Published online Aug 27, 2025. doi: 10.4240/wjgs.v17.i8.109333
Revised: June 9, 2025
Accepted: June 24, 2025
Published online: August 27, 2025
Processing time: 108 Days and 5.1 Hours
Central venous access is essential for administering chemotherapy in patients with gastrointestinal cancer. Peripherally inserted central catheters (PICC) and totally implantable venous access ports (TIVAP) are widely used, but comparative data regarding their impact on catheter-related complications and quality of life (QoL) remain limited.
To evaluate the impact of TIVAPs compared with PICC on catheter-related complications and QoL in patients with gastrointestinal cancer undergoing chemotherapy.
This retrospective study included adults with gastrointestinal cancer who un
A total of 346 patients were analyzed. Baseline demographic, clinical, and cancer characteristics were similar between groups. The TIVAP group demonstrated a significantly lower incidence of catheter-related complications than the PICC group, with no pneumothorax occurring in either group. QoL assessments at baseline were comparable. At one month, the TIVAP group exhibited significantly higher EuroQoL Five Dimensions health state scores and QLQ-C30 global health status scores. Multivariate analysis identified TIVAP use, catheter tip placement in the distal superior vena cava/right atrium, prophylactic antibiotic administration, and antimicrobial dressing application as independent protective factors associated with reduced complications and improved QoL.
In patients with gastrointestinal cancer undergoing chemotherapy, TIVAPs are associated with a lower incidence of catheter-related complications and improved QoL than PICCs. Optimal device selection, precise catheter tip positioning, and effective perioperative management are critical for minimizing complications and enhancing patient-reported outcomes during treatment.
Core Tip: This study demonstrates that totally implantable venous access ports (TIVAP) significantly reduce catheter-related complications and improve quality of life (QoL) compared to peripherally inserted central catheters in gastrointestinal cancer patients undergoing chemotherapy. Key findings include lower complication rates and higher EuroQol Five Dimensions and QoL Questionnaire-Core 30 scores in the TIVAP group, underscoring the importance of optimal device selection for enhancing patient outcomes.
- Citation: Ye XH, Cui RH, Xu L, Ye LR, Wang MJ. Effects of totally implantable venous access ports on complications and quality of life in gastrointestinal cancer chemotherapy. World J Gastrointest Surg 2025; 17(8): 109333
- URL: https://www.wjgnet.com/1948-9366/full/v17/i8/109333.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i8.109333
Gastrointestinal cancers-including malignancies of the esophagus, stomach, and colorectum-constitute the leading causes of cancer-related morbidity and mortality worldwide[1]. Systemic chemotherapy is essential for improving survival, achieving disease control, and enhancing quality of life (QoL) in patients diagnosed with these neoplasms[2]. Despite advances in chemotherapeutic regimens, successful treatment outcomes largely depend on the reliable, sustained, and safe administration of intravenous medications, often requiring long-term central venous access[3]. However, the selection of the most appropriate vascular access device remains a subject of debate due to differences in complication profiles, patient comfort, and health-related QoL (HRQoL)[4].
Central venous access is essential for managing patients undergoing repeated cycles of chemotherapy, as peripheral venous cannulation rapidly becomes impractical due to venous exhaustion, sclerosis, or phlebitis[5]. Over the past decades, various central venous devices have become available, including peripherally inserted central catheters (PICC) and totally implantable venous access ports (TIVAP), each with unique technical and clinical characteristics[6]. PICCs are inserted via peripheral veins in the upper extremity, offering easy access but also increased susceptibility to frequent manipulation and infection risk[7]. By contrast, TIVAPs are entirely implanted beneath the skin, connected to a central vein through a subcutaneous reservoir, and accessed transcutaneously, minimizing external exposure and associated complications[8].
Despite their widespread use, central venous access devices (CVAD) carry the risk of multiple complications[8]. These challenges include infectious events such as local site infections and catheter-related bloodstream infections (CRBSI), mechanical issues such as occlusion or dislodgement, and thrombotic complications such as catheter-related deep vein thrombosis (CR-DVT)[9]. Each of these problems can lead to treatment delays, repeated medical interventions, hospital readmissions, and, in severe cases, increased morbidity or mortality[10]. Consequently, identifying modifiable factors to mitigate these risks is a priority in optimizing oncologic care[11]. Furthermore, the impact of vascular access devices extends beyond safety; convenience, cosmetic factors, maintenance burden, and the cumulative influences of device-related adverse events collectively shape the patient’s QoL[12].
Current literature demonstrates limited consensus regarding the optimal device for patients undergoing prolonged chemotherapy for gastrointestinal cancer[13]. While several studies suggest that TIVAPs may offer lower rates of catheter-related infections and greater patient comfort than PICCs, the evidence remains heterogeneous, traversing various cancer types, institutional protocols, and endpoints[13]. QoL outcomes-critical in advanced malignancies where treatment goals are increasingly palliative-are inconsistently reported or assessed using different instruments[14]. Clinical practice also varies regarding device selection, influenced by physician experience, resource availability, patient comorbidities, and anticipated treatment duration[5]. A systematic comparison of complication incidence and patient-reported outcomes between TIVAPs and PICCs, particularly in the gastrointestinal cancer population, remains highly relevant to clinicians and patients[15].
In the current health care landscape, where patient-centered outcomes are paramount, robust clinical evidence is required for guiding optimal vascular access strategies[15]. HRQoL is increasingly recognized as a critical metric complementing traditional efficacy and safety outcomes, particularly for cancer patients undergoing prolonged therapies and experiencing the cumulative burden of adverse effects[14]. Validated instruments such as the European Organization for Research and Treatment of Cancer QoL Questionnaire-Core 30 (EORTC QLQ-C30), the EuroQol Five Dimensions Questionnaire (EQ-5D), and the visual analogue scale (VAS) are widely used for quantifying patient well-being and play a vital role in evaluating the holistic impact of treatment interventions[16].
Given these contextual considerations, we conducted a retrospective cohort study to systematically compare catheter-related complication rates and quality-of-life outcomes in patients with gastrointestinal cancer undergoing chemotherapy, who received either TIVAPs or PICC as their primary vascular access. Our primary objectives were to: (1) Assess the effect of device selection on catheter-related complication incidence; and (2) Analyze differences in HRQoL using validated instruments, thereby providing clinical evidence to inform vascular access decision-making in gastrointestinal oncology. By deepening the understanding of device-based outcomes in this patient cohort, this study aims to contribute to the refinement of supportive care practices in cancer chemotherapy.
The inclusion criteria for this study were as follows: (1) Adults diagnosed with gastrointestinal cancer[17], requiring intermittent intravenous chemotherapy administration[18]; (2) Expected chemotherapy duration of 12 weeks or longer; (3) Preoperative routine tests confirming no abnormal coagulation, with a platelet count ≥ 50 × 109/L and an absolute neutrophil count ≥ 0.5 × 109/L; (4) Suitability for venous access insertion through an upper-body vein; and (5) Complete medical records without missing data.
The exclusion criteria for this study were as follows: (1) Treatment duration or expected lifespan of less than three months; and (2) Ongoing severe systemic infection, clinically significant upper limb or central deep vein thrombosis, severe coagulation disorders, inability to communicate, or an urgent need for dialysis fistula.
A retrospective analysis was conducted on 346 chemotherapy patients with gastrointestinal cancer who underwent central venous access insertion at the Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, between December 2021 and December 2024. Patients were divided into two groups based on the type of central venous access: The PICC group and the TIVAP group. At the end of the follow-up period, patients were further categorized into four subgroups based on the occurrence catheter-related complications and their QoL scores: The occurrence group, the non-occurrence group, the low QoL group (VAS ≤ 70), and the high QoL group (VAS > 70). Patient data were collected through the case system, including demographic characteristics, gastrointestinal cancer features, insertion procedure details, catheter complication occurrences, and QoL assessment scales. Mechanical events were defined as any unintended physical interaction with the catheter system that could result in malfunction or patient discomfort, including but not limited to catheter displacement, breakage, kinking, or external damage to the catheter line.
PICC: Patients were placed in a supine position, and relevant upper limb veins were identified using ultrasound. The procedure adhered to strict aseptic techniques, including the use of sterile gowns, masks, caps, and sterile gloves by the operators. The surgical area was initially disinfected with chlorhexidine alcohol and then fully covered with sterile drapes. A single type of port made of titanium and silicone rubber (Dome port, C. R. Bard, Inc., United States) was connected to a polyurethane catheter (4 Fr. Groshong NTX Clear VUE, C. R. Bard, Inc., United States). Successful catheter insertion was confirmed by drawing blood from the catheter. At the end of the procedure, a chest X-ray verified accurate PICC tip placement. Post-insertion, the puncture site was inspected daily using a transparent semi-permeable membrane dressing to monitor for signs of infection. Dressings were changed at least once a week to maintain cleanliness and dryness at the puncture site. The catheter was flushed with 10-20 mL of normal saline before and after each infusion and at least once a week when not in use to prevent thrombosis and catheter occlusion.
TIVAP: Contrary to the PICC group, the TIVAP group used a single type of port made of titanium and silicone rubber (Port-A-Cath, Pharmacia, Canada), connected to an 8.5 Fr silicone catheter (Celsite 301/305 S, B. Braun Melsungen AG, Germany). Maintenance was required only once every four weeks.
At baseline and one month after catheter insertion, patients’ health status was assessed using the EuroQol Questionnaire, developed by the EuroQol Group. This questionnaire evaluates five dimensions: Mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, each rated across three levels (no problems, some problems, extreme problems). An overall health status score was determined using the VAS. Health utility values range from 0 to 1, where 0 represents death and 1 indicates full health; lower utility values reflect poorer perceived health status. The intraclass correlation coefficient (ICC) for this scale is 0.88[19].
Pain was assessed using the VAS at one month and three months after catheter insertion. The VAS is a simple linear scale ranging from 0 to 10 or 100, where 0 represents “no pain” or no discomfort, and 10 or 100 indicates “most intense pain” or the maximum symptom severity a patient can perceive. The ICC of this scale is between 0.97 and 0.99[20].
At baseline and one month after catheter insertion, the QoL of gastrointestinal cancer patients was assessed using the QLQ-C30, developed by the European Organization for Research and Treatment of Cancer. This questionnaire includes five functional scales (physical, role, emotional, cognitive, and social), nine symptom scales (such as fatigue, nausea and vomiting, pain, dyspnea, insomnia, loss of appetite, constipation, diarrhea, and financial difficulties), and a global health status score. In the functional scales and the global health status score, higher scores indicate better functioning and QoL. Conversely, in the symptom scales, higher scores reflect greater symptom burden. The Cronbach’s α coefficients for this multi-item scale range from 0.52 to 0.89 prior to treatment and from 0.52 to 0.89 during treatment[21].
This study received formal approval from the Institutional Review Board and Ethics Committee of the Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital. Given that this study uses only de-identified patient data and poses no potential risks or adverse effects to participants, the Institutional Review Board and Ethics Committee jointly waived the requirement for informed consent in accordance with relevant regulatory and ethical standards governing retrospective studies.
Data analysis was conducted using SPSS statistical software (version 29.0; SPSS Inc., Chicago, IL, United States). Categorical variables were reported as frequencies and percentages, i.e., [n (%)], and compared using the χ2 test based on fundamental formulas. All continuous variables were tested for normal distribution using the Shapiro-Wilk test. If the data satisfied normality criteria, then they were reported as mean ± SD. A P value less than 0.05 was considered statistically significant. Univariate and multivariate analyses were performed to determine the incidence of catheter-related complications and the QoL in gastrointestinal cancer patients undergoing chemotherapy.
A total of 346 patients were included in the study, with 199 in the PICC group and 147 in the TIVAP group (Table 1). Baseline demographic and clinical characteristics did not differ significantly between the groups. The mean age was comparable (64.48 ± 13.42 years for the PICC group vs 64.87 ± 12.96 years for the TIVAP group; P = 0.789), as were sex distributions (male/female: 53.77%/46.23% vs 55.10%/44.90%; P = 0.806) and BMI (28.78 ± 5.97 kg/m² vs 27.56 ± 6.14 kg/m²; P = 0.065). No significant differences were found in ethnicity, smoking or drinking history, diabetes or hypertension status, allergies, prior fluorouracil use, history of thrombosis or hemorrhage, CVAD history, or planned treatment modality (all P > 0.05). These findings confirm that the groups were well matched at baseline, providing a comparable foundation for subsequent analyses of catheter-related complications and QoL outcomes.
| Parameters | PICC group (n = 199) | TIVAP group (n = 147) | t/χ2 | P value |
| Age (years) | 64.48 ± 13.42 | 64.87 ± 12.96 | 0.268 | 0.789 |
| Male/female | 107 (53.77)/92 (46.23) | 81 (55.10)/66 (44.90) | 0.061 | 0.806 |
| BMI (kg/m²) | 28.78 ± 5.97 | 27.56 ± 6.14 | 1.853 | 0.065 |
| Ethnicity | 1.512 | 0.219 | ||
| Han | 153 (76.88) | 121 (82.31) | ||
| Others | 46 (23.12) | 26 (17.69) | ||
| Smoking history | 0.217 | 0.641 | ||
| Yes | 83 (41.71) | 65 (44.22) | ||
| No | 116 (58.29) | 82 (55.78) | ||
| Drinking history | 0.073 | 0.787 | ||
| Yes | 134 (67.34) | 101 (68.71) | ||
| No | 65 (32.66) | 46 (31.29) | ||
| Diabetes history | 0.242 | 0.623 | ||
| Yes | 52 (26.13) | 35 (23.81) | ||
| No | 147 (73.87) | 112 (76.19) | ||
| Hypertension history | 0.889 | 0.346 | ||
| Yes | 36 (18.09) | 21 (14.29) | ||
| No | 163 (81.91) | 126 (85.71) | ||
| Allergy history | 1.456 | 0.228 | ||
| Yes | 43 (21.61) | 40 (27.21) | ||
| No | 156 (78.39) | 107 (72.79) | ||
| Taking fluorouracil | 0.013 | 0.910 | ||
| Yes | 122 (61.31) | 91 (61.90) | ||
| No | 77 (38.69) | 56 (38.10) | ||
| Past history of thrombosis | 2.527 | 0.112 | ||
| Yes | 52 (26.13) | 50 (34.01) | ||
| No | 147 (73.87) | 97 (65.99) | ||
| Past history of hemorrhage | 0.257 | 0.612 | ||
| Yes | 35 (17.59) | 29 (19.73) | ||
| No | 164 (82.41) | 118 (80.27) | ||
| CVAD history | 0.035 | 0.851 | ||
| Yes | 168 (84.42) | 123 (83.67) | ||
| No | 31 (15.58) | 24 (16.33) | ||
| Planned treatment mode | 0 | 1.000 | ||
| Inpatient | 6 (3.02) | 5 (3.40) | ||
| Outpatient | 193 (96.98) | 142 (96.60) |
No statistically significant differences were observed in gastrointestinal cancer features between the PICC and TIVAP groups (Table 2). Tumor site distribution was similar, with esophageal, gastric, and colorectal cancers comprising the majority of cases in both groups (P = 0.368). Likewise, tumor staging was comparable, with no significant difference in the proportion of patients staged < 3 and ≥ 3 (P = 0.670). These results demonstrate that both groups were well balanced in terms of primary tumor characteristics at baseline.
| Parameters | PICC group (n = 199) | TIVAP group (n = 147) | χ2 | P value |
| Tumor site | 3.157 | 0.368 | ||
| Esophagus | 52 (26.13) | 45 (30.61) | ||
| Stomach | 59 (29.65) | 40 (27.21) | ||
| Colorectal | 68 (34.17) | 54 (36.73) | ||
| Others | 20 (10.05) | 8 (5.44) | ||
| Tumor staging | 0.181 | 0.670 | ||
| < 3 | 43 (21.61) | 29 (19.73) | ||
| ≥ 3 | 156 (78.39) | 118 (80.27) |
No statistically significant differences in insertion characteristics were found between the PICC and TIVAP groups (Table 3). The right side was the predominant catheter placement site in both groups (P = 0.371), and most patients underwent successful puncture on the first attempt (P = 0.953). Anesthetic approaches, including local anesthesia, with or without sedation or general anesthesia, were comparable (P = 0.076). Tip position, prophylactic antibiotic administration, dressing type, and treatment goal (adjuvant vs palliative) did not differ significantly between groups (all P > 0.05). The mean operating time for PICC and TIVAP placements was similar (43.41 ± 12.28 minutes vs 41.74 ± 12.62 minutes; P = 0.218). These results confirm that insertion procedures were well balanced between the two groups, minimizing procedural bias in subsequent analyses.
| Parameters | PICC group (n = 199) | TIVAP group (n = 147) | t/χ2 | P value |
| Laterality | 0.800 | 0.371 | ||
| Right | 136 (68.34) | 107 (72.79) | ||
| Left | 63 (31.66) | 40 (27.21) | ||
| Puncture attempts | 0.003 | 0.953 | ||
| Yes | 7 (3.52) | 5 (3.40) | ||
| No | 192 (96.48) | 142 (96.60) | ||
| Anesthetic approach | 6.872 | 0.076 | ||
| No LA | 2 (1.01) | 3 (2.04) | ||
| LA only | 168 (84.42) | 135 (91.84) | ||
| LA and sedation | 10 (5.03) | 4 (2.72) | ||
| General anesthetic | 19 (9.55) | 5 (3.40) | ||
| Tip position | 0.035 | 0.852 | ||
| Distal SVC/RA | 165 (82.91) | 123 (83.67) | ||
| Others | 34 (17.09) | 24 (16.33) | ||
| Prophylactic antibiotics given | 1.910 | 0.167 | ||
| Yes | 39 (19.60) | 38 (25.85) | ||
| No | 160 (80.40) | 109 (74.15) | ||
| Type of dressing applied | 1.130 | 0.288 | ||
| Non-antimicrobial | 140 (70.35) | 111 (75.51) | ||
| Antimicrobial | 59 (29.65) | 36 (24.49) | ||
| Treatment goal | 1.025 | 0.311 | ||
| Adjuvant | 155 (77.89) | 121 (82.31) | ||
| Palliative | 44 (22.11) | 26 (17.69) | ||
| Operating time (min) | 43.41 ± 12.28 | 41.74 ± 12.62 | 1.234 | 0.218 |
The incidence of catheter-related complications was significantly lower in the TIVAP group than in the PICC group (at least one complication: 31.29% vs 46.23%, respectively; P = 0.005) (Table 4). No pneumothorax occurred in either group, and hematoma rates were similar (P = 0.794). The TIVAP group exhibited a significantly lower rates of procedure failure (10.20% vs 4.02%; P = 0.022), CR-DVT (3.40% vs 10.55%; P = 0.013), catheter infection (10.88% vs 3.02%; P = 0.003), CRBSI (8.16% vs 2.51%; P = 0.016), catheter occlusion (2.72% vs 11.56%; P = 0.002), and mechanical events (3.40% vs 11.56%; P = 0.006) compared with the PICC group. These findings suggest that TIVAP is associated with a lower risk of catheter-related complications in chemotherapy patients with gastrointestinal cancer.
| Parameters | PICC group (n = 199) | TIVAP group (n = 147) | χ2 | P value |
| Number of complications | 7.869 | 0.005 | ||
| 0 | 107 (53.77) | 101 (68.71) | ||
| ≥ 1 | 92 (46.23) | 46 (31.29) | ||
| Pneumothorax | 0 (0) | 0 (0) | 0 | 1.000 |
| Haematoma | 15 (7.54) | 10 (6.80) | 0.068 | 0.794 |
| Procedure failed | 8 (4.02) | 15 (10.20) | 5.210 | 0.022 |
| CR-DVT | 21 (10.55) | 5 (3.40) | 6.222 | 0.013 |
| Catheter infection | 6 (3.02) | 16 (10.88) | 8.793 | 0.003 |
| CRBSI | 5 (2.51) | 12 (8.16) | 5.778 | 0.016 |
| Catheter occlusion | 23 (11.56) | 4 (2.72) | 9.176 | 0.002 |
| Mechanical event | 23 (11.56) | 5 (3.40) | 7.562 | 0.006 |
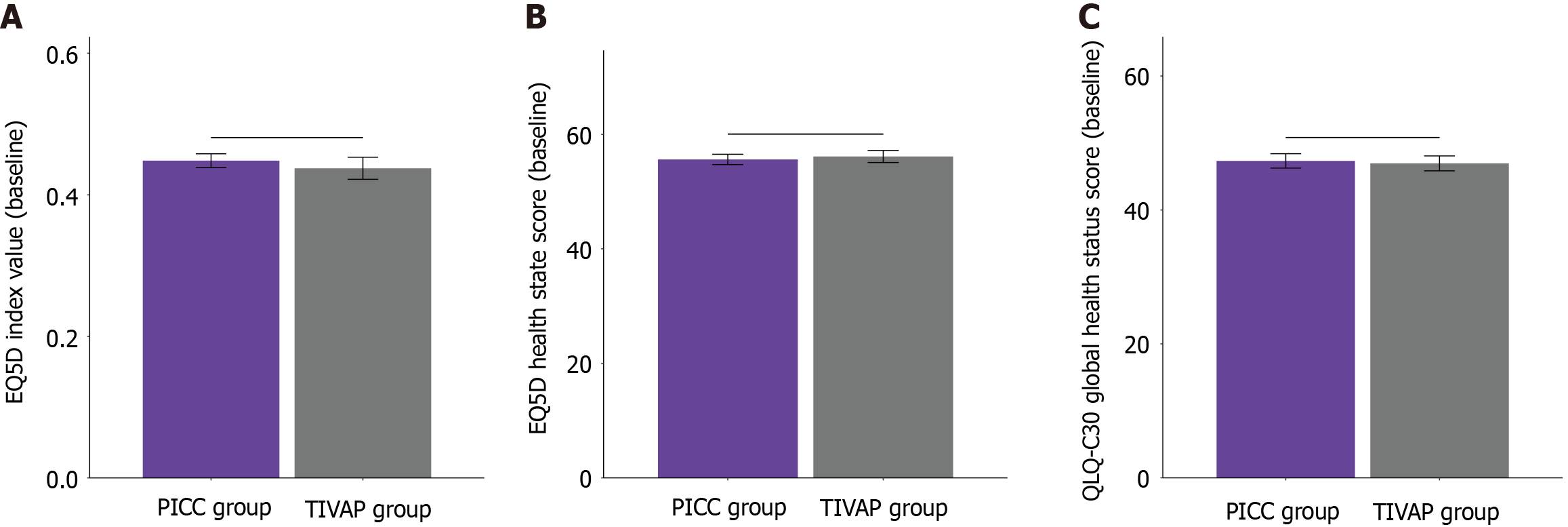
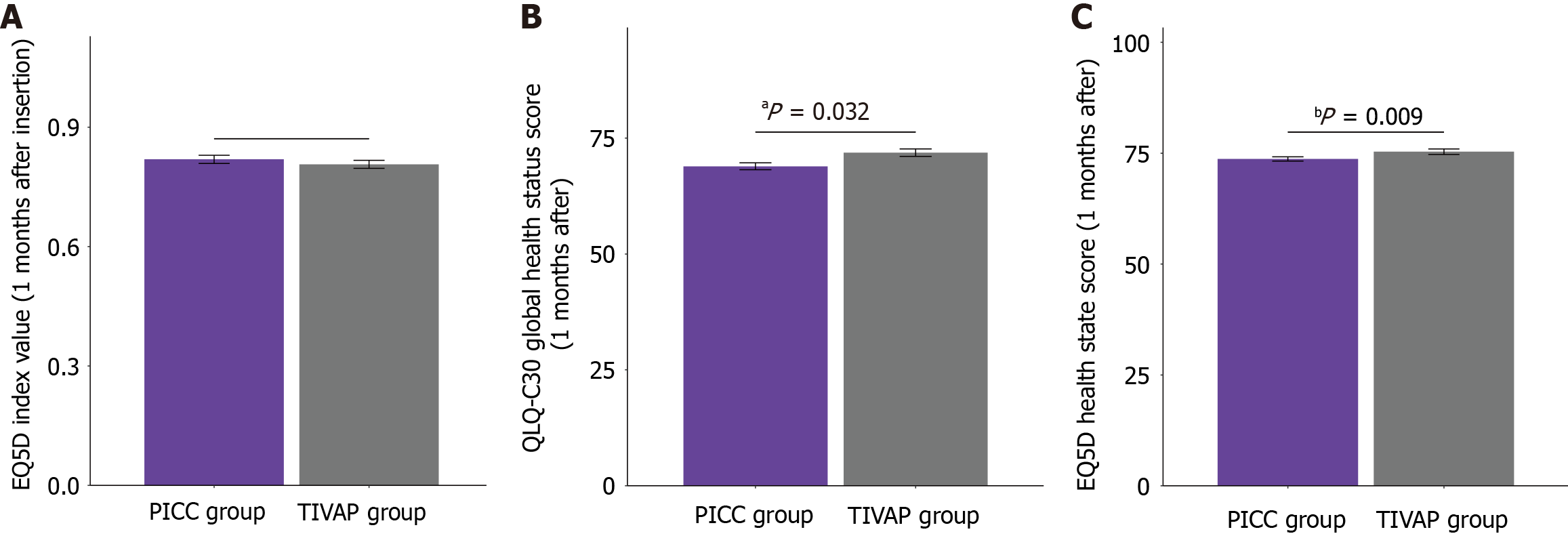
At baseline, no statistically significant differences in QoL scores were found between the PICC and TIVAP groups (Figure 1). The EQ-5D index values were comparable (0.45 ± 0.14 for the PICC group vs 0.44 ± 0.19 for the TIVAP group; P = 0.552), as were the EQ-5D health state scores (55.62 ± 12.84 vs 56.14 ± 12.75; P = 0.710) and QLQ-C30 global health status scores (47.32 ± 15.18 vs 46.96 ± 13.47; P = 0.816). At one month after catheter insertion (Figure 2), the EQ-5D index value remained comparable between the groups (0.82 ± 0.14 vs 0.81 ± 0.12; P = 0.378). However, the TIVAP group demonstrated significantly higher EQ-5D health state scores (75.34 ± 7.52 vs 73.69 ± 6.62; P = 0.032) and QLQ-C30 global health status scores (71.85 ± 9.93 vs 68.91 ± 10.65; P = 0.009) than the PICC group. These findings suggest that TIVAP is associated with better self-reported QoL and global health status at one month after catheter insertion in chemotherapy patients with gastrointestinal cancer.
Univariate and multivariate analyses identified several independent factors associated with catheter-related complications in cancer patients undergoing chemotherapy (Tables 5 and 6). The use of TIVAPs was significantly linked to a reduced risk of complications (multivariate OR, 0.562; 95%CI: 0.355-0.889; P = 0.014). Positioning the catheter tip in the distal superior vena cava/right atrium (SVC/RA) was also an independent protective factor (multivariate OR, 0.478; 95%CI: 0.264-0.868; P = 0.015). Appropriate placement in this location is crucial for minimizing thrombotic and mechanical complications. In addition, prophylactic antibiotic use (multivariate OR, 0.557; 95%CI: 0.314-0.986; P = 0.045) and the application of antimicrobial dressings (multivariate OR, 0.517; 95%CI: 0.305-0.877; P = 0.014) were significantly associated with a reduced incidence of catheter-related complications. These findings underscore the importance of device selection, catheter tip positioning, and perioperative management in mitigating catheter-related risks.
| Parameters | Univariate analysis | Multivariate analysis | ||||
| P value | OR | 95%CI | P value | OR | 95%CI | |
| TIVAP | 0.005 | 0.530 | 0.337-0.825 | 0.014 | 0.562 | 0.355-0.889 |
| Distal SVC/RA | 0.022 | 0.515 | 0.290-0.908 | 0.015 | 0.478 | 0.264-0.868 |
| Prophylactic antibiotics given | 0.011 | 0.487 | 0.274-0.839 | 0.045 | 0.557 | 0.314-0.986 |
| Antimicrobial dressing applied | 0.030 | 0.572 | 0.343-0.939 | 0.014 | 0.517 | 0.305-0.877 |
| Parameters | Univariate analysis | Multivariate analysis | ||||
| P value | OR | 95%CI | P value | OR | 95%CI | |
| TIVAP | 0.005 | 0.522 | 0.330-0.816 | 0.020 | 0.578 | 0.364-0.918 |
| Distal SVC/RA | 0.012 | 0.511 | 0.302-0.862 | 0.026 | 0.542 | 0.317-0.929 |
| Prophylactic antibiotics given | 0.044 | 0.582 | 0.339-0.975 | 0.047 | 0.591 | 0.344-1.015 |
| Antimicrobial dressing applied | 0.019 | 0.554 | 0.335-0.902 | 0.049 | 0.601 | 0.362-0.997 |
Univariate and multivariate analyses revealed that TIVAP use was independently associated with improved QoL in chemotherapy patients with gastrointestinal cancer (multivariate OR, 0.578; 95%CI: 0.364-0.918; P = 0.020). Moreover, catheter tip placement in the distal SVC/RA (multivariate OR, 0.542; 95%CI: 0.317-0.929; P = 0.026), administration of prophylactic antibiotics (multivariate OR, 0.591; 95%CI: 0.344-1.015; P = 0.047), and application of antimicrobial dressings (multivariate OR, 0.601; 95%CI: 0.362-0.997; P = 0.049) were all significantly associated with enhanced QoL. These findings highlight the positive impact of TIVAP use and suggest that optimal catheter management strategies-including precise tip positioning and perioperative care-contribute to improved patient-reported outcomes during chemotherapy.
In this retrospective study, we systematically evaluated the impact of TIVAPs compared with PICCs on catheter-related complications and QoL in patients with gastrointestinal cancer undergoing chemotherapy. The findings indicate that TIVAP use is associated with a lower risk of catheter-related complications and improved patient-reported QoL compared with PICCs. These results reinforce the clinical relevance of device selection, meticulous perioperative management, and optimal catheter tip positioning in achieving safer and more effective central venous access in this vulnerable patient population.
The observed reduction in catheter-related complications among patients with TIVAPs compared with PICCs can be attributed to several interrelated factors rooted in the design, material, and anatomical placement of the devices. Contrary to PICCs, which remain externally accessible and traverse the peripheral venous system prior to reaching central veins, TIVAPs are completely implanted beneath the skin and connect directly to a central vein, typically the SVC via the subclavian or internal jugular vein[22]. The subcutaneous reservoir and absence of an external portion render TIVAPs less susceptible to local and systemic infectious complications[23]. By contrast, repeated manipulation of external catheter segments in PICCs-for infusion, aspiration, or dressing changes-increases the risk of skin colonization and subsequent catheter tract infection[24]. However, TIVAPs are accessed only via transcutaneous puncture using sterile, non-coring needles, reducing the cumulative risk of microbial ingress and local infection[25]. This mechanism aligns with our finding of significantly lower catheter infection and bloodstream infection rates in the TIVAP group.
In addition, the risk of mechanical complications, such as catheter occlusion, fracture, and dislodgement, is generally lower in TIVAPs[26]. The external segment of a PICC is exposed to ongoing limb movement, which can lead to mechanical displacement, kinking, lumen occlusion from extraluminal compression, or material fatigue[27]. The subcutaneous placement of TIVAPs shields the device from these external forces, creating a more stable environment that likely contributes to the reduced incidence of mechanical complications[28]. Furthermore, the use of silicone or polyurethane materials in TIVAPs enhances biocompatibility and flexibility, reducing the likelihood of valve malfunction and thrombotic occlusion[29].
Thrombotic events such as CR-DVT are an important concern in cancer patients with central venous access, given the hypercoagulable state associated with malignancy and cytotoxic chemotherapy[30]. In this study, TIVAPs were associated with a substantially lower incidence of CR-DVT compared with PICCs. Several factors may contribute to this benefit. The larger outer diameter and greater intravascular length of PICCs, particularly when placed in small-caliber basilic or cephalic veins, can disrupt venous hemodynamics, irritate the endothelium, and promote stasis-major components of Virchow’s triad for thrombogenesis[30]. Liu et al[31] noted that the mechanical irritation from PICC can lead to endothelial damage, a crucial factor in Virchow’s triad. This damage triggers a cascade of inflammatory responses, promoting platelet adhesion and activation, thereby increasing thrombus formation risk. TIVAPs, when appropriately positioned in a high-flow central vein, may induce less turbulence and consequently reduce thrombotic risk[32]. Moreover, the lower frequency of manipulation and longer required maintenance intervals for TIVAPs minimize the necessity for repeated flushing or invasive handling, both of which exhibit the potential to precipitate minor endothelial trauma or introduce prothrombotic contaminants[32].
In multivariate modeling, catheter tip positioning, particularly at the distal SVC or RA, emerged as a significant protective factor against complications and diminished QoL. Optimal tip placement minimizes turbulence at the catheter tip and reduces vascular endothelium irritation-key contributors to thrombotic and mechanical sequelae[33]. Malpositioned or migrated catheter tips can lead to infusion extravasation, arrhythmias, or even perforation, thereby amplifying patient morbidity and hospital resource utilization[34]. Thus, meticulous attention to tip location-verified radiographically-remains an essential safeguard in central venous device management.
Perioperative measures, including prophylactic antibiotic administration and antimicrobial dressings, independently mitigated the risk of catheter-related complications, according to our analysis. The protective effect of these strategies stems from their ability to reduce skin colonization and early device infection, particularly during the immediate post-insertion period, when tissue barriers have not fully matured around the device. While routine antibiotic prophylaxis for central venous access is not universally endorsed across all guidelines, our findings suggest its selective use may benefit high-risk patients or align with institutional protocols. Similarly, antimicrobial dressings, whether impregnated with chlorhexidine or other biocidal agents, have demonstrated efficacy in reducing central line-associated bloodstream infections in meta-analyses and clinical practice guidelines[35,36].
The study demonstrates that TIVAP use is associated not only with lower complication rates but also with markedly improved patient-reported QoL. The mechanisms underlying this association are multifaceted and warrant detailed exploration. First, the reduced frequency of device manipulation and fewer required maintenance visits inherent to TIVAPs result in less disruption to patients’ daily routines and fewer hospital or clinic visits[37]. TIVAPs typically require flushing every four weeks, whereas PICCs necessitate at least weekly interventions and more frequent dressing changes[38]. For patients undergoing repeated chemotherapy cycles, this reduced need for ongoing care can offer substantial psychological and logistical benefits-promoting a greater sense of normalcy and patient autonomy[38].
Second, the cosmetic and functional disadvantages of an external catheter segment with a PICC-including local discomfort, activity limitations, aesthetic concerns, and exposure to environmental pathogens-can significantly impact QoL[39]. Many patients feel self-conscious about the visibility of a PICC, and activities such as bathing, exercise, and sleep may be restricted due to the need to protect and monitor the external access[39]. By contrast, TIVAPs, being entirely concealed beneath the skin when not in use, enable a greater degree of social engagement and physical activity. This approach allows individuals to participate in personal, occupational, and recreational activities without constant reminders of their underlying illness[36].
Third, the lower incidence of complications, such as infections, thrombosis, and occlusions, among TIVAP users directly translates to fewer interruptions in cancer therapy, reduced need for re-intervention or hospital admissions, and less cumulative physical discomfort[37]. On a psychosocial level, these tangible benefits alleviate the emotional and cognitive burden associated with cancer therapy-a finding reflected in the significantly higher EORTC QLQ-C30 and EQ-5D scores in our study.
The choice between TIVAPs and PICCs is rarely based solely on theoretical complication risk. Factors including anticipated therapy duration, vascular anatomy, prior device complications, comorbidities, institutional protocols, resource constraints, and patient preferences must all be considered. Patient preferences for TIVAPs vs PICCs vary widely based on comfort, aesthetics, and ease of use. Some patients prefer TIVAPs for their reduced visibility under clothing and lower maintenance requirements, whereas others find the less invasive placement of PICCs more appealing. To enhance the comprehensiveness of our QoL assessment, future studies could incorporate patient preference surveys evaluating subjective factors, such as comfort, cosmetic satisfaction, and overall convenience. These insights provide a more holistic understanding of each device’s impact on patient well-being. Although TIVAPs involve higher upfront costs and require surgical placement under local or general anesthesia, their long-term benefits-demonstrated here-support their preferential consideration for prolonged chemotherapy or when optimizing patient comfort and QoL is paramount.
One significant limitation of this study is the potential for selection bias due to uncontrolled confounding factors. For example, chemotherapy regimens and patient compliance may have influenced the outcomes. Different chemotherapy protocols vary in intensity and side effect profiles, which could affect the incidence of catheter-related complications and overall QoL. In addition, patient adherence to prescribed care routines, including maintenance visits and self-care practices, plays a crucial role in the performance and longevity of CVADs. These variables were not controlled in our analysis, potentially introducing selection bias into our findings.
Our findings should be contextualized within its retrospective design, which carries inherent limitations, including potential unmeasured confounding factors, recall bias, and limitations in data completeness despite rigorous inclusion criteria. Nonetheless, the relatively large sample size, robust statistical analysis, and comprehensive evaluation of clinical and patient-reported outcomes provide compelling evidence supporting the use of TIVAPs in gastrointestinal cancer patients undergoing chemotherapy.
In summary, this study contributes to the growing body of evidence demonstrating that the selection of TIVAPs, combined with meticulous tip positioning and perioperative care, significantly reduces catheter-related complications and enhances HRQoL in patients undergoing chemotherapy for gastrointestinal cancer. These findings underscore the importance of multidisciplinary collaboration in vascular access planning, continuous surveillance for device complications, and patient-centered communication regarding device selection and management.
| 1. | Annetta MG, Marche B, Ortiz Miluy G, Pittiruti M. Totally implanted central venous access devices inserted by the femoral route: A narrative review and the proposal of a novel approach, the FICC-port. J Vasc Access. 2025;26:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Annetta MG, Pinelli F, Ortiz Miluy G, Scoppettuolo G, Pittiruti M. The SaRePo protocol: A seven-step strategy to minimize complications potentially related to the removal of totally implanted central venous access devices. J Vasc Access. 2025;11297298251333863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Arredondo Montero J. Surgical technique: placement of a totally implantable venous access port (TIVAP) through a cephalic vein cutdown in pediatric patients. Transl Pediatr. 2024;13:1820-1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Augustin AM, Kertels O, Wiegering V, Thurner A, Kickuth R. Percutaneous implantation of peripherally inserted totally implantable venous access systems in the forearm in adolescent patients. Pediatr Radiol. 2022;52:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Bailleul A, Fulgencio JP, Vimont S, Mordelet C, Ray B, Lassel L, Lapidus N, Quesnel C, Garnier M. Risk factors and prognostic significance of infection of totally implantable vascular access port in solid tumor patients: A prospective cohort study. Infect Dis Now. 2023;53:104766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Lan Y, Wu L, Guo J, Wang J, Guan H, Li B, Liu L, Zhang L, Hong Y, Deng J, Zhu J, Lu S, Sun F, Huang J, Sun X, Zhang Y, Wang J, Cai R. Risk factors for totally implantable access ports-associated thrombosis in pediatric oncology patients. Sci Rep. 2023;13:3553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Ding W, Qiu L, Li T, Su W, Yu Q, Hu T, Wang C, Fan C, Wang W. Ultrasound-guided totally implantable venous access ports placement via right brachiocephalic vein in pediatric population: A clinical debut. Pediatr Blood Cancer. 2022;69:e29911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Zhen C, Yandong W, Mao Y, Chao L, Jun Z. Internal jugular vein versus external jugular vein as the insertion site for totally implantable venous access ports in children: a randomized comparative study. Pediatr Surg Int. 2025;41:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Chang TC, Yen MH, Kiu KT. Incidence and risk factor for infection of totally implantable venous access port. Langenbecks Arch Surg. 2022;407:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Thiel K, Kalmbach S, Maier G, Wichmann D, Schenk M, Königsrainer A, Thiel C. Standardized procedure prevents perioperative and early complications in totally implantable venous-access ports-a complication analysis of more than 1000 TIVAP implantations. Langenbecks Arch Surg. 2022;407:3755-3762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Guo Y, Wang X, Huang Y, Wu X, Wang Y. Evaluation of the efficacy of totally implantable venous access port in breast cancer patients undergoing chemotherapy. Discov Oncol. 2025;16:383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Wang F, Zhu Y, Wang L, Huang C, Mei R, Deng LE, Yang X, Xu Y, Zhang L, Xu M. Machine learning risk prediction model for bloodstream infections related to totally implantable venous access ports in patients with cancer. Asia Pac J Oncol Nurs. 2024;11:100546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Hataoka T, Sanmoto Y, Kinuta S. Cephalic Vein Cut-Down Method for Totally Implantable Venous Access Ports: A Single-Institution Experience. Ann Vasc Surg. 2024;98:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Vélez ÁB. Cephalic Vein Cut Down for Total Implantable Venous Access Ports: A Retrospective Review of a Single Institution Series. EJVES Vasc Forum. 2023;59:2-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Ross AB, Rouanet E, Murphy AJ, Weldon CB, Weil BR. Complications associated with totally implantable access ports in children less than 1 year of age. J Pediatr Surg. 2022;57:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Chang YL, Sae-Lim C, Lin SL, Lai HW, Huang HI, Lai YC, Chen ST, Chen DR. Scarless totally implantable venous access port (TIVAP) implantation: Surgical technique, preliminary results, learning curve, and patients-reported outcome in 125 breast cancer patients. Surg Oncol. 2024;53:102048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Stanley RJP. World Health Organization classification of tumours. 2000. Available from: https://cir.nii.ac.jp/crid/1571135650366951552. |
| 18. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 607] [Article Influence: 303.5] [Reference Citation Analysis (2)] |
| 19. | Moradi N, Poder TG, Safari H, Mojahedian MM, Ameri H. Psychometric properties of the EQ-5D-5L compared with EQ-5D-3L in cancer patients in Iran. Front Oncol. 2022;12:1052155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1542] [Cited by in RCA: 1937] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 21. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11467] [Article Influence: 358.3] [Reference Citation Analysis (0)] |
| 22. | Zhang H, Li Y, Zhu N, Li Y, Fu J, Liu J. Comparison of peripherally inserted central catheters (PICCs) versus totally implantable venous-access ports in pediatric oncology patients, a single center study. Sci Rep. 2022;12:3510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | Mu C, Zhu Z, Miao D, Wu Q, Chen L, Jin Y. Clinical efficacy and safety of a new single-incision axillary vein puncture technique for totally implantable venous access ports. Sci Rep. 2025;15:7281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Wang M, Tang L, Xu R, Qin S, Zhang S. Clinical application of ultrasound-guided totally implantable venous access ports implantation via the posterior approach of the internal jugular vein. J Chin Med Assoc. 2024;87:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Zhang S, Xiao Z, Yang F. Analysis of related complications of totally implantable venous access ports in children's chemotherapy: Single center experience. Medicine (Baltimore). 2022;101:e29899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Deng XB, Peng L, Zhang J, Kong X, Zhao Z, Wang S, Li C, Du Y, Zhou J, Liu L, Yang C. Dual ultrasound-guided totally implantable venous access ports via the right internal jugular vein in pediatric patients with cancer: a preliminary experience in a single institution. World J Pediatr Surg. 2023;6:e000509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Zhou Y, Lan Y, Zhang Q, Song J, He J, Peng N, Peng X, Yang X. Totally implantable venous access ports: A systematic review and meta-analysis comparing subclavian and internal jugular vein punctures. Phlebology. 2022;37:279-288. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Wu J, Zhang L, Jia X, Mu Y, Ding C, Lou Y. The diverse technical choices during the implantation of the totally implantable venous access ports: A review. Phlebology. 2025;40:300-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Pike S, Tan K, Burbridge B. Complications Associated With Totally Implanted Venous Access Devices in the Arm Versus the Chest: A Short-Term Retrospective Study. Can Assoc Radiol J. 2022;73:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Yang WJ, Song MG, Seo TS, Park SJ. Effectiveness of mechanical recanalization for intraluminal occlusion of totally implantable venous access ports. J Vasc Access. 2023;24:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Liu W, He L, Zhou J, Zeng W, Zeng S, Gong Z. Different diagnostic strategies using D-dimer for peripherally inserted central catheter-related upper extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2023;11:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Hara Y, Sumida Y, Yamazaki S, Takei D, Yamashita M, Fukuda A, Hisanaga M, Tanaka T, Wakata K, Miyazaki T, Araki M, Yano H, Nakamura A. Risk factors for infection of totally implantable central venous access ports among patients requiring port removal. J Vasc Access. 2025;26:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Ikeda M, Matsuzuka T, Kakamu T, Nakaegawa Y, Kawase T, Saito Y, Kubota S, Imaizumi M, Murono S. Feasibility of totally implantable venous access ports in the upper arm for patients with head and neck cancer in the modern era of chemotherapy. J Vasc Access. 2024;11297298241279623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Wu J, Zhang L, Jia X, Mu Y, Lou Y. Application of pocket-first technique for implantation of totally implantable venous access ports. BMC Surg. 2024;24:118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Song X, Chen S, Dai Y, Sun Y, Lin X, He J, Xu R. A novel incision technique of a totally implanted venous access port in the upper arm for patients with breast cancer. World J Surg Oncol. 2023;21:162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Xiao XL, Yang QX, Niu HZ, Li LJ, Xie ZJ. A Retrospective Study of the Use of Antibiotic Lock Therapy and Cluster Nursing Management in Infections in Children with Short Bowel Syndrome or Solid Abdominal Tumours Treated with Totally Implantable Venous Access Ports. J Multidiscip Healthc. 2023;16:431-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Salawu K, Arowojolu O, Afolaranmi O, Jimoh M, Nworgu C, Falase B. Totally implantable venous access ports and associated complications in sub-Saharan Africa: a single-centre retrospective analysis. Ecancermedicalscience. 2022;16:1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Sosnowska-Sienkiewicz P, Moryciński S, Januszkiewicz-Lewandowska D, Michalik K, Madziar K, Kukfisz A, Zielińska D, Mańkowski P. Totally implantable venous ports in infants and children: a single-center retrospective study of indications and safety. Front Oncol. 2024;14:1351630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Huang XM, Li X, Deng J, Chen J, Qian L. Clinical applications and research progress of totally implantable venous access ports: a literature review. Front Oncol. 2024;14:1519728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |