Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.104923
Revised: March 13, 2025
Accepted: May 6, 2025
Published online: June 27, 2025
Processing time: 107 Days and 3.2 Hours
Primary liver cancer (PLC) is characterized by high malignancy, rapid disease progression, and persistent high incidence and mortality rates, posing a sig
To probe the clinical significance of CD3+CD161+NKT cell subsets and AFP and GGT changes in the peripheral blood of individuals with PLC.
The PLC group comprised 30 patients diagnosed with PLC who were admitted to our hospital between July 2022 and December 2023, whereas the control group consisted of 30 healthy individuals undergoing routine physical examinations at our hospital. Peripheral blood samples were harvested from both cohorts of patients. The levels of CD4+NKT, CD8+NKT, CD3+CD56+NKT, CD8+CD56+NKT, CD3+CD161+NKT, and CD3-CD161+NKT were measured by flow cytometry. Serum AFP content was determined using a fully automatic immunoassay ana
No significant disparities were observed in the counts of white blood cells, neutrophils, and platelets, as well as the levels of blood urea nitrogen and serum creatinine between the two groups (P > 0.05). Lymphocytes, red blood cells, hemoglobin, total protein, albumin, and globulin were more attenuated in the PLC group than in the control group, while glutamic-pyruvic transaminase, glutamic oxalacetic transaminase, and carcinoembryonic antigen levels were increased in the PLC cohort compared with the control cohort, with statistical significance (P < 0.05). No substantial difference was discovered in peripheral blood CD4+NKT, CD8+NKT, and CD3+CD56+NKT cells between the two cohorts (P > 0.05). The percentage of CD8+CD56+NKT cells (8.35% ± 1.01%), CD3+CD161+NKT cells (14.36% ± 1.55%), and CD3-CD161+NKT cells (12.08% ± 1.34%) in the PLC group was higher than that in the control group (P < 0.05). The levels of AFP (335.71 ± 20.89 ng/mL) and GGT (136.87 ± 15.62 U/mL) in the PLC cohort were elevated within the PLC cohort compared with the control cohort (P < 0.05). The sensitivity of CD8+CD56+NKT, CD3+CD161+NKT, CD3-CD161+NKT, AFP, and GGT alone for diagnosing PLC was 70.00%, 83.33%, 80.00%, 56.67%, and 53.33%, respectively (P < 0.05), with specificity rates of 66.67%, 80.00%, 76.67%, 76.67%, and 66.67%, res
The levels of CD8+CD56+NKT, CD3+CD161+NKT, CD3-CD161+NKT, AFP, and GGT in the peripheral blood of patients with PLC were markedly elevated. The combined detection of these five indicators can improve the sensitivity and specificity of PLC diagnosis, providing solid evidence for the early clinical diagnosis of PLC.
Core Tip: This investigation determined cluster of differentiation 4-positive (CD4+), CD8+, CD3+CD56+, CD8+CD56+, CD3+CD161+, and CD3-CD161+ natural killer T (NKT) cell, alpha fetoprotein (AFP), and gamma-glutamyl transpeptidase (GGT) levels in the peripheral blood samples of patients with primary liver cancer (PLC) and healthy individuals. The aim was to confirm the diagnostic value of NKT cell subsets, AFP, and GGT levels in the context of PLC. The results revealed a marked increase in the levels of CD8+CD56+NKT, CD3+CD161+NKT, CD3-CD161+NKT, AFP, and GGT in patients with PLC. Combining these five indicators for detection enhances the sensitivity and specificity of PLC diagnosis.
- Citation: Zhou ST, Zhang B, Ma K, Guo J. Clinical significance of immune cell and biomarker changes in liver cancer. World J Gastrointest Surg 2025; 17(6): 104923
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/104923.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.104923
Primary liver cancer (PLC) is a globally prevalent malignancy characterized by high malignancy levels, with its incidence and mortality rates steadily rising over time. The incidence of PLC has risen from 471000 cases in 1990 to 1007800 cases in 2016, marking a 114% increase[1]. Additionally, the global mortality rate of LC was approximately 8.5 deaths per 100000 individuals in 2018[2], posing a significant threat to patient health and life. The etiological factors of PLC are diverse, including genetic factors, alcoholic liver disease, liver cirrhosis, viral hepatitis, and non-alcoholic fatty liver disease, among others[3,4], all of which can induce its onset. PLC often manifests insidiously, progresses rapidly, and is typically diagnosed in the advanced stages when typical symptoms and signs emerge, thus missing the optimal treatment window[5]. Therefore, early detection, diagnosis, and treatment are essential measures to reduce the mortality rate of PLC, prolong patient survival, and enhance prognosis.
Research has unraveled that the progression of cancer bears a relation to defects in both innate and adaptive immune surveillance. Immune B cells, immune T cells, natural killer (NK) cells, and NKT cells in the tumor microenvironment play crucial regulatory roles in tumor nonspecific immune responses[6]. NKT cells represent a lymphocyte subset characterized by the concurrent expression of NK cell markers and T cell receptors. The T cell receptor on the surface of NKT cells recognizes antigens presented by the non-classical antigen-presenting molecule cluster of differentiation 1d (CD1d), which triggers the release of cytokines in an innate immune manner, thereby modulating specific immune functions[7]. CD161, a C-type lectin-like receptor expressed on most NK cell and T cell subsets, has emerged as a recently identified potential inhibitor of tumor-infiltrating T cells. It can synergistically regulate the immune microenvironment together with other immune checkpoints, influencing tumor progression[8]. Hence, we boldly speculate that CD161+T cells in peripheral blood may also play an essential part in PLC occurrence and development.
In the diagnosis of various tumors, the combined detection of different tumor markers or relevant indicators can significantly improve the diagnostic accuracy. For example, in the diagnosis of breast cancer, the combined detection of carcinoembryonic antigen (CEA), carbohydrate antigen 15-3, and other indicators has greatly enhanced the diagnostic rate of early-stage breast cancer compared with single marker detection[9]. During the occurrence and progression of PLC, certain tumor markers begin to appear or exhibit significantly heightened levels compared to normal values. Alpha fetoprotein (AFP) and gamma-glutamyl transpeptidase (GGT) are the two most commonly applied markers for diagnosing PLC in clinical practice, with high sensitivity and specificity[10]. Notwithstanding, single detection often results in missed diagnoses or misdiagnoses.
Based on the above research background and current situation, this study aims to clarify the levels of CD3+CD161+NKT cell subsets, as well as AFP and GGT in the peripheral blood of patients with PLC. It also delves into the application value of their combined detection in the diagnosis of PLC, the evaluation of treatment efficacy, and the prediction of prognosis. We expect that through the combined detection of these indicators, we can leverage the advantages of each indicator, minimize the limitations of single item detection, improve the sensitivity and specificity of PLC diagnosis, and provide a more accurate basis for the formulation of clinical treatment plans.
From July 2022 to December 2023, 30 patients diagnosed with PLC were admitted to our medical institution, constituting the PLC group. This cohort comprised 21 males and 9 females, aged 35 to 62 years, with a mean age of 49.41 ± 3.15 years. Simultaneously, 30 healthy individuals undergoing health examinations at our hospital’s health examination center were selected as the control cohort. The individuals within the control group had not used immunosuppressants, other immunomodulators, or anti-inflammatory mediator antagonists in the past 2 weeks and had no history of specific diseases. Within this group, there were 22 males and 8 females, aged 32 to 69 years, with a mean age of 48.36 ± 3.76 years.
Inclusion criteria: (1) Age greater than or equal to 18 years and less than 70 years; (2) Diagnosis meeting the criteria of the Practice Guidelines for the Pathological Diagnosis of Primary Liver Cancer: 2015 Update[11]; and (3) Not having un
Exclusion criteria: (1) Concurrent with other malignant tumors; (2) Coinfection with human acquired immunodeficiency virus; (3) Concurrent autoimmune disease or long-term use of immunosuppressants; or (4) Pregnant or lactating women.
Following enrollment, 2 mL fasting venous blood was harvested from the elbow in the morning, stored in sterile collection tubes, and added to anticoagulant tubes containing EDTA for flow cytometry detection. Then 5 mL fasting venous blood was harvested from the elbow in the morning, The samples were subjected to 15-minute centrifugation (3000 rpm) with the use of a table model high speed centrifuge. The upper layer of serum and plasma was separated and aliquoted into Eppendorf tubes and then stored in a -70 °C ultra-low temperature freezer for the determination of serum AFP and GGT levels.
According to a study by Winkler et al[12], fluorescence-labeled antibodies were added to the collected blood samples: CD3 APC, CD4 TITC, CD8 PE, CD56 PE-Cy7, and CD161 PerCP-eFluorTM 710 (Becton, Dickinson and Company, Franklin Lakes, NJ, United States). After mixing, the samples underwent 20-minute incubation at room temperature (RT) away from light. Then, 3 mL of 1 times BD FACS Lysing Solution (Becton, Dickinson and Company) was added and allowed to lyse the red blood cells in a dark environment at RT for 10 minutes, followed by centrifugation at 500 g for 5 minutes. Then the supernatant was discarded, and 2 mL phosphate-buffered saline (PBS) was added, mixed, and the sample was centrifuged again. Following removal of the supernatant, 500 μL PBS was added and thoroughly mixed for subsequent flow cytometry analysis. During analysis of the samples, 100000 cells were harvested per round-bottom tube with the BD FACSCanto II Flow Cytometer (Becton, Dickinson and Company), and FlowJo_V10 software was adopted to analyze the flow cytometry data. A scatter diagram was generated with forward scatter on the y-axis and side scatter on the x-axis to evaluate the levels of each NKT cell subset.
To quantify serum AFP levels, 5 mL serum samples were processed using the Cosmai Smart 6500 Electrochemiluminescence Fully Automated Immunoassay Analyzer (Beijing Huaketai Biotechnology Co., Ltd., Beijing, China) as per the instructions of the AFP quantitative electrochemiluminescence assay kit (Roche, Basel, Switzerland).
Serum GGT contents were determined using the Beckman Coulter AU5800 Fully Automated Biochemical Analyzer. The determination was performed strictly following the instructions provided with the GGT Assay Kit (Meikang Biotechnology Co., Ltd., Shanghai, China)[13].
For data processing and analysis in this study, SPSS 22.0 software was utilized. Measurement data are denoted as the mean ± SD and subjected to analysis employing the t-test. Categorical data are displayed as percentages and evaluated through the χ2 test. The Kaplan-Meier method was utilized to plot survival curves, and the log-rank test was adopted to assess patient prognosis. The receiver operating characteristic curve was introduced for evaluation of the diagnostic value of CD3+CD161+NKT cell subsets as well as AFP and GGT changes under PLC circumstances, with statistical significance set at P < 0.05 for all analyses.
Regarding the PLC cohort, there were 21 male and 9 female patients, with an average age of 49.41 ± 3.15 years. Within the control group, there were 22 male and 8 female participants, with an average age of 48.36 ± 3.76 years. The incidence rate of PLC was higher in males (70.0%) vs females (30.0%). The distribution of cases and the male-to-female ratio in both cohorts are illustrated in Figure 1.
The differences in white blood cells, neutrophils, lymphocytes, red blood cells, hemoglobin, platelets, glutamic-pyruvic transaminase (GPT), glutamic oxalacetic transaminase (GOT), total protein, albumin, globulin, blood urea nitrogen (BUN), serum creatinine (Scr), and CEA were compared between the PLC group and the control group. As shown by the data, there were no noticeable statistically significant divergence in indicators such as white blood cells, neutrophils, platelets, BUN, or Scr between the two cohorts (P > 0.05). The lymphocyte count within the PLC group was 1.56 ± 0.45 × 109/L, the red blood cell count was 4.21 ± 0.34 × 1012/L, and the hemoglobin level reached 117.48 ± 12.54 g/L, which were all lower than those within the control group. Regarding the PLC group, the GPT level was 76.54 ± 6.57 U/L, and the GOT level was 68.82 ± 5.33 U/L, which were both higher than those in the control cohort. In the PLC group, the total protein level was 56.37 ± 6.88 g/L, the albumin level reached 25.78 ± 3.13 g/L, and the globulin level was 0.65 ± 0.11 g/L, which were all lower than those within the control group. The CEA level in the PLC cohort was 13.65 ± 1.88 μg/L, conspicuously higher than that within the control group. The differences in the above comparisons showed statistical significance (P < 0.05), as detailed in Table 1. Our findings suggested that patients within the PLC group experienced poor nutritional status, decreased liver function, and dramatically elevated levels of CEA.
| Indicators | PLC group (n = 30) | Control group (n = 30) | t | P value |
| White blood cells (× 109/L) | 5.89 ± 0.65 | 6.02 ± 0.73 | 0.728 | 0.469 |
| Neutrophils (× 109/L) | 3.41 ± 0.29 | 3.35 ± 0.24 | 0.873 | 0.386 |
| Lymphocytes (× 109/L) | 1.56 ± 0.45 | 2.01 ± 0.57 | 3.394 | 0.001 |
| Red blood cells (× 1012/L) | 4.21 ± 0.34 | 4.75 ± 0.39 | 5.716 | < 0.001 |
| Hemoglobin (g/L) | 117.48 ± 12.54 | 146.33 ± 11.48 | 9.294 | < 0.001 |
| Blood platelets (× 109/L) | 199.02 ± 18.36 | 198.46 ± 20.11 | 0.113 | 0.911 |
| GPT (U/L) | 76.54 ± 6.57 | 23.88 ± 3.15 | 39.586 | < 0.001 |
| GOT (U/L) | 68.82 ± 5.33 | 20.46 ± 4.28 | 38.749 | < 0.001 |
| Total protein (g/L) | 56.37 ± 6.88 | 71.52 ± 6.75 | 8.609 | < 0.001 |
| Albumin (g/L) | 25.78 ± 3.13 | 41.33 ± 3.62 | 17.798 | < 0.001 |
| Globulin (g/L) | 0.65 ± 0.11 | 1.03 ± 0.23 | 8.164 | < 0.001 |
| BUN (μmol/L) | 4.61 ± 0.52 | 4.65 ± 0.47 | 0.313 | 0.756 |
| Scr (μmol/L) | 302.58 ± 26.79 | 299.47 ± 23.85 | 0.475 | 0.637 |
| CEA (μg/L) | 13.65 ± 1.88 | 2.84 ± 0.49 | 30.476 | < 0.001 |
The differences in peripheral blood CD4+NKT, CD8+NKT, and CD3+CD56+NKT cells between the two cohorts exhibited no substantial statistical significance (P > 0.05). Within the PLC group, the percentages of CD8+CD56+NKT, CD3+CD161+NKT, and CD3-CD161+NKT cells were 8.35% ± 1.01%, 14.36% ± 1.55%, and 12.08% ± 1.34%, respectively, which were all higher than those in the control cohort, with the differences being statistical significance (P < 0.05), as shown in Table 2. These results suggest that compared to healthy individuals, individuals with PLC displayed notable alterations in the peripheral blood NKT cell subsets, particularly in the levels of CD8+CD56+, CD3+CD161+, and CD3-CD161+ cells, su
| Indicators | PLC group (n = 30) | Control group (n = 30) | t | P value |
| CD4+NKT | 5.52 ± 0.88 | 5.18 ± 0.94 | 1.446 | 0.154 |
| CD8+NKT | 3.12 ± 0.37 | 3.09 ± 0.46 | 0.278 | 0.782 |
| CD3+CD56+NKT | 6.68 ± 0.52 | 6.47 ± 0.63 | 1.408 | 0.165 |
| CD8+CD56+NKT | 8.35 ± 1.01 | 6.77 ± 0.74 | 6.912 | < 0.001 |
| CD3+CD161+NKT | 14.36 ± 1.55 | 12.82 ± 1.87 | 3.473 | 0.001 |
| CD3-CD161+NKT | 12.08 ± 1.34 | 7.69 ± 0.93 | 14.742 | < 0.001 |
Within the PLC cohort, the AFP level was 335.71 ± 20.89 ng/mL, and the GGT level reached 136.87 ± 15.62 U/mL, which were both higher than those within the control cohort with statistical significance (P < 0.05), as displayed in Table 3. These results showed that compared with healthy individuals, patients suffering from PLC exhibited a substantial and abnormal elevation in the levels of tumor markers AFP and GGT in peripheral blood.
| Indicators | PLC group (n = 30) | Control group (n = 30) | t | P value |
| AFP (ng/mL) | 335.71 ± 20.89 | 2.45 ± 0.73 | 87.325 | < 0.001 |
| GGT (U/mL) | 136.87 ± 15.62 | 34.41 ± 4.55 | 34.494 | < 0.001 |
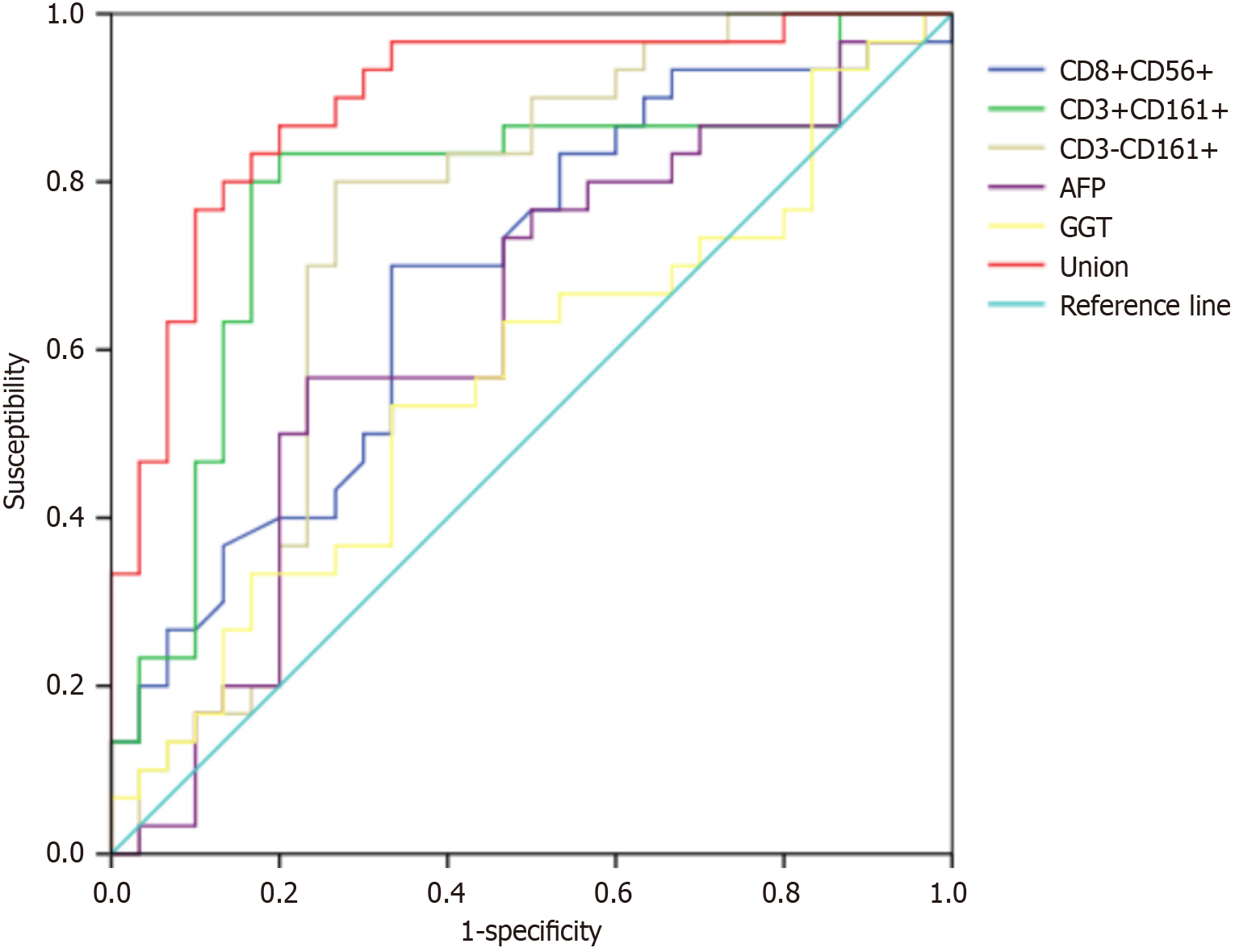
The sensitivity rates of CD8+CD56+NKT, CD3+CD161+NKT, CD3-CD161+NKT, AFP, and GGT for single diagnosis of PLC reached 70.00%, 83.33%, 80.00%, 56.67%, and 53.33%, respectively (P < 0.05), while their specificity rates attained 66.67%, 80.00%, 76.67%, 76.67%, and 66.67%, respectively (P < 0.05). The area under the curve (AUC) of the combined detection was 0.898, with a sensitivity of 86.67% and specificity of 80.00% (P < 0.05), suggesting that combined diagnosis is superior to individual tests, as shown in Table 4 and Figure 2. Our findings revealed that although peripheral blood NKT cell subsets, AFP, and GGT for a single diagnosis of PLC have a certain value, a combined method can efficaciously enhance the sensitivity and specificity for identifying PLC, reducing misdiagnosis and missed diagnosis and achieving better diagnostic efficacy.
| Indicators | Cut-off | AUC | Sensitivity (%) | Specificity (%) | Youden index | 95%CI | P value |
| CD8+CD56+NKT | > 7.30% | 0.686 | 70.00 | 66.67 | 0.367 | 0.550-0.822 | 0.007 |
| CD3+CD161+NKT | > 13.80% | 0.786 | 83.33 | 80.00 | 0.633 | 0.659-0.912 | < 0.001 |
| CD3-CD161+NKT | > 11.01% | 0.758 | 80.00 | 76.67 | 0.567 | 0.627-0.888 | < 0.001 |
| AFP | > 309.65 ng/mL | 0.628 | 56.67 | 76.67 | 0.333 | 0.482-0.774 | 0.086 |
| GGT | > 114.79 U/mL | 0.568 | 53.33 | 66.67 | 0.200 | 0.420-0.716 | 0.370 |
| Combined | - | 0.898 | 86.67 | 80.00 | 0.667 | 0.817-0.979 | < 0.001 |
The liver, as the sixth most vulnerable site for primary cancer in humans, can develop PLC due to inflammation or liver cirrhosis[6]. PLC is highly prevalent in China and worldwide, with hepatocellular carcinoma (HCC) being the most prevalent type. It shows several characteristics, such as high malignancy, rapid progression, and easy dissemination of tumor foci, with a mortality rate increasing at a rate of 2%-3% annually, posing a daunting threat to patient health[14]. As research on PLC progresses, it has been discovered that PLC incidence pertains to geographical factors and regions with a high prevalence of viral hepatitis. Treatment strategies are multimodal, tailored to individual patients based on tumor staging and other health factors[15]. PLC is often diagnosed in the late stages, culminating in high mortality rates[5]. Lymphocytes play a crucial role in the body's immune surveillance against tumor cells. When the lymphocyte count decreases, the body's ability to recognize and eliminate tumor cells weakens, which may lead to the progression of PLC. Meanwhile, elevated liver enzymes indicate that liver cells are damaged. This double whammy situation will accelerate the deterioration of liver function. Therefore, there is an urgent need to identify effective and accurate diagnostic markers to assist clinical treatment and prolong patient survival.
AFP and GGT are widely utilized serum biomarkers for detecting PLC globally. Their secretion bears a relation to the histological grading of malignant tumors and the invasive biological behavior of tumors, playing an indispensable predictive role in the diagnosis of PLC and the recurrence of HCC following liver transplantation[16]. However, a multitude of research has found that AFP and GGT are imperfect biomarkers. They exhibit high false-negative rates in the diagnosis of early-stage and small tumors, and their contents may also be aberrantly augmented in benign liver diseases such as chronic hepatitis and liver cirrhosis[17-19]. Feng et al[20] demonstrated that serum PIVKA-II can complement AFP, significantly improving the diagnostic rate of HCC when applied in conjunction. Zhao et al[21] demonstrated that the ratio of GGT to alanine aminotransferase (ALT) effectively forecasts the severity of HCC, and this ratio is highly correlated with disease progression and prognosis in hepatitis B-infected patients with HCC. High GGT levels relative to ALT predict vascular invasion, tumor volume, pathological differentiation, tumor load, and poor survival rates of HCC. Apart from some serum tumor markers, an increasing body of evidence suggests that immune regulatory cells, immune tolerance mechanisms, and immune checkpoints are also involved in the growth and differentiation processes of tumors such as PLC[22,23]. Existing evidence denotes that NKT cells, as unconventional T lymphocytes, play a crucial regulatory part in glioblastoma by recognizing lipoidal antigens presented by CD1d molecules. Brettschneider and Terabe[24] revealed that the activation of type I NKT cells boosts the immune response against glioblastoma, whereas type II NKT cells cross-regulate the activity of type I NKT cells to exert immunosuppressive functions in various cancer cells. CD161 has been discovered to be expressed on cells such as NKT cells, CD4+T cells, and CD8+T cells and functions as an inhibitory receptor[25]. Here, we hypothesize that CD3+CD161+NKT cell subsets may also undergo abnormal alterations in the development of PLC, and in conjunction with AFP and GGT, investigate the clinical significance of their level changes in the context of PLC.
Currently, there is limited research on CD3+CD161+NKT cells in patients with PLC both domestically and internationally. However, based on similar prior studies, we made bold hypotheses and conducted experiments to validate them. The experimental outcomes showed that compared to healthy individuals, patients with PLC exhibited no remarkable differences in the levels of peripheral blood CD4+NKT, CD8+NKT, and CD3+CD56+NKT cells (P > 0.05). Nonetheless, CD8+CD56+NKT, CD3+CD161+NKT, and CD3-CD161+NKT cell levels were dramatically heightened, along with a notable uplift in serum AFP and GGT levels (P < 0.05). Given the above findings, CD4+NKT, CD8+NKT, CD3+
Due to the sophisticated interplay among multiple cytokines and cell subsets during the development of PLC, this research only examined peripheral blood specimens from 30 patients with PLC treated at our hospital. This may introduce bias due to the limited sample size. Therefore, further expansion of sample size and sampling areas is needed to clarify the characteristics and functional status of various cytokines and immune cell subsets in the peripheral blood and liver tissues of patients with PLC. This will facilitate a deeper understanding and exploration of the specific regulatory pathways of immune modulation mechanisms within the local tissue environment. In our current cross-sectional study, limited by time and resources, we focused on single point biomarker status. Based on these results, a follow-up longitudinal study is planned. Moreover, the study did not explore the mechanisms of these cells in promoting tumor development or immune evasion, leading to an incomplete understanding of NKT cell subsets' roles in PLC. Future research can target uncovering the molecular mechanisms of NKT cell subsets in PLC tumors, such as signaling pathway activation and cytokine secretion regulation. This will clarify PLC pathogenesis and support new immunotherapy target development.
In conclusion, patients with PLC displayed markedly elevated levels of CD8+CD56+NKT, CD3+CD161+NKT, CD3-CD161+NKT, AFP, and GGT in the peripheral blood. The combined detection using these five indicators can boost the early diagnosis rate of PLC, aiding clinicians in identifying PLC at an earlier stage with greater precision. Our experimental findings are expected to provide new laboratory diagnostic evidence for the clinical diagnosis and treatment of PLC, with potential for wide-ranging applications.
| 1. | Liu Z, Jiang Y, Yuan H, Fang Q, Cai N, Suo C, Jin L, Zhang T, Chen X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 517] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 2. | Shi JF, Cao M, Wang Y, Bai FZ, Lei L, Peng J, Feletto E, Canfell K, Qu C, Chen W. Is it possible to halve the incidence of liver cancer in China by 2050? Int J Cancer. 2021;148:1051-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 3. | Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, Li HL, Xiang YB. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. 2020;124:330-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Xing QQ, Li JM, Dong X, Zeng DY, Chen ZJ, Lin XY, Pan JS. Socioeconomics and attributable etiology of primary liver cancer, 1990-2019. World J Gastroenterol. 2022;28:2361-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Salazar J, Le A. The Heterogeneity of Liver Cancer Metabolism. Adv Exp Med Biol. 2021;1311:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. 2021;21:541-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 345] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 7. | Issazadeh-Navikas S. NKT cell self-reactivity: evolutionary master key of immune homeostasis? J Mol Cell Biol. 2012;4:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Zhou X, Du J, Liu C, Zeng H, Chen Y, Liu L, Wu D. A Pan-Cancer Analysis of CD161, a Potential New Immune Checkpoint. Front Immunol. 2021;12:688215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Yonemori K, Katsumata N, Noda A, Uno H, Yunokawa M, Nakano E, Kouno T, Shimizu C, Ando M, Tamura K, Takeuchi M, Fujiwara Y. Development and verification of a prediction model using serum tumor markers to predict the response to chemotherapy of patients with metastatic or recurrent breast cancer. J Cancer Res Clin Oncol. 2008;134:1199-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Hou SC, Xiao MB, Ni RZ, Ni WK, Jiang F, Li XY, Lu CH, Chen BY. Serum GP73 is complementary to AFP and GGT-II for the diagnosis of hepatocellular carcinoma. Oncol Lett. 2013;6:1152-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, Chen J; Guideline Committee. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279-9287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 185] [Cited by in RCA: 291] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 12. | Winkler I, Woś J, Bojarska-Junak A, Semczuk A, Rechberger T, Baranowski W, Markut-Miotła E, Tabarkiewicz J, Wolińska E, Skrzypczak M. An association of iNKT+/CD3+/CD161+ lymphocytes in ovarian cancer tissue with CA125 serum concentration. Immunobiology. 2020;225:152010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Saeed U, Uppal MR, Uppal MS, Uppal R, Khan AA, Hassan A, Piracha ZZ. Hepatitis C virus associated ALT, AST, GGT, Bili T, HB, HBA1C, CREAT, PT, aPPT, AFP, CEA, CA 125, CA 19-9, iPTH biomarkers, computed tomography and HCV burden of disease during pre COVID-19 era (2018-2019) and post COVID-19 era (2020-2022) in Pakistan. Braz J Biol. 2023;84:e271451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Wang W, Wei C. Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis. 2020;7:308-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 15. | Liu CY, Chen KF, Chen PJ. Treatment of Liver Cancer. Cold Spring Harb Perspect Med. 2015;5:a021535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 16. | Özdemir F, Baskiran A. The Importance of AFP in Liver Transplantation for HCC. J Gastrointest Cancer. 2020;51:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Norman JS, Li PJ, Kotwani P, Shui AM, Yao F, Mehta N. AFP-L3 and DCP strongly predict early hepatocellular carcinoma recurrence after liver transplantation. J Hepatol. 2023;79:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 71] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 18. | Jia Y, Xing Y, Yang M. Efficacy of Sorafenib Combined with Interventional Therapy on Primary Liver Cancer Patients and Its Effect on Serum AFP, VEGF, and GGT. J Oncol. 2021;2021:9120265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Liu H, Wei D, Yan Z, Cui B, Song Y, Yin H. Analyzing the Role of Septin9 Gene Methylation in the Diagnosis and Treatment of Primary Liver Cancer in the Elderly. Altern Ther Health Med. 2023;29:194-199. [PubMed] |
| 20. | Feng H, Li B, Li Z, Wei Q, Ren L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021;21:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 21. | Zhao Z, Zhu Y, Ni X, Lin J, Li H, Zheng L, Zhang C, Qi X, Huo H, Lou X, Fan Q, Bao Y, Luo M. Serum GGT/ALT ratio predicts vascular invasion in HBV-related HCC. Cancer Cell Int. 2021;21:517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Zheng Y, Wang S, Cai J, Ke A, Fan J. The progress of immune checkpoint therapy in primary liver cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, Zhang Z, Xie J, Wang C, Chen D, Huang Y, Wei X, Shi Y, Zhao Z, Li Y, Guo Z, Yu Q, Xu L, Volpe G, Qiu S, Zhou J, Ward C, Sun H, Yin Y, Xu X, Wang X, Esteban MA, Yang H, Wang J, Dean M, Zhang Y, Liu S, Yang X, Fan J. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184:404-421.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 528] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 24. | Brettschneider EES, Terabe M. The Role of NKT Cells in Glioblastoma. Cells. 2021;10:1641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Park Y, Lim J, Kim SY, Kwon GC, Koo SH, Kim J. Changes of frequency and expression level of CD161 in CD8(+) T cells and natural killer T cells in peripheral blood of patients with systemic lupus erythematosus. Microbiol Immunol. 2020;64:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Yamagiwa S, Matsuda Y, Ichida T, Honda Y, Takamura M, Sugahara S, Ishikawa T, Ohkoshi S, Sato Y, Aoyagi Y. Sustained response to interferon-alpha plus ribavirin therapy for chronic hepatitis C is closely associated with increased dynamism of intrahepatic natural killer and natural killer T cells. Hepatol Res. 2008;38:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Tsumura R, Haruta M, Kuwano M, Yasunaga M. Expansion of mixed immune cells using CD3/CD161 co-stimulation for the treatment of cancer. Sci Rep. 2023;13:6803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M, Chen CL, Kawano K, Kitano S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother. 2003;52:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |