Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1442
Peer-review started: February 13, 2023
First decision: March 28, 2023
Revised: April 11, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: July 27, 2023
Processing time: 157 Days and 21.4 Hours
Indocyanine green (ICG) fluorescence played an important role in tumor localization and margin delineation in hepatobiliary surgery. However, the preoperative regimen of ICG administration was still controversial. Factors associated with tumor fluorescence staining effect were unclear.
To investigate the preoperative laboratory indexes corelated with ICG fluorescence staining effect and establish a novel laboratory scoring system to screen specifical patients who need ICG dose adjustment.
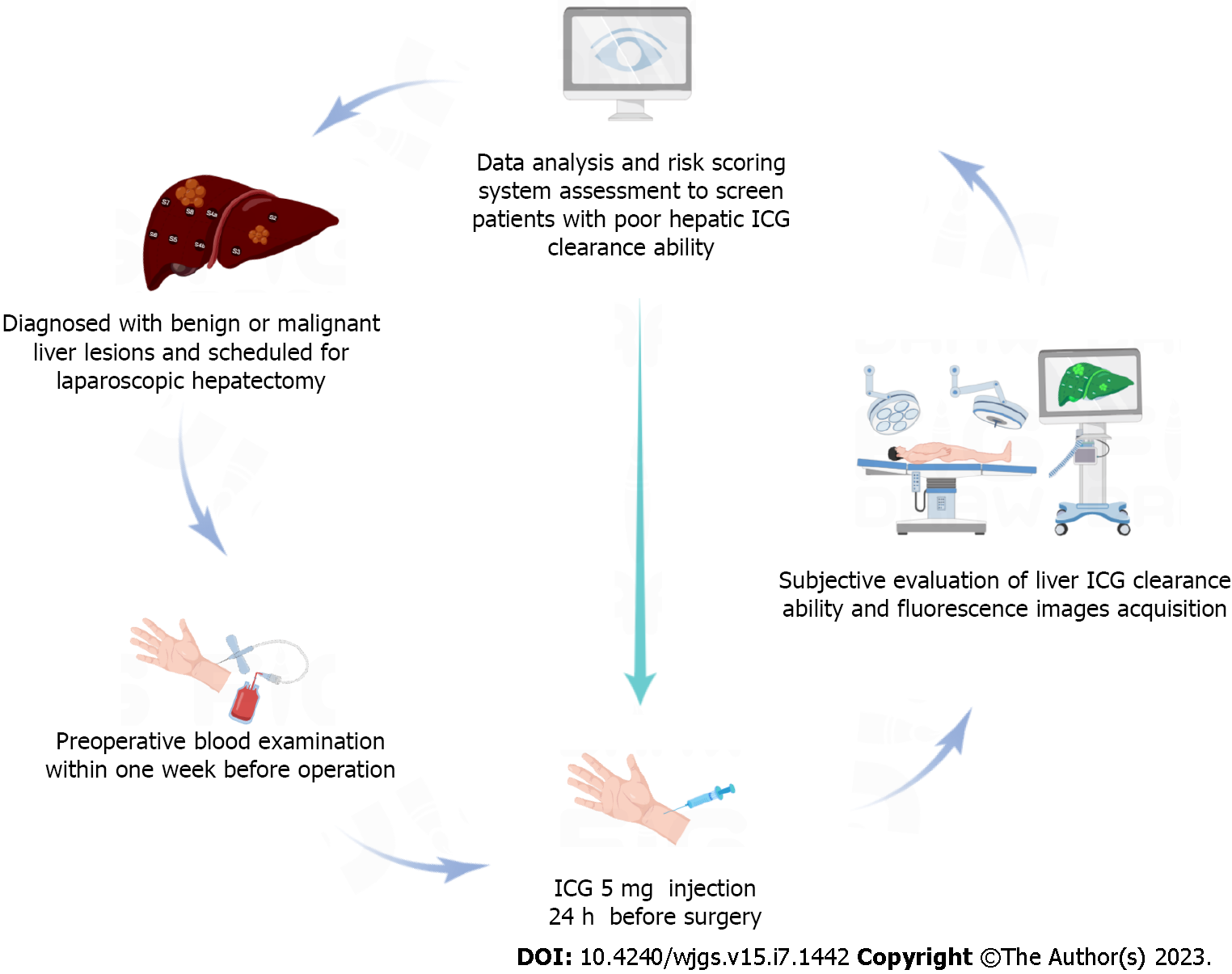
To investigate the predictive indicators of ICG fluorescence characteristics in patients undergoing laparoscopic hepatectomy from January 2018 to January 2021 were included. Blood laboratory tests were completed within 1 wk before surgery. All patients received 5 mg ICG injection 24 h before surgery for preliminary tumor imaging. ImageJ software was used to measure the fluorescence intensity values of regions of interest. Correlation analysis was used to identify risk factors. A laboratory risk model was established to identify individuals at high risk for high liver background fluorescence.
There were 110 patients who were enrolled in this study from January 2019 to January 2021. The mean values of fluorescence intensity of liver background (FI-LB), fluorescence intensity of gallbladder, and fluorescence intensity of target area were 18.87 ± 17.06, 54.84 ± 33.29, and 68.56 ± 36.11, respectively. The receiver operating characteristic (ROC) curve showed that FI-LB was a good indicator for liver clearance ability [area under the ROC curve (AUC) = 0.984]. Correlation analysis found pre-operative aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, adenosine deaminase, and lactate dehydrogenase were positively associated with FI-LB and red blood cell, cholinesterase, and were negatively associated with FI-LB. Total laboratory risk score (TLRS) was calculated according to ROC curve (AUC = 0.848, sensitivity = 0.773, specificity = 0.885). When TLRS was greater than 6.5, the liver clearance ability of ICG was considered as poor.
Preoperative laboratory blood indicators can predict hepatic ICG clearance ability. Surgeons can adjust the dose and timing of ICG preoperatively to achieve better liver fluorescent staining.
Core Tip: Indocyanine green (ICG) fluorescence plays an important role in tumor localization and edge delineation in hepatobiliary surgery. However, the preoperative medication scheme of ICG is still controversial. Factors related to the effect of tumor fluorescence staining are still unclear. The purpose of this study is to investigate the preoperative laboratory indicators related to the effect of ICG fluorescence staining, and establish a new risk scoring system to screen specific patients who need to adjust the ICG dose. This scoring system will provide a new method and idea, making it possible to individualize the usage and dosage of ICG staining.
- Citation: Chen ZR, Zeng QT, Shi N, Han HW, Chen ZH, Zou YP, Zhang YP, Wu F, Xu LQ, Jin HS. Laboratory scoring system to predict hepatic indocyanine green clearance ability during fluorescence imaging-guided laparoscopic hepatectomy. World J Gastrointest Surg 2023; 15(7): 1442-1453
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1442.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1442
Indocyanine green (ICG) was a water-soluble, non-toxic sodium cyanide compound, approved for clinical use by the United States Food and Drug Administration since 1954[1]. It was not until the 1970s that researchers discovered that ICG could bind to proteins in blood vessels and be excited by light in the 750-810 nm wavelength band, which then emits near-infrared light with a wavelength close to 840 nm[2]. This particular optical signal could be displayed as a green fluorescent image by an acquisition system during laparoscopic surgery. ICG injected into the body can be highly selectively absorbed by hepatocytes, excreted from the bile duct into the intestine and finally excreted into the feces[3]. Different from the rapid excretion of ICG in normal liver tissue, the excretion of ICG in tumor or adjacent compressed tissue was relatively slow, which made ICG an effective navigation and localization tool for hepatobiliary surgery[4].
A previous study has found that the fluorescent signal can appear in the common bile duct a few minutes after ICG injection[5]. The residual ICG in blood vessels could be excreted out in about 20 min in patients with normal liver function, but it took 30 min or longer for patients with abnormal liver function or biliary obstruction[6]. In 2017, researchers in Japan indicated that the expression of Na+/taurocholate co-transport polypeptide (NTCP) and organic anti-transport peptide 8 (OATP8) genes and proteins in cancerous cells affected the retention time of ICG in liver compared with adjacent tissues[7]. Therefore, ICG retention time will be longer in hepatocellular carcinoma (HCC) or non-cancerous cells with higher levels of NTCP and OATP8 expression, resulting in a different type of fluorescent signal.
The applications of ICG real-time fluorescence in hepatobiliary surgery included: (1) Preliminary liver tumor location discovery; (2) Tumor border display; (3) Positive or negative liver segment staining; (4) Biliary tract imaging; and (5) Bile leakage detection, etc[8]. Recently, experts and scholars around the world published a consensus on the ICG in hepatobiliary surgery, and put forward preliminary recommendations on the dosage, timing, and injection route of ICG for different surgical purposes[9]. However, according to previous studies, the overall ICG fluorescence staining of liver was not ideal, mainly due to various influencing factors such as liver cirrhosis, drug-induced liver damage, tumor (lesion) type and location, and presence or absence of intrahepatic biliary obstruction[10]. Inaccurate assessment of preoperative ICG dose and timing will result in excessive intraoperative ICG fluorescence intensity against the background of the liver, or poor fluorescence visualization of liver tumors, rendering the navigational function of this technique almost useless[11]. To date, there is no specific individualized ICG dosing regimen.
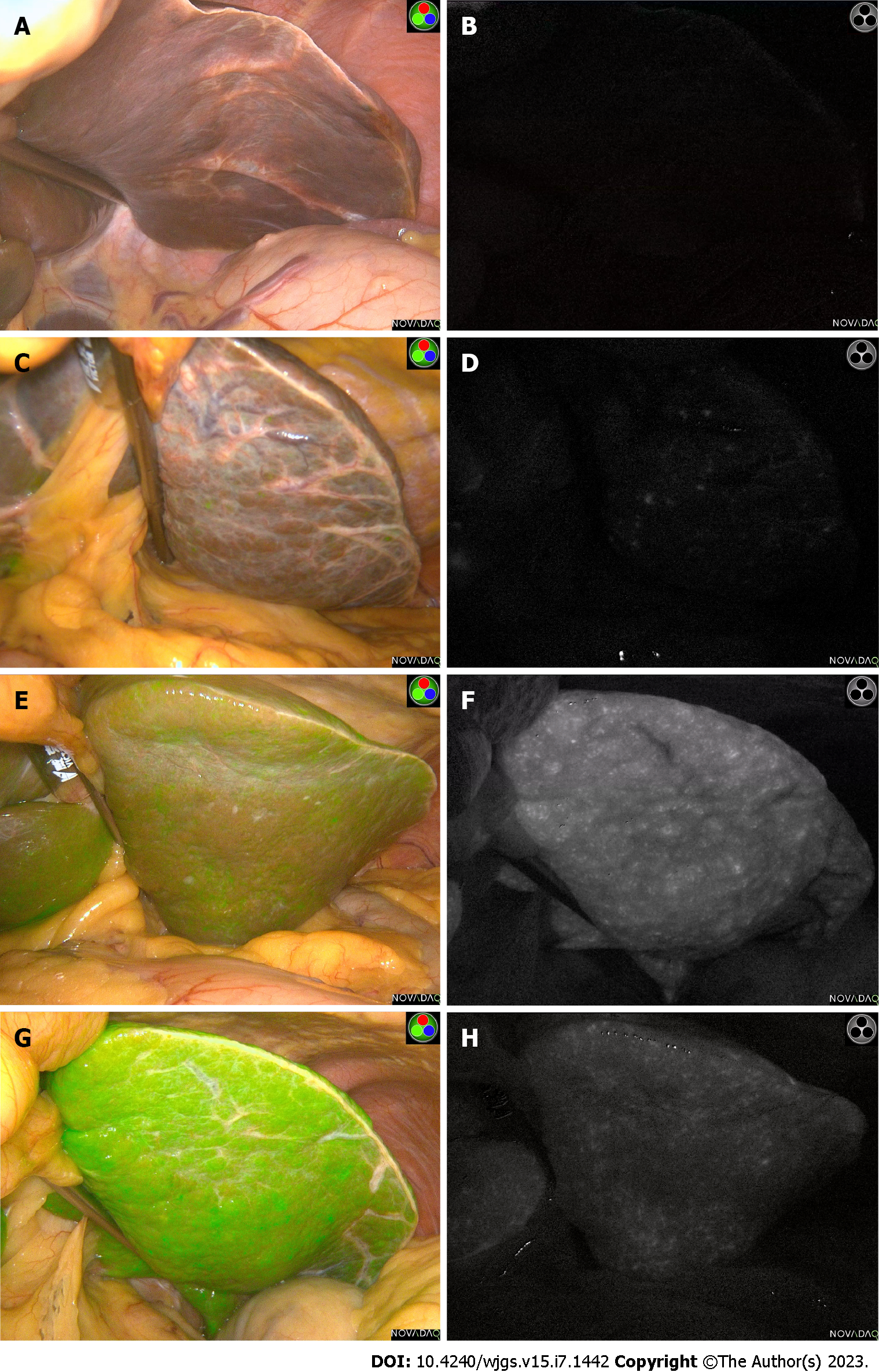
After our center found that long-term (> 7 d) injection of ICG before operation will make ICG excretion more complete, but it may be greatly affected by other subsequent drug injection and the change of operation time. And we found that a small dose of ICG (5 mg) approximately 24 h preoperatively was sufficient for tumor initial localization and boundary determination after nearly 5 years exploration. This protocol was highly feasible to implement and could be dynamically adjusted, but the specific individualized adjustment of the dose needed to be further explored. In a previous ICG fluorescence-related study, researchers found that 10 mg ICG injection 24 h before surgery provided the best biliary-liver background contrast, but most of the livers were normal in this study[12]. Interestingly, in patients undergoing laparoscopic partial hepatectomy with the same preoperative ICG regimen, there were significant differences in fluorescence intensity in the same area between patients, which may be due to different degrees of liver structure or dysfunction (Figure 1).
Therefore, we designed this study to explore the relationship between intraoperative fluorescence characteristics and preoperative routine common laboratory blood indexes, and to find some effective clinical indicators to adjust and intervene the use of preoperative ICG scheme, and finally achieve better staining effect.
From January 2018 to January 2022, patients with benign and malignant liver diseases proposed for liver resection at the Guangdong Provincial People’s Hospital in China were recruited. Inclusion criteria were: (1) Age 18-75 years; (2) Diagnosis of benign and malignant liver diseases with proposed laparoscopic partial hepatectomy; and (3) Preoperative evaluation showed that only one side of the liver had major lesions. Exclusion criteria were: (1) Repeated preoperative ICG use (interval, < 7 d) or preoperative ICG dose > 5 mg; (2) Inability to undergo hepatectomy due to serious diseases (severe cardiopulmonary insufficiency, renal insufficiency, and hematologic disorders); (3) Liver lesions of similar size on both sides of the liver, which may affect the measurement of liver background; or (4) ICG allergy or metabolic disorders.
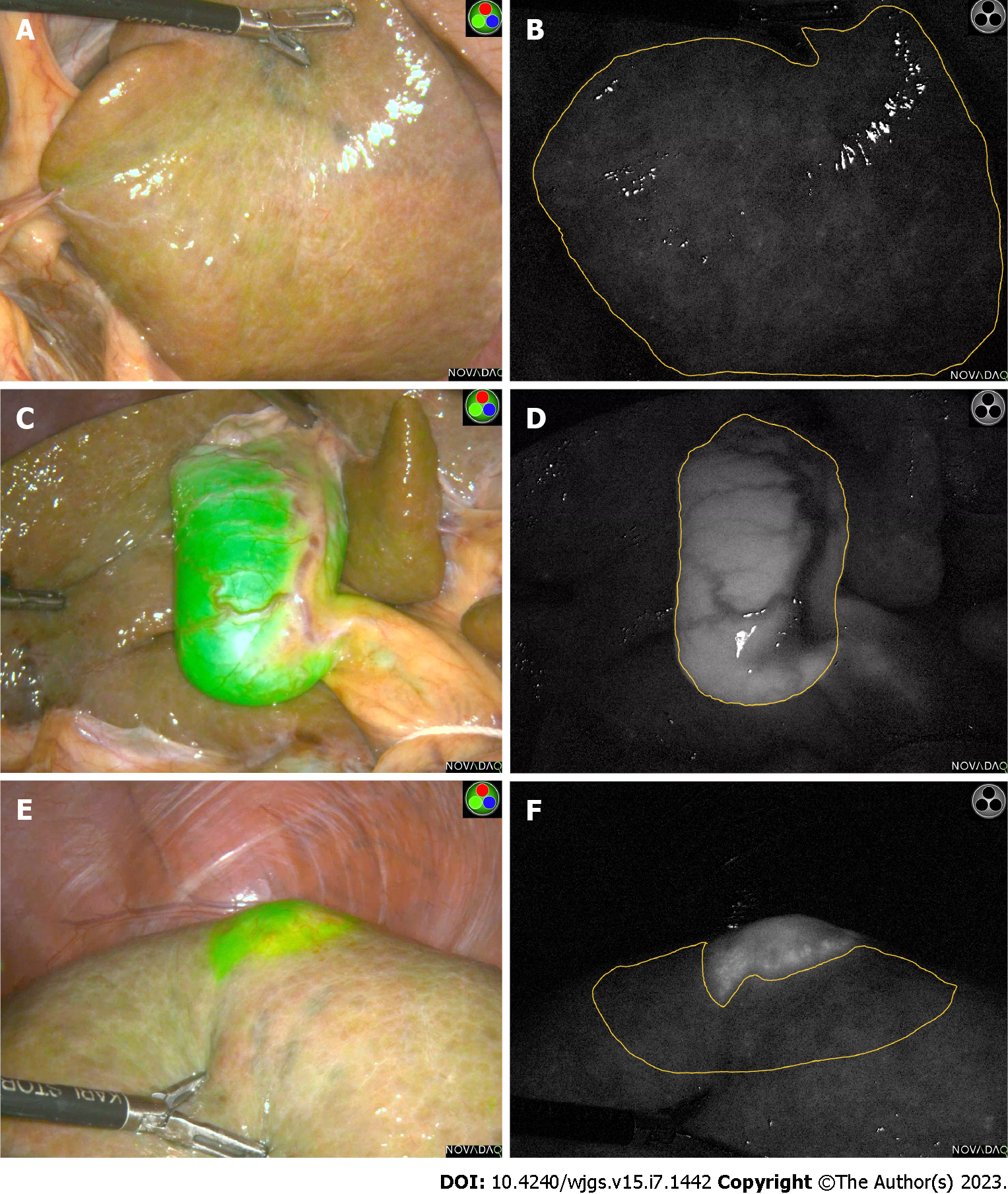
The ICG dosage was manufactured by Dandong Pharmaceuticals (Dandong, China) with a manufacturing approval number H20073073. Routine blood tests, liver function and coagulation indexes were completed within 1 wk preoperatively. After the surgery date was scheduled, all patients received 5 mg ICG intravenously for initial tumor staining approximately 24 h prior to the scheduled time. All operations were performed by equally qualified surgeons. The observation hole of the laparoscopic lens is located at the lower edge of the umbilicus. After entering the abdominal cavity, the surgeons first evaluated and recorded the liver background, major target lesion areas, and gallbladder fluorescence staining effects with the naked eye. Then, the assistant switched the laparoscopic lens to the ICG spy display mode and placed the liver background, gallbladder and major liver lesion areas to observe the center of the surgical field. In addition, the surgical assistant carefully approached the target area with a handheld lens until the target area surface was completely aligned. The observation camera was then retracted 10 cm and held for 5 to 10 s to ensure clear images were captured (Figure 2).
The Potplayer video player was used to display surgery videos and to capture the clearest images (PNG format) in spy display mode. The most representative images were manually selected by more than 2 investigators to delineate ROIs. Finally, average fluorescence intensity (Gray value) measurements were performed using ImageJ software (https://imagej.nih.gov/ij/).
Liver background is defined as the flat, largest area of the liver surface on the side without the hepatic lesion. The target area is defined as the fluorescence area of the primary lesion or tumor for this procedure (the lesion with the largest volume and diameter). Fluorescence intensity of liver background (FI-LB) is the FI-LB, fluorescence intensity of target area (FI-T) is the fluorescence intensity of the primary target lesion/tumor during the procedure, and fluorescence intensity of gallbladder (FI-G) is the FI-G. Target area - liver background fluorescence intensity ratio (TLR) is considered to be an important parameter for the overall effect of liver fluorescence staining (Figure 3). A laparoscopic operating system with ICG fluoroscopic navigation function (PINPOINT, Novadaq Technologies Inc. Canada) was used for all procedures.
Correlation analysis identified blood laboratory tests associated with FI-LB and assigned scores based on the magnitude of the correlation coefficient. The score values of all indicators were summed to obtain the total laboratory risk score (TLRS). Optimal laboratory risk score cut-off values were obtained using receiver operating characteristic (ROC) curve analysis. Grouping based on cut-off values was used to identify individuals at high risk for low hepatic ICG clearance.
Software of IBM SPSS Statistics 26 (IBM Corp., Armonk, NY, United States) was used for statistical analysis and evaluating the findings obtained in the study. Continuous data were described as mean ± SD. Correlation analysis was performed to explore the correlation between the FI-LB, gallbladder and lesion areas and pre-operative blood indexes. Pearson correlation analysis was used for normally distributed data, and Spearman’s correlation analysis was used for non-normally distributed data. ROC curves were used to evaluate the degree of conformity between subjective and objective evaluations.
A total of 110 patients were included in this study. The mean values of FI-LB, FI-G, and FI-T were 18.87 ± 17.06, 54.84 ± 33.29, and 68.56 ± 36.11, respectively. Other basic information was shown in Table 1.
| Characteristics | Patients, n = 110 |
| Sex, male, n (%) | 79 (71.80%) |
| Age in yr | 54.09 ± 11.80 |
| Height in cm | 165.47 ± 7.349 |
| Weight in kg | 64.21 ± 11.64 |
| Poor liver ICG clearance ability, subjective evaluation | 23 (20.90%) |
| FI-LB | 18.87 ± 17.06 |
| FI-G | 54.84 ± 33.29 |
| FI-T | 68.56 ± 36.11 |
| TLR | 14.05 ± 62.72 |
| Cirrhosis | 43 (39.10%) |
| Diabetes | 11 (10.20%) |
| Liver cirrhosis | 43 (39.80%) |
| First abdominal surgery | 66 (61.10%) |
| Postoperative pathology diagnosis | |
| HCC | 67 (60.90%) |
| ICC | 7 (6.40%) |
| Hepatic metastases | 15 (13.60%) |
| Hepatic hemangioma | 7 (6.40%) |
| FNH | 3 (2.70%) |
| Tuberculosis lesions, etc. | 11 (10.00%) |
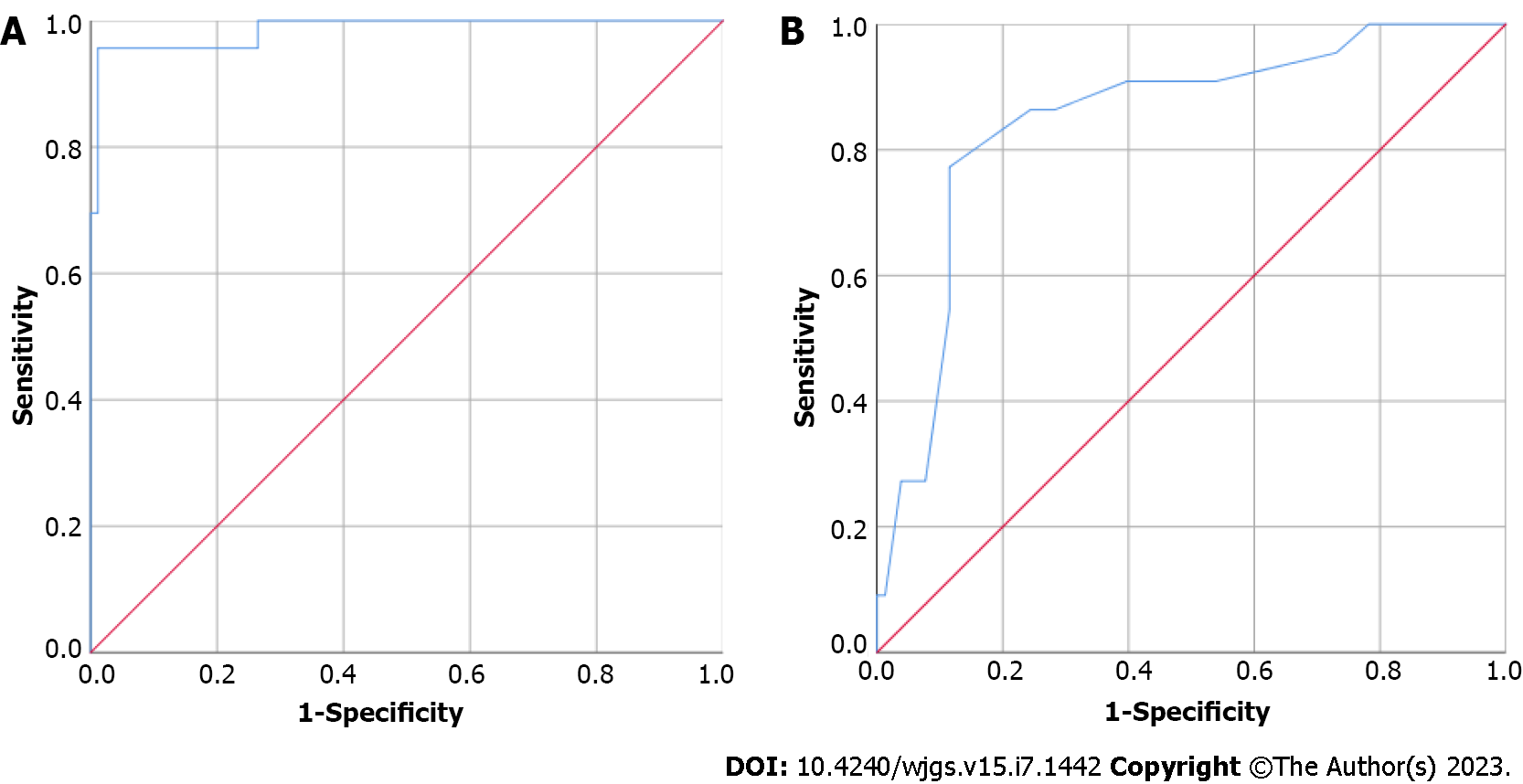
The subjective evaluation brightness and FI-LB of the liver background were included in the ROC curve analysis, and the area under the ROC curve (AUC) value was 0.986, indicating a high concordance between subjective evaluation (hepatic ICG clearance ability good or poor) and objective fluorescence intensity (gray value). The cut-off FI-LB value was 24.892 (Figure 4).
Correlation analysis showed that there was a positive correlation between FI-LB and FI-T (correlation coefficient = 0.518, P value < 0.001) and between FI-LB and FI-G (correlation coefficient = 0.302, P = 0.003). Also, TLR was not correlated with FI-G (P = 0.745). To further explain the relationship between the average intensity of different fluorescent regions. We took the high and low values of FI-LB as the boundary to test and analyse FI-G and FI-T. The student-t test results showed that there was a significant difference in FI-T between the high FI-LB group and the low FI-LB group (P value < 0.001), but there was no difference in FI-G between the two groups (P = 0.193).
Spearman’s correlation analysis revealed that pre-operative aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), adenosine deaminase (ADA), and lactate dehydrogenase (LDH) were positively associated with fluorescence intensity of background liver (FI-LB) and pre-operative red blood cell (RBC), cholinesterase (CHE), and were negatively associated with FI-LB. In the analysis with FI-T, GGT and LDH were positively correlated, while total bilirubin (TBil) and activated partial thromboplastin time (APTT) were negatively correlated. Interestingly, among the blood indicators involved in our study, ADA were found to be significantly positively associated with the mean FI-G, negative correlations were found for RBC, hemoglobin, and CHE. TLR, an important fluorescent staining composite parameter, had not been found to be significantly associated with any enrolled preoperative laboratory blood indicators in this study (Table 2).
| Factor | FI-LB | FI-T | FI-G | TLR | ||||
| Correlation coefficient | P value | Correlation coefficient | P value | Correlation coefficient | P value | Correlation coefficient | P value | |
| APRI-Score | 0.136 | 0.160 | 0.005 | 0.957 | 0.016 | 0.878 | -0.148 | 0.135 |
| RBC | -0.234a | 0.014 | -0.099 | 0.313 | -0.273b | 0.008 | 0.113 | 0.250 |
| Hb | -0.154 | 0.109 | -0.093 | 0.344 | -0.247a | 0.016 | 0.035 | 0.721 |
| Plt | -0.038 | 0.695 | 0.063 | 0.521 | -0.139 | 0.182 | 0.054 | 0.583 |
| TP | -0.050 | 0.605 | 0.049 | 0.620 | -0.001 | 0.992 | 0.019 | 0.843 |
| ALB | -0.084 | 0.384 | 0.016 | 0.870 | -0.158 | 0.129 | 0.001 | 0.989 |
| TBil | -0.026 | 0.791 | -0.192a | 0.048 | 0.052 | 0.621 | -0.037 | 0.705 |
| DBil | 0.078 | 0.438 | -0.105 | 0.302 | 0.052 | 0.635 | -0.071 | 0.489 |
| TA | -0.058 | 0.558 | -0.049 | 0.629 | 0.045 | 0.677 | 0.045 | 0.657 |
| AST | 0.191a | 0.048 | 0.074 | 0.454 | -0.066 | 0.530 | -0.168 | 0.088 |
| ALT | 0.253b | 0.009 | 0.093 | 0.349 | 0.114 | 0.282 | -0.191 | 0.053 |
| GGT | 0.197a | 0.044 | 0.231a | 0.020 | 0.133 | 0.211 | -0.003 | 0.978 |
| ALP | 0.050 | 0.612 | 0.057 | 0.574 | -0.106 | 0.321 | -0.031 | 0.758 |
| CHE | -0.224a | 0.020 | -0.144 | 0.144 | -0.225a | 0.031 | 0.053 | 0.591 |
| LDH | 0.233a | 0.017 | 0.269b | 0.007 | 0.105 | 0.331 | -0.045 | 0.656 |
| ADA | 0.200a | 0.045 | 0.053 | 0.604 | 0.216a | 0.046 | -0.112 | 0.275 |
| INR | -0.072 | 0.457 | -0.106 | 0.280 | -0.043 | 0.684 | 0.074 | 0.448 |
| PTA | 0.073 | 0.448 | 0.108 | 0.271 | 0.044 | 0.675 | -0.072 | 0.465 |
| PT | -0.030 | 0.756 | -0.066 | 0.499 | 0.017 | 0.874 | 0.028 | 0.775 |
| APTT | -0.168 | 0.080 | -0.248a | 0.010 | 0.030 | 0.773 | -0.095 | 0.330 |
| Fib | 0.086 | 0.373 | 0.118 | 0.227 | 0.038 | 0.713 | -0.064 | 0.516 |
| TT | 0.109 | 0.256 | 0.008 | 0.936 | 0.014 | 0.892 | -0.055 | 0.574 |
According to the results of correlation analysis, the preoperative RBC, AST, ALT, GGT, CHE, LDH and ADA were included in the scoring system. The optimal cut-off values for the above blood indicators were calculated and grouped using ROC curve analysis (Supplementary materials). A score of 0-2 was assigned according to the grouping as well as the correlation coefficient. The rules of the laboratory risk score for each blood indicator were shown in Table 3, and other situations not mentioned were all assigned a 0 score. The TLRS was the sum of the laboratory risk scores for all selected single blood indicators.
| Variables’ relationship | Correlation coefficient | Cut-off group | Score |
| Positive correlation | 0.1-0.2 | High | 1 |
| Positive correlation | > 0.2 | High | 2 |
| Negative correlation | 0.1-0.2 | Low | 1 |
| Negative correlation | > 0.2 | Low | 2 |
Using the FI-LB grouping (cut-off = 24.892) as the state variable, the area under the curve is 0.848 (P < 0.001) for the TLRS. Sensitivity is 0.773, specificity is 0.885, 95% confidence interval: 0.755-0.0941, cut-off point was 6.5. In patients with TRLS scores greater than 6.5, the timing and dose of ICG needed be adjusted (24 h) before laparoscopic hepatectomy because of low hepatic ICG clearance ability.
With the widespread use of ICG fluorescence staining in hepatobiliary surgery, surgeons can more accurately delineate tumor margins and perform anatomic segmental resections[13]. Previous clinical studies have focused on analyzing the effects of ICG administration time and dose on fluorescence, while other factors associated with the overall staining effect have not been investigated[14]. In China, Liang et al[15] suggested that better tumor staining effects could be obtained based on the results of preoperative ICG R15 tests. Some scholars also pointed out that preoperative ICG administration for 0 to 3 or 4 to 7 d did not affect intraoperative fluorescence imaging[16]. In general, opinions on the specific ICG dosing regimen before laparoscopic hepatectomy were not yet standardized.
The main metabolic pathway of ICG in the body is blood-bile-feces. Studies have clarified the process of ICG uptake and metabolism in the liver. Normal hepatocytes can rapidly absorb ICG and completely excrete it from the biliary system in approximately 20 h[17]. Previous studies have shown that taurocholic acid cotransport polypeptide (NTCP) and organic anion transporting polypeptide 1B3 (OATP1B3), which are expressed in adjacent normal liver cells or tumor cells, are involved in the uptake and excretion of ICG. Well-differentiated HCC cells with higher expression of the above-mentioned transporters can often show uniform cancer fluorescence, whereas poorly differentiated HCC cells and other types of non-HCC tumor cells usually have poor ICG uptake ability, so that the tumor itself shows little or no fluorescent signal in this area[7]. However, these tumors have a relatively intact capsule and swollen growth that compresses the peritumoral capillary bile ducts, resulting in impaired excretion of ICG, which is present in the liver tissue around the tumor, forming annular or sheet-like fluorescent rings or plaques. Patients undergoing liver surgery usually have cirrhosis and biliary obstruction, and the short-term use of ICG prior to surgery results in a high false positive rate of fluorescence. Although liver cirrhosis has been considered as an important factor affecting the quality of liver ICG staining, we found that many patients with severe liver cirrhosis (severe cirrhotic nodules in the surgical field) are not always accompanied by severe liver background fluorescence interference (ICG retention), there is no obvious linear relationship between the degree of macroscopic liver cirrhosis and the degree of liver background interference fluorescence, which may be related to the low dosage of our drug or the combination of other influencing factors. Therefore, the discovery and verification of other potential influencing factors affecting ICG metabolism in the liver is crucial for ICG in liver surgery, but there are no more related studies and discoveries.
In addition to the main blood bile fecal pathway, ICG can also enter the lymphatic system through blood circulation[18], playing a role in lymph node tracing in breast surgery[19], gastrointestinal surgery[20], and other procedures. However, as we all know, the capillary blood flow velocity in the circulatory system is much faster than that in the lymphatic system[21], and the total protein content in the lymph is significantly lower than that in the blood[22]. This has an important influence on the binding degree and distribution of ICG and protein components in human body fluids. Therefore, when we apply small doses of ICG intravenously before surgery, there may be no obvious tracking effect on lymph nodes adjacent to the liver.
Of note, we developed a novel TLRS scoring system that can be used to assess hepatic ICG clearance to identify cases with possible fluorescence interference prior to surgery. With an AUC of 0.848, a sensitivity of 0.773 and a specificity of 0.885 by ROC curve analysis, we believe that this scoring system has a good identification ability. It has a relatively important clinical application for f-hepatectomy patients who are prepared for initial tumor staining with ICG within 24 h or even 48 h before surgery in the clinic. Interestingly, the student t-test in this study also showed that patients with body mass index > 24 (overweight) and ≤ 24 (normal weight/overweight) had no statistical difference between high and low FI-LB groups. Therefore, ICG administration based on body weight alone and ignoring other indicators may not be a wise option.
Through this study, we found that the indicators with correlation with FI-LB and FI-T can be divided into two categories, which were liver injury and validation indicators, liver functional reserve and synthetic indicators. Among them, both LDH and GGT were shown, and they may be more closely related to the possible overall liver tumor staining. LDH is an important enzyme in the glycolytic process, and hepatocellular injury of any cause increases LDH levels[23]. Previous studies have shown that LDH5 is mainly expressed in the liver[24]. The rate of glycolysis is significantly higher in tumor tissue than in normal tissue, and serum LDH activity is significantly increased in patients with hepatocellular carcinoma[25]. The main source of serum GGT is the mitochondria of hepatocytes, and GGT is mainly secreted through the bile; elevated GGT levels reflect massive hepatocyte necrosis or bile duct obstruction[26]. AST and ALT are the most commonly used indicators for clinical assessment of liver function and are significantly elevated after hepatocellular injury; ADA is also an important enzyme responsible for purine nucleotide metabolism, catalyzing the formation of hypoxanthine from purine nucleotides, in autoimmune hepatitis. CHE is a hydrolase with a half-life of 10 d, is synthesised and secreted by the liver, and can be used as a direct indicator of reserve liver function and synthetic capacity[27]. RBC was another independent influencing factor in addition to LDH, which was negatively correlated with FI-LB. It may be related to increased ICG metabolism in the liver due to increased ICG and hemoglobin binding. Notably, APTT may be limited by sample size issues in the analysis of correlation with FI-LB and did not show statistical significance in the present analysis (P = 0.063), but there was a phase relationship in the correlation analysis with FI-T (P = 0.007), which may also be a potential indicator that can be used to predict the effect of liver staining. As for FIG, the main relevant indicators were erythrocyte count, hemoglobin, and CHE (the liver reserve indicator), suggesting that gallbladder fluorescence intensity is more closely related to the synthetic function of the liver. Tbil and direct bilirubin (Dbil) are blood indicators closely related to bile excretion and obstruction. However, no correlation between Tbil, Dbil and FI-T, FI-LB was observed in our study, and no correlation was found in FI-G analysis, which may be related to the insufficient sample size of ICG used in this study and the small dose of ICG used, because the main object of our current study was the underlying staining of preoperative liver tumor lesions, and the main object was not bile staining.
Our study demonstrated the feasibility of low-dose and short-interval (24 h) ICG administration for basal staining of liver tumors/lesions. Notably, the present study has the following unavoidable limitations: (1) The sample size was not large enough to allow subgroup analysis based on tumor or lesion type, and other ICG dose controls were not used for comparison, which should be improved in future studies; (2) Only a few preoperative blood indicators were included in this study, tumor indicators and other potentially relevant blood indicators were not fully included; Therefore, the predictive power of the model was insufficient; (3) All patients enrolled in this study had Child-Pugh grade A or B liver function assessment, and those with inoperable Child-Pugh grade C were not enrolled; therefore, we were unable to obtain fluorescence images of their livers via the surgical route; and (4) We still lack a specific and scientific staining scoring system. In the study, we only take the average fluorescence intensity as the main object of analysis and do not include other important parameters such as the area of staining. A bias was observed in the overall patient selection. In addition, with the deepening of imaging research, it has been pointed out that the degree of liver cirrhosis can be evaluated by the functional liver imaging score. In the follow-up study, we can combine imaging scores, laboratory blood indicators and other clinical information to establish a more complete prediction model of fluorescence staining effect and improve the overall predictive ability of the model.
Compared with the results of previous relevant studies, a preoperative 5 mg/d regimen was ideal for most ICG-guided laparoscopic procedures for hepatic tumors/lesions. However, a higher dose may be required to achieve better results when used for clear fluorescence visualization of the gallbladder and biliary tract. Several factors affect the fluorescence effect of ICG-guided laparoscopic liver surgery. Preoperative blood indicators can be used as a useful tool to predict different intraoperative fluorescence characteristics and to guide the adjustment of the ICG dosing regimen for better overall staining results.
Preoperative blood laboratory indexes can potentially predict indicators of liver background fluorescence intensity in ICG-guided laparoscopic hepatectomy. Surgeons can adjust the preoperative drug regimen of liver ICG based on preoperative blood indexes to reduce intraoperative fluorescence interference and achieve a more satisfactory tumor fluorescence staining effect.
The preoperative regimen of indocyanine green (ICG) administration in laparoscopic hepatectomy was still controversial. Factors associated with tumor fluorescence staining effect were unclear.
Establish a novel laboratory scoring system to screen specifical patients who need ICG dose adjustment.
Establish a risk model that can predict the effect of liver ICG preoperative staining through clinical indicators, and reduce the probability of ICG staining failure events.
All enrolled patients received 5 mg ICG 24 h before laparoscopic hepatectomy, then investigate the predictive indicators of ICG fluorescence characteristics and established a laboratory risk model to identify individuals at high risk for high liver background fluorescence.
Pre-operative aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, adenosine deaminase, and lactate dehydrogenase were positively associated with fluorescence intensity of background liver (FI-LB) and pre-operative red blood cell, cholinesterase, and were negatively associated with FI-LB. Total laboratory risk score (TLRS) was calculated according to receiver operating characteristic (ROC) curve [area under the ROC curve (AUC) = 0.848, sensitivity = 0.773, specificity = 0.885]. When TLRS was greater than 6.5, the liver clearance ability of ICG was considered as poor.
Common preoperative laboratory blood parameters can be used to predict liver ICG clearance. The established risk prediction model helps to identify individuals who may be at risk of fluorescent staining failure. Surgeons can calculate the risk value of high liver background fluorescence intensity based on relevant blood indicators, so as to adjust the dose and time of ICG before operation, and finally obtain better liver fluorescence staining.
More effective clinical indicators (including preoperative ultrasound or radiographic examination characteristics, blood tumor indicators, etc.) are expected to be added to the model for predicting liver ICG fluorescence staining, further improving the accuracy of the model, and thus obtaining greater clinical application value.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goja S, India; Hori T, Japan S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Chen YL
| 1. | Slakter JS, Yannuzzi LA, Guyer DR, Sorenson JA, Orlock DA. Indocyanine-green angiography. Curr Opin Ophthalmol. 1995;6:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Juszczyk J, Dybizbańska A, Kryska A, Adamek J, Czajka B. [Binding of indocyanine green (ICG) with serum proteins in various liver diseases]. Pol Arch Med Wewn. 1979;62:305-314. [PubMed] |
| 3. | Nakagawa S, Nagata K, Araki K, Nagashima H. Investigation of the hepatic excretion mechanism of indocyanine green in patients with liver disease. Gastroenterol Jpn. 1976;11:237-245. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Li J, Li X, Zhang X, Wang H, Li K, He Y, Liu Z, Zhang Z, Yuan Y. Indocyanine green fluorescence imaging-guided laparoscopic right posterior hepatectomy. Surg Endosc. 2022;36:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Vlek SL, van Dam DA, Rubinstein SM, de Lange-de Klerk ESM, Schoonmade LJ, Tuynman JB, Meijerink WJHJ, Ankersmit M. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc. 2017;31:2731-2742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Wiegand BD, Ketterer SG, Rapaport E. The use of indocyanine green for the evaluation of hepatic function and blood flow in man. Am J Dig Dis. 1960;5:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Ishizawa T, Masuda K, Urano Y, Kawaguchi Y, Satou S, Kaneko J, Hasegawa K, Shibahara J, Fukayama M, Tsuji S, Midorikawa Y, Aburatani H, Kokudo N. Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma. Ann Surg Oncol. 2014;21:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr. 2016;5:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, Panganiban KM, Perini MV, Shah SR, Wang H, Xu Y, Suh KS, Kokudo N. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg. 2021;274:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 10. | Digital MAOCMA, Digital ISPCOCRH, Liver CPCOCMDA, Clinical PMPC, Medical IAEPCOCGS, Molecular IPCOCBS. [Guidelines for application of computer-assisted indocyanine green molecular fluorescence imaging in diagnosis and surgical navigation of liver tumors (2019)]. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:1127-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Wakabayashi T, Cacciaguerra AB, Abe Y, Bona ED, Nicolini D, Mocchegiani F, Kabeshima Y, Vivarelli M, Wakabayashi G, Kitagawa Y. Indocyanine Green Fluorescence Navigation in Liver Surgery: A Systematic Review on Dose and Timing of Administration. Ann Surg. 2022;275:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 12. | Verbeek FP, Schaafsma BE, Tummers QR, van der Vorst JR, van der Made WJ, Baeten CI, Bonsing BA, Frangioni JV, van de Velde CJ, Vahrmeijer AL, Swijnenburg RJ. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc. 2014;28:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Aoki T, Yasuda D, Shimizu Y, Odaira M, Niiya T, Kusano T, Mitamura K, Hayashi K, Murai N, Koizumi T, Kato H, Enami Y, Miwa M, Kusano M. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J Surg. 2008;32:1763-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Tanaka T, Takatsuki M, Hidaka M, Hara T, Muraoka I, Soyama A, Adachi T, Kuroki T, Eguchi S. Is a fluorescence navigation system with indocyanine green effective enough to detect liver malignancies? J Hepatobiliary Pancreat Sci. 2014;21:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Liang X, Zhai S, Liang Y, Jiang G, Mao Q, Xie Y, Cai X. [Laparoscopic liver tumor resection under indocyanine green fluorescent navigation: A single center experience of 60 patients to study the optimal preoperative injection timing of indocyanine green]. Chin J Hepatobil Sur. 2019;25:90-93. [DOI] [Full Text] |
| 16. | Lu H, Gu J, Qian XF, Dai XZ. Indocyanine green fluorescence navigation in laparoscopic hepatectomy: a retrospective single-center study of 120 cases. Surg Today. 2021;51:695-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Chen Q, Zhou R, Weng J, Lai Y, Liu H, Kuang J, Zhang S, Wu Z, Wang W, Gu W. Extrahepatic biliary tract visualization using near-infrared fluorescence imaging with indocyanine green: optimization of dose and dosing time. Surg Endosc. 2021;35:5573-5582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Kraft JC, Treuting PM, Ho RJY. Indocyanine green nanoparticles undergo selective lymphatic uptake, distribution and retention and enable detailed mapping of lymph vessels, nodes and abnormalities. J Drug Target. 2018;26:494-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Zhang L, Cheng M, Lin Y, Zhang J, Shen B, Chen Y, Yang C, Yang M, Zhu T, Gao H, Ji F, Li J, Wang K. Ultrasound-assisted carbon nanoparticle suspension mapping versus dual tracer-guided sentinel lymph node biopsy in patients with early breast cancer (ultraCars): phase III randomized clinical trial. Br J Surg. 2022;109:1232-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Müller D, Stier R, Straatman J, Babic B, Schiffmann L, Eckhoff J, Schmidt T, Bruns C, Fuchs HF. ICG-Lymphknoten-Mapping in der Tumorchirurgie des oberen Gastrointestinaltrakts. Chirurgie. 2022;93:925-933. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 485] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 22. | Nanjee MN, Cooke CJ, Olszewski WL, Miller NE. Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans. Associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J Lipid Res. 2000;41:1317-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Kuwano A, Kurokawa M, Kohjima M, Imoto K, Tashiro S, Suzuki H, Tanaka M, Okada S, Kato M, Ogawa Y. Microcirculatory disturbance in acute liver injury. Exp Ther Med. 2021;21:596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Puri BK, Kingston MC, Monro JA. Fructose-associated hepatotoxicity indexed by the lactate dehydrogenase isoenzyme LDH-5. Med Hypotheses. 2019;124:40-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Yada M, Miyazaki M, Motomura K, Masumoto A, Nakamuta M, Kohjima M, Sugimoto R, Aratake Y, Higashi N, Morizono S, Takao S, Yamashita N, Satoh T, Yamashita S, Kuniyoshi M, Kotoh K. The prognostic role of lactate dehydrogenase serum levels in patients with hepatocellular carcinoma who are treated with sorafenib: the influence of liver fibrosis. J Gastrointest Oncol. 2016;7:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Hanigan MH. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Adv Cancer Res. 2014;122:103-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 27. | Tan L, Meng Y, Zeng T, Wang Q, Long T, Wu S, Guan X, Fu H, Zheng W, Tian Y, Chen J, Yu J, Wu Y, Li H, Cao L. Clinical diagnostic significance of prealbumin, cholinesterase and retinol binding protein in liver cirrhosis combined with encephalopathy. Br J Biomed Sci. 2019;76:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |