Published online Jun 26, 2025. doi: 10.4330/wjc.v17.i6.105330
Revised: April 16, 2025
Accepted: June 3, 2025
Published online: June 26, 2025
Processing time: 151 Days and 7.4 Hours
Development of pericardial effusion in patients with left ventricular assist devices (LVADs) can be detrimental to health outcomes. This study aims to elucidate the prevalence and risk factors for pericardial effusion in patients with LVADs.
To elucidate risk factors associated with the presence of pericardial effusion in patients with LVADs and compare the clinical outcomes of those with and with
Data were obtained from the National Inpatient Sample database between 2016 and 2018. Statistical analysis was performed using Pearson χ2 test and multivariate logistic regression analysis to determine clinical outcomes of pericardial effusion and to identify variables associated with pericardial effusion in LVAD patients, respectively.
The prevalence of LVAD was 9850 (0.01%) among total study patients (n = 98112095). The incidence of pericardial effusion among LVAD patients was 640 (6.5%). The prevalence of liver disease (26.6% vs 17.4%), chronic kidney disease (CKD; 54.6% vs 49.4%), hypothyroidism (21.9% vs 18.1%), congestive heart failure (98.4% vs 96.5%), atrial fibrillation (Afib; 58.59% vs 50.5%), coronary artery disease (CAD; 11.7% vs 4.4%), dyslipidemia (31.3% vs 39.3%), and having undergone percutaneous coronary intervention (PCI; 1.6% vs 0.7%) was higher in the pericardial effusion cohort vs the non-pericardial effusion cohort. Multivariate regression analysis demonstrated that CAD (OR = 2.89) and PCI (OR = 2.2) had the greatest association with pericardial effusion in patients with LVADs. These were followed by liver disease (OR = 1.72), hypothyroidism (OR = 1.2), electrolyte derangement (OR = 1.2), Afib (OR = 1.1), and CKD (OR = 1.05). Among patients with LVADs, the median length of stay (33 days vs 27 days) and hospitalization cost (847525 USD vs 792616 USD) were significantly higher in the pericardial effusion cohort com
This study shows that liver disease, CKD, PCI, hypothyroidism, electrolyte derangement, Afib, and CAD had a significant association with pericardial effusion in LVAD patients. Hospitalization cost and length of stay were higher in the pericardial effusion group, but mortality was the same.
Core Tip: Pericardial effusions in patients with left ventricular assist devices (LVADs) can lead to prolonged hospitalization and increased healthcare costs. Risk factors associated with the development of pericardial effusions include: Liver disease, chronic kidney disease (CAD), hypothyroidism, electrolyte derangement, atrial fibrillation, with the highest association found with CAD and percutaneous coronary intervention. While the presence of pericardial effusions did not have significant impact on mortality in our study, further studies are needed to elucidate whether the optimization of these co-morbidities would reduce the incidence of pericardial effusion in patients with LVADs.
- Citation: Khan MZ, Brailovsky Y, Bhuiyan MAN, Marhefka G, Faisal ASM, Sircar A, O'Neill P, Rame JE, Franklin S, Waqas M, Shah H, Rajapreyar I, Alvarez RJ. Incidence, risk factors and clinical outcomes of pericardial effusion in left ventricular assist device patients. World J Cardiol 2025; 17(6): 105330
- URL: https://www.wjgnet.com/1949-8462/full/v17/i6/105330.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i6.105330
Patients suffering from advanced end-stage heart failure who do not meet the criteria for heart transplantation can now benefit from mechanical circulatory support therapies such as left ventricular assist devices (LVADs). These devices improve quality of life and delay end-organ dysfunction; they can be used as bridging therapy until transplantation, or as destination therapy for patients who are ineligible for transplantation[1]. In the recent years, the use of LVADs as destination therapy has vastly increased, accounting for about 46% of all LVAD placements[1]. In the United States, the amount of LVAD implants is now approaching the number heart transplants[2]. As a result, the prevalence of LVADs and their associated complications are rapidly increasing. Topilsky et al[3] found that up to 60% of patients receiving LVADs experience an LVAD-related complication within 6 months of implantation, and 80% experience an adverse event within 2 years of implantation. Another study by Hillebrand et al[4] found that on average, patients were re-admitted 2.2 times in 11 months post-implant, with the median time to re-admission being just 35 days after initial discharge.
The most common LVAD-related complications include bleeding, device thrombosis, ischemic and hemorrhagic stro
Pericardial effusions can be found in patients with an initial myocardial infarction (MI) and are more often found in patients with an anterior ST segment MI, larger infarctions and congestive heart failure[6-8]. Pericardial effusions usually present within the first 5 days and slowly resolve over weeks to months[9]. In patients with acute MI or percutaneous coronary intervention (PCI), hemorrhagic pericardial effusions and cardiac tamponade may develop from coronary artery perforation during PCI, as well as hemorrhagic pericarditis, and cardiac rupture due to left ventricular free wall rupture.
Pericardial effusion is the abnormal accumulation of fluid within the pericardial cavity, and may be classified accor
LVAD placement carries a high risk of pericardial bleeding and cardiac tamponade[13]. Furthermore, LVADs confer a greater risk of bleed due to acquired von Willebrand syndrome, platelet dysfunction, arteriovenous malformation and angiodysplasia[14]. Additional risk of bleed is secondary to aggressive anticoagulation that is administered for the risk of pump thrombosis.
This study aims to elucidate risk factors associated with the presence of pericardial effusion in patients with LVADs and compare the clinical outcomes of those with and without pericardial effusion. The secondary goal is to determine the incidence of pericardiocentesis and pericardial window placement in patients with LVADs experiencing pericardial effusion.
This study was conducted using the National Inpatient Sample (NIS) database which is part of the Healthcare Cost and Utilization Project, developed by the Agency for Healthcare Research and Quality[15]. The NIS is representative of over 97% of the human population, and the data encompasses an average of 7-8 million discharges per year, spanning 48 states. NIS data estimates more than 35 million hospitalizations nationally. Each of these admissions contain a myriad of patient information, including demographics, comorbidities, complications, the primary and secondary discharge diag
This study uses the International Classification of Disease (ICD), 10th revision, and Clinical Modification (ICD 10-CM) codes to identify diagnoses in the NIS database and included data obtained between January 2016 and December 2018. Only patients over the age of 18 years with were included in the study. Subjects excluded were patients under the age of 18 years, those heart transplant, intra-aortic balloon pump, and Impella device. ICD 10-CM codes were utilized to extract patients with LVAD from the NIS database. From within this subset of patients, ICD codes were again utilized to delineate those who had pericardial effusion and no pericardial effusion, as well as to identify those with specific comorbidities and baseline characteristics. This study was considered exempt from the formal approval of the Institutional Review Board, as the study cohort was derived from a publicly available database containing non-identifiable patient information.
Diagnosis code for LVAD, pericardial effusion, and other co-morbidities: The NIS data provides up to 30 Clinical Classifications Software (CCS) diagnoses for each inpatient visit. The CCS codes used for this study were: LVAD, pericar
The data analysis and extraction were done using SAS statistical software version 9.4. All continuous variables were compared using Student's t-test. These variables were presented as a mean ± SD for normally distributed variables. Median and interquartile ranges were used for non-Gaussian distributed variables. Categorical variables were analyzed using the Pearson χ2 test. These variables were presented as a weighted frequency in percentages. A P value of < 0.05 was considered statistically significant. In the Table 1, age was analyzed using the Student's t-test, while the rest of variables in Table 1 were analyzed using the Pearson’s χ2 test. In Table 2, mortality was analyzed using Pearson’s χ2 test while length of stay and hospitalization cost were analyzed using the Student's t-test.
| Characteristics | Pericardial effusion | No pericardial effusion | P value |
| Total number | 640.00 | 9210.00 | |
| Gender | |||
| Male | 445 (69.53) | 7245 (78.66) | 0.001 |
| Female | 195 (30.47) | 1965 (21.34) | |
| Age (years) | 57.57 ± 2.54 | 57.03 ± 3.63 | 0.649 |
| Race | |||
| White | 345 (57.02) | 5365 (62.21) | 0.031 |
| Black | 165 (27.27) | 2135 (24.75) | |
| Others | 95 (15.7) | 1125 (13.04) | |
| Co-morbidities | |||
| Liver disease | 170 (26.56) | 1600 (17.37) | 0.001 |
| Obesity | 150 (23.44) | 1910 (20.74) | 0.115 |
| Chronic kidney disease | 350 (54.69) | 4550 (49.41) | 0.010 |
| Chronic obstructive pulmonary disease | 90 (14.06) | 1480 (16.07) | 0.198 |
| Carotid artery disease | 10 (1.56) | 1020 (11.07) | 0.978 |
| Percutaneous coronary intervention | 10 (1.56) | 65 (0.71) | 0.029 |
| CABG | 5 (0.78) | 135 (1.47) | 0.214 |
| Hypertension | 65 (10.16) | 1020 (11.07) | 0.514 |
| Peripheral vascular disease | 15 (2.34) | 370 (4.02) | 0.044 |
| Hypothyroidism | 140 (21.88) | 1665 (18.08) | 0.018 |
| Diabetes mellitus types 1&2 | 190 (29.69) | 3555 (38.6) | 0.001 |
| Congestive heart failure | 630 (98.44) | 8890 (96.53) | 0.013 |
| Electrolyte derangement | 455 (71.09) | 6040 (65.58) | 0.005 |
| Smoking | 25 (3.91) | 520 (5.65) | 0.076 |
| Anemia | 505 (78.91) | 7055 (76.6) | 0.198 |
| Afib | 375 (58.59) | 4650 (50.49) | 0.001 |
| CAD | 75 (11.72) | 405 (4.41) | 0.001 |
| Dyslipidemia | 200 (31.25) | 3620 (39.31) | 0.001 |
| Comorbidity | Odds ratio (95%CI) | P value |
| Liver disease | 1.721 (1.429- 2.063) | 0.001 |
| Chronic kidney disease | 1.235 (1.052- 1.452) | 0.009 |
| Percutaneous coronary intervention | 2.263 (1.083 -4.232) | 0.015 |
| Peripheral vascular disease | 0.579 (0.328 -0.942) | 0.034 |
| Hypothyroidism | 1.269 (1.041- 1.538) | 0.016 |
| Diabetes mellitus types 1&2 | 0.671 (0.563 -0.798) | 0.001 |
| Congestive heart failure | 2.232 (1.249 -4.523) | 0.009 |
| Electrolyte derangement | 1.290 (1.083-1.541) | 0.004 |
| Afib | 1.387 (1.179-1.633) | 0.001 |
| CAD | 2.890 (2.212-3.729) | 0.001 |
| Dyslipidemia | 0.702 (0.590- 0.833) | 0.001 |
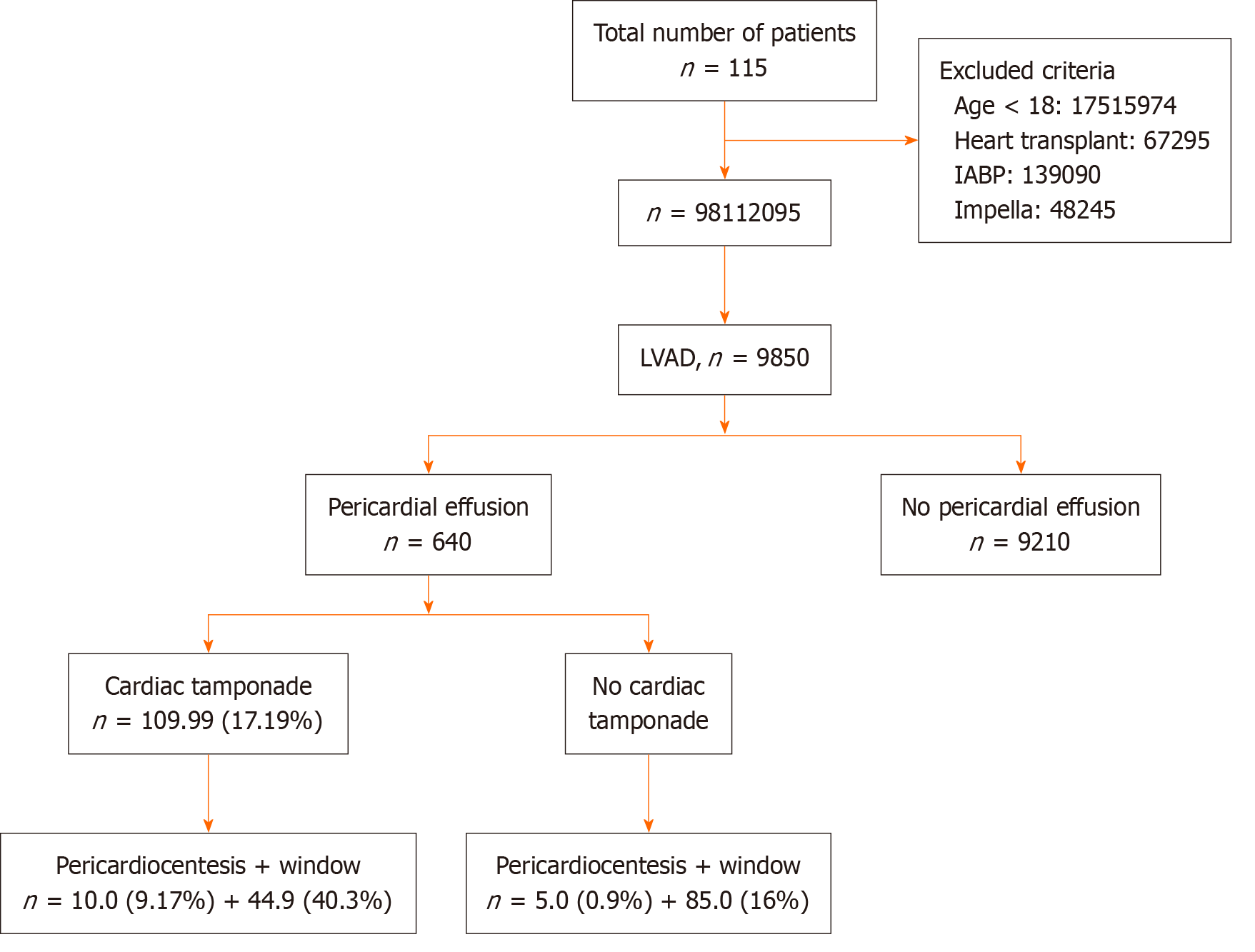
Between 2016 and 2018, there was a total of 115882699 hospitalizations in the United States. After excluding patients under 18 years (n = 17515974), patients with heart transplants (n = 67295), patients with intra-aortic balloon pumps (n = 139090) and patients with Impella heart pumps (n = 48245), 98112095 patients were eligible to be included in the study. Of the included patients, 9850 had LVADs with 640 (6.5%) patients having experienced a pericardial effusion (Figure 1).
Our results demonstrated a statistically significant correlation between multiple co-morbidities and the presence of pericardial effusion in patients with LVADs (Table 1).
These co-morbidities (percentage of pericardial effusion cohort vs percentage of no pericardial effusion cohort) in
Multivariate regression analysis demonstrated that CAD (OR = 2.89, 95%CI = 2.21-3.72) and PCI (OR = 2.2, 95%CI = 1.08-4.23) had the greatest association with pericardial effusion in patients with LVADs. These were followed by liver disease (OR = 1.72, 95%CI = 1.42-2.06), hypothyroidism (OR = 1.26, 95%CI = 1.04-1.53), electrolyte derangement (OR = 1.2, 95%CI = 1.08-1.54), Afib (OR = 1.38, 95%CI = 1.17 -1.63), and CKD (OR = 1.23, 95%CI = 1.08-1.45; Table 2).
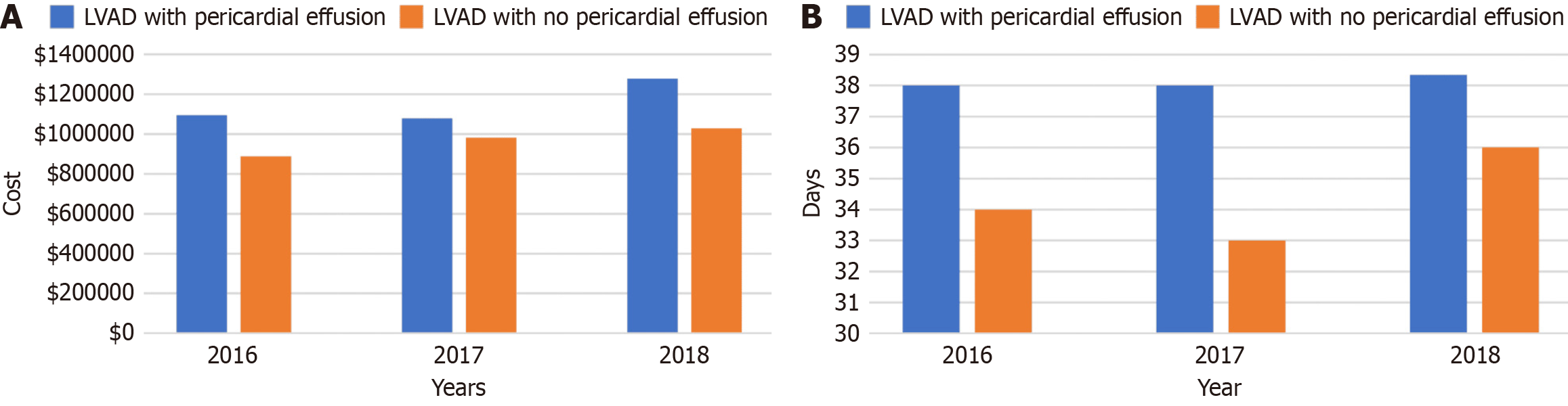
Patients with LVADs who developed pericardial effusion experienced longer durations of hospitalization and higher hospitalization costs than patients without pericardial effusions. The median length of stay in LVAD patients with a pericardial effusion was 33 days compared to 27 days in those without a pericardial effusion (Table 3). The average hospitalization cost of LVAD patients with pericardial effusion was 847525 USD compared to 792616 USD in patients without pericardial effusion. Interestingly, there was no significant difference in mortality with a mortality of 9.38% in patients with pericardial effusion compared to 9.34% of patients without pericardial effusion. The annual trends of mean hospitalization cost and length of stay in the hospital were compared between pericardial effusion in patients with LVADs vs no pericardial effusion (Figure 2).
| Pericardial effusion | No pericardial effusion | P value | |
| Length of stay (days) | 40.52 ± 6.06 | 34.08 ± 1.39 | 0.043 |
| Hospitalization cost (USD) | 847525 ± 93380 | 792616 ± 37959 | 0.035 |
| Mortality | 9.38 ± 2.26 | 9.34% ± 0.40 | 1 |
Of the 9850 patients with implanted LVADs, 640 (6.5%) experienced a pericardial effusion. Within the pericardial effusion cohort, 109 (17.0%) patients developed cardiac tamponade. Ten of these patients (9.2%) underwent pericardiocentesis and 45 patients (40.3%) received pericardial windows. Five-hundred and thirty-one patients experienced pericardial effusion without tamponade, of which 5 (0.9%) underwent pericardiocentesis, and 85 (16.3%) received pericardial windows.
This study demonstrates that LVAD patients with pericardial effusion experience longer hospitalization courses and higher hospitalization costs, however, there is no significant effect on mortality. Additionally, our results highlight a significant association between pericardial effusion and the presence of liver disease, CKD, hypothyroidism, electrolyte derangement, Afib, and CAD in LVAD patients.
To date, there are no large trials detailing the rate at which patients with LVADs develop pericardial effusion, how
One of the major concerns with developing pericardial effusion in a patient with an LVAD is that the device can mask the symptoms associated with cardiac effusion and tamponade, such as jugular venous distension, pulsus paradoxus, hypotension, and rest dyspnea[17]. This may be due to the set values of the LVAD preventing tachycardia or pulsus paradoxus, and is further complicated by the blurred echocardiogram windows in patients due to the LVAD flow, making rapid diagnosis difficult[18]. Both factors increase the risk of delayed diagnosis and management, leading to worse clinical outcomes. This was demonstrated by Al Shakaki et al’s case report of a patient with a ventricular assist device who suffered a massive pericardial effusion that was initially misdiagnosed as a thrombus of the outflow graft due to flow-limiting formation seen on CT. The patient received lysis therapy and subsequently clinically deteriorated, requiring emergent re-sternotomy and pericardiocentesis[19]. Our study relied upon ICD-10 coding of pericardial effu
This study found statistically significant correlations between a myriad of clinical conditions and the presence of pericardial effusion in patients with LVADs, including liver disease, CKD, hypothyroidism, electrolyte derangement, Afib, CAD, dyslipidemia, and those undergoing PCI. This correlation is not isolated to patients with LVADs and has been demonstrated to increase the risk of pericardial effusion in patients without mechanical circulatory support. Ashikhmina et al[20] found that patients who underwent cardiac surgery were at increased risk of developing a pericardial effusion if they had a history of a high body mass index, pulmonary thromboembolism, hypertension, immunosuppression, or renal failure. Further research is required to determine whether optimization of these co-morbidities can reduce the incidence of pericardial effusion. It seems likely that a multidisciplinary approach to the management of patients with LVADs would lead to decreased incidence of pericardial effusion and improved clinical outcomes.
Pericardial effusion in patients with LVADs leads to an increased burden on the healthcare system through prolonging hospitalization and increasing the cost of healthcare delivery. Our study demonstrated that patients with pericardial effusion spent a median of 6 days longer in hospital and cost an additional 54909 USD per hospital stay compared to LVAD patients without pericardial effusion. These findings may be confounded by the fact that many of the co-morbi
Our study has several limitations. First of all, our data relied upon the correct diagnosis and use of ICD-10 codes by physicians across the country which may have led to some disease misclassification, as we were unable to validate the signs and symptoms experienced by the patients included in this study. Another limitation is that the study population is confined to inpatients and our results may not correlate with outcomes in patients with subclinical pericardial effusion who are managed in an outpatient setting. The NIS database provides information on hospital admissions and not individual patients. As a result, our data may be skewed by patients being admitted multiple times and artificially increasing the prevalence of pericardial effusion in our study population. In addition, LVAD type, implantation duration and anticoagulation therapy were not included in our study, and their inclusion could potentially influence the occur
The development of pericardial effusion in patients with LVADs leads to prolonged hospitalization and increased healthcare costs, while the presence of pericardial effusion did not have significant impacts on mortality. The presence of liver disease, CKD, hypothyroidism, electrolyte derangement, Afib, CAD, and undergoing PCI have a significant asso
| 1. | Fukunaga N, Rao V. Left ventricular assist device as destination therapy for end stage heart failure: the right time for the right patients. Curr Opin Cardiol. 2018;33:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Mancini D, Colombo PC. Left Ventricular Assist Devices: A Rapidly Evolving Alternative to Transplant. J Am Coll Cardiol. 2015;65:2542-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Topilsky Y, Price TN, Atchison FW, Joyce LD. Atypical tamponade hemodynamic in a patient with temporary left ventricular assist device. Interact Cardiovasc Thorac Surg. 2011;12:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Hillebrand J, Hoffmeier A, Djie Tiong Tjan T, Sindermann JR, Schmidt C, Martens S, Scherer M. Minimally Invasive Implantation of HeartWare Assist Device and Simultaneous Tricuspid Valve Reconstruction Through Partial Upper Sternotomy. Artif Organs. 2017;41:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kilic A, Acker MA, Atluri P. Dealing with surgical left ventricular assist device complications. J Thorac Dis. 2015;7:2158-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 6. | Mega JL, Morrow DA. ST elevation myocardial infarction: management. In: Mann DL, Zipes DP, Libby P, Bonow RO, editors. Braunwald’s heart disease: a textbook of cardiovascular medicine. 10th ed. Philadelphia: Elsevier Saunders, 2015: 1095–1154. |
| 7. | Galve E, Garcia-Del-Castillo H, Evangelista A, Batlle J, Permanyer-Miralda G, Soler-Soler J. Pericardial effusion in the course of myocardial infarction: incidence, natural history, and clinical relevance. Circulation. 1986;73:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 65] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, Cowley MJ, Raymond RE, Safian RD, Whitlow PL. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. 1994;90:2725-2730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 466] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 9. | Widimský P, Gregor P. Pericardial involvement during the course of myocardial infarction. A long-term clinical and echocardiographic study. Chest. 1995;108:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34:1186-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristić AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W. 2015 ESC Guidelines for the Diagnosis and Management of Pericardial Diseases. Rev Esp Cardiol (Engl Ed). 2015;68:1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Lazaros G, Imazio M, Brucato A, Tousoulis D. Untying the Gordian knot of pericardial diseases: A pragmatic approach. Hellenic J Cardiol. 2016;57:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Kohmoto T, Oz MC, Naka Y. Late bleeding from right internal mammary artery after HeartMate left ventricular assist device implantation. Ann Thorac Surg. 2004;78:689-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | den Exter PL, Beeres SLMA, Eikenboom J, Klok FA, Huisman MV. Anticoagulant treatment and bleeding complications in patients with left ventricular assist devices. Expert Rev Cardiovasc Ther. 2020;18:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | AHRQ. Agency for Healthcare Research and Quality. [cited 20 January 2025] Available from: www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. |
| 16. | Foo J. Left Ventricular Assist Device (LVAD) in Singapore: A Nation's 10 Year Experience. J Heart Lung Transpl. 2020;39:S393. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Zalawadiya SK, Lindenfeld J, DiSalvo T. Rapid diagnosis of cardiac tamponade using pulsatility index variability in a patient with a HeartWare ventricular assist device. Circulation. 2015;131:e387-e388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Akin S, Ince C, Struijs A, Caliskan K. Case Report: Early Identification of Subclinical Cardiac Tamponade in a Patient With a Left Ventricular Assist Device by the Use of Sublingual Microcirculatory Imaging: A New Diagnostic Imaging Tool? Front Cardiovasc Med. 2022;9:818063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Al Shakaki M, Dell'Aquila AM, Rukosujew A. Symptoms of massive cardiac tamponade during support of biventricular assist device. Int J Artif Organs. 2018;41:245-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Ashikhmina EA, Schaff HV, Sinak LJ, Li Z, Dearani JA, Suri RM, Park SJ, Orszulak TA, Sundt TM 3rd. Pericardial effusion after cardiac surgery: risk factors, patient profiles, and contemporary management. Ann Thorac Surg. 2010;89:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Cotts WG, McGee EC Jr, Myers SL, Naftel DC, Young JB, Kirklin JK, Grady KL. Predictors of hospital length of stay after implantation of a left ventricular assist device: an analysis of the INTERMACS registry. J Heart Lung Transplant. 2014;33:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Briasoulis A, Chehab O, Alvarez P. In-hospital Outcomes of Left Ventricular Assist Devices (LVAD) Patients Undergoing Noncardiac Surgery. ASAIO J. 2021;67:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |