Published online Aug 27, 2025. doi: 10.4240/wjgs.v17.i8.108579
Revised: May 30, 2025
Accepted: June 30, 2025
Published online: August 27, 2025
Processing time: 125 Days and 21.8 Hours
No reports have been published on the use of iodine-125 (125I) seed strips com
To evaluate effectiveness and safety of 125I seed trips combined with double SEMS in treating Bismuth type III and IV hilar MBO.
This was a retrospective, observational study conducted from April 2017 to December 2022. Patients with Bismuth-Corlette type III and IV hilar MBO who underwent 125I seed strip implantation combined with double SEMS placement were analyzed. Patient demographics, clinical characteristics, SEMS implantation methods, procedural and clinical outcomes, overall survival, stent patency duration, and complications were evaluated.
Four types of stent implantation were utilized: (1) Type X; (2) Type T; (3) Type Y; and (4) Tandem type. The technical success rate was 94.1% (16/17), and the clinical success rate was 100% (17/17). The median overall survival time was 189.00 days ± 47.27 days (95%CI: 96.35-281.66). The median stent fluency time was 154.00 days ± 12.19 days (95%CI: 130.11-177.89). No serious complications were observed.
This retrospective, observational study suggests that the combination of 125I seed strips with double SEMS may be a safe and potentially effective approach for managing type III and IV hilar MBO patients.
Core Tip: This study explores the innovative use of iodine-125 seed strips combined with double self-expandable metallic stent for treating Bismuth type III and IV hilar malignant biliary obstruction. In a retrospective analysis of 17 patients from April 2017 to December 2022, we achieved a technical success rate of 94.1% and a clinical success rate of 100%. The median overall survival was 189 days, with stent patency lasting an average of 154 days. No serious complications were reported, indicating that this combination therapy is a safe and effective treatment option for these patients.
- Citation: Zhou CG, Zhang Y, Li H, Liu KY, Yang XY, Gao K. Outcomes of iodine-125 seed strips combined with double self-expandable metallic stent for Bismuth type III and IV malignant biliary obstruction. World J Gastrointest Surg 2025; 17(8): 108579
- URL: https://www.wjgnet.com/1948-9366/full/v17/i8/108579.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i8.108579
Hilar malignant biliary obstruction (MBO) is commonly caused by hilar cholangiocarcinoma (HC), primary hepatocellular carcinoma, gallbladder cancer, liver metastases, or metastatic lymph node compression[1]. Due to its silent clinical progression, hilar MBO is rarely diagnosed at an early stage. Fewer than 20% of patients undergo surgical treatment, and the five-year survival rate remains below 30%[2]. Among the various causes of hilar MBO, HC (Klatskin tumor) is the most common, accounting for approximately 60% of all cholangiocarcinomas[3].
According to the Bismuth-Corlette classification, hilar MBO is categorized into four types (I-IV)[4]. Type III and IV involve the confluence of the left and right hepatic ducts as well as the invasion of the left and/or right secondary bile duct branches. Treating type III and IV hilar MBO poses significant challenges, is particularly challenging, with survival in patients with HC estimated at approximately 5.9 months[5].
Current treatment strategies for hilar MBO include surgery, chemotherapy (gemcitabine combined with cisplatin), radiotherapy, biliary self-expandable metallic stent (SEMS) implantation, photodynamic therapy, intraductal radiofrequency, hepatic artery infusion chemotherapy (HAIC), and transcatheter arterial chemoembolization (TACE). Surgical treatment of hilar MBO requires achieving R0 resection margins, which is technically demanding and associated with a morbidity rate ranging from 10% to 50.8% in patients with postoperative type IV disease[6]. Most patients with hilar MBO are diagnosed at an advanced stage, making surgical intervention unfeasible. In such cases, systemic chemotherapy with gemcitabine and cisplatin is the standard first-line treatment, though its effectiveness remains uncertain[7].
For patients with unresectable MBO and an expected survival of more than three months, SEMS implantation is the primary treatment approach. However, stent restenosis remains a major limitation[1]. The use of radioactive iodine-125 (125I) seed therapy has demonstrated promising therapeutic effects in MBO treatment[8-11]. Previous studies have explored the application of double 125I seed strips[12,13] and 125I seed drainage tubes (where the 125I seeds are removed 1-1.5 months post-implantation) in combination with SEMS. However, there is currently no clinical evidence is available on the efficacy of 125I seed strip therapy combined with double SEMS for treating Bismuth type III and IV hilar MBO.
Therefore, this study presents the first retrospective analysis evaluating the effectiveness and safety of 125I strip therapy combined with double SEMS for managing Bismuth type III and IV hilar MBO.
This was a retrospective, observational study conducted at Beijing Chaoyang Hospital, Capital Medical University, Beijing, China from April 2017 to December 2022. The medical records of 195 patients with MBO were reviewed. Based on the inclusion and exclusion criteria, 17 patients with type III and IV hilar MBO who received 125I seed strip combined with double SEMS implantation were included in the study. The remaining patients with type III and IV hilar MBO, type I and II hilar MBO, and biliary-enteric anastomotic stenosis were treated with 125I seed strip combined with single SEMS implantation.
Inclusion criteria: (1) Age between 18 years and 90 years; (2) Diagnosis of hilar MBO confirmed by luminal biliary biopsy pathology or clinical course; (3) Bismuth-Corlette type III and IV unresectable hilar MBO; (4) No history of radiotherapy or chemotherapy; and (5) Karnofsky Performance Status (KPS) scale score > 70.
Exclusion criteria: (1) Bismuth-Corlette type I and II or distal biliary obstruction; (2) Prior treatment with SEMS or endoscopic retrograde cholangiopancreatography; (3) Concomitant malignancies; (4) Massive ascites or severe coa
In this study, the 125I seeds (CIAE-6711), provided by Beijing Zhibo Hi-tech Biotechnology Co., Ltd. (No. H20083013), were used for the procedure. A percutaneous transhepatic biliary drainage (PTBD) puncture kit and a 7.0-8.5 French (7.0-8.5F) biliary drainage catheter were provided by Cook Medical. Additionally, an uncovered nitinol SEMS was used for stent implantation, also supplied by Cook Medical. Luminal biopsy forceps, manufactured by Micro-Tech Co., Ltd., were used for tissue sampling. Various interventional catheters and guidewires were employed as needed throughout the procedure to facilitate accurate placement and deployment of the devices.
Luminal biliary biopsy: A 7F sheath was inserted into the intrahepatic bile duct through a guidewire. Cholangiography was performed through the sheath to identify the location of the biliary obstruction. Following this, a 6F guiding catheter was inserted along a hydrophilic guidewire, and luminal biological forces were introduced through the 6F guiding catheter. The bile duct tissue was clamped 2-3 times at the obstruction site using biopsy forceps. The tissue was then soaked in formaldehyde solution and sent for pathological examination.
125I seed strips preparation: The 125I seed radioactive source used in the procedure was a silver wire containing 125I with a half-life of 60.1 days. The radioactive seed was encapsulated within a laser-sealed medical titanium alloy tube. Each 125I seed measured 4.5 mm ± 0.5 mm in length and 0.8 mm in diameter. The required number of 125I seeds for the procedure was calculated using the following formula: Length of biliary stricture (mm)/4.5 + 4. A 4F catheter (Cook Medical) was heat-sealed at one end, and the calculated number of 125I seeds was inserted into the catheter. The catheter was then heat-sealed at the other end, completing the 125I seed strip. The estimated radiation dose from the 125I seed strips was calculated based on the AAPM Task Group No. 43 Update Report (TG-43U1)[14].
125I seed strip and double SEMS implantation: The 125I seed strip and double SEMS implantation were based on several considerations: Implantation for all patients. The procedure was performed using the double-guidewire technique: (1) One guidewire was used to release the SEMS; and (2) The other was employed to position the 6F guiding catheter at the site of biliary obstruction. The 125I seed strip was deployed through the preset 6F guiding catheter. The handling of the 125I seeds followed the recommendations of the International Commission on Radiological Protection[15]. At the conclusion of the procedure, a 7.0-8.5F drainage catheter was deployed for bile drainage. Four weeks later, the biliary drainage catheter was removed, provided that cholangiography showed SEMS fluency.
Preoperative and postoperative management: Routine preoperative examinations included blood tests, biochemical parameters, coagulation function, tumor markers, myocardial enzymes, blood amylase, computed tomography (CT)/magnetic resonance imaging (MRI) scans, and magnetic resonance cholangiopancreatography (MRCP). Liver protection was administered using 150 mg of magnesium isoglycyrrhizinate. Gastric acid inhibition was achieved with 40 mg of pantoprazole, and anti-infection treatments included 6 g (divided into three doses) of cefoxitin sodium.
Postoperatively, 5 mg morphine was administered subcutaneously to relieve pain. If the SEMS crossed the ampulla of the duodenum, blood amylase levels were monitored. If amylase levels increased, octreotide was administered to inhibit pancreatic enzyme secretion, and rehydrating nutritional support was provided.
Data was collected from both outpatient and inpatient medical records. The primary endpoint of the study was clinical success, defined as a decrease of at least 75% in total bilirubin levels one month postoperatively, compared to pre-operative levels, and the return of normal stool color indicating bile entry into the intestinal circulation.
The secondary endpoint was technical success, defined as complete coverage of the bile duct obstruction segment by the SEMS, with residual biliary stenosis of less than 30% following SEMS implantation. Postoperative cholangiography confirmed that the contrast compound smoothly entered the distal bile duct and duodenum through the SEMS. The 125I seed strip was required to completely cover the bile duct obstruction segment, with both ends extending approximately 1 cm beyond the obstruction segment.
Additional secondary endpoints included SEMS restenosis, stent fluency time, and overall survival. SEMS restenosis was defined as the recurrence of elevated bilirubin levels following SEMS implantation, accompanied by symptoms of cholangitis, such as fever and abdominal pain. It was diagnosed via CT, MRI, MRCP, or cholangiography.
Stent fluency time was defined as the time interval from double SEMS implantation to the occurrence of stent restenosis. In the absence of biliary stent obstruction at the time of patient death, the patency time was considered equivalent to survival time but was censored.
Overall survival was defined as the time interval from the implantation of double SEMS and the 125I seed strip to the time of patient death or the last follow-up.
All patients were followed up through outpatient clinic visits or telephone interviews every 3-6 months. The final follow-up was conducted on July 31, 2023.
All statistical analyses were performed using Statistical Package for the Social Sciences 24.0 statistical software (IBM Corp., Armonk, NY, United States). Continuous variables following a normal distribution are presented as mean ± SD and were compared using Student’s t-test. Continuous variables that did not follow a normal distribution are expressed as median (interquartile range) and were compared using the Mann-Whitney U-test. Categorical variables were analyzed using the χ² test or Fisher’s exact test, as appropriate. The overall survival time and stent patency time were evaluated using Kaplan-Meier survival analysis. A P value < 0.05 was considered statistically significant.
Seventeen patients (6 males, 11 females) with type III and IV hilar MBO underwent 125I seed strip implantation combined with double SEMS placement (Table 1). The mean age of the patients was 69.71 years ± 11.6 years (range: 49-90 years). Among the 195 MBO cases retrospectively reviewed, 118 cases had type I and II hilar MBO, 58 cases had type III and IV hilar MBO, and 19 cases had biliary-enteric anastomotic stenosis. Based on the inclusion and exclusion criteria, 17 patients with type III and IV hilar MBO who received the combined therapy were included in this study.
| Patients | Gender | Age | Primary disease | Bismuth typing | Biopsy | SEMS parameters (mm) | SEMS implantation methods | Number of iodine-125 seeds | Technical success | Clinical success |
| Case 1 | F | 63 | HC | III | No | 8 × 60/8 × 40 | Tandem | 20 | Yes | Yes |
| Case 2 | F | 66 | HC | IV | No | 8 × 60/8 × 60 | Type T | 11 + 13 | Yes | Yes |
| Case 3 | M | 81 | HC | III | No | 8 × 60/8 × 40 | Type T | 12 + 12 | Yes | Yes |
| Case 4 | M | 67 | HC | IV | No | 10 × 40/8 × 60 | Tandem | 16 | Yes | Yes |
| Case 5 | F | 75 | HC | III | No | 8 × 60/8 × 60 | Type X | 15 + 15 | Yes | Yes |
| Case 6 | M | 64 | HC | IV + distal | Heterotypic cells | 8 × 60/8 × 40 | Tandem | 22 | Yes | Yes |
| Case 7 | F | 80 | HC | III | No | 8 × 40/8 × 40 | Type Y | 12 + 12 | Yes | Yes |
| Case 8 | F | 86 | HC | IV | No | 7 × 60/6 × 40 | Type Y | 12 + 12 | Yes | Yes |
| Case 9 | F | 90 | HC | IV | No | 8 × 80/6 × 40 | Type Y | 18 + 8 | Yes | Yes |
| Case 10 | F | 76 | HC | III | Adenocarcinoma | 8 × 60/8 × 60 | Type Y | 14 + 14 | Yes | Yes |
| Case 11 | M | 75 | Cardiac cancer, lymph node metastasis | IV | No | 8 × 80/8 × 60 | Tandem | 24 | Yes | Yes |
| Case 12 | F | 82 | HC | IV | Adenocarcinoma | 8 × 40/8 × 60 | Tandem | 16 | Yes | Yes |
| Case 13 | F | 54 | HC | IV | Adenocarcinoma | 8 × 60/8 × 60/8 × 40 | Type Y | 18 + 18 | Yes | Yes |
| Case 14 | M | 56 | Gallbladder cancer, liver metastasis | IV | Negative | 8 × 80/8 × 80 | Type Y | 20 + 20 | Yes | Yes |
| Case 15 | F | 49 | HC | IV | Adenocarcinoma | 8 × 80/8 × 80 | Type Y | 20 + 20 | Yes | Yes |
| Case 16 | M | 61 | Gastric cancer, liver metastasis | IV | Adenocarcinoma | 8 × 80/8 × 60 | Type T | 16 + 14 | Yes | Yes |
| Case 17 | F | 60 | Gallbladder cancer, liver metastasis | IV | Adenocarcinoma | 8 × 80/8 × 60 | Type X | 16 | No | Yes |
Diagnosis was based on clinical characteristics and disease progression (these include skin itching, scleral icterus, elevated bilirubin levels, elevated tumor markers, enhanced CT or MRCP showing bile duct dilation, and progressive tumor enlargement with metastasis) for 9 patients, 7 of whom underwent an assessment before 2020, when luminal biliary biopsy had not yet been implemented in our department. Two additional patients declined biopsy due to advanced age and systemic metastases. Among the 8 patients who underwent intraluminal biliary biopsy, adenocarcinoma was confirmed in 6 cases, heterotypic cells were identified in one case, and one biopsy result was negative, yielding a positive diagnostic rate of 87.5%.
Among the 17 patients, 13 were diagnosed with HC, 1 with cardiac cancer and lymph node metastasis, 2 with gallbladder cancer and liver metastases, and 1 with gastric cancer and liver metastases. According to the Bismuth classification, 5 patients had type III disease, while 12 had type IV. Additionally, six patients received systemic anticancer therapy, including lenvatinib, HAIC, or TACE.
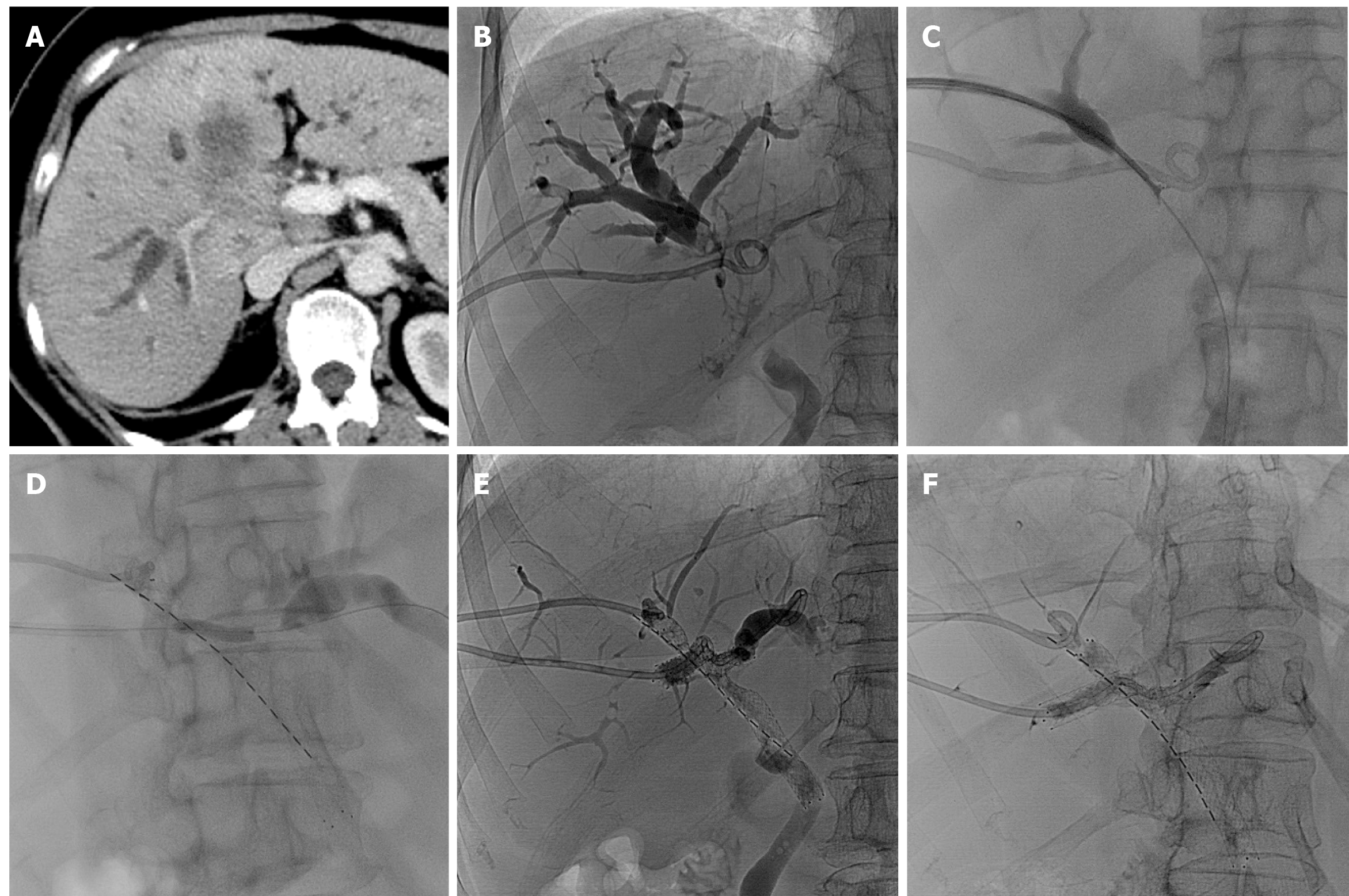
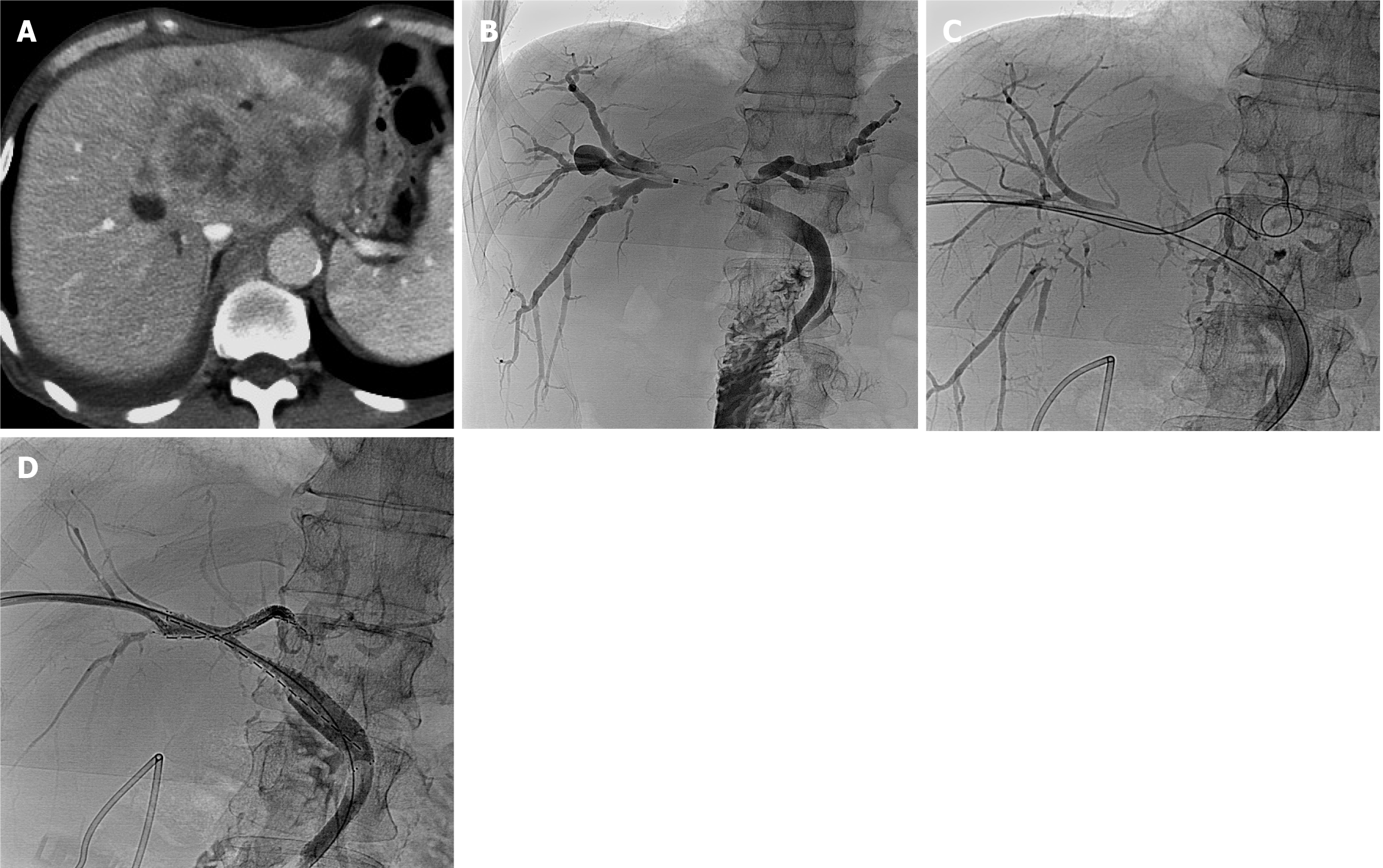
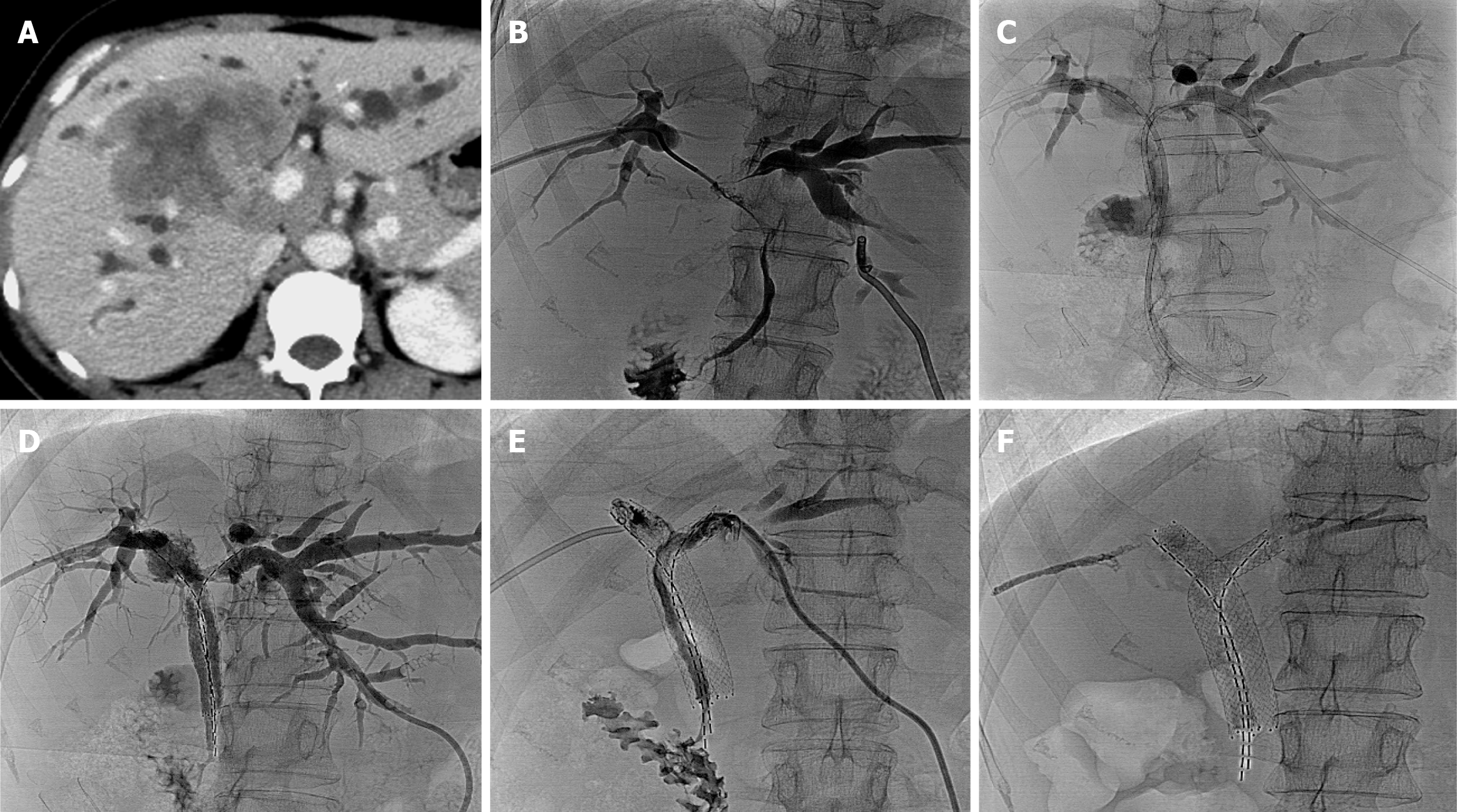
A total of 35 stents were implanted, with diameters of 6-8 mm and lengths ranging from 40-80 mm, were implanted. One patient required three stents. The stent configurations varied, including type X (2 patients; Figure 1), type T (3 patients; Figure 2), type Y (7 patients; Figure 3), and tandem-type stents (5 patients). Due to severe bile duct stenosis, 5 patients required balloon pre-dilation before stent deployment. In four patients, the stents extended beyond the ampulla.
Among the 17 patients, 5 received a single 125I seed strip, while 12 received double seed strips. The number of 125I seed per patient ranged from 16 to 40, with a mean of 25.88 ± 7.50, totaling 440 seeds. Each 125I seed had a radioactivity level of 0.6 mCi. One patient with a type X stent could not receive 125I seed strips due to tortuosity from the right posterior lobe bile duct to the left lobe bile duct. Additionally, in 1 type Y case, a third stent was required as two stents failed to fully cover the narrowed intrahepatic bile duct segment. The distribution of stent placement types was as follows: (1) Type X (11.8%, 2/17); (2) Type T (17.6%, 3/17); (3) Type Y (41.2%, 7/17); and (4) Tandem (29.4%, 5/17).
The procedure demonstrated a technical success rate of 94.1% (16/17) and a clinical success rate of 100% (17/17). The dose reference point was set at 5 mm from the seed axis, with an initial dose rate of 8.34-8.47 cGy/hour and a cumulative absorbed dose (over one half-life) of 82.3-83.6 Gy.
Postoperative biochemical parameters, including total bilirubin, direct bilirubin, alkaline phosphatase, gamma-glutamyl transferase, aspartate aminotransferase, alanine aminotransferase, significantly decreased compared to preoperative values. However, no significant differences were observed in tumor marker levels, including alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 125, before and after the procedure.
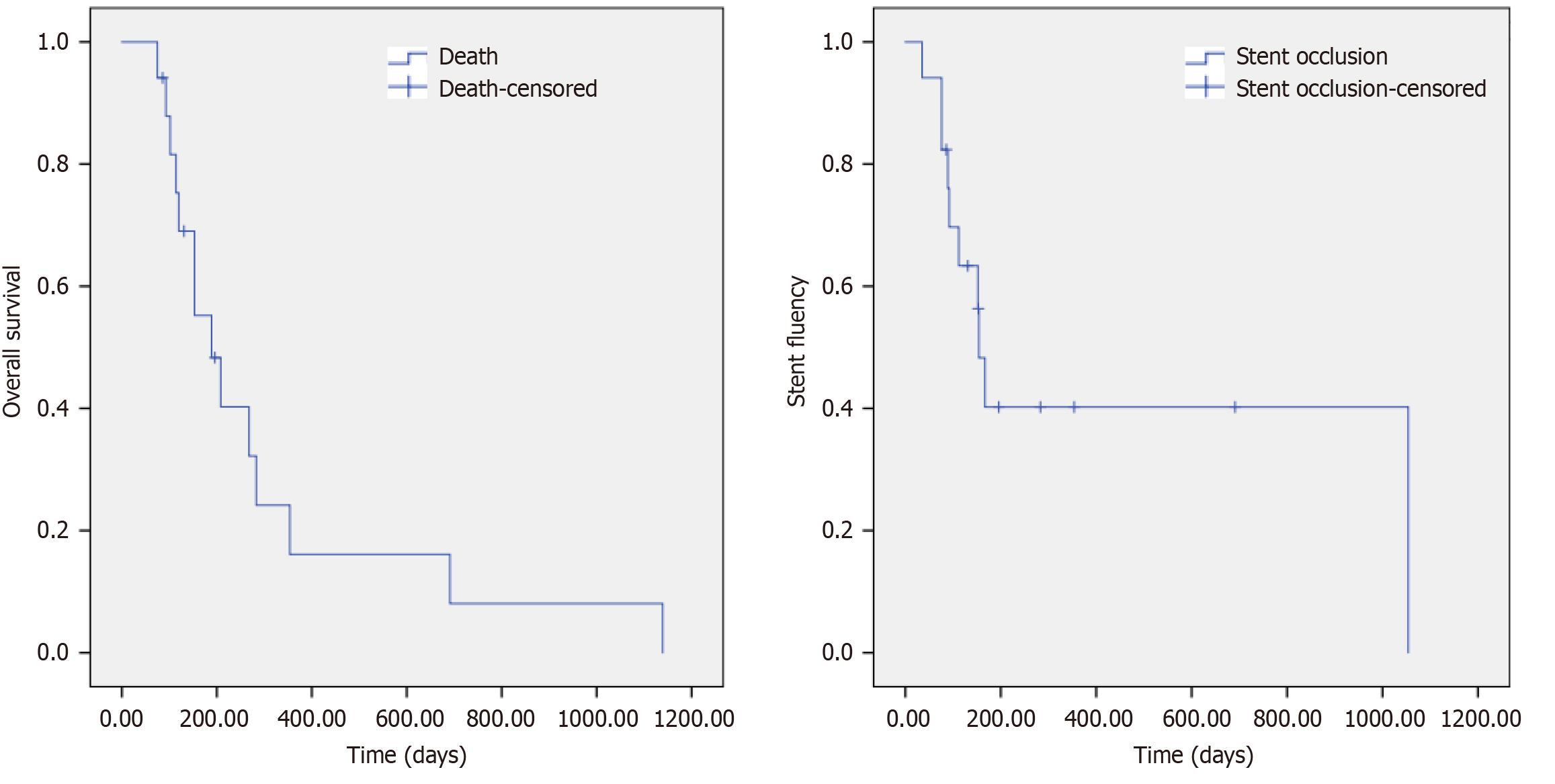
By July 31, 2023, 14 out of 17 patients had died, while 3 remained alive. The causes of death included multiple organ failure in 5 patients, tumor metastases in 6 patients, gastrointestinal bleeding in 2 patients, and cerebrovascular accident in 1 patient. No deaths occurred within 30 days post-procedure. Among the 14 deceased patients, four had functional stents with normal serum bilirubin levels at the time of death, and the three surviving patients continued to have patent stents. The median overall survival was 189.00 ± 47.27 days (95%CI: 96.35-281.66), while the median stent patency duration was 154.00 ± 12.19 days (95%CI: 130.11-177.89; Figure 4).
Early complications included pain in 29.4% (5/17) of patients, fever in 11.7% (2/17), minor biliary bleeding in 11.7% (2/17), asymptomatic amylase elevation in 5.9% (1/17), and transient acute renal failure in 5.9% (1/17). The only late complication observed was 125I seed strip migration in one patient (5.9%, 1/17). No cases of stent fracture, severe biliary bleeding, perforation, severe cholangitis, or other serious complications were reported.
Cholangiocarcinoma is a heterogeneous group of neoplasms originating from the bile duct, classified into intrahepatic, hilar, and extrahepatic types. The latest research shows that immune cell phenotypes and gut microbiota environment may be closely related to the occurrence of cholangiocarcinoma[16,17]. Traditional treatment options include systemic chemotherapy with gemcitabine and cisplatin, surgical resection, and liver transplantation. However, due to the typically advanced stage at diagnosis, fewer than 33% of patients are eligible for surgery, which is associated with a high incidence of complications. In patients with resected cholangiocarcinoma, circulating tumor DNA (ctDNA) status and dynamics have important predictive value for tumor recurrence[18]. In cases where it is challenging to obtain tumor tissue, ctDNA can be used as a substitute and has good consistency with tissue genomic profiling[19]. In recent studies, novel therapeutic approaches, such as targeted therapy and immune checkpoint inhibitors (including programmed death receptor-1, programmed cell death-ligand 1, and cytotoxic T-lymphocyte-associated antigen 4 inhibitors), have emerged as first-line treatment for biliary tract cancers. Despite these advancements, the overall prognosis remains poor[7]. This study contributes to the existing literature by evaluating the combination of 125I seed strip therapy and double SEMS implantation in patients with advanced type III and IV hilar MBO, providing a potential alternative with promising outcomes. Our findings, in line with previous research, show that 125I seed therapy offers a non-invasive treatment option that could be considered in cases where traditional treatments are not feasible.
Based on the level of bile duct obstruction, hilar MBO is classified into four types according to the Bismuth classification (I-IV). Among these, Bismuth type III and IV involve tumor invasion into the second-level intrahepatic bile duct branches, making endoscopic stent placement particularly challenging. For these cases, PTBD has been shown to provide more effective symptom relief than endoscopic approaches[20]. PTBD, along with the implantation of SEMS, is effective in reducing bilirubin levels. However, tumor ingrowth and overgrowth often result in stent re-obstruction within a short period. Various adjunctive therapies, such as intraductal ablation, photodynamic therapy, radiotherapy, and paclitaxel-coated stents[21], have been employed to prolong stent patency, though with limited success.
Radioactive 125I seed implantation is an established treatment for prostate cancer and has also been widely used for solid tumors, including hepatocellular carcinoma, lung cancer, and metastatic tumors[22,23]. Intraluminal brachytherapy with 125I seed strips has shown promising results in managing MBO and portal vein tumor thrombosis. A multicenter, open label, randomized phase III trial in China evaluated 328 patients with unresectable MBO, comparing 125I seed stents (ISG) with uncovered SEMS (USG). The ISG group demonstrated a lower stent restenosis rate at 90, 180, and 360 days (9%, 16%, and 21%, respectively) compared to the USG group (15%, 27%, and 33%, respectively). Additionally, the ISG group exhibited prolonged survival without an increase in treatment-related complications[24]. Retrospective studies from our medical center further suggest that SEMS combined with 125I seed strands is a safe and effective option for treating hilar MBO (Bismuth type I-IV), with potential benefits in extending stent patency and overall survival[11].
A meta-analysis of 11 randomized controlled trials (2012-2018) involving 767 patients compared survival, stent patency, and complications between 125I-stent combination therapy and stent monotherapy. The combination therapy demonstrated lower stent restenosis rates at 3 months, 6 months, 9 months, and 12 months, along with improved mean survival. Importantly, there were no statistically significant differences in complications such as abdominal pain, hemobilia, pancreatitis, cholangitis, or cholecystitis between the two groups[25]. However, this study did not specifically evaluate Bismuth type III and IV patients, limiting its applicability to this subgroup.
Currently, limited data exist comparing the efficacy and safety of 125I therapy vs SEMS in Bismuth type III and IV MBO. A retrospective study[26] of 59 patients (91.5% with type III and IV MBO) compared CT-guided intratumoral 125I seed implantation combined with SEMS to stent monotherapy. The combination therapy significantly prolonged median stent patency (289 days vs 88 days, P = 0.001) and median overall survival (221 days vs 78 days, P = 0.001). Another study[27] comparing radiation-emitting metallic stents (REMS) with SEMS in 59 patients (34 type III and 25 type IV) reported significantly longer median overall survival (338 days vs 141 days, P < 0.001) and stent patency (385 days vs 142 days, P < 0.001) with REMS.
The primary methods for 125I implantation in hilar MBO include: (1) 125I combined with biliary drainage tubes[28]; (2) 125I strips combined with SEMS[11,29]; (3) REMS, 125I seeds loaded stents[27]; and (4) CT-guided 125I implantation[26]. Currently, 125I strips combined with SEMS is the most commonly used approach, offering a high procedure success rate and low complication incidence. However, a major limitation is the inability to replace the 125I strip once it becomes non-functional.
This study is the first to report the efficacy and safety of 125I seed strips combined with double SEMS implantation for treating Bismuth type III and IV hilar MBO. In patients with a performance status score > 70 and a life expectancy > 3 months, 125I strips combined with double SEMS offers a promising treatment approach by ensuring optimal bile drainage and brachytherapy effects. This method contrasts with the treatment for Bismuth type I and II hilar MBO, where a single SEMS combined with 125I strips is commonly used. The choice of stent implantation configuration was tailored to the type of bile duct obstruction, based on four different stent placement strategies (X, T, Y, and Tandem). These configurations help ensure the most effective bile duct clearance and minimize the risk of complications such as stent occlusion, which remains a challenge in advanced hilar MBO cases.
Our results, showing an average of 25.88 ± 7.50 seeds per patient, align with previous studies suggesting that the total number of seeds (ranging from 16 to 40) can effectively provide the necessary radiation dose for adenocarcinoma treatment. The initial dose rate and cumulative absorbed dose fall within safe and effective thresholds, as evidenced by the median overall survival of 189.00 ± 47.27 days and median stent patency of 154.00 ± 12.19 days. These findings further support the viability of 125I seed therapy in prolonging stent patency and improving survival outcomes. While minor complications such as pain, fever, and biliary bleeding were observed, there were no severe complications, which reinforces the safety profile of this treatment modality, consistent with previous reports.
This study has some limitations. First, previous data on double SEMS for treating type III and IV hilar MBO are incomplete, preventing the inclusion of a control group. Second, most patients with type III and IV hilar MBO typically receive bile drainage catheters or 125I strips combined with single SEMS due to poor physical status, making cases suitable for 125I strips combined with double SEMS relatively rare. Consequently, the sample size was small. Third, due to the retrospective nature of the study, six patients also received sequential systemic anticancer therapy, such as lenvatinib, HAIC, and TACE, which could have influenced survival outcomes. Additionally, 125I implantation may trigger antitumor immune responses[30], potentially improving overall survival and reducing stent occlusion rates.
In conclusion, tailored stent implantation strategies were used based on hilar MBO classification. The findings suggest that 125I seed strips combined with double SEMS implantation provide a safe and effective treatment option for Bismuth type III and IV hilar MBO.
| 1. | Kapoor BS, Mauri G, Lorenz JM. Management of Biliary Strictures: State-of-the-Art Review. Radiology. 2018;289:590-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Pu LZ, Singh R, Loong CK, de Moura EG. Malignant Biliary Obstruction: Evidence for Best Practice. Gastroenterol Res Pract. 2016;2016:3296801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Madariaga JR, Iwatsuki S, Todo S, Lee RG, Irish W, Starzl TE. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg. 1998;227:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 170] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 239] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, Eum J, Lee SS, Seo DW, Lee SK. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver. 2009;3:298-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Ku D, Tang R, Pang T, Pleass H, Richardson A, Yuen L, Lam V. Survival outcomes of hepatic resections in Bismuth-Corlette type IV cholangiocarcinoma. ANZ J Surg. 2020;90:1604-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, Cristoferi L, Ciaccio A, Cortinovis DL, Invernizzi P, Massironi S. Clinical treatment of cholangiocarcinoma: an updated comprehensive review. Ann Hepatol. 2022;27:100737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 8. | Hasimu A, Gu JP, Ji WZ, Zhang HX, Zhu DW, Ren WX. Comparative Study of Percutaneous Transhepatic Biliary Stent Placement with or without Iodine-125 Seeds for Treating Patients with Malignant Biliary Obstruction. J Vasc Interv Radiol. 2017;28:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Zhou WZ, Fu YM, Yang ZQ, Shi HB, Liu S, Xia JG, Zhou CG. Study of Percutaneous Stent Placement with Iodine-125 Seed Strand for Malignant Biliary Obstruction. Cardiovasc Intervent Radiol. 2019;42:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Jiao D, Wu G, Ren J, Han X. Study of self-expandable metallic stent placement intraluminal (125)I seed strands brachytherapy of malignant biliary obstruction. Surg Endosc. 2017;31:4996-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Zhou C, Li H, Huang Q, Wang J, Gao K. Biliary self-expandable metallic stent combined with Iodine-125 seeds strand in the treatment of hilar malignant biliary obstruction. J Int Med Res. 2020;48:300060519887843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Li ZM, Jiao DC, Han XW, Lei QY, Zhou XL, Xu M. Preliminary application of brachytherapy with double-strand (125)I seeds and biliary drainage for malignant obstructive jaundice. Surg Endosc. 2022;36:4932-4938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Li Z, Jiao D, Han X, Liu Z. A Comparative Study of Self-Expandable Metallic Stent Combined with Double (125)I Seeds Strands or Single (125)I Seeds Strand in the Treatment of Advanced Perihilar Cholangiocarcinoma with Malignant Obstructive Jaundice. Onco Targets Ther. 2021;14:4077-4086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Rivard MJ, Coursey BM, DeWerd LA, Hanson WF, Huq MS, Ibbott GS, Mitch MG, Nath R, Williamson JF. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004;31:633-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1254] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 15. | International Commission on Radiological Protection. Radiation safety aspects of brachytherapy for prostate cancer using permanently implanted sources. A report of ICRP Publication 98. Ann ICRP. 2005;35:iii-ivi, 3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Hu Y, Wang K, Chen Y, Jin Y, Guo Q, Tang H. Causal relationship between immune cell phenotypes and risk of biliary tract cancer: evidence from Mendelian randomization analysis. Front Immunol. 2024;15:1430551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 17. | Wang K, Wang S, Qin X, Chen Y, Chen Y, Wang J, Zhang Y, Guo Q, Zhou C, Zou D. The causal relationship between gut microbiota and biliary tract cancer: comprehensive bidirectional Mendelian randomization analysis. Front Cell Infect Microbiol. 2024;14:1308742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Yoo C, Jeong H, Jeong JH, Kim KP, Lee S, Ryoo BY, Hwang DW, Lee JH, Moon DB, Kim KH, Lee SS, Song TJ, Oh D, Lee MA, Chon HJ, Lee JS, Laliotis G, Rivero-Hinojosa S, Spickard E, Renner D, Dutta P, Palsuledesai CC, Sharma S, Malhotra M, Jurdi A, Liu MC. Circulating tumor DNA status and dynamics predict recurrence in patients with resected extrahepatic cholangiocarcinoma. J Hepatol. 2025;82:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Hwang S, Woo S, Kang B, Kang H, Kim JS, Lee SH, Kwon CI, Kyung DS, Kim HP, Kim G, Kim C, Chon HJ. Concordance of ctDNA and tissue genomic profiling in advanced biliary tract cancer. J Hepatol. 2025;82:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Dondossola D, Ghidini M, Grossi F, Rossi G, Foschi D. Practical review for diagnosis and clinical management of perihilar cholangiocarcinoma. World J Gastroenterol. 2020;26:3542-3561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (2)] |
| 21. | Boulay BR, Birg A. Malignant biliary obstruction: From palliation to treatment. World J Gastrointest Oncol. 2016;8:498-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Routman DM, Funk RK, Stish BJ, Mynderse LA, Wilson TM, McLaren R, Harmsen WS, Mara K, Deufel CL, Furutani KM, Haddock MG, Pisansky TM, Choo CR, Davis BJ. Permanent prostate brachytherapy monotherapy with I-125 for low- and intermediate-risk prostate cancer: Outcomes in 974 patients. Brachytherapy. 2019;18:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Zhang W, Li J, Li R, Zhang Y, Han M, Ma W. Efficacy and safety of iodine-125 radioactive seeds brachytherapy for advanced non-small cell lung cancer-A meta-analysis. Brachytherapy. 2018;17:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Zhu HD, Guo JH, Huang M, Ji JS, Xu H, Lu J, Li HL, Wang WH, Li YL, Ni CF, Shi HB, Xiao EH, Lv WF, Sun JH, Xu K, Han GH, Du LA, Ren WX, Li MQ, Mao AW, Xiang H, Zhang KX, Min J, Zhu GY, Su C, Chen L, Teng GJ. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: A multicenter trial. J Hepatol. 2018;68:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Xiang Y, Lu S, Li Y, Liu Z, Wang W. Iodine-125 Seeds Combined With Biliary Stent Placement Versus Stent Placement Alone For Unresectable Malignant Biliary Obstruction: A Meta-Analysis Of Randomized Controlled Trials. J Cancer. 2021;12:1334-1342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Sheng Y, Fu X, Wang G, Mu M, Jiang W, Chen Z, Qi H, Gao F. Safety and efficacy of self-expandable metallic stent combined with (125)I brachytherapy for the treatment of malignant obstructive jaundice. Cancer Imaging. 2023;23:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Lu J, Guo JH, Zhu HD, Zhu GY, Wang Y, Zhang Q, Chen L, Wang C, Pan TF, Teng GJ. Palliative treatment with radiation-emitting metallic stents in unresectable Bismuth type III or IV hilar cholangiocarcinoma. ESMO Open. 2017;2:e000242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Li S, Li B, Li L, Yang X, Xu F, Wang W. The efficacy of the combination of percutaneous transhepatic biliary drainage and (125)I stranded seeds for malignant bile duct obstruction treatment. J Contemp Brachytherapy. 2020;12:225-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | An R, Zhang H, Yu J, Cao Y, Ren J, Guo W, Luo Z. Self-expandable metallic stent with (125)I seed strand in malignant biliary obstruction: a self-made delivery system and novel implantation method. Ann Transl Med. 2021;9:1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Xiang GA, Chen KY, Wang HN, Xiao JF. [Immunological influence of iodine-125 implantation in patients with hepatocellular carcinoma resection]. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:292-294. [PubMed] |