Published online Oct 26, 2010. doi: 10.4331/wjbc.v1.i10.313
Revised: August 19, 2010
Accepted: August 26, 2010
Published online: October 26, 2010
AIM: To study the localization and function of a eukaryotic initiation factor 2 (eIF2α)-associated 67-kDa glycoprotein (p67).
METHODS: Immunofluorescence staining, 35S-Met/Cys metabolic labeling, Western blotting analysis, sucrose gradient centrifugation and high speed centrifugation were used to determine the localization of proteins in transiently transfected COS-1 cells. Transient co-transfection followed by co-immunoprecipitation was used to study the interaction between p67 and double-stranded RNA (dsRNA)-dependent protein kinase (PKR). Wheat germ agglutinin agarose beads were used to absorb glycosylated proteins. In vivo32P-labeling followed by immunoprecipitation and Western blotting were used to measure PKR autophosphorylation, eIF2α phosphorylation, and p67 expression in normal and breast cancer cells.
RESULTS: The image from immunofluorescence staining showed that p67 was overexpressed in the cytosol but not in the nucleus. In a sucrose gradient, approximately 30% of the overexpressed p67 was bound with ribosomes. p67 interacted with the kinase domain, but not the dsRNA-binding domains of PKR. Only the glycosylated p67 was associated with the ribosome, and p67 did not compete with PKR for ribosome binding. In breast cancer cells, there was increased autophosphorylation of PKR but no phosphorylation of eIF2α, compared with normal breast cells.α The ratio of glycosylated/deglycosylated p67 was altered in breast cancer cells.
CONCLUSION: Glycosylation of p67 is required for its ribosomal association and can potentially inhibit PKR via interaction with the kinase domain of PKR.
- Citation: Wu S. Localization and function of a eukaryotic-initiation-factor-2-associated 67-kDa glycoprotein. World J Biol Chem 2010; 1(10): 313-320
- URL: https://www.wjgnet.com/1949-8454/full/v1/i10/313.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i10.313
Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α) is an important regulatory mechanism in the initiation of protein synthesis[1-3]. Under certain physiological conditions, eIF2α-specific kinases (EIF2AKs) phosphorylate eIF2α and inhibit protein synthesis[1-3]. An eIF2-associated 67-kDa glycoprotein (p67) protects the eIF2α-subunit from inhibitory phosphorylation by eIF2 kinases, and this promotes protein synthesis in the presence of active EIF2AKs[4-7]. p67 is a glycoprotein that contains 12 O-linked N-acetyl-glucosamine (GlcNAc) residues[8]. The glycosyl residues on p67 are required for the activity of p67 in protecting eIF2α from EIF2AK-catalyzed phosphorylation. Under external stimuli, such as heme-deprivation or serum-starvation, p67 is deglycosylated and subsequently degraded, which lead to eIF2α phosphorylation and protein synthesis inhibition[9]. p67 deglycosylase regulates p67 activity[4,10], and the balance between glycosylated and deglycosylated p67 has been shown to regulate cell transformation or apoptosis[11].
Our previous study has indicated that p67 protects cells from double-stranded RNA (dsRNA) dependent protein kinase (PKR)-catalyzed eIF2α phosphorylation[12]. In most cells and tissues, PKR is expressed and exists in a latent form[13]. PKR expression is induced by interferon[14,15], and its activation is dependent upon dsRNA[16]. Many of the antiviral activities of interferon are mediated by PKR. In addition to its role in the antiviral activity of interferon, PKR also plays an important role in the regulation of cell growth. Overexpression of wild-type PKR inhibits protein synthesis and cell growth[14,15,17], whereas overexpression of a dominant negative PKR inhibits endogenous PKR activity and causes NIH 3T3 cell transformation[18,19]. These results implicate PKR as a tumor suppressor gene. However, some studies have indicated that elevated eIF2α phosphorylation and increased expression and activity of PKR are observed in human breast cancer, melanoma and colon cancer cells[20,21]. In the present study, we determined the role of p67 glycosylation in regulation of its localization. We also demonstrated that the ratio of deglycosylated/glycosylated p67 was altered, and PKR autophosphorylation, but not eIF2α phosphorylation, was increased in human breast cancer cells.
COS-1 cells were cultured in Dulbecco’s Minimal Essential Medium that contained 10% fetal calf serum (FCS) and penicillin/streptomycin (100 U/mL). MCF-10A cells were cultured in serum-free Ham’s F-12 medium with insulin, hydrocortisone and epidermal growth factor (EGF). SUM52 cells were cultured in Ham’s F-12 medium supplemented with 5% fetal bovine serum, insulin and hydrocortisone. SUM102 cells were cultured in serum-free Ham’s F-12 medium with insulin, hydrocortisone and EGF. SUM149 cells were cultured in serum-free Ham’s F-12 medium with insulin and hydrocortisone.
The expression plasmid pETFVA- has been described previously[22]. It contained a transcription unit that utilized the adenovirus major late promoter and simian virus 40 (SV40) enhancer element. In addition, the vectors contained the SV40 origin for replication in COS-1 cells. The K296P expression vectors that encoded K296P PKR (pETFVA--K296P), the PKR dsRNA binding domain (BD, amino acids 1-243) (pETFVA--BD), the mutant PKR kinase domain (amino acids 228-551) (pETFVA--K296P-KD) and p67 (pETFVA--p67) have also been described previously[12,22]. All the PKR mutants contained a T7 epitope (MASMTGGQQMG) at the C-terminal for T7 antibody binding.
COS-1 monkey kidney cells were transfected by the DEAE-dextran procedure[16]. For each 100-mm plate, 4 mg plasmid DNA was used in 4 mL transfection medium. At 48 h post-transfection, the cells were fixed in a 4% paraformaldehyde solution and permeabilized with PBS that contained 0.1% saponin. These cells were incubated in mouse anti-p67 monoclonal antibody or anti-mouse IgG polyclonal antibody (Pharmingen, San Diego, CA, USA) for 1 h at 37°C. The cells were washed with PBS and incubated in fluorescein-conjugated goat anti-mouse antibody (Vector Laboratories, Burlingame, CA, USA). Finally, fluorescent microscopy of p67 was performed using a Nikon Labphot-2 lance (Nikon, Melville, NY, USA).
Cell extracts were prepared by lysing the cells with 500 mL ribosome binding solution (RBS; 10 mmol/L KCl, 1.5 mmol/L MgCl2 and 10 mmol/L Tris-HCl, pH 7.5) that contained 0.5% NP-40. The nuclei and debris were removed by centrifugation at 5000 rpm at 4°C in an Eppendorf 5415C microcentrifuge apparatus. The S100 of the cell extracts was prepared by ultracentrifugation at 100 000 g at 4°C using an SW-60 swing bucket rotor in a ultracentrifuge (Beckman, Arlington Heights, IL, USA). The supernatant was collected and the pellets were washed twice with PBS. The pellets were resuspended with 200 mL RBS and dissolved overnight at 4°C. The protein concentration of the samples was determined using a Bio-Rad protein assay kit. The assayed samples were measured in a spectrophotometer at an absorbance of 520 nm.
COS-1 cells were transfected with mammalian expression vectors. These cells were labeled with 35S-Met/Cys (100 μCi/mL, Amersham Life Science, Arlington Heights, IL, USA) for 20 min in methionine/cysteine-free minimal essential media (Life Technologies Inc., Gaithersburg, MD, USA). These cells were lysed using 0.5% NP-40 in RBS. The cell extracts were collected and loaded onto a 4-mL 10%-40% linear sucrose gradient. The samples were centrifuged at 150 000 g for 2 h and collected into nine fractions. These fractions (20 μL) were resolved using SDS-PAGE. The gels were fixed in a solution of 30% methanol and 10% acetic acid. The fixed gels were then treated with En3Hance (New England Nuclear Co., Boston, MA, USA) and dried with a gel dryer (Savant Instruments, New York, NY, USA). The dried gels were autoradiographed using BioMAX MR film (Kodak-Eastman, Rochester, NY, USA). Ribosome-associated p67 levels were quantified using NIH-Image v. 1.57 (National Institutes of Health, Bethesda, MD, USA).
The level of p67 was measured in cell extracts obtained as described above. The total cell extract (10 mg), supernatants (10 mg) and S100 (2 mg) proteins were resolved by SDS-PAGE and electroblotted onto nitrocellulose membranes. These membranes were initially blotted with Tris-buffered saline/Tween 20 (TBST) that contained 2% non-fat dry milk. The membranes were subsequently blotted with anti-p67 monoclonal antibody for 3 h, washed in TBST, and blotted with anti-mouse IgG for 1 h. The nitrocellulose membranes were then developed using a solubilized alkaline phosphatase substrate (Promega Corp., Madison, WI, USA).
COS-1 cells were transfected as described above. After 48 h, the cells were labeled with Pro-Mix L-[35S] cell labeling mix (100 mCi/mL; 1000 Ci/mmol; Amersham Life Science) for 20 min in methionine/cysteine-free minimal essential medium (GIBCO BRL, Gaithersburg, MD, USA). Cell extracts were prepared by lysis in NP-40 lysis buffer. Proteins were immunoprecipitated using a T7-tag monoclonal antibody (Novagen Co., Madison, WI, USA) or a monoclonal antibody against p67[12]. The immunoprecipitants were analyzed by SDS-PAGE. The gels were fixed, prepared for fluorography by treatment with En3Hance (New England Nuclear Corp.), dried, and autoradiographed using Kodak XAR-5 film.
Total cell extracts transfected with p67 expression vectors were mixed with wheat germ agglutinin (WGA) agarose beads (Vector Laboratories). After incubation, the WGA was collected and resolved with SDS-PAGE. The resultant gel was electroblotted onto a nitrocellulose membrane and incubated with anti-p67 monoclonal antibodies for 3 h. This membrane was blotted with anti-mouse IgG for 1 h and visualized with alkaline phosphatase colored substrate.
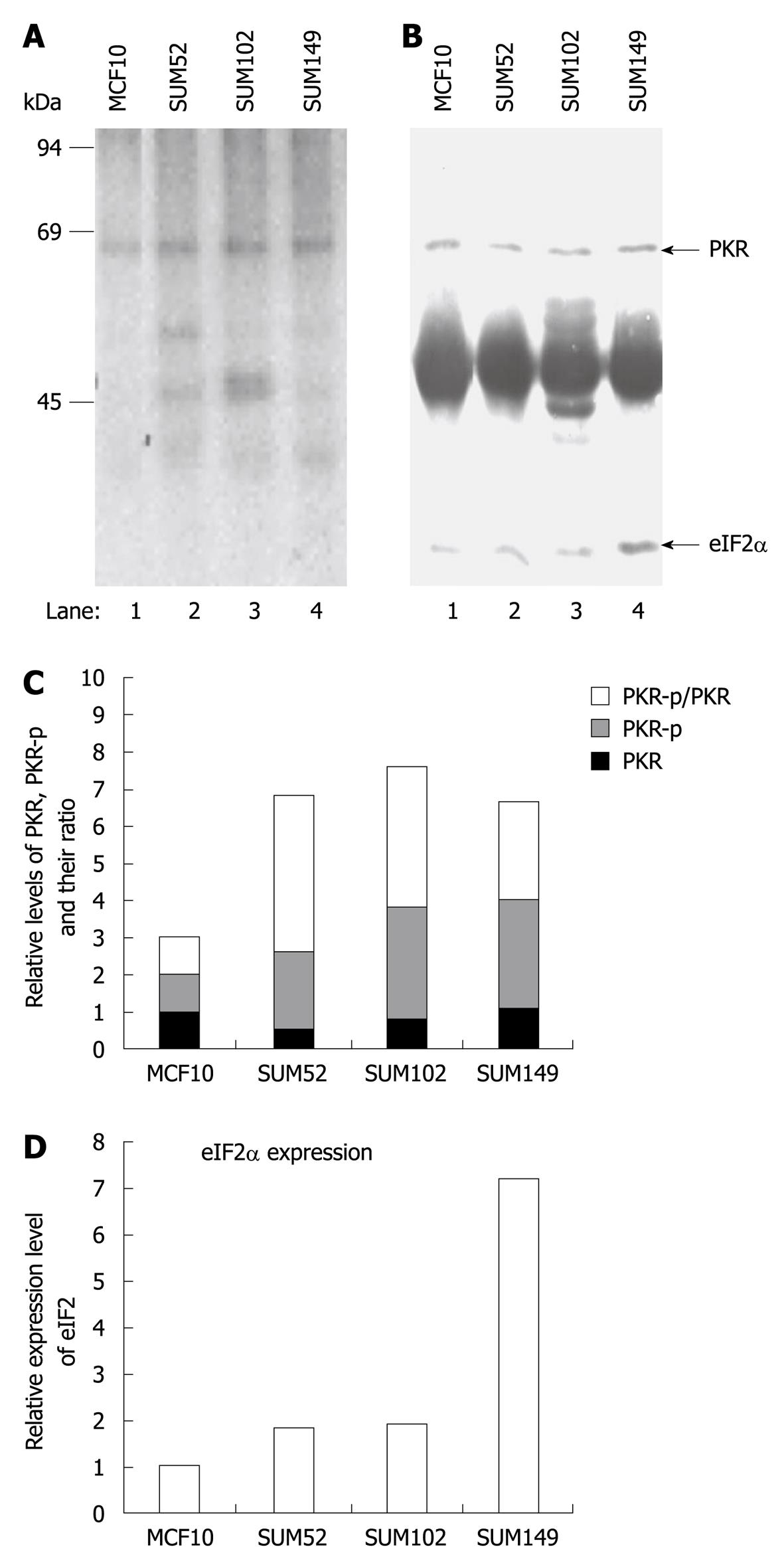
To monitor in vivo phosphorylation of endogenous PKR and the eIF2α subunit, the cultured cells were labeled with 32P-phosphoric acid (200 mCi/mL, NEN, Boston, MA, USA) for 4 h in phosphate-free medium (Life Technologies) with 2% FCS. Cell extracts were prepared by lysis in NP-40 lysis buffer. The labeled PKR and eIF2α was immunoprecipitated with anti-PKR polyclonal antibody and anti-eIF2α monoclonal antibody, resolved by SDS-PAGE, and electroblotted to nitrocellulose. The membrane was immunoblotted with an anti-PKR polyclonal antibody and an anti-eIF2α monoclonal antibody. The PKR and eIF2α protein levels were visualized by the alkaline-phosphatase immunoblotting detection system (Promega Co.). 32PO4 incorporation was quantitated by autoradiography of the nitrocellulose membrane using Kodak BioMax film (Eastman Kodak, Rochester, NY, USA) and a Dupont Cronex Lightning-Plus screen.
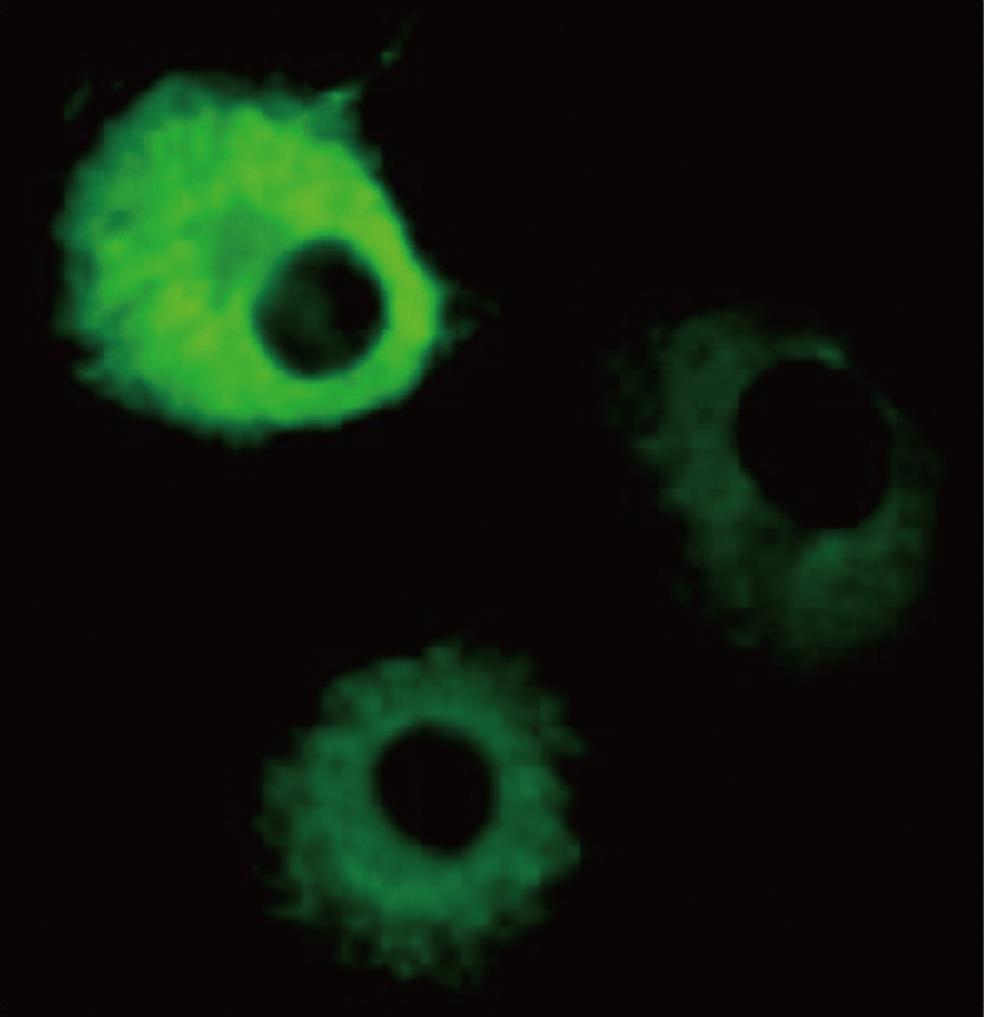
Using immunofluorescence detection, it was found that overexpressed p67 was present exclusively in the cytosol of COS-1 cells. COS-1 cells transfected with a p67 expression vector showed increased fluorescence in the cytosol (Figure 1), whereas the nucleus was almost completely devoid of fluorescent materials (Figure 1). Fluorescence was not detected with the non-transfected COS-1 cells, which were incubated with an anti-mouse IgG polyclonal antibody (data not shown). Our result indicated that the overexpressed p67 only existed in the cytosol. This result agrees with the previous observation that a green fluorescence (GFP)-tagged p67 is found only in the cytosol[11].
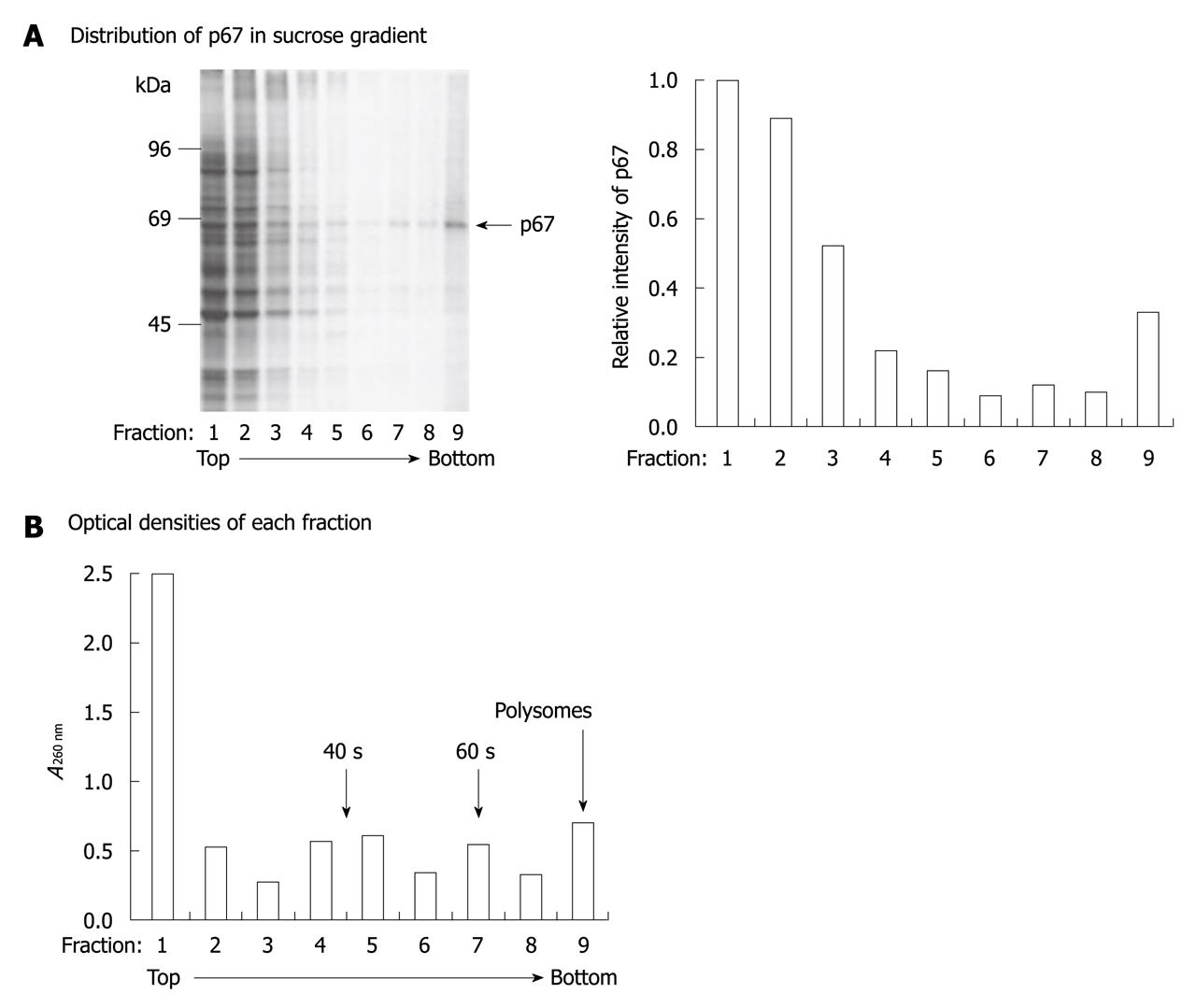
To study further the localization of p67, we analyzed the interaction of p67 with ribosomes in COS-1 cells. Expression vector that encoded p67 was transiently transfected into COS-1 cells, and the cells were pulse-labeled with [35S]-methionine/cysteine and chased for 1 h in medium that contained excess unlabeled methionine and cysteine to ensure completion of radiolabeled nascent polypeptide chains. Extracts were fractionated by centrifugation at 100 000 g, and the S100 supernatant and the S100 pellet samples were analyzed by SDS-PAGE. The ribosomal association of the expressed proteins was measured by analyzing the co-sedimentation of p67 with ribosomes. As previously reported[12], the strong 67-kDa protein band was detected in the p67-transfected cell lysate (Figure 2A), but not in the non-transfected cell lysate (data not shown). Our data also showed that the overexpressed p67 was detected in every fraction of the gradient (Figure 2A). Based on the optical density of each fraction at 260 nm, most p67 was found in the cytosolic fractions 1 and 2. By taking fractions 4-9 as polysomal fractions, we estimated that approximately 30% of p67 was associated with the polysomes (Figure 2). These results indicate that p67 is strongly associated with the translation apparatus.
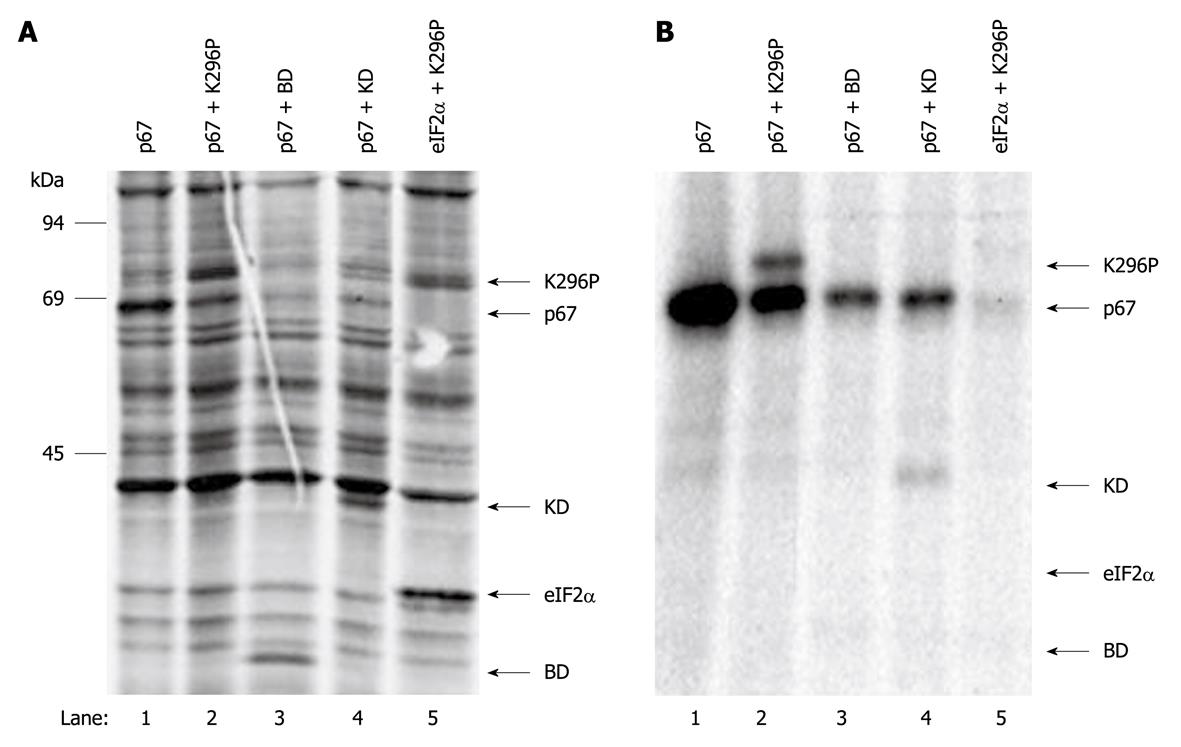
To investigate the mechanism by which p67 protects eIF2α from phosphorylation, a co-immunoprecipitation assay was established to measure the interaction of p67 and PKR. Direct studies on the wild-type PKR in vivo have been limited due to the inhibition of protein synthesis observed in the presence of active PKR. We used the catalytically inactivated intact PKR (K296P) in this study. p67 was co-transfected with either the K296P mutant of PKR (K296P), the dsRNA binding domains of PKR (BD) or the K296P mutated kinase domain of PKR (KD) into COS-1 cells. K296P and eIF2α were also co-transfected in the absence of p67 into COS-1 cells as a control. After labeling the cells with 35S-Met/Cys, the cell extracts were prepared with NP-40 lysis buffer, and p67 was immunoprecipitated with anti-p67 monoclonal antibody. The data showed that all the transfected vectors expressed their corresponding genes (Figure 3A). The co-immunoprecipitation revealed that p67 interacted with intact PKR (Figure 3B, lane 2) and the kinase domain of PKR (Figure 3B, lane 4), but did not interact with the dsRNA-binding domain of PKR (Figure 3B, lane 3). The co-immunoprecipitated PKR was specific because it was not co-immunoprecipitated without the presence of p67 (Figure 3B, lane 5). These results suggest that p67 interacts with the kinase domain of PKR.
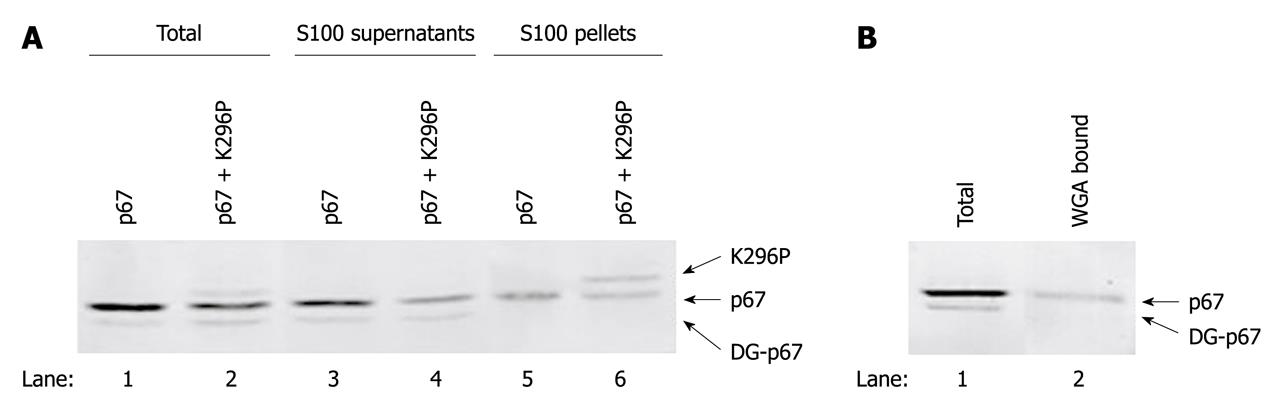
Our previous study demonstrated that overexpressed PKR mainly existed in polysomal fractions of sucrose gradient centrifugation[23]. To determine whether p67 inhibited PKR by competing for the binding of polysomes, we co-overexpressed p67 with PKR in COS-1 cells, and analyzed their ribosomal associations using S100 centrifugation. As in the study above, we used the catalytically inactivated intact PKR (K296P) in this study. The data showed that p67 was highly expressed in the presence or absence of the expression of K296P (Figure 4A, lanes 1 and 2). Comparison of S100 supernatant with the S100 pellet demonstrated that p67 was present in the cytosolic and polysomal fractions (Figure 4A, lanes 3 and 5), which agrees with the sucrose gradient data (Figure 2). However, the overexpressed K296P was only detected in the S100 pellet in the presence of expressed p67, and was not detected in the S100 supernatants (Figure 4A, lane 6 vs 4). These results suggest that overexpression of p67 does not interfere with the polysomal association of PKR.
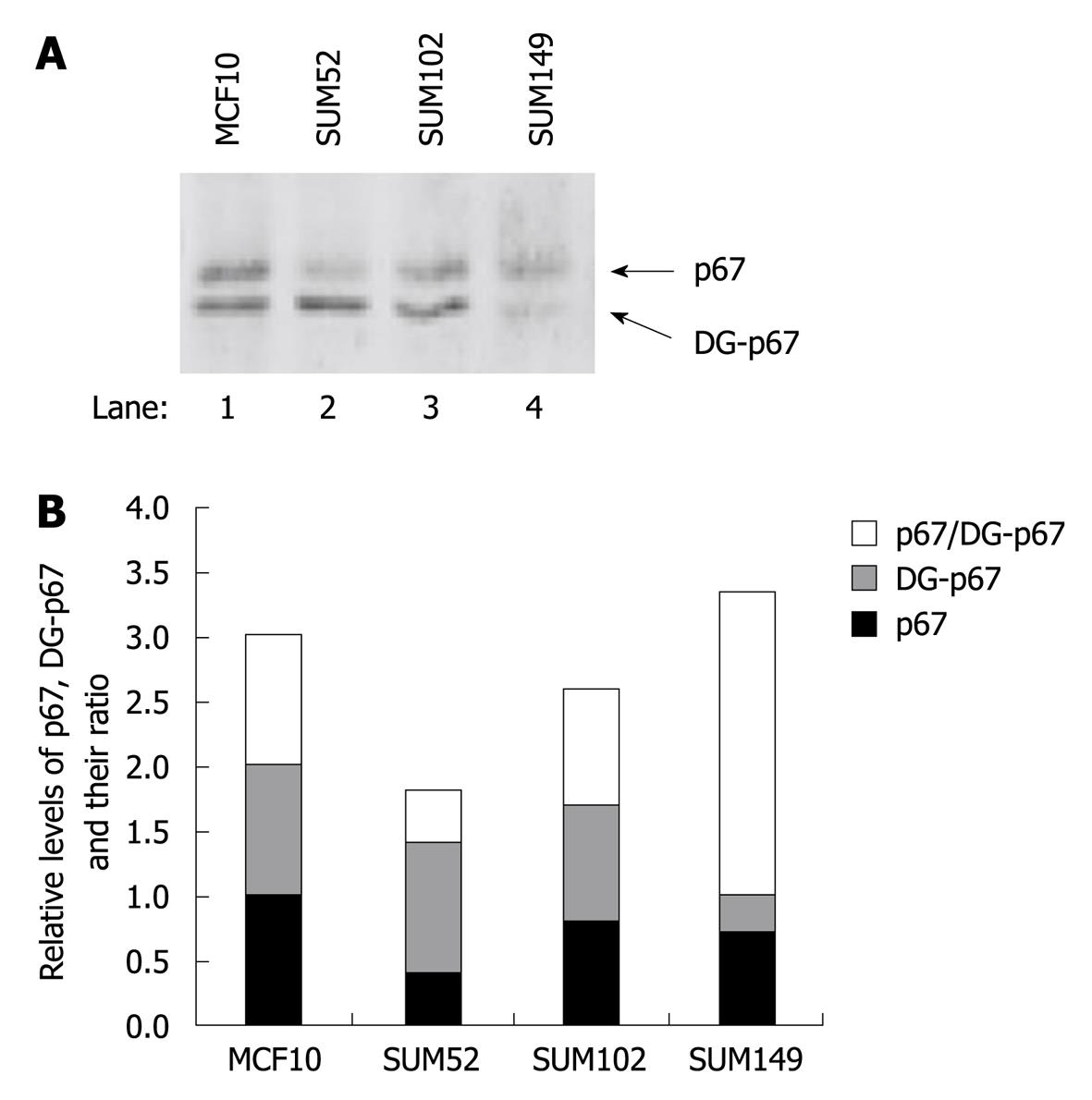
Western blotting analysis detected two bands in the 67 kDa region (Figure 4A). The higher band existed in the S100 supernatant and pellet, whereas the lower band existed only in the supernatant. A previous study has indicated that deglycosylated p67 moves slightly faster than glycosylated p67 on SDS-PAGE[4,7,8], therefore, we determined whether the non-polysomal associated lower band was the deglycosylated p67. WGA-conjugated agarose beads were incubated with the total cell extract that overexpressed p67. The total glycosylated proteins bound to the beads were resolved with SDS-PAGE and p67 was probed by antibody. The data showed that the WGA exclusively bound to the higher band but not to the lower band of p67 (Figure 4B). This result indicated that the higher band was the glycosylated p67 and suggested that glycosylation was required for polysomal association of p67.
A previous study has indicated that elevated eIF2α phosphorylation and expression and activity of PKR are observed in human breast cancer, melanoma and colon cancer cells[20,21]. To determine whether p67 plays a role in regulation of PKR activity and eIF2α phosphorylation in cancer cells, we analyzed PKR autophosphorylation, eIF2α phosphorylation, and p67 expression in a normal breast cell line MCF10 and three breast cancer cell lines, SUM52, SUM102 and SUM149[24-26]. By using an in vivo autophosphorylation assay[12,22], we showed that the background autophosphorylation of PKR was elevated in all the breast cancer cell lines (Figure 5A, lanes 2-4 vs 1), whereas the expression levels of PKR were not increased (Figure 5B, lanes 2-4 vs 1). The autophosphorylated PKR without external stimuli did not lead to a detectable amount of phosphorylation of eIF2α (Figure 5A), when the expression of eIF2α was increased in the cancer cells (Figure 5B, lanes 2-4 vs 1). The expression of p67 was detected in normal and cancer cell lines (Figure 6). A decreased ratio of glycosylated/deglycosylated p67 was correlated to an increased ratio of phosphorylated PKR/PKR in two breast cancer cell lines, SUM52 and SUM102 (Figures 5C and 6B). In the other breast cancer cell line, SUM149, an increased ratio of glycosylated/deglycosylated p67 (Figure 6B) was correlated with a sevenfold increase of eIF2α expression (Figure 5D) in the presence of a higher ratio of phosphorylated PKR/PKR (Figure 5C). These observations indicate that p67 might coordinate with other PKR regulators, such as p58[27], in regulation of PKR activation, eIF2α expression, and phosphorylation in cancer cells without external stimuli.
p67 can be isolated from rabbit reticulocyte lysates, and remains associated with reticulocyte eIF2 during several purification steps until it is freed of eIF2 upon further purification[8,9,28]. p67 protects eIF2α from eIF2-kinase-catalyzed phosphorylation and thus promotes protein synthesis in the presence of active eIF2 kinase[8,9]. Expression of p67 is regulated at both transcriptional and translational levels[5,29], and cells that express higher levels of p67 are more resistant to viral infection[30]. p67 in cultured cells can be induced by the mitogen phorbol 12-myristate 13 acetate (PMA). After addition of PMA to serum-starved cells in culture, p67 appears at high levels and is accompanied with an increase in protein synthesis activity in the cells[9]. p67 is a glycosylated protein, and the GlcNAc residues are necessary for its protection of eIF2α from inhibitory phosphorylation by eIF2 kinase[8]. A p67-deglycosylase has been isolated from rabbit reticulocytes, and an increase in p67-deglycosylase activity leads to deglycosylation of p67, which is correlated with a decrease in protein synthesis[10]. However, little is known about the relationship between p67 glycosylation and its polysomal association. In this study, we showed that p67 is a cytosolic protein (Figure 1) and approximately 30% of expressed p67 is associated with polysomes (Figure 2). A higher ratio of non-polysomal/polysomal association of p67 could explain our previous observation that, although p67 is slightly more effective than another known PKR inhibitor, namely K3L, in inhibition of PKR-catalyzed eIF2α phosphorylation, it is significantly less effective than K3L in reversal of PKR inhibition of protein synthesis[22]. PKR is located on the polysomes and is locally activated by specific mRNA, therefore, the mis-location of p67 can affect its ability to inhibit locally activated PKR and reverse PKR-catalyzed protein synthesis inhibition[16,23].
In contrast to the low efficiency of rescuing the translational inhibition by activated PKR, p67 efficiently protects eIF2α phosphorylation in transfected COS-1 cells[12]. This might be due to the ability of p67 to interact with the kinase domain of PKR (Figure 3). One of the mechanisms of PKR activation is through the release of the caspase-3-generated kinase domain of PKR, which is able to phosphorylate eIF2α[31-33]. Overexpressed p67 can interact with the 43-kDa kinase domain of PKR, which is released from intact PKR via caspase 3 digestion[31]. The ribosomal association of p67 does not disrupt the ribosome-binding of PKR and requires the glycosyl residues on p67 (Figure 4). In comparison with normal breast cells, breast cancer cells had increased autophosphorylation of PKR and eIF2α expression, but not eIF2α phosphorylation (Figure 5). The ratio of glycosylated/deglycosylated p67 was also changed in cancer cells in comparison with that in normal cells (Figure 6). The results further suggest that glycosylation, and hence polysome association, might play a role in regulation of local activation of PKR and therefore the expression of certain PKR-regulated genes. These findings could provide us with a new approach to regulate PKR activity that has been shown to affect the efficacy of cancer chemotherapy[34].
The eukaryotic initiation factor 2 (eIF2) is a heterotrimeric protein, which plays a significant role in regulation of protein synthesis in mammalian cells. The phosphorylation of the serine 51 on the α subunit of eIF2 (eIF2α) is inhibited in cells. The eIF2-associated 67-kDa glycoprotein (p67) has been shown to protect eIF2 (eIF2α) from inhibitory phosphorylation by eIF2 kinase, and thus promotes protein synthesis in the presence of active eIF-2 kinase.
Cells respond to environmental stimuli through rapid and reversible covalent modification of translation initiation factors, to mediate immediate increases or decreases in protein synthesis. Deregulation of the phosphorylation of eIF2α has been associated with many human diseases such as viral infection and cancer. Understanding the regulatory mechanism of eIF2α can potentially lead to identification of new targets for treating these diseases.
The present study is believed to be the first to show that p67, a protein that protects eIF2α from phosphorylation, can interact with the kinase domain of double-stranded RNA (dsRNA) dependent protein kinase (PKR); one of the four eIF2α kinases. The phosphorylation of PKR was increased in breast cancer cells. However, phosphorylation of eIF2α was not detectably increased, which indicates that PKR activity might be inhibited in cancer cells.
Each of the eIF2α kinases has a kinase domain and a regulatory domain, which responds to kinase activators. As for PKR, the regulatory domain binds to dsRNA, and therefore it has been named as the dsRNA-binding domain.
The experiments are in general well executed, although not very well presented, and the conclusions are supported by the data. The results of the research seem to me important and novel enough to significantly advance the knowledge in the field.
Peer reviewer: Gaetano Cairo, PhD, Professor, Department Human Morphology and Biomedical Sciences-Città Studi-Via Mangiagalli 31, Milano, 20133, Italy
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
| 1. | Kaufman RJ. The Double-stranded RNA-activated Protein Kinase PKR. Translational control of gene expression. Cold Spring Harbor, New York: CSHL Press 2000; 503-528. |
| 2. | Pain VM. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747-771. |
| 3. | Samuel CE. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993;268:7603-7606. |
| 4. | Saha D, Wu S, Bose A, Chatterjee N, Chakraborty A, Chatterjee M, Gupta NK. Viral infection. II. Hemin induces overexpression of p67 as it partially prevents appearance of an active p67-deglycosylase in baculovirus-infected insect cells. Arch Biochem Biophys. 1997;342:373-382. |
| 5. | Gupta S, Bose A, Chatterjee N, Saha D, Wu S, Gupta NK. p67 transcription regulates translation in serum-starved and mitogen-activated KRC-7 cells. J Biol Chem. 1997;272:12699-12704. |
| 6. | Gil J, Esteban M, Roth D. In vivo regulation of the dsRNA-dependent protein kinase PKR by the cellular glycoprotein p67. Biochemistry. 2000;39:16016-16025. |
| 7. | Datta B, Datta R, Ghosh A, Majumdar A. Eukaryotic initiation factor 2-associated glycoprotein, p67, shows differential effects on the activity of certain kinases during serum-starved conditions. Arch Biochem Biophys. 2004;427:68-78. |
| 8. | Datta B, Ray MK, Chakrabarti D, Wylie DE, Gupta NK. Glycosylation of eukaryotic peptide chain initiation factor 2 (eIF-2)-associated 67-kDa polypeptide (p67) and its possible role in the inhibition of eIF-2 kinase-catalyzed phosphorylation of the eIF-2 alpha-subunit. J Biol Chem. 1989;264:20620-20624. |
| 9. | Ray MK, Datta B, Chakraborty A, Chattopadhyay A, Meza-Keuthen S, Gupta NK. The eukaryotic initiation factor 2-associated 67-kDa polypeptide (p67) plays a critical role in regulation of protein synthesis initiation in animal cells. Proc Natl Acad Sci USA. 1992;89:539-543. |
| 10. | Datta B, Datta R, Mukherjee S, Zhang Z. Increased phosphorylation of eukaryotic initiation factor 2alpha at the G2/M boundary in human osteosarcoma cells correlates with deglycosylation of p67 and a decreased rate of protein synthesis. Exp Cell Res. 1999;250:223-230. |
| 11. | Datta B. MAPs and POEP of the roads from prokaryotic to eukaryotic kingdoms. Biochimie. 2000;82:95-107. |
| 12. | Wu S, Rehemtulla A, Gupta NK, Kaufman RJ. A eukaryotic translation initiation factor 2-associated 67 kDa glycoprotein partially reverses protein synthesis inhibition by activated double-stranded RNA-dependent protein kinase in intact cells. Biochemistry. 1996;35:8275-8280. |
| 14. | Thomis DC, Doohan JP, Samuel CE. Mechanism of interferon action: cDNA structure, expression, and regulation of the interferon-induced, RNA-dependent P1/eIF-2 alpha protein kinase from human cells. Virology. 1992;188:33-46. |
| 15. | Thomis DC, Samuel CE. Mechanism of interferon action: autoregulation of RNA-dependent P1/eIF-2 alpha protein kinase (PKR) expression in transfected mammalian cells. Proc Natl Acad Sci USA. 1992;89:10837-10841. |
| 16. | Wu S, Kaufman RJ. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem. 1997;272:1291-1296. |
| 17. | Chong KL, Feng L, Schappert K, Meurs E, Donahue TF, Friesen JD, Hovanessian AG, Williams BR. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 1992;11:1553-1562. |
| 18. | Donzé O, Jagus R, Koromilas AE, Hershey JW, Sonenberg N. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 1995;14:3828-3834. |
| 19. | Meurs EF, Galabru J, Barber GN, Katze MG, Hovanessian AG. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1993;90:232-236. |
| 20. | Kim SH, Forman AP, Mathews MB, Gunnery S. Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene. 2000;19:3086-3094. |
| 21. | Kim SH, Gunnery S, Choe JK, Mathews MB. Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon-inducible protein kinase, PKR. Oncogene. 2002;21:8741-8748. |
| 22. | Wu S, Kaufman RJ. Double-stranded (ds) RNA binding and not dimerization correlates with the activation of the dsRNA-dependent protein kinase (PKR). J Biol Chem. 1996;271:1756-1763. |
| 23. | Wu S, Kumar KU, Kaufman RJ. Identification and requirement of three ribosome binding domains in dsRNA-dependent protein kinase (PKR). Biochemistry. 1998;37:13816-13826. |
| 24. | Moffa AB, Tannheimer SL, Ethier SP. Transforming potential of alternatively spliced variants of fibroblast growth factor receptor 2 in human mammary epithelial cells. Mol Cancer Res. 2004;2:643-652. |
| 25. | Sartor CI, Zhou H, Kozlowska E, Guttridge K, Kawata E, Caskey L, Harrelson J, Hynes N, Ethier S, Calvo B. Her4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Mol Cell Biol. 2001;21:4265-4275. |
| 26. | Willmarth NE, Ethier SP. Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J Biol Chem. 2006;281:37728-37737. |
| 27. | Polyak SJ, Tang N, Wambach M, Barber GN, Katze MG. The P58 cellular inhibitor complexes with the interferon-induced, double-stranded RNA-dependent protein kinase, PKR, to regulate its autophosphorylation and activity. J Biol Chem. 1996;271:1702-1707. |
| 28. | Ray MK, Chakraborty A, Datta B, Chattopadhyay A, Saha D, Bose A, Kinzy TG, Wu S, Hileman RE, Merrick WC. Characteristics of the eukaryotic initiation factor 2 associated 67-kDa polypeptide. Biochemistry. 1993;32:5151-5159. |
| 29. | Chatterjee N, Zou C, Osterman JC, Gupta NK. Cloning and characterization of the promoter region of a gene encoding a 67-kDa glycoprotein. J Biol Chem. 1997;272:12692-12698. |
| 30. | Bose A, Saha D, Gupta NK. Viral infection. I. Regulation of protein synthesis during vaccinia viral infection of animal cells. Arch Biochem Biophys. 1997;342:362-372. |
| 31. | Saelens X, Kalai M, Vandenabeele P. Translation inhibition in apoptosis: caspase-dependent PKR activation and eIF2-alpha phosphorylation. J Biol Chem. 2001;276:41620-41628. |
| 32. | Suen KC, Yu MS, So KF, Chang RC, Hugon J. Upstream signaling pathways leading to the activation of double-stranded RNA-dependent serine/threonine protein kinase in beta-amyloid peptide neurotoxicity. J Biol Chem. 2003;278:49819-49827. |
| 33. | Wu S, Kaufman RJ. trans-Autophosphorylation by the isolated kinase domain is not sufficient for dimerization or activation of the dsRNA-activated protein kinase PKR. Biochemistry. 2004;43:11027-11034. |
| 34. | Pataer A, Swisher SG, Roth JA, Logothetis CJ, Corn PG. Inhibition of RNA-dependent protein kinase (PKR) leads to cancer cell death and increases chemosensitivity. Cancer Biol Ther. 2009;8:245-252. |