Published online Sep 26, 2021. doi: 10.4330/wjc.v13.i9.503
Peer-review started: March 6, 2021
First decision: March 31, 2021
Revised: June 22, 2021
Accepted: August 4, 2021
Article in press: August 4, 2021
Published online: September 26, 2021
Processing time: 195 Days and 19.9 Hours
Red blood cell distribution width (RDW) is elevated in patients with cardio
To determine RDW values and impact of CV and non-CV coexisting morbidities in elderly patients hospitalized with chronic CVD.
This prospective study included 204 consecutive elderly patients (age 77.5 [7.41] years, female 94 [46%], left ventricular ejection fraction 53.00% [37.50, 55.00]) hospitalized with chronic CVD at the Cardiology Department of Larissa University General Hospital (Larissa, Greece) from January 2019 to April 2019. Elderly patients were selected due to the high prevalence of coexisting morbidities in this patient population. Hospitalized patients with acute CVD (acute coronary syndromes, new-onset heart failure [HF], and acute pericarditis/myocarditis), primary isolated valvular heart disease, sepsis, and those with a history of blood transfusions or cancer were excluded. The evaluation of the patients within 24 h from admission included clinical examination, laboratory blood tests, and echocardiography.
The most common cardiac morbidities were hypertension and coronary artery disease, with acutely decompensated chronic heart failure (ADCHF) and atrial fibrillation (AF) also frequently being present. The most common non-cardiac morbidities were anemia and chronic kidney disease followed by diabetes mellitus, chronic obstructive pulmonary disease, and sleep apnea. RDW was significantly elevated 15.48 (2.15); 121 (59.3%) of patients had RDW > 14.5% which represents the upper limit of normal in our institution. Factors associated with RDW in stepwise regression analysis were ADCHF (coefficient: 1.406; 95% confidence interval [CI]: 0.830-1.981; P < 0.001), AF (1.192; 0.673 to 1.711; P < 0.001), and anemia (0.806; 0.256 to 1.355; P = 0.004). ADCHF was the most significant factor associated with RDW. RDW was on average 1.41 higher for patients with than without ADCHF, 1.19 higher for patients with than without AF, and 0.81 higher for patients with than without anemia. When patients were grouped based on the presence or absence of anemia, ADCHF and AF, heart rate was not increased in those with anemia but was significantly increased in those with ADCHF or AF.
RDW was elevated in elderly hospitalized patients with chronic CVD. Factors associated with RDW were anemia and CV factors associated with elevated heart rate (ADCHF, AF), suggesting sympathetic overactivity.
Core Tip: This was a prospective observational study with 204 consecutive elderly hospitalized patients seeking to evaluate the impact of cardiovascular (CV) and non-CV coexisting morbidities on red blood cell distribution width (RDW). RDW was significantly elevated and factors associated with RDW were anemia as well as CV factors associated with elevated heart rate (acutely decompensated chronic heart failure and atrial fibrillation), suggesting sympathetic overactivity.
- Citation: Xanthopoulos A, Tryposkiadis K, Dimos A, Bourazana A, Zagouras A, Iakovis N, Papamichalis M, Giamouzis G, Vassilopoulos G, Skoularigis J, Triposkiadis F. Red blood cell distribution width in elderly hospitalized patients with cardiovascular disease. World J Cardiol 2021; 13(9): 503-513
- URL: https://www.wjgnet.com/1949-8462/full/v13/i9/503.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i9.503
Red blood cell (RBC) distribution width (RDW) is calculated as the standard deviation in RBC size divided by the mean corpuscular volume (MCV), and represents an expression of the variation in size of the RBC (anisocytosis) that make up the total population in an individual patient[1]. Emerging evidence suggests that, besides RBC abnormalities, diverse human pathologies have been frequently associated with anisocytosis. In this regard, increased RDW is associated with adverse events and mortality in many cardiovascular diseases (CVDs) such as ischemic cerebrovascular disease, peripheral artery disease, atrial fibrillation (AF), heart failure (HF), and hypertension (HTN)[2,3]. This study evaluated RDW and the impact of coexisting morbidities in elderly patients hospitalized with chronic CVD[4].
This prospective study included 204 consecutive elderly (> 65 years) patients admitted to the Cardiology Department of Larissa University General Hospital (Larissa, Greece) from January 2019 to April 2019. Elderly patients were selected due to the high prevalence of coexisting morbidities in this patient population. Patients hospitalized for acute CVD (acute coronary syndromes [n = 49], acute de novo HF (n = 18), acute pericarditis/myocarditis [n = 15]), primary isolated valvular heart disease (n = 9), sepsis (n = 27), and those with a history of blood transfusions (n = 17) or cancer (n = 25) were excluded. The study complied with the Declaration of Helsinki and the study protocol was approved by the institutional ethical committee. There was no need for written informed consent as the study was observational. All authors had full access to the data, take responsibility for its integrity, contributed to the writing of the manuscript, and agree to this report as written.
The evaluation of the patients within 24 h from admission included clinical examination, laboratory blood tests, and echocardiography. Levels of hemoglobin (Hb) and RDW were measured with the use of the Siemens Advia 2120 (Siemens Healthcare Diagnostics, INC, Deerfield, IL, United States). NT-pro B-type natriuretic peptide (NT-proBNP) was measured with the use of Siemens Advia Centaur (Siemens Healthcare Diagnostics), while urea, creatinine, and electrolyte levels with Siemens Dimension (Siemens Healthcare Diagnostics). Echocardiography was performed and reviewed by two independent echocardiographers, with the use of General Electric Vivid 7 machine (GE Healthcare, Horten, Norway). The left ventricular ejection fraction (LVEF) was calculated with the use of two-dimensional echocardiography by implementing the biplane method of disks summation technique[5].
(1) Coronary artery disease (CAD): history of typical angina in subjects with risk factors, history of myocardial infarction, history of hospitalization for angina, history of percutaneous coronary intervention or coronary bypass grafting and accordant medical prescription list; (2) HTN: history of HTN treatment within the past 3 years; (3) Acutely decompensated chronic HF (ADCHF): deterioration of preexisting chronic HF resulting in an unplanned hospitalization; (4) AF: electrocardiographic findings of AF at admission and/or history of treatment for AF; (5) Diabetes mellitus (DM): treatment with anti-hyperglycemic agents including insulin, within the past 3 years; (6) Chronic obstructive pulmonary disease (COPD): history of dyspnea, chronic cough or sputum production, or history of recurrent lower respiratory tract infections in a patient receiving COPD treatment the past 3 years; (7) Anemia: self-reported anemia and relevant treatment within the past 3 years or hemoglobin (Hb) < 130 g/L for men and < 120 g/L for women at admission; (8) Chronic kidney disease (CKD): elevated creatinine ( ≥ 1.2 mg/dL) in three consecutive measurements in the past 3 years and confirmed at admission; and (9) Sleep apnea: Sleep apnea treatment with continuous positive airway pressure (CPAP) within the past 3 years.
Descriptive statistics are presented for the study population. Continuous variables exhibiting a normal distribution are summarized as the mean and standard deviation (SD), whereas continuous variables exhibiting a non-normal distribution are presented as the median and interquartile range (IQR). The distribution of each continuous variable was visually examined through histograms. Categorical variables are presented as frequencies and percentages. A linear regression model was employed to identify factors associated with the elevation of RDW. Univariate analysis was initially carried out to explore the independent association of each variable with RDW. Any such factor subsequently entered a stepwise forward selection procedure to obtain the multivariate model fitting the data best. Factors were added one-at-a-time, starting from the one indicated as the most significant in the univariate analysis, until none yielded any further improvement in the data fit. This was judged using the likelihood ratio test, a frequently used test that compares the change in deviance in nested models, with the level of significance for addition to the model set at 10%.All estimates generated from linear regression analyses were presented along with 95% confidence intervals (CIs) and P values. P values will be reported from two-sided tests at the 5% significance level. All analyses were carried out with STATA 15 (StataCorp LLC; College Station, TX, United States).
The characteristics of the patients enrolled in this study are presented in Table 1. Patients were elderly, and approximately half were females. Most patients suffered from HTN and CAD, with ADCHF and AF also being frequently present. The most common non-cardiac morbidities were anemia and CKD followed by DM, COPD, and sleep apnea. The RDW values of the study population appeared to be elevated (mean [SD] = 15.48 [2.15], median [IQR] = 14,9 [2.7]) compared to the upper limit of normal of our institution (14.50%). In total, 121 (59.3%) of patients had RDW > 14.5%.
| Demographic/clinical | |
| Age (mean ± SD, yr) | 77.50 ± 7.41 |
| Female sex, n (%) | 94 (46) |
| Body weight (mean ± SD, kg) | 76.28 ± 14.22 |
| Height (mean ± SD, m) | 1.66 ± 0.09 |
| Systolic blood pressure (mmHg) | 135(34.8) |
| Diastolic blood pressure (mmHg) | 79 (21) |
| Heart rate (beats/minute) | 73.5 (19) |
| Left ventricular ejection fraction (%) (median, IQR) | 53.00 (37.50, 55.00) |
| Laboratory | |
| RDW1 (mean ± SD) | 15.48% ± 2.15% |
| C-reactive protein2 (mg/L) (median, IQR) | 0.48 (0.16, 1.37) |
| Hemoglobin3 (mean ± SD, g/dL) | 12.25 ± 1.92 |
| Hematocrit3 (mean ± SD) | 37.89% ± 6.04% |
| White blood cells3 (K/μL) | 8.31 (2.85) |
| Urea (mg/dL) (median, IQR) | 51.70 (38.60, 70.50) |
| Creatinine3 (mean ± SD, mg/dL) | 1.21 (0.49) |
| SGOT4 (IU/L) (median, IQR) | 20.15 (16.55, 25.65) |
| SGPT4 (IU/L) (median, IQR) | 16.75 (11.65, 22.90) |
| K+4 (mean ± SD, mmol/L) | 4.20 ± 0.56 |
| Na+1 (mmol/L) (median, IQR) | 140.00 (137.50, 142.00) |
| Non-cardiac conditions/morbidities, n (%) | |
| Diabetes mellitus | 68 (33) |
| Chronic obstructive pulmonary disease | 37 (18) |
| Chronic kidney disease | 84 (41) |
| Sleep apnea | 15 (7) |
| Anemia | 85 (42) |
| Cardiac conditions/morbidities, n (%) | |
| Acutely decompensated chronic heart failure | 104 (51) |
| Coronary artery disease | 133 (65) |
| Hypertension | 152 (75) |
| Atrial fibrillation | 97 (47.5) |
| Medications (discharge), n (%) | |
| Renin-angiotensin-aldosterone system inhibitors | 159 (78) |
| Beta-blockers | 145 (71) |
| Diuretics | 137 (67) |
The results obtained from the univariate linear regression analysis are presented in Table 2. The presence of ADCHF appeared to be the most important independent factor associated with the elevation of RDW, followed by the presence of AF, anemia, CKD, COPD, increased urea values, reduced LVEF, and a higher age. Other factors found to be significant at the 5% level were the presence of DM, the presence of sleep apnea, and increased C-reactive protein (CRP).
| Factor | Coefficient | 95%CI | P value |
| Age | 0.072 | (0.033, 0.111) | < 0.001 |
| Sex (males vs females) | -0.520 | (-1.115, 0.075) | 0.09 |
| Weight | -0.014 | (-0.035, 0.007) | 0.18 |
| White blood cell count | 0.081 | (-0.024, 0.185) | 0.13 |
| Urea | 0.022 | (0.014, 0.030) | < 0.001 |
| C-reactive protein1 | 0.085 | (0.001, 0.169) | 0.05 |
| Left ventricular ejection fraction | -0.057 | (-0.073, -0.038) | < 0.001 |
| Acutely decompensated chronic heart failure | 2.220 | (1.708, 2.732) | < 0.001 |
| Coronary artery disease | 0.039 | (-0.573, 0.650) | 0.9 |
| Hypertension | -0.519 | (-1.203, 0.165) | 0.14 |
| Atrial fibrillation | 1.862 | (1.322, 2.402) | < 0.001 |
| Diabetes mellitus | 0.854 | (0.230, 1.477) | 0.01 |
| Chronic obstructive pulmonary disease | 1.569 | (0.810, 2.327) | < 0.001 |
| Anemia | 1.682 | (1.123, 2.242) | < 0.001 |
| Chronic kidney disease | 1.590 | (1.022, 2.158) | < 0.001 |
| Sleep apnea | 1.448 | (0.289, 2.606) | 0.02 |
The results obtained from the model selection procedure are presented in Table 3. ADCHF appeared to be the most important factor alone (change in deviance compared to the null model: 62.86, P < 0.001), and hence was contained in all sets of models considered for investigation of the best data fit. The presence of AF in the model yielded greater improvement compared to any other factor when included in the model jointly with ADCHF (change in deviance compared to model including ADCHF only: 19.33, P < 0.001). Thus, AF was retained in the model alongside ADCHF. Anemia provided the most significant improvement compared to any other factor when included in addition to ADCHF and AF (change in deviance compared to model including ADCHF and AF: 8.35, P = 0.004), and hence was retained in the model along with ADCHF and AF. No further improvement was observed when all factors excluded during the selection procedure re-entered the model, one-at-a-time, along with ADCHF, AF, and anemia (P > 0.15). Therefore, the best model included ADCHF, AF, and anemia.
| Explanatory factors of RDW | Change in deviance1 | P value |
| Models including 1 factor2 | ||
| Age | 12.84 | < 0.001 |
| Urea | 26.50 | < 0.001 |
| C-reactive protein | 4.01 | 0.05 |
| LVEF | 30.42 | < 0.001 |
| ADCHF | 62.86 | < 0.001 |
| Atrial fibrillation | 42.01 | < 0.001 |
| Diabetes mellitus | 7.23 | 0.007 |
| COPD | 16.14 | < 0.001 |
| Anemia | 32.69 | < 0.001 |
| CKD | 28.60 | < 0.001 |
| Sleep apnea | 6.04 | 0.01 |
| Models including 2 factors3 | ||
| ADCHF + Age | 0.15 | 0.70 |
| ADCHF + Urea | 3.03 | 0.08 |
| ADCHF + C-reactive protein | 0.30 | 0.59 |
| ADCHF + LVEF | 0.06 | 0.80 |
| ADCHF + Atrial fibrillation | 19.33 | < 0.001 |
| ADCHF + Diabetes mellitus | 3.08 | 0.08 |
| ADCHF + COPD | 2.39 | 0.12 |
| ADCHF + Anemia | 7.74 | 0.005 |
| ADCHF + CKD | 0.85 | 0.36 |
| ADCHF + Sleep apnea | 0.34 | 0.56 |
| Models including 3 factors3 | ||
| ADCHF + Atrial fibrillation + Age | 0.08 | 0.78 |
| ADCHF + Atrial fibrillation + Urea | 2.10 | 0.15 |
| ADCHF + Atrial fibrillation + C-reactive protein | 0.54 | 0.46 |
| ADCHF + Atrial fibrillation + LVEF | 0.75 | 0.39 |
| ADCHF + Atrial fibrillation + Diabetes mellitus | 2.30 | 0.13 |
| ADCHF + Atrial fibrillation + COPD | 0.89 | 0.35 |
| ADCHF + Atrial fibrillation + Anemia | 8.35 | 0.004 |
| ADCHF + Atrial fibrillation + CKD | 0.73 | 0.39 |
| Models including 4 factors3 | ||
| ADCHF + Atrial fibrillation + Anemia + Age | 0.50 | 0.48 |
| ADCHF + Atrial fibrillation + Anemia + Urea | 1.16 | 0.28 |
| ADCHF + Atrial fibrillation + Anemia + C-reactive protein | 0.34 | 0.56 |
| ADCHF + Atrial fibrillation + Anemia + LVEF | 1.86 | 0.17 |
| ADCHF + Atrial fibrillation + Anemia + Diabetes mellitus | 0.92 | 0.34 |
| ADCHF + Atrial fibrillation + Anemia + COPD | 0.77 | 0.38 |
| ADCHF + Atrial fibrillation + Anemia + CKD | 0.26 | 0.61 |
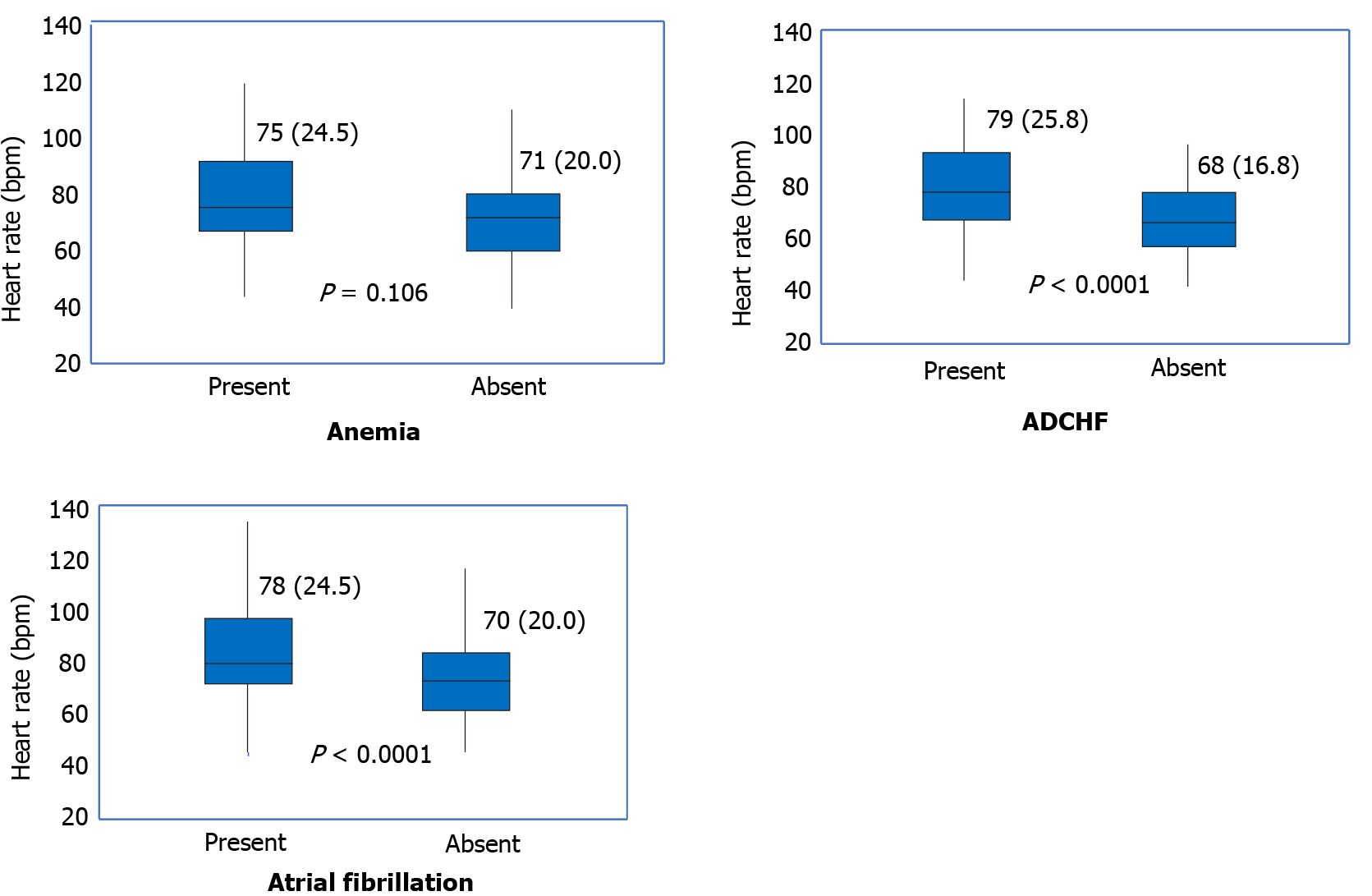
The results obtained from the multivariate linear regression analysis are presented in Table 4. ADCHF was again the most significant factor associated with RDW, with an average increase of 1.41 noted for patients with ADCHF compared with those without. Furthermore, the RDW was on average 1.19 higher for patients with AF compared to patients without AF, and on average 0.81 higher for anemic patients compared to non-anemic. It is noteworthy that when patients were grouped based on the presence or absence of anemia, ADCHF and AF, heart rate was not increased in those with anemia but was significantly increased in those with ADCHF or AF (Figure 1).
| Factor | Coefficient | 95%CI | P value |
| Acutely decompensated chronic heart failure | 1.406 | (0.830, 1.981) | < 0.001 |
| Atrial fibrillation | 1.192 | (0.673, 1.711) | < 0.001 |
| Anemia | 0.806 | (0.256, 1.355) | 0.004 |
In this study that included elderly patients hospitalized with CVD, RDW was significantly elevated. ADCHF was the most significant factor associated with RDW, whereas other important factors were AF and anemia. RDW was significantly higher than the RDW of a subgroup of elderly (i.e. 71-85-years-old) (n = 1479) of a historical cohort including a total of 8089 individuals (15.48 ± 2.15 vs 12.6±0.8, respectively; P < 0.0001)[6].
An increased RDW mirrors a profound deregulation of erythrocyte homeostasis involving both impaired erythropoiesis and abnormal RBC survival and is used along with other RBC indices to help determine the causes of anemia[7]. A high RDW provides a clue for anisocytosis and/or the presence of two red cell populations, since other RBC indices (e.g., MCV or mean corpuscular hemoglobin concentration [MCH]) reflect average values and may not adequately reflect RBC changes where mixed RBC populations are present (e.g., dimorphic RBC populations in sideroblastic anemia or combined iron deficiency anemia (decreased MCV and MCH) and megaloblastic anemia (increased MCV).
RDW has additionally been used as a prognosticator in diverse pathologies including CVD[8,9]. Many of the conditions for which an increase in RDW was observed are associated with systemic inflammation and critical illness, but the exact pathophysiologic mechanisms underlying the association of increase in RDW with morbidity and mortality remains unclear[10]. Given that erythropoietin is a key determinant of the RDW, it has been postulated that any condition affecting erythropoietin activity (e.g., inflammation, primary renal disease, HF, bone marrow failure) may potentially lead to increased RDW values[11-13]. Another consideration could be nutritional imbalance, often present in patients with chronic diseases or critical illness, expressed by micronutrient deficiencies (e.g., iron, vitamin B12, or folate deficiency) that are associated with anisocytosis[14], and excess of macronutrients. On the other hand, higher RDW has been associated with the metabolic syndrome (MS)[15,16]. Proinflammatory cytokines inhibit erythropoietin-induced erythrocyte maturation, which may lead to increased RDW[16]. Macronutrient surplus causes lipotoxicity in healthy non-adipose tissues, and induces tissue damage[17]. Other physiologic determinants that are associated with RDW changes include aging, Black ethnicity, and physical exercise[6,18].
The findings of this study regarding the inflammatory nature of RDW elevation are contradictory. Inflammatory diseases[19] were both included in (e.g., AF and ADCHF) and excluded from (e.g., CKD, and COPD) the final model. Moreover, biomarkers of inflammation were unrelated (white blood cells) or weakly related (CRP) to RDW in univariable analysis and both were not included in the final model. The results of the studies on the relationship between RDW and inflammatory biomarkers have been conflicting. In the study of Lippi and colleagues including 3845 outpatients, when participants were grouped according to RDW quartiles, there were strong, graded increases in erythrocyte sedimentation rate and hsCRP (P < 0.001), both parameters being up to 3-fold higher in the fourth vs the first quartile[20]. In contrast, Lappe and colleagues observed a significant but meaningless correlation between RDW and high-sensitivity CRP (r = 0.181; P < 0.001) in 1489 patients with CAD[21].
Inflammatory processes are present during the development and complications of CVD. However, although there is a wealth of information about the role of inflammatory cells and pathways during acute injury and the reparative processes that are subsequently activated, little is known about the contribution of the immune system once the trajectory has been set, and chronic CVD has been established—which clinically represents the majority of patients[22]. The causative role inflammation plays in disease progression is not well defined, and the majority of clinical trials that target aspects of inflammation in patients with chronic CVD have largely been negative[23,24]. This may be partly due to the fact that the tools currently used to measure “inflammation” are insufficiently precise and do not provide information about disease site, activity, or discrimination between functionally important activation pathways[23,25].
Anisocytosis can be produced by any factor that increases erythropoiesis. In the present study, in contrast to anemia the two most important non-CV factors inducing anisocytosis, ADCHF and AF were associated with increased heart rate confirming that both are hyper-catecholaminergic states[26,27]. The nervous system emerges as a critical regulatory player of the bone marrow, the primary site of postnatal hematopoiesis and hematopoietic stem cell maintenance, both under homeostatic and pathologic settings, with essential roles in cellular anchorage and egress, stem cell differentiation, and endothelial cell permeability[28,29]. Factors involved in erythropoiesis appear to revolve around the nervous system, and catecholamines seem to be the centerpiece. Several studies support the central role of the sympathetic nervous system (SNS) in the regulation of hematopoiesis[30,31]. Norepinephrine (NE) is delivered to the bone marrow (BM) by the sympathetic nerve in a circadian (diurnal) manner[32]. A close communication exists between the SNS and the BM and dysregulation in this communication may lead to aberrant hematopoietic and immune system responses[33].
This study had several limitations. (1) The study enrolled elderly patients (≥ 65-years-old) and therefore the results should be interpreted with caution in younger populations. As previously mentioned the decision to include only elderly patients was based on the fact that these patients usually suffer from several coexisting morbidities enabling us to study their potential impact on RDW. (2) A control group was lacking to compare RDW. However, a normal range of RDW value of 14.5% representing the upper normal limit is widely accepted[1,6] and RDW was compared with an aged-matched historical control[6]; (3) The cause of anemia was not determined. Iron deficiency anemia is diagnosed in 16.6%–25% of non-hospitalized older adults, 22%–40% of institutionalized older adults, and 15%–65% of hospitalized older adults[34]. Iron deficiency and iron deficiency anemia are common problems in patients with CVD[35]. Therefore, it is reasonable to assume that the study findings were driven by iron deficiency anemia. And (4) By assessing heart rate, we achieved information predominantly on the cardiac sympathetic drive. However, differentiation of sympathetic responses means that no simple test can ever represent each and every sympathetic outflow[36]. Nevertheless, the presence of sympathetic overactivity in ADCHF and AF has been demonstrated in numerous studies.
In elderly patients hospitalized with chronic CVD, RDW was elevated and associated both with anemia and factors unrelated to anemia such as ADCHF and AF. It is of interest that ADCHF and AF shared a common characteristic, namely heart rate elevation, which is suggestive of SNS overactivity a well-known regulator of BM. Further studies are necessary to establish the relationship between RDW and SNS.
An increased red blood cell distribution width (RDW) is associated with poor outcomes in patients with several cardiovascular diseases (CVDs).
Data on the pathophysiology of RDW increase in hospitalized patients with CVD are limited.
The current study explored the impact of CV and non-CV coexisting morbidities in elderly patients hospitalized with chronic CVD.
This prospective observational study included 204 consecutive elderly (> 65 years) patients admitted to a tertiary university hospital of Greece. Elderly patients were selected due to the high prevalence of coexisting morbidities in this patient population.
Factors associated with RDW were anemia, acutely decompensated chronic heart failure (ADCHF), and atrial fibrillation (AF).
ADCHF and AF shared a common characteristic, namely heart rate elevation, which suggests sympathetic nervous system (SNS) overactivity, a well-known regulator of bone marrow.
Further studies will establish the relationship between RDW and SNS.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Teragawa H, Yu L S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis. 2015;7:E402-E411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 100] [Reference Citation Analysis (0)] |
| 3. | Xanthopoulos A, Giamouzis G, Melidonis A, Kitai T, Paraskevopoulou E, Paraskevopoulou P, Patsilinakos S, Triposkiadis F, Skoularigis J. Red blood cell distribution width as a prognostic marker in patients with heart failure and diabetes mellitus. Cardiovasc Diabetol. 2017;16:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Pearson-Stuttard J, Ezzati M, Gregg EW. Multimorbidity-a defining challenge for health systems. Lancet Public Health. 2019;4:e599-e600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3897] [Cited by in RCA: 5428] [Article Influence: 542.8] [Reference Citation Analysis (0)] |
| 6. | Hoffmann JJ, Nabbe KC, van den Broek NM. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med. 2015;53:2015-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 700] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 8. | Fava C, Cattazzo F, Hu ZD, Lippi G, Montagnana M. The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: useful or hype? Ann Transl Med. 2019;7:581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169:588-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 10. | Li N, Zhou H, Tang Q. Red Blood Cell Distribution Width: A Novel Predictive Indicator for Cardiovascular and Cerebrovascular Diseases. Dis Markers. 2017;2017:7089493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 11. | Silva Litao MK, Kamat D. Back to Basics: Red Blood Cell Distribution Width: Clinical Use beyond Hematology. Pediatr Rev. 2018;39:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Miyamoto K, Inai K, Takeuchi D, Shinohara T, Nakanishi T. Relationships among red cell distribution width, anemia, and interleukin-6 in adult congenital heart disease. Circ J. 2015;79:1100-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 13. | Rodríguez-Carrio J, Alperi-López M, López P, Alonso-Castro S, Ballina-García FJ, Suárez A. Red cell distribution width is associated with cardiovascular risk and disease parameters in rheumatoid arthritis. Rheumatology (Oxford). 2015;54:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | García-Escobar A, Grande Ingelmo JM. Red Cell Volume Distribution Width as Another Biomarker. Card Fail Rev. 2019;5:176-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Sánchez-Chaparro MA, Calvo-Bonacho E, González-Quintela A, Cabrera M, Sáinz JC, Fernández-Labandera C, Aguado LQ, Meseguer AF, Valdivielso P, Román-García J; Ibermutuamur CArdiovascular RIsk Assessment Study Group. Higher red blood cell distribution width is associated with the metabolic syndrome: results of the Ibermutuamur CArdiovascular RIsk assessment study. Diabetes Care. 2010;33:e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Laufer Perl M, Havakuk O, Finkelstein A, Halkin A, Revivo M, Elbaz M, Herz I, Keren G, Banai S, Arbel Y. High red blood cell distribution width is associated with the metabolic syndrome. Clin Hemorheol Microcirc. 2015;63:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Garbarino J, Sturley SL. Saturated with fat: new perspectives on lipotoxicity. Curr Opin Clin Nutr Metab Care. 2009;12:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Tajuddin SM, Nalls MA, Zonderman AB, Evans MK. Association of red cell distribution width with all-cause and cardiovascular-specific mortality in African American and white adults: a prospective cohort study. J Transl Med. 2017;15:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Triposkiadis F, Starling RC, Boudoulas H, Giamouzis G, Butler J. The cardiorenal syndrome in heart failure: cardiac? Heart Fail Rev. 2012;17:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 703] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 21. | Lappé JM, Horne BD, Shah SH, May HT, Muhlestein JB, Lappé DL, Kfoury AG, Carlquist JF, Budge D, Alharethi R, Bair TL, Kraus WE, Anderson JL. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta. 2011;412:2094-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Dick SA, Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ Res. 2016;119:159-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 495] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 23. | Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 350] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 24. | Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 478] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 25. | Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 2667] [Article Influence: 444.5] [Reference Citation Analysis (0)] |
| 26. | Carnagarin R, Kiuchi MG, Ho JK, Matthews VB, Schlaich MP. Sympathetic Nervous System Activation and Its Modulation: Role in Atrial Fibrillation. Front Neurosci. 2018;12:1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Pepper GS, Lee RW. Sympathetic activation in heart failure and its treatment with beta-blockade. Arch Intern Med. 1999;159:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Leitão L, Alves CJ, Sousa DM, Neto E, Conceição F, Lamghari M. The alliance between nerve fibers and stem cell populations in bone marrow: life partners in sickness and health. FASEB J. 2019;33:8697-8710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Récalde A, Richart A, Guérin C, Cochain C, Zouggari Y, Yin KH, Vilar J, Drouet I, Lévy B, Varoquaux O, Silvestre JS. Sympathetic nervous system regulates bone marrow-derived cell egress through endothelial nitric oxide synthase activation: role in postischemic tissue remodeling. Arterioscler Thromb Vasc Biol. 2012;32:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | del Toro R, Méndez-Ferrer S. Autonomic regulation of hematopoiesis and cancer. Haematologica. 2013;98:1663-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Hanoun M, Maryanovich M, Arnal-Estapé A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 32. | Méndez-Ferrer S, Chow A, Merad M, Frenette PS. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Ahmari N, Hayward LF, Zubcevic J. The importance of bone marrow and the immune system in driving increases in blood pressure and sympathetic nerve activity in hypertension. Exp Physiol. 2020;105:1815-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Joosten E. Iron deficiency anemia in older adults: A review. Geriatr Gerontol Int. 2018;18:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol. 2015;12:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 36. | Esler M, Lambert G, Esler D, Ika Sari C, Guo L, Jennings G. Evaluation of elevated heart rate as a sympathetic nervous system biomarker in essential hypertension. J Hypertens. 2020;38:1488-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |