Published online Sep 26, 2019. doi: 10.4330/wjc.v11.i9.213
Peer-review started: March 28, 2019
First decision: August 2, 2019
Revised: August 14, 2019
Accepted: August 21, 2019
Article in press: August 21, 2019
Published online: September 26, 2019
Processing time: 183 Days and 22.9 Hours
Takotsubo syndrome is a wide spectrum disease with a dramatic clinical presentation mimicking acute coronary syndrome albeit without obstructive coronary disease and typically manifests in the backdrop of intense emotional or physical trigger. Pathophysiology is incompletely understood with multifactorial mechanistic pathways circling around a heart-brain-endocrine axis. Several anatomic and phenotypic variants exist with varied clinical manifestations. The aftermath of Takotsubo syndrome is not always benign and both short- and long-term complications can occur which may impact its prognosis. Several gaps in knowledge exist providing an impetus for tremendous future research opportunities.
Core tip: Further research is necessary in order to better understand the underlying triggers and pathophysiologic principles of Takotsubo syndrome which will help optimize both in-hospital acute and long-term management pathways.
- Citation: Khalid N, Sareen P, Ahmad SA, Chhabra L. Takotsubo syndrome: The past, the present and the future. World J Cardiol 2019; 11(9): 213-216
- URL: https://www.wjgnet.com/1949-8462/full/v11/i9/213.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i9.213
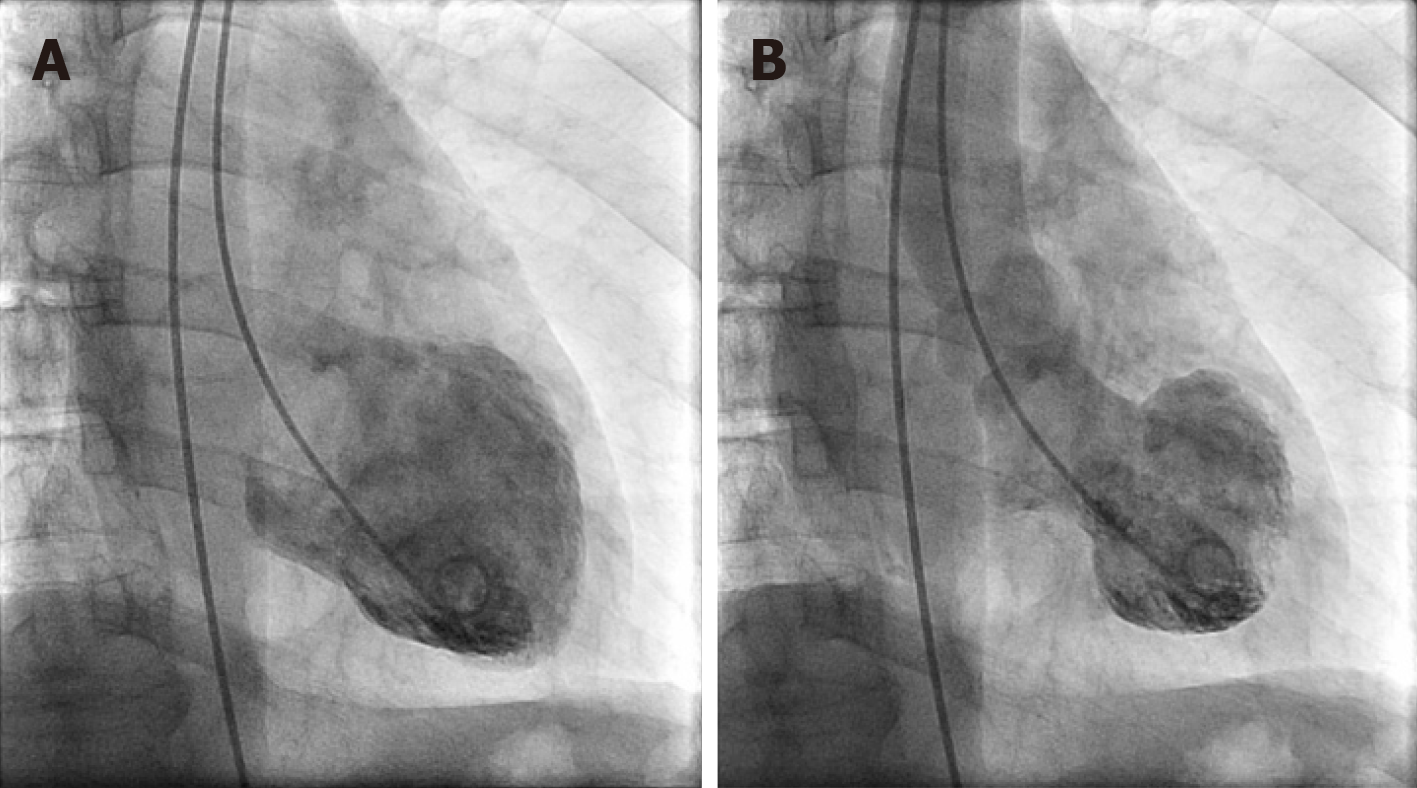
Chest pain and dyspnea are ubiquitous and common clinical symptoms for patients presenting to the Emergency Department and majority of these patients are initially labelled with the diagnosis of coronary artery disease, heart failure or pulmonary disease. Hitherto less well known clinical entity - Takotsubo syndrome (TTS), first described in Japan, is becoming increasingly recognized in the Western world and shares many clinical features indistinguishable from acute coronary syndrome (ACS) or acute heart failure. TTS is a heterogenous entity characterized by transient wall motion abnormalities (WMA) of the left ventricle typically without angiographically significant epicardial coronary artery disease or acute plaque rupture, manifesting with chest pain, dynamic reversible ST segment and T wave abnormalities, and modest elevation of cardiac biomarkers disproportionate to the extent of WMA[1-4] - thus mimicking ACS in many ways (Figure 1). It has also been described as an acute heart failure syndrome characterized by left ventricular systolic and diastolic function, myocardial strain abnormalities, and significant elevation of beta natriuretic peptide. Over the past three decades, our understanding of the pathophysiologic mechanisms of this disease has improved; thanks to the widespread availability of urgent coronary angiography and technological advances in the imaging arena such as modern echocardiography, speckle strain imaging, cardiac magnetic resonance imaging, single-photon emission computed tomography and positron emission tomography, however, several knowledge gaps still remain. What has become clear now is that TTS is much more common than previously anticipated. It predominantly affects post-menopausal women[5] and portends significant morbidity and mortality approaching that of ACS, although still underappreciated. A hallmark feature of TTS is its association with a preceding negative stressful trigger (emotional or physical) - the so-called “broken heart syndrome” or “stress-induced cardiomyopathy”. However, in some cases no stressors may be identified and in few the trigger could even be a positive emotion - the soi-disant “happy heart syndrome”.
Electrocardiographic manifestations of TC patients progress through similar evolutionary pattern as the ECG staging in pericarditis[6]. Stage 1 demonstrates ST segment elevation, followed by normalization of ST segment in stage 2. T-wave inversions develop in stage 3, with subsequent normalization of T waves or rarely persistence of T-wave inversions noted in stage 4[6]. Certainly, an overlap between these changes may exist, whereas some patients may not demonstrate all evolutionary stage changes. Several anatomic and phenotypic variants of TTS have been described with varied clinical manifestations. The most common form is the typical apical ballooning which occurs in 75%-80% of patients; it's easily recognized and is associated with typical complications including thrombus formation due to apical akinesis and left ventricular outflow tract obstruction due to basal hyperkinesis[7]. Other less common types include midventricular, basal or inverted, biventricular, right ventricular, or focal dysfunction[7]. Numerous putative mechanisms have been proposed for development of TTS - these include coronary vasospasm, microvascular spasm or dysfunction [as demonstrated by abnormal Thrombolysis in Myocardial Infarction (TIMI) Frame Count or TIMI perfusion grade], neurogenic stunned myocardium with underlying enhanced sympathetic activity, elevated levels of circulating plasma catecholamines and its metabolites, inflammation, estrogen deficiency, and spontaneously aborted myocardial infarction[8-12]. A possible autoimmune and/or autoinflammatory component has also been hypothesized for TTS, akin to myocardial infarction, thereby providing an impetus to explore long-term immunological effects of TTS[13]. Associated comorbidities and risk factor profile is similar to coronary artery disease although some reports suggest that diabetes mellitus is noted less frequently in patients with TTS suggesting a possible protective mechanism[14,15].
The most commonly applied diagnostic criteria include the Revised Mayo Clinic Criteria[16], International Takotsubo Diagnostic Criteria (InterTAK)[17], and the Heart Failure Association-European Society of Cardiology Criteria[18]. Transthoracic echocardiography with color and tissue Doppler is the preferred noninvasive imaging modality for patients suspected of TTS but most of these patients undergo emergent coronary angiography to rule out ACS. Correct diagnosis is critical since TTS is not a benign condition and is associated with potentially serious short- and long-term complications such as ventricular arrhythmias, dynamic left ventricular outflow tract obstruction, pump failure with cardiogenic shock, thromboembolic sequelae, intramyocardial hemorrhage and rupture, pulmonary edema and others[7]. In-hospital mortality remains high (about 5%) and acute management focuses on the specific complications[7]. Physical triggers, acute neurologic or psychiatric illnesses, elevated cardiac biomarkers (troponin), and a low left ventricular ejection fraction on admission were independent predictors for in-hospital complications[19]. Currently no evidence exists for long-term management of TTS. Nonetheless, beta-blockers are advocated especially in patients with increased sympathetic tone[7]. Angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers have demonstrated a marginal benefit at 1-year[19]. Recurrence rate is reported at 1.8% where the trigger is typically different, and the recurrence can occur at any time[19].
TTS presents with symptoms similar to ACS characterized by transient left ventricular dysfunction typically manifesting in the setting of stressful triggers. Dynamic reversible ST segment and T wave abnormalities, modest elevation of troponin, significant elevation of beta natriuretic peptide, several anatomic variants, potentially serious short- and long-term complications, prognosis similar to ACS are some important features of this entity. Our current understanding of the pathophysiologic underpinnings has improved compared to its first description in 1990 yet there are several knowledge gaps that need to be addressed. Future potential research opportunities include exploring reasons for gender predilection, triggering factors and their role in the development and prognosis of TTS, different phenotypes of TTS, intracellular and intercellular mechanisms involved, genetic predisposition, exact pathophysiologic mechanism, specific acute- and long-term management and the role of animal models. Future larger randomized controlled studies will help resolve these queries.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kharlamov AN, Petix NR, Teragawa H, Ueda H S-Editor: Ma RY L-Editor: A E-Editor: Qi LL
| 1. | Mejía-Rentería HD, Núñez-Gil IJ. Takotsubo syndrome: Advances in the understanding and management of an enigmatic stress cardiomyopathy. World J Cardiol. 2016;8:413-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: Pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6:602-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (3)] |
| 3. | Khalid N, Iqbal I, Coram R, Raza T, Fahsah I, Ikram S. Thrombolysis In Myocardial Infarction Frame Count in Takotsubo Cardiomyopathy. Int J Cardiol. 2015;191:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Khalid N, Chhabra L. Takotsubo cardiomyopathy and microcirculatory dysfunction. Nat Rev Cardiol. 2015;12:497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Khalid N, Ahmad SA, Shlofmitz E, Chhabra L. Racial and gender disparities among patients with Takotsubo syndrome. Clin Cardiol. 2019;42:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Chhabra L, Khalid N, Sareen P. Extremely Low Prevalence of Takotsubo Cardiomyopathy and Transient Cardiac Dysfunction in Stroke Patients With T-wave Abnormalities. Am J Cardiol. 2019;123:1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:1955-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 382] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 8. | Gopalakrishnan P, Zaidi R, Sardar MR. Takotsubo cardiomyopathy: Pathophysiology and role of cardiac biomarkers in differential diagnosis. World J Cardiol. 2017;9:723-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Khalid N, Ahmad SA, Umer A. Mechanisms of Takotsubo cardiomyopathy; role of microcirculatory dysfunction. Int Cardiovas Forum J. 2016;5:30-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Chhabra L, Khalid N, Kluger J, Spodick DH. Lupus myopericarditis as a preceding stressor for takotsubo cardiomyopathy. Proc (Bayl Univ Med Cent). 2014;27:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Khalid N, Ahmad SA, Umer A, Chhabra L. Takotsubo cardiomyopathy and myopericarditis: Unraveling the inflammatory hypothesis. Int J Cardiol. 2015;196:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Khalid N, Chhabra L. Takotsubo Cardiomyopathy and Viral Myopericarditis: An Association Which Should be Considered in the Differential Diagnosis. Angiology. 2016;67:398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Sattler S, Couch LS, Harding SE. Takotsubo Syndrome: Latest Addition to the Expanding Family of Immune-Mediated Diseases? JACC Basic Transl Sci. 2018;3:779-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Khalid N, Ahmad SA, Umer A, Chhabra L. Role of Microcirculatory Disturbances and Diabetic Autonomic Neuropathy in Takotsubo Cardiomyopathy. Crit Care Med. 2015;43:e527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Khalid N, Chhabra L, Ahmad SA, Sareen P, Spodick DH. Autonomic Dysfunction and Takotsubo Cardiomyopathy. Am J Med. 2015;128:e45-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1020] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 17. | Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J. 2018;39:2032-2046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 1021] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 18. | Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 799] [Article Influence: 79.9] [Reference Citation Analysis (1)] |
| 19. | Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1401] [Cited by in RCA: 1718] [Article Influence: 171.8] [Reference Citation Analysis (1)] |