Published online Sep 26, 2021. doi: 10.4330/wjc.v13.i9.416
Peer-review started: March 25, 2021
First decision: May 13, 2021
Revised: May 24, 2021
Accepted: July 22, 2021
Article in press: July 22, 2021
Published online: September 26, 2021
Processing time: 176 Days and 8.6 Hours
Evaluation of acute percutaneous coronary intervention (PCI) results and long-term follow-up remains challenging with ongoing stent designs. Several imaging tools have been developed to assess native vessel atherosclerosis and stent expansion, improving overall PCI results and reducing adverse cardiac events. Quantitative coronary analysis has played a crucial role in quantifying the extent of coronary artery disease and stent results. Digital stent enhancement methods have been well validated and improved stent strut visualization. Intravascular imaging remains the gold standard in PCI guidance but adds costs and time to the procedure. With a recent shift towards non-invasive imaging assessment and coronary computed tomography angiography imaging have shown promising results. We hereby review novel stent visualization techniques used to guide PCI and assess stent patency in the modern PCI era.
Core Tip: Evaluation of acute and long-term percutaneous coronary intervention (PCI) results remains challenging. We hereby review current available tools for guiding and assessing PCI results.
- Citation: Ghafari C, Carlier S. Stent visualization methods to guide percutaneous coronary interventions and assess long-term patency. World J Cardiol 2021; 13(9): 416-437
- URL: https://www.wjgnet.com/1949-8462/full/v13/i9/416.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i9.416
Atherosclerosis is a slow-progressing multifocal immunoinflammatory disease of medium and large-sized arteries[1]. Exposure to established risk factors triggers an oxidative response leading to the formation of fatty streaks within the vessel wall. Phagocytosis of these lipid rich proteins by macrophages induces cell apoptosis leading to cellular debris which, along with necrotic endothelial and smooth muscle cells, form the principal constituent of the lipid-rich plaque core. The deposit of calcium and phosphate-rich hydroxyapatite crystals occurs as a macrophage-mediated response to oxidation and endothelial dysfunction[2]. Coronary artery disease (CAD) secondary to atherosclerosis remains the most common cause of death across the globe, imposing a major health and economic burden on nations[3,4]. Despite major technological and therapeutic advances, the prevalence of CAD is expected to continue to rise secondary to an increase in the aging population[5]. Coronary artery angiography (CAG), despite being invasive, remains the gold standard technique to assess significant coronary artery disease and to guide percutaneous coronary intervention (PCI). PCI reduces major cardiac events in acute coronary syndrome events[6] and improves the quality of life in chronic coronary syndrome[7]. The first human cardiac catheterization dates back to 1929 when Dr. Forssmann[8] auto-measured the pressure in his cardiac chambers using a urinary catheter. Cournand et al[9] and Nossaman et al[10] later developed right heart catheterization with a standard diagnostic tool, and in 1956, along with Dr. Forssmann[8], were awarded the Nobel Prize in Medicine for their work. Dr. Seldinger[11] developed his safe percutaneous catheterization technique in 1953 followed by Dr. Sones[12] who performed the first selective coronary angiogram in 1958. In 1964 the first peripheral angioplasty was performed by Drs. Dotter and Judkins[13]. Since then, PCI has undergone a rapid technological evolution, beginning with Turina et al[14] in 1979 who developed the initial coronary balloon angioplasty, later referred to as plain old balloon angioplasty (POBA). POBA was the first step for modern coronary intervention and was limited by the risk of acute thrombosis (3%-8%)[15,16] and early vessel recoil (5%-10%), along with a high rate of restenosis (33%)[17-22]. Nevertheless, POBA paved the way to the first coronary stents that were implanted in 1986 by Sigwart et al[23]. Although the recent ISCHEMIA trial did not show evidence of a reduced risk of ischemic cardiovascular events or death for an initial invasive strategy as compared with optimal medical treatment among patients with stable angina and moderate or severe ischemia[7], only 4% of contemporary real-world patients would be eligible for the trial[24]. Using coronary physiology guidance, PCI also decreases cardiac death and myocardial infarction in chronic coronary symptoms[25]. Several trials (BENESTENT, STRESS, CAVEAT) have laid the cornerstone upon which angiographic guidelines have been proposed, and coronary stent implantation after PCI has been shown to improve short- and long-term clinical outcomes[26-28]. However, a coronary stent should meet numerous design criteria in order to fulfill its function, including the fact that the stent platform must be radio-opaque enough to provide the required visibility for correct placement and proper expansion in order to ensure successful stent deployment[29,30]. In addition, a stent must have a narrow profile when collapsed for easy deliverability[31,32]. Strut thickness has been a key element of stent design, with thinner struts associated with greater deliverability. Several studies have also shown a lower rate of restenosis associated with thinner stent’ struts[31,33,34]. Consequently, a move towards thinner struts stents has been made, with current stent strut thickness being between 60-100 µm. Initial stents were made of nitinol and were self-expandable. These were later replaced by balloon expandable stents.
Atherosclerosis is an intimal focal disease-causing connective tissue proliferation and lipid accumulation. In order to preserve their lumen, atherosclerotic vessels dilate in a remodeling response and only after reaching the capacity of this response does stenosis occur. These facts imply that an accurate assessment of coronary artery stenosis and reference diameter is of major importance. Lesion severity evaluation by visual estimation remains subjective and has been shown to be inadequate secondary to a high degree of intra-observer and inter-observer variability[35]. In fact, the lumen in eccentric plaques alters in shape and size, and the reference segment (deemed as normal) may be either narrowed or dilated. For decades, PCI guidance and results have been evaluated by visual assessment, nevertheless, angiography alone does not provide sufficient information in several scenarios presenting a major risk factors of stent thrombosis, such as stent fracture, stent malapposition or stent overlap[29,36,37]. To study PCI efficacy and results, several carefully acquired coronary angiographic films need to be interpreted in detail pre- and post-intervention, as well as at follow-up. Alternative imaging techniques have been developed to overcome these limitations. We hereby review the coronary stent visualization methods currently used for the assessment of PCI results.
Since visual assessment of lumen diameter and stenosis is scarcely sensitive, quantitative coronary analysis (QCA) was first described for a theoretical objective evaluation of coronary artery stenosis and lumen diameter in clinical settings. Since then, the field has grown substantially, with several methods and algorithms being developed since the late 1970s[38]. The European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions Task Force on the Evaluation of Coronary Stents in Europe recommended a mandatory assessment by offline QCA core lab analysis in case of comparative studies[39], and QCA was endorsed by the Academic Research Consortium 2[40]. QCA relies on coronary angiography in order to obtain objective parameters and can also be used to assess immediate and long-term PCI results[38]. It is based on the use of dedicated software allowing the precise determination of specific parameters in an operator-independent manner and can serve either a clinical purpose (on-line during a procedure) or a research one (off-line)[41]. With ongoing research, X-ray imaging has significantly progressed, leading to many available computer-integrated applications that allow the quantification of coronary stenosis leading to QCA validation and incorporation in various systems[42]. Multiple validated software are currently available on the market, among which the most widely used are CAAS II (PIE Medical, Maastricht, The Netherlands) and QAngio XA (Medis, Leiden, The Netherlands)[43-45].
QCA requires an optical magnification of the angiographic image. A digital cineangiogram is generated on an image processor after image acquisition. Using an integrated software and analysis system, digital quantification of selected frames can be easily performed with or without magnification. After digitalization, and prior to computer analysis, the images are stored in a specifically designed image processing system.
The real challenge of QCA is the selection of the coronary angiography sequence with a minimal foreshortening and minimal overlap with nearby structures after intracoronary nitroglycerine administration in order to reduce any vasospasm. In addition, the accuracy and reliability of the analysis is improved by increasing the distinction between the contrast-filled coronary artery and the background (best achieved while the patient is in deep inspiration). A frame including a completely contrast-filled catheter is first selected and a central line is hand-drawn along the tip of catheter. Once the image has been acquired and processed, boundary delineation within the area of interest is performed by the computer. QCA usually focuses on one or more coronary segments and is generally carried out in the case of an ambiguous coronary lesion. The area of interest can be identified automatically by the computer software or manually. The operator indicates the window of interest, an approximation of the borders or points along the vessel’s central line[46,47]. The software automatically recognizes its margins and performs calibration. In order to derive quantitative information from the analysis, a calibration converts measured pixels to in vivo millimeters by using the contrast-filled known catheter diameter as a reference standard or using an automatic calibration obtained from recent systems[48]. By assuming a homogeneous distribution of contrast within the vessel lumen, the errors within the edge definition are minimized in eccentric lesions by densitometry[38]. Then, an appropriate frame that includes the segment of interest is selected during end-diastole when coronaries are least subjected to myocardial contractions. Similar to the calibration procedure, a central line is drawn in order for the software to generate automatic contours. The frames are then automatically transferred to a digital lossless compression file that generates a series of diameters and parameters along the vessel line expressed in millimeters and percentages. This also allows an automatic reconstruction of the vessel lumen and interpolates the diseased segment to the proximal and distal references considered disease-free using an algorithm based on the calculation of mean diameter values at different points along the segment of interest (Figure 1). Edge definition, although more important than quantification, is harder to accomplish[48]. The difference in luminal cross-sectional area can then be compared between normal and diseased segments in addition to the assessment of PCI results. The analysis can also be performed after stent implantation in order to compare several parameters pre- and post-stent implantation. Hence, QCA permits the evaluation of the minimal lumen diameter (MLD, the smallest diameter within the segment studied), the reference vessel diameter (RVD, averaged diameter of the coronary assumed disease-free), the diameter stenosis percentage, the lesion length (LL, measured by two points where the margins change direction), the acute gain (post-procedural MLD-pre-procedural MLD), and late loss (post-procedural MLD-follow-up MLD for example at 6 mo). On-line digital systems and off-line computer processing systems have become widely available. These systems facilitate the accurate clinical analysis of vessel diameter, percent stenosis, stent deployment, and other endovascular interventions. One of the major coronary angiography drawbacks is its two-dimensional luminogram of a three-dimensional structure, as well as vessel overlap and foreshortening during acquisition. Furthermore, QCA is influenced by the frame selection, vessel movement, end-diastolic phase and calibration[49,50].
Currently, 2-dimensional QCA remains the most commonly used and validated technique despite its drawbacks. However, as more complex lesions are treated more frequently, 3-dimensional QCA analysis software is a valuable tool. Three-dimensional QCA is based on standard 2-dimensional angiography images obtained in two views at least 30 degrees apart and with as much minimal vessel overlap. Several 3D QCA programs are currently available, among which, the most commonly employed and validated are CAAS 5 (Pie medical Imaging, Maastricht, The Netherlands) and CardiOp-B (Paieon Medical Ltd. Park Afek, Israel)[51-53]. Although these applications defer in their respective calibration methods, nevertheless they allow a better understanding and visualization of coronary anatomy and help the operator find the optimal working angle[54,55]. Three-dimensional QCA software also allows the assessment of bifurcating lesions and helps choose the appropriate treatment strategy[56].
Although 3-dimensional QCA programs are readily available and have already been validated[55,57,58], they fail to improve accuracy by resolving the problem of vessel foreshortening. Several comparative studies between 2-dimensional and 3-dimensional QCA have led to mixed results with regards to the accuracy of this technology[55,57,59-61].
Visual assessment of coronary lesions tends to underestimate < 50% stenoses and to overestimate those > 50%[62,63], while QCA tends more to overestimate lesions < 50% than those between 50% and 75% and underestimate stenoses > 75%[41,64]. QCA may be easily and rapidly employed in everyday clinical practice to objectively, precisely and independently assess coronary lesion severity. Hence, QCA may direct treatment of a stenosis if a functional assessment is not available. Additionally, QCA instantly assesses objective parameters, such as RVD and lesion length, allowing for a more precise choice of treatment material as well as PCI result assessment. QCA has also played a pivotal role in clinical research through its reproducibility and independence. It has become a fundamental core laboratory tool for the off-line assessment of devices and/or the progression of atherosclerosis following pharmacological therapies[65].
Although QCA has been around for more than 30 years now, it still presents multiple limitations. Nevertheless, it remains an easy-to-use tool, both when used in clinical research and as a complementary tool in practice alongside angiography.
Stent positioning and deployment is carried out under angiography guidance and hence relies on the radiopaque nature of the material used for visualization. Long-term safety concerns of implanted coronary stents have led to increased research on the improvement of stent technology. It has been stipulated that thinner struts are associated with a better outcome in drug-eluting stents (DES). The ISAR STEREO trials have identified thicker struts thickness to be an independent predictor of in-stent restenosis[33,34,66]. Furthermore, the use of thinner-strut stents has been associated with a significant reduction in myocardial infarction[67]. In addition, inadequate stent deployment and underexpansion has been shown to be a major factor for stent thrombosis associated with a high death rate and non-fatal myocardial infarction[29,68]. With recent advancements in stent design technology, DES has become the standard-of-care for patients with acute and chronic coronary syndromes. The first DES consisted of a stainless-steel backbone rendering them radiopaque with good visibility on angiography. However, given the inverse relationship between strut thickness and fibrous hyperplasia, newer second-generation DES have switched to cobalt chromium or platinum chromium, along with a trend towards thinner struts decreasing stent radiopacity, all while preserving radial strength. Although the incidence of stent thrombosis has decreased during the first year after implantation as compared to BMS, its rate remains high[69]. Stent architecture assessment is also important in order to assess PCI results and complications. With the trend toward the use of lower X-ray power during procedures at 7.5 frames per second, and despite the use of radiopaque materials in stent construction, the visualization of stents remains challenging and insufficient for stent expansion assessment. Stent visibility is further altered with stent motion within the sequence as well as in larger patients due to X-ray scattering.
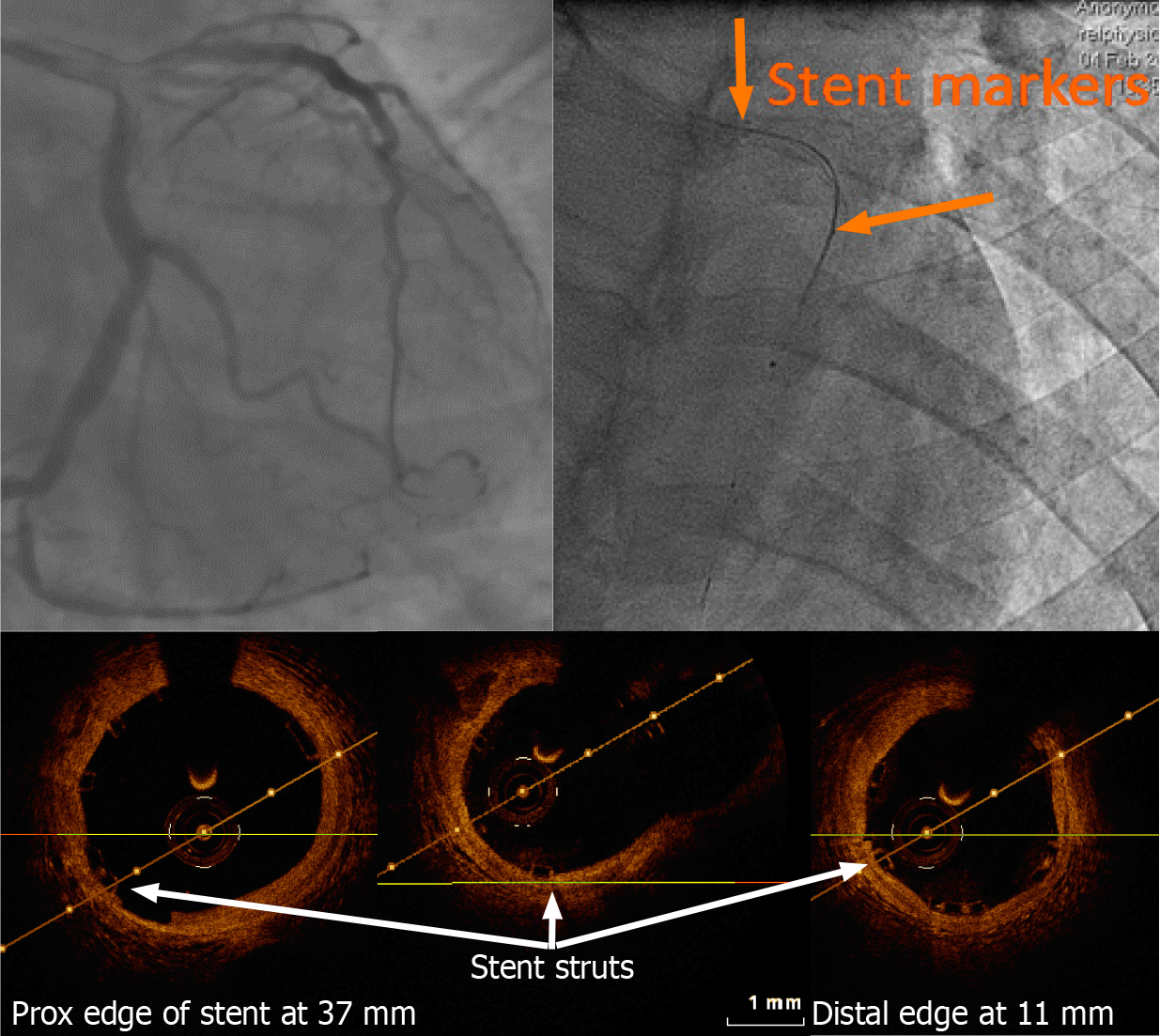
Since the early 2000s, a new image processing technique called digital stent enhancement (DSE) has been developed, specifically tailored for stent visualization. In order to produce an enhanced image of an implanted stent, DSE uses a motion-corrected X-ray image sequence making it reliable during the normal workflow during coronary angioplasties[70]. Due to the ease of use of this technique, several manufacturers have become interested in developing and validating new systems.
Although they cannot bring direct information regarding stent apposition onto the vessel wall, DSE systems can provide relevant images for assessing deployment irregularities, lesion treatment, and potentially measuring stent expansion[71]. Several case reports and case series have validated the use of the different DSE systems in different clinical settings. Additionally, a decrease in late loss and binary restenosis, along with a lower incidence of target lesion revascularization at 6 and 12 mo respectively, were found in patients where DSE was used as compared to patients treated without DSE[72].
StentBoost Subtract System® (Philips Healthcare, Best, The Netherlands) is currently the most used technique and is extensively validated[73-77]. StentBoost Subtract System has also been shown to have high enough specificity as compared to intravascular imaging[73,78]. StentViz (GE Healthcare, Milwaukee, WI, United States), StentOptimizer (Paieon, Rosh Haayin, Israel) and ClearStent (Siemens Healthcare, Munich, Germany) have also been studied and validated[79-81]. Nevertheless, these systems all depend on the X-ray angiographic system vendor. StentEnhancer (Pie Medical, Maastricht, The Netherlands) is a recently added DSE with the advantage of being vendor independent.
DSE improves the fluoroscopic image quality by reducing the influence of background noise. It consists of a short X-ray sequence where the stent is moving with each heartbeat and during breathing. This is based on the fact that over a whole sequence, the sum of the images of the stent is displayed with a much higher contrast to noise ratio. Hence, the DSE algorithm “tracks” the stent motion along the sequence and automatically integrates the value of the stent’ pixels along their trajectories. As a result, the overall sum of the pixels is displayed in an enhanced still-image.
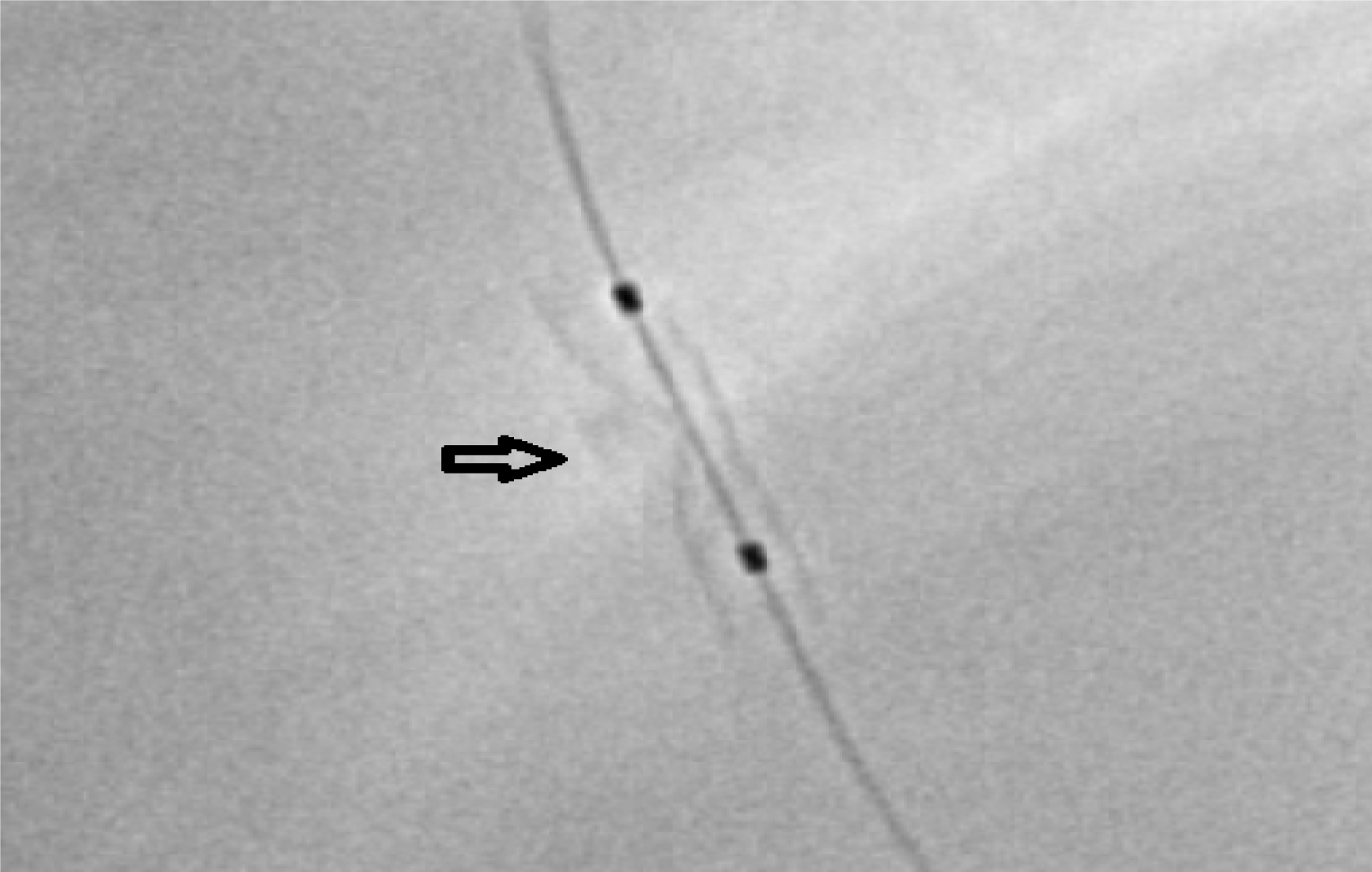
After stent deployment, the stent delivery balloon is kept in place deflated and cineacquisition of the stented segment is performed without contrast injection. Stent-motion is detected using the two balloon radiopaque markers[70,82]. Forty-five frames are acquired and automatically transferred to a workstation where software corrects for motion-compensated temporal averaging[83,84]. Immediately after, an enhanced image of the stent is displayed with an improved resolution and a superior signal-to-noise ratio (Figure 2). These steps may be repeated with a post-dilation balloon. A modified DSE technique, aimed at visualizing the stent in relation to the vessel, is also available. This differs from the abovementioned technique by the contrast injection[84]. The resulting image can be used for stent expansion assessment, visually and quantitatively, much like during QCA analysis[85-87]. Its ease-of-use and availability, along with the high-quality stent images delivered, have led to DSE being an important alternative or complement to stent visualization techniques during PCI. DSE systems are rapid, cost-effective, do not require contrast injection and can be used during complex PCI cases, such as ostial or bifurcating lesions[74,88]. However, an increase in radiation exposure has been reported, but this had no significant impact on patient radiation dose[89].
Coronary angiography only provides a planar, 2-dimensional evaluation of the contrast-filled coronary lumen and fails to evaluate the atherosclerotic plaque located within the arterial wall, hence often underestimating the degree of intraluminal stenosis[35]. The addition of quantitative digital assessment (QCA and DSE) improves the overall coronary assessment but still presents pitfalls. In fact, extensive coronary disease with positive remodeling may appear “normal”, leading to a false feeling of reassurance. This is especially apparent with patients presenting with extensive disease on computed tomography angiography but found to have minimal disease on angiography. In order to bypass these limitations, the idea of intravascular imaging emerged in 1971, thanks to Bom et al[90] in Rotterdam, and Yock et al[91] later recorded the first greyscale transluminal ultrasound images of human arteries. Since then, major breakthroughs have been achieved including intravascular ultrasound (IVUS) radiofrequency tissue characterization and optical coherence tomography (OCT). Intravascular imaging has the advantage of capturing plaques from close proximity. These techniques have been a valuable tool in everyday practice shedding light on whether the plaque is at risk of progression, vessel diameter size, acute PCI results and reasons behind target lesion failure and revascularization.
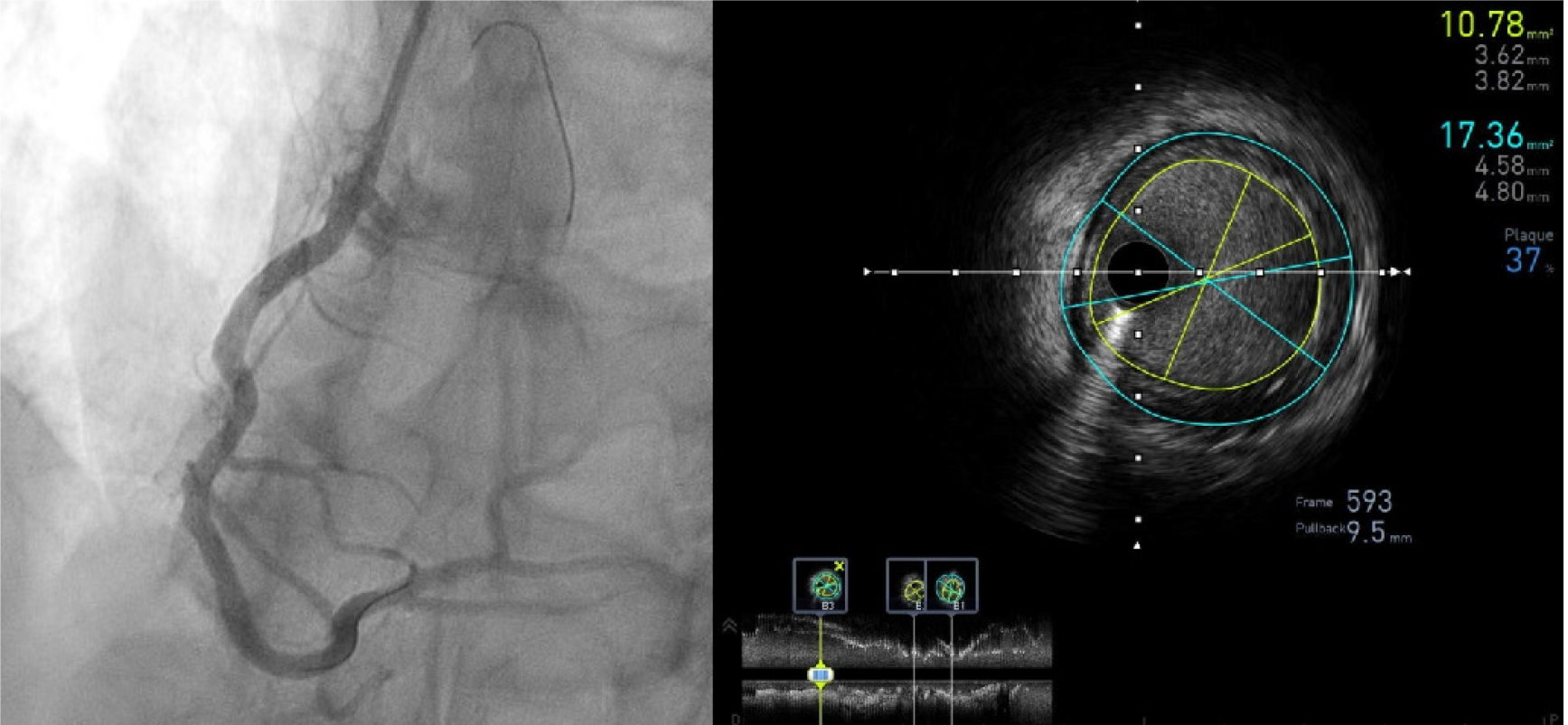
IVUS imaging relies on the properties of ultrasound waves which are produced by the oscillatory movement of a transducer[92]. A piezoelectric crystal inside a transducer, attached to a catheter, produces high frequency sound waves which penetrate tissue, reflect off vascular structures and return to the transducer, which then transmits the information to a dedicated system for processing. These catheters are 3.2 to 3.5 French in size and compatible with 5 French guiding catheters over 0.014 in guidewires.
The first ultrasound imaging catheters, designed by Bom and colleagues, were used for cardiac chamber and valve assessment. It was not until 1988 that the first intracoronary images were recorded[93]. Since then, IVUS has been extensively used and developed, and has been the cornerstone of major interventional trials, from the bare metal stents era to the newer bio-absorbable stents (BVS). In addition, IVUS has allowed a better understanding of CAD progression, vessel reaction to PCI, as well as long- and short-term vessel remodeling after stent implantation.
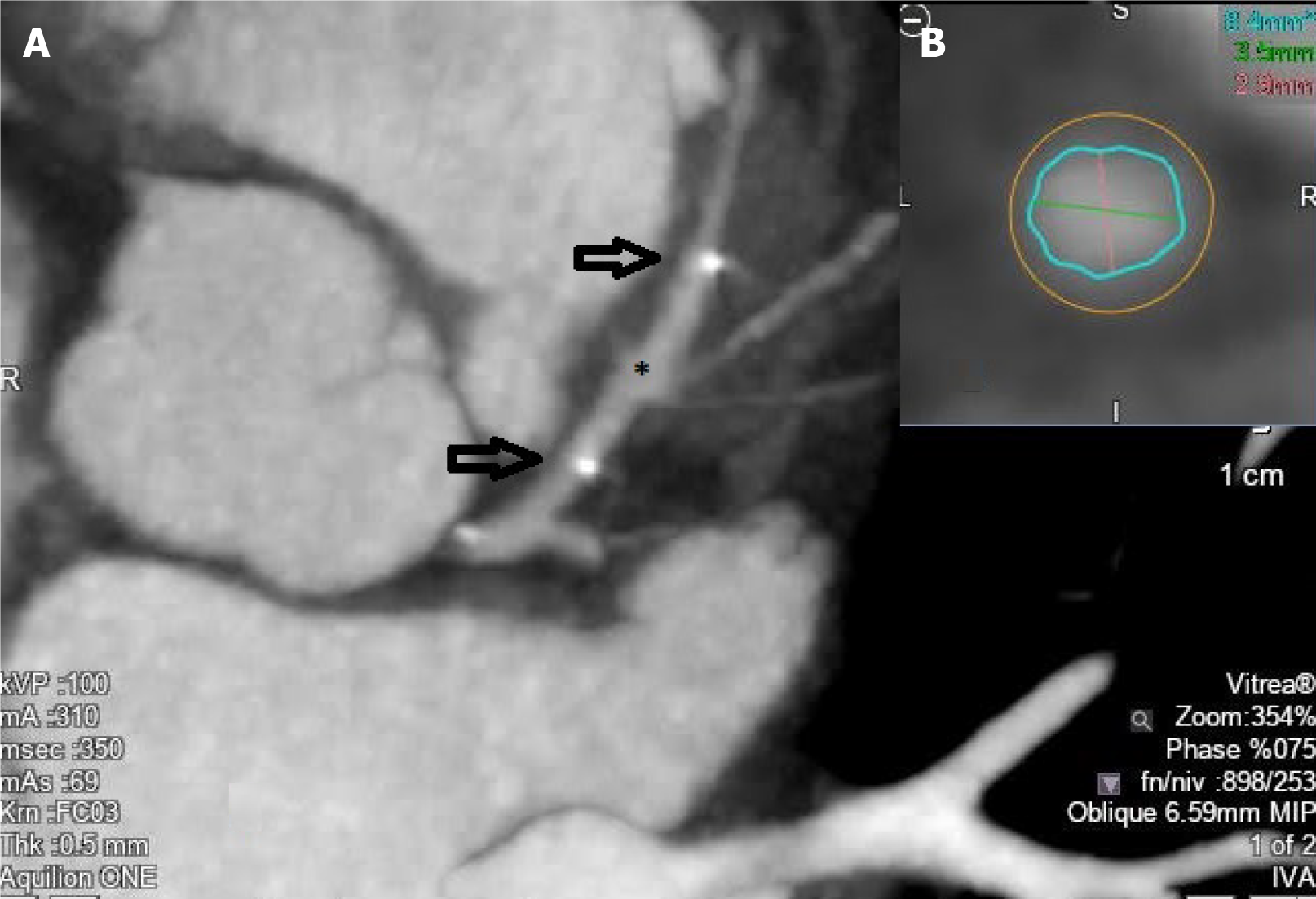
In order to increase radial resolution, IVUS catheters use higher frequencies than non-invasive echocardiography, typically between 20 and 60 MHz. Resolution increases with increased frequency, however penetration decreases. Currently, 2 types of IVUS catheter designs exist on the market: a solid-state, phased array one and a rotational, mechanical one. Solid-state catheters (Volcano, Philips Healthcare, Best, The Netherlands) consist of multiple transducer elements mounted circumferentially at the tip of the catheter. The phased-array transducers are activated in groups in a rotational manner. The collected information is then transferred to a computer system which computes a cross sectional image of the vessel. Volcano catheters also offer kind of virtual histology, (VH-IVUS). The advantage of a solid-state system is its prepackaged ease of setup along with the lack of guidewire artefacts. This is because the catheter is advanced on the wire itself, meaning that there are no non-uniform rotational distortion (NURD) artefacts, which usually result from mechanical binding, as in this case there is no friction on the catheter. On the other hand, solid-state catheters have a low resolution, running at 20 MHz, and a blind spot all around the imaging ring, with a need for masking this “ring down” zone by digital subtraction before acquiring images. Rotational systems (Boston Scientific, Santa Clara, CA, United States; Terumo Corporation, Tokyo, Japan; Philips Healthcare, Best, The Netherlands; Acist Medical Systems Inc., Eden Prairie, MN, United States) use a single transducer element at the tip of the catheter rotated by an external motor drive attached to the catheter. Similar to solid-state systems, a cross sectional image is displayed after gathering echoes while the catheter rotates. Rotational systems have the advantage of having a significantly higher axial resolution with transducers up to 60 MHz with a large bandwidth. Nevertheless, rotational system setup is somewhat more cumbersome with the need to flush the catheter. They use a short monorail with, consequently, an artefact of the wire running along the imaging crystal. However, this offers a more stable automated pull-back of the imaging tip inside a sheath for length measurements of vessel structures. In order to perform imaging, the catheter is advanced beyond the area of interest over a 0.014 in. guidewire after a 100-200 mcg intracoronary nitroglycerin injection in order to minimize vasospasm and to have maximal vasodilation. The catheter is then pulled back in order to obtain the desired images either manually or automatically (motorized) at a standardized speed (up to 10 mm/s). Motorized pull-back has the advantage of providing longitudinal information of the area of interest in order to assess lesion length, while manual pull-back provides a more detailed and prolonged view of the area of interest. Regardless of the system used, proper identification of the three histologic vessel layers is critical for an accurate interpretation (Figure 3). The major advantage of IVUS imaging is its ability to see both intraluminal and extra-luminal elements in the vessel (coronary thrombus, edge dissection after stenting, etc.). It also allows for accurate and reproducible measurements of several segments of the vessel. In fact, the proximal and distal reference vessel segments can be identified and measured, along with the minimal lumen area (MLA), minimal and maximal lumen diameter, lumen eccentricity, area stenosis, cross section area (CSA), plaque area and burden, and the remodeling index can be computed. IVUS can also guide PCI, evaluate stent expansion, assess side branch compromise, determine the underlying mechanism of stent thrombosis/underexpansion and evaluate tears of the vessel from coronary dissections[94]. Although IVUS presents many advantages, several pitfalls remain. The displayed image relies on the absorption and reflection of ultrasound waves from tissues and, in the presence of calcium, which is a high reflector, all the ultrasound waves are reflected and do not penetrate beyond the underlying tissue. This reveals reverberation artefacts and shadowing behind the calcium with no possibility to analyze the extent of plaque and vessel structure behind the calcium. A wire artefact during IVUS images analysis may result in shadowing and difficulty seeing beyond the wire. Accurate analysis, especially with longitudinal measurements, can be hindered between systole and diastole secondary to the catheter movement[95]. In addition, blood speckle artefacts increase as blood flow decreases or the transducer frequency increases, hampering the ability to differentiate lumen from tissue, especially in the case of soft plaques and thrombus.
IVUS guided PCI has been shown to increase the choice of balloon and stent size by the PCI operator, increase the length of the stented segment, and increase the minimal lumen diameter and stent CSA at the end of the procedure as compared to angiography alone. Mixed results were found in multiple prospective studies during the bare metal stent era as to whether this increase improved clinical outcome[96-99]. In a meta-analysis, Parise et al[100] found a reduced 6-month angiographic binary restenosis with a significant reduction in revascularization rate and major adverse cardiac events in IVUS-guided PCI patients. Several retrospective and prospective studies were conducted during the DES era showing a net benefit of IVUS on overall clinical outcomes[101,102]. Several meta-analyses highlighted the net benefit of IVUS-guided PCI using DES with a significant reduction in major adverse cardiac events, myocardial infarction, target vessel revascularization, target lesion revascularization and stent thrombosis as compared to angiography guided PCI[103-105]. Recently, the 3-year follow-up of the ULTIMATE trial showed a reduction in cardiovascular death, target vessel failure and stent thrombosis in patients who underwent IVUS-guided PCI as compared to patients who underwent angiography-guided PCI alone[106,107]. With the treatment of more complex lesions, IVUS has been shown to improve mortality in left main stenting, probably due to a better stent apposition and lower rate of underexpansion[108-110], as well as in bifurcating lesions in order to predict side branch closure[111]. Moreover, IVUS is currently recommended for evaluating and guiding chronic total occlusion procedures[112,113].
VH-IVUS uses advanced backscatter signal analysis in order to try to characterize plaque composition. VH-IVUS limitations are the lack of thrombus detection and plaque characterization in the presence of calcium shadowing, and a limited resolution.
Despite the introduction of high definition IVUS catheters, the major limitation of IVUS imaging remains its limited spatial resolution, making the assessment of stent-strut tissue coverage challenging.
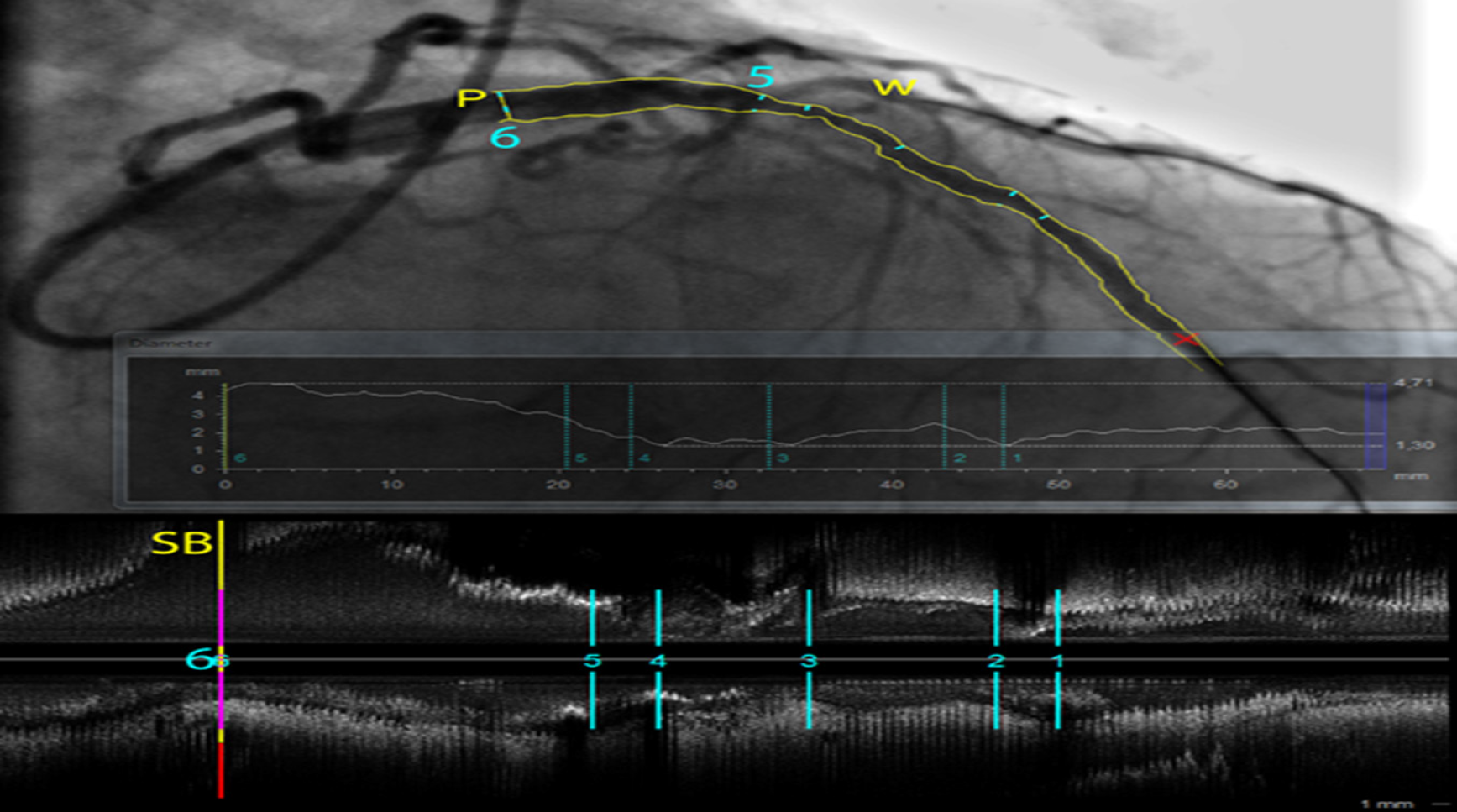
OCT is a light-based intravascular imaging technique that provides a high-resolution image of the tissue microstructure by using backscattering and near-infrared light reflections from a fiber optic wire coupled with an imaging lens while simultaneously being pulled back and rotated. The first OCT clinical application on coronary arteries was reported in 1991 after the addition of transverse scanning[114]. The rapidly evolving technique was named by James Fujimoto and made way to contemporary OCT catheters that use a central light wavelength range of 1300 nm, which limits tissue penetration to 1-3 mm as compared to IVUS. With an axial resolution of 10-15 µm, OCT offers a very detailed picture of the vessel while the imaging optics of the catheter allow for a lateral resolution of 20-40 µm.
The first available OCT systems from LightLab Imaging (Westford, Massachusetts, United States) used an old OCT technology, called time-domain (TD) OCT, based on a broadband light source emitting through a fiberoptic coupler. The main disadvantage of this system was the slow speed of acquisition, which resulted in the need of total vessel flush for a long period. Newer generation OCT systems, termed Fourier-domain OCT (FD-OCT), use a fixed mirror and light is emitted by a “swept laser” source with a variable frequency. This allows for a faster frame acquisition rate (up to 100 frames/s) with only a short vessel flush duration. Continuous flush of contrast fluid is required in order to clear intraluminal blood since blood causes significant signal attenuation. The first FD-OCT was developed by St. Jude Medical (St Jude Medical, St. Paul, Minnesota; United States). It is a 2.5 Fr catheter compatible with 5 Fr guiding catheters. Currently a 2.4 Fr catheter is also available from Terumo (Terumo Corporation, Tokyo, Japan). The OCT catheter is advanced over a 0.014 inch wire to the distal vessel and an automated pull-back is then performed at a rate of up to 20-40 mm/s during short iodinated contrast injection (4 cc/s) in order to clear blood. During pull-back, a long spiral scan is created within less than 3 s in order to map the vessel and create the required frames. The emitted light splits into 2 parts: the first sample arm travels to the patient while the second reference arm travels a predefined distance. The two arms are then collected and an interferometer is used to measure the light reflected (back-scattered) from tissues in order to determine the depth of the tissue (A line). The system then processes the reflected light and computes a very high-resolution image due to the shorter wavelength of light and 3D tissue morphology.
Each image is made up of lines and pixels; the higher the lines/frames ratio is, the finer the image, and the higher the frame rate, the higher the longitudinal resolution. Artefacts associated with OCT include light attenuation from incomplete blood flushing, artefacts secondary to the guidewire, distortion of stent reflections from eccentric wire position (sunflower effect), air bubble artefacts, and stitch artefacts secondary to rapid catheter or wire movements (sew-up effect). These can be minimized through a proper positioning of the guidewire and appropriate contrast injection[115]. Even macrophage accumulation in thin-cap fibroatheroma or small intraluminal thrombi can be seen[116]. Plaque characterization can be achieved by OCT combining backscattering and attenuation measurements[117].
Currently, limited data for OCT-guided vs. angiography-guided interventions are available, while ongoing randomized clinical trials are running. A reduced rate of cardiac death and major adverse cardiac events has been reported in patients who underwent OCT-guided stenting[118], as well as larger final in-stent minimal lumen diameter[119] and a reduction in the number of stents implanted[120]. OCT-guided PCI has also been associated with better clinical outcomes in acute coronary syndrome patients[121,122]. In addition, a better stent coverage at 3 mo was found in OCT-guided PCI chronic CAD patients[123].
OCT delivers high-resolution intravascular images which are used in clinical practice, but mainly in the research field[124,125]. As compared to IVUS, OCT showed comparable stent expansions and comparative efficacy in the ILLUMIEN II and III studies respectively[126,127]. OCT was also shown to have non-inferior clinical outcomes as compared to IVUS in the OPINION[128] study. The latest European Society of Cardiology guidelines have included IVUS and OCT imaging as a class IIa recommendation[129]. Although both imaging modalities share multiple similarities, some controversy remains regarding which modality is to be used in specific cases. The need of contrast injection during OCT imaging tends to be a trigger towards IVUS use in patients with renal failure. The very high resolution of OCT images enables a more detailed evaluation at the endoluminal level and superficial plaques, and better stent implantation short- and long-term results[130]. However, OCT has lower penetration depth as compared to IVUS, which makes larger vessel visualization more challenging, in addition to the external elastic membrane-based approach for vessel sizing. IVUS images are significantly altered by the presence of calcium whereas OCT recognizes calcium plaques as well-demarcated low-intensity structures. The relatively smaller size of OCT may reduce the incidence of coronary spasm, and can identify coronary thrombosis and dissection easily. However, OCT fails to assess aorto-ostial lesions. Several clinical studies have demonstrated the non-inferiority of OCT imaging as compared to IVUS (OPINION, ILLUMEN III)[127,131].
Co-registration of both OCT and IVUS with angiography has been developed[132] adding to the precision during PCI and reducing the risks of geographic miss[94,115]. An OCT/IVUS hybrid technology (Novasight) has been recently introduced, and further developed by Conavi Medical Inc. (Toronto, Canada), and this was reported in 2018[133]. A second hybrid system is currently under development by Terumo (Terumo, Tokyo, Japan) and is yet to be released.
Intravascular imaging has marked its place during PCI offering multiple benefits over angiography alone. Multiple meta-analyses and registries have proven its improved procedural and clinical outcome. However, intravascular usage rate remains low probably due to the increased cost, time and unfamiliarity of the operators[134]. With increasing PCI complexity, intravascular imaging adds a clinically proven indispensable benefit for successful intervention.
Coronary computed tomography angiography (CCTA) has recently emerged as a precise non-invasive tool for the detection and evaluation of CAD[135,136]. Computed tomography (CT) technology has advanced significantly since its first use, by Sir Godfrey Hounsfield in 1970, followed by the introduction of electron beam computed tomography by Douglas Boyd improving temporal resolution. A major breakthrough came in the 1990s with the introduction of multidetector computed tomography that has the ability of improving a 360° spatial resolution. CT images depict tissue densities and are the product of X-ray attenuation as they pass through tissue. A CT detector measures and converts the X-ray photons exiting the patient into light. This light is then transformed into an electrical signal by a photodiode and converted into a digital signal by a computer system. The image is an overall estimation of the attenuation of X-rays as they pass through tissues of different densities represented by Hounsfield units.
Currently, multidetector computed tomography scanners include 320-detector rings and two X-ray sources, allowing for the acquisition of submillimeter resolution over large volumes in milliseconds. Improvements in detector characteristics have increased the x, y and z axis spatial resolution, which allows for successful imaging of the coronary arteries while reducing radiation dose[137]. Three steps are required to successfully create a CCTA image: data acquisition, reconstruction and image display. Data acquisition involves scanning portions of the heart with each gantry rotation and requires proper patient selection, preparation, appropriate imaging protocols and contrast injection techniques. Effective heart rate control, proper timing of the scan after contrast injection and minimizing patient movement are all crucial during data acquisition. It is therefore advisable for patients known to have arrhythmias to be deferred from CCTA. CCTA has the advantage of visualizing the entire vessel, and can accurately evaluate stenoses severity and plaque morphology.
Along with CCTA already being established for the detection of CAD and confirming the ability of calcium score in providing a prognostic value in multiple large studies[137-139], it is an excellent diagnostic tool for bypass graft imaging. Moreover, CCTA has been validated and used for the assessment of coronary artery stent with recent technical refinements showing negative predictive values >90% for the exclusion of in-stent restenosis[138,140-142]. However, a blooming effect secondary to the stent struts and beam-hardening artefacts remain a challenge for proper evaluation[143,144]. This is also true for patients known to have excessive coronary calcium, as calcifications may interfere with the accurate interpretation of CCTA. Since coronary stenosis alone does not predict the risk of future cardiovascular events, the coronary artery calcium (CAC) score estimates the overall calcification burden and predicts future ischemic events without contrast use. With the introduction of bioresorbable stents (Figures 4 and 5), this set-back has been overcome[145,146]. Contrary to magnetic resonance imaging (MRI), pacemakers and defibrillators do not preclude the use of CCTA, however it should be noted that their leads may create artefacts.
CCTA has been shown to be a better predictor of obstructive CAD compared to traditional functional testing. The EVINCI study showed the superior accuracy of CCTA for the detection of CAD as compared to single photon emission CT (SPECT), position emission tomography (PET), echocardiography and cardiac MRI[147]. The prognostic value of CCTA was also demonstrated in the PROMISE and SCOT-HEART studies which showed high CCTA prediction of cardiovascular events as compared to functional testing and standard care[139,148]. A meta-analysis showed a significant reduction of myocardial infarction as well as an increased incidence of coronary revascularization with no effect on all-cause mortality using CCTA in patients with chronic coronary syndrome as compared to usual care[149].
Observer variability and expertise remain a challenge for CCTA interpretation. Dedicated software programs for increasing automation and identifying high-risk plaques have been validated as well as newer technologies, including fractional flow reserve (FFR) derived from CCTA (FFRCT), perivascular fat attenuation index (FAI) and wall shear stress (WSS)[136]. Advances in imaging modalities permitted a 3-dimensional reconstruction of the coronary artery tree and a decrease in radiation exposure[150]. Myocardial perfusion by CCTA is also possible and allows for the assessment of the physiologic consequences of stenoses[136]. It has evolved as a valuable tool in guiding PCI and assessing lesion characteristics, especially in chronic total occlusion (CTO) lesions using co-registration with angiography[151]. In the acute disease setting, CCTA fails to differentiate between a thrombus and acute plaque hemorrhage.
CCTA technology still has a lot to offer in the future. It has been a useful and promising tool in the assessment and tracking of atheromatous plaques, improving understanding of early atherosclerosis.
The success of stent implantation during PCI procedures depends on proper positioning of the stent, expansion and apposition, all of which reduce the rate of major adverse cardiac events. Traditional two-dimensional angiography alone fails to fully assess the result of stent implantation. Pre-procedural measurement of the vessel diameter along with post-procedural evaluation of the strut-level and minimal lumen area and diameter improve clinical outcomes. Despite the substantial technological advances, a growing need for newer and more accurate tools for PCI guidance and assessment has emerged with the increasing number of complex procedures performed worldwide and ongoing stent technology advances.
Quantitative coronary analysis has developed substantially since its early beginnings due to its research and clinical uses both on-line and off-line. Recent 3-dimensional QCA systems have also emerged, providing more reliable assessment of lumen dimensions[152]. The ease-of-use and reproducibility of QCA analysis has made it widely available within PCI centers worldwide. However, several limitations persist and QCA measurements are lower compared with other imaging modalities[153]. Furthermore, QCA measurements are limited for diffuse lesions, as the reference diameter tends to be underestimated[50].
Digital stent enhancement imaging has been thoroughly validated for stent underexpansion. It allows for easy visualization of areas of stent underdeployment for positioning of post-dilation balloons. DSE has been reported to be associated with better angiographic and clinical outcomes[72] as well as stent fracture identification. DSE adds little additional time to the procedure, provides a better image resolution as compared to angiography with no risk of mechanical complications while using less contrast. It has been found to be particularly useful for obese patients, long lesions, in-stent restenosis and bifurcating lesions[77,86,154]. Although the accuracy and resolution of DSE is lower than intravascular imaging, measurement of stent diameters correlated well[71,73,87,153,155]. Its main drawback could be the increased radiation dose, however this does not have a significant impact on the patient[86]. DSE remains a useful and rapid tool that can be used in conjunction with intravascular imaging during PCI.
Intravascular imaging remains the gold standard during PCI and has demonstrated low complication rates. Several randomized controlled trials and observational studies have established the role of intravascular imaging-guided stent implantation during PCI with a reduction in major cardiac events and target vessel revascularization[105]. Compared to angiography, intravascular imaging-guided PCI has been shown to have a relative reduction of cardiovascular death, a lower risk of myocardial infarction, a decreased risk of target vessel revascularization and a reduction in the rate of stent thrombosis[105]. In addition, the greatest benefit of intravascular imaging guidance may be in complex lesions and stent failure patients. The non-inferiority of OCT as compared to IVUS in guiding PCI has been demonstrated in several clinical trials and meta-analyses[128,131,137,153,156]. Clear differences exist between the two technologies. OCT offers a higher resolution along with finer details, whereas IVUS has a better penetration and does not require flushing the lumen with contrast. IVUS and angiography co-registration systems have been tested and validated and provide valuable information. Furthermore, more recently, a 3-dimensional reconstruction of the coronary tree using IVUS co-registration allowed for further detailed information about vessel size, plaque size, shear stress and hemodynamic studies at every position along the vessel[152].
Thus, intracoronary imaging provides an accurate evaluation of coronary anatomy and plaque evaluation during PCI allowing for optimized stent sizing while avoiding stent malaposition, underexpansion and stent edge dissection, all in addition to an accurate assessment of the stent result. However, its routine use in clinical practice remains low and highly variable, mainly due to the added procedure cost and time. Progress is still ongoing within the field, with further advances on the way using OCT/IVUS hybrid console systems.
With the ongoing paradigm shift towards non-invasive imaging, new reconstruction techniques have significantly improved coronary artery stent imaging by CCTA. The European Society of Cardiology recommends the use of CCTA for the evaluation of CAD for low to intermediate risk patients in the setting of chronic and acute coronary syndromes[157,158]. With its high specificity (97%), CCTA could be used to exclude in-stent restenosis[159]. The CAC score used during CCTA remains an important tool for future cardiovascular ischemic events. Artefacts secondary to the stent struts hinder the accurate evaluation of the stent lumen, however, with higher scan-detector row systems and thinner stent struts, an improved image quality can be achieved using CCTA. In addition, in the PLATFORM trial[160], the introduction of FFRCT reduced the number of patients referred for invasive angiography by 61%. CMR allows the reduction of the contrast dose without compromising image quality. Both CCTA and CMR can easily and accurately assess coronary bypass grafts along with performing myocardial perfusion and a 3-dimensional reconstruction of the vessels. Furthermore, the introduction and on-going progress of newer generation bioresorbable stents have made CCTA an attractive tool for the evaluation of PCI results, as artefacts are greatly decreased[149,161].
In conclusion, invasive angiography remains the gold standard tool for the treatment of CAD and the visual guidance and evaluation of PCI results however, the conjunction and advances of several readily available alternative and complementary tools improve its sensitivity and accuracy.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kashiwagi M S-Editor: Ma YJ L-Editor: A P-Editor: Wang LYT
| 1. | Teague HL, Ahlman MA, Alavi A, Wagner DD, Lichtman AH, Nahrendorf M, Swirski FK, Nestle F, Gelfand JM, Kaplan MJ, Grinspoon S, Ridker PM, Newby DE, Tawakol A, Fayad ZA, Mehta NN. Unraveling Vascular Inflammation: From Immunology to Imaging. J Am Coll Cardiol. 2017;70:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Syed MB, Fletcher AJ, Forsythe RO, Kaczynski J, Newby DE, Dweck MR, van Beek EJ. Emerging techniques in atherosclerosis imaging. Br J Radiol. 2019;92:20180309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P; European Society of Cardiology. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J. 2020;41:12-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 676] [Article Influence: 135.2] [Reference Citation Analysis (1)] |
| 4. | Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139-e596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 5494] [Article Influence: 1098.8] [Reference Citation Analysis (1)] |
| 5. | Laslett LJ, Alagona P Jr, Clark BA 3rd, Drozda JP Jr, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60:S1-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 519] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 6. | Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease : A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Nucl Cardiol. 2017;24:1759-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, Chaitman BR, Senior R, López-Sendón J, Alexander KP, Lopes RD, Shaw LJ, Berger JS, Newman JD, Sidhu MS, Goodman SG, Ruzyllo W, Gosselin G, Maggioni AP, White HD, Bhargava B, Min JK, Mancini GBJ, Berman DS, Picard MH, Kwong RY, Ali ZA, Mark DB, Spertus JA, Krishnan MN, Elghamaz A, Moorthy N, Hueb WA, Demkow M, Mavromatis K, Bockeria O, Peteiro J, Miller TD, Szwed H, Doerr R, Keltai M, Selvanayagam JB, Steg PG, Held C, Kohsaka S, Mavromichalis S, Kirby R, Jeffries NO, Harrell FE Jr, Rockhold FW, Broderick S, Ferguson TB Jr, Williams DO, Harrington RA, Stone GW, Rosenberg Y; ISCHEMIA Research Group. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1755] [Article Influence: 351.0] [Reference Citation Analysis (0)] |
| 8. | Forssmann W. Die Sondierung des Rechten Herzens. Klin Wochenschr. 1929;8:2085-2087. [RCA] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 259] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Cournand A, Ranges HA. Catheterization of the Right Auricle in Man. Proc Soc Exp Biol Med. 1941;46:462-466. [RCA] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 255] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Nossaman BD, Scruggs BA, Nossaman VE, Murthy SN, Kadowitz PJ. History of right heart catheterization: 100 years of experimentation and methodology development. Cardiol Rev. 2010;18:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2369] [Cited by in RCA: 2049] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 12. | Sones FM Jr. Cine-cardio-angiography. Pediatr Clin North Am. 1958;5:945-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Dotter CT, Judkins MP. Transluminal treatment of arteriosclerotic obstruction. description of a new technic and a preliminary report of its application. Circulation. 1964;30:654-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 902] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | Turina M, Grüntzig A, Krayenbühl C, Senning A. Percutaneous transluminal dilatation of coronary artery stenosis. Thorac Cardiovasc Surg. 1979;27:199-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Lincoff AM, Popma JJ, Ellis SG, Hacker JA, Topol EJ. Abrupt vessel closure complicating coronary angioplasty: clinical, angiographic and therapeutic profile. J Am Coll Cardiol. 1992;19:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 264] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Huber MS, Mooney JF, Madison J, Mooney MR. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol. 1991;68:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 295] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Nobuyoshi M, Kimura T, Nosaka H, Mioka S, Ueno K, Yokoi H, Hamasaki N, Horiuchi H, Ohishi H. Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J Am Coll Cardiol. 1988;12:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 647] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 18. | Holmes DR Jr, Vlietstra RE, Smith HC, Vetrovec GW, Kent KM, Cowley MJ, Faxon DP, Gruentzig AR, Kelsey SF, Detre KM. Restenosis after percutaneous transluminal coronary angioplasty (PTCA): a report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am J Cardiol. 1984;53:77C-81C. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 804] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 19. | de la Torre Hernandez JM, Puri R, Alfonso F. Drug-Coated Balloon: "Scoring to Win". JACC Cardiovasc Interv. 2017;10:1341-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Schmidt T, Abbott JD. Coronary Stents: History, Design, and Construction. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Iqbal J, Gunn J, Serruys PW. Coronary stents: historical development, current status and future directions. Br Med Bull. 2013;106:193-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 22. | Serruys PW, Rutherford JD. The Birth, and Evolution, of Percutaneous Coronary Interventions: A Conversation With Patrick Serruys, MD, PhD. Circulation. 2016;134:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1377] [Cited by in RCA: 1173] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 24. | De Luca L, Uguccioni M, Meessen J, Temporelli PL, Tomai F, De Rosa FM, Passamonti E, Formigli D, Riccio C, Gabrielli D, Colivicchi F, Gulizia MM, Perna GP. External applicability of the ISCHEMIA trial: an analysis of a prospective, nationwide registry of patients with stable coronary artery disease. EuroIntervention. 2020;16:e966-e973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Zimmermann FM, Omerovic E, Fournier S, Kelbæk H, Johnson NP, Rothenbühler M, Xaplanteris P, Abdel-Wahab M, Barbato E, Høfsten DE, Tonino PAL, Boxma-de Klerk BM, Fearon WF, Køber L, Smits PC, De Bruyne B, Pijls NHJ, Jüni P, Engstrøm T. Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: meta-analysis of individual patient data. Eur Heart J. 2019;40:180-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 26. | Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3153] [Cited by in RCA: 2973] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 27. | Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3312] [Cited by in RCA: 3111] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 28. | Topol EJ, Leya F, Pinkerton CA, Whitlow PL, Hofling B, Simonton CA, Masden RR, Serruys PW, Leon MB, Williams DO. A comparison of directional atherectomy with coronary angioplasty in patients with coronary artery disease. The CAVEAT Study Group. N Engl J Med. 1993;329:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 490] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 29. | Fujii K, Carlier SG, Mintz GS, Yang YM, Moussa I, Weisz G, Dangas G, Mehran R, Lansky AJ, Kreps EM, Collins M, Stone GW, Moses JW, Leon MB. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45:995-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 602] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 30. | Fujii K, Mintz GS, Kobayashi Y, Carlier SG, Takebayashi H, Yasuda T, Moussa I, Dangas G, Mehran R, Lansky AJ, Reyes A, Kreps E, Collins M, Colombo A, Stone GW, Teirstein PS, Leon MB, Moses JW. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation. 2004;109:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Morton AC, Crossman D, Gunn J. The influence of physical stent parameters upon restenosis. Pathol Biol (Paris). 2004;52:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Lau KW, Johan A, Sigwart U, Hung JS. A stent is not just a stent: Stent construction and design do matter in its clinical performance. Singapore Med J. 2004;45:305-11; quiz 312. [PubMed] |
| 33. | Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schühlen H, Neumann FJ, Fleckenstein M, Pfafferott C, Seyfarth M, Schömig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 522] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 34. | Pache J, Kastrati A, Mehilli J, Schühlen H, Dotzer F, Hausleiter J, Fleckenstein M, Neumann FJ, Sattelberger U, Schmitt C, Müller M, Dirschinger J, Schömig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 402] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 35. | Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 687] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 36. | Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv. 2014;7:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 37. | Cheneau E, Leborgne L, Mintz GS, Kotani J, Pichard AD, Satler LF, Canos D, Castagna M, Weissman NJ, Waksman R. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003;108:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 347] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 38. | Serruys PW, Reiber JH, Wijns W, van den Brand M, Kooijman CJ, ten Katen HJ, Hugenholtz PG. Assessment of percutaneous transluminal coronary angioplasty by quantitative coronary angiography: diameter vs densitometric area measurements. Am J Cardiol. 1984;54:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 212] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Byrne RA, Serruys PW, Baumbach A, Escaned J, Fajadet J, James S, Joner M, Oktay S, Jüni P, Kastrati A, Sianos G, Stefanini GG, Wijns W, Windecker S. Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J. 2015;36:2608-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 40. | Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW; Academic Research Consortium. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 547] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 41. | Garrone P, Biondi-Zoccai G, Salvetti I, Sina N, Sheiban I, Stella PR, Agostoni P. Quantitative coronary angiography in the current era: principles and applications. J Interv Cardiol. 2009;22:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Gavit L, Carlier S, Hayase M, Burkhoff D, Leon MB. The evolving role of coronary angiography and fluoroscopy in cardiac diagnosis and intervention. EuroIntervention. 2007;2:526-532. [PubMed] |
| 43. | Reiber JH, van der Zwet PM, Koning G, von Land CD, van Meurs B, Gerbrands JJ, Buis B, van Voorthuisen AE. Accuracy and precision of quantitative digital coronary arteriography: observer-, short-, and medium-term variabilities. Cathet Cardiovasc Diagn. 1993;28:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | van der Zwet PM, Reiber JH. A new approach for the quantification of complex lesion morphology: the gradient field transform; basic principles and validation results. J Am Coll Cardiol. 1994;24:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Beauman GJ, Reiber JH, Koning G, Vogel RA. Comparisons of angiographic core laboratory analyses of phantom and clinical images: interlaboratory variability. Cathet Cardiovasc Diagn. 1996;37:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Ellis S, Sanders W, Goulet C, Miller R, Cain KC, Lesperance J, Bourassa MG, Alderman EL. Optimal detection of the progression of coronary artery disease: comparison of methods suitable for risk factor intervention trials. Circulation. 1986;74:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Nichols AB, Gabrieli CF, Fenoglio JJ Jr, Esser PD. Quantification of relative coronary arterial stenosis by cinevideodensitometric analysis of coronary arteriograms. Circulation. 1984;69:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 138] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Brown BG, Bolson E, Frimer M, Dodge HT. Quantitative coronary arteriography: estimation of dimensions, hemodynamic resistance, and atheroma mass of coronary artery lesions using the arteriogram and digital computation. Circulation. 1977;55:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 495] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 49. | Ito S, Kinoshita K, Endo A, Nakamura M. Impact of Cine Frame Selection on Quantitative Coronary Angiography Results. Clin Med Insights Cardiol. 2019;13:1179546819838232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Suzuki N, Asano T, Nakazawa G, Aoki J, Tanabe K, Hibi K, Ikari Y, Kozuma K. Clinical expert consensus document on quantitative coronary angiography from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther. 2020;35:105-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 51. | Andrikos IO, Sakellarios AI, Siogkas PK, Tsompou PI, Kigka VI, Michalis LK, Fotiadis DI. A new method for the 3D reconstruction of coronary bifurcations pre and post the angioplasty procedure using the QCA. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:5757-5760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Ng VG, Lansky AJ. Novel QCA methodologies and angiographic scores. Int J Cardiovasc Imaging. 2011;27:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Tomaniak M, Masdjedi K, van Zandvoort LJ, Neleman T, Tovar Forero MN, Vermaire A, Kochman J, Kardys I, den Dekker W, Wilschut J, Diletti R, de Jaegere P, Van Mieghem NM, Zijlstra F, Daemen J. Correlation between 3D-QCA based FFR and quantitative lumen assessment by IVUS for left main coronary artery stenoses. Catheter Cardiovasc Interv. 2021;97:E495-E501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Agostoni P, Biondi-Zoccai G, Van Langenhove G, Cornelis K, Vermeersch P, Convens C, Vassanelli C, Van Den Heuvel P, Van Den Branden F, Verheye S. Comparison of assessment of native coronary arteries by standard vs three-dimensional coronary angiography. Am J Cardiol. 2008;102:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Dvir D, Marom H, Guetta V, Kornowski R. Three-dimensional coronary reconstruction from routine single-plane coronary angiograms: in vivo quantitative validation. Int J Cardiovasc Intervent. 2005;7:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Galassi AR, Tomasello SD, Capodanno D, Seminara D, Canonico L, Occhipinti M, Tamburino C. A novel 3-d reconstruction system for the assessment of bifurcation lesions treated by the mini-crush technique. J Interv Cardiol. 2010;23:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Tsuchida K, van der Giessen WJ, Patterson M, Tanimoto S, García-García HM, Regar E, Ligthart JM, Maugenest AM, Maatrijk G, Wentzel JJ, Serruys PW. In vivo validation of a novel three-dimensional quantitative coronary angiography system (CardiOp-B): comparison with a conventional two-dimensional system (CAAS II) and with special reference to optical coherence tomography. EuroIntervention. 2007;3:100-108. [PubMed] |
| 58. | Schuurbiers JC, Lopez NG, Ligthart J, Gijsen FJ, Dijkstra J, Serruys PW, Van der Steen AF, Wentzel JJ. In vivo validation of CAAS QCA-3D coronary reconstruction using fusion of angiography and intravascular ultrasound (ANGUS). Catheter Cardiovasc Interv. 2009;73:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Ramcharitar S, Daeman J, Patterson M, van Guens RJ, Boersma E, Serruys PW, van der Giessen WJ. First direct in vivo comparison of two commercially available three-dimensional quantitative coronary angiography systems. Catheter Cardiovasc Interv. 2008;71:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Meerkin D, Marom H, Cohen-Biton O, Einav S. Three-dimensional vessel analyses provide more accurate length estimations than the gold standard QCA. J Interv Cardiol. 2010;23:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Tu S, Koning G, Jukema W, Reiber JH. Assessment of obstruction length and optimal viewing angle from biplane X-ray angiograms. Int J Cardiovasc Imaging. 2010;26:5-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Fleming RM, Kirkeeide RL, Smalling RW, Gould KL. Patterns in visual interpretation of coronary arteriograms as detected by quantitative coronary arteriography. J Am Coll Cardiol. 1991;18:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 126] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Zhang H, Mu L, Hu S, Nallamothu BK, Lansky AJ, Xu B, Bouras G, Cohen DJ, Spertus JA, Masoudi FA, Curtis JP, Gao R, Ge J, Yang Y, Li J, Li X, Zheng X, Li Y, Krumholz HM, Jiang L; China PEACE Collaborative Group. Comparison of Physician Visual Assessment With Quantitative Coronary Angiography in Assessment of Stenosis Severity in China. JAMA Intern Med. 2018;178:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 64. | Kalbfleisch SJ, McGillem MJ, Pinto IM, Kavanaugh KM, DeBoe SF, Mancini GB. Comparison of automated quantitative coronary angiography with caliper measurements of percent diameter stenosis. Am J Cardiol. 1990;65:1181-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Serruys PW, Luijten HE, Beatt KJ, Geuskens R, de Feyter PJ, van den Brand M, Reiber JH, ten Katen HJ, van Es GA, Hugenholtz PG. Incidence of restenosis after successful coronary angioplasty: a time-related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3, and 4 mo. Circulation. 1988;77:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 641] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 66. | Colombo A, Hall P, Nakamura S, Almagor Y, Maiello L, Martini G, Gaglione A, Goldberg SL, Tobis JM. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation. 1995;91:1676-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 914] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 67. | Iantorno M, Lipinski MJ, Garcia-Garcia HM, Forrestal BJ, Rogers T, Gajanana D, Buchanan KD, Torguson R, Weintraub WS, Waksman R. Meta-Analysis of the Impact of Strut Thickness on Outcomes in Patients With Drug-Eluting Stents in a Coronary Artery. Am J Cardiol. 2018;122:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 68. | Okabe T, Mintz GS, Buch AN, Roy P, Hong YJ, Smith KA, Torguson R, Gevorkian N, Xue Z, Satler LF, Kent KM, Pichard AD, Weissman NJ, Waksman R. Intravascular ultrasound parameters associated with stent thrombosis after drug-eluting stent deployment. Am J Cardiol. 2007;100:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 69. | Piccolo R, Bonaa KH, Efthimiou O, Varenne O, Baldo A, Urban P, Kaiser C, Remkes W, Räber L, de Belder A, van 't Hof AWJ, Stankovic G, Lemos PA, Wilsgaard T, Reifart J, Rodriguez AE, Ribeiro EE, Serruys PWJC, Abizaid A, Sabaté M, Byrne RA, de la Torre Hernandez JM, Wijns W, Jüni P, Windecker S, Valgimigli M; Coronary Stent Trialists' Collaboration. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2019;393:2503-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 70. | Close RA, Abbey CK, Whiting JS. Improved image guidance of coronary stent deployment. In: Mun SK. Medical Imaging 2000. Image Display and Visualization, 2000: 301-304. |
| 71. | Sanidas EA, Maehara A, Barkama R, Mintz GS, Singh V, Hidalgo A, Hakim D, Leon MB, Moses JW, Weisz G. Enhanced stent imaging improves the diagnosis of stent underexpansion and optimizes stent deployment. Catheter Cardiovasc Interv. 2013;81:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Oh DJ, Choi CU, Kim S, Im SI, Na JO, Lim HE, Kim JW, Kim EJ, Han SW, Rha SW, Park CG, Seo HS. Effect of StentBoost imaging guided percutaneous coronary intervention on mid-term angiographic and clinical outcomes. Int J Cardiol. 2013;168:1479-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 73. | Mishell JM, Vakharia KT, Ports TA, Yeghiazarians Y, Michaels AD. Determination of adequate coronary stent expansion using StentBoost, a novel fluoroscopic image processing technique. Catheter Cardiovasc Interv. 2007;69:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Silva JD, Carrillo X, Salvatella N, Fernandez-Nofrerias E, Rodriguez-Leor O, Mauri J, Bayes-Genis A. The utility of stent enhancement to guide percutaneous coronary intervention for bifurcation lesions. EuroIntervention. 2013;9:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Davies AG, Conway D, Reid S, Cowen AR, Sivananthan M. Assessment of coronary stent deployment using computer enhanced x-ray images-validation against intravascular ultrasound and best practice recommendations. Catheter Cardiovasc Interv. 2013;81:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Blicq E, Georges JL, Elbeainy E, Gibault-Genty G, Benjemaa K, Jerbi B, Livarek B. Detection of Stent Underdeployment by StentBoost Imaging. J Interv Cardiol. 2013;26:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Fysal Z, Hyde T, Barnes E, McCrea W, Ramcharitar S. Evaluating stent optimisation technique (StentBoost®) in a dedicated bifurcation stent (the Tryton™). Cardiovasc Revasc Med. 2014;15:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Tanaka N, Pijls NH, Koolen JJ, Botman KJ, Michels HR, Brueren BR, Peels K, Shindo N, Yamashita J, Yamashina A. Assessment of optimum stent deployment by stent boost imaging: comparison with intravascular ultrasound. Heart Vessels. 2013;28:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Nazif TM, Weisz G, Moses JW. Clinical applications of a new Enhanced Stent Imaging technology. Catheter Cardiovasc Interv. 2013;82:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | McBeath KCC, Rathod KS, Cadd M, Beirne A, Guttmann O, Knight CJ, Amersey R, Bourantas CV, Wragg A, Smith EJ, Baumbach A, Mathur A, Jones DA. Use of enhanced stent visualisation compared to angiography alone to guide percutaneous coronary intervention. Int J Cardiol. 2020;321:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Biscaglia S, Tumscitz C, Tebaldi M, Andrenacci E, Pavasini R, Campo G, Ferrari R. Enhanced stent visualization systems during PCI: A case series and review of literature. J Cardiol Cases. 2015;12:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Florent R, Lucile Nosjean L, Pierre Lelong N, Rongen PMJ. Medical viewing system and method for enhancing structures in noisy images. US Pat. 2008;2:7415169. |
| 83. | Close RA, Whiting JS. Decomposition of coronary angiograms into non-rigid moving layers. Med Imag. 1999;3661:1515-1520. [DOI] [Full Text] |
| 84. | Rogers RK, Michaels AD. Enhanced x-ray visualization of coronary stents: clinical aspects. Cardiol Clin. 2009;27:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Córdova J, Aleong G, Colmenarez H, Cruz A, Canales E, Jimenez-Quevedo P, Hernández R, Alfonso F, Macaya C, Bañuelos C, den Hartog W, Escaned J. Digital enhancement of stent images in primary and secondary percutaneous coronary revascularisation. EuroIntervention. 2009;5 Suppl D:D101-D106. [PubMed] |
| 86. | Mutha V, Asrar Ul Haq M, Sharma N, den Hartog WF, van Gaal WJ. Usefulness of enhanced stent visualization imaging technique in simple and complex PCI cases. J Invasive Cardiol. 2014;26:552-557. [PubMed] |
| 87. | Cura F, Albertal M, Candiello A, Nau G, Bonvini V, Tricherri H, Padilla LT, Belardi JA. StentBoost Visualization for the Evaluation of Coronary Stent Expansion During Percutaneous Coronary Interventions. Cardiol Ther. 2013;2:171-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Zhang J, Duan Y, Jin Z, Wei Y, Yang S, Luo J, Ma D, Jing L, Liu H. Stent boost subtract imaging for the assessment of optimal stent deployment in coronary ostial lesion intervention: comparison with intravascular ultrasound. Int Heart J. 2015;56:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Jin Z, Yang S, Jing L, Liu H. Impact of StentBoost subtract imaging on patient radiation exposure during percutaneous coronary intervention. Int J Cardiovasc Imaging. 2013;29:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Bom N, Lancée CT, Van Egmond FC. An ultrasonic intracardiac scanner. Ultrasonics. 1972;10:72-76. [PubMed] [DOI] [Full Text] |
| 91. | Yock PG, Fitzgerald PJ, Linker DT, Angelsen BA. Intravascular ultrasound guidance for catheter-based coronary interventions. J Am Coll Cardiol. 1991;17:39B-45B. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 92] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 92. | Garcìa-Garcìa HM, Gogas BD, Serruys PW, Bruining N. IVUS-based imaging modalities for tissue characterization: similarities and differences. Int J Cardiovasc Imaging. 2011;27:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 93. | Yock PG, Linker DT. Intravascular ultrasound. Looking below the surface of vascular disease. Circulation. 1990;81:1715-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Sanidas EA, Carlier SG. Clinical Utility of Intravascular Ultrasound, In: Balocco S. Intravascular Ultrasound From Acquisition to Advanced Quantitative Analysis. Elsevier, 2020. |
| 95. | Arbab-Zadeh A, DeMaria AN, Penny WF, Russo RJ, Kimura BJ, Bhargava V. Axial movement of the intravascular ultrasound probe during the cardiac cycle: implications for three-dimensional reconstruction and measurements of coronary dimensions. Am Heart J. 1999;138:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Fitzgerald PJ, Oshima A, Hayase M, Metz JA, Bailey SR, Baim DS, Cleman MW, Deutsch E, Diver DJ, Leon MB, Moses JW, Oesterle SN, Overlie PA, Pepine CJ, Safian RD, Shani J, Simonton CA, Smalling RW, Teirstein PS, Zidar JP, Yeung AC, Kuntz RE, Yock PG. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation. 2000;102:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 256] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 97. | Mudra H, di Mario C, de Jaegere P, Figulla HR, Macaya C, Zahn R, Wennerblom B, Rutsch W, Voudris V, Regar E, Henneke KH, Schächinger V, Zeiher A; OPTICUS (OPTimization with ICUS to reduce stent restenosis) Study Investigators. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS Study). Circulation. 2001;104:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 98. | Oemrawsingh PV, Mintz GS, Schalij MJ, Zwinderman AH, Jukema JW, van der Wall EE; TULIP Study. Thrombocyte activity evaluation and effects of Ultrasound guidance in Long Intracoronary stent Placement. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP Study). Circulation. 2003;107:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 99. | Russo RJ, Silva PD, Teirstein PS, Attubato MJ, Davidson CJ, DeFranco AC, Fitzgerald PJ, Goldberg SL, Hermiller JB, Leon MB, Ling FS, Lucisano JE, Schatz RA, Wong SC, Weissman NJ, Zientek DM; AVID Investigators. A randomized controlled trial of angiography vs intravascular ultrasound-directed bare-metal coronary stent placement (the AVID Trial). Circ Cardiovasc Interv. 2009;2:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 100. | Parise H, Maehara A, Stone GW, Leon MB, Mintz GS. Meta-analysis of randomized studies comparing intravascular ultrasound vs angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am J Cardiol. 2011;107:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 101. | Chieffo A, Latib A, Caussin C, Presbitero P, Galli S, Menozzi A, Varbella F, Mauri F, Valgimigli M, Arampatzis C, Sabate M, Erglis A, Reimers B, Airoldi F, Laine M, Palop RL, Mikhail G, Maccarthy P, Romeo F, Colombo A. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 102. | Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Brodie BR, Stuckey TD, Mazzaferri EL Jr, Xu K, Parise H, Mehran R, Mintz GS, Stone GW. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 312] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 103. | Jang JS, Song YJ, Kang W, Jin HY, Seo JS, Yang TH, Kim DK, Cho KI, Kim BH, Park YH, Je HG, Kim DS. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 104. | Zhang Y, Farooq V, Garcia-Garcia HM, Bourantas CV, Tian N, Dong S, Li M, Yang S, Serruys PW, Chen SL. Comparison of intravascular ultrasound vs angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 105. | Darmoch F, Alraies MC, Al-Khadra Y, Moussa Pacha H, Pinto DS, Osborn EA. Intravascular Ultrasound Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2020;9:e013678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 106. | Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, Tian N, Lin S, Lu Q, Wu X, Li Q, Liu Z, Chen Y, Qian X, Wang J, Chai D, Chen C, Li X, Gogas BD, Pan T, Shan S, Ye F, Chen SL. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J Am Coll Cardiol. 2018;72:3126-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 445] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 107. | Gao XF, Ge Z, Kong XQ, Kan J, Han L, Lu S, Tian NL, Lin S, Lu QH, Wang XY, Li QH, Liu ZZ, Chen Y, Qian XS, Wang J, Chai DY, Chen CH, Pan T, Ye F, Zhang JJ, Chen SL; ULTIMATE Investigators. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc Interv. 2021;14:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 108. | Park SJ, Kim YH, Park DW, Lee SW, Kim WJ, Suh J, Yun SC, Lee CW, Hong MK, Lee JH, Park SW; MAIN-COMPARE Investigators. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;2:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 109. | de la Torre Hernandez JM, Baz Alonso JA, Gómez Hospital JA, Alfonso Manterola F, Garcia Camarero T, Gimeno de Carlos F, Roura Ferrer G, Recalde AS, Martínez-Luengas IL, Gomez Lara J, Hernandez Hernandez F, Pérez-Vizcayno MJ, Cequier Fillat A, Perez de Prado A, Gonzalez-Trevilla AA, Jimenez Navarro MF, Mauri Ferre J, Fernandez Diaz JA, Pinar Bermudez E, Zueco Gil J; IVUS-TRONCO-ICP Spanish study. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease: pooled analysis at the patient-level of 4 registries. JACC Cardiovasc Interv. 2014;7:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 110. | Puri R, Kapadia SR, Nicholls SJ, Harvey JE, Kataoka Y, Tuzcu EM. Optimizing outcomes during left main percutaneous coronary intervention with intravascular ultrasound and fractional flow reserve: the current state of evidence. JACC Cardiovasc Interv. 2012;5:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 111. | Kim JS, Hong MK, Ko YG, Choi D, Yoon JH, Choi SH, Hahn JY, Gwon HC, Jeong MH, Kim HS, Seong IW, Yang JY, Rha SW, Tahk SJ, Seung KB, Park SJ, Jang Y. Impact of intravascular ultrasound guidance on long-term clinical outcomes in patients treated with drug-eluting stent for bifurcation lesions: data from a Korean multicenter bifurcation registry. Am Heart J. 2011;161:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 112. | Fujii K, Ochiai M, Mintz GS, Kan Y, Awano K, Masutani M, Ashida K, Ohyanagi M, Ichikawa S, Ura S, Araki H, Stone GW, Moses JW, Leon MB, Carlier SG. Procedural implications of intravascular ultrasound morphologic features of chronic total coronary occlusions. Am J Cardiol. 2006;97:1455-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Song L, Maehara A, Finn MT, Kalra S, Moses JW, Parikh MA, Kirtane AJ, Collins MB, Nazif TM, Fall KN, Hatem R, Liao M, Kim T, Green P, Ali ZA, Batres C, Leon MB, Mintz GS, Karmpaliotis D. Intravascular Ultrasound Analysis of Intraplaque Versus Subintimal Tracking in Percutaneous Intervention for Coronary Chronic Total Occlusions and Association With Procedural Outcomes. JACC Cardiovasc Interv. 2017;10:1011-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 114. | Attizzani GF, Patrício L, Bezerra HG. Optical coherence tomography assessment of calcified plaque modification after rotational atherectomy. Catheter Cardiovasc Interv. 2013;81:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Nguyen P, Seto A. Contemporary practices using intravascular imaging guidance with IVUS or OCT to optimize percutaneous coronary intervention. Expert Rev Cardiovasc Ther. 2020;18:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 116. | Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Halpern EF, Bouma BE. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 477] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 117. | Xu C, Schmitt JM, Carlier SG, Virmani R. Characterization of atherosclerosis plaques by measuring both backscattering and attenuation coefficients in optical coherence tomography. J Biomed Opt. 2008;13:034003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 118. | Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, Burzotta F, Trani C, Porto I, Ramazzotti V, Imola F, Manzoli A, Materia L, Cremonesi A, Albertucci M. Angiography alone vs angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 119. | Sheth TN, Kajander OA, Lavi S, Bhindi R, Cantor WJ, Cheema AN, Stankovic G, Niemelä K, Natarajan MK, Shestakovska O, Tittarelli R, Meeks B, Jolly SS. Optical Coherence Tomography-Guided Percutaneous Coronary Intervention in ST-Segment-Elevation Myocardial Infarction: A Prospective Propensity-Matched Cohort of the Thrombectomy Versus Percutaneous Coronary Intervention Alone Trial. Circ Cardiovasc Interv. 2016;9:e003414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 120. | Iannaccone M, D'Ascenzo F, Frangieh AH, Niccoli G, Ugo F, Boccuzzi G, Bertaina M, Mancone M, Montefusco A, Amabile N, Sardella G, Motreff P, Toutouzas K, Colombo F, Garbo R, Biondi-Zoccai G, Tamburino C, Omedè P, Moretti C, D'amico M, Souteyrand G, Meieir P, Lüscher TF, Gaita F, Templin C. Impact of an optical coherence tomography guided approach in acute coronary syndromes: A propensity matched analysis from the international FORMIDABLE-CARDIOGROUP IV and USZ registry. Catheter Cardiovasc Interv. 2017;90:E46-E52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 121. | Antonsen L, Thayssen P, Maehara A, Hansen HS, Junker A, Veien KT, Hansen KN, Hougaard M, Mintz GS, Jensen LO. Optical Coherence Tomography Guided Percutaneous Coronary Intervention With Nobori Stent Implantation in Patients With Non-ST-Segment-Elevation Myocardial Infarction (OCTACS) Trial: Difference in Strut Coverage and Dynamic Malapposition Patterns at 6 Months. Circ Cardiovasc Interv. 2015;8:e002446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 122. | Meneveau N, Souteyrand G, Motreff P, Caussin C, Amabile N, Ohlmann P, Morel O, Lefrançois Y, Descotes-Genon V, Silvain J, Braik N, Chopard R, Chatot M, Ecarnot F, Tauzin H, Van Belle E, Belle L, Schiele F. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation. 2016;134:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 250] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 123. | Lee SY, Kim JS, Yoon HJ, Hur SH, Lee SG, Kim JW, Hong YJ, Kim KS, Choi SY, Shin DH, Nam CM, Kim BK, Ko YG, Choi D, Jang Y, Hong MK. Early Strut Coverage in Patients Receiving Drug-Eluting Stents and its Implications for Dual Antiplatelet Therapy: A Randomized Trial. JACC Cardiovasc Imaging. 2018;11:1810-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 124. | Mehnert A, Glaesmer H. [Patient-centered care--daily routine or future vision? Psychother Psychosom Med Psychol. 2014;64:339-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 125. | Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv. 2009;2:1035-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 467] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 126. | Maehara A, Ben-Yehuda O, Ali Z, Wijns W, Bezerra HG, Shite J, Généreux P, Nichols M, Jenkins P, Witzenbichler B, Mintz GS, Stone GW. Comparison of Stent Expansion Guided by Optical Coherence Tomography Versus Intravascular Ultrasound: The ILUMIEN II Study (Observational Study of Optical Coherence Tomography [OCT] in Patients Undergoing Fractional Flow Reserve [FFR] and Percutaneous Coronary Intervention). JACC Cardiovasc Interv. 2015;8:1704-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 127. | Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, Guagliumi G, Meraj PM, Alfonso F, Samady H, Akasaka T, Carlson EB, Leesar MA, Matsumura M, Ozan MO, Mintz GS, Ben-Yehuda O, Stone GW; ILUMIEN III: OPTIMIZE PCI Investigators. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 489] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 128. | Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, Shite J, Fusazaki T, Otake H, Kozuma K, Ioji T, Kaneda H, Serikawa T, Kataoka T, Okada H, Akasaka T; OPINION Investigators. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38:3139-3147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 129. | Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2722] [Cited by in RCA: 4508] [Article Influence: 901.6] [Reference Citation Analysis (0)] |
| 130. | Ono M, Kawashima H, Hara H, Gao C, Wang R, Kogame N, Takahashi K, Chichareon P, Modolo R, Tomaniak M, Wykrzykowska JJ, Piek JJ, Mori I, Courtney BK, Wijns W, Sharif F, Bourantas C, Onuma Y, Serruys PW. Advances in IVUS/OCT and Future Clinical Perspective of Novel Hybrid Catheter System in Coronary Imaging. Front Cardiovasc Med. 2020;7:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 131. | Habara M, Nasu K, Terashima M, Kaneda H, Yokota D, Ko E, Ito T, Kurita T, Tanaka N, Kimura M, Kinoshita Y, Tsuchikane E, Asakura K, Asakura Y, Katoh O, Suzuki T. Impact of frequency-domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance. Circ Cardiovasc Interv. 2012;5:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 132. | Carlier S, Didday R, Slots T, Kayaert P, Sonck J, El-Mourad M, Preumont N, Schoors D, Van Camp G. A new method for real-time co-registration of 3D coronary angiography and intravascular ultrasound or optical coherence tomography. Cardiovasc Revasc Med. 2014;15:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 133. | Li J, Ma T, Mohar D, Steward E, Yu M, Piao Z, He Y, Shung KK, Zhou Q, Patel PM, Chen Z. Ultrafast optical-ultrasonic system and miniaturized catheter for imaging and characterizing atherosclerotic plaques in vivo. Sci Rep. 2015;5:18406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 134. | Rahim HM, Shlofmitz E, Gore A, Hakemi E, Mintz GS, Maehara A, Jeremias A, Ben-Yehuda O, Stone GW, Shlofmitz RA, Ali ZA. IVUS- Versus OCT-Guided Coronary Stent Implantation: a Comparison of Intravascular Imaging for Stent Optimization. Curr Cardiovasc Imaging Rep. 2018;11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 135. | Dębski M, Kruk M, Bujak S, Dzielińska Z, Demkow M, Kępka C. Coronary computed tomography angiography equals invasive angiography for the prediction of coronary revascularization. Postepy Kardiol Interwencyjnej. 2019;15:308-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 136. | Abdelrahman KM, Chen MY, Dey AK, Virmani R, Finn AV, Khamis RY, Choi AD, Min JK, Williams MC, Buckler AJ, Taylor CA, Rogers C, Samady H, Antoniades C, Shaw LJ, Budoff MJ, Hoffmann U, Blankstein R, Narula J, Mehta NN. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:1226-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 137. | Chang SM, Bhatti S, Nabi F. Coronary computed tomography angiography. Curr Opin Cardiol. 2011;26:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 138. | Carbone I, Francone M, Algeri E, Granatelli A, Napoli A, Kirchin MA, Catalano C, Passariello R. Non-invasive evaluation of coronary artery stent patency with retrospectively ECG-gated 64-slice CT angiography. Eur Radiol. 2008;18:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 139. | SCOT-HEART investigators. . CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 750] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 140. | Ebersberger U, Tricarico F, Schoepf UJ, Blanke P, Spears JR, Rowe GW, Halligan WT, Henzler T, Bamberg F, Leber AW, Hoffmann E, Apfaltrer P. CT evaluation of coronary artery stents with iterative image reconstruction: improvements in image quality and potential for radiation dose reduction. Eur Radiol. 2013;23:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 141. | Sun Z, Almutairi AM. Diagnostic accuracy of 64 multislice CT angiography in the assessment of coronary in-stent restenosis: a meta-analysis. Eur J Radiol. 2010;73:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 142. | Rixe J, Achenbach S, Ropers D, Baum U, Kuettner A, Ropers U, Bautz W, Daniel WG, Anders K. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur Heart J. 2006;27:2567-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 143. | Chung SH, Kim YJ, Hur J, Lee HJ, Choe KO, Kim TH, Choi BW. Evaluation of coronary artery in-stent restenosis by 64-section computed tomography: factors affecting assessment and accurate diagnosis. J Thorac Imaging. 2010;25:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 144. | Sheth T, Dodd JD, Hoffmann U, Abbara S, Finn A, Gold HK, Brady TJ, Cury RC. Coronary stent assessability by 64 slice multi-detector computed tomography. Catheter Cardiovasc Interv. 2007;69:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 145. | Feren I, Mester A, Chiu M, Benedek A, Raiu M, Hodas R, Benedek I. CTA Evaluation of Bioresorbable Scaffolds vs Metallic Coronary Stents – a Feasibility Study. J Interdiscip Med. 2018;3:152-159. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 146. | Salinas P, Pozo-Osinalde E, Cerrato E, Garcia-Blas S, Vaudano GP, Parrilla C, Sanchis J, Varbella F, Escaned J. Cardiac computed tomography angiography tomography follow-up of resorbable magnesium scaffolds. Cardiovasc Revasc Med. 2020;29:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 147. | Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguadé-Bruix S, Pizzi MN, Todiere G, Gimelli A, Schroeder S, Drosch T, Poddighe R, Casolo G, Anagnostopoulos C, Pugliese F, Rouzet F, Le Guludec D, Cappelli F, Valente S, Gensini GF, Zawaideh C, Capitanio S, Sambuceti G, Marsico F, Perrone Filardi P, Fernández-Golfín C, Rincón LM, Graner FP, de Graaf MA, Fiechter M, Stehli J, Gaemperli O, Reyes E, Nkomo S, Mäki M, Lorenzoni V, Turchetti G, Carpeggiani C, Marinelli M, Puzzuoli S, Mangione M, Marcheschi P, Mariani F, Giannessi D, Nekolla S, Lombardi M, Sicari R, Scholte AJ, Zamorano JL, Kaufmann PA, Underwood SR, Knuuti J; EVINCI Study Investigators. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging. 2015;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 270] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 148. | Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, Huang M, Pencina M, Mark DB, Heitner JF, Fordyce CB, Pellikka PA, Tardif JC, Budoff M, Nahhas G, Chow B, Kosinski AS, Lee KL, Douglas PS; PROMISE Investigators. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation. 2017;135:2320-2332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 357] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 149. | Bittencourt MS, Hulten EA, Murthy VL, Cheezum M, Rochitte CE, Di Carli MF, Blankstein R. Clinical Outcomes After Evaluation of Stable Chest Pain by Coronary Computed Tomographic Angiography Versus Usual Care: A Meta-Analysis. Circ Cardiovasc Imaging. 2016;9:e004419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 150. | Stocker TJ, Deseive S, Leipsic J, Hadamitzky M, Chen MY, Rubinshtein R, Heckner M, Bax JJ, Fang XM, Grove EL, Lesser J, Maurovich-Horvat P, Otton J, Shin S, Pontone G, Marques H, Chow B, Nomura CH, Tabbalat R, Schmermund A, Kang JW, Naoum C, Atkins M, Martuscelli E, Massberg S, Hausleiter J; PROTECTION VI investigators. Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur Heart J. 2018;39:3715-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 151. | Velagapudi P, Abbott JD, Mamas M, Blankstein R, Chatzizisis YS, Brilakis ES, Jaffer FA. Role of Coronary Computed Tomography Angiography in Percutaneous Coronary Intervention of Chronic Total Occlusions. Curr Cardiovasc Imaging Rep. 2020;13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 152. | Kubo T, Akasaka T, Shite J, Suzuki T, Uemura S, Yu B, Kozuma K, Kitabata H, Shinke T, Habara M, Saito Y, Hou J, Suzuki N, Zhang S. OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. JACC Cardiovasc Imaging. 2013;6:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 153. | Eng MH, Klein AP, Wink O, Hansgen A, Carroll JD, Garcia JA. Enhanced stent visualization: a case series demonstrating practical applications during PCI. Int J Cardiol. 2010;141:e8-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 154. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9319] [Article Influence: 1863.8] [Reference Citation Analysis (0)] |
| 155. | Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-Guided Versus OCT-Guided Coronary Stent Implantation: A Critical Appraisal. JACC Cardiovasc Imaging. 2017;10:1487-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 156. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 4539] [Article Influence: 907.8] [Reference Citation Analysis (0)] |
| 157. | Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2569] [Cited by in RCA: 3162] [Article Influence: 790.5] [Reference Citation Analysis (0)] |
| 158. | Sun Z, Davidson R, Lin CH. Multi-detector row CT angiography in the assessment of coronary in-stent restenosis: a systematic review. Eur J Radiol. 2009;69:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 159. | Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Chiswell K, Cyr D, Wilk A, Wang F, Rogers C, De Bruyne B; PLATFORM Investigators. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J. 2015;36:3359-3367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 452] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 160. | von Zur Mühlen C, Reiss S, Krafft AJ, Besch L, Menza M, Zehender M, Heidt T, Maier A, Pfannebecker T, Zirlik A, Reinöhl J, Stachon P, Hilgendorf I, Wolf D, Diehl P, Wengenmayer T, Ahrens I, Bode C, Bock M. Coronary magnetic resonance imaging after routine implantation of bioresorbable vascular scaffolds allows non-invasive evaluation of vascular patency. PLoS One. 2018;13:e0191413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 161. | Reiber JH, Tu S, Tuinenburg JC, Koning G, Janssen JP, Dijkstra J. QCA, IVUS and OCT in interventional cardiology in 2011. Cardiovasc Diagn Ther. 2011;1:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |