Published online Apr 26, 2022. doi: 10.4330/wjc.v14.i4.220
Peer-review started: December 10, 2021
First decision: February 15, 2022
Revised: February 17, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: April 26, 2022
Processing time: 129 Days and 4 Hours
In the heart, aldosterone (Aldo) binds the mineralocorticoid receptor (MR) to exert damaging, adverse remodeling-promoting effects. We recently showed that G protein-coupled receptor-kinase (GRK)-5 blocks the cardiac MR by directly phosphorylating it, thereby repressing its transcriptional activity. MR antagonist (MRA) drugs block the cardiac MR reducing morbidity and mortality of advanced human heart failure. Non-steroidal MRAs, such as finerenone, may provide better cardio-protection against Aldo than classic, steroidal MRAs, like spironolactone and eplerenone.
To investigate potential differences between finerenone and eplerenone at engaging GRK5-dependent cardiac MR phosphorylation and subsequent blockade.
We used H9c2 cardiomyocytes, which endogenously express the MR and GRK5.
GRK5 phosphorylates the MR in H9c2 cardiomyocytes in response to finerenone but not to eplerenone. Unlike eplerenone, finerenone alone potently and efficiently suppresses cardiac MR transcriptional activity, thus displaying inverse agonism. GRK5 is necessary for finerenone’s inverse agonism, since GRK5 genetic deletion renders finerenone incapable of blocking cardiac MR transcriptional activity. Eplerenone alone does not fully suppress cardiac MR basal activity regardless of GRK5 expression levels. Finally, GRK5 is necessary for the anti-apoptotic, anti-oxidative, and anti-fibrotic effects of both finerenone and eplerenone against Aldo, as well as for the higher efficacy and potency of finerenone at blocking Aldo-induced apoptosis, oxidative stress, and fibrosis.
Finerenone, but not eplerenone, induces GRK5-dependent cardiac MR inhibition, which underlies, at least in part, its higher potency and efficacy, compared to eplerenone, as an MRA in the heart. GRK5 acts as a co-repressor of the cardiac MR and is essential for efficient MR antagonism in the myocardium
Core Tip: G protein-coupled receptor-kinase (GRK)-5 blocks the cardiac actions of aldosterone via phosphorylation of the mineralocorticoid receptor (MR). We show here that the non-steroidal MR antagonist (MRA) finerenone may provide better cardio-protection against aldosterone than classic, steroidal MRAs, like eplerenone, thanks to induction of GRK5’s phosphorylation and subsequent blockade of cardiac MR. GRK5 is necessary for the anti-apoptotic, anti-oxidative, and anti-fibrotic effects of both finerenone and eplerenone against aldosterone but also for the higher efficacy/potency of the former drug at producing all these effects in cardiomyocytes. Thus, GRK5 acts as a co-repressor of the cardiac MR and is essential for efficient MR antagonism in the myocardium.
- Citation: Pollard CM, Suster MS, Cora N, Carbone AM, Lymperopoulos A. GRK5 is an essential co-repressor of the cardiac mineralocorticoid receptor and is selectively induced by finerenone. World J Cardiol 2022; 14(4): 220-230
- URL: https://www.wjgnet.com/1949-8462/full/v14/i4/220.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i4.220
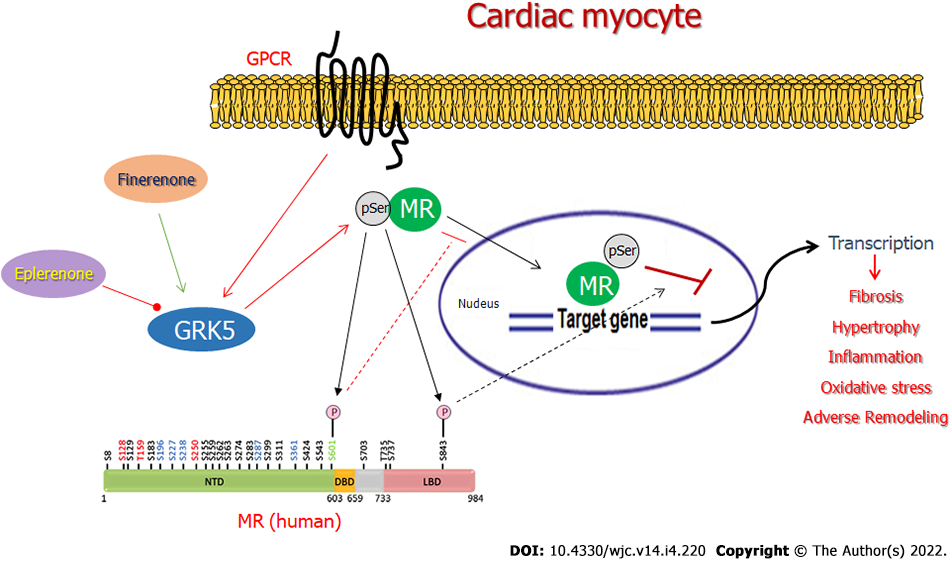
Aldosterone (Aldo) is one of several cardio-toxic hormones, whose elevated circulating levels significantly confound and aggravate heart disease, including hypertension and chronic heart failure (CHF)[1-4]. The mineralocorticoid receptor (MR), a cytosolic transcription factor that, upon activation, translocates to the nucleus to activate gene transcription, is the main receptor mediating Aldo’s adverse remodeling effects in the failing heart[1-5]. GRK2 and GRK5 are the most abundant cardiac G protein-coupled receptor (GPCR)-kinase (GRK) isoforms. Both phosphorylate GPCRs but also non-GPCR substrates[6-10]. We recently showed that GRK5 blocks the cardio-toxic MR-dependent effects of aldosterone in the heart by directly phosphorylating the cardiac MR and inhibiting its transcriptional activity[11].
MR antagonist (MRA) drugs are beneficial in human advanced CHF thanks to their blockade of the MR in various cardiovascular tissues, including in cardiomyocytes and cardiac fibroblasts[3,12]. Novel, non-steroidal MRAs, such as finerenone, may provide better cardio-protection against aldosterone’s cardio-toxic actions than the classic steroidal MRAs, such as sprironolactone and eplerenone[13,14]. Indeed, finerenone was recently shown to be a more potent and efficacious inverse agonist at the MR, compared to eplerenone, in terms of cardiac fibrosis/adverse remodeling attenuation[15]. This prompted us to investigate the effects of these two MRAs on GRK5-dependent cardiac MR phosphorylation and subsequent suppression, in an effort to delineate potential molecular mechanisms underlying their differences in cardiac MR blocking efficacy. Indeed, we found that finerenone, but not eplerenone, promotes the inhibitory action of GRK5 on cardiac MR, which may underlie finerenone’s significantly greater efficacy/potency as an inverse agonist at this receptor. Moreover, GRK5 is necessary for both MRA drugs’ cardioprotective actions against Aldo in cardiac myocytes.
All methods were carried out in accordance with the relevant guidelines and regulations.
All drugs/chemicals were from Sigma-Aldrich (St. Louis, MO, United States), except for finerenone (BAY94-8862) which was purchased from MedKoo Biosciences, Inc. (Cat. #319698, Morrisville, NC, United States).
The H9c2 rat cardiomyoblast cell line was purchased from American Type Culture Collection (Manassas, VA, United States) and cultured as previously described[11,16-18]. Recombinant lentiviruses encoding for wild-type full-length GRK5 or for empty vector (control) (OriGene Technologies, Rockville, MD, United States) were propagated and purified via CsCl density gradient ultracentrifugation, as described previously[11,19]. For CRISPR/Cas9-mediated GRK5 gene deletion, a gRNA sequence was custom-synthesized by Sigma-Aldrich (target ID: RN0000391809, target sequence: 5’-GTGGTT
Cell extracts were prepared, as described previously[11,20], in a 20-mmol/L Tris pH 7.4 buffer containing 137 mmol/L NaCl, 1% Nonidet P-40, 20% glycerol, 10 mmol/L phenylmethylsulfonylfluoride (PMSF), 1 mmol/L Na3VO4, 10 mmol/L NaF, 2.5 µg/mL aprotinin, and 2.5 µg/mL leupeptin. Protein concentration was determined (Pierce BCA Protein Assay Kit, Thermo Scientific, Waltham, MA, United States), and equal amounts of protein per sample were used for Immunoprecipitation (IP) or western blotting. MR was immunoprecipitated by overnight incubation of extracts with an anti-MR antibody (#ab62532; Abcam, Cambridge, MA, United States), attached to Protein A/G-Sepharose beads (Sigma-Aldrich). The IPs were then subjected to immunoblotting for GRK5 (#sc-565; Santa Cruz Biotechnology, Santa Cruz, CA, United States) or for phosphoserine (#AB1603; Millipore-Sigma, Burlington, MA, United States) to measure the pSer content of the immunoprecipitated MR. Finally, an anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (#sc-25778; Santa Cruz Biotechnology) was used to control for protein loading. All immunoblots were revealed by enhanced chemiluminescence (ECL, Life Technologies, Grand Island, NY, United States) and visualized in the FluorChem E Digital Darkroom (Protein Simple, San Jose, CA, United States), as described previously[21].
Luciferase reporter activity assay was performed, as described previously, by transfecting the cells with the LightSwitch™ luciferase reporter gene vector under the influence of the MR promoter (Active Motif, Inc., Carlsbad, CA, United States)[11]. The measurements were done the next day with the manu
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay to measure apoptotic cell death was done as described[22]. Briefly, cells were fixed with 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5-µm thickness. DNA fragmentation was detected in situ in deparaffinized sections using the ApopTag peroxidase in situ apoptosis detection Kit (Millipore-Sigma) and according to the manufacturer’s instructions. The total number of nuclei was determined by manual counting of 4’,6’-diamidino-2-phenylindole (DAPI)-stained nuclei in six random fields per section. All terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL)-positive nuclei were counted in each section.
Real-time PCR for rat plasminogen activator inhibitor (PAI)-1 and rat fibronectin mRNA levels in total RNA isolated from cells was done as described previously[16]. Briefly, quantitative real-time PCR was performed using a MyIQ Single-Color Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA, United States) using SYBR Green Supermix (Bio-Rad) and 100 nmol/L of gene-specific oligonucleotides. Quantification of mRNA included normalization to 18s rRNA levels. No bands were seen in control reactions in the absence of reverse transcriptase. Primer pairs used were: 5’-TTCCTCCACAGCCATTCTAGTCT-3’ and 5’-GAAAGGATCGGTCTAAAACCATCTC-3’ for PAI-1; 5’-CGAGGTGACAGAGACCACAA-3’ and 5’-CTGGAGTCAAGCCAGACACA-3’ for fibronectin; and 5’-TCGATGCTCTTAGCTGAGTG-3’ and 5’-TGATCGTCTTCGAACCTCC-3’ for 18S rRNA.
To determine reactive oxygen species (ROS) production, the 2′,7′-dichlorofluorescein diacetate (DCFDA) dye-based assay kit from Molecular Probes (Cat. #C13293; Eugene, OR, United States) was used and the measurements were done according to manufacturer's instructions and as previously described[11]. Briefly, cell extracts were incubated with 2 µmol/L DCFDA for 20 min and ROS production was monitored by determining the fluorescence intensity using a fluorescent plate reader in which excitation and emission wavelengths were set at 495 and 520 nm, respectively. The fluorescence OD values obtained were normalized with protein determination and expressed as % of the values obtained upon 100 nmol/L Aldo treatment (1 mmol/L DMSO was used as vehicle treatment).
Student’s t test and one- or two-way ANOVA with Bonferroni test were used for statistical comparisons, unless otherwise indicated. For multiple group analyses, Dunnett’s test with SAS version 9 software (Cary, NC, United States) was also used. A P value of < 0.05 indicated statistical significance.
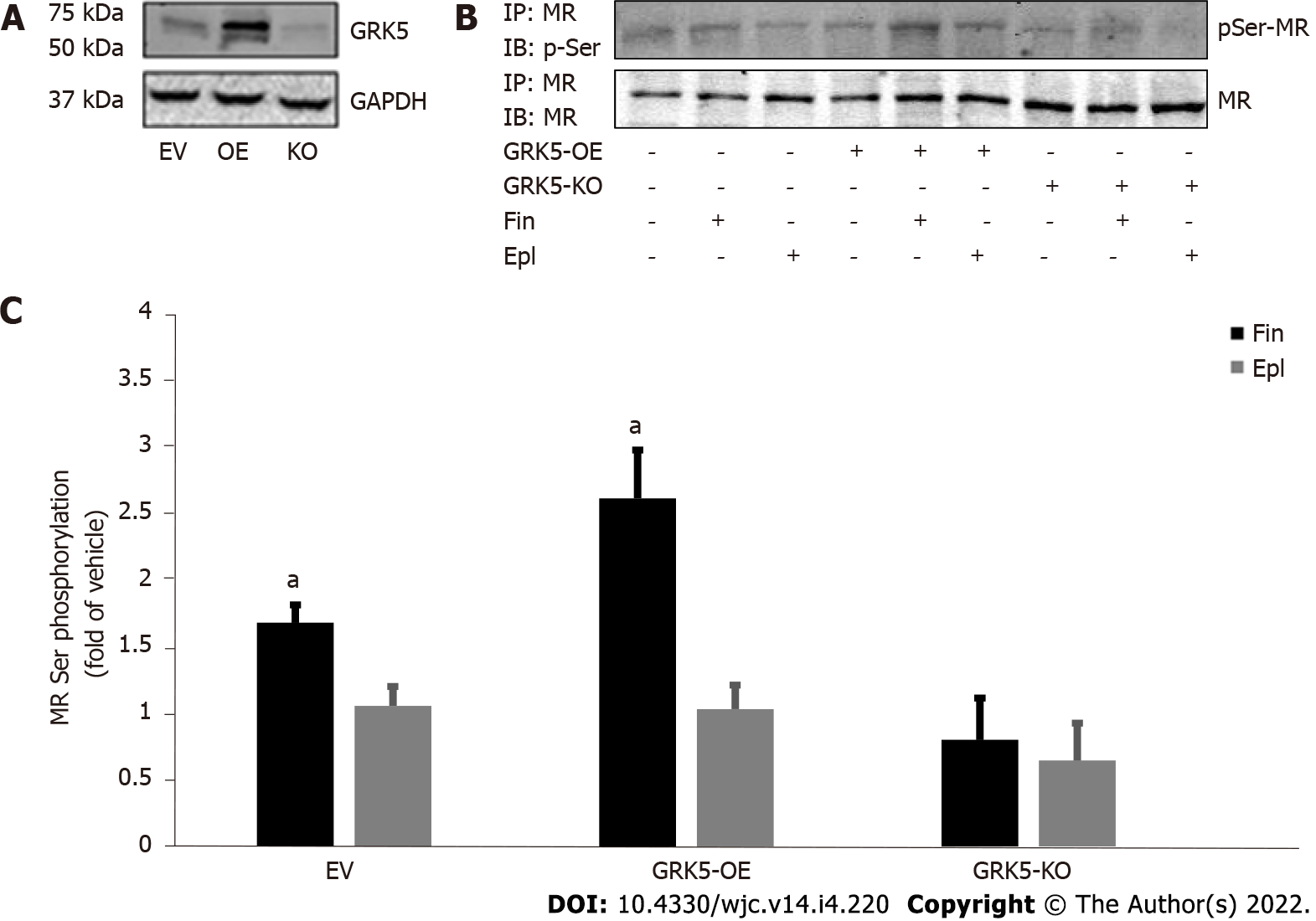
We recently reported that GRK5 selectively phosphorylates and inhibits the cardiac MR[11]. Based also on recent evidence suggesting greater potency for finerenone, compared to eplerenone, at inhibiting the cardiac MR and its downstream fibrosis[15], we hypothesized, in the present study, that the higher efficacy/potency of finerenone over eplerenone might be due (at least in part) to differences in modulation of the GRK5 inhibitory action on the cardiac MR. Thus, in a first series of experiments, we overexpressed or knocked out (via CRISPR) GRK5 in H9c2 cardiac myocytes (Figure 1A), which endogenously express both GRK5 and MR[11,23], and checked for the effects of the two MRA drugs on MR serine phosphorylation. GRK5, being a Ser/Thr kinase, likely phosphorylates multiple Ser and Thr residues of the MR protein, with phosphorylations of Ser601 and Ser843 (in the human orthologue sequence), in particular, resulting in significant functional inhibition of the MR, courtesy of cytosolic retention and transcriptional activity suppression, respectively[24,25]. After preliminary concentration-response experiments (not shown), and based on the associated literature, we chose a 10 mmol/L concentration for both drugs throughout the experiments of our study, as this concentration (10 mmol/L) is quite close to both drugs’ effective IC50 values[12,15]. As shown in Figure 1B and C, finerenone led to much higher phosphorylation (pSer content) of the MR than eplerenone did in control H9c2 cardiomyocytes (mock virus-EV lanes). This finerenone-induced MR phosphorylation was significantly enhanced upon GRK5 overexpression but essentially abrogated in GRK5-depleted H9c2 cardiomyocytes (Figure 1B and C). Notably, eplerenone essentially failed to elicit any appreciable MR Ser phosphorylation in H9c2 cardiomyocytes (Figure 1B and C), irrespective of GRK5 expression levels [eplerenone-induced phosphorylation: 1.2 ± 0.25-fold of vehicle in EV cells; 1.23 ± 0.27-fold of vehicle in GRK5-OE cells; 0.6 ± 0.55-fold of vehicle in GRK5-KO cells; i.e., non-significant vs vehicle, in all three clones at P = 0.05 (n = 3); Figure 1C]. Although we cannot account for the potential of some extent of Thr phosphorylation of the MR induced by the two drugs, these results strongly suggest that only finerenone (not eplerenone) induces GRK5-mediated phosphorylation of the MR in H9c2 cardiac myocytes.
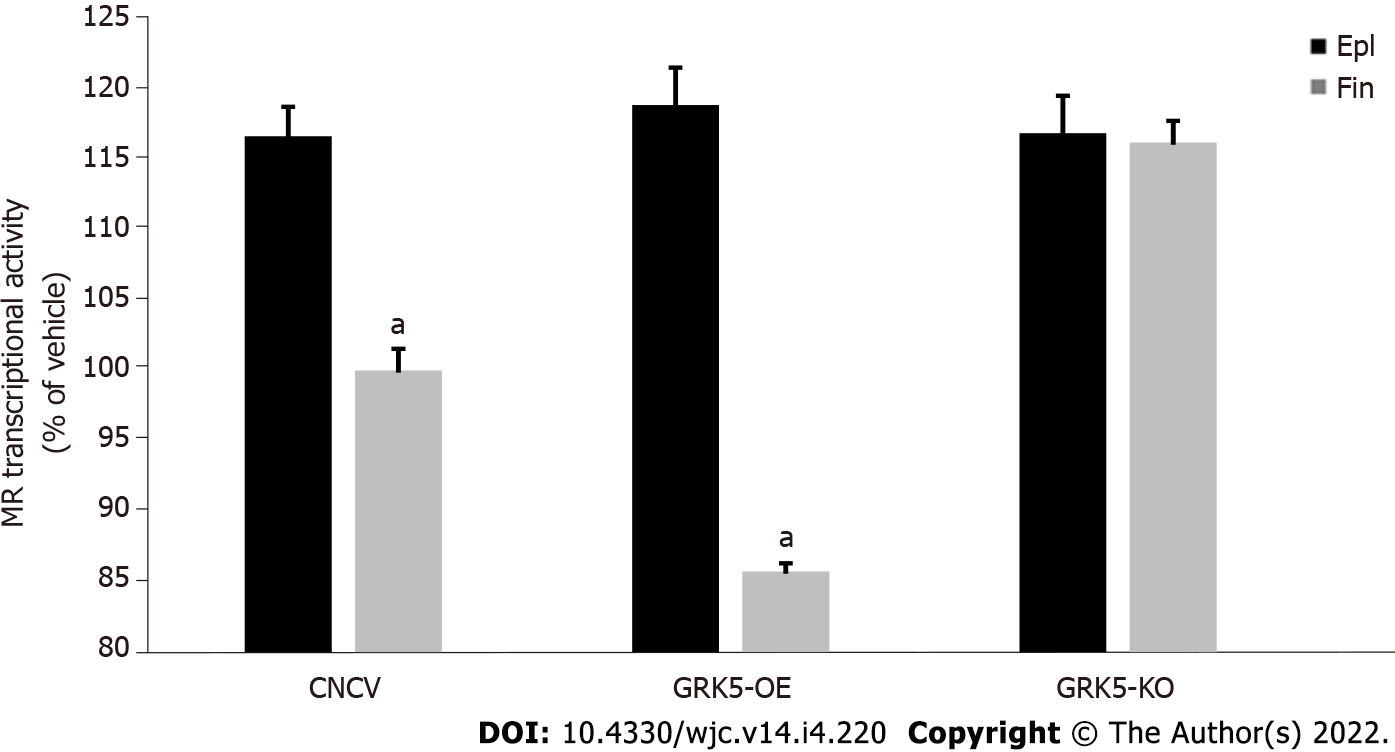
Since GRK5-induced phosphorylation translates into transcriptional repression of the cardiac MR[11], we next examined the impact of the finerenone-induced, GRK5-mediated MR phosphorylation on the transcriptional activity of the receptor. In contrast with eplerenone, finerenone lacks agonist activity at the MR in control (CNCV) H9c2 cardiomyocytes, i.e., no increase in MR basal transcriptional activity (in the absence of Aldo) is observed with finerenone (Figure 2). In the absence of GRK5 however, finerenone loses the ability to keep the MR transcriptionally inactive, i.e., the MR displays significant basal activity in GRK5-KO H9c2 cardiomyocytes (Figure 2). Upon GRK5 overexpression, this picture is reversed, i.e., finerenone acts as potent inverse agonist at the MR, markedly suppressing MR basal transcriptional activity in GRK5-overexpressing (GRK5-OE) cardiomyocytes (Figure 2). In contrast, eplerenone allows for substantial MR basal transcriptional activity, regardless of GRK5 expression levels (Figure 2). Taken together, these results indicate that GRK5 is essential for finerenone’s inverse agonism at the cardiac MR, while eplerenone is essentially a partial agonist (mixed agonist/antagonist) at this receptor in the heart, a finding consistent with the literature[12,15]. GRK5 is unable to affect eplerenone’s actions on the cardiac MR, probably because this MRA agent cannot induce the inhibitory phosphorylation of this receptor by GRK5 in cardiac myocytes (see above, Figure 1).
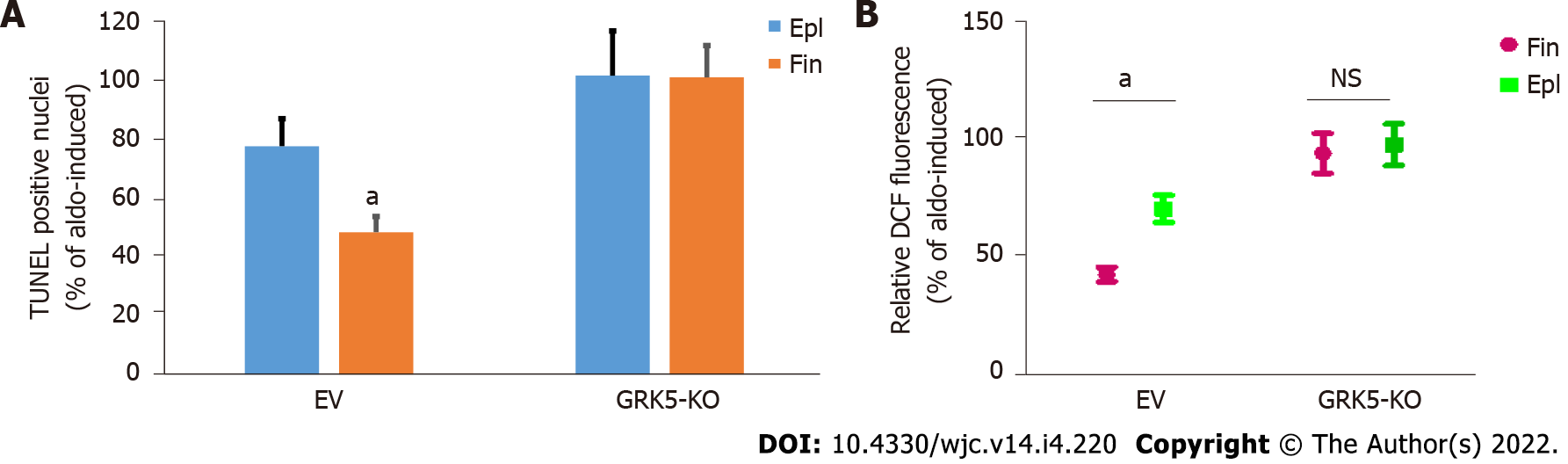
Next, we compared the cardio-protective efficacies of the two MRA drugs against the deleterious actions of Aldo. Finerenone was much more effective than eplerenone at suppressing Aldo-induced apoptosis (Figure 3A) and oxidative stress (Figure 3B), in control myocytes. However, upon GRK5 genetic deletion, both MRAs failed completely to block these two cardiac adverse remodeling-promoting Aldo effects (Figures 3A and B). This strongly suggests that GRK5 is essential for the anti-apoptotic and anti-oxidative effects of MRAs against Aldo in the heart, as well as for the better cardioprotective efficacy of finerenone vs eplerenone against Aldo.
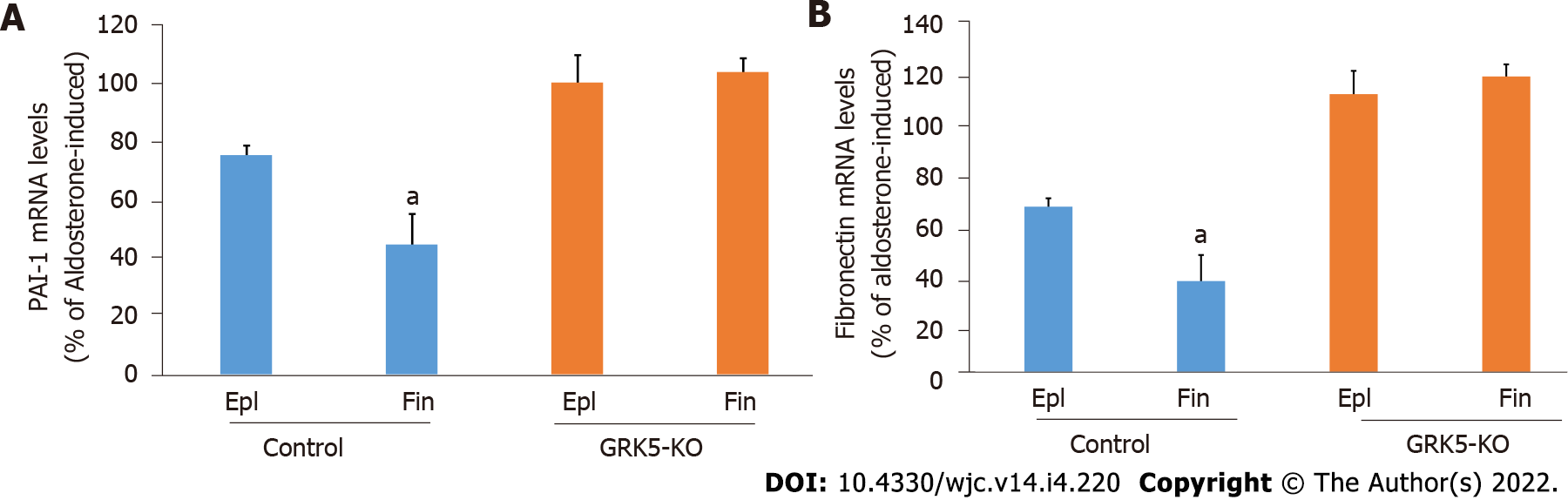
In addition to apoptosis and oxidative stress, we compared the two MRAs in terms of Aldo-induced fibrosis inhibition in cardiac myocytes. Assessment of Aldo-dependent mRNA induction of two major pro-fibrotic stimuli, PAI-1 and fibronectin, both of which are immediate/early MR-responsive genes[1,3,16], revealed that finerenone was more effective than eplerenone at suppressing both PAI-1 (Figure 4A) and fibronectin (Figure 4B) mRNA inductions by Aldo in control cells. Again however, neither drug was effective at all when GRK5 was absent (Figure 4A and B, compare with GRK5-KO bars). Thus, GRK5 is essential also for the anti-fibrotic effects of MRAs in cardiac myocytes.
The MR has long been established as an important molecular culprit in heart disease progression[1-5], including a recent study in transgenic mice showing that, unlike its closely related glucocorticoid receptor, the MR promotes cardiac dysfunction even in the absence of a cardiac insult or injury[26]. Indeed, the well-documented deleterious effects of the cardiac MR have provided the pharmacological basis for the use of MRA drugs in advanced stage human CHF and other heart diseases[1-5,27,28]. The MRA drug class, which began with the approval and marketing of spironolactone more than 60 years ago, now encompasses several agents, with some already in clinical use and some in clinical trials. The MRAs are broadly divided to traditional, steroidal MRAs, like spironolactone and eplerenone currently in clinical use, and later generation, non-steroidal agents. Among the latter is finerenone (formerly BAY 94-8862), a third generation, non-steroidal, dihydropyridine-derived MRA currently in phase III clinical trials[3,12].
Despite being very potent and effective aldosterone antagonists with salutary effects in the heart and kidneys, the currently available steroidal MRAs are hampered by several limiting side effects, most prominent of which are hyperkalemia, renal function deterioration, and gynecomastia. These are generally thought to be due to their binding to other types of steroid receptors (e.g., estrogen receptor, glucocorticoid receptor, etc.) exactly because of their steroidal structure[3,12,29]. Thus, non-steroidal MRAs have been developed, currently headlined by finerenone. Finerenone has shown advantageous pharmacological and therapeutic profiles, compared to the steroidal MRAs. It has demonstrated improved therapeutic properties in heart failure animal models in head-to-head comparisons with eplerenone[12,15] and leads to bigger improvements in HFrEF (heart failure with reduced ejection fraction) confounded by diabetes or chronic kidney disease[12,14]. In addition to its much higher selectivity for the MR over other steroid receptors, finerenone is also at least one log scale more potent at MR antagonism than eplerenone and spironolactone, both of which are competitive MR antagonists[3]. Furthermore, finerenone displays inverse agonist activity at the MR, whereas the steroidal MRAs are only partial MR antagonists[3,12]. This means that, depending on the activity status of the MR, spironolactone and eplerenone may actually promote the activity of the MR rather than inhibiting it[12,14,15]. In other words, eplerenone inhibits the MR when the receptor is activated by Aldo but it may actually promote the activity of the MR when bound alone to the receptor (in the absence of Aldo). Finerenone, thanks to the non-steroidal nature of its structure, appears to be devoid of any agonist activity at the MR and thus, has strong potential to provide better cardiovascular and renal outcomes, especially in diseases severely affected by hyperaldosteronism.
One of the most important parameters affecting the selectivity of a particular MRA for the MR vs other steroid receptors, as well as tissue specificity for MR antagonism (inhibition of the cardiac MR vs inhibition of the MR in other tissues), is the identity/identities of the receptor’s co-factors activated or repressed by the MRA agent, which ultimately affects the MRA drug’s potency and efficacy[1,3,15,25]. In other words, how good a particular MRA is at blocking the cardiac MR depends strongly on which co-activators of the MR the drug inhibits and/or which co-repressors of the MR it activates inside the cardiac myocyte[3]. Indeed, a recent study in mice reported much higher potency and inverse agonism of finerenone, relative to eplerenone, in terms of cardiac fibrosis suppression and suggested that the pharmacological difference between these two MRAs was probably due to differential cardiac MR co-factor regulation/engagement[15]. We recently uncovered that GRK5 is an important co-repressor of the cardiac MR, via its direct binding to, and phosphorylation of the MR that results in cytosolic retention of the phosphorylated receptor and thus, MR transcriptional repression[11]. Our present data strongly suggest that finerenone selectively activates this kinase in cardiac myocytes to potently inhibit/repress the cardiac MR. In contrast, eplerenone is incapable of this action (GRK5 activation) and thus, is a much weaker MR antagonist in the myocardium.
There are a few very important questions emanating from our present work that await delineation in future studies. First, does finerenone activate GRK5 to suppress MR activity only in the heart or in other tissues, as well (e.g., kidneys)? Another critical question is whether this property is shared by other non-steroidal MRAs or it is specific to finerenone. Finally, there is also the obvious mechanistic question of how exactly finerenone, not known to be a GPCR agonist, induces GRK5, normally activated by a GPCR, such as the b2-adrenergic receptor (Figure 5)[8,11], to phosphorylate and inhibit the MR in the cytosol of a cardiac myocyte. Nevertheless, these salient questions will be the focus of our future investigations, along with our already ongoing efforts to map the specific phosphorylation sites of GRK5 on the human MR protein and to characterize the functional impact for the receptor of each one of them.
In summary, our present study reinforces the emerging and therapeutically very intriguing notion that GRK5, acting as a cardiac MR co-repressor in this instance, may actually be beneficial in the myocardium[11,31-33], contrary to its counterpart GRK2 that is generally considered deleterious in the heart[7,10]. Importantly, we have identified GRK5 as a potential co-factor of the cardiac MR that is differentially regulated by finerenone and eplerenone, which may underlie the higher potency/efficacy (and inverse agonism) of finerenone at the MR. To our knowledge, cardiac GRK5 is the first such MR co-factor to be shown as differentially modulated/stimulated among different individual MRA drugs. Finally, from the therapeutic standpoint, we provide evidence that GRK5 is indispensable for MRAs’ cardioprotective actions against Aldo (e.g., anti-apoptosis, anti-oxidant action, anti-fibrosis) and, importantly, this applies to both steroidal (eplerenone) and non-steroidal (finerenone) MRA agents alike.
In the present study, we report that finerenone is a more potent and efficacious cardiac MR blocker than eplerenone, thanks, at least in part, to stimulation of GRK5-dependent cardiac MR phosphorylation, which eplerenone is incapable of inducing (Figure 5). This non-canonical effect of GRK5 on the cardiac MR is essential for efficient blockade of Aldo’s deleterious actions in the heart, such as apoptosis, oxidative stress, fibrosis, and probably other adverse remodeling-associated effects (Figure 5). Therefore, GRK5-dependent inhibitory phosphorylation is a key molecular mechanism for cardiac MR inverse agonism and needs to be considered in the design and development of novel, more effective MRA drugs for heart disease (e.g., CHF, hypertension, renal insufficiency, etc.) treatment.
Cardiac GRK5 is an essential mediator of the general cardio-protection afforded by MRA drugs against the cardio-toxic effects of excess Aldo, e.g., during CHF and other chronic cardiac diseases. This is due to the inhibitory phosphorylation GRK5 performs on the cardiac MR. This non-canonical (given the substrate is not a GPCR), co-repressor effect of GRK5 on cardiac MR is also (at least partly) responsible for the inverse agonism properties of finerenone at this receptor that bestow this non-steroidal MRA with superior potency and efficacy, compared to eplerenone, at protecting the heart against the damaging effects of Aldo. Finally, since GRK5 is a co-repressor of the MR, at least in the myocardium, its stimulation (or potentiation) should be a desired property of every novel MRA drug designed and developed for improved cardiovascular pharmacotherapy.
Different mineralocorticoid receptor (MR) antagonists (MRAs) have different potencies at the cardiac MR blockade. G protein-coupled receptor kinase (GRK)-5 phosphorylates the MR in the heart and inhibits its transcriptional activity.
The authors wanted to compare two different MRAs, eplerenone and finerenone, in their ability to stimulate GRK5-dependent MR inhibition in cardiac myocytes.
The authors sought to identify a mechanism for the increased effectiveness of finerenone over eplerenone at blocking cardiac MR.
The authors studied MR phosphorylation and activity in cardiomyocytes in response to eplerenone or finerenone treatments.
GRK5 is necessary for the anti-apoptotic, anti-oxidative, and anti-fibrotic effects of both finerenone and eplerenone against Aldo, as well as for the higher efficacy and potency of finerenone at blocking Aldo-induced apoptosis, oxidative stress, and fibrosis.
Finerenone, but not eplerenone, induces GRK5-dependent cardiac MR inhibition, which underlies, at least in part, its higher potency and efficacy, compared to eplerenone, as an MRA in the heart.
GRK5 is an essential mediator of finerenone’s effects on cardiac aldosterone antagonism.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Nova Southeastern University, Nova Southeastern University; American Heart Association, No. 20038364.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balbaa ME, Egypt; Emran TB, Bangladesh S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Parker BM, Wertz SL, Pollard CM, Desimine VL, Maning J, McCrink KA, Lymperopoulos A. Novel Insights into the Crosstalk between Mineralocorticoid Receptor and G Protein-Coupled Receptors in Heart Adverse Remodeling and Disease. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Luther JM. Is there a new dawn for selective mineralocorticoid receptor antagonism? Curr Opin Nephrol Hypertens. 2014;23:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Lother A, Moser M, Bode C, Feldman RD, Hein L. Mineralocorticoids in the heart and vasculature: new insights for old hormones. Annu Rev Pharmacol Toxicol. 2015;55:289-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 494] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 5. | Mihailidou AS, Funder JW. Nongenomic effects of mineralocorticoid receptor activation in the cardiovascular system. Steroids. 2005;70:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Lymperopoulos A, Bathgate A. Pharmacogenomics of the heptahelical receptor regulators G-protein-coupled receptor kinases and arrestins: the known and the unknown. Pharmacogenomics. 2012;13:323-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol Rev. 2015;95:377-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Komolov KE, Benovic JL. G protein-coupled receptor kinases: Past, present and future. Cell Signal. 2018;41:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | McCrink KA, Brill A, Lymperopoulos A. Adrenal G protein-coupled receptor kinase-2 in regulation of sympathetic nervous system activity in heart failure. World J Cardiol. 2015;7:539-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Siryk-Bathgate A, Dabul S, Lymperopoulos A. Current and future G protein-coupled receptor signaling targets for heart failure therapy. Drug Des Devel Ther. 2013;7:1209-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Maning J, McCrink KA, Pollard CM, Desimine VL, Ghandour J, Perez A, Cora N, Ferraino KE, Parker BM, Brill AR, Aukszi B, Lymperopoulos A. Antagonistic Roles of GRK2 and GRK5 in Cardiac Aldosterone Signaling Reveal GRK5-Mediated Cardioprotection via Mineralocorticoid Receptor Inhibition. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Kolkhof P, Jaisser F, Kim SY, Filippatos G, Nowack C, Pitt B. Steroidal and Novel Non-steroidal Mineralocorticoid Receptor Antagonists in Heart Failure and Cardiorenal Diseases: Comparison at Bench and Bedside. Handb Exp Pharmacol. 2017;243:271-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Sueta D, Yamamoto E, Tsujita K. Mineralocorticoid Receptor Blockers: Novel Selective Nonsteroidal Mineralocorticoid Receptor Antagonists. Curr Hypertens Rep. 2020;22:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Rico-Mesa JS, White A, Ahmadian-Tehrani A, Anderson AS. Mineralocorticoid Receptor Antagonists: a Comprehensive Review of Finerenone. Curr Cardiol Rep. 2020;22:140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Grune J, Beyhoff N, Smeir E, Chudek R, Blumrich A, Ban Z, Brix S, Betz IR, Schupp M, Foryst-Ludwig A, Klopfleisch R, Stawowy P, Houtman R, Kolkhof P, Kintscher U. Selective Mineralocorticoid Receptor Cofactor Modulation as Molecular Basis for Finerenone's Antifibrotic Activity. Hypertension. 2018;71:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 16. | Pollard CM, Desimine VL, Wertz SL, Perez A, Parker BM, Maning J, McCrink KA, Shehadeh LA, Lymperopoulos A. Deletion of Osteopontin Enhances β₂-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | McCrink KA, Maning J, Vu A, Jafferjee M, Marrero C, Brill A, Bathgate-Siryk A, Dabul S, Koch WJ, Lymperopoulos A. β-Arrestin2 Improves Post-Myocardial Infarction Heart Failure via Sarco(endo)plasmic Reticulum Ca2+-ATPase-Dependent Positive Inotropy in Cardiomyocytes. Hypertension. 2017;70:972-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | McCrink KA, Brill A, Jafferjee M, Valero TR, Marrero C, Rodriguez MM, Hale GM, Lymperopoulos A. β1-adrenoceptor Arg389Gly polymorphism confers differential β-arrestin-binding tropism in cardiac myocytes. Pharmacogenomics. 2016;17:1611-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Pollard CM, Ghandour J, Cora N, Perez A, Parker BM, Desimine VL, Wertz SL, Pereyra JM, Ferraino KE, Patel JJ, Lymperopoulos A. GRK2-Mediated Crosstalk Between β-Adrenergic and Angiotensin II Receptors Enhances Adrenocortical Aldosterone Production In Vitro and In Vivo. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Nguyen K, Kassimatis T, Lymperopoulos A. Impaired desensitization of a human polymorphic α2B-adrenergic receptor variant enhances its sympatho-inhibitory activity in chromaffin cells. Cell Commun Signal. 2011;9:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Salazar NC, Vallejos X, Siryk A, Rengo G, Cannavo A, Liccardo D, De Lucia C, Gao E, Leosco D, Koch WJ, Lymperopoulos A. GRK2 blockade with βARKct is essential for cardiac β2-adrenergic receptor signaling towards increased contractility. Cell Commun Signal. 2013;11:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Koch WJ. Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels. J Am Coll Cardiol. 2011;57:356-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Ashton AW, Le TY, Gomez-Sanchez CE, Morel-Kopp MC, McWhinney B, Hudson A, Mihailidou AS. Role of Nongenomic Signaling Pathways Activated by Aldosterone During Cardiac Reperfusion Injury. Mol Endocrinol. 2015;29:1144-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Faresse N. Post-translational modifications of the mineralocorticoid receptor: How to dress the receptor according to the circumstances? J Steroid Biochem Mol Biol. 2014;143:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Fuller PJ. Novel interactions of the mineralocorticoid receptor. Mol Cell Endocrinol. 2015;408:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Oakley RH, Cruz-Topete D, He B, Foley JF, Myers PH, Xu X, Gomez-Sanchez CE, Chambon P, Willis MS, Cidlowski JA. Cardiomyocyte glucocorticoid and mineralocorticoid receptors directly and antagonistically regulate heart disease in mice. Sci Signal. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 27. | Markan U, Pasupuleti S, Pollard CM, Perez A, Aukszi B, Lymperopoulos A. The place of ARBs in heart failure therapy: is aldosterone suppression the key? Ther Adv Cardiovasc Dis. 2019;13:1753944719868134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Lymperopoulos A, Aukszi B. Angiotensin receptor blocker drugs and inhibition of adrenal beta-arrestin-1-dependent aldosterone production: Implications for heart failure therapy. World J Cardiol. 2017;9:200-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1262] [Cited by in RCA: 1224] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 30. | Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18:660-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Eijgelsheim M, Visser LE, Uitterlinden AG, Stricker BH. Protective effect of a GRK5 polymorphism on heart failure and its interaction with beta-adrenergic receptor antagonists. Pharmacogenomics. 2008;9:1551-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Wu JH, Zhang L, Fanaroff AC, Cai X, Sharma KC, Brian L, Exum ST, Shenoy SK, Peppel K, Freedman NJ. G protein-coupled receptor kinase-5 attenuates atherosclerosis by regulating receptor tyrosine kinases and 7-transmembrane receptors. Arterioscler Thromb Vasc Biol. 2012;32:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Montó F, Oliver E, Vicente D, Rueda J, Agüero J, Almenar L, Ivorra MD, Barettino D, D'Ocon P. Different expression of adrenoceptors and GRKs in the human myocardium depends on heart failure etiology and correlates to clinical variables. Am J Physiol Heart Circ Physiol. 2012;303:H368-H376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |