-1 via type A endothelin receptors induces expression of adhesion molecules and neutrophil adhesion to endothelial cells, and promotes cytokine and ROS generation[82-84]. Elevated endothelin-1 blood levels can be seen in atherosclerosis[85], pulmonary hypertension[86] and heart failure[87,88], which may account for the development of endothelial dysfunction under these circumstances. Although the activation of Type B endothelin receptors generally induces vasodilation, these receptors appear to mediate endothelin-1-induced ROS production and contribute to endothelial dysfunction in obese rats[82].
Endothelial cells can produce vasoconstrictor prostaglandin H2 (PGH2), thromboxane A2 (TXA2) and PGF2α. The production of these prostanoids is enhanced in hypertension, hypercholesterolemia, diabetes and vitamin E deficiency[89], which in turn upregulates NAPDH oxidase and type 4 and type 5 phosphodiesterases (PDE4 and PDE5)[90,91], resulting in increases in ROS production, cAMP and cGMP degradation, and vasoconstriction. TXA2 is also a potent activator of platelets, while activated platelets in turn release a large amount of TXA2 to promote thrombosis. Furthermore, TXA2 interacts with EDHF by inhibiting potassium channels, EETs and gap junction-mediated signaling pathways[27], which could account for the development of endothelial dysfunction.
Clinically, endothelial dysfunction is characterized by impaired endothelium-dependent vasorelaxation in response to endothelium-dependent agonists such as acetylcholine and bradykinin, or to maneuvers that increase shear stress such as flow-mediated dilatation. Although mechanisms leading to endothelial dysfunction are multiple, a reduction in NO bioavailability is largely observed in many cardiovascular disorders. As shown in Figure 1, reduced NO bioavailability can be the consequence of decreased L-arginine availability[92], increased ADMA[1,2], altered interaction with hsp90[93] and phosphorylation of eNOS[94], as well as increased NO scavenging by excessive ROS generated by NADPH and xanthine oxidases[10,11,95] and eNOS uncoupling. It is worth noting that changes in caveolin-1[96], BH4[97], S-glutathionylation of eNOS[15,98] and OxLDL[12] are all involved in eNOS uncoupling. Importantly, a reduction in eNOS protein expression also leads to impaired eNOS activity and NO production, which can be observed in different cardiovascular diseases such as atherosclerosis, acute myocardial infarction and heart failure in animals and in humans[99-103]. However, mechanisms underlying endothelial dysfunction in different cardiovascular disorders may be different, depending on risk factors contributing to the development of specific disease.
Atherosclerosis is a chronic arterial disease involving the formation of multiple atheromatous plaques within arteries by accumulation of lipids due to the inability to remove LDL from macrophages. In this process, endothelial dysfunction related to hypercholesterolemia plays a pivotal role in the development of atherosclerosis. Hypercholesterolemia induces endothelial cell activation, leukocyte recruitment and adherence, platelet activation and adhesion within the vasculature, reflecting an inflammatory response and high thrombotic state that may cause endothelial dysfunction. Hypercholesterolemia increases superoxide and hydrogen peroxide production by increasing NAD(P)H oxidases[79], xanthine oxidase[95], and myeloperoxidase[104]. Increased superoxide reacts with NO, resulting in the formation of RNS and reduced eNOS-derived NO bioavailability. ROS induce oxidation of lipids, proteins and DNA, which cause cell damage, necrosis and cell apoptosis. Increased RNS induce nitrosylation reactions that modify the structure and function of proteins. Hypercholesterolemia increases caveolin-1 levels[105], also contributing to impaired eNOS activity. In addition, hypercholesterolemia disturbs reactions between oxygen radicals or enzymatic oxidation and lipoproteins, particularly LDL phospholipids and results in the production of oxidized phospholipids. These phospholipids contain arachidonic acid and bind to their membrane receptors, resulting in their accumulation within the cellular membrane, immune and inflammatory responses and ROS generation, which in turn induces eNOS uncoupling that impairs endothelium-dependent vasodilation induced by endogenous vasodilator such as kinins but enhances the role of endogenous vasoconstrictors such as angiotensin II and endothelin-1, and promote endothelial dysfunction[106,107]. Therefore, endogenous vasoactive substances such as NO, prostanoids, ROS, RNS, AM, angiotensin II, endothelin-1 and other substances interact and reduced NO bioavailability due to eNOS uncoupling is a key event contributing to the development of endothelial dysfunction and ultimately atherosclerosis.
Homocysteine is a non-protein α-amino acid synthesized from methionine. Hyperhomocysteinemia is observed in patients with coronary disease and is correlated with endothelial dysfunction[108]. Homocysteine causes endothelial dysfunction through NO inhibition, vasoconstrictor prostanoid production, EDHF inhibition[109,110], angiotensin AT1 receptor activation, and ROS generation[111]. Homocysteine reduces eNOS activity by increasing asymmetric dimethylarginine production[112] and eNOS uncoupling via decreasing intracellular do novel synthesis of BH4[97], leading to decreased NO bioavailability and increased ROS generation. Furthermore, homocysteine downregulates eNOS expression in human endothelial cells[113], and induces endothelial loss, vascular deendothelialization and increases platelet adherence and consumption in baboons[114]. Homocysteine also increases ROS generation by phosphorylating NAPDH oxidase[115], and/or by increasing ACE activity via ACE homocysteinylation to generate angiotensin II that activates NAPDH oxidase[116].
Endothelial dysfunction is associated with both insulin-dependent and independent diabetes mellitus. In this setting, hyperglycemia increases ROS generation through activating protein kinase C-mediated NAD(P)H oxidases[117] and peroxynitrite-mediated eNOS uncoupling[118], which also leads to reduced NO bioavailability. In addition, hyperglycemia increases iNOS expression and iNOS-derived NO and peroxynitrite production, leading to increased ROS and RNS levels and pancreatic islet endothelial cell apoptosis[119]. Moreover, hyperglycemia promotes platelet aggregation by increasing expression and circulating levels of endothelial adhesion molecules through protein kinase C-NFκB signaling pathway[120-122], and increases endothelial apoptosis[123]. All of these effects of hyperglycemia contribute to endothelial dysfunction observed in diabetes mellitus. Otherwise, increased release of vasoconstrictors such as prostanoids and endothelin-1 through protein kinase C-mediated pathway in response to hyperinsulinemia and hyperglycemia appears to precede changes in vascular complication or NO production[124,125]. Changes in EDHF also contribute to endothelial dysfunction, especially in type 2 diabetes as suggested in rat models in which an impaired EDHF-mediated vasorelaxation was observed before marked alteration in NO-mediated responses[126-129]. Therefore, altered NO bioavailability in type 2 diabetes appears to be a relatively late event worsening endothelial dysfunction.
An impaired endothelium-dependent vasorelaxation has been observed in patients with essential hypertension[130] and in several animal models of hypertension. This is related to a lower production of endothelium-derived vasodilators and/or over production of vasoconstrictors. An increased endothelin-1 production also plays a role in endothelial function, especially in pulmonary hypertension as lung is an important metabolic organ of circulating peptides such as adrenomodullin and endothelin-1. In this regard, pulmonary endothelin-1 extraction affects the incremental resistance of pulmonary vascular bed in response to increased cardiac work[131] and plasma endothelin-1 levels are closely related to clinical worsening of patients with pulmonary hypertension[132]. An impaired NO and EDHF-mediated vasorelaxation linked to an increased ADMA that inhibit eNOS and downregulates SKca in endothelial cells has been reported in hypertensive patients and in spontaneous hypertensive rats[133]. Interestingly, alterations in EDHF appears to occur before alterations in NO pathways in different rat models of hypertension[134]. An reduced vasodilator response to AM has been observed in hypertensive patients[45]. However, an increased eNOS expression is generally observed in animal models of hypertension associated with angiotensin II. In this case, angiotensin II-induced oxidative stress and increases in the production of vasoconstrictor prostanoids and cytokines may account for the development of endothelial dysfunction. Furthermore, a reduced NO bioavailability has been reported in some models of hypertension. This appears to be linked to reduced substrate availability due to L-arginine deficiency and changed L-arginine transport[92] and to eNOS uncoupling due to oxidation of BH4 and/or S-glutathionylation, leading to increased ROS production[4,15]. Thus, although altered NO bioavailability may not be an initial event to induce endothelial dysfunction, it participates in its progression in hypertensive subjects.
Endothelial dysfunction is one of the primary damages induced by cigarette smoke. Circulating cigarette toxins such as free radicals and reactive glycation products can react with endothelial cells and cause vascular impairment[135]. Cigarette smoking induces inflammatory state as indicated by elevation of white blood cells, adhesion molecules and cytokines, and increases ROS production and lipid peroxidation[136-141]. These mechanisms may contribute to impaired endothelium-dependent vasodilation observed in active smokers, even at young healthy adult, and in passive smokers[142,143]. However, despite a reduced NO bioavailability, eNOS expression has been shown to be increased in different endothelial cells or decreased in platelets in response to cigarette smoke[144,145]. Cigarette smoke extracts inhibits eNOS activity of pulmonary arterial endothelial cells through modifying eNOS phosphorylation pattern, which cannot be protected by antioxidants such as vitamin E and C[146,147]. In this setting, decreased NO bioavailability is probably the consequence of decreased eNOS activity due to modified eNOS phosphorylation and uncoupling as well as NO scavenging by increased ROS.
Endothelial cells produce inflammatory and immune mediators (Table 1) and undergo morphological modifications in response to inflammatory stimuli. The inflammatory and immune mediators increase endothelial permeability and promote adhesion of leukocyte to endothelial cells and interactions between chemokine receptors on leukocyte and proteoglycans on endothelial cells, leading to leukocyte transendothelial migration to inflammation sites. Inflammation induced endothelial dysfunction is often associated with impaired NO bioavailability. For example, typhoid vaccination induced an inflammatory response as indicated by increased cytokines and oxidative stress as well as a decreased endothelium-dependent vasodilation that was partially restored by antioxidant vitamin C[148]. In patients with viral myocarditis, acetylcholine induced a coronary vasoconstriction rather vasodilation[149]. Similar responses were also observed in mice with virus-induced myocarditis, which was attributed to reduced eNOS activity and expression[150]. In some autoimmune diseases, anti-endothelial antibodies cause abnormal immune activation that activates endothelial cells to release adhesion molecules and cytokines, leading to inflammation, increased permeability of the endothelium, thrombosis and cell apoptosis[151-153], which are, at least in part, responsible for endothelial dysfunction in this setting. Patients with rheumatoid arthritis have increased levels of cytokines and ADMA and impaired flow-mediated dilation[154]. Similarly, increased arterial stiffness is closely correlated with ADMA blood levels in systemic lupus erythematosus patients[155]. The increase in ADMA levels may account for reduced NO bioavailability in these autoimmune diseases.
In some cases, an over production of NO occurs in response to inflammation. Septic shock associated with a severe infection and sepsis is characterized by a profound hypotension, widespread endothelial injury and activation, multiple organ failure and death. In this setting, toxic microbe products, including endotoxins (bacterial membrane lipopolysaccharides, LPS) of gram-negative bacteria and analogous molecules in the walls of gram-positive bacteria and fungi, dramatically activate mononuclear cells to release cytokines[156] that upregulate bradykinin B1 receptors[157,158], inducible NO synthase[159] and COX-2[160], which increase NO and prostaglandin E2. In this regard, blocking or deleting bradykinin B1 receptors might yield benefits for the treatment of septic shock. However, experimental studies showed conflicting results regarding the role of kinins in septic shock in animals. Mice with overexpression of B1 receptors exhibited an increased susceptibility to develop septic shock and mice lacking B1 receptors or both B1 and B2 receptors had an enhanced resistance to LPS-induced sepsis[161-163], whereas mice lacking B1 receptors had an higher mortality in response to LPS[164] and additional B1 receptor blockade suppressed the beneficial effect of B2 receptor blockade[165]. Similarly, B2 receptor blockade showed no effect or amelioration in porcine sepsis[165,166]. Results regarding the role of NO, particularly iNOS in septic shock are also elusive. Experiments in rats and in human blood cells showed that iNOS expression is correlated with cell apoptosis in septic shock[167,168]. Selective iNOS inhibition improved hemodynamics and mortality in nondiabetic rats with LPS-induced sepsis but not in diabetic rats[169], whereas depletion of iNOS resulted in increased dysfunctional mitochondria, IL-1β production and caspase-1 activation in response to LPS in myeloid cells from both mice and humans and increased NLRP3 inflammasome-mediated cytokine production and mortality in mice with LPS-induced sepsis, which was prevented by NLRP3 deficiency[170]. Although treatment with methylene blue that has the ability to scavenge NO and to inhibit NO synthase showed a transient and reproducible beneficial effect on systemic vascular resistance, arterial pressure and organ function in patients with septic shock, but its effect on mortality remains unknown[171,172].
Aging is accompanied by complex structural and functional modifications of the vasculature, leading to dysfunction of both the endothelium and smooth muscle cells. Changes in aged smooth muscle cells are characterized by changed migration, proliferative and apoptotic behavior, increased response to vasoconstrictors and decreased expression of Ca2+-activated K+ channels in coronary arteries[173,174]. Aged endothelial cells are associated with decreased NO synthesis and sensitivity to agonist and mechanic stimuli that promote eNOS expression but increased sensitivity to be apoptotic[175,176]. Loss of PI3K/Akt-dependent eNOS phosphorylation seems to be a main mechanism explaining the reduction in NO production in old rats[94]. In addition, aging of endothelial cells is associated with increased production of vasoconstrictor prostanoids, endothelin-1 and ROS[176-178]. ROS are mainly produced by mitochondrial respiratory chain and NADPH oxidases, although eNOS uncoupling my also contribute to increased ROS during aging[179].
In animals, endothelial dysfunction can be measured by examining vasodilator responses to endothelium-dependent substances such as acetylcholine, bradykinin and serotonin in comparison with responses to endothelium-independent molecules such as NO donor in the absence and presence of NOS inhibitor and COX inhibitor in vivo[180,181] and in isolated vessels[19,182].
The methods used in clinical practice to measure endothelial dysfunction are detailed elsewhere[183]. This includes invasive methods by using quantitative angiography and intracoronary Doppler wire within coronary circulation and non-invasive methods, including venous occlusion plethysmography to measure forearm blood flow, flow-mediated dilatation in brachial artery, and peripheral arterial tonometry measuring pulsatile volume changes in the distal digit[183].
In addition, some circulating biomarkers such as endothelin-1, E-selectin, von Willebrand factor, thrombomodulin, intercellular adhesion molecules and vascular cell adhesion molecules can also be analyzed to detect endothelial dysfunction, although none of them are specific[183].
Experimental and clinical studies have shown that numerous currently used or investigational drugs can improve endothelial function, although they have different structure and mechanisms of actions.
Since the success of ACE inhibitors in the treatment of heart failure and discovery of their multiple actions, ACE inhibitors and AT1 blockers are widely used to the treatment of hypertension, arthrosclerosis, diabetes and some autoimmune diseases. It is well established that ACE inhibitors can improve endothelial function in animals with heart failure[184] and in patients with coronary artery disease[185,186]. This effect is related to both reduction in angiotensin II and increase in bradykinin accumulation. In addition, ACE inhibitors upregulate eNOS expression in animals[102,187]. The effect of ACE inhibitors on eNOS expression is mediated by bradykinin B2 receptors, which can be blocked by B2 receptors blockers[102,187]. ACE inhibitors and AT1 blockers also inhibit ROS production and COX-2-derived vasoconstrictors, which contribute to endothelial protective effects of these drugs[188]. It appears that the combination of both ACE inhibitor and AT1 blocker does not produce more beneficial effects on endothelial dysfunction than monotherapy in a murine model of atheroclerosis[189], whereas the combination of a stain with an ACE inhibitor or an AT1 blocker produces additive effects on systemic inflammation biomarkers[190]. Also, the combination of ramilapril with felodipine, an calcium channel blocker does not induce more effect on endothelium-dependent vasodilation than each drug alone but increases endothelium-independent vasodilation in spontaneous hypertensive rats[191].
Several substances having very different molecular structure and proprieties, such as vitamin C and E, N-acetylcysteine and genistein exert antioxidant effects through different mechanisms.
Vitamin C can improve endothelium-dependent response in circumstances such as chronic smoking, diabetes mellitus, hypercholesterolemia and hypertension[136,192-195]. Vitamin C protects the endothelium by scavenging superoxide, which in turn prevents NO scavenging, lipid peroxidation, platelet and neutrophil activation, and adhesion molecule upregulation[136,196]. Vitamin C scavenges peroxidase-generated reactive nitrogen species and inhibits myeloperoxidase/H2O2/nitrite-mediated LDL oxidation[197]. Vitamin E also exerts endothelial-protective effects in smoking and hypercholesterolemia[194,198] but its effects in diabetes remains controversial[199,200]. Vitamin E acts as a lipid soluble antioxidant, scavenging hydroperoxyl radicals in lipid milieu[201].
N-acetylcysteine is a non-essential amino acid, essentially used in the treatment of cough. However, experimental studies have demonstrated that N-acetylcysteine is a potent antioxidant. It acts on the production of glutathione, which protects the cardiovascular system from harmful effects of TNF-α that induces glutathione depletion and ROS production via NADPH oxidase and ceramide[202-206]. For example, N-acetylcysteine improves coronary and peripheral vascular endothelium-dependent responses in patients with or without atherosclerosis[203]. The effect of N-acetylcysteine on endothelial dysfunction is associated with inhibition of NADPH oxidase expression, leukocyte adhesion and inflammatory cytokine secretion[204]. In addition, N-acetylcysteine inhibits von Willebrand factor dependent platelet aggregation and collagen binding in human plasma and in mice[207], attenuates MMPs expression in microvascular endothelial cells and in rats[202,208], and inhibits caveolin-1 upregulation and improves endothelial barrier function in mice[209], which may also contribute to the endothelial protective effect of N-acetylcysteine. N-acetylcysteine interacts with endogenous and exogeneous vasodilators. For example, in patients with systemic sclerosis, N-acetylcysteine induces vasodilation in association with a reduction in plasma AM concentrations[210] and potentiates hypotensive effects of ACE inhibitors in hypertensive patients[211].
Genistein is a soya-derived phytoestrogen and exerts an antioxidant effect. Genistein attenuates endothelial dysfunction in hypertensive rats and hyperhomocysteinemic rats. This endothelial protective effect appears to be due to increases in eNOS activity and expression and decreases in cytokine and ROS generation[212-215]. Genistein also improves endothelium-dependent vasodilator response in healthy postmenopausal women, increases plasma nitrite/nitrate concentration but decreases plasma endothelin-1 levels[216]. In this regard, genistein may be useful for the treatment of endothelial dysfunction associated with atherosclerosis and hypertension.
Some beta blockers, particularly the β1-selective beta blockers exert endothelial protective effects. Nebivolol, a β1-antagonist with β2,3-agonist property, improves endothelium-dependent vasodilator responses in patients with essential hypertension[217,218] and in smokers[219]. Nebivolol also improves endothelial function, which is associated with reduced vascular remodeling and expression of endothelin-1 and cytokines in rats with pulmonary hypertension and in endothelial cells taken from these rats[220]. The effect of Nebivolol on endothelial function appears to be mediated by increasing NO release and reducing prothrombotic blood levels of fibrinogen, homocysteine and plasminogen activator inhibitor-1, especially in smokers[218,219]. Carvedilol, a non-selective β1- and β2 antagonist with α-antagonist property, also improves endothelium-dependent responses in patients with essential hypertension but this seems to be related to its antioxidant capacity[218]. The combination of carvedilol with an ACE inhibitor produces more beneficial effect on endothelial function than each drug alone in hypertensive patients with obesity[221]. Thus, this type of beta blockers and its combination are suitable for the treatment of endothelial dysfunction associated with hypertension, atherosclerosis, and probably diabetes.
Nicardipine and nifedipine protect against ROS-induced endothelial cell death and lose of glutathione in cultured cells[222]. Benidipine exerts an endothelial protective effect against OxLDL induced ROX generation in human endothelial cells[223]. Israpidine improves endothelial function in cholesterol-fed rabbit[224]. Thus, the endothelial protective effect of dihydropyridine calcium channel blockers is mainly mediated by their antioxidant actions related to reduction in lipid peroxidation and associated ROS generation[222,225]. In addition, some dihydropyridines such as, amlodipine, azelnidipine and nifedipine were shown to exert an antiinflammatory action as indicated by decreased C-reactive protein and interleukin-6 levels as well as leucocyte activation[226,227]. Amlodipine or in combination with an renin inhibitor improves endothelial dysfunction in hypertensive patients, which seems to be linked to its NO-releasing action and anti-inflammatory effect[181,228-230]. In addition, the combination of amlodipine with a statin induces more favorable vascular effects than each drug alone in rats with hypertension or diabetes[231,232]. Thus, in addition to hypertension, dihydropyridines may also be useful for the treatment of endothelial dysfunction in diabetes.
Phosphodiesterase-5 (PDE5) is a cytosolic enzyme localized in vascular smooth muscle, heart, skeletal muscle, platelet, placenta, brain, kidney, liver, pancreas, gastrointestinal tissues and lung[233]. In vasculature, the primary action of PDE5 is to degrade cGMP and thereby induces vasoconstriction. PDE5 inhibitors are a class of drugs used to improve erectile dysfunction. These drugs block PDE5-induced cGMP degradation, leading to tissue cGMP accumulation and vasodilation[234]. PDE5 inhibitors upregulate eNOS expression and thereby increase NO release[235,236], which may contribute to long-term vasodilator effects of PDE5 inhibitors. PDE5 inhibitors also exert other initially unexpected effects. For example, in mouse hind limb ischemia model, treatment with sildenafil not only improves blood flow recovery but also increases capillary density and endothelial progenitor cell mobilization[237]. In patients with vasculogenic erectile dysfunction, daily treatment with vardenafil reduces both arterial stiffness and plasma AM level[238]. These effects may also account for the effects of chronic PDE5 inhibition. In addition to erectile dysfunction, PDE5 inhibitors can improve endothelial dysfunction in other circumstances. For example, PDE5 inhibition improves coronary and peripheral vascular endothelial function, and inhibits platelet activation in patients with coronary artery disease[239] or with congestive heart failure[240-242], and improves endothelium-dependent vasorelaxation in rats with experimental diabetes mellitus[243]. PDE5 inhibitors also improves erectile function in patients with systemic sclerosis and reduces plasma endothelin-1 concentration[244]. Similarly, PDE5 inhibitors improve Raynaud’s phenomenon characterized by reduced blood flow to fingers and toes in response to cold and stress, probably through decreasing plasma endothelin-1 and improving microcirculation[245]. However, the mechanism underlying endothelin-1-reducing effect of PDE5 inhibitors remains to be determined.
Statins, inhibitors of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase are a class of drugs utilized to reduce hypercholesterolemia, especially LDL cholesterol. In 1994, pravastatin was shown to improve endothelium-dependent response of coronary and peripheral arteries in patients with hypercholesterolemia[246], which was confirmed later by other studies[247]. The beneficial effect of statins on endothelial function involves multiple mechanisms. Statins improving endothelial dysfunction is partly due to their lowering LDL cholesterol effect, while native LDL and OxLDL reduce eNOS expression[248,249] and increase levels of caveolin-1[250]. Statins also exert direct antioxidant effects on LDL to reduce electronegative form of LDL[251,252]. Statins increase NO bioavailability by activating eNOS via the PI3K/Akt signaling pathway[253], agonist-stimulated eNOS-hsp90 interaction[250], and BH4-mediated eNOS coupling. This latter was demonstrated in patients with atherosclerosis[254] and in rat model of insulin resistance of diabetes[231]. These studies showed that atorvastatin increased vascular BH4 content and NO bioavailability and reduced O2- production via upregulating GTP-cyclohydrolase I gene expression and activity. These effects occurred rapidly in patients with atherosclerosis and could be reversed by mevalonate, indicating a direct effect of vascular HMG-CoA reductase inhibition[254]. In addition, statins upregulate eNOS expression through enhancing eNOS mRNA stability. Indeed, statins increase eNOS mRNA polyadenylation through Rho-mediated changes in the actin cytoskeleton[255,256]. However, a study showed that statins can increase eNOS gene transcription by upregulating Kruppel-like factor 2 through inhibition of Rho pathway[257]. The effect of statins on eNOS expression may account for the long-term effect of stains on endothelial function. Statins also exerts antiinflammatory effects[258]. For example, atorvastatin treatment reduces proinflammatory cytokines (TNF-α, IL-1 and IL-6), intercellular adhesion molecules and C-reactive protein blood levels in hypercholesterolemic patients[259], while rapid withdrawal of statin treatment increases proinflammatory and prothrombotic biomarkers[260]. Statins were also shown vascular benefice in other inflammatory diseases such as rheumatoid arthritis[261]. Otherwise, statins increase circulating endothelial progenitor cells, likely through the PI3K/Akt pathway[262], which could contribute to long-term effects of statins on endothelial function.
Another type of LDL-lowering drugs, the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, may be expected to improve endothelial function. In humans, PCSK9 mutation is closely correlated with LDL cholesterol levels and inhibition of PSCK9 with a monoclonal antibody reduces LDL cholesterol levels[263] and enhances the LDL cholesterol-lowering effect of atorvastatin[264]. Studies in cells and animals have shown that PCSK9 is associated with inflammation and endothelial cell apoptosis. In mice, systolic inflammation and OxLDL upregulate PCSK9, whereas PCSK9 interacts with macrophage, leading to NF-κB activation[265,266]. Knockdown of PCSK9 with PCSK9 siRNA or induction of gain of function mutant D374Y-PCSK9 reduces expression of stress-response genes and specific inflammation pathways, inflammation pathway activation and OxLDL-induced endothelial apoptosis[266-268]. Nonetheless, the effects of PCSK9 inhibition on human endothelial function are not yet explored.
Angiotensin-(1-7) is a metabolite of angiotensin I under the action of various enzymes, including neutral endopeptidase, prolylendopeptidase, aminopeptidase A and neprilysin[36]. It can also be generated from angiotensin II by prolylcarboxypeptidase[269] and carboxypeptidase (ACE2)[270]. In endothelial cells, angiotensin-(1-7) activates eNOS via the Mas/PI3K/Akt pathway and inhibits angiotensin II-induced NAD(P)H oxidase activation[271,272]. Chronic treatment with angiotensin-(1-7) improves renal endothelial dysfunction associated with apolipoprotein E-deficiency[273] and diet-induced obesity in mice[274], which is likely mediated by increasing NO release[275] and eNOS expression[276,277]. Otherwise, angiotensin-(1-7) restores vascular ACE2-angiotensin-(1-7)-Mas receptor axis function that impairs ROS production by angiotensin AT1 receptor-activated NAD(P)H oxidases in hypertensive or diabetic rats[278,279]. Angiotensin-(1-7) restores NO/cGMP production and migration, decreases NADPH oxidase activity, and enhances survival and proliferation of endothelial progenitor cells isolated from the blood of diabetic patients in a Mas/PI3K/Akt-dependent manner[280]. Interestingly, overexpression of angiotensin-(1-7) gene restores the vasoreparative function of endothelial progenitor cells in mice[280]. Despite these encouraging results in cells and in animals, the information regarding the effects of angiotensin-(1-7) on human endothelial function remains lacking.
As discussed above, endogenous bradykinin exerts multiple actions that affect endothelial function. It is worth noting that bradykinin as an investigational drug protects against ROS- and toxin-induced microvascular endothelial cell death[281], and chronic treatment with bradykinin not only preserves eNOS expression in dogs with pacing-induced heart failure[101], but also upregulates eNOS and nNOS expression in vessels and in the heart of dogs with dystrophin-deficiency cardiomyopathy[19,282]. However, due to the very short half-life and implication of bradykinin in the inflammation[283] and cancers[284,285], the clinical use of bradykinin remains a challenge.
Interestingly, specific targeting eNOS transcription with a chemical compound, AVE3085, increases eNOS expression but reduces oxidative stress and platelet activation, which is associated with improved endothelium-dependent relaxation and cardiac function in animals with different experimental diseases[286-289]. This compound also prevents the inhibitory effect of ADMA on endothelium-dependent vasodilation in human internal thoracic artery rings and in pig coronary artery rings[290,291]. Thus, this compound showed a potential for the treatment of endothelial dysfunction although its effects in human clinical situations remain to be demonstrated.
If current is an inward current carried by Na+ and K+, activated by hyperpolarization and conducted by hyperpolarization-activated cyclic nucleotide-gated channels (f-channels)[292]. If current participates in the spontaneous depolarization during Phase 4 of the action potential and plays a crucial role in the pacemaker activity of pacemaker cells located in the sinus node and atrioventricular node. Inhibition of this current by ivabradine slows down heart rate and exerts cardioprotective effects[293-296], which may involves pleiotropic actions of ivabradine[297]. Among them, beneficial effects of ivabradine on the endothelium-dependent vasodilation and on the expression of eNOS expression in both animals and humans have been reported[298-300]. Nevertheless, the effects of ivabradine on human endothelial dysfunction are controversial. Several studies did not observe significant improvement in flow-mediated vasodilation by ivabradine in patients with microvascular angina pectoris[301] or stable coronary heart disease[302] and in patients with type II diabetes[303]. In addition, in patients with stable of coronary disease without heart failure, the additional ivabradine plus standard treatment did not improve outcome but was associated with increased frequency of atrial fibrillation, questioning the utility of this drug in the treatment of stable coronary disease[304].
Sphingosine-1-phosphate (S1P), a signaling sphingolipid formed by sphingosine kinase in the blood and in tissues, regulates different biological responses such as angiogenesis, vascular permeability and trafficking of T- and B-cells. S1P enhances endothelial barrier function[305,306], stimulates endothelial NO release through Akt-mediated phosphorylation of eNOS[307], and reconstitutes high density lipoproteins[308]. S1P also has antiinflammatory properties and exerts protective effect against endotoxin-induced lung injury[309,310]. Moreover, S1P exhibits a potent effect on the differentiation of adipose-derived stem cells into endothelial-like cells and upregulation of eNOS in these cells[311]. All of these properties of S1P may contribute to its endothelial protective effects. Interestingly, an orally active of S1P analogue, FTY720 also shows similar effects[312]. Thus, S1P and analogues may be used to improve endothelial function, especially in atherosclerosis and acute lung injury where presents an impairment of endothelial barrier function[313].
Endothelial dysfunction is a common mechanism involved in many cardiovascular diseases, although in some diseases such as atherosclerosis, endothelial dysfunction plays a critical role in the development of diseases, whereas in others such as essential hypertension and type II diabetes, endothelial dysfunction generally occurs as a complication but thereafter contributes to the development and progression of organ damages. Clearly, multiple mechanisms such as inflammation, increased ROS and RNS, cellular apoptosis, increased vasoconstrictor production, decreased vasodilator production and vascular remodeling are involved in endothelial dysfunction and a specific pathology may involve more or less them as described above. However, a decreased NO bioavailability appears to play a central role because in many pathologies such as atherosclerosis, diabetes, essential and pulmonary hypertension and heart failure except for septic shock where there is a overproduction of NO, a reduction in NO bioavailability occurs sooner or later in response to different risk factors. This may explain the beneficial effects of some drugs in the treatment of a variety of cardiovascular disorders. It appears that a drug with endothelium-protective property may yield more therapeutic benefits than that without such feature. For this reason, the evaluation of endothelium-improving action may be helpful for the development of a novel cardiovascular drug. Moreover, due to the differences in risk factors contributing to the different cardiovascular diseases and the differences in mechanisms of action, treatment of endothelial dysfunction with drugs needs to be carried out according to specific mechanisms underlying endothelial dysfunction of the disease.
P- Reviewer: Lee TS, Letizia C, Li YL S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1547] [Cited by in RCA: 1541] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 2. | Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 826] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 3. | Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220-9225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1122] [Cited by in RCA: 1061] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 4. | Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 526] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 5. | Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522-18525. [PubMed] |
| 6. | García-Cardeña G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 772] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 7. | Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2723] [Cited by in RCA: 2749] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 8. | Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2015] [Cited by in RCA: 2066] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 9. | Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA. 2004;101:2619-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994;266:H2568-H2572. [PubMed] |
| 11. | Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1762] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 12. | Fleming I, Mohamed A, Galle J, Turchanowa L, Brandes RP, Fisslthaler B, Busse R. Oxidized low-density lipoprotein increases superoxide production by endothelial nitric oxide synthase by inhibiting PKCalpha. Cardiovasc Res. 2005;65:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Heeba G, Hassan MK, Khalifa M, Malinski T. Adverse balance of nitric oxide/peroxynitrite in the dysfunctional endothelium can be reversed by statins. J Cardiovasc Pharmacol. 2007;50:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 453] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 16. | Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 386] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 737] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 18. | Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2000;97:13818-13823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 337] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Dabiré H, Barthélémy I, Blanchard-Gutton N, Sambin L, Sampedrano CC, Gouni V, Unterfinger Y, Aguilar P, Thibaud JL, Ghaleh B. Vascular endothelial dysfunction in Duchenne muscular dystrophy is restored by bradykinin through upregulation of eNOS and nNOS. Basic Res Cardiol. 2012;107:240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Camacho M, López-Belmonte J, Vila L. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity. Circ Res. 1998;83:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Blanco-Rivero J, Cachofeiro V, Lahera V, Aras-Lopez R, Márquez-Rodas I, Salaices M, Xavier FE, Ferrer M, Balfagón G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Taylor SG, Weston AH. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988;9:272-274. [PubMed] |
| 23. | Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005;111:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Edwards G, Félétou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 286] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 25. | Félétou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond). 2009;117:139-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 370] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 27. | Ellinsworth DC, Shukla N, Fleming I, Jeremy JY. Interactions between thromboxane A₂, thromboxane/prostaglandin (TP) receptors, and endothelium-derived hyperpolarization. Cardiovasc Res. 2014;102:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Fleming I, Michaelis UR, Bredenkötter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 305] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 29. | Fisslthaler B, Michaelis UR, Randriamboavonjy V, Busse R, Fleming I. Cytochrome P450 epoxygenases and vascular tone: novel role for HMG-CoA reductase inhibitors in the regulation of CYP 2C expression. Biochim Biophys Acta. 2003;1619:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Rummery NM, Hill CE. Vascular gap junctions and implications for hypertension. Clin Exp Pharmacol Physiol. 2004;31:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Young EJ, Hill MA, Wiehler WB, Triggle CR, Reid JJ. Reduced EDHF responses and connexin activity in mesenteric arteries from the insulin-resistant obese Zucker rat. Diabetologia. 2008;51:872-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Mombouli JV, Vanhoutte PM. Kinins and endothelial control of vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1995;35:679-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 141] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Carretero OA, Scicli AG. The kallikrein-kinin system. Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE, editors. New York: Raven Press Ltd 1992; 1851-1874. |
| 34. | Su JB. Kinins and cardiovascular diseases. Curr Pharm Des. 2006;12:3423-3435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Su JB, Hoüel R, Héloire F, Barbe F, Beverelli F, Sambin L, Castaigne A, Berdeaux A, Crozatier B, Hittinger L. Stimulation of bradykinin B(1) receptors induces vasodilation in conductance and resistance coronary vessels in conscious dogs: comparison with B(2) receptor stimulation. Circulation. 2000;101:1848-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Su JB. Different cross-talk sites between the renin-angiotensin and the kallikrein-kinin systems. J Renin Angiotensin Aldosterone Syst. 2014;15:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Kakoki M, Sullivan KA, Backus C, Hayes JM, Oh SS, Hua K, Gasim AM, Tomita H, Grant R, Nossov SB. Lack of both bradykinin B1 and B2 receptors enhances nephropathy, neuropathy, and bone mineral loss in Akita diabetic mice. Proc Natl Acad Sci USA. 2010;107:10190-10195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Wende AR, Soto J, Olsen CD, Pires KM, Schell JC, Larrieu-Lahargue F, Litwin SE, Kakoki M, Takahashi N, Smithies O. Loss of bradykinin signaling does not accelerate the development of cardiac dysfunction in type 1 diabetic akita mice. Endocrinology. 2010;151:3536-3542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Côté J, Savard M, Neugebauer W, Fortin D, Lepage M, Gobeil F. Dual kinin B1 and B2 receptor activation provides enhanced blood-brain barrier permeability and anticancer drug delivery into brain tumors. Cancer Biol Ther. 2013;14:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1585] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 41. | Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, Eto T, Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 417] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 42. | Passaglia P, Gonzaga NA, Tirapelli DP, Tirapelli LF, Tirapelli CR. Pharmacological characterisation of the mechanisms underlying the relaxant effect of adrenomedullin in the rat carotid artery. J Pharm Pharmacol. 2014;66:1734-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Kamitani S, Asakawa M, Shimekake Y, Kuwasako K, Nakahara K, Sakata T. The RAMP2/CRLR complex is a functional adrenomedullin receptor in human endothelial and vascular smooth muscle cells. FEBS Lett. 1999;448:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Frayon S, Cueille C, Gnidéhou S, de Vernejoul MC, Garel JM. Dexamethasone increases RAMP1 and CRLR mRNA expressions in human vascular smooth muscle cells. Biochem Biophys Res Commun. 2000;270:1063-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Terata K, Miura H, Liu Y, Loberiza F, Gutterman DD. Human coronary arteriolar dilation to adrenomedullin: role of nitric oxide and K(+) channels. Am J Physiol Heart Circ Physiol. 2000;279:H2620-H2626. [PubMed] |
| 46. | Nishimatsu H, Suzuki E, Nagata D, Moriyama N, Satonaka H, Walsh K, Sata M, Kangawa K, Matsuo H, Goto A. Adrenomedullin induces endothelium-dependent vasorelaxation via the phosphatidylinositol 3-kinase/Akt-dependent pathway in rat aorta. Circ Res. 2001;89:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Ross GR, Yallampalli C. Endothelium-independent relaxation by adrenomedullin in pregnant rat mesenteric artery: role of cAMP-dependent protein kinase A and calcium-activated potassium channels. J Pharmacol Exp Ther. 2006;317:1269-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Yoshimoto T, Gochou N, Fukai N, Sugiyama T, Shichiri M, Hirata Y. Adrenomedullin inhibits angiotensin II-induced oxidative stress and gene expression in rat endothelial cells. Hypertens Res. 2005;28:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Shimosawa T, Ogihara T, Matsui H, Asano T, Ando K, Fujita T. Deficiency of adrenomedullin induces insulin resistance by increasing oxidative stress. Hypertension. 2003;41:1080-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Iwase T, Nagaya N, Fujii T, Itoh T, Ishibashi-Ueda H, Yamagishi M, Miyatake K, Matsumoto T, Kitamura S, Kangawa K. Adrenomedullin enhances angiogenic potency of bone marrow transplantation in a rat model of hindlimb ischemia. Circulation. 2005;111:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Kong XQ, Wang LX, Yang CS, Chen SF, Xue YZ, Liu YH. Effects of adrenomedullin on the cell numbers and apoptosis of endothelial progenitor cells. Clin Invest Med. 2008;31:E117-E122. [PubMed] |
| 52. | Temmesfeld-Wollbrück B, Hocke AC, Suttorp N, Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb Haemost. 2007;98:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 53. | Honda M, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Nagata I, Niwa M. Adrenomedullin improves the blood-brain barrier function through the expression of claudin-5. Cell Mol Neurobiol. 2006;26:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Dohgu S, Sumi N, Nishioku T, Takata F, Watanabe T, Naito M, Shuto H, Yamauchi A, Kataoka Y. Cyclosporin A induces hyperpermeability of the blood-brain barrier by inhibiting autocrine adrenomedullin-mediated up-regulation of endothelial barrier function. Eur J Pharmacol. 2010;644:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Onur OE, Guneysel O, Akoglu H, Denizbasi A, Onur E. Adrenomedullin reduces the severity of cerulein-induced acute pancreatitis. Peptides. 2007;28:2179-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, Krüll M, Seybold J, Seeger W, Rascher W, Schütte H. Adrenomedullin reduces endothelial hyperpermeability. Circ Res. 2002;91:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Maki T, Ihara M, Fujita Y, Nambu T, Miyashita K, Yamada M, Washida K, Nishio K, Ito H, Harada H. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke. 2011;42:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Rauma-Pinola T, Pääkkö P, Ilves M, Serpi R, Romppanen H, Vuolteenaho O, Ruskoaho H, Hautala T. Adrenomedullin gene transfer induces neointimal apoptosis and inhibits neointimal hyperplasia in injured rat artery. J Gene Med. 2006;8:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Nagaya N, Kangawa K. Adrenomedullin in the treatment of pulmonary hypertension. Peptides. 2004;25:2013-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Kato J, Kitamura K, Eto T. Plasma adrenomedullin level and development of hypertension. J Hum Hypertens. 2006;20:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Kato J, Tsuruda T, Kita T, Kitamura K, Eto T. Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:2480-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Ishimitsu T, Nishikimi T, Saito Y, Kitamura K, Eto T, Kangawa K, Matsuo H, Omae T, Matsuoka H. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest. 1994;94:2158-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 341] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 63. | Nishikimi T, Saito Y, Kitamura K, Ishimitsu T, Eto T, Kangawa K, Matsuo H, Omae T, Matsuoka H. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol. 1995;26:1424-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 216] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 64. | Iacobellis G, di Gioia CR, Di Vito M, Petramala L, Cotesta D, De Santis V, Vitale D, Tritapepe L, Letizia C. Epicardial adipose tissue and intracoronary adrenomedullin levels in coronary artery disease. Horm Metab Res. 2009;41:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Florio P, Abella R, Marinoni E, Di Iorio R, Letizia C, Meli M, de la Torre T, Petraglia F, Cazzaniga A, Giamberti A. Adrenomedullin blood concentrations in infants subjected to cardiopulmonary bypass: correlation with monitoring parameters and prediction of poor neurological outcome. Clin Chem. 2008;54:202-206. [PubMed] |
| 66. | Tsuruda T, Kato J, Kitamura K, Kuwasako K, Imamura T, Koiwaya Y, Tsuji T, Kangawa K, Eto T. Adrenomedullin: a possible autocrine or paracrine inhibitor of hypertrophy of cardiomyocytes. Hypertension. 1998;31:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Øie E, Vinge LE, Andersen GØ, Yndestad A, Krobert KA, Sandberg C, Ahmed MS, Haug T, Levy FO, Skomedal T. RAMP2 and RAMP3 mRNA levels are increased in failing rat cardiomyocytes and associated with increased responsiveness to adrenomedullin. J Mol Cell Cardiol. 2005;38:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Cueille C, Pidoux E, de Vernejoul MC, Ventura-Clapier R, Garel JM. Increased myocardial expression of RAMP1 and RAMP3 in rats with chronic heart failure. Biochem Biophys Res Commun. 2002;294:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Nouguerède E, Berenguer C, Garcia S, Bennani B, Delfino C, Nanni I, Dahan L, Gasmi M, Seitz JF, Martin PM. Expression of adrenomedullin in human colorectal tumors and its role in cell growth and invasion in vitro and in xenograft growth in vivo. Cancer Med. 2013;2:196-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Berenguer-Daizé C, Boudouresque F, Bastide C, Tounsi A, Benyahia Z, Acunzo J, Dussault N, Delfino C, Baeza N, Daniel L. Adrenomedullin blockade suppresses growth of human hormone-independent prostate tumor xenograft in mice. Clin Cancer Res. 2013;19:6138-6150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Liu AG, Zhang XZ, Li FB, Zhao YL, Guo YC, Yang RM. RNA interference targeting adrenomedullin induces apoptosis and reduces the growth of human bladder urothelial cell carcinoma. Med Oncol. 2013;30:616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Li DY, Zhang YC, Philips MI, Sawamura T, Mehta JL. Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ Res. 1999;84:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Watanabe T, Barker TA, Berk BC. Angiotensin II and the endothelium: diverse signals and effects. Hypertension. 2005;45:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 74. | Tamarat R, Silvestre JS, Durie M, Levy BI. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Lab Invest. 2002;82:747-756. [PubMed] |
| 75. | Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-alpha. Am J Physiol Cell Physiol. 2004;286:C779-C784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Ridker PM, Gaboury CL, Conlin PR, Seely EW, Williams GH, Vaughan DE. Stimulation of plasminogen activator inhibitor in vivo by infusion of angiotensin II. Evidence of a potential interaction between the renin-angiotensin system and fibrinolytic function. Circulation. 1993;87:1969-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 263] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 77. | Kossmann S, Hu H, Steven S, Schönfelder T, Fraccarollo D, Mikhed Y, Brähler M, Knorr M, Brandt M, Karbach SH. Inflammatory monocytes determine endothelial nitric-oxide synthase uncoupling and nitro-oxidative stress induced by angiotensin II. J Biol Chem. 2014;289:27540-27550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 78. | Levy BI, Benessiano J, Henrion D, Caputo L, Heymes C, Duriez M, Poitevin P, Samuel JL. Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J Clin Invest. 1996;98:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Warnholtz A, Nickenig G, Schulz E, Macharzina R, Bräsen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 428] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 80. | Marsen TA, Egink G, Suckau G, Baldamus CA. Tyrosine-kinase-dependent regulation of the nitric oxide synthase gene by endothelin-1 in human endothelial cells. Pflugers Arch. 1999;438:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Zhang M, Luo B, Chen SJ, Abrams GA, Fallon MB. Endothelin-1 stimulation of endothelial nitric oxide synthase in the pathogenesis of hepatopulmonary syndrome. Am J Physiol. 1999;277:G944-G952. [PubMed] |
| 82. | Sánchez A, Martínez P, Muñoz M, Benedito S, García-Sacristán A, Hernández M, Prieto D. Endothelin-1 contributes to endothelial dysfunction and enhanced vasoconstriction through augmented superoxide production in penile arteries from insulin-resistant obese rats: role of ET(A) and ET(B) receptors. Br J Pharmacol. 2014;171:5682-5695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 83. | Callera GE, Montezano AC, Touyz RM, Zorn TM, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor mediates altered leukocyte-endothelial cell interaction and adhesion molecules expression in DOCA-salt rats. Hypertension. 2004;43:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Helset E, Sildnes T, Konopski ZS. Endothelin-1 Stimulates Monocytes in vitro to Release Chemotactic Activity Identified as Interleukin-8 and Monocyte Chemotactic Protein-1. Mediators Inflamm. 1994;3:155-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 674] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 86. | Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114:464-469. [PubMed] |
| 87. | Choussat R, Hittinger L, Barbe F, Maistre G, Carayon A, Crozatier B, Su J. Acute effects of an endothelin-1 receptor antagonist bosentan at different stages of heart failure in conscious dogs. Cardiovasc Res. 1998;39:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Stewart DJ, Cernacek P, Costello KB, Rouleau JL. Elevated endothelin-1 in heart failure and loss of normal response to postural change. Circulation. 1992;85:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 160] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Davidge ST. Prostaglandin H synthase and vascular function. Circ Res. 2001;89:650-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 90. | Muzaffar S, Shukla N, Bond M, Sala-Newby GB, Newby AC, Angelini GD, Jeremy JY. Superoxide from NADPH oxidase upregulates type 5 phosphodiesterase in human vascular smooth muscle cells: inhibition with iloprost and NONOate. Br J Pharmacol. 2008;155:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Muzaffar S, Jeremy JY, Angelini GD, Shukla N. NADPH oxidase 4 mediates upregulation of type 4 phosphodiesterases in human endothelial cells. J Cell Physiol. 2012;227:1941-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, Kaye DM. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004;110:3680-3686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Ou J, Ou Z, McCarver DG, Hines RN, Oldham KT, Ackerman AW, Pritchard KA. Trichloroethylene decreases heat shock protein 90 interactions with endothelial nitric oxide synthase: implications for endothelial cell proliferation. Toxicol Sci. 2003;73:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Smith AR, Hagen TM. Vascular endothelial dysfunction in aging: loss of Akt-dependent endothelial nitric oxide synthase phosphorylation and partial restoration by (R)-alpha-lipoic acid. Biochem Soc Trans. 2003;31:1447-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Scheuer H, Gwinner W, Hohbach J, Gröne EF, Brandes RP, Malle E, Olbricht CJ, Walli AK, Gröne HJ. Oxidant stress in hyperlipidemia-induced renal damage. Am J Physiol Renal Physiol. 2000;278:F63-F74. [PubMed] |
| 96. | Darblade B, Caillaud D, Poirot M, Fouque M, Thiers JC, Rami J, Bayard F, Arnal JF. Alteration of plasmalemmal caveolae mimics endothelial dysfunction observed in atheromatous rabbit aorta. Cardiovasc Res. 2001;50:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Topal G, Brunet A, Millanvoye E, Boucher JL, Rendu F, Devynck MA, David-Dufilho M. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radic Biol Med. 2004;36:1532-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 98. | Palmer L, Kavoussi P, Lysiak J. S-Nitrosylation of endothelial nitric oxide synthase alters erectile function. Nitric Oxide. 2012;27 Supplement:S22-S23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 99. | Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Lüscher TF. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation. 1998;97:2494-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 263] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 100. | Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 101. | Tonduangu D, Hittinger L, Ghaleh B, Le Corvoisier P, Sambin L, Champagne S, Badoual T, Vincent F, Berdeaux A, Crozatier B. Chronic infusion of bradykinin delays the progression of heart failure and preserves vascular endothelium-mediated vasodilation in conscious dogs. Circulation. 2004;109:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Fujii M, Wada A, Tsutamoto T, Ohnishi M, Isono T, Kinoshita M. Bradykinin improves left ventricular diastolic function under long-term angiotensin-converting enzyme inhibition in heart failure. Hypertension. 2002;39:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | de Frutos L, Farré J, Gómez J, Romero J, Marcos-Alberca P, Nuñez A, Rico L, López-Farré A. Expression of an endothelial-type nitric oxide synthase isoform in human neutrophils: modification by tumor necrosis factor-alpha and during acute myocardial infarction. J Am Coll Cardiol. 2001;37:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Pignatelli P, Loffredo L, Martino F, Catasca E, Carnevale R, Zanoni C, Del Ben M, Antonini R, Basili S, Violi F. Myeloperoxidase overexpression in children with hypercholesterolemia. Atherosclerosis. 2009;205:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Zhu Y, Liao HL, Wang N, Yuan Y, Ma KS, Verna L, Stemerman MB. Lipoprotein promotes caveolin-1 and Ras translocation to caveolae: role of cholesterol in endothelial signaling. Arterioscler Thromb Vasc Biol. 2000;20:2465-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Kuga T, Egashira K, Mohri M, Tsutsui H, Harasawa Y, Urabe Y, Ando S, Shimokawa H, Takeshita A. Bradykinin-induced vasodilation is impaired at the atherosclerotic site but is preserved at the spastic site of human coronary arteries in vivo. Circulation. 1995;92:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 107. | Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 493] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 108. | Woo KS, Chook P, Lolin YI, Cheung AS, Chan LT, Sun YY, Sanderson JE, Metreweli C, Celermajer DS. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation. 1997;96:2542-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 109. | Heil SG, De Vriese AS, Kluijtmans LA, Mortier S, Den Heijer M, Blom HJ. The role of hyperhomocysteinemia in nitric oxide (NO) and endothelium-derived hyperpolarizing factor (EDHF)-mediated vasodilatation. Cell Mol Biol (Noisy-le-grand). 2004;50:911-916. [PubMed] |
| 110. | Cheng Z, Jiang X, Kruger WD, Praticò D, Gupta S, Mallilankaraman K, Madesh M, Schafer AI, Durante W, Yang X. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118:1998-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 111. | Cheng Z, Yang X, Wang H. Hyperhomocysteinemia and Endothelial Dysfunction. Curr Hypertens Rev. 2009;5:158-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 112. | Stühlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104:2569-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 447] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 113. | Zhang JG, Wang LZ, Han XQ, Jiang YD, Zhang RM, Wang SR. The pathogenic mechanism of homocysteine -induced endothelial nitric oxide synthase dysfunction and the antagonistic effects by folic acid. Fenzi Xibao Shengwu Xuebao. 2007;40:17-23. [PubMed] |
| 114. | Harker LA, Ross R, Slichter SJ, Scott CR. Homocystine-induced arteriosclerosis. The role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976;58:731-741. [PubMed] |
| 115. | Siow YL, Au-Yeung KK, Woo CW, O K. Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activation. Biochem J. 2006;398:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 116. | Huang A, Pinto JT, Froogh G, Kandhi S, Qin J, Wolin MS, Hintze TH, Sun D. Role of homocysteinylation of ACE in endothelial dysfunction of arteries. Am J Physiol Heart Circ Physiol. 2015;308:H92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1132] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 118. | Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, Belin de Chantemele E, Feher A, Romero MJ, Bagi Z. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes. 2014;63:1381-1393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 119. | Gong L, Liu FQ, Wang J, Wang XP, Hou XG, Sun Y, Qin WD, Wei SJ, Zhang Y, Chen L. Hyperglycemia induces apoptosis of pancreatic islet endothelial cells via reactive nitrogen species-mediated Jun N-terminal kinase activation. Biochim Biophys Acta. 2011;1813:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Marfella R, Esposito K, Giunta R, Coppola G, De Angelis L, Farzati B, Paolisso G, Giugliano D. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101:2247-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 121. | Zhu M, Chen J, Jiang H, Miao C. Propofol protects against high glucose-induced endothelial adhesion molecules expression in human umbilical vein endothelial cells. Cardiovasc Diabetol. 2013;12:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101:1905-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 319] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 123. | Zhu M, Wen M, Sun X, Chen W, Chen J, Miao C. Propofol protects against high glucose-induced endothelial apoptosis and dysfunction in human umbilical vein endothelial cells. Anesth Analg. 2015;120:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 124. | Park JY, Takahara N, Gabriele A, Chou E, Naruse K, Suzuma K, Yamauchi T, Ha SW, Meier M, Rhodes CJ. Induction of endothelin-1 expression by glucose: an effect of protein kinase C activation. Diabetes. 2000;49:1239-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 125. | Schneider JG, Tilly N, Hierl T, Sommer U, Hamann A, Dugi K, Leidig-Bruckner G, Kasperk C. Elevated plasma endothelin-1 levels in diabetes mellitus. Am J Hypertens. 2002;15:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 126. | Gao X, Martinez-Lemus LA, Zhang C. Endothelium-derived hyperpolarizing factor and diabetes. World J Cardiol. 2011;3:25-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Katakam PV, Ujhelyi MR, Miller AW. EDHF-mediated relaxation is impaired in fructose-fed rats. J Cardiovasc Pharmacol. 1999;34:461-467. [PubMed] |
| 128. | Miller AW, Katakam PV, Ujhelyi MR. Impaired endothelium-mediated relaxation in coronary arteries from insulin-resistant rats. J Vasc Res. 1999;36:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 129. | Diamant M, Tvede N, Prause JU, Oxholm P. Soluble interleukin-2 receptors in serum from patients with primary Sjögren’s syndrome. Scand J Rheumatol. 1991;20:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 130. | Panza JA, García CE, Kilcoyne CM, Quyyumi AA, Cannon RO. Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 210] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 131. | Vizza CD, Letizia C, Badagliacca R, Sciomer S, Poscia R, Della Rocca G, Iacoboni C, Leonardo de L, Quattrucci S, Dario C. Plasma adrenomedullin and endothelin-1 concentration during low-dose dobutamine infusion: Relationship between pulmonary uptake and pulmonary vascular pressure/flow characteristics. Regul Pept. 2006;136:85-91. [PubMed] |
| 132. | Vizza CD, Letizia C, Badagliacca R, Poscia R, Pezzuto B, Gambardella C, Nona A, Papa S, Marcon S, Mancone M. Relationship between baseline ET-1 plasma levels and outcome in patients with idiopathic pulmonary hypertension treated with bosentan. Int J Cardiol. 2013;167:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 133. | Li J, Zhou Z, Jiang DJ, Li D, Tan B, Liu H, Li YJ. Reduction of NO- and EDHF-mediated vasodilatation in hypertension: role of asymmetric dimethylarginine. Clin Exp Hypertens. 2007;29:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 134. | Wu X, Mäkynen H, Kähönen M, Arvola P, Pörsti I. Mesenteric arterial function in vitro in three models of experimental hypertension. J Hypertens. 1996;14:365-372. [PubMed] |
| 135. | Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci USA. 1997;94:13915-13920. [PubMed] |
| 136. | Heitzer T, Just H, Münzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 345] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 137. | Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 394] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 138. | Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, Lerman LO, Lerman A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 139. | Barbieri SS, Zacchi E, Amadio P, Gianellini S, Mussoni L, Weksler BB, Tremoli E. Cytokines present in smokers’ serum interact with smoke components to enhance endothelial dysfunction. Cardiovasc Res. 2011;90:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 140. | Yamaguchi Y, Haginaka J, Morimoto S, Fujioka Y, Kunitomo M. Facilitated nitration and oxidation of LDL in cigarette smokers. Eur J Clin Invest. 2005;35:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 141. | Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1016] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 142. | Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 982] [Cited by in RCA: 1011] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 143. | Jefferis BJ, Lowe GD, Welsh P, Rumley A, Lawlor DA, Ebrahim S, Carson C, Doig M, Feyerabend C, McMeekin L. Secondhand smoke (SHS) exposure is associated with circulating markers of inflammation and endothelial function in adult men and women. Atherosclerosis. 2010;208:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 144. | Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107:2342-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 145. | Shimasaki Y, Saito Y, Yoshimura M, Kamitani S, Miyamoto Y, Masuda I, Nakayama M, Mizuno Y, Ogawa H, Yasue H. The effects of long-term smoking on endothelial nitric oxide synthase mRNA expression in human platelets as detected with real-time quantitative RT-PCR. Clin Appl Thromb Hemost. 2007;13:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 146. | Su Y, Han W, Giraldo C, De Li Y, Block ER. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am J Respir Cell Mol Biol. 1998;19:819-825. [PubMed] |
| 147. | Wagner L, Laczy B, Tamaskó M, Mazák I, Markó L, Molnár GA, Wagner Z, Mohás M, Cseh J, Fekete A. Cigarette smoke-induced alterations in endothelial nitric oxide synthase phosphorylation: role of protein kinase C. Endothelium. 2007;14:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 148. | Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, MacAllister RJ. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 149. | Klein RM, Schwartzkopff B, Strauer BE. Evidence of endothelial dysfunction of epicardial coronary arteries in patients with immunohistochemically proven myocarditis. Am Heart J. 1998;136:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 150. | Choy JC, Lui AH, Moien-Afshari F, Wei K, Yanagawa B, McManus BM, Laher I. Coxsackievirus B3 infection compromises endothelial-dependent vasodilation of coronary resistance arteries. J Cardiovasc Pharmacol. 2004;43:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 151. | Bordron A, Dueymes M, Levy Y, Jamin C, Leroy JP, Piette JC, Shoenfeld Y, Youinou PY. The binding of some human antiendothelial cell antibodies induces endothelial cell apoptosis. J Clin Invest. 1998;101:2029-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 152. | Pierangeli SS, Espinola RG, Liu X, Harris EN. Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule-1, vascular cell adhesion molecule-1, and P-selectin. Circ Res. 2001;88:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 153. | Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527-3561. [PubMed] |
| 154. | Antoniades C, Demosthenous M, Tousoulis D, Antonopoulos AS, Vlachopoulos C, Toutouza M, Marinou K, Bakogiannis C, Mavragani K, Lazaros G. Role of asymmetrical dimethylarginine in inflammation-induced endothelial dysfunction in human atherosclerosis. Hypertension. 2011;58:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 155. | Perna M, Roman MJ, Alpert DR, Crow MK, Lockshin MD, Sammaritano L, Devereux RB, Cooke JP, Salmon JE. Relationship of asymmetric dimethylarginine and homocysteine to vascular aging in systemic lupus erythematosus patients. Arthritis Rheum. 2010;62:1718-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 156. | Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence. 2014;5:213-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 157. | Schanstra JP, Bataillé E, Marin Castaño ME, Barascud Y, Hirtz C, Pesquero JB, Pecher C, Gauthier F, Girolami JP, Bascands JL. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-kappaB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J Clin Invest. 1998;101:2080-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 147] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 158. | Passos GF, Fernandes ES, Campos MM, Araújo JG, Pesquero JL, Souza GE, Avellar MC, Teixeira MM, Calixto JB. Kinin B1 receptor up-regulation after lipopolysaccharide administration: role of proinflammatory cytokines and neutrophil influx. J Immunol. 2004;172:1839-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 159. | Balligand JL, Ungureanu-Longrois D, Simmons WW, Pimental D, Malinski TA, Kapturczak M, Taha Z, Lowenstein CJ, Davidoff AJ, Kelly RA. Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Characterization and regulation of iNOS expression and detection of iNOS activity in single cardiac myocytes in vitro. J Biol Chem. 1994;269:27580-27588. [PubMed] |
| 160. | Kettelhut IC, Fiers W, Goldberg AL. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc Natl Acad Sci USA. 1987;84:4273-4277. [PubMed] |
| 161. | Merino VF, Todiras M, Campos LA, Saul V, Popova E, Baltatu OC, Pesquero JB, Bader M. Increased susceptibility to endotoxic shock in transgenic rats with endothelial overexpression of kinin B(1) receptors. J Mol Med (Berl). 2008;86:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 162. | Cayla C, Todiras M, Iliescu R, Saul VV, Gross V, Pilz B, Chai G, Merino VF, Pesquero JB, Baltatu OC. Mice deficient for both kinin receptors are normotensive and protected from endotoxin-induced hypotension. FASEB J. 2007;21:1689-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 163. | Pesquero JB, Araujo RC, Heppenstall PA, Stucky CL, Silva JA, Walther T, Oliveira SM, Pesquero JL, Paiva AC, Calixto JB. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci USA. 2000;97:8140-8145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 298] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 164. | Seguin T, Buleon M, Destrube M, Ranera MT, Couture R, Girolami JP, Tack I. Hemodynamic and renal involvement of B1 and B2 kinin receptors during the acute phase of endotoxin shock in mice. Int Immunopharmacol. 2008;8:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 165. | Siebeck M, Spannagl E, Schorr M, Stumpf B, Fritz H, Whalley ET, Cheronis JC. Effect of combined B1 and B2 kinin receptor blockade in porcine endotoxin shock. Immunopharmacology. 1996;33:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 166. | Barratt-Due A, Johansen HT, Sokolov A, Thorgersen EB, Hellerud BC, Reubsaet JL, Seip KF, Tønnessen TI, Lindstad JK, Pharo A. The role of bradykinin and the effect of the bradykinin receptor antagonist icatibant in porcine sepsis. Shock. 2011;36:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 167. | Pei H, Zhang Y, Wu H, Ren L, Jia X, Zhang Y, Chen M. Relationship between iNOS expression and apoptosis in cerebral tissue, and the effect of sini injection in endotoxin shock rats. J Tradit Chin Med. 2013;33:486-491. [PubMed] |
| 168. | Khan R, Kirschenbaum LA, LaRow C, Berna G, Griffin K, Astiz ME. Augmentation of platelet and endothelial cell eNOS activity decreases sepsis-related neutrophil-endothelial cell interactions. Shock. 2010;33:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 169. | Kadoi Y, Goto F. Effects of selective iNOS inhibition on systemic hemodynamics and mortality rate on endotoxic shock in streptozotocin-induced diabetic rats. Shock. 2007;28:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 170. | Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang B, He Z, Zeng Y, Hu Y, Sun S. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013;23:201-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 171. | Heemskerk S, van Haren FM, Foudraine NA, Peters WH, van der Hoeven JG, Russel FG, Masereeuw R, Pickkers P. Short-term beneficial effects of methylene blue on kidney damage in septic shock patients. Intensive Care Med. 2008;34:350-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 172. | Kwok ES, Howes D. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med. 2006;21:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 173. | Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res. 2001;88:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 174. | Herrera MD, Mingorance C, Rodríguez-Rodríguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010;9:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 175. | Hoffmann J, Haendeler J, Aicher A, Rössig L, Vasa M, Zeiher AM, Dimmeler S. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 254] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 176. | Matz RL, Schott C, Stoclet JC, Andriantsitohaina R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol Res. 2000;49:11-18. [PubMed] |
| 177. | de Sotomayor MA, Pérez-Guerrero C, Herrrera MD, Jimenez L, Marín R, Marhuenda E, Andriantsitohaina R. Improvement of age-related endothelial dysfunction by simvastatin: effect on NO and COX pathways. Br J Pharmacol. 2005;146:1130-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 178. | Sato I, Kaji K, Morita I, Nagao M, Murota S. Augmentation of endothelin-1, prostacyclin and thromboxane A2 secretion associated with in vitro ageing in cultured human umbilical vein endothelial cells. Mech Ageing Dev. 1993;71:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 179. | El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. 2013;65:380-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 180. | Su JB, Barbe F, Houel R, Guyene TT, Crozatier B, Hittinger L. Preserved vasodilator effect of bradykinin in dogs with heart failure. Circulation. 1998;98:2911-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 181. | Champagne S, Hittinger L, Héloire F, Suto Y, Sambin L, Crozatier B, Su JB. Reduced coronary vasodilator responses to amlodipine in pacing-induced heart failure in conscious dogs: role of nitric oxide. Br J Pharmacol. 2002;136:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 182. | Kaiser L, Spickard RC, Olivier NB. Heart failure depresses endothelium-dependent responses in canine femoral artery. Am J Physiol. 1989;256:H962-H967. [PubMed] |
| 183. | Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 184. | Varin R, Mulder P, Tamion F, Richard V, Henry JP, Lallemand F, Lerebours G, Thuillez C. Improvement of endothelial function by chronic angiotensin-converting enzyme inhibition in heart failure: role of nitric oxide, prostanoids, oxidant stress, and bradykinin. Circulation. 2000;102:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 185. | Mancini GB, Henry GC, Macaya C, O’Neill BJ, Pucillo AL, Carere RG, Wargovich TJ, Mudra H, Lüscher TF, Klibaner MI. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 637] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 186. | Ceconi C, Condorelli E, Quinzanini M, Rodella A, Ferrari R, Harris P. Noradrenaline, atrial natriuretic peptide, bombesin and neurotensin in myocardium and blood of rats in congestive cardiac failure. Cardiovasc Res. 1989;23:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 187. | Bachetti T, Comini L, Pasini E, Cargnoni A, Curello S, Ferrari R. Ace-inhibition with quinapril modulates the nitric oxide pathway in normotensive rats. J Mol Cell Cardiol. 2001;33:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 188. | Mukai Y, Shimokawa H, Higashi M, Morikawa K, Matoba T, Hiroki J, Kunihiro I, Talukder HM, Takeshita A. Inhibition of renin-angiotensin system ameliorates endothelial dysfunction associated with aging in rats. Arterioscler Thromb Vasc Biol. 2002;22:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 189. | Lee YH, Ahn DS, Song HJ, Kim YH, Kim HS, Ahn SH, Kang BS. Effects of Na+, K(+)-pump inhibitors on acetylcholine-induced relaxation in the rabbit aorta. Yonsei Med J. 1992;33:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 190. | Grothusen C, Bley S, Selle T, Luchtefeld M, Grote K, Tietge UJ, Drexler H, Schieffer B. Combined effects of HMG-CoA-reductase inhibition and renin-angiotensin system blockade on experimental atherosclerosis. Atherosclerosis. 2005;182:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 191. | Mervaala EM, Teräväinen TL, Malmberg L, Laakso J, Vapaatalo H, Karppanen H. Cardiovascular effects of a low-dose combination of ramipril and felodipine in spontaneously hypertensive rats. Br J Pharmacol. 1997;121:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 192. | Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 550] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 193. | Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95:2617-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 201] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 194. | Engler MM, Engler MB, Malloy MJ, Chiu EY, Schloetter MC, Paul SM, Stuehlinger M, Lin KY, Cooke JP, Morrow JD. Antioxidant vitamins C and E improve endothelial function in children with hyperlipidemia: Endothelial Assessment of Risk from Lipids in Youth (EARLY) Trial. Circulation. 2003;108:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 195. | Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 470] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 196. | Matsumoto T, D’uscio LV, Eguchi D, Akiyama M, Smith LA, Katusic ZS. Protective effect of chronic vitamin C treatment on endothelial function of apolipoprotein E-deficient mouse carotid artery. J Pharmacol Exp Ther. 2003;306:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 197. | Carr AC, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection. Arterioscler Thromb Vasc Biol. 2000;20:1716-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 246] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 198. | Neunteufl T, Priglinger U, Heher S, Zehetgruber M, Söregi G, Lehr S, Huber K, Maurer G, Weidinger F, Kostner K. Effects of vitamin E on chronic and acute endothelial dysfunction in smokers. J Am Coll Cardiol. 2000;35:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 199. | Economides PA, Khaodhiar L, Caselli A, Caballero AE, Keenan H, Bursell SE, King GL, Johnstone MT, Horton ES, Veves A. The effect of vitamin E on endothelial function of micro- and macrocirculation and left ventricular function in type 1 and type 2 diabetic patients. Diabetes. 2005;54:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 200. | Skyrme-Jones RA, O’Brien RC, Berry KL, Meredith IT. Vitamin E supplementation improves endothelial function in type I diabetes mellitus: a randomized, placebo-controlled study. J Am Coll Cardiol. 2000;36:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 201. | Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 757] [Cited by in RCA: 596] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 202. | Bourraindeloup M, Adamy C, Candiani G, Cailleret M, Bourin MC, Badoual T, Su JB, Adubeiro S, Roudot-Thoraval F, Dubois-Rande JL. N-acetylcysteine treatment normalizes serum tumor necrosis factor-alpha level and hinders the progression of cardiac injury in hypertensive rats. Circulation. 2004;110:2003-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 203. | Andrews NP, Prasad A, Quyyumi AA. N-acetylcysteine improves coronary and peripheral vascular function. J Am Coll Cardiol. 2001;37:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 204. | Scioli MG, Bielli A, Agostinelli S, Tarquini C, Arcuri G, Ferlosio A, Costanza G, Doldo E, Orlandi A. Antioxidant treatment prevents serum deprivation- and TNF-α-induced endothelial dysfunction through the inhibition of NADPH oxidase 4 and the restoration of β-oxidation. J Vasc Res. 2014;51:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 205. | Adamy C, Le Corvoisier P, Candiani G, Kirsch M, Pavoine C, Defer N, Bourin MC, Su JB, Vermes E, Hittinger L. Tumor necrosis factor alpha and glutathione interplay in chronic heart failure. Arch Mal Coeur Vaiss. 2005;98:906-912. [PubMed] |
| 206. | Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 277] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 207. | Chen J, Reheman A, Gushiken FC, Nolasco L, Fu X, Moake JL, Ni H, López JA. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J Clin Invest. 2011;121:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 208. | Moshal KS, Sen U, Tyagi N, Henderson B, Steed M, Ovechkin AV, Tyagi SC. Regulation of homocysteine-induced MMP-9 by ERK1/2 pathway. Am J Physiol Cell Physiol. 2006;290:C883-C891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 209. | Beauchesne E, Desjardins P, Butterworth RF, Hazell AS. Up-regulation of caveolin-1 and blood-brain barrier breakdown are attenuated by N-acetylcysteine in thiamine deficiency. Neurochem Int. 2010;57:830-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 210. | Salsano F, Letizia C, Proietti M, Rossi C, Proietti AR, Rosato E, Pisarri S. Significant changes of peripheral perfusion and plasma adrenomedullin levels in N-acetylcysteine long term treatment of patients with sclerodermic Raynauds phenomenon. Int J Immunopathol Pharmacol. 2005;18:761-770. [PubMed] |
| 211. | Barrios V, Calderón A, Navarro-Cid J, Lahera V, Ruilope LM. N-acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Press. 2002;11:235-239. [PubMed] |
| 212. | Vera R, Sánchez M, Galisteo M, Villar IC, Jimenez R, Zarzuelo A, Pérez-Vizcaíno F, Duarte J. Chronic administration of genistein improves endothelial dysfunction in spontaneously hypertensive rats: involvement of eNOS, caveolin and calmodulin expression and NADPH oxidase activity. Clin Sci (Lond). 2007;112:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 213. | Si H, Liu D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J Nutr. 2008;138:297-304. [PubMed] |
| 214. | Cho HY, Park CM, Kim MJ, Chinzorig R, Cho CW, Song YS. Comparative effect of genistein and daidzein on the expression of MCP-1, eNOS, and cell adhesion molecules in TNF-α-stimulated HUVECs. Nutr Res Pract. 2011;5:381-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 215. | Zhen P, Zhao Q, Hou D, Liu T, Jiang D, Duan J, Lu L, Wang W. Genistein attenuates vascular endothelial impairment in ovariectomized hyperhomocysteinemic rats. J Biomed Biotechnol. 2012;2012:730462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 216. | Squadrito F, Altavilla D, Morabito N, Crisafulli A, D’Anna R, Corrado F, Ruggeri P, Campo GM, Calapai G, Caputi AP. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 217. | Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double-blind, crossover study. Circulation. 2001;104:511-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 218. | Zepeda RJ, Castillo R, Rodrigo R, Prieto JC, Aramburu I, Brugere S, Galdames K, Noriega V, Miranda HF. Effect of carvedilol and nebivolol on oxidative stress-related parameters and endothelial function in patients with essential hypertension. Basic Clin Pharmacol Toxicol. 2012;111:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 219. | Vyssoulis GP, Marinakis AG, Aznaouridis KA, Karpanou EA, Arapogianni AN, Cokkinos DV, Stefanadis CI. The impact of third-generation beta-blocker antihypertensive treatment on endothelial function and the prothrombotic state: effects of smoking. Am J Hypertens. 2004;17:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 220. | Perros F, Ranchoux B, Izikki M, Bentebbal S, Happé C, Antigny F, Jourdon P, Dorfmüller P, Lecerf F, Fadel E. Nebivolol for improving endothelial dysfunction, pulmonary vascular remodeling, and right heart function in pulmonary hypertension. J Am Coll Cardiol. 2015;65:668-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 221. | Kelly AS, Gonzalez-Campoy JM, Rudser KD, Katz H, Metzig AM, Thalin M, Bank AJ. Carvedilol-lisinopril combination therapy and endothelial function in obese individuals with hypertension. J Clin Hypertens (Greenwich). 2012;14:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 222. | Mak IT, Boehme P, Weglicki WB. Antioxidant effects of calcium channel blockers against free radical injury in endothelial cells. Correlation of protection with preservation of glutathione levels. Circ Res. 1992;70:1099-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 223. | Matsubara M, Hasegawa K. Benidipine, a dihydropyridine-calcium channel blocker, prevents lysophosphatidylcholine-induced injury and reactive oxygen species production in human aortic endothelial cells. Atherosclerosis. 2005;178:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 224. | Habib JB, Bossaller C, Wells S, Williams C, Morrisett JD, Henry PD. Preservation of endothelium-dependent vascular relaxation in cholesterol-fed rabbit by treatment with the calcium blocker PN 200110. Circ Res. 1986;58:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 225. | Napoli C, Salomone S, Godfraind T, Palinski W, Capuzzi DM, Palumbo G, D’Armiento FP, Donzelli R, de Nigris F, Capizzi RL. 1,4-Dihydropyridine calcium channel blockers inhibit plasma and LDL oxidation and formation of oxidation-specific epitopes in the arterial wall and prolong survival in stroke-prone spontaneously hypertensive rats. Stroke. 1999;30:1907-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 226. | Fukao K, Shimada K, Hiki M, Kiyanagi T, Hirose K, Kume A, Ohsaka H, Matsumori R, Kurata T, Miyazaki T. Effects of calcium channel blockers on glucose tolerance, inflammatory state, and circulating progenitor cells in non-diabetic patients with essential hypertension: a comparative study between azelnidipine and amlodipine on glucose tolerance and endothelial function--a crossover trial (AGENT). Cardiovasc Diabetol. 2011;10:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 227. | Yasu T, Kobayashi M, Mutoh A, Yamakawa K, Momomura S, Ueda S. Dihydropyridine calcium channel blockers inhibit non-esterified-fatty-acid-induced endothelial and rheological dysfunction. Clin Sci (Lond). 2013;125:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 228. | Celık T, Balta S, Karaman M, Ahmet Ay S, Demırkol S, Ozturk C, Dınc M, Unal HU, Yılmaz MI, Kılıc S. Endocan, a novel marker of endothelial dysfunction in patients with essential hypertension: comparative effects of amlodipine and valsartan. Blood Press. 2015;24:55-60. [PubMed] |
| 229. | He Y, Si D, Yang C, Ni L, Li B, Ding M, Yang P. The effects of amlodipine and S(-)-amlodipine on vascular endothelial function in patients with hypertension. Am J Hypertens. 2014;27:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 230. | Fukutomi M, Hoshide S, Mizuno H, Kario K. Differential effects of aliskiren/amlodipine combination and high-dose amlodipine monotherapy on endothelial function in elderly hypertensive patients. Am J Hypertens. 2014;27:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 231. | Okamura T, Tawa M, Geddawy A, Shimosato T, Iwasaki H, Shintaku H, Yoshida Y, Masada M, Shinozaki K, Imamura T. Effects of atorvastatin, amlodipine, and their combination on vascular dysfunction in insulin-resistant rats. J Pharmacol Sci. 2014;124:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 232. | Zhou MS, Tian R, Jaimes EA, Raij L. Combination therapy of amlodipine and atorvastatin has more beneficial vascular effects than monotherapy in salt-sensitive hypertension. Am J Hypertens. 2014;27:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 233. | Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res. 2007;101:1084-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 234. | Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47-52. [PubMed] |
| 235. | De Young LX, Domes T, Lim K, Carson J, Brock GB. Endothelial rehabilitation: the impact of chronic PDE5 inhibitors on erectile function and protein alterations in cavernous tissue of diabetic rats. Eur Urol. 2008;54:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 236. | Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 237. | Dussault S, Maingrette F, Ménard C, Michaud SE, Haddad P, Groleau J, Turgeon J, Perez G, Rivard A. Sildenafil increases endothelial progenitor cell function and improves ischemia-induced neovascularization in hypercholesterolemic apolipoprotein E-deficient mice. Hypertension. 2009;54:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 238. | Aversa A, Letizia C, Francomano D, Bruzziches R, Natali M, Lenzi A. A spontaneous, double-blind, double-dummy cross-over study on the effects of daily vardenafil on arterial stiffness in patients with vasculogenic erectile dysfunction. Int J Cardiol. 2012;160:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 239. | Halcox JP, Nour KR, Zalos G, Mincemoyer RA, Waclawiw M, Rivera CE, Willie G, Ellahham S, Quyyumi AA. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol. 2002;40:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 240. | Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 268] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 241. | Bocchi EA, Guimarães G, Mocelin A, Bacal F, Bellotti G, Ramires JF. Sildenafil effects on exercise, neurohormonal activation, and erectile dysfunction in congestive heart failure: a double-blind, placebo-controlled, randomized study followed by a prospective treatment for erectile dysfunction. Circulation. 2002;106:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 242. | Katz SD, Balidemaj K, Homma S, Wu H, Wang J, Maybaum S. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 2000;36:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 243. | Schäfer A, Fraccarollo D, Pförtsch S, Flierl U, Vogt C, Pfrang J, Kobsar A, Renné T, Eigenthaler M, Ertl G. Improvement of vascular function by acute and chronic treatment with the PDE-5 inhibitor sildenafil in experimental diabetes mellitus. Br J Pharmacol. 2008;153:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 244. | Proietti M, Aversa A, Letizia C, Rossi C, Menghi G, Bruzziches R, Merla A, Spera G, Salsano F. Erectile dysfunction in systemic sclerosis: effects of longterm inhibition of phosphodiesterase type-5 on erectile function and plasma endothelin-1 levels. J Rheumatol. 2007;34:1712-1717. [PubMed] |
| 245. | Rosato E, Letizia C, Proietti M, Aversa A, Menghi G, Rossi C, Torella E, Cotesta D, Petramala L, Bruzziches R. Plasma adrenomedullin and endothelin-1 levels are reduced and Raynaud’s phenomenon improved by daily tadalafil administration in male patients with systemic sclerosis. J Biol Regul Homeost Agents. 2009;23:23-29. [PubMed] |
| 246. | Egashira K, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Inou T, Takeshita A. Reduction in serum cholesterol with pravastatin improves endothelium-dependent coronary vasomotion in patients with hypercholesterolemia. Circulation. 1994;89:2519-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 377] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 247. | Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481-487. [PubMed] [DOI] [Full Text] |
| 248. | Martínez-González J, Raposo B, Rodríguez C, Badimon L. 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition prevents endothelial NO synthase downregulation by atherogenic levels of native LDLs: balance between transcriptional and posttranscriptional regulation. Arterioscler Thromb Vasc Biol. 2001;21:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 249. | Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1223] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 250. | Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 301] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 251. | Sánchez-Quesada JL, Otal-Entraigas C, Franco M, Jorba O, González-Sastre F, Blanco-Vaca F, Ordóñez-Llanos J. Effect of simvastatin treatment on the electronegative low-density lipoprotein present in patients with heterozygous familial hypercholesterolemia. Am J Cardiol. 1999;84:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 252. | Aviram M, Hussein O, Rosenblat M, Schlezinger S, Hayek T, Keidar S. Interactions of platelets, macrophages, and lipoproteins in hypercholesterolemia: antiatherogenic effects of HMG-CoA reductase inhibitor therapy. J Cardiovasc Pharmacol. 1998;31:39-45. [PubMed] |
| 253. | Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1098] [Cited by in RCA: 1113] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 254. | Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang MH, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation. 2011;124:335-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 255. | Kosmidou I, Moore JP, Weber M, Searles CD. Statin treatment and 3’ polyadenylation of eNOS mRNA. Arterioscler Thromb Vasc Biol. 2007;27:2642-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 256. | Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res. 2005;97:1232-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 371] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 257. | Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 270] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 258. | Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18:1519-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 334] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 259. | Ascer E, Bertolami MC, Venturinelli ML, Buccheri V, Souza J, Nicolau JC, Ramires JA, Serrano CV. Atorvastatin reduces proinflammatory markers in hypercholesterolemic patients. Atherosclerosis. 2004;177:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 260. | Lai WT, Lee KT, Chu CS, Voon WC, Yen HW, Tsai LY, Sheu SH. Influence of withdrawal of statin treatment on proinflammatory response and fibrinolytic activity in humans: an effect independent on cholesterol elevation. Int J Cardiol. 2005;98:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 261. | McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, Capell HA, Sattar N. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 586] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 262. | Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 263. | Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 264. | Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 370] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 265. | Feingold KR, Moser AH, Shigenaga JK, Patzek SM, Grunfeld C. Inflammation stimulates the expression of PCSK9. Biochem Biophys Res Commun. 2008;374:341-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 266. | Tang Z, Jiang L, Peng J, Ren Z, Wei D, Wu C, Pan L, Jiang Z, Liu L. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-κB activation in THP-1-derived macrophages. Int J Mol Med. 2012;30:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 267. | Ranheim T, Mattingsdal M, Lindvall JM, Holla OL, Berge KE, Kulseth MA, Leren TP. Genome-wide expression analysis of cells expressing gain of function mutant D374Y-PCSK9. J Cell Physiol. 2008;217:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 268. | Wu CY, Tang ZH, Jiang L, Li XF, Jiang ZS, Liu LS. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem. 2012;359:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 269. | Mallela J, Perkins R, Yang J, Pedigo S, Rimoldi JM, Shariat-Madar Z. The functional importance of the N-terminal region of human prolylcarboxypeptidase. Biochem Biophys Res Commun. 2008;374:635-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 270. | Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 253] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 271. | Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 418] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 272. | Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 273. | Stegbauer J, Potthoff SA, Quack I, Mergia E, Clasen T, Friedrich S, Vonend O, Woznowski M, Königshausen E, Sellin L. Chronic treatment with angiotensin-(1-7) improves renal endothelial dysfunction in apolipoproteinE-deficient mice. Br J Pharmacol. 2011;163:974-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 274. | Beyer AM, Guo DF, Rahmouni K. Prolonged treatment with angiotensin 1-7 improves endothelial function in diet-induced obesity. J Hypertens. 2013;31:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 275. | Trask AJ, Ferrario CM. Angiotensin-(1-7): pharmacology and new perspectives in cardiovascular treatments. Cardiovasc Drug Rev. 2007;25:162-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 276. | Zhang Y, Lu J, Shi J, Lin X, Dong J, Zhang S, Liu Y, Tong Q. Central administration of angiotensin-(1-7) stimulates nitric oxide release and upregulates the endothelial nitric oxide synthase expression following focal cerebral ischemia/reperfusion in rats. Neuropeptides. 2008;42:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 277. | Costa MA, Lopez Verrilli MA, Gomez KA, Nakagawa P, Peña C, Arranz C, Gironacci MM. Angiotensin-(1-7) upregulates cardiac nitric oxide synthase in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H1205-H1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 278. | Pernomian L, Gomes MS, Restini CB, de Oliveira AM. MAS-mediated antioxidant effects restore the functionality of angiotensin converting enzyme 2-angiotensin-(1-7)-MAS axis in diabetic rat carotid. Biomed Res Int. 2014;2014:640329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 279. | Fraga-Silva RA, Costa-Fraga FP, Murça TM, Moraes PL, Martins Lima A, Lautner RQ, Castro CH, Soares CM, Borges CL, Nadu AP. Angiotensin-converting enzyme 2 activation improves endothelial function. Hypertension. 2013;61:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 280. | Jarajapu YP, Bhatwadekar AD, Caballero S, Hazra S, Shenoy V, Medina R, Kent D, Stitt AW, Thut C, Finney EM. Activation of the ACE2/angiotensin-(1-7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes. 2013;62:1258-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 281. | Bovenzi V, Savard M, Morin J, Cuerrier CM, Grandbois M, Gobeil F. Bradykinin protects against brain microvascular endothelial cell death induced by pathophysiological stimuli. J Cell Physiol. 2010;222:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 282. | Su JB, Cazorla O, Blot S, Blanchard-Gutton N, Ait Mou Y, Barthélémy I, Sambin L, Sampedrano CC, Gouni V, Unterfinger Y. Bradykinin restores left ventricular function, sarcomeric protein phosphorylation, and e/nNOS levels in dogs with Duchenne muscular dystrophy cardiomyopathy. Cardiovasc Res. 2012;95:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 283. | Chen BC, Yu CC, Lei HC, Chang MS, Hsu MJ, Huang CL, Chen MC, Sheu JR, Chen TF, Chen TL. Bradykinin B2 receptor mediates NF-kappaB activation and cyclooxygenase-2 expression via the Ras/Raf-1/ERK pathway in human airway epithelial cells. J Immunol. 2004;173:5219-5228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 284. | Yu HS, Lin TH, Tang CH. Bradykinin enhances cell migration in human prostate cancer cells through B2 receptor/PKCδ/c-Src dependent signaling pathway. Prostate. 2013;73:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 285. | Montana V, Sontheimer H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J Neurosci. 2011;31:4858-4867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 286. | Westermann D, Riad A, Richter U, Jäger S, Savvatis K, Schuchardt M, Bergmann N, Tölle M, Nagorsen D, Gotthardt M. Enhancement of the endothelial NO synthase attenuates experimental diastolic heart failure. Basic Res Cardiol. 2009;104:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 287. | Yang Q, Xue HM, Wong WT, Tian XY, Huang Y, Tsui SK, Ng PK, Wohlfart P, Li H, Xia N. AVE3085, an enhancer of endothelial nitric oxide synthase, restores endothelial function and reduces blood pressure in spontaneously hypertensive rats. Br J Pharmacol. 2011;163:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 288. | Schäfer A, Fraccarollo D, Widder J, Eigenthaler M, Ertl G, Bauersachs J. Inhibition of platelet activation in rats with severe congestive heart failure by a novel endothelial nitric oxide synthase transcription enhancer. Eur J Heart Fail. 2009;11:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 289. | Cheang WS, Wong WT, Tian XY, Yang Q, Lee HK, He GW, Yao X, Huang Y. Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovasc Res. 2011;92:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 290. | Xuan C, Chang FJ, Liu XC, Bai XY, Liao XL, He GW, Ou JS. Endothelial nitric oxide synthase enhancer for protection of endothelial function from asymmetric dimethylarginine-induced injury in human internal thoracic artery. J Thorac Cardiovasc Surg. 2012;144:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 291. | Xue HM, Yu CM, Underwood MJ, Huang JH, Yang Q. AVE3085 protects coronary endothelium from the impairment of asymmetric dimethylarginine by activation and recoupling of eNOS. Cardiovasc Drugs Ther. 2012;26:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 292. | DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 402] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 293. | Reil JC, Tardif JC, Ford I, Lloyd SM, O’Meara E, Komajda M, Borer JS, Tavazzi L, Swedberg K, Böhm M. Selective heart rate reduction with ivabradine unloads the left ventricle in heart failure patients. J Am Coll Cardiol. 2013;62:1977-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 294. | Sargento L, Satendra M, Longo S, Lousada N, dos Reis RP. Heart rate reduction with ivabradine in patients with acute decompensated systolic heart failure. Am J Cardiovasc Drugs. 2014;14:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 295. | Fox K, Ford I, Steg PG, Tendera M, Ferrari R. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 783] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 296. | Rienzo M, Melka J, Bizé A, Sambin L, Jozwiak M, Su JB, Hittinger L, Berdeaux A, Ghaleh B. Ivabradine improves left ventricular function during chronic hypertension in conscious pigs. Hypertension. 2015;65:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 297. | Su JB. Cardioprotective effects of the Ιf current inhibition by ivabradine during cardiac dysfunction. Curr Pharm Biotechnol. 2014;14:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 298. | Bolduc V, Drouin A, Gillis MA, Duquette N, Thorin-Trescases N, Frayne-Robillard I, Des Rosiers C, Tardif JC, Thorin E. Heart rate-associated mechanical stress impairs carotid but not cerebral artery compliance in dyslipidemic atherosclerotic mice. Am J Physiol Heart Circ Physiol. 2011;301:H2081-H2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 299. | Orea-Tejeda A, Balderas-Muñoz K, Castillo-Martínez L, Infante-Vázquez O, Martínez Memije R, Keirns-Davis C, Dorantes-García J, Narváez-David R, Vázquez-Ortíz Z. Effect of ivabradine on endothelial function in diastolic and right heart failure patients. Cardiol Res Pract. 2013;2013:603913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 300. | Musikhina NA, Gapon LI, Utesheva AB, Petelina TI, Kolesnikova SN. Cerebral blood flow and endothelial functional activity in patients with coronary heart disease and arterial hypertension during therapy with ivabradine in combination with perindopril. Ter Arkh. 2012;84:30-34. [PubMed] |
| 301. | Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, Di Monaco A, Sarullo FM, Lanza GA, Crea F. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 302. | Jochmann N, Schröter F, Knebel F, Hättasch R, Gericke C, Stangl K, Baumann G, Stangl V. Effect of ivabradine-induced heart rate reduction on flow-mediated dilation measured with high-sensitivity ultrasound in patients with stable coronary heart disease. Cardiovasc Ultrasound. 2014;12:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 303. | Nerla R, Di Franco A, Milo M, Pitocco D, Zaccardi F, Tarzia P, Sarullo FM, Villano A, Russo G, Stazi A. Differential effects of heart rate reduction by atenolol or ivabradine on peripheral endothelial function in type 2 diabetic patients. Heart. 2012;98:1812-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 304. | Fox K, Ford I, Ferrari R. Ivabradine in stable coronary artery disease. N Engl J Med. 2014;371:2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 305. | Wilkerson BA, Argraves KM. The role of sphingosine-1-phosphate in endothelial barrier function. Biochim Biophys Acta. 2014;1841:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 306. | Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 701] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 307. | Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001;276:12420-12426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 308. | Tong X, Lv P, Mathew AV, Liu D, Niu C, Wang Y, Ji L, Li J, Fu Z, Pan B. The compensatory enrichment of sphingosine -1- phosphate harbored on glycated high-density lipoprotein restores endothelial protective function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2014;13:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 309. | Lucke S, Levkau B. Endothelial functions of sphingosine-1-phosphate. Cell Physiol Biochem. 2010;26:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 310. | Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 417] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 311. | Arya D, Chang S, DiMuzio P, Carpenter J, Tulenko TN. Sphingosine-1-phosphate promotes the differentiation of adipose-derived stem cells into endothelial nitric oxide synthase (eNOS) expressing endothelial-like cells. J Biomed Sci. 2014;21:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 312. | van der Giet M, Tölle M, Kleuser B. Relevance and potential of sphingosine-1-phosphate in vascular inflammatory disease. Biol Chem. 2008;389:1381-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 313. | Natarajan V, Dudek SM, Jacobson JR, Moreno-Vinasco L, Huang LS, Abassi T, Mathew B, Zhao Y, Wang L, Bittman R. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am J Respir Cell Mol Biol. 2013;49:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |