Published online Aug 26, 2025. doi: 10.4330/wjc.v17.i8.107811
Revised: May 7, 2025
Accepted: July 10, 2025
Published online: August 26, 2025
Processing time: 145 Days and 13.5 Hours
Extracorporeal membrane oxygenation (ECMO) is mainly applied to patients with significant cardiorespiratory failure who do not respond to existing conventional treatments. Patients that are supported with veno-arterial ECMO (VA-ECMO) are considered very-high risk patients to participate in any type of physical therapy (PT) or mobilization. However, cumulative evidence suggests that early mobilization of critically ill patients is feasible, safe, and efficient under certain circumstances.
To summarize the existing evidence on the impact of early mobilization and physiotherapy on VA-ECMO patients.
This is a scoping review that used systematic electronic literature searches (from inception until January 2025) on MEDLINE (PubMed), PEDro, DynaMed, CINAHL, Scopus, Science direct and Hellenic Academic Libraries. Snowball searching method was also applied. Eligible studies included those reporting patients on VA-ECMO who participated in early mobilization or PT, published in English and utilized any primary evidence study design. Studies on children, animals and patients placed on any other ECMO, secondary evidence, and ‘grey’ literature were excluded.
A total of 316 articles were retrieved and 13 were included in the study. Of those, 1 study was a randomized control trial, 4 retrospective studies, 4 retrospective cohort studies, 1 case series and 3 case reports. The sample size of the included studies ranged from 1 to 104 VA-ECMO patients, who were ambulated or received PT inter
Early mobilization in VA-ECMO seems to be safe and can potentially help reduce vasoconstrictors and speed up rehabilitation times. High quality research on early mobilization in VA-ECMO patients is warranted.
Core Tip: This scoping review summarizes evidence of the effects of early mobilization on extracorporeal membrane oxygenation (ECMO) and veno-arterial ECMO (VA-ECMO) patients. VA-ECMO patients can safely participate in early mobilization and physical therapy (PT) interventions under certain criteria. Mobilization of VA-ECMO patients can be considered safe regardless of the anatomical insertion of the cannula. Mobilization and application of PT in VA-ECMO patients seems to contribute to improved respiratory volume and respiratory rate, as well as to the stabilization or reduction of inotropes. Specific PT and early mobilization intervention guidelines for VA-ECMO patients need to be developed.
- Citation: Kanellou V, Kaliarntas K, Dounavi DM, Patsaki I, Kalpaxis D, Kourek C, Dimopoulos S. Early mobilization in patients on venoarterial extracorporeal membrane oxygenation: A scoping review. World J Cardiol 2025; 17(8): 107811
- URL: https://www.wjgnet.com/1949-8462/full/v17/i8/107811.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i8.107811
Extracorporeal membrane oxygenation (ECMO) is mainly applied to patients with significant cardiorespiratory failure who do not respond to existing conventional treatments[1,2]. The ECMO circuit consists of a control console, a centrifugal pump, a membrane oxygenator, and two large cannulas[3]. There are two main types of ECMO, depending on the system that is supported. Specifically, veno-venous ECMO (VV-ECMO), is used to support only the respiratory system, while veno-arterial ECMO (VA-ECMO), is used to support both the circulation and heart[4].
Typically, those who require ECMO support are bedridden critically ill patients. Therefore, the establishment of intensive care unit acquired neuromuscular weakness (ICU-AW) is almost inevitable[5], as well as its consequences, which affect patients’ prognosis. ICU-AW occurs in 30%-50% of critically ill patients and up to 67% in critically ill patients with sepsis[6]. ICU-AW is also associated with significant physical and cognitive deficits occurring in approximately 25%-80% of cases[7,8]. Consequently, patients have longer hospitalization, increased mortality (46%)[9] and poor quality of life[10]. The deterioration of physical, mental, and cognitive function of critically ill patients is collectively described as Post Intensive Care Syndrome[11]. Recent literature suggests that early mobilization is safe, feasible and may prevent ICU-AW[12].
VA-ECMO patients are considered very high-risk to participate in any type of physical therapy (PT) or mobilization. There are practical aspects, such as the insertion sites of the cannulas (e.g., femoral cannula), which are very prone to accidental dislocation during mobilization. Other common barriers that prevent early mobilization are the deep sedation and the myorelaxants used in cardiopulmonary supported patients, and the lack of experienced and skilled staff to provide safe mobilization[13].
Early mobilization has been proposed as a safe and feasible preventive tool for ICU-AW in ECMO patients[14] after thorough initial risk assessment and screening with the appropriate PT planning and exercise monitoring. However, there is limited evidence about early mobilization of VA-ECMO patients, who are considered as the most critically ill patients due to the high risk of destabilization and the risk of peripheral arterial cannula dislocation. The reviews that have investigated the effects of mobilization on ECMO patients[15] mainly concern VV-ECMO patients or consider mixed populations of VV-ECMO and VA-ECMO patients[16-20].
In this scoping review, studies with VA-ECMO patients will be presented separately from studies with a mixed popu
Therefore, the aim of this study is to conduct a scoping review to identify, critically evaluate and synthesize current evidence regarding the effects of early mobilization on ECMO and more specifically on VA-ECMO patients.
More specifically, this study aims to address the following questions: (1) Has early mobilization been used on VA-ECMO patients and if yes, what mobilization protocols have been used; (2) What are the optimal mobilization parameters for VA-ECMO patients; (3) Does early mobilization improve the respiratory and hemodynamic parameters of VA-ECMO patients; (4) Is early mobilization safe for VA-ECMO patients; and (5) What are the criteria for participation in early mobilization of VA-ECMO patients.
This is a scoping review, and electronic searches were conducted using the following databases: (1) Electronic databases of MEDLINE (PubMed), PEDro, DynaMed, CINAHL; (2) Electronic libraries Scopus, Science direct; and (3) The Hellenic Academic Libraries (HeAL). Additionally, manual reference list searches of the included studies were also performed (snowball technique). DynaMed, CINAHL, Scopus, Science direct and HeAL were searched through the library of the National and Kapodistrian University of Athens. The literature searches and screening were performed by the main author (Kanellou V), the identified studies were screened by a second author (Kaliarntas K) and the appraisal of the evidence was performed by a third author (Dounavi DM). The study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyse (PRISMA) guidelines[21] and in line with a preregistered protocol https://doi.org/10.17605/OSF.IO/3U6YD.
The keywords used were: “Physical therapy”, “Early ambulation”, and “Extracorporeal membrane oxygenation”, “Physiotherapy”, “Early mobilization”. A combination of these keywords with Boolean operators were applied in diffe
Only studies with VA-ECMO or hybrid forms of arterial ECMO venovenous-venoarterial ECMO, venovenous-arterial ECMO (VV-A-ECMO) patients participating in mobilization interventions, were included. Studies with a mixed ECMO population were also considered and were presented separately. All searches were applied from inception to January 2025. There were no exclusions based on age, gender or the reason for admission to VA-ECMO. Only papers published in English and all types of study designs presenting primary evidence were accepted.
Studies on children and animals, book chapters, secondary and grey literature were excluded.
The assessment of the methodological quality of the literature is not necessary in scoping reviews[22]. However, in this review, the quality of the included studies was appraised. The only randomized control trial (RCT) that was found and included in this review was quality appraised with the PEDro scale[23]. The Newcastle Ottawa Scale (NOS)[24] was used to evaluate the observational studies, and the case studies were appraised by a 18-item scale[25].
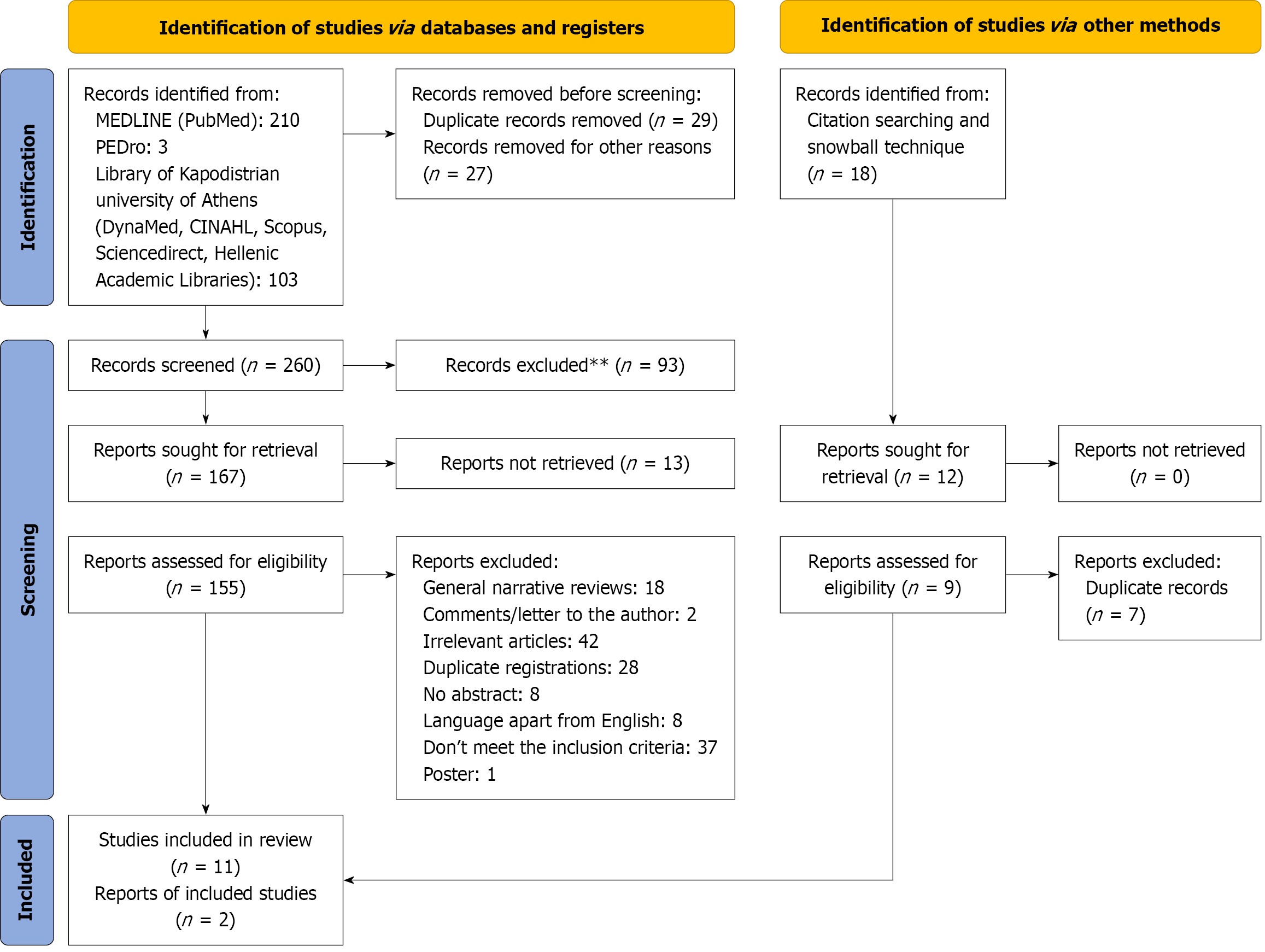
The search strategy revealed 316 articles in total. After screening the titles and the abstracts, 56 articles were excluded, as they were not relevant or were duplicates. The full texts of the 167 articles were retrieved, and 13 were found to meet the inclusion criteria (Figure 1)[21].
Studies that reported data only on VA-ECMO patients are presented separately. Studies with mixed ECMO popula
The studies included consisted of: (1) 1 randomized controlled clinical trial[3]; (2) 4 retrospective observational studies[26-29]; (3) 4 retrospective cohort studies[2,13,24,30]; (4) 3 case studies[31-33]; and (5) 1 case series[34].
The randomized clinical trial[3] was considered of “good” methodological quality (7/10 on the PEDro scale; Table 1). The methodological shortcomings are the blinding of the sample, of the examiners and of the evaluators.
| Study | Hayes et al[3] |
| Eligibility criteria | Yes |
| Random allocation | Yes |
| Concealed allocation | Yes |
| Baseline comparability | Yes |
| Blind subjects | No |
| Blind therapists | No |
| Blind assessors | No |
| Adequate follow-up | Yes |
| Intention-to-treat analysis | Yes |
| Between-group comparisons | Yes |
| Point estimates and variability | Yes |
| Total score | 7 |
The 8 observational studies were assessed with the NOS and they all were of low to moderate methodological quality (Table 2)[2,13,26-30,35]. Two retrospective studies[2,28] did not meet the criteria for “representativeness of the exposed cohort” of the NOS scale whereas two other retrospective observational studies[26,28] did not meet the criteria for “follow up”. None of the included observational studies[2,13,26-30,35] met the “selection of the non-exposed cohort” criterion; however they did assess their outcome.
| Ref. | Selection | Comparability | Exposure | Score | ||||||
| Representa-tiveness of the exposed cohort | Selection of the non-exposed cohort | Ascertain-ment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design | Comparability of cohorts on the basis of the analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Abrams et al[30] | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Abrams et al[2] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Braune et al[26] | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 4 |
| Ko et al[27] | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Mayer et al[28] | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
| Munshi et al[13] | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 5 |
| Pasrija et al[29] | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Wells et al[35] | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
Two out of 3 case studies[29,31] had low methodological quality, as they met only 4 out of the 18 criteria[25]. The case study by Orozco-Hernandez et al[33] met 7 out of the 18 criteria (39% of the scale’s criteria) and had good methodological quality. The case series[34] had high methodological quality and met 14 (78%) out of the 18 criteria (Table 3)[31-33].
| Ref. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Score |
| Case studies | |||||||||||||||||||
| Rinewalt et al[31] | Yes | PS | No | PS | N/A | N/A | Yes | Yes | Yes | Uncertain | Uncertain | N/A | No | N/A | N/A | No | PS | No | 4 |
| Shudo et al[32] | Yes | PS | No | PS | N/A | N/A | Yes | Yes | Yes | Uncertain | No | N/A | No | N/A | N/A | No | PS | Uncertain | 4 |
| Orozco-Hernandez et al[33] | Yes | Yes | N/A | N/A | N/A | N/A | Yes | PS | Yes | Uncertain | No | No | Yes | No | N/A | Yes | PS | Yes | 7 |
| Case series | |||||||||||||||||||
| Whitlock et al[34] | Yes | PS | No | Yes | Uncertain | Yes | Yes | PS | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 14 |
The study samples ranged from 1 to 104 adult patients who were mobilized or received PT interventions (Table 4)[2,3,13,26-32,35]. Eleven studies[3,13,26-28,30-35] assessed 225 patients, which were mobilized on VA-ECMO. In one study, the number of patients on VA-ECMO who received PT intervention or were mobilized is not reported[2]. Similarly, in the study by Pasrija et al[29], there is no clarity on how many patients on VA-ECMO were mobilized and received PT. In addition, there was no precise data about the gender or age of the VA-ECMO patients. Approximately 60% of patients on VA-ECMO, were male and 40% female. The age of the patients included ranged between 16-83 years[2,3,13,26-36].
| Authors/date/study type/appraisal | Sample/reason for VA-ECMO | Mobilization and PT | Frequency | Results | Critique | Complications during mobilization |
| Abrams et al[30]; retrospective cohort study; NOS: 6; separate data on VA-ECMO: No | A total of 100 ECMO patients. A total of 35 patients were immobilized (12 VA-ECMO patients/23 VV-ECMO patients), 5 VA-ECMO patients and 14 VV-ECMO patients for transplantation. A total of 7 VA-ECMO patients and 9 VV-ECMO patients for recovery | No mobilization or passive range of motion of extremities; turning in bed (including active-assisted range of motion of extremities); sitting in bed with the head of bed elevated; sitting on the edge of the bed with feet on floor; sitting in a chair; standing; marching on the spot; ambulation | 7.2 ± 6.5 PT sessions while on ECMO; 2.8 PT sessions per week | During PT sessions, 18 patients (51%) ambulated [Distance: 53.34 m (IQR 11.43-86.87)]. During ECMO, 23 (66%) patients were liberated from invasive mechanical ventilation. Of the 16 recovery patients, 14 (88%) survived to discharge; 10 transplant patients (53%) survived to transplantation. 90% of survivors were discharged. In total, of the 23 survivors, 13 (57%) went home, 8 (35%) to acute rehabilitation, and 2 (9%) to subacute rehabilitation | Mobilization of patients on ECMO is safe and contributes to faster weaning from invasive mechanical ventilation and faster recovery | There were no PT-related complications |
| Abrams et al[2]; retrospective cohort study; NOS: 6; separate data on VA-ECMO: No | A total of 511 ECMO patients (184 VA-ECMO patients and 327 VV-ECMO patients). A total of 177 patients were immobilized, 33/177 VA-ECMO patient with femoral cannula. A total of 124/177 for transplantation and 53/177 for recovery | Nothing (lying in bed); sitting in bed; passively moved to chair; sitting over edge of bed; standing weight bearing through the feet in the standing position; transferring bed to chair; marching on spot; walking with assistance of 2 or more people; walking with assistance of 1 person; walking independently with a gait aid; walking independently without a gait aid | A total of 2.706 PT sessions; 108 (61%) patients, who walked participated in 1.284. The 34 patients, with femoral cannulas, participated in 250 sessions | BTT (OR = 17.2, 95%CI: 4.12–72.1), VV-ECMO (OR = 2.83, 95%CI: 1.29–6.22) and higher Charlson comorbidity index (OR = 1.53, 95%CI: 1.07–2.19) were associated with increased odds of achieving out-of-bed exercises vs in-bed PT. Invasive mechanical ventilation (OR = 0.11, 95%CI: 0.05–0.25) and femoral cannulation (OR = 0.19, 95%CI: 0.04–0.92) were associated with decreased odds of performing out-of-bed activities. IMS score on ECMO: BTT 7 (5–9); bridge to recovery 3 (1–5), (P= 0.001) | ECMO patients’ mobilization is safe. VenoVenous ECMO, and higher Charlson comorbidity index were associated with increased odds of achieving out-of-bed vs in-bed PT. However, invasive mechanical ventilation and femoral cannulation were associated with decreased odds of performing out-of-bed activities | A total of 13 isolated bleeding events at the cannula insertion site, (10 involved femoral cannulas. Stroke in 2 patients cardiac arrest in 1 VV-ECMO patient |
| Braune et al[26]; prospective observational study; NOS: 4; separate data on VA-ECMO: No | A total of 115 ECMO patients [VA-ECMO (n = 63), VV-ECMO (n = 26), veno-venous extracorporeal CO2 removal (n = 12), veno-arterial-extracorporeal CO2 removal (n = 10)]. A total of 43 patients mobilized, 17 VA-ECMO patients. Bridge for transplantation: 2 patients | Functional strengthening, breathing exercises, active upper and lower limb exercises, endurance exercises, and progressing functional mobility; IMS | Total mobilization sessions IMS ≥ 3, during ECMO: 72 sessions on VA-ECMO patients. Mobilization median IMS ≥ 3 was 130 minutes (IQR 44–215) | A total of 43 (37.4%) ECMO patients were actively immobilized, with an activity level on the IMS of ≥ 3. The 108 patients (93.9%) had femoral cannulation. Duration of activities (IMS ≥ 3): 130 minutes (IQR: 44–215). All mobilizations were applied by a multi-professional ECLS team. A total of 3 members involved (IQR: 3–4). Mobilization was significantly associated with the severity of illness (P ≤ 0.01) | ECMO patients’ early mobilization is safe and require multidiscipline team. Sedation was the main reason for non-mobilization | Bleeding from cannulation site during mobilization: 6.9%. During mobilization, one accidental episode of the femoral cannula displacement |
| Hayes et al[3]; pilot randomized controlled trial; PEDro scale: 7; separate data on VA-ECMO: Yes for the sessions frequency | A total of 15 ECMO patients [VA-ECMO (n = 10), VV-ECMO (n = 5)]. A total of 7 patients participated in an intense rehabilitation program and 8 in classic PT. Bridge for recovery | Intervention program: Progression of exercises to the highest level of mobility that the patient could tolerate. Classic PT program: Passive range of motion exercises to unstable or sedated patients. To the stable patients, rehabilitation consisted of resistance and active exercises and if was capable, sitting on the edge of the bed, standing and ambulation | The intervention program was applied for 7 days. Rehabilitation was applied for about 1 hour per day and there was a minimum of 20 minutes for passive exercise and 30 minutes for active exercise | There was no difference between the groups concerning the respiratory, hemodynamic, or ECMO parameters. The intensive rehabilitation group was exercised more than the standard care group (mean = 28.7 minutes vs 4.2 minutes, P < 0.0001). Treatment group [12.9 (7.2-16.7) days] had lower length of stay in relation with the standard care group [21.4 (15.5-38.5) days] (P= 0.05). Respiratory rate [r (SE): | The physiological parameters associated with the highest level of mobility (IMS) during PT, were respiratory rate and maximum tidal volume. Generally, early mobilization has low impact on physiological factors of ECMO patients | No complications mentioned |
| Ko et al[27]; retrospective study; NOS: 6; separate data on VA-ECMO: Yes | A total of 8 ECMO patients. A total of 8 patients were mobilized (1 VA-ECMO patient and 7 VV-ECMO patients). The 1 VA-ECMO (central) patients and 2 VV-ECMO for transplantation. A total of 5 VV-ECMO for recovery | A total of 62 sessions: 31 sessions (50%) of passive mobilization (VV-ECMO and VA-ECMO) and electrical muscle stimulation (VV-ECMO); 17 sessions (27.4%) of sitting in bed or on the edge of bed (VV-ECMO and VA-ECMO); 2 sessions (3.2%) of strengthening while sitting (VV-ECMO); 11 sessions (18%) of standing or marching in place (VV-ECMO); 1 session (2%) for walking (VV-ECMO) | A total of 62 sessions. The sessions’ frequency and duration it is not mentioned | The early mobilization of ECMO patients is safe and beneficial. It contributes to the better functionality and physical situation of the ECMO patients. The ECMO flow rate was higher during PT (2.53 ± 0.71) in contrast before (2.36 ± 0.65) (P = 0.013). However, the sweep gas flow rate of ECMO was not different before PT (4.89 ± 1.78) and during PT (4.90 ± 1.78) (P = 0.321) | The early mobilization of ECMO patients is safe and contributes to faster rehabilitation | Three sessions (5%) were interrupt due to tachycardia (n = 1) and tachypnea (n = 2). There was no clinically significant adverse event |
| Mayer et al[28]; retrospective study; NOS: 6; separate data on VA-ECMO: Yes for the sessions frequency | A total of 315 ECMO patients. A total of 218 patients were mobilized (venoarterial: 84 patients, venovenous single lumen: 53 patients, venovenous double lumen: 62 patients. Hybrid (venoarterial-venovenous): 19 patients). Bridge for recovery | IMS; nothing (lying in bed); sitting in bed; passively moved to chair; sitting over edge of bed; standing; transferring bed to chair; marching on spot; walking with assistance of 2 or more people; walking with assistance of 1 person; walking independently with a gait aid; walking independently without a gait aid | Sessions’ frequency: 4-7 times per week. Sessions’ average: 7.4 (SD: 12.7). Sessions’ frequency on ECMO: Mean: 0.41 (SD: 0.24). Occupational therapy: Session number on ECMO: Mean: 2.2 (SD: 5.5). Sessions’ frequency on ECMO: Mean: 0.24 (SD: 0.14) | A total of 218 patients (69%) had at least 1 PT session. A total of 70 patients (22%) received rehabilitation after ECMO therapy and 27 patients (9%) did not participate in rehabilitation. Survivors had mobility levels and more positive rate of change in mobility over the first 4 sessions than individuals who died in the hospital (2.8 vs 0.38, df = 199, t = 8.24, P < 0.001). The patients that could sit on the edge of the bed and walk for > 45 m were more likely to survive (47% vs 13%, χ2 = 156, P < 0.0001) than those who did not (26% vs 3.5%, χ2 = 80, P < 0.0001) | The ECMO survivors who participated in early rehabilitation achieved higher mobility levels and faster rehabilitation | No adverse events reported |
| Munshi et al[13]; retrospective cohort study; NOS: 5; separate data on VA-ECMO: Yes for the mobilization protocols | A total of 107 patients on ECMO. A total of 61 Acute Respiratory Distress Syndrome patients on ECMO. A total of 57 on VV-ECMO and 4 on V- VA-ECMO. A total of 50 patients (47 on VV-ECMO and 3 on VV-A-ECMO) were mobilized. Bridge: 61 patients for recovery | Passive mobilization; active mobilization; sitting; standing; IMS | It is not described | The 18 patients achieved an activity level of 2 or higher (active exercises in bed), and 8 patients achieved an activity level 4 or higher (actively sitting over the side of the bed). ICU physiotherapy (OR = 0.19, 95%CI: 0.04–0.98), Acute Physiology and Chronic Health Evaluation II score (OR = 1.13, 95%CI: 1.01–1.26), and sex (OR = 8.4, 95%CI: 1.71–41.7) were significantly associated with ICU mortality | The early mobilization of the ECMO patients is safe and was related with lower mortality | No adverse events reported |
| Pasrija et al[29]; retrospective study; NOS: 6; separate data on VA-ECMO: Yes | A total of 104 VA-ECMO patients. A total of 15 patients were mobilized out of bed. Bridge for recovery | Strength exercises; bed to chair transferring; walking | It is not described | Of the 104 VA-ECMO patients, 15 were immobilized with a femoral arterial cannula. Time duration from cannulation to out of bed exercises was 3 (0-26) days. Time duration from cannulation to initial mobilization was 4 (1-42) days. Median distance of the first postcannulation walk: 91.44 m. There wasn’t any decrease in ECMO flow and ECMO speed during or after ambulation. ICU stay: 12 days. Hospitalization: 21 days. One-year survival was 100% for ambulating patients | Selected VA-ECMO patients can be safely ambulated even though they have arterial cannulation | A total of 3 minor bleeding events (20%). There were no major bleeding episodes, vascular complications, or cannula displacement events related to mobilization |
| Rinewalt et al[31]; case report; 18-criteria checklist by the Delphi panel: 4 yes; separate data on VA-ECMO: Yes | A total of 1 VA-ECMO patient. Bridge for heart transplantation | Standing and isometric upper body exercises according to his personalized program; flexion of the femoral region (sitting, walking) was avoided | It is not described | The patient underwent VA-ECMO as a bridge to heart transplantation. While on ECMO, he participated in a pt. program that included tilt bed with lower body support and excluded hip flexing to secure ECMO cannulas. The patient was discharged home 21 days after heart transplantation, due to his aggressive rehabilitation while he was on VA-ECMO support | Early mobilization contributed to the faster recovery of the transplanted patient | No complications mentioned |
| Shudo et al[32]; case report; 18-criteria checklist by the Delphi panel: 4 yes; separate data on VA-ECMO: Yes | A total of 1 VA-ECMO (central) patient. Bridge for heart and lung transplantation | Tilt Bed: Day 1: 45° incline and 40% weight bearing (30’). After 7 days: 180° incline and full weight bearing (30’). After 10 days: Standing and walking beside the bed with assistance. After 14 days: Walking (30’) with minimal assistance and strengthening exercises | 19 days | Successful heart-lung transplant after 19 days of VA-ECMO support. Discharged from hospital 12 days after transplant | Mobilization led to better recovery of the patient after heart transplantation | No complications mentioned |
| Wells et al[35]; retrospective cohort study; NOS: 6; separate data on VA-ECMO: No | A total of 254 ECMO patients. A total of 167 patients were immobilized (98/167 on VV-ECMO and 69/167 on VA-ECMO). Bridge: 167/167 recovery | Range of motion strength and endurance exercises, stretching, breathing exercises. Mobilization in bed; activities and sitting at the edge of the bed; sitting, standing and transferring; transfers to other surfaces; standing activities; walking (gait training, speed, endurance) | A total of 268 therapeutic exercises; 170 bed mobilization; 100 bedside mobilization; 106 sitting–standing interventions; 39 standing– transfers; 98 standing activity interventions; 37 walking | The 667% of the ECMO patients were mobilized. A total of 607 physiotherapy sessions were performed. A total of 134 patients (80.2%) had at least one femoral cannula during their mobilization. A total of 25 patients (15%) participated in standing or walking. A total of 8 patients (4.8%) were ambulated 91.44 (9.144-304.8) m. A total of 75 patients (68.8%) who were successfully weaned from ECMO were discharged to a rehabilitation unit and another 26 patients (23.8%) went home | Early mobilization of ECMO patients is safe. Patients who were early mobilized had higher IMS scores and greater functional capacity during and after weaning from ECMO support compared to patients who started rehabilitation after ECMO weaning | There were 3 minor events (< 0.5%). A total of 2 arrhythmia episodes; 1 hypotension event |
The reasons for VA-ECMO use are mentioned in 11 of the 13 studies[3,13,26-34] and mainly concern heart failure and cardiogenic shock. Other reasons[2,3,26-28] were pulmonary hypertension[29], post-cardiac surgery, pulmonary embo
Eleven patients on VA-ECMO, who were mobilized and received PT[27], were waiting for transplantation[30-34], and 190 patients on VA-ECMO who were mobilized and receiving PT used the membrane oxygenator as a bridge to recovery[3,28-30,35]. In four studies, it is unclear how many patients on VA-ECMO, who mobilized and received PT, were candidates for transplantation or recovery[2,13,26,29].
Additionally, in most studies[2,3,13,26,27] the population was mixed[28,30,34,35] (for example individuals with respi
The included studies used a range of outcomes (Table 5) and were grouped according to the present study’s questions[2,3,13,26-35].
| Ref. | Sedation | Mobility level | Severity of illness | Other parameters |
| Abrams et al[30] | X | Validated IMS | APACHE II | ECMO blood flow rate, ECMO sweep gas flow rate by monitoring. Vasopressor dosage during Physical therapy |
| Abrams et al[2] | X | IMS | APACHE II, Charlson comorbidity index, SOFA | Arterial blood gas analyses by blood drawing, vasopressors dosage, ECMO settings by monitoring, days of hospitalization |
| Braune et al[26] | RASS | IMS | Simplified Acute Physiology Score-II, APACHE II, SOFA | Hemodynamic parameters and ECMO settings by monitoring |
| Hayes et al[3] | RASS | IMS | APACHE II, APACHE III, Clinical Frailty Scale | Patients’ functionality by Borg scale, Katz Index of Independence in Activities of Daily living score, Functional Comorbidity Index. Respiratory, hemodynamic, and ECMO parameter by monitoring |
| Ko et al[27] | RASS | Modified IMS | X | Respiratory, hemodynamic, and ECMO parameter by monitoring |
| Mayer et al[28] | RASS | IMS | SOFA | Days of hospitalization, discharge destination, and 30-day readmission rates were recorded |
| Munshi et al[13] | SAS | IMS | X | ECMO data, ventilator data |
| Orozco-Hernandez et al[33] | X | X | X | Respiratory and hemodynamic parameters |
| Pasrija et al[29] | X | X | X | Intubation duration, ECMO duration and settings, days of hospitalization |
| Rinewalt et al[31] | X | X | X | X |
| Shudo et al[32] | X | X | X | X |
| Wells et al[35] | RASS | IMS | X | Days of ECMO support, and survival data |
| Whitlock et al[34] | X | Activity Measure for Post-Acute Care "6-clicks" | APACHE-IV | Hospital length of stay, intensive care unit Length of stay, hemodynamic and physiological responses to exercise |
Studies on VA ECMO patients: Altogether, 17 VA-ECMO patients, (15 for recovery and 2 for heart transplantation) participated in strengthening and functional exercises[29,32,33]. Moreover, all referred patients sat and walked[29,33] apart from 1 patient[31] who had femoral cannula. Also, 1 patient on central VA-ECMO was gradually mobilized on a reclining bed and finally walked[32].
Studies with mixed ECMO populations but with separate data on VA-ECMO patients: Ko et al[27] reported that their single VA-ECMO patient was seated on the bed. Additionally, Munshi et al[13] mobilized 2 VV-A-ECMO patients from 0 to 1 [intensive care unit mobility scale (IMS)] and 1 VV-A-ECMO patient greater than 2 (IMS).
Studies with mixed ECMO populations, but without separate data on VA-ECMO patients: Abrams et al[30] mobilized 32% of ECMO patients actively and passively on the bed, 9% in a sitting position, 9% in standing and 51% ambulated. In another retrospective cohort study, 96 ECMO patients were passively mobilized on the bed, 2 patients participated in active exercises on the bed, 28 patients sat on the edge of the bed, 11 patients stood up, 12 patients achieved bed-to-chair transfers, 6 patients marched, 7 patients walked with the assistance of 2 people and 8 patients walked with the help of 1 person[35]. Braune et al[26] reported that 43 (37.4%) ECMO patients were actively mobilized (IMS ≥ 3). Specifically, 13.6% of ECMO patients sat on the edge of the bed, 6.3% stood up and 0.6% walked with assistance[26]. Additionally, Hayes et al[3] reported that 3 of 7 VA-ECMO patients in the intervention group (43%) and none in the control group were able to be mobilized out of bed. In the study by Abram et al[2], 177 (35%) ECMO patients underwent active kinesiotherapy, 138 were mobilized out of bed and 108 walked. Similarly, Mayer et al[28] mobilized 84 patients on VA-ECMO and 19 on VA-VV ECMO and reported that 188 patients sat on the edge, 169 stood, 129 walked longer than 1.5 m, and 95 walked longer than 4.5 m. Finally, Whitlock et al[34] mobilized 8 ECMO patients according to their ability and physiological condition. The median number of ambulation while on ECMO was 17 m [interquartile range (IQR): 17.5]. The median distance of walking during their last session was 228.6 m (IQR: 320.0) vs zero during the initial session.
There was variation in the parameters of the mobilization and PT sessions used in VA-ECMO patients.
Studies on VA ECMO patients: In the case study of Shudo et al[32], each session lasted 30 minutes for 19 days (1 per day). Orozco-Hernandez et al[33] applied PT sessions 4 to 5 times per week, depending on the patient’s mobilization progress. There is no reference to the mobilization parameters and PT in the other 2 studies[29,31].
Studies with mixed ECMO populations with separate data on VA-ECMO patients: Ko et al[27] reported that their VA-ECMO patient participated in a total of 23 mobilization sessions, 7 sitting sessions, and 31 passive PT sessions. Addi
Studies with mixed ECMO populations, but without separate data on VA-ECMO patients: Abrams et al[30] reported a total of 7.2 ± 6.5 PT sessions per patient and 2.8 PT sessions per patient per week. Moreover, Wells et al[35], recorded a total of 607 PT sessions. Braune et al[26] counted 332 sessions at mobilization level (IMS) ≥ 3 during 1242 (26.7%) days of ECMO application. Abrams et al[2] counted 2706 PT sessions, with a median of 8 (IQR: 2.0–21.0) sessions for each patient. Finally, Whitlock et al[34] applied PT 3-6 days per week depending on each patients’ condition. They performed 246 sessions on ECMO patients and advanced ambulation sessions while on ECMO on 129 occasions, whereas the also used 116 non-ambulation PT sessions.
Studies on VA-ECMO patients: In the study of Pasrija et al[29], ECMO speed and flow and the hemodynamic parame
During PT: Regarding ECMO parameters, Hayes et al[3] recorded lower ECMO gas mixture flow, and Abrams et al[30] reported no statistical difference in ECMO gas mixture flow. However, Ko et al[27] found higher blood flow (P = 0.013) in ECMO, while Abrams et al[30] found no statistical difference before and during PT. ECMO sweep gas flow rate was not statistically different before or during PT (P = 0.321) in Ko et al[27]. Also, Hayes et al[3] found a statistically significant association among the respiratory rate and the maximal tidal volume (P ≤ 0.006) with the higher mobility level (IMS). Abrams et al[30] found no difference between the amount of conventional ventilation support before and during therapy PT. Finally, PT application was associated with lower sedation level[3,13,28] and stable or lower vasopressor dosage[3,30].
Effects of mobilization: Hayes et al[3] reported that higher mobilization levels (IMS) were associated with longer exercise durations. Mayer et al[28] reported similar survival rates for patients mobilized during ECMO and those who were mobilized after ECMO (P= 0.161). Moreover, Munshi et al[13] found a positive association between the application of PT and mortality reduction [odds ratio (OR) = 0.19, 95%CI: 0.04–0.98]. Finally, Abrams et al[30] reported that 66% of the patients who were mobilized during ECMO were weaned from invasive mechanical ventilation.
Early mobilization on ECMO patients[2,13,26] is considered safe and feasible on carefully selected ECMO patients[27,29,30,33,35].
Studies on VA-ECMO patients and studies with mixed population with separate data on VA-ECMO patients: Pasrija et al[29] described three minor cases (20%) of bleeding around the cannulae. Orozco-Hernandez et al[33], Rinewalt et al[31] and Shudo et al[32] did not record any complications related to ambulation. Ko et al[27] reported that three sessions (5%) were interrupted due to tachycardia (n = 1) and tachypnea (n = 2).
Studies with mixed ECMO populations, but without separate data on VA-ECMO patients: Abrams et al[2] reported that complications occurred in 2% of all mobilization sessions. Braune et al[26] reported a femoral cannula dislocation and a bleeding event at the cannula insertion in 6.9% of actively mobilized and 15.3% of non-mobilized patients. In addition, Wells et al[35] described 3 (< 0.5%) minor events in VV-ECMO patients, a hypotensive episode during preparation for the sitting position and two episodes of unsustained ventricular tachycardia while standing. Hayes et al[3] did not record any complications. Finally, Whitlock et al[34] reported three events of ECMO flow decrease and 20 events of hemodynamic changes.
In most studies (8/13), the predominant reason that patients were not mobilized was hemodynamic instability, deep sedation or neuromuscular blockers[3,13,26-28,30,32,33]. Additional reasons are described below.
Studies on VA-ECMO patients: Pasrija et al[29] used criteria such as neurological alertness, respiratory stability and muscle weakness. Also, Shudo et al[32] and Orozco-Hernandez et al[33] had patients weaned from the ventilator to ambulate them.
Studies with mixed ECMO populations, but without separate data on VA-ECMO patients: Abrams et al[30] reported the large dose of vasoconstrictors and severe hypoxemia as reasons for exclusion from mobilization despite extracorporeal support. Also, Abram et al[2] correlated the application of mechanical invasive ventilation (OR = 0.11, 95%CI: 0.05–0.25) and the presence of a femoral cannula with reduced odds of out-of-bed activity (OR = 0.19, 95%CI: 0.04–0.92, OR = 0.23, 95%CI: 0.06–0.91 for patients with a femoral cannula). Furthermore, Braune et al[26] described some additional restricting factors such as poor general condition of the patient, coma without sedation, lack of specialized staff, respiratory instability and the existence of a femoral cannula. Hayes et al[3], Ko et al[27] and Mayer et al[28] also cited the instability of the patients' vital signs, which prohibited mobilization. Finally, Whitlock et al[34] and Wells et al[35], mobilized patients when they were medically stable, under stable ECMO flows, and secured cannulas.
The current review demonstrates that there is a lack of robust evidence to draw firm conclusions on early mobilization in VA-ECMO patients. However, it was shown that VA-ECMO patients can safely participate in early mobilization interventions and PT sessions under certain conditions. Respiratory and cardiovascular stability are key assessment criteria for mobilization of VA-ECMO patients. Based on the current evidence, mobilization of VA-ECMO patients is feasible and safe regardless of the cannula’s insertion location, if it is preceded by a thorough screening and risk stratification. Mobilization and application of PT in VA-ECMO patients also seems to contribute to improved respiratory volume and respiratory rate, as well as to the stabilization or reduction of inotropes, during mobilization. To the authors’ knowledge, this is the first scoping review that summarizes separate evidence of the effects of mobilization on VA-ECMO patients.
Early mobilization is defined as any activity in which patients use their muscles actively or passively within 2-5 days of critical illness[12]. The characteristics of the exercise, regarding the intensity, duration and type, can produce various changes on the body. Physical activity affects genetics, cell signaling pathways, tissue maintenance and organ system function[37]. It has been suggested that early mobilization reduces the occurrence of ICU-AW (33.1% vs 51.9% of control group), the length of ventilation [weighted mean difference (WMD) = -0.2 days], hospital stay (WMD = −3.9 days) and the occurrence of ventilator-associated pneumonia (3.2% vs 12.3% of control group), while it enhances patients' response to inflammation[12,38]. It is well known that skeletal muscle is responsible for all bodily movements and regulates body metabolism[37,39]. When skeletal muscle contracts during physical activity, the cardiac factors such as stroke volume, preload, heart rate and cardiac output, increase to support the metabolic changes of the activated skeletal muscle[37,39]. The respiratory system accompanies the cardiovascular system to provide O2 to the tissues[37,39]. During muscle exercise, the respiratory system increases pulmonary ventilation[37,39]. The respiratory centers of the brainstem are stimulated by the motor cortex and the muscle and joint proprioceptors[37,39]. In addition, the production of CO2 surplus, the increase of the hydrogen ions, and the rise of the body temperature accelerate respiratory rate[37,39].
It is important to emphasize that despite the scarcity of high quality evidence, several benefits of PT in critically ill patients have been reported, including the reduction of hospitalization and health care costs (22%)[40], shorter duration of invasive ventilation, decrease in morbidity and mortality rates[13,41], with decrease in myopathy incidence[41] and increase in functional capacity. The limited data from the current review in VA-ECMO patients does not allow any firm conclusions on the benefits of early mobilization and PT in such a population.
Specifically, evidence in the current review showed that 2 VA-ECMO patients listed for transplantation were able to ambulate successfully[32,33], another one stood by their bed[31] and one more also waiting for transplantation, was able to sit while was supported by central VA-ECMO[27]. Pasrija et al[29] mobilized 14% of their VA-ECMO patients who were supported for recovery, and Munshi et al[13] had 3 patients with ARDS on VV-A-ECMO, where 2 of them received passive mobilization and 1 was also seated.
Early rehabilitation of ECMO patients[3,34,35,42] seems to be beneficial, as the patients can score higher in the IMS and, therefore, have shorter hospitalization and ECMO duration and fewer complications[27,29]. Whitlock et al[34] (case series, 14 yes–high quality in 18 criteria checklist by the Delphi panel; Table 4) and Mayer et al[28] (retrospective study, 6 fair quality in NOS; Table 4) reported that early mobilization increases functional mobility while on ECMO and after ECMO. Nevertheless, these findings have limited ecological validity and cannot be generalized to VA-ECMO populations because of the evidence quality and their heterogeneity (concerning patients’ diagnosis, severity of illness, ECMO type and configuration, mobilization interventions and methodological protocols).
The scarcity and low quality of evidence about the effects of early mobilization on ECMO patients increase the need for further research. First, it should be clearly defined which ECMO subgroup may benefit by early ambulation. Subgroups include either the different diagnosis of the ECMO patients or the different modes of applied ECMO (such as VA-ECMO etc.) or the severity of illness. The degree of recovery[2,3] and the rehabilitation procedure depend on the patient’s diagnosis, illness severity and the type of ECMO[13,27,28,30,34]. Consequently, patients who are candidates for trans
Moreover, the relationship between the mortality of ECMO patients and mobilization is controversial. Mayer et al[28] (retrospective study, 6 fair quality in NOS; Table 4) found no statistically significant effects between early mobilization of ECMO patients and survival odds. On the contrary, Munshi et al[13] (retrospective cohort study, 5 fair quality in NOS; Table 4) associated the consultation with the intensive care unit (ICU) physiotherapy team with decreased ICU mortality. However, Wang et al[43] reported that there was no effect of early ambulation of the critically ill patients on mortality. Even though early ambulation has been reported to be very beneficial in critically ill patients, it is probably difficult to impact the life expectancy on patients with high scores of illness severity[43]. There is a wide range of parameters that may affect mortality, such as starting time, frequency, dose, type and level of mobilization and the patient’s physical condition and illness severity. Thus, further research should be conducted to define to which extent early mobilization can affect prognosis.
Similarly, evidence regarding the duration of mechanical ventilation during extracorporeal oxygenation and ambu
Notably, there are conflicting results regarding the adjustment of ECMO parameters, such as blood flow and sweep gas during mobilization. Ko et al[27] (retrospective study, 6 fair quality in NOS; Table 4) and Pasrija et al[29] (retrospective study, 6 fair quality in NOS; Table 4) suggested a need for increased blood flow on ECMO, without an increase in sweep gas flow rate, while Munshi et al[13] (retrospective cohort study, 5 fair quality in NOS; Table 4) reported a decrease in blood flow rate and sweep gas flow rate on ECMO. Hayes et al[3] (pilot study, RCT, 7 good quality in PEDro Scale, Table 4) recorded a gradual reduction of ECMO fresh gas flow and Abrams et al[30] (retrospective cohort study, 6 fair quality in NOS; Table 4) reported that ECMO blood flow and sweep gas flow rate were stable during ambulation. This variation in ECMO parameters is probably related to the type of ECMO supporting the patient, the intensity of exercise, as well as the clinical characteristics of the patients. Therefore, more research is needed in this area.
However, none of the studies described the protocol of respiratory PT used on these patients. In addition, there is scarce data on respiratory PT in ECMO patients, with relative information emerging mainly from the 'ECMO Technical Specialist Manual' from the University of Michigan[48].
Recently, research has been focused on alternative modalities of mobilization, such as Neuromuscular Electrical Stimulation (NMES), for patients who cannot be ambulated due to their severity of illness, low consciousness, and deep sedation. These patients can only receive passive mobilization (e.g., stretching passive movements; continuous passive motion; functional electrical stimulation of lower limb muscles and passive cycling and NMES) and respiratory physiotherapy[36]. A recent meta-analysis by Nakanishi et al[49] reported that NMES on critically ill patients could prevent ICU-acquired muscle weakness and enhance muscle mass and muscle strength. VA-ECMO patients are the most critically ill patients, and, according to authors’ knowledge, no such alternative modality has been used in these patients so far. In the only safety and feasibility study, Kourek et al[36] investigated the application of NMES on 16 ECMO patients (13 were on VA-ECMO). They reported that NMES does not affect the hemodynamic and metabolic parameters in ECMO patients during and after NMES, and also reported no adverse events. Therefore, NMES may be a safe and feasible intervention and might be an alternative form of early mobilization in ECMO patients[36].
This is the first review that systematically researched and summarized the evidence regarding the early mobilization and application of PT in VA-ECMO patients. PRISMA guidelines were followed, and studies were selected according to the review’s criteria. To eliminate risk of bias, appraisal of the studies’ methodology was performed by 2 reviewers, despite not being a prerequisite for a scoping review.
Most of the selected studies were of moderate methodological quality although methods and particularly the interventions were not clearly described in most included studies. Consequently, the low to moderate methodological quality, the different study designs of the studies included and the lack of RCTs, increase the risk of bias. After all, 91% of the studies included were observational and case studies, and there was only 1 RCT.
The studies included had small sample sizes of VA-ECMO patients, and most of them had mixed and heterogenous populations (different diseases, different types of ECMO, different therapeutic approaches), with non-separate data of VA-ECMO population. The exact number of VA-ECMO patients who were mobilized was also unclear. Additionally, the lack of control groups and randomization procedures reduced the ability to draw firm conclusions. Moreover, no studies compared the results of mobilization either among patients on VA ECMO and on VV ECMO or among VA-ECMO patients with the same disease.
The selected studies are characterized by lack of replicability, as they did not clearly describe their interventions according to current guidelines[50]. The protocols of mobilizations and PT sessions were reported in only four out of thirteen studies, were heterogeneous and lacked specificity.
Finally, the 18-criteria scale was chosen for the appraisal of the case studies. This scale was constructed by the modified Delphi method and was originally designed to assess case series and may not be ideal for the methodological quality appraisal of the case studies[25].
Future research should be performed to investigate the optimal balance between the maximal level of mobilization of VA-ECMO patients and the derived benefits, as well as the optimal protocols (Frequency, Intensity, Duration) for the maximum therapeutic effect.
Powered RCTs should be conducted to study more homogeneous ECMO subgroups. In addition, the methodological quality of clinical trials should be high to draw safe conclusions, with the ability of generalization to the VA-ECMO population. Also, comparisons between VV-ECMO patients and VA-ECMO patients, regarding mobilization, would be possible in larger and adequately powered studies. Finally, NMES might be a promising alternative technique for mobilization of VA-ECMO patients who cannot be physically mobilized. Therefore, more research is required to provide more robust evidence.
Future clinical studies should be designed to assess the possible benefits of PT and mobilization in VA-ECMO patients, focusing on the duration of mechanical ventilation, muscle strength and mass, functional capacity and lung function and the length of hospital stay and mortality.
Current evidence indicates that early individualized mobilization is feasible and may be applied with safety in VA-ECMO patients after thorough screening and risk stratification. Early mobilization requires hemodynamic and res
| 1. | Allen S, Holena D, McCunn M, Kohl B, Sarani B. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J Intensive Care Med. 2011;26:13-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Abrams D, Madahar P, Eckhardt CM, Short B, Yip NH, Parekh M, Serra A, Dubois RL, Saleem D, Agerstrand C, Scala P, Benvenuto L, Arcasoy SM, Sonett JR, Takeda K, Meier A, Beck J, Ryan P, Fan E, Hodgson CL, Bacchetta M, Brodie D; MORE-PT Investigators. Early Mobilization during Extracorporeal Membrane Oxygenation for Cardiopulmonary Failure in Adults: Factors Associated with Intensity of Treatment. Ann Am Thorac Soc. 2022;19:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Hayes K, Holland AE, Pellegrino VA, Young M, Paul E, Hodgson CL. Early rehabilitation during extracorporeal membrane oxygenation has minimal impact on physiological parameters: A pilot randomised controlled trial. Aust Crit Care. 2021;34:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Vyas A, Bishop MA. Extracorporeal Membrane Oxygenation in Adults. 2023 Jun 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 5. | Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, Himmelfarb CR, Desai SV, Ciesla N, Herridge MS, Pronovost PJ, Needham DM. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 438] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 6. | Zhou J, Zhang C, Zhou JD, Zhang CK. Effect of early progressive mobilization on intensive care unit-acquired weakness in mechanically ventilated patients: An observational study. Medicine (Baltimore). 2022;101:e31528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Geense WW, Zegers M, Peters MAA, Ewalds E, Simons KS, Vermeulen H, van der Hoeven JG, van den Boogaard M. New Physical, Mental, and Cognitive Problems 1 Year after ICU Admission: A Prospective Multicenter Study. Am J Respir Crit Care Med. 2021;203:1512-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 8. | Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1965] [Article Influence: 140.4] [Reference Citation Analysis (0)] |
| 9. | Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, Nollet F, van Schaik IN, Schultz MJ, Horn J, van der Schaaf M. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care. 2015;19:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 10. | Adler J, Malone D. Early mobilization in the intensive care unit: a systematic review. Cardiopulm Phys Ther J. 2012;23:5-13. [PubMed] |
| 11. | Harrold ME, Salisbury LG, Webb SA, Allison GT; Australia and Scotland ICU Physiotherapy Collaboration. Early mobilisation in intensive care units in Australia and Scotland: a prospective, observational cohort study examining mobilisation practises and barriers. Crit Care. 2015;19:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Rosa D, Negro A, Marcomini I, Pendoni R, Albabesi B, Pennino G, Terzoni S, Destrebecq A, Villa G. The Effects of Early Mobilization on Acquired Weakness in Intensive Care Units: A Literature Review. Dimens Crit Care Nurs. 2023;42:146-152. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Munshi L, Kobayashi T, DeBacker J, Doobay R, Telesnicki T, Lo V, Cote N, Cypel M, Keshavjee S, Ferguson ND, Fan E. Intensive Care Physiotherapy during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. Ann Am Thorac Soc. 2017;14:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Kourek C, Nanas S, Kotanidou A, Raidou V, Dimopoulou M, Adamopoulos S, Karabinis A, Dimopoulos S. Modalities of Exercise Training in Patients with Extracorporeal Membrane Oxygenation Support. J Cardiovasc Dev Dis. 2022;9:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Hayes K, Hodgson CL, Webb MJ, Romero L, Holland AE. Rehabilitation of adult patients on extracorporeal membrane oxygenation: A scoping review. Aust Crit Care. 2022;35:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Haji JY, Mehra S, Doraiswamy P. Awake ECMO and mobilizing patients on ECMO. Indian J Thorac Cardiovasc Surg. 2021;37:309-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Ferreira DDC, Marcolino MAZ, Macagnan FE, Plentz RDM, Kessler A. Safety and potential benefits of physical therapy in adult patients on extracorporeal membrane oxygenation support: a systematic review. Rev Bras Ter Intensiva. 2019;31:227-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Polastri M, Loforte A, Dell'Amore A, Nava S. Physiotherapy for Patients on Awake Extracorporeal Membrane Oxygenation: A Systematic Review. Physiother Res Int. 2016;21:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Cucchi M, Mariani S, De Piero ME, Ravaux JM, Kawczynski MJ, Di Mauro M, Shkurka E, Hoskote A, Lorusso R. Awake extracorporeal life support and physiotherapy in adult patients: A systematic review of the literature. Perfusion. 2023;38:939-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 20. | Chatziefstratiou AA, Fotos NV, Giakoumidakis K, Brokalaki H. The Early Mobilization of Patients on Extracorporeal Membrane Oxygenation: A Systematic Review. Nurs Rep. 2023;13:751-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40456] [Article Influence: 10114.0] [Reference Citation Analysis (2)] |
| 22. | Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22118] [Cited by in RCA: 18480] [Article Influence: 2640.0] [Reference Citation Analysis (1)] |
| 23. | Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713-721. [PubMed] |
| 24. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24830] [Article Influence: 1773.6] [Reference Citation Analysis (3)] |
| 25. | Moga C, Guo B, Schopflocher D, Harstall C. Development of a quality appraisal tool for case series studies using a modified Delphi technique. Edmonton AB: Institute of Health Economics, 2012. |
| 26. | Braune S, Bojes P, Mecklenburg A, Angriman F, Soeffker G, Warnke K, Westermann D, Blankenberg S, Kubik M, Reichenspurner H, Kluge S. Feasibility, safety, and resource utilisation of active mobilisation of patients on extracorporeal life support: a prospective observational study. Ann Intensive Care. 2020;10:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Ko Y, Cho YH, Park YH, Lee H, Suh GY, Yang JH, Park CM, Jeon K, Chung CR. Feasibility and Safety of Early Physical Therapy and Active Mobilization for Patients on Extracorporeal Membrane Oxygenation. ASAIO J. 2015;61:564-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Mayer KP, Pastva AM, Du G, Hatchett SP, Chang M, Henning AN, Maher B, Morris PE, Zwischenberger JB. Mobility Levels With Physical Rehabilitation Delivered During and After Extracorporeal Membrane Oxygenation: A Marker of Illness Severity or an Indication of Recovery? Phys Ther. 2022;102:pzab301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Pasrija C, Mackowick KM, Raithel M, Tran D, Boulos FM, Deatrick KB, Mazzeffi MA, Rector R, Pham SM, Griffith BP, Herr DL, Kon ZN. Ambulation With Femoral Arterial Cannulation Can Be Safely Performed on Venoarterial Extracorporeal Membrane Oxygenation. Ann Thorac Surg. 2019;107:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 31. | Rinewalt D, Shudo Y, Kawana M, Woo YJ. Physical therapy in successful venoarterial extracorporeal membrane oxygenation bridge to orthotopic heart transplantation. J Card Surg. 2019;34:1390-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Shudo Y, Wang H, Ha RV, Hayes AD, Woo YJ. Heart transplant after profoundly extended ambulatory central venoarterial extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2018;156:e7-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Orozco-Hernandez EJ, Melnikoff B, Lusby M, Tallaj J, Hoopes CW. Peripheral femoral venoarterial extracorporeal membrane oxygenation as bridge to heart-lung transplant omne iter incipit primus. J Card Surg. 2020;35:2077-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Whitlock K, Rzewnicki D, Krieger B, Miller C, Creel-Bulos C. "Beyond waking and walking. Intensive rehabilitation in patients requiring extended durations of advanced mechanical circulatory support: A case series". Perfusion. 2024;39:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Wells CL, Forrester J, Vogel J, Rector R, Tabatabai A, Herr D. Safety and Feasibility of Early Physical Therapy for Patients on Extracorporeal Membrane Oxygenator: University of Maryland Medical Center Experience. Crit Care Med. 2018;46:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Kourek C, Raidou V, Antonopoulos M, Dimopoulou M, Koliopoulou A, Karatzanos E, Pitsolis T, Ieromonachos K, Nanas S, Adamopoulos S, Chamogeorgakis T, Dimopoulos S. Safety and Feasibility of Neuromuscular Electrical Stimulation in Patients with Extracorporeal Membrane Oxygenation. J Clin Med. 2024;13:3723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 37. | Matthews MJ, Kanungo S, Baker RJ, Kenter K. Exercise Physiology: A Review of Established Concepts and Current Questions. Physiologia. 2024;4:202-212. [DOI] [Full Text] |
| 38. | Zang K, Chen B, Wang M, Chen D, Hui L, Guo S, Ji T, Shang F. The effect of early mobilization in critically ill patients: A meta-analysis. Nurs Crit Care. 2020;25:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 39. | Patel PN, Horenstein MS, Zwibel H. Exercise Physiology. 2024 Oct 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 40. | Bain JC, Turner DA, Rehder KJ, Eisenstein EL, Davis RD, Cheifetz IM, Zaas DW. Economic Outcomes of Extracorporeal Membrane Oxygenation With and Without Ambulation as a Bridge to Lung Transplantation. Respir Care. 2016;61:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Rehder KJ, Turner DA, Hartwig MG, Williford WL, Bonadonna D, Walczak RJ Jr, Davis RD, Zaas D, Cheifetz IM. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care. 2013;58:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Bonizzoli M, Lazzeri C, Drago A, Tadini Boninsegni L, Donati M, Di Valvasone S, Pesenti A, Peris A. Effects of a physiotherapic program in patients on veno-venous extracorporeal membrane oxygenation: an 8-year single-center experience. Minerva Anestesiol. 2019;85:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Wang J, Ren D, Liu Y, Wang Y, Zhang B, Xiao Q. Effects of early mobilization on the prognosis of critically ill patients: A systematic review and meta-analysis. Int J Nurs Stud. 2020;110:103708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 44. | Hayes K, Hodgson CL, Pellegrino VA, Snell G, Tarrant B, Fuller LM, Holland AE. Physical Function in Subjects Requiring Extracorporeal Membrane Oxygenation Before or After Lung Transplantation. Respir Care. 2018;63:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Batycka-Stachnik D, Piwoda A, Darocha T, Spiewak M, Kosinski S, Jarosz A, Hymczak H, Sanak T, Galazkowski R, Piatek J, Konstanty-Kalandyk J, Drwila R. Problems and challenges in the early period of rehabilitating patients with severe hypothermia treated using ecmo support. Wiad Lek. 2016;69:489-494. [PubMed] |
| 46. | Hayes K, Holland AE, Pellegrino VA, Mathur S, Hodgson CL. Acute skeletal muscle wasting and relation to physical function in patients requiring extracorporeal membrane oxygenation (ECMO). J Crit Care. 2018;48:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | ECMO-PT Study Investigators; International ECMO Network. Early mobilisation during extracorporeal membrane oxygenation was safe and feasible: a pilot randomised controlled trial. Intensive Care Med. 2020;46:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 48. | Kurusz M. Ecmo: An Overview for Perfusionists. In: Cernaianu AC, DelRossi AJ, editors. Cardiac Surgery. Berlin: Springer, 1992. [DOI] [Full Text] |
| 49. | Nakanishi N, Yoshihiro S, Kawamura Y, Aikawa G, Shida H, Shimizu M, Fujinami Y, Matsuoka A, Watanabe S, Taito S, Inoue S. Effect of Neuromuscular Electrical Stimulation in Patients With Critical Illness: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit Care Med. 2023;51:1386-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5117] [Cited by in RCA: 6093] [Article Influence: 553.9] [Reference Citation Analysis (1)] |