Published online Jun 26, 2013. doi: 10.4330/wjc.v5.i6.196
Revised: April 23, 2013
Accepted: May 16, 2013
Published online: June 26, 2013
Processing time: 127 Days and 13.2 Hours
AIM: To derive and validate a score for the prediction of mid-term bleeding events following discharge for myocardial infarction (MI).
METHODS: One thousand and fifty patients admitted for MI and followed for 19.9 ± 6.7 mo were assigned to a derivation cohort. A new risk model, called BLEED-MI, was developed for predicting clinically significant bleeding events during follow-up (primary endpoint) and a composite endpoint of significant hemorrhage plus all-cause mortality (secondary endpoint), incorporating the following variables: age, diabetes mellitus, arterial hypertension, smoking habits, blood urea nitrogen, glomerular filtration rate and hemoglobin at admission, history of stroke, bleeding during hospitalization or previous major bleeding, heart failure during hospitalization and anti-thrombotic therapies prescribed at discharge. The BLEED-MI model was tested for calibration, accuracy and discrimination in the derivation sample and in a new, independent, validation cohort comprising 852 patients admitted at a later date.
RESULTS: The BLEED-MI score showed good calibration in both derivation and validation samples (Hosmer-Lemeshow test P value 0.371 and 0.444, respectively) and high accuracy within each individual patient (Brier score 0.061 and 0.067, respectively). Its discriminative performance in predicting the primary outcome was relatively high (c-statistic of 0.753 ± 0.032 in the derivation cohort and 0.718 ± 0.033 in the validation sample). Incidence of primary/secondary endpoints increased progressively with increasing BLEED-MI scores. In the validation sample, a BLEED-MI score below 2 had a negative predictive value of 98.7% (152/154) for the occurrence of a clinically significant hemorrhagic episode during follow-up and for the composite endpoint of post-discharge hemorrhage plus all-cause mortality. An accurate prediction of bleeding events was shown independently of mortality, as BLEED-MI predicted bleeding with similar efficacy in patients who did not die during follow-up: Area Under the Curve 0.703, Hosmer-Lemeshow test P value 0.547, Brier score 0.060; low-risk (BLEED-MI score 0-3) event rate: 1.2%; intermediate risk (score 4-6) event rate: 5.6%; high risk (score ≥ 7) event rate: 12.5%.
CONCLUSION: A new bedside prediction-scoring model for post-discharge mid-term bleeding has been derived and preliminarily validated. This is the first score designed to predict mid- term hemorrhagic risk in patients discharged following admission for acute MI. This model should be externally validated in larger cohorts of patients before its potential implementation.
Core tip: Prediction of mid- to long-term clinically significant bleeding following discharge for a myocardial infarction has received scarce attention from the scientific community. The BLEED-myocardial infarction (MI) prediction model is the first score designed to predict mid-term hemorrhagic risk in these patients. Easy to use and comprising clinical and analytical items that can be collected in a few minutes, BLEED-MI showed good calibration, accuracy and discriminative performance for predicting post-discharge hemorrhagic episodes and a composite endpoint of bleeding events plus all-cause mortality. Importantly, an accurate prediction of bleeding events was shown independently of mortality. Furthermore, a progressively increasing risk of the primary and secondary endpoints was seen with increasing BLEED-MI scores and our results suggested a very high capability of the BLEED-MI rule in identifying low-risk patients. Depending on its potential external validation in larger cohorts of patients, the BLEED-MI score may eventually help tailor therapeutic decisions
- Citation: Barra S, Providência R, Caetano F, Almeida I, Paiva L, Dinis P, Leitão Marques A. BLEED-Myocardial Infarction Score: Predicting mid-term post-discharge bleeding events. World J Cardiol 2013; 5(6): 196-206
- URL: https://www.wjgnet.com/1949-8462/full/v5/i6/196.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i6.196
Bleeding has emerged as a predictor of early and late mortality in patients with a myocardial infarction (MI)[1-5]. Extensive data indicate that bleeding complications occur with relative frequency (up to 11.4% of patients depending on the type of MI, comorbid illnesses, performance of coronary revascularization procedures or whether patient was given thrombolytic therapy[6-9]), independently affect outcomes, carry similar importance in adversely influencing mortality risk as ischemic events, can be grossly predicted by recognizing patient, presentation, treatment and procedural risk factors for hemorrhagic complications and may be prevented by pharmacologic or nonpharmacologic measures[10].
Despite the proven benefits of anti-platelet or anti-thrombotic drugs, they are mechanistically linked to an increased risk of bleeding. Newer, more potent, agents may decrease risk of further ischemic events at a cost of increased bleeding risk, which may decrease compliance[11,12].
A thorough understanding of the prediction of hemorrhagic complications following discharge for acute coronary syndromes is therefore a particularly sensitive concern, as we pursue our common goal of maximizing efficacy of antithrombotic drugs while minimizing bleeding risk. Multiple studies have addressed the prediction of bleeding events in the acute/sub-acute phases of a MI or early post-discharge period (30 d within admission) or in patients undergoing percutaneous coronary interventions[7,8,10,13-15]. However, prediction of mid- and long-term hemorrhagic events following an acute coronary syndrome has received surprisingly scarce attention from the scientific community. To the best of our knowledge, to this date no risk score has been developed for predicting the mid-term risk of bleeding complications following discharge for a MI. In the context of bleeding assessment, evidence-based decision making should lead to selection of appropriate pharmacologic and nonpharmacologic treatments, invasive or conservative strategies that may offer the best balance of benefit and risk. Furthermore, identification of those patients at highest hemorrhagic risk allows application of more aggressive preventive strategies and potential optimization of outcomes.
The purpose of this investigation was to derive and preliminarily validate a new risk score for the prediction of mid-term bleeding events in patients discharged following admission for a MI.
We included all patients admitted at our hospital’s Acute Coronary Care Unit (ACCU) with a diagnosis of MI between December 1, 2006 and August 31, 2009 in a derivation cohort. Using collected baseline data at the time of MI diagnosis and outcome data from this cohort, we developed a new algorithm for the prediction of post-discharge bleeding events-BLEED-MI score. This model was evaluated for its overall predictive performance, discriminatory power and calibration in the derivation sample and in a different cohort comprising patients admitted at our institution for a MI between September 1, 2009 and September 30, 2011.
One thousand and fifty patients consecutively admitted to the ACCU of a tertiary referral hospital and university centre with a MI were included in the derivation sample, while 852 patients admitted at a later date to the ACCU with a MI were assigned to the validation cohort. Eligible patients were required to have a diagnosis of MI according to the Universal Definition of MI[16]. Patients were classified as having acute MI with ST-segment elevation (STEMI) or MI without ST-segment elevation (NSTEMI). Patients with previously known left bundle branch block or ventricular pacemaker rhythm were included in the NSTEMI group.
The following data were collected: demographic features, cardiovascular risk factors and previous medical history, physical examination (including weight, height, body mass index, blood pressure and heart rate) and analytical study at admission (including complete blood count, glycaemia, NT-proBNP, C-reactive protein, creatinine, urea, troponin I), maximum troponin I levels, results of coronary angiography and eventual revascularization procedures, inhospital bleeding complications, pre-discharge thoracic echocardiogram (when performed) and post-discharge antithrombotic therapies. Glomerular filtration rate (GFR by MDRD formula) and the GRACE scores for intrahospital and 6-mo post-discharge mortality were calculated for all patients.
The primary endpoint of this study was the occurrence of clinically significant bleeding events during follow-up. In-hospital bleeding events were censored, as only post-discharge hemorrhage was considered. Clinical significance of a documented hemorrhage was analyzed according to its severity, localization and associated hemodynamic compromise. Heterogeneous definitions are frequently observed in the trials assessing the benefits of antithrombotic drugs in acute coronary syndromes (ACS), with the Thrombolysis in MI (TIMI) and GUSTO being the two bleeding definitions most commonly used in trials on ACS[17,18].
Therefore, clinically significant hemorrhage included: (1) major, severe or life-threatening bleeding events, namely those at intracerebral location, those resulting in substantial hemodynamic compromise requiring treatment or in reduction of hemoglobin of 5 g/dL or more (or > 15% in hematocrit); and (2) moderate bleeding, defined by the need for transfusion, a drop in hemoglobin of 3-5 g/dL (or in hematocrit from 10% to 15%) from previous blood tests to the time of admission, the occurrence of spontaneous gross hematuria or hematemesis even in the absence of hemoglobin drop higher than 3 g/dL, or unobserved loss of 4 g/dL or more in hemoglobin
Minor bleeding, referring to hemorrhagic events not included in the previous categories, nor requiring transfusion or causing hemodynamic compromise or substantial fall in haemoglobin levels, was not assigned to the primary endpoint. Also, blood loss attributable to new revascularization or other surgical procedures was not included.
The secondary endpoint of this study was a composite outcome of post-discharge clinically significant bleeding event plus all-cause mortality.
Patients assigned to the derivation cohort were followed for 19.9 ± 6.7 mo following their discharge, while those in the validation sample were followed for a mean period of 13.4 ± 8.1 mo. Follow-up data was obtained from clinical records from outpatient clinic and hospital ward and emergency department admission(s), and through phone calls by the end of a 2-year period after discharge for patients not followed at our hospital.
Statistical analysis was done using SPSS, v.17.0. When needed, baseline characteristics are described with mean ± SD for continuous data and counts and proportions for categorical data. The Kolmogorov-Smirnov test was used to test the normal distribution of continuous variables. A model for the prediction of post-discharge mid-term clinically significant bleeding episode was developed in the derivation cohort, comprising several parameters that have been shown before to predict bleeding events in different clinical contexts: age, hemoglobin at admission, GFR by MDRD formula at admission, blood urea nitrogen at admission, history of stroke, bleeding event during hospital stay for the index MI or history of major hemorrhage (defined as non-fatal hemorrhagic stroke or history of serious bleeding requiring transfusion), signs of heart failure before discharge, previously known hypertension, diabetes mellitus, smoking habits and post-discharge treatment with anti-platelet or anticoagulant agents. Gender was indirectly considered, as it is one of the parameters used for the GFR calculation with the MDRD formula. Type of MI, performance of revascularization procedures, implanted stent type per se and atrial fibrillation at admission have not been consistently shown before to predict mid to long-term bleeding events following a MI. Furthermore, as these parameters did not help predict the occurrence of bleeding events during follow-up in univariate analysis, they were not included in our model. Table 1 unveils predictors of clinically significant hemorrhage in univariate analysis and Table 2 illustrates BLEED-MI score calculation.
| Bleeding event | No bleeding event | P value | |
| Age (yr) | 74.6 | 67.2 | < 0.001 |
| Female gender | 41.40% | 34.50% | 0.292 |
| NSTEMI | 67.20% | 57.50% | 0.095 |
| Diabetes mellitus | 50.00% | 33.50% | 0.011 |
| Previous arterial hypertension | 84.50% | 73.80% | 0.072 |
| Smoking habits | 29.30% | 17.20% | 0.049 |
| History of stroke/TIA | 17.50% | 8.10% | 0.015 |
| Atrial fibrillation at admission | 16.70% | 13.50% | 0.516 |
| Bleeding during hospitalization | 19.30% | 7.10% | 0.001 |
| Maximum killip class | 1.62 | 1.39 | 0.004 |
| Hemoglobin at admission (g/dL) | 12.0 | 13.5 | < 0.001 |
| GFR at admission (mL/min) | 55.0 | 71.6 | < 0.001 |
| Blood urea nitrogen at admission (mg/dL) | 13.5 | 8.7 | < 0.001 |
| Submitted to revascularization procedures | 58.60% | 63.90% | 0.422 |
| Variable | Points assigned |
| Age (yr) | |
| < 65 | 0 |
| 65-74 | 1 |
| ≥ 75 | 2 |
| GFR at admission (MDRD formula, mL/min) | |
| ≥ 60 | 0 |
| 30-59.9 | 1 |
| < 30 | 2 |
| History of stroke or transient ischemic attack1 | |
| No | 0 |
| Yes | 1 |
| Heart failure during hospitalization2 | |
| No | 0 |
| Yes | 1 |
| History of hypertension | |
| No | 0 |
| Yes | 1 |
| Antithrombotic therapy3 | |
| 1 agent | 1 |
| 2 agents | 2 |
| 3 agents | 3 |
| Hemoglobin at admission (g/dL) | |
| ≥ 12 | 0 |
| 10-11.9 | 1 |
| < 10 | 2 |
| Blood urea nitrogen at admission (mg/dL) | |
| < 10 | 0 |
| 10-25 | 1 |
| > 25 | 2 |
| History of major hemorrhage or bleeding event during hospitalization4 | |
| No | 0 |
| Yes | 1 |
| Smoking habits (until hospitalization) | |
| No | 0 |
| Yes | 1 |
| History of diabetes mellitus | |
| No | 0 |
| Yes | 1 |
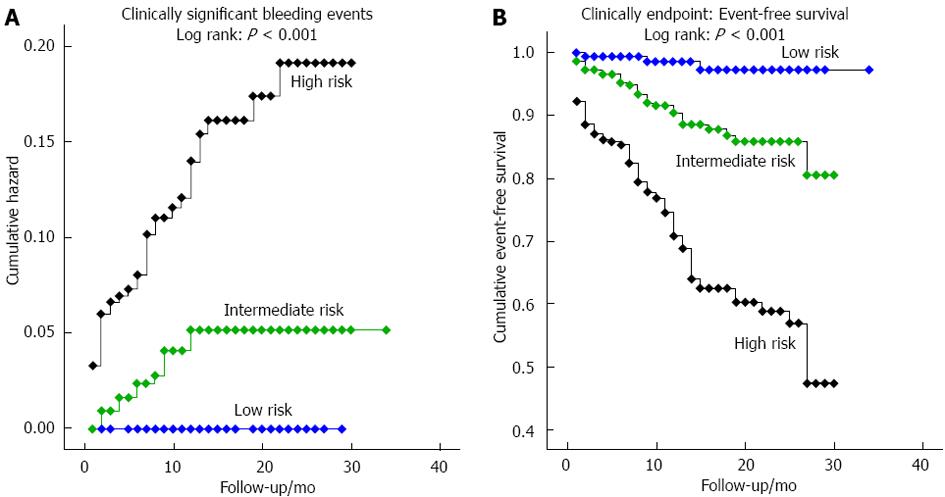
Patients were divided into three risk categories: (1) BLEED-MI score 0-3: Low risk; (2) BLEED-MI score 4-6: Intermediate risk; and (3) BLEED-MI score ≥ 7: High risk.
In both the derivation and validation cohorts, we assessed the discriminatory power of the BLEED-MI model by calculating the area under each receiver operating characteristic (ROC) curve [area under the curve (AUC)]. Discrimination, measured in terms of the AUC, refers to BLEED-MI score’s ability to assign a higher probability to patients with hemorrhagic events than to those without bleeding episodes. The same analysis was performed for the secondary endpoint, post-discharge all-cause mortality.
Binary logistic regression was performed including the BLEED-MI model exclusively to obtain estimated probabilities of significant bleeding event. Thereafter, the accuracy of the score was analyzed through the Brier score[19]. Accuracy is a measure of the average distance (residual) between the observed outcome and its predicted probability for each individual patient. A popular accuracy measure is the Brier score, which is the squared mean of the residual values. The Brier score is sensitive to both discrimination as well as calibration of the predicted probabilities and describes how well a particular model predicts the likelihood of an outcome in an individual patient (a score of 0.0 implies perfect prediction, while a Brier score of 0.25 suggests lack of utility in endpoint prediction).
The overall tendency of increasing event rates with increasing risk score was tested using chi-square for trend (gamma) and Kaplan-Meier curves were created in the validation sample to evaluate bleeding risk during follow-up and overall event-free survival in each risk category.
Finally, comparison through ROC curve analysis and the integrated discrimination improvement index (IDI) was performed between the BLEED-MI model and the CRUSADE score[20]. The IDI, which may be seen as a continuous form of the net reclassification improvement index, assesses improvement in risk discrimination by estimating the change in the difference in the mean predicted probabilities of the outcome between those with and without the outcome in question. This comparison was performed in the validation sample only.
Table 3 describes both study samples. Of the 1050 patients assigned to the derivation cohort, 91 (8.6%) died during hospitalization, 62 (6.2%) and 200 (21.8%) reached the primary and secondary endpoints during the 19.9 ± 6.7 mo follow-up, respectively. Significant bleeding events occurred in 7.5% (n = 60) of patients included in the validation cohort, while 15.6% (n = 124) reached the secondary outcome during a 13.4 ± 8.1 mo follow-up.
| Characteristic | Derivation sample (n = 1050) | Validation sample (n = 852) |
| Age (yr) | 67.9 ± 13.5 | 67.9 ± 13.6 |
| Male gender | 686 (64.7) | 578 (68.0) |
| Type of myocardial infarction | ||
| STEMI | 42.1% | 38.8% |
| NSTEMI | 57.9% | 61.2% |
| Diabetes mellitus | 380 (35.9) | 266 (31.2) |
| Previous hypertension | 796 (75.2) | 631 (74.2) |
| Hyperlipidemia | 59629 (56.3) | 475 (59.6) |
| Smoking habits | 287 (27.1) | 281 (33.1) |
| Previously known coronary disease | 283 (26.7) | 243 (28.6) |
| History of stroke/TIA | 94 (9.0) | 77 (9.1) |
| Atrial fibrillation at admission | 144 (13.7) | 99 (12.4) |
| Admission killip class | 1.40 ± 0.6 | 1.36 ± 0.7 |
| Maximum killip class | 1.56 ± 0.8 | 1.46 ± 0.8 |
| Average number of vessels with significant lesions | 1.60 ± 0.97 | 1.54 ± 0.99 |
| GFR at admission | 68.6 ± 38.4 | 72.6 ± 32.0 |
| BUN at admission (mmol/L) | 9.58 ± 6.81 | 8.85 ± 6.20 |
| Hemoglobin at admission (mg/dL) | 13.3 ± 2.1 | 13.8 ± 6.14 |
| NT-proBNP at admission (ng/L) | 4202 ± 13400 | 6393 ± 15950 |
| Submitted to revascularization procedures | 645 (61.4) | 663 (77.8) |
| Clinically significant bleeding during hospitalization | 87 (8.3) | 55 (6.5) |
| Average GRACE score for intrahospital mortality | 153.9 (P25 124; P50 151; P75 179) | 145.6 (P25 114; P50 143; P75 173) |
| Average GRACE score for 6-mo mortality | 128.0 (P25 102; P50 125; P75 149) | 121.0 (P25 94; P50 118; P75 145) |
| Moderate-severe left ventricular systolic dysfunction | 19.50% | 23.00% |
| Discharged on dual anti-platelet therapy | 818 (89.2) | 723 (90.6) |
| Discharged on anticoagulant treatment | 36 (3.9) | 37 (4.6) |
| Intrahospital mortality | 8.60% | 6.10% |
| Post-discharge mortality (mo) | 165 (16.5) (Follow-up: 19.9 ± 6.7) | 88 (11.0) (Follow-up: 13.4 ± 8.1) |
| Bleeding events during follow-up (mo) | 62 (6.8) (Follow-up: 19.9 ± 6.7) | 60 (7.5) (Follow-up: 13.4 ± 8.1) |
Fifteen point seven percent of patients in the derivation sample were assigned to the low-risk category, while 49.9% and 34.4% were included in the intermediate and high risk strata, respectively. Similarly, 22.9% of patients in the validation sample were assigned to the low risk sub-group, while 39.4% and 37.7% were included in the intermediate and high risk categories, respectively.
Derivation sample: The P value for the Hosmer and Lemeshow goodness-of-fit test confirmed the good calibration of BLEED-MI model (P = 0.371), indicating that the overall model fit was good.
Incidence of the primary and secondary endpoints according to risk category is reported on Table 4.
| Category | Low risk | Intermediate risk | High risk | Gamma for trend | P value | |
| Clinically significant bleeding events | ||||||
| Derivation cohort (follow-up: 19.9 ± 6.7 mo) | Incidence | 0.80% | 3.40% | 14.40% | 0.70 ± 0.08 | < 0.001 |
| Validation cohort (follow-up: 13.4 ± 8.1 mo) | Incidence | 1.30% | 5.00% | 14.10% | 0.61 ± 0.08 | < 0.001 |
| Composite endpoint (bleeding + all-cause mortality) | ||||||
| Derivation cohort (follow-up: 19.9 ± 6.7 mo) | Incidence | 3.10% | 11.40% | 45.70% | 0.76 ± 0.04 | < 0.001 |
| Validation cohort (follow-up: 13.4 ± 8.1 mo) | Incidence | 1.30% | 9.30% | 31.30% | 0.73 ± 0.05 | < 0.001 |
Mean BLEED-MI score in patients reaching the primary endpoint was 7.9 ± 2.4 (vs 5.6 ± 2.2 for those without significant hemorrhage, P < 0.001). Brier score analysis using this model demonstrated a mean value of 0.061, which suggests a high predictive capacity within individual patients. BLEED-MI score’s discriminatory power was assessed by calculating the AUC for the occurrence of significant hemorrhagic events or the composite endpoint of post-discharge bleeding event plus all-cause mortality: (1) Bleeding event: AUC 0.753 ± 0.032, 95%CI: 0.690-0.816, P < 0.001; and (2) Composite endpoint: AUC 0.808 ± 0.018, 95%CI: 0.772-0.844, P < 0.001.
A BLEED-MI score below 4 had a negative predictive value of 99.2% for the occurrence of a clinically significant hemorrhagic episode during follow-up and for the composite endpoint of post-discharge hemorrhage plus all-cause mortality.
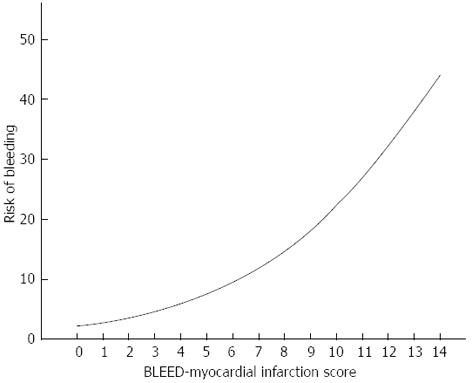
Incidence of primary and secondary endpoints increased progressively with increasing BLEED-MI scores, as shown in Table 5.
| Sample | BLEED-MI score | Bleeding event rate | Composite endpoint event rate |
| Derivation sample | 0-1 | 0.80% | 3.10% |
| 2-3 | 2.70% | 10.00% | |
| 4-5 | 7.90% | 19.10% | |
| 6-7 | 13.60% | 50.40% | |
| 8-9 | 20.00% | 65.90% | |
| 10-11 | 25.00% | 71.40% | |
| Gamma for trend | 0.60 ± 0.07 | 0.70 ± 0.04 | |
| P value | < 0.001 | < 0.001 | |
| Validation sample | 0-1 | 0.00% | 0.00% |
| 2-3 | 1.20% | 1.80% | |
| 4-5 | 5.40% | 8.80% | |
| 6-7 | 6.50% | 16.10% | |
| 8-9 | 13.90% | 25.70% | |
| 10-11 | 17.80% | 39.70% | |
| 12-13 | 23.10% | 48.00% | |
| 14-15 | - | 60.00% | |
| Gamma for trend | 0.52 ± 0.07 | 0.63 ± 0.05 | |
| P value | < 0.001 | < 0.001 |
The BLEED-MI score predicted ischaemic events (non-fatal reinfarction and ischaemic stroke) with reasonable, yet lower, discriminative performance (AUC 0.682 ± 0.028, 95%CI: 0.627-0.738, P < 0.001), suggesting a higher utility in the prediction of bleeding. In addition, it was useful in the evaluation of the net clinical risk (composite of death, non-fatal reinfarction, stroke and significant bleeding): AUC 0.760 ± 0.018, 95%CI: 0.724-0.797, P < 0.001.
Validation sample: The Hosmer-Lemeshow test confirmed that there were no statistically significant differences between observed and expected post-discharge hemorrhages across risk groups (P = 0.444).
Incidence of the primary and secondary endpoints according to risk category is reported on Table 6.
| Time (mo) | Low risk | Intermediate risk | High risk |
| 0-3 | 1 | 4 | 21 |
| 4-6 | 0 | 5 | 3 |
| 7-9 | 1 | 4 | 6 |
| 10-12 | 0 | 3 | 4 |
| 13-15 | 0 | 0 | 3 |
| 16-18 | 0 | 0 | 0 |
| 19-21 | 0 | 0 | 1 |
| 22-24 | 0 | 0 | 1 |
Mean BLEED-MI score in patients reaching the primary endpoint was 8.0 ± 2.7 (vs 5.8 ± 2.8 for those without significant hemorrhage, P < 0.001). Brier score analysis using this score demonstrated a mean value of 0.067, suggesting high predictive capacity within each individual patient. BLEED-MI score’s discriminatory power was assessed by calculating the AUC for the occurrence of significant hemorrhagic events or the composite endpoint of post-discharge bleeding event plus all-cause mortality: (1) Bleeding event: AUC 0.718 ± 0.033, 95%CI: 0.652-0.783, P < 0.001; and (2) Composite endpoint: AUC 0.774 ± 0.022, 95%CI: 0.731-0.818, P < 0.001.
A BLEED-MI score below 4 had a negative predictive value of 98.9% for the occurrence of a clinically significant hemorrhagic episode during follow-up and for the composite endpoint of post-discharge hemorrhage plus all-cause mortality.
Incidence of primary and secondary endpoints increased progressively with increasing BLEED-MI scores, as shown in Table 5.
Kaplan-Meier curves illustrate the occurrence of the primary endpoint during follow-up and event-free survival (Figure 1) according to risk-group stratification by the BLEED-MI model. As suggested in Figure 1A and Table 6 (which reveals number of patients reaching the primary endpoint at different time points), all bleeding events in the low and intermediate risk categories occurred in the first 12 mo, while hemorrhagic events in the high risk strata were strongly concentrated in the first trimester but otherwise seen until the end of the second year of follow-up. Figure 2 shows the curvilinear change in expected risk of bleeding with increasing BLEED-MI scores (as mentioned before, Table 5 illustrates the actual risk of bleeding in the validation sample).
In patients who did not die during follow-up, the BLEED-MI predicted bleeding with similar efficacy: AUC 0.703, Hosmer-Lemeshow test P value 0.547, Brier score 0.060. Low-risk (BLEED-MI score 0-3) event rate: 1.2%; intermediate risk (score 4-6) event rate: 5.6%; high risk (score ≥ 7) event rate: 12.5%. In patients who died during follow-up, no clinically significant non-fatal bleeding event occurred in patients assigned to BLEED-MI low and intermediate risk categories, while BLEED-MI high risk patients had a 20.7% bleeding rate.
The BLEED-MI model was superior to the CRUSADE score in the prediction of post-discharge mid-term bleeding events (AUC 7.18 ± 0.033 vs AUC 0.696 ± 0.036, respectively). The IDI and relative IDI were 0.024 and 15.6%, respectively, translating significant improvement in risk classification. BLEED-MI was also more effective in predicting inhospital major hemorrhage when both scores were calculated at admission (AUC 7.19 ± 0.032 vs AUC 0.642 ± 0.038, respectively).
The BLEED-MI score predicted ischaemic events (non-fatal reinfarction and ischaemic stroke) with reasonable, yet lower, discriminative performance (AUC 0.670 ± 0.029, 95%CI: 0.612-0.727, P < 0.001), suggesting a higher utility in the prediction of bleeding, similar to what had been reported in the derivation sample. In addition, it was useful in the evaluation of the net clinical risk (composite of death, non-fatal reinfarction, stroke and significant bleeding): AUC 0.736 ± 0.020, 95%CI: 0.696-0.776, P < 0.001.
We have derived and preliminarily validate a new bedside prediction-scoring model for clinically significant bleeding events following discharge for acute MI. The score is easy to use and comprises clinical and analytical items that can be collected in a few minutes. The BLEED-MI rule showed good calibration, accuracy and discriminative performance for predicting post-discharge hemorrhagic episodes and a composite endpoint of bleeding events plus all-cause mortality. Importantly, an accurate prediction of bleeding events was shown independently of mortality. Furthermore, a progressively increasing risk of the primary and secondary endpoints was seen with increasing BLEED-MI scores and our results suggested a very high capability of the BLEED-MI rule in identifying low-risk patients, which may be of particular clinical utility.
To the best of our knowledge, this is the first score designed to predict mid-term hemorrhagic risk in patients discharged following admission for acute MI. Other risk scores have been developed to evaluate bleeding risk, but they were designed for patients with atrial fibrillation on oral anticoagulants[21], for the prediction of inhospital hemorrhages in individuals with ACS[20] or following percutaneous coronary interventions[15], or for stable outpatients with or at risk or atherothrombosis-the REACH score[22]. The utility and reliability of the REACH score for the prediction of post-discharge bleeding was recently and preliminarily evaluated in a contemporary cohort of patients with acute coronary syndrome. It showed good calibration and reasonable discriminative performance (c-statistic values of 0.65 in the whole population (1548 patients), 0.63 for those without coronary revascularization and 0.67 for those treated with PCI[23].
All risk factors included in the BLEED-MI score have been demonstrated before to predict hemorrhagic risk in different or similar clinical contexts: (1) Smoking increases the risk of hemorrhagic stroke both in men[24] and women[25], with a graded increase in risk proportional to how many cigarettes are smoked, and is also considered a risk factor for bleeding and perforated peptic ulcers[26]. The REACH risk score, developed for evaluation of the risk of hemorrhagic episodes in stable outpatients with or at risk of atherothrombosis, included “smoking” as one of its variables[22]; (2) A recently published population-based cohort study demonstrated diabetes mellitus was independently associated with an increased risk of major bleeding episodes[27]. The CRUSADE Bleeding score, developed for the prediction of inhospital major bleeding, incorporates Diabetes[21]. The REACH risk score included diabetes mellitus as well[22]; (3) Age, history of stroke, bleeding history or predisposition and arterial hypertension have been included in the HAS-BLED[28] and HEMORR2HAGES risk scores[21], created for the prediction of bleeding events in patients with atrial fibrillation. Age, hypertension and history of stroke are also among the nine-item REACH risk score[22]; (4) The association between renal dysfunction and bleeding is well documented[7,21,29-31], although a complete understanding of the underlying patophysiology is still lacking. Impaired platelet function, uremic toxins and anemia are some of the determinants of uremic bleeding. Renal dysfunction is also a predictor of hemorrhagic episodes in patients with atrial fibrillation, justifying its inclusion in HAS-BLED (defined as the presence of chronic dialysis or renal transplantation or serum creatinine ≥ 200 μmol/L)[28] and HEMORR2HAGES (defined as a creatinine clearance < 30 mL/min)[21] risk scores; and (5) A low baseline haemoglobin level is an independent predictor of the risk of major bleeding in ACS as well as of the risk of death[32]. Some authors have proposed a reverse J-shaped relationship between baseline hemoglobin values and major adverse cardiovascular events[33], but whether this J-shaped relationship applies to bleeding events as well is still unknown.
Some risk factors for bleeding previously identified in studies of hospitalized patients were not included in this outpatient score. For example, type of MI (STEMI vs NSTEMI) and anthropometric variables such as weight and body mass index did not help predict hemorrhagic episodes in univariate analysis and were therefore excluded from the model. This decision was substantiated by the lack of studies demonstrating a potential association between the type of MI and mid to long-term hemorrhagic risk and the fact that the inclusion of anthropometric variables or “type of MI” considerably lowered the c-statistic for post-discharge bleed prediction in both the derivation and validation samples.
The BLEED-MI model can accurately predict post-discharge bleeding events when it is calculated at the patient’s admission, before treatment decisions that affect outcome are made. However, as the occurrence of heart failure or bleeding events during hospitalization and the type of antithrombotic therapies prescribed at discharge are also strong predictors of post-discharge bleeding events, they were incorporated in the score as well. Therefore, the BLEED-MI may be calculated any time during hospitalization, depending on the clinical progress and potential complications such as heart failure or significant bleeding.
Depending on its potential external validation in larger cohorts of patients, the BLEED-MI score may eventually help tailor therapeutic decisions, which include the choice of invasive vs conservative strategies, the selection of the most appropriate revascularization modality or stent, the prescription of long-term dual-antiplatelet therapy or anti-coagulation or the selection of the best candidates for gastroprotection with proton pump inhibitors. Beyond its potential value in ascertaining relative changes in the risk of bleeding depending on the choice of therapy by including anti-coagulation and anti-platelet therapy in its construction, the BLEED-MI score helps estimate the baseline risk for future treatment decisions.
The c-statistic of BLEED-MI for predicting post-discharge hemorrhage might not be considered particularly impressive. However, performance of a score is evaluated by its discrimination, accuracy and calibration, which were rather good in both the derivation and validation samples. Even so, our c-statistic (0.753 in the derivation cohort, 0.718 in the validation sample) was higher than that of the CRUSADE (0.71)[20], HEMORR2HAGES (0.67)[21], TIMI (0.65)[17] and REACH (0.68)[22] risk scores, and similar to the c-statistic of the HAS-BLED model[28].
Additional considerations concerning the secondary endpoint must be stated. Patients at risk for bleeding events are also at higher post-discharge mortality risk. Although the BLEED-MI model predicted bleeding independently of mortality, major bleeding also identifies patients with an underlying risk for mortality. The true incidence of hemorrhagic events may be underestimated, as patients at higher hemorrhagic risk may die before actually having a significant hemorrhage. Also, some deaths could have been caused by a severe bleed. However, as many patients were not autopsied, it is impossible to know whether a bleeding event was responsible for the death. Therefore, we considered important to test the BLEED-MI rule as a predictor of a composite endpoint of significant bleed plus all-cause mortality. Our model performed even better for this particular endpoint, which reinforces its clinical applicability.
The moderate size of our derivation and validation samples should be considered the main limitation of this study. In fact, the relatively low absolute number of bleeding events during follow-up (62 in the derivation cohort, 60 in the validation sample) and the low event-per-variable ratio posing the risk of over-fitting[34] reinforces the need for external validation in larger cohorts of patients. However, as no other post-discharge mid-term hemorrhage prediction score has been developed to this date, a comparison between derivation cohorts is not possible.
Another limitation of this investigation concerns the different lengths of follow-up in the derivation (19.9 ± 6.7 mo) and validation (13.4 ± 8.1 mo) samples, which was due to the later admission to our hospital of patients assigned to the validation cohort. This explains why post-discharge mortality rate was slightly higher in the derivation sample compared to the validation cohort. However, this limitation is mitigated by the fact that the majority of hemorrhagic episodes occurred in the first year following the MI index (as expected). Also, as most patients stop dual anti-platelet therapy at the end of the 12th month, their bleeding risk is very likely to decrease. Considering the length of follow-up in the derivation sample was > 1 full year, this limitation did not significantly influence the validation of the model.
An internationally accepted, meaningful and standardized approach for reporting bleeding events is lacking. A fixed definition may not work for all disease states throughout ACS and percutaneous revascularization procedures. Definitions of bleeding overlap to a degree but still differ substantially, which may lead to markedly different conclusions regarding incidence of hemorrhagic episodes, predictors and magnitude of short- and long-term prognostic impact. The clinically important goal of identifying patients at very low or high risk of post-discharge bleeding events increases the need for standardized bleeding definitions. The definition of significant hemorrhagic events used in this study partially overlaps with those of the TIMI[17] and GUSTO[18] trials, but it is unclear whether these definitions remain clinically relevant in the era of routine PCI and aggressive antithrombotic therapy[35]. This should be considered a limitation of the present investigation. Also, our study and model is not yet powered to prediction of clinically significant hemorrhages according to severity (life-threatening vs moderate episodes), due to the overall low number of events in each isolated category.
Recurrent bleeds were not counted and minor bleeding during follow-up was not systematically assessed. This could be viewed as a limitation of the present study, as minor bleeding also affects quality of life and increases health care costs.
A lower rate of revascularization was reported in the derivation group (61% vs 78%), which adds some imbalance to our study populations and may have affected statistical analysis.
Furthermore, although we validated the BLEED-MI score in an independent patient sample and demonstrated its overall applicability, internal validation cannot control for unrecognized biases in different institutions. This model should be externally validated in larger cohorts of patients, preferably involving multicentre and prospective registries, before its potential implementation. As external validation requires a second large population for whom all necessary data and long-term outcomes are available, we encourage other institutions to test our score in their populations.
In conclusion, a new risk score for predicting post-discharge mid-term hemorrhagic risk has been derived and preliminarily validated in an independent patient sample. The BLEED-MI model has good calibration, accuracy and discriminatory performance in the prediction of bleeding events or a composite endpoint of bleeding plus all-cause mortality. As it is both easy to use and easy to calculate from routinely available clinical data, it may eventually help clinicians take the most appropriate therapeutic decisions in patients with a MI. Nevertheless, the BLEED-MI score needs external validation in larger cohorts of patients before its potential implementation. We encourage other investigators or institutions to test our model in their patients.
Bleeding has emerged as a predictor of early and late mortality in patients with a myocardial infarction (MI). However, prediction of mid- to long-term haemorrhagic risk following an acute coronary syndrome has received scarce attention, as, to this date, no risk score has been developed for this purpose. In the context of bleeding assessment, evidence-based decision making should lead to selection of appropriate pharmacologic and non-pharmacologic treatments, invasive or conservative strategies that may offer the best balance of benefit and risk. The identification of those patients at highest hemorrhagic risk allows application of more aggressive preventive strategies and potential optimization of outcomes.
Haemorrhagic events predict early and late mortality in most cardiovascular conditions. Several risk scores have been developed for the prediction of bleeding risk in different clinical contexts. In the area of prediction of bleeding risk in patients with a MI, the research hotspot is how to identify those patients at highest haemorrhagic risk who could eventually benefit from a more conservative strategy regarding revascularization and antithrombotic therapy, and those individuals at lower bleeding risk who may be safely submitted to more aggressive antithrombotic treatment. Optimization of outcomes through efficient thrombotic and haemorrhagic risk stratification is a major research field.
This is the first score designed to predict mid-term hemorrhagic risk in patients discharged following admission for acute MI. Their new bedside prediction-scoring model is easy to use and comprises clinical and analytical items that can be collected in a few minutes. It has shown to be reliable and accurate in the prediction of post-discharge hemorrhagic episodes and a composite endpoint of bleeding events plus all-cause mortality. Importantly, an accurate prediction of bleeding events was shown independently of mortality. Furthermore, a progressively increasing risk of the primary and secondary endpoints was seen with increasing BLEED-MI scores and our results suggested a very high capability of the BLEED-MI rule in identifying low-risk patients, which may be of particular clinical utility. The BLEED-MI model’s c-statistic (0.753 in the derivation cohort, 0.718 in the validation sample) was higher than that of the CRUSADE (0.71), HEMORR2HAGES (0.67), TIMI (0.65) and REACH (0.68) risk scores in their respective clinical contexts, and similar to the c-statistic of the HAS-BLED model.
Depending on its potential external validation in larger cohorts of patients, the BLEED-MI score may eventually help tailor therapeutic decisions, which include the choice of invasive vs conservative strategies, the selection of the most appropriate revascularization modality or stent, the prescription of long-term dual-antiplatelet therapy or anti-coagulation or the selection of the best candidates for gastroprotection with proton pump inhibitors. Beyond its potential value in ascertaining relative changes in the risk of bleeding depending on the choice of therapy by including anti-coagulation and anti-platelet therapy in its construction, the BLEED-MI score may help estimate the baseline risk for future treatment decisions.
The definition of significant hemorrhagic events used in this study partially overlaps with those of the TIMI and GUSTO trials. Therefore, clinically significant hemorrhage included any major, severe or life-threatening bleeding event, namely those at intracerebral location, those resulting in substantial hemodynamic compromise requiring treatment or in reduction of hemoglobin of 5 g/dL or more (or > 15% in hematocrit). They also included moderate bleeding, defined by the need for transfusion, a drop in hemoglobin of 3-5 g/dL (or in hematocrit from 10% to 15%) from previous blood tests to the time of admission, the occurrence of spontaneous gross hematuria or hematemesis even in the absence of hemoglobin drop higher than 3 g/dL, or unobserved loss of 4 g/dL or more in hemoglobin.
This is an interesting study developing and validating a novel risk score for post-discharge bleeding in patients with acute MI.
P- Reviewer Biondi-Zoccai GGL S- Editor Wen LL L- Editor A E- Editor Ma S
| 1. | Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 633] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 2. | Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 985] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 3. | Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, Caixeta A, Feit F, Manoukian SV, White H. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 315] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 4. | Lopes RD, Subherwal S, Holmes DN, Thomas L, Wang TY, Rao SV, Magnus Ohman E, Roe MT, Peterson ED, Alexander KP. The association of in-hospital major bleeding with short-, intermediate-, and long-term mortality among older patients with non-ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:2044-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Rao SV, O’Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, Mahaffey KW, Califf RM, Harrington RA. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 6. | Mehran R, Pocock SJ, Stone GW, Clayton TC, Dangas GD, Feit F, Manoukian SV, Nikolsky E, Lansky AJ, Kirtane A. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J. 2009;30:1457-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 7. | Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 8. | Berkowitz SD, Granger CB, Pieper KS, Lee KL, Gore JM, Simoons M, Armstrong PW, Topol EJ, Califf RM. Incidence and predictors of bleeding after contemporary thrombolytic therapy for myocardial infarction. The Global Utilization of Streptokinase and Tissue Plasminogen activator for Occluded coronary arteries (GUSTO) I Investigators. Circulation. 1997;95:2508-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Spencer FA, Moscucci M, Granger CB, Gore JM, Goldberg RJ, Steg PG, Goodman SG, Budaj A, FitzGerald G, Fox KA. Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction. Circulation. 2007;116:2793-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Manoukian SV. Predictors and impact of bleeding complications in percutaneous coronary intervention, acute coronary syndromes, and ST-segment elevation myocardial infarction. Am J Cardiol. 2009;104:9C-15C. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4971] [Cited by in RCA: 4922] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 12. | Becker RC, Bassand JP, Budaj A, Wojdyla DM, James SK, Cornel JH, French J, Held C, Horrow J, Husted S. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2011;32:2933-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 13. | Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 405] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 14. | Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, Topol EJ, Manoukian SV. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol. 2007;100:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | Nikolsky E, Mehran R, Dangas G, Fahy M, Na Y, Pocock SJ, Lincoff AM, Stone GW. Development and validation of a prognostic risk score for major bleeding in patients undergoing percutaneous coronary intervention via the femoral approach. Eur Heart J. 2007;28:1936-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2313] [Article Influence: 177.9] [Reference Citation Analysis (0)] |
| 17. | Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, Dodge HT, Francis CK, Hillis D, Ludbrook P. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1704] [Cited by in RCA: 1686] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 18. | An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2804] [Cited by in RCA: 2631] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 19. | Graf E, Schmoor C, Sauerbrei W, Schumacher M. Assessment and comparison of prognostic classification schemes for survival data. Stat Med. 1999;18:2529-2545. [PubMed] |
| 20. | Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 736] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 21. | Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 720] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 22. | Ducrocq G, Wallace JS, Baron G, Ravaud P, Alberts MJ, Wilson PW, Ohman EM, Brennan DM, D’Agostino RB, Bhatt DL. Risk score to predict serious bleeding in stable outpatients with or at risk of atherothrombosis. Eur Heart J. 2010;31:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Abu-Assi E, Raposeiras Roubin S, Agra-Bermejo RM, Cabanas-Grandio P, Gestal Romari S, Pereira Lopez E, Martinez Cereijo JM, Garcia Acuna JM, Pena Gil C, Gonzalez-Juanatey JR. Utility and reliability of the REACH risk score in evaluating the risk of post-discharge bleeding in a contemporary cohort of patients with ACS patients. European Heart Journal. 2011;32:Abstract Supplement 735. |
| 24. | Kurth T, Kase CS, Berger K, Schaeffner ES, Buring JE, Gaziano JM. Smoking and the risk of hemorrhagic stroke in men. Stroke. 2003;34:1151-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Kurth T, Kase CS, Berger K, Gaziano JM, Cook NR, Buring JE. Smoking and risk of hemorrhagic stroke in women. Stroke. 2003;34:2792-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Andersen IB, Jørgensen T, Bonnevie O, Grønbaek M, Sørensen TI. Smoking and alcohol intake as risk factors for bleeding and perforated peptic ulcers: a population-based cohort study. Epidemiology. 2000;11:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (35)] |
| 27. | De Berardis G, Lucisano G, D’Ettorre A, Pellegrini F, Lepore V, Tognoni G, Nicolucci A. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2971] [Cited by in RCA: 3359] [Article Influence: 223.9] [Reference Citation Analysis (0)] |
| 29. | Attallah N, Yassine L, Fisher K, Yee J. Risk of bleeding and restenosis among chronic kidney disease patients undergoing percutaneous coronary intervention. Clin Nephrol. 2005;64:412-418. [PubMed] |
| 30. | Fox KA, Antman EM, Montalescot G, Agewall S, SomaRaju B, Verheugt FW, Lopez-Sendon J, Hod H, Murphy SA, Braunwald E. The impact of renal dysfunction on outcomes in the ExTRACT-TIMI 25 trial. J Am Coll Cardiol. 2007;49:2249-2255. [PubMed] |
| 31. | Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, Dalby AJ, Montalescot G, Braunwald E. Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel--Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38). Circulation. 2011;123:2681-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 32. | Bassand JP, Afzal R, Eikelboom J, Wallentin L, Peters R, Budaj A, Fox KA, Joyner CD, Chrolavicius S, Granger CB. Relationship between baseline haemoglobin and major bleeding complications in acute coronary syndromes. Eur Heart J. 2010;31:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, Gibson CM, Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 510] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 34. | Biondi-Zoccai G, Romagnoli E, Agostoni P, Capodanno D, Castagno D, D’Ascenzo F, Sangiorgi G, Modena MG. Are propensity scores really superior to standard multivariable analysis. Contemp Clin Trials. 2011;32:731-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 35. | Wallace TW, Rao SV. The challenge of defining bleeding among patients with acute coronary syndromes. Clin Cardiol. 2007;30:II16-II23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |