Published online May 26, 2025. doi: 10.4330/wjc.v17.i5.106123
Revised: April 6, 2025
Accepted: April 28, 2025
Published online: May 26, 2025
Processing time: 96 Days and 2.4 Hours
Hypertrophic cardiomyopathy (HCM) is characterized by left ventricular hypertrophy and interstitial fibrosis, which contribute to adverse outcomes such as heart failure and sudden cardiac death. While cardiac magnetic resonance (CMR) imaging is commonly used to detect myocardial fibrosis, circulating microRNAs (miRNAs) have emerged as promising noninvasive biomarkers for this condition due to their stability in blood plasma and resistance to pH and temperature variance.
To explore the role of specific circulating miRNAs in identifying myocardial fibrosis in patients with HCM.
Using PubMed/MEDLINE and Google Scholar, we reviewed studies from 2014 to 2024 examining the link between circulating miRNAs and myocardial fibrosis in HCM. We included studies measuring miRNA expression in blood samples from HCM patients and assessing fibrosis via imaging, mostly CMR. Data extraction concentrated on the population, methodology, and findings related to the correlation between miRNA levels and fibrosis.
Seven studies involving 365 HCM patients with a mean age of 49.37 ± 10.5 years, 116 (31.78%) females, and one animal study identified miR-21, miR-29a, miR-133, miR-4454, and miR-221 as frequently dysregulated markers associated with fibrosis. Elevated levels of miR-21 and miR-29a correlated with more extensive fibrosis, as assessed by late gadolinium enhancement in CMR imaging, with miR-29a consistently linked to both fibrosis and hypertrophy across the studies.
Circulating miRNAs, particularly miR-21, miR-29a, and miR-221, show significant potential as biomarkers for myocardial fibrosis in HCM. Further research should validate these findings and investigate the clinical application of miRNA-based diagnostics in HCM.
Core Tip: Circulating microRNAs (miRNA) show promise as non-invasive biomarkers for myocardial fibrosis in hypertrophic cardiomyopathy (HCM). Our systematic review of eight studies identified miRNAs miR-21, miR-29a, and miR-221 as frequently dysregulated, with elevated miR-21 and miR-29a correlating with increased fibrosis detected by cardiac magnetic resonance imaging. These findings suggest that miRNAs could be a less invasive approach to the detection of fibrosis in HCM. However, the clinical validation of these findings has to be further validated with further research.
- Citation: Mylavarapu M, Kodali LSM, Vempati R, Nagarajan JS, Vyas A, Desai R. Circulating microRNAs in predicting fibrosis in hypertrophic cardiomyopathy: A systematic review. World J Cardiol 2025; 17(5): 106123
- URL: https://www.wjgnet.com/1949-8462/full/v17/i5/106123.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i5.106123
Hypertrophic cardiomyopathy (HCM) is a common inherited cardiac genetic disorder but does not require a positive family history, and the prevalence of unexplained asymptomatic cardiac hypertrophy has been reported to be in the range of 1: 500. The true burden of symptomatic hypertrophy is higher than that found through medical claims data, which estimates prevalence to be < 1: 3000[1]. HCM is inherited as an autosomal dominant pattern, with incomplete penetrance and variable expressivity[2,3], and involves mutations in one of the genes encoding structural proteins of sarcomere, proteins regulating sarcomere function, and calcium homeostasis[4-8]. Pathophysiology of HCM involves asymptomatic left ventricular hypertrophy (LVH) along with cardiomyocyte hypertrophy, sarcomere disarray, and myocardial fibrosis at the cellular level[9], which leads to diastolic dysfunction, mitral regurgitation, dynamic left ventricular outflow tract obstruction, myocardial ischemia, arrhythmias, metabolic and energetic abnormalities, potential autonomic dysfunction[1].
Myocardial fibrosis is characterized by excessive deposition of extracellular matrix (ECM) proteins, consisting of fibrillar proteins like collagen type I and III, and non-fibrillar proteins including glycoproteins, glycosaminoglycans, and proteoglycans[10,11]. Myocardial fibrosis in HCM involves the septum, right ventricle, and right ventricle insertion points[12]. Color tissue Doppler echocardiography can be utilized to diagnose and grade diastolic dysfunction in HCM. Cardiac magnetic resonance imaging (MRI) has been recognized as superior in confirming and more accurately quantifying the severity of LVH and left ventricular parameters, assessing the extent of myocardial fibrosis, and also for serial monitoring among HCM patients. Late gadolinium enhancement (LGE) provides an assessment of myocardial fibrosis and resultant diastolic dysfunction[13]. Quantitative LGE by cardiac magnetic resonance (CMR) exhibited substantial prognostic value in predicting sudden cardiac death (SCD) events, independent of baseline characteristics. The assessment of LGE can be utilized as an effective tool for risk-stratifying patients with HCM[14]. Cardiac MRI has limitations, including the relatively long duration of CMR examinations, high costs, and various patient factors that can affect comfort and safety during MRI procedures[15].
MicroRNAs (miRNA) are small, noncoding, single-stranded ribonucleic acid (RNA) molecules that act as post-transcriptional regulators of gene expression. They have been studied as biomarkers of myocardial injury and heart failure and are involved in myocardial hypertrophy, fibrosis, and apoptosis[16,17]. Due to their stability in blood plasma and resistance to pH and temperature variance[17], miRNAs are good candidates for clinical biomarkers. Our systematic review aims to study the role of miRNA as a biomarker and its prognostic significance as a predictor of fibrosis in patients with HCM.
Our systematic review has been conducted with adherence to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[18].
Original articles from PubMed/MEDLINE, Google Scholar, and EMBASE databases were searched to identify articles from 2014 to 2024 to identify articles that studied the role of miRNA as a biomarker of myocardial fibrosis among patients with HCM. A snowballing strategy was used to make the search comprehensive.
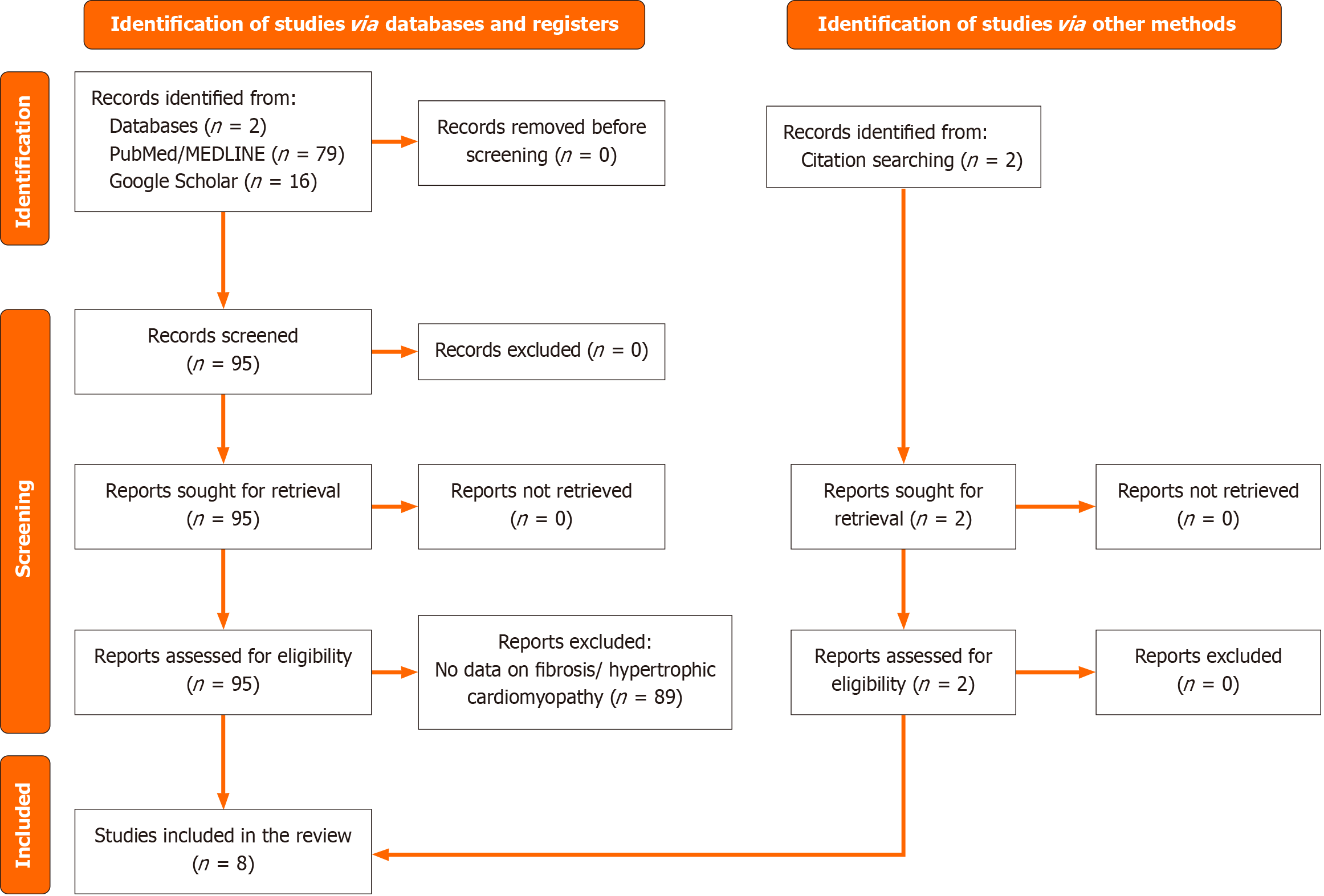
The articles obtained were screened to eliminate duplicates, and inclusion and exclusion criteria were applied during the title review. An abstract and full-text screening conducted by two independent reviewers was utilized to narrow down the selected articles. Studies involving human subjects, with quantitative assessments of circulating miRNAs and fibrosis in patients with HCM, were included. Studies focusing on non-circulating miRNA, animal or in vitro research, conference abstracts, editorials, and review articles were excluded. Supplementary Tables 1 and 2 provide a detailed list of the search strategy and inclusion and exclusion criteria, respectively. Figure 1 illustrates the detailed searching and screening strategy[19].
Study details, including author, year, study design, sample size, patient characteristics, methods for miRNA measurements, fibrosis assessment, and key findings, were extracted into spreadsheets. Data extraction and management were conducted in Microsoft Excel. Quality assessment was conducted using the Newcastle-Ottawa Scale[20]. The majority (6/8) were found to have a low risk of bias. Supplementary Figure 3 outlines a detailed assessment of the risk of bias[21].
This study was based on a systematic review of previously published articles, so no ethical approval was required. All included studies were assumed to have appropriate ethical approval.
A total of eight studies[16,22-28] were included in our review. All studies included human subjects (365) with a mean age of 49.37 ± 10.5 years, 116 (31.78%) females, and were case-control studies except one[22], which included murine mouse models. All studies applied reverse transcription polymerase chain reaction (RT-PCR) assays for miRNA detection except one[25] that used a microarray kit. Most of the studies (6 out of 8)[16,23-26,28] used CMR with LGE images to detect fibrosis, and one study[27] made a symptom-based assessment. Table 1 shows the characteristics of selected studies, including sample size, detection methods of miRNAs and fibrosis, and key findings.
| Ref. | Study design | Sample size | Population | Method of miRNA detection | Method of fibrosis assessment | Key findings |
| Human studies | ||||||
| Angelopoulos et al[23] | Case-control study | 50 | 27 HCM patients, 13 hypertensive cardiomyopathy patients, 10 controls | miR-21 and -29 assessed by qRT-PCR | LGE by CMR | miR-21 upregulated in HCM and correlated with extensive myocardial fibrosis; miR-29 did not differ between groups |
| Zhang et al[25] | Case-control study | 25 | 21 HCM patients, 4 controls | 68 miRNAs detected by Agilent Human miRNA Microarray Kit | LGE by CMR | 22 miRNAs increased and 46 miRNAs decreased in HCM patients; miR-3960 and miR-652-3p levels correlated with LV hypertrophy; miR-642a-3p levels correlated with LV fibrosis |
| Thottakara et al[16] | Case-control study | 40 | 24 HCM patients, 11 controls | 6 miRNAs quantified by Human v3 miRNA Expression Assay Kit Code set and validated by RT-PCR | LGE by CMR | Elevated miR-4454 levels correlated with fibrosis in HCM patients |
| Huang et al[24] | Case-control study | 72 | 42 HOCM patients and 30 controls | 11 miRNAs initially detected by qRT-PCR; 2 further studied | Echocardiography and LGE by CMR | miR-221 correlated with myocardial fibrosis and hypertrophy in HOCM |
| Zhou et al[27] | Case-control study | 69 | 30 HCM with fibrosis and 39 HCM without fibrosis patients | miR-29a assessed by qRT-PCR | Symptomatic assessment | lncRNA-MIAT associated with fibrosis in HCM by regulating miR-29a |
| Fang et al[28] | Case-control study | 55 | 55 HCM patients | 16 miRNAs validated using Taqman PCR | LGE by CMR | 14 miRNAs upregulated in HCM patients; combination of 8 miRNAs showed high diagnostic value for diffuse myocardial fibrosis in HCM |
| Roncarati et al[26] | Case-control study | 82 | 41 HCM patients, 41 controls | 21 miRNAs assessed by qRT-PCR | LGE by CMR | 12 miRNAs upregulated in HCM; 3 correlated with hypertrophy; only miR-29a correlated with hypertrophy and fibrosis |
| Animal studies | ||||||
| Zalivina et al[22] | Animal study | - | Murine mouse model | miR-199a-3p assessed by qPCR | N/A | miR-199a-3p contributed to cardiac fibrosis in HCM mice |
A total of 7 miRNAs were reported to be associated with myocardial fibrosis in HCM patients, including miR-199a-3p, miR-21, miR-4454, miR-221, miR-29a, miR-642a-3p, and miR-133a-3p.
Zalivina et al[22] reported on the role of miR-199a-3p in cardiac fibrosis associated with HCM using a murine model. The levels of miR-199a-3p were significantly elevated by two-fold (P = 0.0006) in the hearts of HCM mice and did not correlate with cardiac hypertrophy in HCM[22]. They also found that treatment with lock-nucleic acid (LNA) antimiR-199a-3p significantly reduced cardiac fibrosis, indicating that miR-199a-3p may promote fibrosis by modulating the expression of profibrotic genes[22]. Furthermore, miR-199a-3p was shown to regulate the PI3K/AKT pathway in cardiac fibroblasts[22].
A study by Angelopoulos et al[23] reported that circulating miR-21 levels were 5.57 times higher in HCM patients compared to healthy controls. The study also reported that delta computed tomography miR-21 values were lower in HCM patients with LGE-detected myocardial fibrosis in more than 4 out of 17 myocardial segments than those with myocardial LGE in 3 or fewer myocardial segments[23]. These findings indicate that a higher expression of miR-21 was associated with more extensive myocardial fibrosis, as is evident from CMR imaging[23].
Thottakara et al[16] evaluated miR-4454 concerning ventricular fibrosis in patients with HCM. They found that miR-4454 levels were 2.1-fold higher in HCM patients compared to control individuals (P = 0.03). Furthermore, levels of miR-4454 showed a correlation with the extent of myocardial fibrosis, as detected by LGE on MRI (r = 0.56, P = 0.001) and septal left ventricle (LV) wall thickness (r = 0.38, P = 0.03). A direct correlation was identified between the extent of fibrosis and miR-4454 expression in HCM patients (r = 0.4856, P = 0.0256, R2 = 0.2358), indicating its potential as a biomarker for myocardial fibrosis in HCM[16].
Huang et al[24] reported a correlation between miR-221 and myocardial fibrosis in patients with hypertrophic obstructive cardiomyopathy (HOCM). The expression levels miR-221 were significantly increased in these patients (z = -2.249, P = 0.024), indicating their involvement in myocardial remodeling. Furthermore, they found that circulating miR-221 had a significant positive correlation with collagen volume fraction (CVF) (r = 0.454, P = 0.002), LGE (r = 0.630, P = 0.004), maximum interventricular septal thickness measured by echocardiography (r = 0.318, P = 0.042) and CMR (r = 0.342, P = 0.027), as well as left ventricular mass index (LVMI) measured by echocardiography (r = 0.638, P < 0.001) and CMR (r = 0.725, P < 0.001)[24]. The area under the curve (AUC) for circulating miR-221 was reported to be 0.764, higher than other miRNAs, highlighting its diagnostic value[24]. Consequently, elevated levels of miR-221 were positively correlated with myocardial fibrosis and hypertrophy and left ventricular ejection fraction (LVEF), suggesting its role in regulating myocardial hypertrophy, fibrosis, and cardiac function[24]. These findings suggest that circulating miR-221 could be a potential biomarker for myocardial hypertrophy and fibrosis in HOCM[24].
A study by Zhang et al[25] reported that miR-642a-3p expression was positively correlated with LV fibrosis and significantly correlated with LGE quantification (r = 0.467, P = 0.028). Furthermore, myocardial miR-642-3p was significantly down-regulated in HCM compared to other miRNAs[25]. Another important finding of this study is the significant association of myocardial miR-3960 and miR-652-3p with LV hypertrophy[25]. These findings suggest that miR-642a-3p could be a biomarker for LV fibrosis.
Roncarati et al[26] reported that among three miRNAs associated with LV hypertrophy in HCM patients, only miR-29a showed a significant positive correlation with cardiac fibrosis as evaluated by CMR (r = 0.691, P = 0.003). Additionally, they discovered that miR-29a was fibrosis-specific in HCM since its levels were not elevated in concentric LVH caused by severe aortic stenosis. Because of its ability to precisely detect fibrosis, miR-29a may serve as a potential biomarker for evaluating myocardial remodeling in HCM[26].
Another study by Zhou et al[27] demonstrated the molecular mechanism behind the association of miR-29a with fibrosis in patients with HCM. miR-29a was overexpressed in HCM patients with fibrosis, suggesting its involvement in the pathological mechanism of fibrosis. Conversely, the long non-coding RNA known as myocardial infarction-associated transcript (LncRNA-MIAT) was significantly elevated in patients with HCM who did not have fibrosis. Additionally, it was concluded that MIAT was negatively correlated with miR-29a (r = -0.539, P < 0.0001). A luciferase assay further confirmed a negative correlation between MIAT and miR-29a, verifying their involvement in the fibrosis of HCM patients[27]. Furthermore, the AUC analysis for MIAT and miR-29a was reported as 0.885 and 0.810, respectively, indicating their potential as predictive biomarkers for risk stratification of fibrosis in HCM patients[27].
A study by Fang et al[28] revealed that 14 circulating miRNAs were upregulated and associated with cardiac fibrosis in patients with HCM. They reported that these 14 individual miRNAs had moderate diagnostic values (AUC: 0.663-0.742) for myocardial fibrosis, while a combination of 8 miRNAs enhanced the diagnostic value (AUC: 0.87) in HCM patients. miR-133a-3p, along with miR-29a-3p, was found to be significantly elevated in patients with diffuse fibrosis. Furthermore, individual miR-133a-3p showed a slightly lower (AUC: 0.663, P = 0.038) diagnostic value compared to the combination of 8 miRNAs[28]. Table 2 outlines key findings of miRNAs linked to fibrosis in HCM.
| miRNA | Role in fibrosis | Diagnostic/prognostic potential |
| miR-199a-3p | Promotes fibrosis by modulating profibrotic genes and regulating the PI3K/AKT pathway | Potential therapeutic target to reduce cardiac fibrosis |
| miR-21 | Associated with extensive myocardial fibrosis evident from imaging studies | Biomarker for assessing fibrosis severity through non-invasive imaging |
| miR-4454 | Correlates with ventricular fibrosis and septal wall thickness | Biomarker for detecting fibrosis extent and ventricular structural changes |
| miR-221 | Involved in myocardial remodeling, hypertrophy, and fibrosis regulation | Biomarker for myocardial hypertrophy, fibrosis, and cardiac function |
| miR-642a-3p | Positively correlated with LV fibrosis | Biomarker for LV fibrosis detection |
| miR-29a | Specific for fibrosis in HCM; regulates pathological fibrosis mechanisms | Diagnostic biomarker for assessing myocardial remodeling: AUC = 0.810 |
| miR-133a-3p | Plays a role in diffuse fibrosis in HCM | Diagnostic biomarker for myocardial fibrosis; better utility in combination with other miRNAs |
Two studies[22,27] in our review examined the mechanistic roles of miRNAs in patients with HCM fibrosis. Using mouse models, Zalivina et al[22] demonstrated that miR-199a-3p was upregulated in HCM mice by regulating ECM gene and protein expression pathways in cardiac fibroblasts. Its inhibition during mild-to-moderate disease with a specific LNA-antimiR oligonucleotide reduces fibrotic remodeling. Zhou et al[27] found that lncRNA-MIAT diminishes fibrotic changes in HCM by inhibiting miR-29a expression, which is elevated in HCM fibrosis patients, as evidenced by their negative correlation.
All these studies suggest that miRNAs play a role as biomarkers for cardiac fibrosis in patients with HCM. miR-221, miR-29a, and miR-133a-3p demonstrated significant diagnostic value according to receiver operating characteristic analysis. Conversely, other studies reported that miR-4454, miR-642a-3p, and miR-29a exhibited a significant positive correlation with fibrosis in HCM patients. When combined with existing imaging techniques like CMR, miRNAs have the potential to act as biomarkers for the early detection of fibrosis in HCM patients.
Our review emphasizes the growing evidence supporting the role of miRNAs as promising biomarkers for fibrosis in HCM. Among the identified miRNAs, miR-199a-3p, miR-21, miR-4454, miR-221, miR-29a, miR-642a-3p, and miR-133a-3p were consistently linked with myocardial fibrosis across multiple studies. These miRNAs exhibited diagnostic and prognostic potential through their significant correlations with fibrosis-specific parameters, including LGE detected via CMR, CVF, and LVMI. Furthermore, the mechanistic insights into miR-199a-3p and miR-29a, encompassing their regulation of ECM and interaction with long non-coding RNAs, provide a molecular foundation for their role in fibrotic remodeling. The consistent use of advanced detection techniques, such as RT-PCR and CMR imaging, across studies strengthens the validity of these findings. However, certain miRNAs, like miR-29a and miR-4454, displayed fibrosis-specific characteristics, distinguishing HCM fibrosis from other cardiac conditions. These distinctions enhance their potential as precise biomarkers for disease monitoring and therapeutic targeting.
While the findings largely support the role of miRNAs as biomarkers for fibrosis in HCM, certain discrepancies require attention. For example, miR-21 and miR-4454 were linked to different degrees of fibrosis severity based on LGE imaging, with miR-21 associated with more extensive fibrotic involvement. Likewise, the diagnostic value of miR-133a-3p was moderate when evaluated individually but improved significantly when assessed as part of a miRNA panel. The study by Zhang et al[25] noted a downregulation of miR-642a-3p in HCM compared to other miRNAs, highlighting variability in miRNA expression patterns. These differences may arise from heterogeneity in study populations, miRNA detection methodologies, or criteria for assessing fibrosis[17,26,28-30].
Furthermore, including a murine model in one study (miR-199a-3p) highlights translational gaps between experimental and clinical findings[22]. While murine models provide critical mechanistic insights, their direct applicability to human subjects remains limited. These inconsistencies underscore the need for standardized protocols and larger multicenter studies to validate miRNAs' diagnostic and prognostic utility in HCM fibrosis.
Invasive tests have traditionally detected myocardial fibrosis. However, recent studies have shown that CMR is a non-invasive modality for diagnosing this condition. Nevertheless, the use of CMR has been restricted due to its high cost, limited availability of CMR facilities and expertise, and specific contraindications. Circulating miRNAs present an alternative for assessing myocardial fibrosis, given their easy accessibility, reliability, and specificity. A study by Fang et al[28] demonstrated that 14 miRNAs, including miR-29a-3p, were upregulated in diffuse fibrosis among HCM patients. Roncarati et al[26] studied 12 miRNAs and found that only 3 (miR-199a-5p, -27a, and -29a) correlated with hypertrophy; more importantly, only miR-29a correlated with fibrosis, suggesting that circulating miRNA can serve as a viable alternative to CMR for detecting diffuse fibrosis in patients with HCM.
Circulating miRNA has been positively linked to the degree of myocardial fibrosis. In a study by Thottakara et al[16], MiR-4454 was correlated with the extent of fibrosis in HCM patients, indicating that it could be a marker of fibrosis in HCM patients. In another study by Angelopoulos et al[23], patients with HCM exhibited a higher expression of circulating miR-21, which was associated with more extensive myocardial fibrosis. This finding further reinforces the positive correlation between miRNA levels and the degree of fibrosis.
Considering that myocardial fibrosis is well-documented as being related to cardiac arrhythmias and is a key predictor of SCD in HCM, miR-4454 may serve as a novel biomarker for fibrosis and SCD in HCM patients. Furthermore, Huang et al[24] found that increased miR-221 expression was positively associated with both the degree of myocardial fibrosis and the parameters of myocardial hypertrophy and LVEF, suggesting its influence on these parameters and cardiac function. Therefore, miR-221 could also be utilized as a potential biomarker for myocardial hypertrophy.
Sonsöz et al[29] highlighted a potential association between circulating miR-29a and QRS width in HCM, suggesting that increased miR-29a levels and increased myocardial fibrosis in HCM could delay the conduction time of impulse, resulting in a wide QRS complex. Sucharov et al[30] investigated the role of circulating miRNAs as biomarkers for clinical stratification and found that miRNA profiles distinguished patients with clinical HCM and subclinical HCM from healthy controls. The ability to differentiate patients with subclinical HCM from healthy controls is of great importance as it provides an opportunity for early identification and management of patients at risk of developing HCM. miRNAs can be used as potential therapeutic agents by modifications to either restore the miRNA function through miRNA mimics or by miRNA silencing with the addition of synthetic oligonucleotides called anti-miRNAs. Several in vivo studies explored the possibility of using miRNA therapy for treating HCM[31-33].
However, our review has its limitations. Differences in study designs, participant groups, interventions, and outcome measures can create inconsistencies across research studies, a phenomenon known as heterogeneity. These variabilities can complicate combining or synthesizing results from multiple studies, undermining the generalizability of findings to all contexts or populations. Fluctuations in miRNA levels across samples or individuals-caused by technical differences in extraction and quantification, biological variation, and miRNA instability in certain bodily fluids-can further complicate the accurate interpretation of miRNA expression, mainly when used as biomarkers. In addition, discrepancies in myocardial fibrosis measurements stem from several factors, including the imaging technique used, the specific region of the heart analyzed, inter-observer variability in image interpretation, and the underlying pathology causing the fibrosis. These factors can lead to differences in quantifying fibrosis even among similar patients. The variability in miRNA measurement and myocardial fibrosis assessments reduces the generalizability of our study findings. It underscores the importance of standardized miRNA analysis protocols and the need for larger, multi-center studies[34].
The miRNAs have the potential to serve as important biomarkers, assisting in the diagnostic process, patient sub-classification, and outcome prediction. Circulating biomolecules that reflect myocardial disease processes may improve the early detection of HCM. However, there is a lack of longitudinal studies to validate miRNAs as biomarkers for myocardial fibrosis. Additionally, miRNAs could contribute to the development of targeted treatment strategies for individuals with cardiovascular diseases, highlighting the need for clinical trials to evaluate therapeutic interventions targeting miRNA modulation. The complexity and high costs associated with analyzing miRNA expression pose challenges for both research and clinical application. Nevertheless, the creation of simpler miRNA biomarker kits, driven by advancing technologies, is expected to facilitate their integration into clinical practice. The origin of circulating miRNAs is still unclear, as they may also derive from other cell types, such as endothelial cells, blood cells, and immune cells. Further research is essential to examine the relationship between circulating miRNAs and myocardial miRNAs.
Several circulating miRNAs, including miR-199a-3p, miR-21, miR-4454, miR-221, miR-29a, miR-642a-3p, and miR-133a-3p, demonstrated significant associations with fibrosis-related parameters, such as LGE detected via CMR, CVF, and LVMI. Furthermore, mechanistic studies revealed critical insights into the molecular pathways modulated by these miRNAs, such as regulating ECM remodeling and interactions with long non-coding RNAs.
Despite their promise, variations in expression patterns, study designs, and diagnostic accuracy emphasize the need for further standardization of detection protocols and validation in larger, multicenter studies. Translational gaps between experimental animal models and clinical findings also warrant closer scrutiny to enhance the applicability of preclinical data. Additional research focusing on integrating miRNA panels into clinical workflows is also required to enhance diagnostic precision and guide personalized therapeutic strategies for patients with HCM.
| 1. | Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, Dearani JA, Epps KC, Evanovich L, Ferrari VA, Joglar JA, Khan SS, Kim JJ, Kittleson MM, Krittanawong C, Martinez MW, Mital S, Naidu SS, Saberi S, Semsarian C, Times S, Waldman CB; Peer Review Committee Members. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1239-e1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 181] [Article Influence: 181.0] [Reference Citation Analysis (0)] |
| 2. | Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 863] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 3. | Moody WE, Elliott PM. Changing concepts in heart muscle disease: the evolving understanding of hypertrophic cardiomyopathy. Heart. 2022;108:768-773. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Ingles J, Burns C, Bagnall RD, Lam L, Yeates L, Sarina T, Puranik R, Briffa T, Atherton JJ, Driscoll T, Semsarian C. Nonfamilial Hypertrophic Cardiomyopathy: Prevalence, Natural History, and Clinical Implications. Circ Cardiovasc Genet. 2017;10:e001620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 5. | Aziz A, Musiol SK, Moody WE, Pickup L, Cooper R, Lip GYH. Clinical prediction of genotypes in hypertrophic cardiomyopathy: A systematic review. Eur J Clin Invest. 2021;51:e13593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Alfares AA, Kelly MA, McDermott G, Funke BH, Lebo MS, Baxter SB, Shen J, McLaughlin HM, Clark EH, Babb LJ, Cox SW, DePalma SR, Ho CY, Seidman JG, Seidman CE, Rehm HL. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet Med. 2015;17:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 341] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 7. | Marian AJ. Molecular Genetic Basis of Hypertrophic Cardiomyopathy. Circ Res. 2021;128:1533-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 8. | Gerull B, Klaassen S, Brodehl A. The Genetic Landscape of Cardiomyopathies. Card Vasc Biol. 2019;. [DOI] [Full Text] |
| 9. | Schlittler M, Pramstaller PP, Rossini A, De Bortoli M. Myocardial Fibrosis in Hypertrophic Cardiomyopathy: A Perspective from Fibroblasts. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 10. | Chute M, Aujla P, Jana S, Kassiri Z. The Non-Fibrillar Side of Fibrosis: Contribution of the Basement Membrane, Proteoglycans, and Glycoproteins to Myocardial Fibrosis. J Cardiovasc Dev Dis. 2019;6:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Frangogiannis N. Transforming growth factor-β in tissue fibrosis. J Exp Med. 2020;217:e20190103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 743] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 12. | Eijgenraam TR, Silljé HHW, de Boer RA. Current understanding of fibrosis in genetic cardiomyopathies. Trends Cardiovasc Med. 2020;30:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | O'Brien AC, MacDermott R, Keane S, Ryan DT, McVeigh N, Durand R, Ferre M, Murphy DJ, Teekakirikul P, Keane D, McDonald K, Ledwidge M, Dodd JD. Cardiac MRI e-prime predicts myocardial late gadolinium enhancement and diastolic dysfunction in hypertrophic cardiomyopathy. Eur J Radiol. 2022;149:110192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, He Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1392-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 15. | Siddiqui TA, Chamarti KS, Tou LC, Demirjian GA, Noorani S, Zink S, Umair M. The Merits, Limitations, and Future Directions of Cost-Effectiveness Analysis in Cardiac MRI with a Focus on Coronary Artery Disease: A Literature Review. J Cardiovasc Dev Dis. 2022;9:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Thottakara T, Lund N, Krämer E, Kirchhof P, Carrier L, Patten M. A Novel miRNA Screen Identifies miRNA-4454 as a Candidate Biomarker for Ventricular Fibrosis in Patients with Hypertrophic Cardiomyopathy. Biomolecules. 2021;11:1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Scolari FL, Faganello LS, Garbin HI, Piva E Mattos B, Biolo A. A systematic review of microRNAs in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2021;327:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39931] [Article Influence: 9982.8] [Reference Citation Analysis (2)] |
| 19. | Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. 2022;18:e1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 843] [Article Influence: 281.0] [Reference Citation Analysis (0)] |
| 20. | Wells G, Wells G, Shea B, O’Connell D, Peterson J, Welch, Losos M, Tugwell P, Ga SW, Zello G, Petersen J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Accessed December 30, 2024. Available from: https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-(NOS)-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf. |
| 21. | McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 2524] [Article Influence: 504.8] [Reference Citation Analysis (0)] |
| 22. | Zalivina I, Barwari T, Yin X, Langley SR, Barallobre-Barreiro J, Wakimoto H, Zampetaki A, Mayr M, Avkiran M, Eminaga S. Inhibition of miR-199a-3p in a murine hypertrophic cardiomyopathy (HCM) model attenuates fibrotic remodeling. J Mol Cell Cardiol Plus. 2023;6:100056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 23. | Angelopoulos A, Oikonomou E, Antonopoulos A, Theofilis P, Zisimos K, Katsarou O, Gazouli M, Lazaros G, Papanikolaou P, Siasos G, Tousoulis D, Tsioufis K, Vlachopoulos C. Expression of Circulating miR-21 and -29 and their Association with Myocardial Fibrosis in Hypertrophic Cardiomyopathy. Curr Med Chem. 2024;31:3987-3996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 24. | Huang D, Chen Z, Wang J, Chen Y, Liu D, Lin K. MicroRNA-221 is a potential biomarker of myocardial hypertrophy and fibrosis in hypertrophic obstructive cardiomyopathy. Biosci Rep. 2020;40:BSR20191234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Zhang C, Zhang H, Zhao L, Wei Z, Lai Y, Ma X. Differential Expression of microRNAs in Hypertrophied Myocardium and Their Relationship to Late Gadolinium Enhancement, Left Ventricular Hypertrophy and Remodeling in Hypertrophic Cardiomyopathy. Diagnostics (Basel). 2022;12:1978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Roncarati R, Viviani Anselmi C, Losi MA, Papa L, Cavarretta E, Da Costa Martins P, Contaldi C, Saccani Jotti G, Franzone A, Galastri L, Latronico MV, Imbriaco M, Esposito G, De Windt L, Betocchi S, Condorelli G. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2014;63:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 27. | Zhou J, Zhou Y, Wang CX. LncRNA-MIAT regulates fibrosis in hypertrophic cardiomyopathy (HCM) by mediating the expression of miR-29a-3p. J Cell Biochem. 2019;120:7265-7275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Fang L, Ellims AH, Moore XL, White DA, Taylor AJ, Chin-Dusting J, Dart AM. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J Transl Med. 2015;13:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 29. | Sonsöz MR, Yilmaz M, Cevik E, Orta H, Bilge AK, Elitok A, Onur I, Komurcu-Bayrak E. Circulating Levels of MicroRNAs in Hypertrophic Cardiomyopathy: The Relationship With Left Ventricular Hypertrophy, Left Atrial Dilatation and Ventricular Depolarisation-Repolarisation Parameters. Heart Lung Circ. 2022;31:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Sucharov CC, Neltner B, Pietra AE, Karimpour-Fard A, Patel J, Ho CY, Miyamoto SD. Circulating MicroRNAs Identify Early Phenotypic Changes in Sarcomeric Hypertrophic Cardiomyopathy. Circ Heart Fail. 2023;16:e010291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1367] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 32. | Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1893] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 33. | Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012;3:1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 499] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 34. | Felekkis K, Papaneophytou C. The Circulating Biomarkers League: Combining miRNAs with Cell-Free DNAs and Proteins. Int J Mol Sci. 2024;25:3403. [PubMed] [DOI] [Full Text] |