Published online Jun 5, 2025. doi: 10.4331/wjbc.v16.i2.106850
Revised: March 31, 2025
Accepted: April 9, 2025
Published online: June 5, 2025
Processing time: 82 Days and 9.8 Hours
Parkinson’s disease (PD) is a progressive neurodegenerative disorder marked by the loss of dopaminergic neurons in the substantia nigra that leads to reduced dopamine levels and impaired motor function. Current treatments only provide temporary symptom relief without addressing the underlying neuronal loss. A promising new approach for treating PD is stem cell therapy, particularly induced pluripotent stem cells and human pluripotent stem cells. They have the ability to differentiate into various neural cells, offering potential for neuronal replacement and restoration of brain function. Induced pluripotent stem cells are derived from reprogramming adult cells and present advantages such as genetic compatibility and reduced immune rejection, overcoming ethical concerns associated with embryonic stem cells. Preclinical studies show promising results, demonstrating that stem cells can differentiate into dopaminergic neurons and improve motor function in animal models. These advancements pave the way for clinical trials and potential long-term solutions for patients with PD. This review highlighted the significance of stem cell therapy in neuroregeneration and addressed pre
Core Tip: Parkinson’s disease (PD) is a neurodegenerative disorder caused by the loss of dopamine neurons in the substantia nigra. Currently, there is no treatment for PD, while stem cell therapy represents a new therapeutic approach to restore lost neural function. This manuscript focused on the potential of stem cell therapy, particularly induced pluripotent stem cells and human pluripotent stem cells, in treating PD, offering potential for neuronal replacement and restoration of brain function. Induced pluripotent stem cells are derived from reprogramming adult cells, which are genetically compatible and cause reduced immune rejection, overcoming ethical concerns associated with embryonic stem cells.
- Citation: Mokhtari YG, Varnava I, Kyrgiannis K, Ampatsidou V, Giakoumettis D. Stem cell therapy for Parkinson’s disease: A new hope for neural regeneration. World J Biol Chem 2025; 16(2): 106850
- URL: https://www.wjgnet.com/1949-8454/full/v16/i2/106850.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v16.i2.106850
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that primarily affects motor function. It is characterized by the loss of dopaminergic neurons in the substantia nigra of the brain, a region crucial for movement control. This neuronal loss leads to a significant reduction in dopamine levels, causing the main symptoms of PD, such as tremor, bradykinesia (slowness of movement), rigidity, and postural instability[1]. Although current treatments, such as levodopa and dopaminergic agonists, can temporarily relieve symptoms, they do not provide a long-term solution or cure for the disease. This necessity drives the search for new therapeutic approaches to address underlying neuronal loss and offer long-term patient improvement.
One of the most promising approaches for treating PD is the use of stem cells; in this case a graft containing dopamine-producing cells transplanted into the striatum so that dopamine is released directly in a manner mimicking the normal physiology of the brain[2]. Stem cells have the ability to differentiate into various cell types, including neural cells, and can replace damaged or lost cells in the brain[3]. This therapeutic approach offers a new perspective for restoring neu
Furthermore, recent advancements in induced pluripotent stem cell (iPSC) technology provide a promising source for generating neural cells that can be used in cell replacement therapies[4]. iPSCs are created by reprogramming adult cells into a pluripotent state, allowing for the unlimited production of neural cells that are genetically compatible with the patient. This reduces the risk of immune rejection and offers a safer and more effective therapeutic option. Research in this field has already shown promising results in preclinical models of PD. Studies have demonstrated that stem cells can differentiate into dopaminergic neurons and restore motor function in animal models[5]. These preclinical successes pave the way for clinical trials in humans, with the hope that stem cell therapies can provide a real cure for patients with PD in the future. The potential to restore lost neural cells and improve brain function is exciting. Stem cells may offer a revolutionary solution for a disease that currently remains incurable.
PD is a neurodegenerative disorder caused by the loss of dopamine neurons, while stem cell therapy represents a new therapeutic approach to restore lost neural function. This review highlighted recent advances and discussed significant preclinical studies that demonstrate a recovery in PD models. By addressing challenges like long-term safety and ethical considerations, stem cell therapies show potential in revolutionizing PD treatment and improving patient outcomes.
Stem cells are cells with the ability to self-renew and differentiate into various cell types. This characteristic makes them valuable for regenerative medicine and research as they can be used to replace damaged or diseased tissues.
Stem cells also have the ability to directly affect their environment by demonstrating autocrine and paracrine effects by secreting various molecules such as growth factors or cytokines. These effects achieve the self-renewal of stem cells and the maintenance of their differentiation within the tissue and promote processes such as angiogenesis and inflammation reduction[6]. There are findings that suggest that the therapeutic benefits of stem cells are mediated by structures called extracellular vesicles formed by direct budding of the plasma membrane or by multivesicular bodies in the endolysosomal pathway that fuse with the stem cell membrane, which is filled with various biomolecules such as proteins and RNA transcripts that travel to neighboring cells causing changes in their phenotype[7].
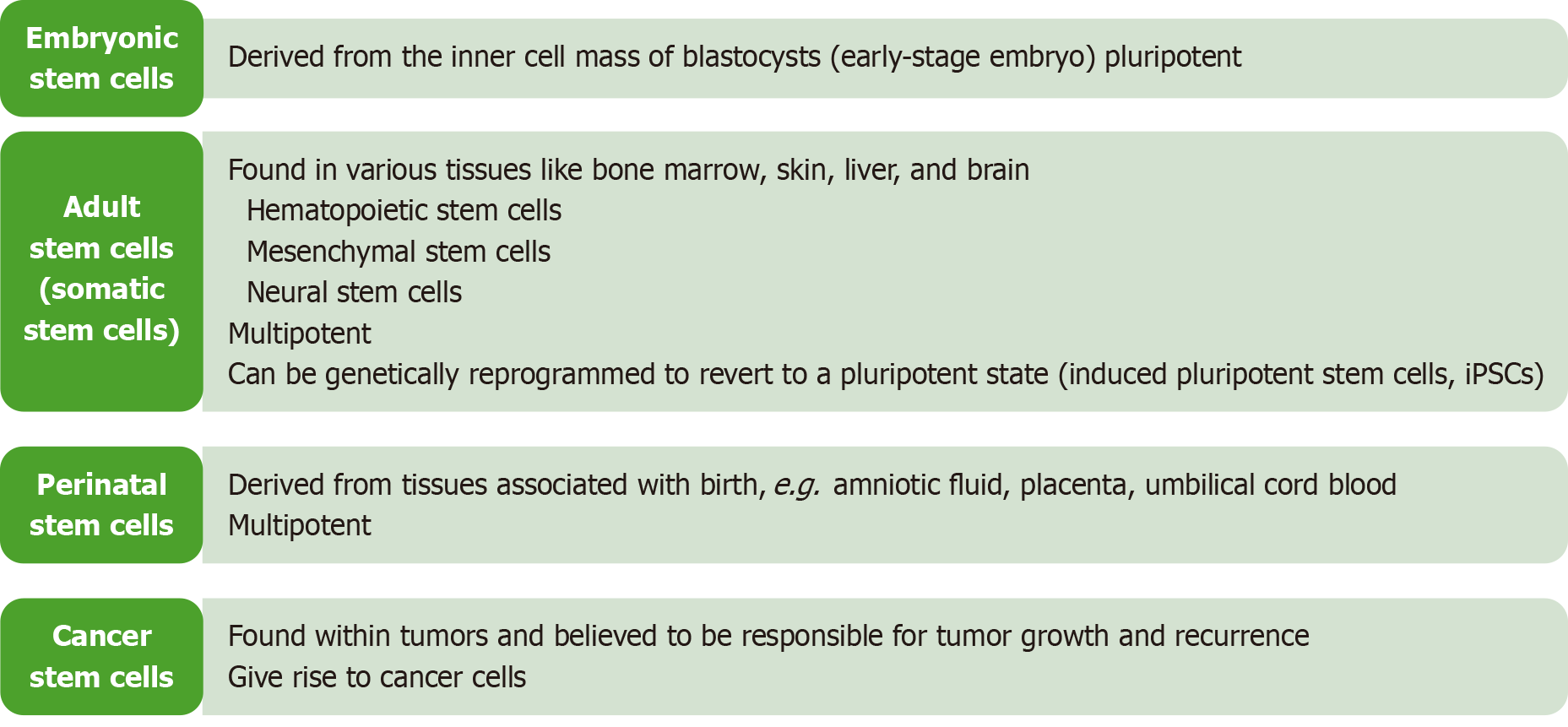
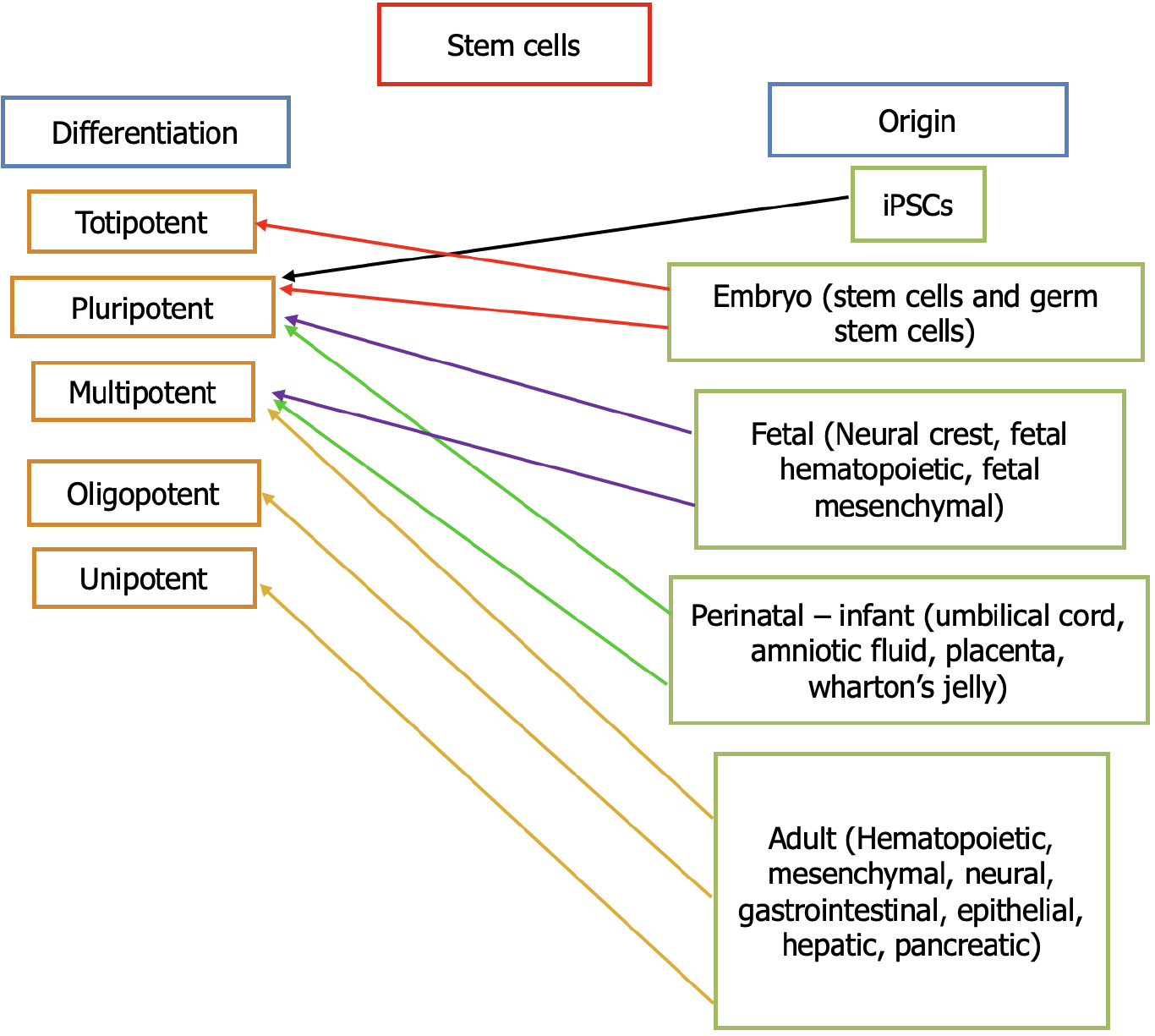
Stem cells are categorized into two large families: (1) Embryonic stem cells (ESCs) derived from embryos; and (2) Adult stem cells, which are found in most body tissues albeit in smaller quantities and with limited differentiation potential[8]. There are also fetal and perinatal stem cells with multipotency and pluripotency[9] (Figure 1). This means that not all stem cells have the same potential to give rise to similar cell types. Therefore, in terms of potency, they are divided into multipotent and pluripotent (Figure 2). The former refers to the stem cells that can differentiate to make at least two different lineages, usually from the same embryonic germ layer. On the other hand, pluripotent stem cells can make multiple lineages from all three embryonic germ layers. The study and use of stem cells have opened new horizons in medical research, with promising prospects for treating diseases such as diabetes, PD, and cardiovascular disorders.
Human pluripotent stem cells (hPSCs) encompass two main types: ESCs and iPSCs. The human ESCs (hESCs) originate from the inner cell mass of the blastocyst, a stage of the preimplantation embryo[10], whereas iPSCs are generated by reprogramming adult cells to revert to an embryonic-like state using specific genes[11]. Both hESCs and iPSCs possess the remarkable ability to differentiate into any cell type of the three primary germ layers (endoderm, mesoderm, ectoderm), offering immense potential for applications in regenerative medicine, disease modeling, and drug discovery[12,13].
One of the significant advantages of iPSCs is their capacity to be personalized by deriving them from a patient’s own cells. This feature reduces the risk of immune rejection when used for therapeutic purposes and sidesteps ethical concerns associated with hESCs, which involve the destruction of embryos. They are particularly valuable in medical research and treatment due to their potential to regenerate damaged tissues and organs, model diseases for research purposes, and serve as platforms for testing new treatments[14]. The ability to derive iPSCs from a patient’s own cells further enhances their applicability in personalized medicine, underscoring their promise in advancing therapeutic approaches while addressing ethical considerations associated with traditional ESCs.
iPSCs are generated by reprogramming adult cells to revert to an embryonic-like pluripotent state. This groundbreaking technology was first developed by Takahashi and Yamanaka[15] in 2006. They demonstrated that introducing specific genes encoding transcription factors into adult cells can reprogram them to become pluripotent. Much like ESCs, iPSCs can differentiate into any cell type in the body, making them a powerful tool for regenerative medicine, disease modeling, and drug discovery[4,15].
One of the significant advantages of iPSCs is that they can be derived from a patient’s own cells, which minimizes the risk of immune rejection when used for therapeutic purposes. Another important feature is that iPSCs bypass the ethical concerns associated with using ESCs as they do not require the destruction of embryos[16]. It is important to note that there are reports about a possible higher incidence of tumorigenesis and genomic instability related to iPSCs when compared with hESCs due to the reprogramming process[17].
These concerns are challenged by the generation of iPSCs with a low incidence of DNA sequence variations by optimizing the reprogramming protocols (e.g., optimizing reprogramming factors by replacing transcription factors such as c-Myc with small molecules like C6FZ, implementing DNA-free strategies such as recombinant protein transductions and gene editing using nanomaterials like lipid nanoparticles for cell delivery[18] thus eliminating the use of viral vector-based gene transductions). Other techniques to optimize reprogramming protocols include gene editing with the CRISPR-Cas9 system or transcription activator-like effector nucleases to develop suicide safety switches using an anti-CD20 monoclonal antibody to eliminate aberrant iPSCs before transplantation.
The purity of iPSCs can be increased by using specific staining and markers like TRA-1–60 or SSEA4 antibodies for correct iPSCs selection[19]. Research involving iPSCs holds great promise for understanding complex diseases, developing personalized medicine approaches, and potentially offering cures for conditions such as PD, heart disease, and diabetes.
Multipotent stem cells are like pluripotent stem cells, but they are more restricted. They have the ability to become cells that belong to the same specific tissue. Multipotent stem cells are capable of differentiating into adipogenic, chondro
According to recent in vivo studies conducted over the last decade, several intriguing findings have emerged regarding the use of stem cells for PD therapy. Specifically, research has demonstrated promising outcomes in both hPSCs and iPSCs in various experimental models of the disease. In experiments with mice, as illustrated in the study with Xu et al[22] in 2022, hPSCs were used, and the severity of the damage was assessed through the amphetamine-induced rotation test. Meanwhile, research with laboratory animals (rats), as depicted in the study with Hiller et al[23] in 2022, was conducted through unilateral injections of 6-hydroxydopamine (6-OHDA) into the right medial forebrain bundle were followed by iPSC-mDA progenitor transplantation. Over time, these interventions demonstrated promising results in mitigating motor disturbances as evidenced by behavioral analyses.
Similarly, iPSCs have shown potential in alleviating PD symptoms. Notably, Hiller et al[23] observed that the rat models exhibited complete normalization of movement asymmetry following iPSC transplantation. Additionally, confirmed long-term survival and functional recovery were observed in athymic nude mice in the study by Lian et al[24] in 2021 after unilateral 6-OHDA injection. The differentiation of stem cells and their functional status were crucial aspects addressed in these studies. iPSC-mDA progenitors were transplanted into specific anatomical locations, such as the right medial forebrain bundle in mice, and unilateral injections of 6-OHDA targeted the same region in rats. Long-term monitoring revealed positive outcomes, including the complete normalization of movement asymmetry in rat models and improved motor function in SCID beige mice.
In other studies, hESCs and iPSCs were employed in rat models, showing complete functional recovery after the induction of PD[25]. Additionally, hADSCs were used in Parkinsonian animal models. Transplantation of these cells led to improvement in motor performance and symptoms of the disease[24]. Finally, ESCs were utilized in animal models of PD, resulting in complete functional recovery and long-term survival of transplanted cells[26]. The analysis of these studies demonstrates the potential of stem cells to provide therapeutic effectiveness in PD with prospects for long-term improvement in motor function and symptom reduction.
The utilization of hPSCs, including hESCs and iPSCs, represents a significant advancement in medical research, particularly in the context of treating PD. Both hESCs and iPSCs possess the remarkable ability to differentiate into various cell types, making them invaluable for regenerative medicine and disease modeling. The studies reviewed demonstrate promising outcomes in animal models, suggesting a potential therapeutic role for these stem cells in PD. The studies highlighted various methodologies and approaches in utilizing hPSCs for PD therapy. For instance, the transplantation of iPSC-mDA progenitors into specific brain regions of rodent models showed substantial improvement in motor function[23]. Similarly, studies involving hESCs and iPSCs demonstrated significant motor function recovery and long-term survival of transplanted cells in Parkinsonian models[24,25]. These findings underscore the therapeutic potential of hPSCs in addressing the motor symptoms associated with PD.
However, challenges remain in translating these findings from preclinical studies to clinical applications. One critical aspect is the long-term efficacy and safety of stem cell transplantation in human patients. While animal studies show promise, the complexity of human neurobiology and the potential immune response to transplanted cells necessitate further investigation. Additionally, optimizing the differentiation protocols of iPSCs into midbrain dopaminergic neurons and ensuring their survival and integration post-transplantation are crucial for achieving sustained therapeutic benefits in patients.
Ethical considerations also play a significant role, particularly in the context of using hESCs vs iPSCs. iPSCs, derived from a patient’s own cells, offer a personalized approach that minimizes ethical concerns associated with hESCs involving embryo destruction (Harvard Stem Cell Institute, 2023). This personalized medicine approach not only enhances therapeutic efficacy but also addresses ethical dilemmas associated with traditional stem cell therapies.
The research on hPSCs, including hESCs and iPSCs, and their applications in treating PD, presents promising avenues for future therapeutic interventions. The ability of these stem cells to differentiate into specific neuronal subtypes and inte
| 1. | Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386:896-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3143] [Cited by in RCA: 4056] [Article Influence: 405.6] [Reference Citation Analysis (39)] |
| 2. | Stoker TB. Stem Cell Treatments for Parkinson’s Disease. In: Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]. Brisbane (AU): Codon Publications; 2018-Dec-21. [PubMed] |
| 3. | Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10 Suppl:S42-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 678] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 4. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14297] [Article Influence: 841.0] [Reference Citation Analysis (0)] |
| 5. | Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1289] [Cited by in RCA: 1430] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 6. | Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 634] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 7. | Hur YH, Cerione RA, Antonyak MA. Extracellular vesicles and their roles in stem cell biology. Stem Cells. 2020;38:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 9. | Barzegar M, Kaur G, Gavins FNE, Wang Y, Boyer CJ, Alexander JS. Potential therapeutic roles of stem cells in ischemia-reperfusion injury. Stem Cell Res. 2019;37:101421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5956] [Cited by in RCA: 5429] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 11. | Guan J, Wang G, Wang J, Zhang Z, Fu Y, Cheng L, Meng G, Lyu Y, Zhu J, Li Y, Wang Y, Liuyang S, Liu B, Yang Z, He H, Zhong X, Chen Q, Zhang X, Sun S, Lai W, Shi Y, Liu L, Wang L, Li C, Lu S, Deng H. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature. 2022;605:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 222] [Article Influence: 74.0] [Reference Citation Analysis (18)] |
| 12. | Roobrouck VD, Clavel C, Jacobs SA, Ulloa-Montoya F, Crippa S, Sohni A, Roberts SJ, Luyten FP, Van Gool SW, Sampaolesi M, Delforge M, Luttun A, Verfaillie CM. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells. 2011;29:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci USA. 2006;103:6907-6912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 855] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 15. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18157] [Article Influence: 955.6] [Reference Citation Analysis (0)] |
| 16. | Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 525] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 17. | Bai Q, Desprat R, Klein B, Lemaître JM, De Vos J. Embryonic stem cells or induced pluripotent stem cells? A DNA integrity perspective. Curr Gene Ther. 2013;13:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Breda L, Papp TE, Triebwasser MP, Yadegari A, Fedorky MT, Tanaka N, Abdulmalik O, Pavani G, Wang Y, Grupp SA, Chou ST, Ni H, Mui BL, Tam YK, Weissman D, Rivella S, Parhiz H. In vivo hematopoietic stem cell modification by mRNA delivery. Science. 2023;381:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 155] [Article Influence: 77.5] [Reference Citation Analysis (37)] |
| 19. | Zhong C, Liu M, Pan X, Zhu H. Tumorigenicity risk of iPSCs in vivo: nip it in the bud. Precis Clin Med. 2022;5:pbac004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (37)] |
| 20. | Cheng JW, Sadeghi Z, Levine AD, Penn MS, von Recum HA, Caplan AI, Hijaz A. The role of CXCL12 and CCL7 chemokines in immune regulation, embryonic development, and tissue regeneration. Cytokine. 2014;69:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5012] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 22. | Xu P, He H, Gao Q, Zhou Y, Wu Z, Zhang X, Sun L, Hu G, Guan Q, You Z, Zhang X, Zheng W, Xiong M, Chen Y. Human midbrain dopaminergic neuronal differentiation markers predict cell therapy outcomes in a Parkinson's disease model. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (37)] |
| 23. | Hiller BM, Marmion DJ, Thompson CA, Elliott NA, Federoff H, Brundin P, Mattis VB, McMahon CW, Kordower JH. Optimizing maturity and dose of iPSC-derived dopamine progenitor cell therapy for Parkinson's disease. NPJ Regen Med. 2022;7:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (37)] |
| 24. | Lian C, Huang Q, Zhong X, He Z, Liu B, Zeng H, Xu N, Yang Z, Liao C, Fu Z, Guo H. Pentraxin 3 secreted by human adipose-derived stem cells promotes dopaminergic neuron repair in Parkinson's disease via the inhibition of apoptosis. FASEB J. 2021;35:e21748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (37)] |
| 25. | Shrigley S, Nilsson F, Mattsson B, Fiorenzano A, Mudannayake J, Bruzelius A, Ottosson DR, Björklund A, Hoban DB, Parmar M. Grafts Derived from an α-Synuclein Triplication Patient Mediate Functional Recovery but Develop Disease-Associated Pathology in the 6-OHDA Model of Parkinson's Disease. J Parkinsons Dis. 2021;11:515-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (37)] |
| 26. | Adler AF, Cardoso T, Nolbrant S, Mattsson B, Hoban DB, Jarl U, Wahlestedt JN, Grealish S, Björklund A, Parmar M. hESC-Derived Dopaminergic Transplants Integrate into Basal Ganglia Circuitry in a Preclinical Model of Parkinson's Disease. Cell Rep. 2019;28:3462-3473.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (37)] |