Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1294
Peer-review started: January 21, 2023
First decision: April 13, 2023
Revised: April 17, 2023
Accepted: May 8, 2023
Article in press: May 8, 2023
Published online: July 27, 2023
Processing time: 180 Days and 21.8 Hours
Magnetic compression anastomosis (MCA) is a simple procedure contributing to a reliable anastomosis. However, digestive-tract reconstruction after total gastrectomy using MCA has not yet been reported.
To investigate the feasibility of MCA for simultaneous esophagojejunostomy and jejunojejunostomy after total gastrectomy using beagle dogs.
Sixteen beagles were randomly divided into an MCA group (study group, n = 8) and a manual-suture anastomosis group (control group, n = 8). Two different magnetic anastomosis devices were used in the study group for esophagojejunal and jejunojejunal anastomoses. Both devices included a pair of circular daughter and parent magnets each. The time of esophagojejunostomy and jejunojejunostomy, postoperative complications, and survival rate of the two groups were compared. The dogs were sacrificed one month after the operation and their anastomotic specimens were obtained. Healing was observed by the naked eye and a light microscope.
Digestive-tract reconstruction after total gastrectomy was successfully completed in both groups (survival rate = 100%). In the study group, esophagojejunal and jejunojejunal anastomoses took 6.13 ± 0.58 and 4.06 ± 0.42 min, respectively, significantly lower than those in the control group (15.63 ± 1.53 min, P < 0.001 and 10.31 ± 1.07 min, P < 0.001, respectively). Complications such as bleeding, anastomotic leakage, and anastomotic stenosis were not observed. In the study group, the magnets did not interfere with each other. Discharge time of the jejunojejunal magnetic anastomosis device was 10.75 ± 1.28 d, while that of the esophagojejunal magnetic anastomosis device was 12.25 ± 1.49 d. Residual silk was found in the control group. The study group showed a greater smoothness of the anastomosis than that of the control group. All layers of anastomosis healed well in both groups.
MCA is a safe and feasible procedure for digestive-tract reconstruction after total gastrectomy in this animal model.
Core Tip: Magnetic compression anastomosis (MCA) is a new type of anastomosis and a simple procedure affording a reliable anastomosis effect. We proposed the application of MCA in digestive tract reconstruction after total gastrectomy in dogs, and set up a control group to compare with manual suture anastomosis. The results showed that MCA was superior to manual suture anastomosis in anastomosis time and anastomosis effect. Further optimization of magnet design and surgical methods can make it have clinical application prospects.
- Citation: Zhang MM, Li CG, Xu SQ, Mao JQ, Zhang YH, Shi AH, Li Y, Lyu Y, Yan XP. Magnetic compression anastomosis for reconstruction of digestive tract after total gastrectomy in beagle model. World J Gastrointest Surg 2023; 15(7): 1294-1303
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1294.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1294
Gastric cancer (GC) is one of the most serious and common malignancies of the digestive system worldwide. Approximately 1.08 million new cases of GC and 769000 deaths globally were reported in 2020[1]. Borderline gastroesophageal tumors, cardiac cancer, and fundus tumors are treated by performing total gastrectomy and reconstruction of the digestive tract. After total gastrectomy for GC, Roux-en-Y esophagojejunal anastomosis is commonly used for reconstruction. However, complications of esophagojejunostomy and jejunojejunostomy after total gastrectomy, the most common of which are anastomotic stricture and anastomotic leakage, are closely related to the prognosis of patients. Some examples of common esophagogastrostomy methods are hand-sewn and mechanical anastomoses. Studies have reported an incidence rate of 2.1%–14.6% for esophagojejunal anastomotic leakage and a high mortality rate of 50%[2-5]. Hence, it is necessary to study whether other anastomotic methods can be used for digestive tract reconstruction after total gastrectomy.
Magnetic compression anastomosis (MCA) is a new type of anastomosis, characterized by a “no-contact” magnetic field force between magnetic materials. It uses magnetic force for non-penetrating anastomosis reconstruction of tissues at both ends of the anastomosis, without any sutures and staples[6,7]. Obora was the first to carry out research on MCA. He showed that magnetic ring anastomosis has the advantages of a simple operation and a reliable anastomosis effect[8]. At present, MCA is applied to esophageal anastomosis[9,10], gastrointestinal anastomosis[11], small intestine anastomosis[12], colorectal anastomosis[13,14]. In addition, MCA can be used for vascular anastomosis[15,16] and cystostomy[17]. However, there is no relevant report on the application of MCA in esophagojejunostomy and jejunojejunostomy after total gastrectomy.
This study investigates the safety and reliability of MCA for digestive-tract reconstruction (esophagojejunostomy and jejunojejunostomy) after total gastrectomy in beagle dogs. It also compares the results with those of hand-sewn anastomosis.
The experimental protocol was approved by the Committee for Ethics of Animal Experiments of Xi’an Jiaotong University (license No. 2022-1451). Sixteen beagles (male = 8, female = 8) were obtained from the Laboratory Animal Center of the Xi’an Jiaotong University (Xi’an, China). The research protocol and all the experimental procedures were strictly in accordance with the Guidelines for the Care and Use of Experimental Animals, issued by the Xi’an Jiaotong University Medical Center.
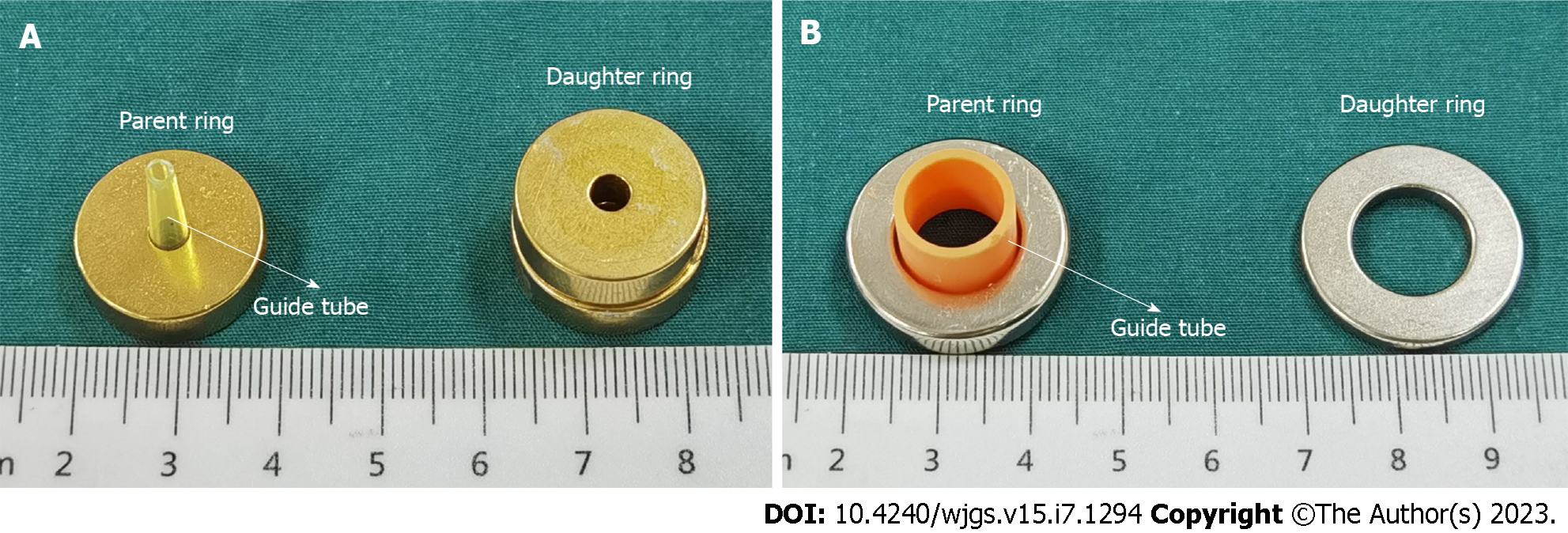
Since esophagojejunostomy and jejunojejunostomy are required for Roux-en-Y digestive-tract reconstruction after total gastrectomy, we used two pairs of magnetic anastomosis rings: An end-to-end esophagojejunal magnetic anastomosis ring (EJ-MAR) and an end-to-side jejunojejunal magnetic anastomosis ring (JJ-MAR). The EJ-MAR comprises a pair of cylindrical magnetic rings (parent ring and daughter ring) with a central hole. The guide tube is fixed at the center of the parent ring. The JJ-MAR also comprises a pair of circular rings (parent ring and daughter ring). The parent magnetic ring is fixed with a T-shaped guide tube. Both the EJ-MAR and JJ-MAR are made of N40 sintered NdFeB with axial saturation magnetization. The surfaces of the EJ-MAR and JJ-MARare protected by titanium nitride coating and nickel coating respectively. The magnetic anastomosis device was designed by the authors of this paper. Figure 1 shows the two types of magnetic anastomosis rings. Table 1 Lists the physical parameters of these two rings.
| Device | OD, mm | ID, mm | Thickness, mm | MFD, mT | Weight, g | GTL, mm | |
| EJ-MAR | PR | 20 | 5 | 5 | 338 | 11.05 | 20 |
| DR | 20 | 5 | 10 | 436 | 21.04 | ||
| JJ-MAR | PR | 25 | 14 | 5 | 267 | 12.39 | 14 |
| DR | 25 | 14 | 3 | 202 | 7.74 | ||
Sixteen beagles > 1 year old and weighing 12–15 kg were randomly divided into two groups: The MCA group (female = 4, male = 4) and the hand-sewn group (female = 4, male = 4). The study group was given end-to-end esophagojejunostomy and end-to-side jejunojejunostomy with the EJ-MAR and JJ-MAR, respectively. The control group was given hand-sewn anastomosis with 4-0 interrupted non-absorbable sutures. The anastomotic time, postoperative complications, and survival rate of the two groups were compared. In the study group, the time that the magnet rings took to be expelled from the body was recorded. One month after the operation, the anastomosis specimens were examined.
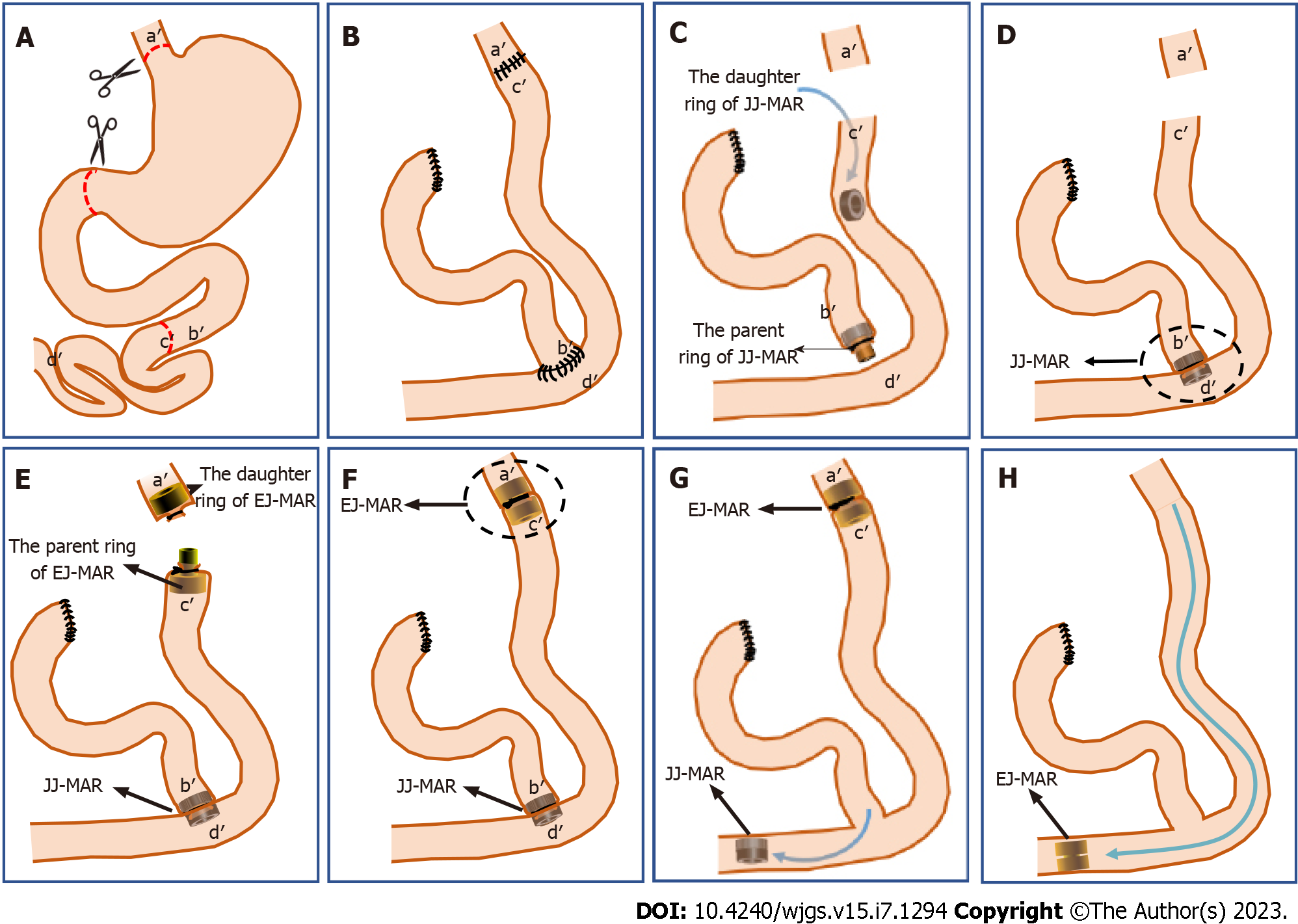
The beagles were kept on a fast 12 h before surgery and were not fed any solids. They were anesthetized by intravenous injection of 3% pentobarbital sodium (1 mL/kg) and fixed in the supine position. An approximately 20-cm incision was made in the middle of the upper abdomen. The left and right gastroepiploic vessels were cut with hemostatic forceps clips, and the broken ends of the vessels were ligated with 4-0 silk sutures. The duodenum was cut just below the anterior pyloric vein and the stump was closed by purse-string sutures. The ligaments of the spleen and stomach were separated, the short gastric vein was cut off, and the broken end of the blood vessels was ligated with a 4-0 silk thread. The left gastric vessels and fundic veins were divided along the gastric wall and ligated with the 4-0 silk thread. The esophagus was cut above the cardia incisura. After total gastrectomy, the jejunum was incised approximately 12 cm distal to the Treitz ligament (Figure 2A).
In the control group: End-to-end interrupted full-thickness hand-sutured anastomosis was performed between the esophageal stump and the distal jejunum (a'-c') using 4-0 silk sutures. The proximal jejunal stump was anastomosed with the distal jejunum (b'-d') by end-to-side interrupted full-thickness manual suture using 4-0 silk sutures (Figure 2B). To avoid differences in surgical outcomes caused by human factors, all surgeries were performed by the same surgeon.
In the study group: The parent ring of the JJ-MAR was placed at the proximal jejunal stump, which was then ligated to the guide tube of the abovementioned ring with a 4-0 non-absorbable silk suture. The daughter magnet ring of the JJ-MAR was pushed to the appropriate position at the distal end of the jejunum (Figure 2C), so that it was automatically aligned with the parent ring of the JJ-MAR, and the end-to-side magnetic anastomosis between the proximal jejunal stump and the distal jejunum (b'-d') was completed (Figure 2D). The same method was used to place the parent and daughter rings of the EJ-MAR in the distal jejunal stump and the esophageal stump, respectively. The esophageal stump and the distal jejunal stump were ligated using a purse-string suture (Figure 2E). The daughter and parent rings of the EJ-MAR were placed close to each other so that they were automatically attracted to each other, and the end-to-end magnetic anastomosis between the esophageal stump and the distal jejunal stump (a'-c') was completed (Figure 2F). After using the continuous magnetic force for a certain period, the JJ-MAR rings were first detached from the anastomosis, and the jejunal end-to-side anastomosis was established (Figure 2G). Next, the EJ-MAR rings were detached from the anastomosis and the end-to-end esophagojejunal anastomosis was established (Figure 2H).
All dogs were managed in a single cage after emergence from anesthesia. Pethidine hydrochloride (1 mg/kg) was injected intramuscularly every 12 h for 3 d after operation for analgesia. Cefotiam (0.5 g) was given intravenously every 12 h for 3 d after surgery to fight infection. The dogs in the control group were given a non-residue liquid diet for the first 5 d after operation, and then placed back to the normal diet before operation. The dogs in the study group were fasted without drinking and were given glucose and an electrolyte solution intravenously every day. The study group underwent abdominal X-ray examination every other day to monitor the position of the magnet. When the X-ray examination showed that the magnet had detached from the anastomosis, the normal diet for the dogs was resumed.
All dogs were euthanized one month after operation, and the esophagojejunal anastomosis and jejunojejunal anastomosis specimens were obtained. The healing of the anastomosis was observed by the naked eye. Next, the anastomotic specimen was soaked overnight in 10% formalin. After fixation, the specimen was embedded in paraffin and a 4-μm-thick section was prepared from the anastomosis. The sections were stained with hematoxylin and eosin (H&E) and Masson trichrome and examined under a bright-field microscope.
SPSS statistical 20.0 software was used for data analysis. The quantitative data of normal distribution are described by the mean ± SD, while those of non-normal distribution are described by the median. Differences between the groups were compared by an independent sample t-test or a nonparametric test. P < 0.05 indicated a significant difference.
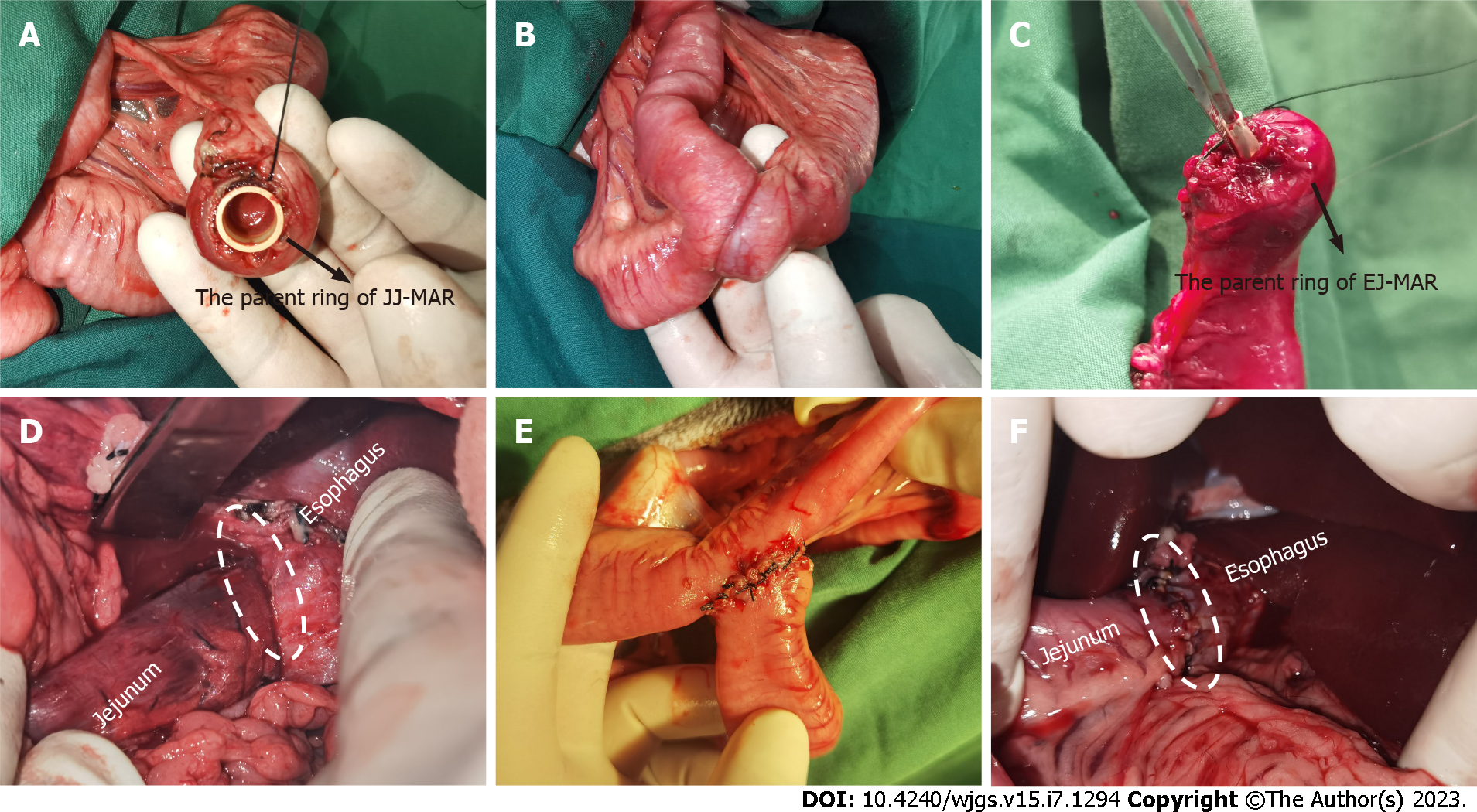
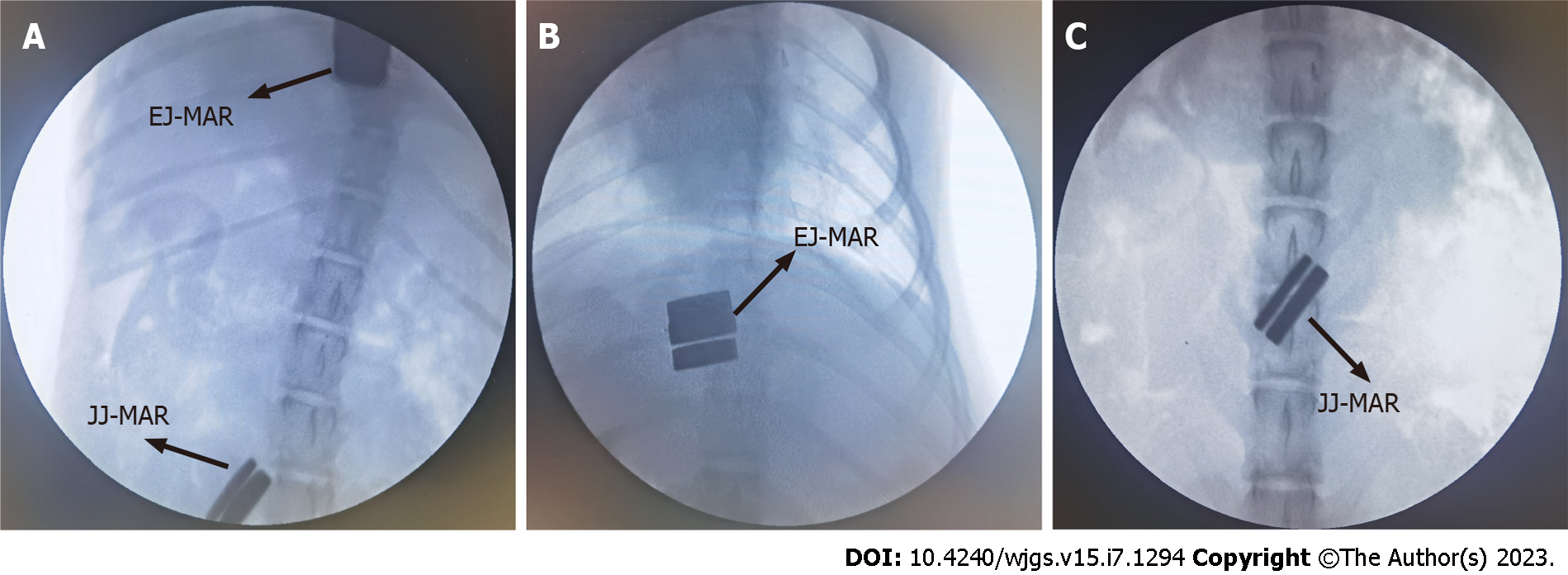
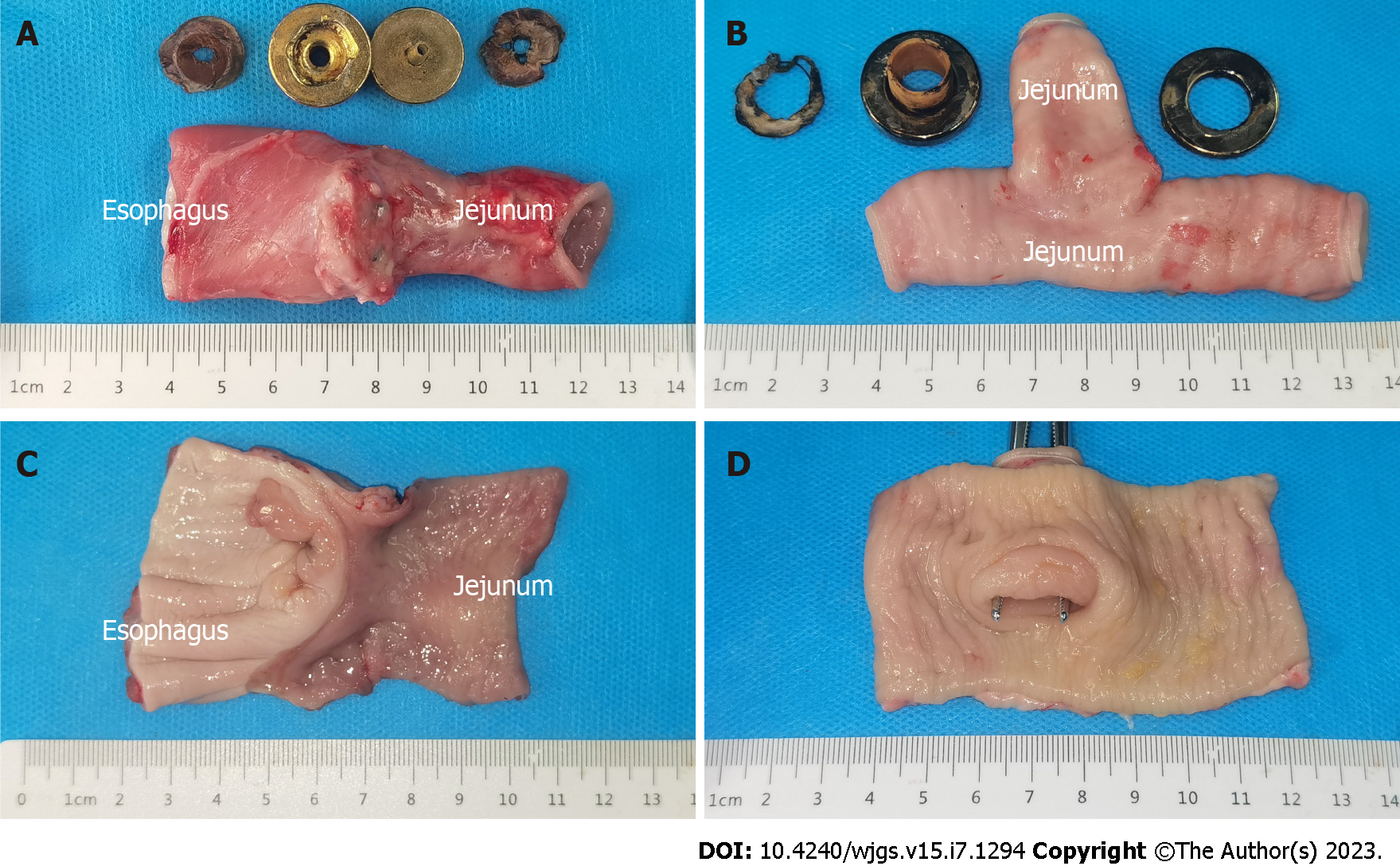
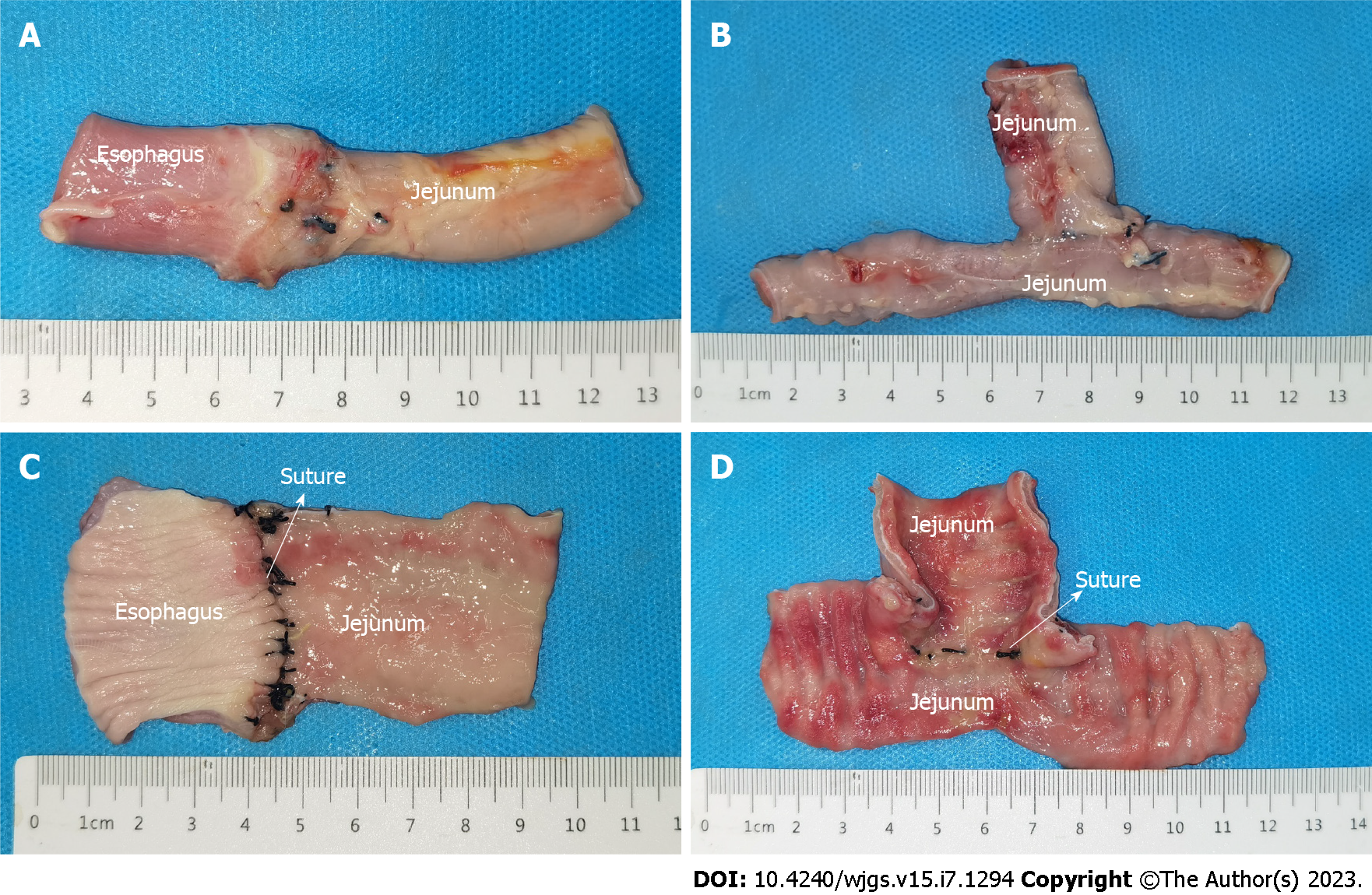
All dogs successfully underwent digestive-tract reconstruction after total gastrectomy using MCA and manual-suture anastomosis in the study group (Figure 3A-D) and the control group (Figure 3E and F), respectively. Postoperative abdominal X-ray examination of the study group showed that the two pairs of magnets were well attracted to each other (Figure 4). In the study group, esophagojejunal and jejunojejunal anastomoses took 6.13 ± 0.58 and 4.06 ± 0.42 min, respectively, significantly lower than those in the control group (15.63 ± 1.53 min, P < 0.001 and 10.31 ± 1.07 min, P < 0.001, respectively). In the study group, the mean time for expelling the JJ-MAR from the anus was 10.75 ± 1.28 d (range 9–13 d), and that for the EJ-MAR was 12.25 ± 1.49 d (range 10–15 d).
All animals in both groups survived to 1 mo after operation, with a survival rate of 100%. No bleeding, anastomotic leakage, digestive-tract obstruction, and other postoperative complications occurred.
The serosal layer of esophagojejunal and jejunojejunal anastomoses of both groups healed well (Figures 5A and B and 6A and B). No obvious stenosis in the anastomosis was observed, except for the residual suture in the anastomosis of the control group (Figure 6C and D). No residual foreign body was observed in the anastomosis of the study group (Figure 5C and D). H&E and Masson staining showed that all layers of the anastomosis of the two groups healed well (Figure 7). The mucosal layer of the anastomosis in the study group was smoother than that in the control group.
Some studies have confirmed that MCA can be applied to end-to-end and end-to-side anastomosis in digestive tract[10,18]. However, there is no relevant report on the application of MCA for digestive-tract reconstruction after total gastrectomy. This study is the first to use MCA for total magnetic anastomosis reconstruction after total gastrectomy. The following study results were obtained: (1) MCA can significantly reduce the anastomosis time compared with that needed for manual sutures in both esophagojejunostomy and jejunojejunostomy, which is consistent with the results of previous studies; (2) Despite significant differences in the histological structure of the esophagus and gastrointestinal tract, MCA can still establish a good anastomosis effect; and (3) The magnetic compression of jejunojejunal and esophagojejunal anastomoses was established sequentially, and the magnets were discharged in batches to avoid their unplanned absorption in the digestive tract.
MCA is different from staple anastomosis and manual-suture anastomosis. MCA is a type of “structure immediate anastomosis and function delayed anastomosis” which means that even after the operation, the patency of the anastomosis could not be established before the magnet was detached from the anastomosis. Therefore, the animals in the study group were unable to eat during this period and needed to rely on intravenous nutritional support. Although the esophagojejunal and jejunojejunal anastomoses can be blocked for a short period, during which time they do not undertake the task of letting food pass, it is still necessary to ensure a safe passage for digestive fluids such as saliva, bile, pancreatic juice, and intestinal juice. Therefore, in this study, the parent magnets of the EJ-MAR and JJ-MAR were fixed with a guide tube, which plays a role in draining the digestive fluid before the anastomosis was established.
In this study, the design of the EJ-MAR was kept different from that of the JJ-MAR for two reasons. First, the daughter magnetic ring of the former adopts the scheme of superposition of two magnetic rings, mainly because the retraction occurs after the esophagus is severed and the tension at the anastomosis is large. Therefore, a stronger magnetic force is needed, which can be obtained by increasing the number of magnetic rings. Second, the wall of the esophageal stump is thick. For a better compression anastomosis effect, the central hole of the EJ-MAR should be kept small, which not only facilitates the purse-string suture of the esophageal and jejunal stumps, but also ensures that there is enough wall tissue between the daughter and parent magnets to ensure the quality of anastomosis. Of course, because of the thin guide tube of the EJ-MAR, the dogs in the study group need parenteral nutrition support after operation, which is one of its shortcomings. Therefore, the future research should focus on optimizing the design of the EJ-MAR and increasing the diameter of the guide tube. This will allow the study-group dogs to receive a slag-free liquid diet after surgery.
Poor anastomotic blood supply, high tension, and long operative time may increase the incidence of anastomotic leakage[19,20]. In this study, we demonstrated that the application of MCA significantly reduced the operative time for gastrointestinal anastomosis compared to that needed for hand-sewn anastomosis. Shortening the anastomosis operation and simplifying the anastomosis technique reduce the destruction of anastomotic submucosal vessels and facilitate the healing of the anastomotic tissue. Hand-sewn anastomosis is used to connect tissues at two ends of anastomotic stoma through suture, so as to ensure the continuity of the gastrointestinal tract and making the anastomosis successful. When sutures penetrate tissues, they form microgaps with tissues through which bacteria and digestive juices invade the tissues, increasing the potential for anastomotic leakage. MCA is a “non-penetrating” mode of anastomosis, also known as “the third mode of anastomosis” after hand-sewn anastomosis and mechanical stapling anastomosis. MCA creates a uniform compression force between the two ends of the anastomosis, which can effectively avoid tearing of the anastomotic tissue caused by the uneven tensile force between different points in the suture anastomosis, so as to effectively improve the quality of anastomosis. Moreover, under the action of the magnetic force of the parent and daughter magnets, the pathological process of ischemia–necrosis–shedding occurred at the target anastomosis site, while adhesion–repair–healing occurred in the tissues adjacent to the anastomosis. Discharge of the magnets from the anastomosis indicated that the anastomosis was successfully established and no foreign body was present in the tissue.
Examination of gross specimens and histological sections revealed that the mucosa on the anastomotic surface was smoother in the study group than it was in the control group. This could be because sutures retained in the tissue as foreign bodies cause a chronic inflammatory response in the tissue, resulting in inflammatory-cell infiltration, excessive fibroblast proliferation, and scarring of the anastomotic tissue; in severe cases, it may cause anastomotic stenosis or even occlusion. This situation can be avoided to a great extent using MCA[19]. The study has the following limitations: (1) A small sample size of the experimental animals; and (2) Lack of observation over a longer duration in both groups of anastomoses. In this study, the two pairs of magnets in the study group did not attract each other. Of course, it cannot be denied that the adverse events of the two magnets attraction were not observed due to the small sample size. Therefore, we suggest that necessary protective measures should be taken for the possible attraction of the two pairs of magnets in the next research, such as magnetic shielding measures for the two pairs of magnets.
This study demonstrates the feasibility of MCA in digestive-tract reconstruction after total gastrectomy, especially when there are two sets of magnets in the body. Further optimization of the magnet design and surgical procedure would allow the application of MCA in clinical total gastrectomy.
Magnetic compression anastomosis (MCA) has been reported in gastrointestinal anastomosis and small intestine anastomosis, however, there is no relevant report on the application of MCA in esophagojejunostomy and jejunojejunostomy after total gastrectomy.
To investigate the feasibility of total magnetic anastomosis reconstruction of digestive tract after total gastrectomy.
To design the magnet anastomosis rings for reconstruction of digestive tract magnamosis after total gastrectomy and investigate its feasibility.
Sixteen beagle dogs were randomly assigned to the MCA group and the manual suture group. The operation time, postoperative complication and anastomosis specimens were examined between the two groups.
The results showed that the anastomosis time of MCA group was significantly shorter than that of manual suture anastomosis group. The study group showed a greater smoothness of the anastomosis than that of the control group.
MCA is safe and feasible for digestive-tract anastomosis reconstruction after total gastrectomy.
Animal experiments show that MCA has advantages for digestive-tract anastomosis reconstruction after total gastrectomy, which can be applied in clinic after further optimization of magnet design.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mishra TS, India; Tharavej C, Thailand; Yu HG, China S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64702] [Article Influence: 16175.5] [Reference Citation Analysis (177)] |
| 2. | Makuuchi R, Irino T, Tanizawa Y, Bando E, Kawamura T, Terashima M. Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg Today. 2019;49:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Migita K, Takayama T, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Nakajima Y. Risk factors for esophagojejunal anastomotic leakage after elective gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1659-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Carboni F, Valle M, Federici O, Levi Sandri GB, Camperchioli I, Lapenta R, Assisi D, Garofalo A. Esophagojejunal anastomosis leakage after total gastrectomy for esophagogastric junction adenocarcinoma: options of treatment. J Gastrointest Oncol. 2016;7:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | El-Sourani N, Bruns H, Troja A, Raab HR, Antolovic D. Routine Use of Contrast Swallow After Total Gastrectomy and Esophagectomy: Is it Justified? Pol J Radiol. 2017;82:170-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Jamshidi R, Stephenson JT, Clay JG, Pichakron KO, Harrison MR. Magnamosis: magnetic compression anastomosis with comparison to suture and staple techniques. J Pediatr Surg. 2009;44:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Yan XP, Shang P, Shi AH, Liu WY, Liu YX, Lv Y. [Exploration and establishment of magnetic surgery]. Kexue Tongbao. 2019;64:815-826. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Obora Y, Tamaki N, Matsumoto S. Nonsuture microvascular anastomosis using magnet rings: preliminary report. Surg Neurol. 1978;9:117-120. [PubMed] |
| 9. | Bruns NE, Glenn IC, Craner DR, Schomisch SJ, Harrison MR, Ponsky TA. Magnetic compression anastomosis (magnamosis) in a porcine esophagus: Proof of concept for potential application in esophageal atresia. J Pediatr Surg. 2019;54:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Slater BJ, Borobia P, Lovvorn HN, Raees MA, Bass KD, Almond S, Hoover JD, Kumar T, Zaritzky M. Use of Magnets as a Minimally Invasive Approach for Anastomosis in Esophageal Atresia: Long-Term Outcomes. J Laparoendosc Adv Surg Tech A. 2019;29:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Kawabata H, Nakase K, Okazaki Y, Yamamoto T, Yamaguchi K, Ueda Y, Miyata M, Motoi S. Endoscopic ultrasonography for pre-operative local assessment and endoscopic ultrasonography-guided marking before gastrojejunostomy for duodenal obstruction using magnetic compression anastomosis. J Clin Transl Res. 2021;7:621-624. [PubMed] |
| 12. | Toselli L, Martinez-Ferro M, Cervio G, Kwiat D, Imamura-Ching J, Graves CE, Gaston B, Harrison M. Magnetic Compression Anastomosis (Magnamosis) for Functional Undiversion of Ileostomy in Pediatric Patients. J Laparoendosc Adv Surg Tech A. 2017;27:1314-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Russell KW, Rollins MD, Feola GP, Scaife ER. Magnamosis: a novel technique for the management of rectal atresia. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Kamada T, Ohdaira H, Hoshimoto S, Narihiro S, Suzuki N, Marukuchi R, Takeuchi H, Yoshida M, Yamanouchi E, Suzuki Y. Magnetic compression anastomosis with atypical anastomosis for anastomotic stenosis of the sigmoid colon: a case report. Surg Case Rep. 2020;6:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Vicol C, Eifert S, Oberhoffer M, Boekstegers P, Reichart B. Mid-term patency after magnetic coupling for distal bypass anastomosis in coronary surgery. Ann Thorac Surg. 2006;82:1452-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Zhang M, Ma J, An Y, Lyu Y, Yan X. Construction of an intrahepatic portosystemic shunt using the magnetic compression technique: preliminary experiments in a canine model. Hepatobiliary Surg Nutr. 2022;11:611-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Uygun I, Okur MH, Cimen H, Keles A, Yalcin O, Ozturk H, Otcu S. Magnetic compression ostomy as new cystostomy technique in the rat: magnacystostomy. Urology. 2012;79:738-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Fan C, Ma J, Zhang HK, Gao R, Li JH, Yu L, Wu Z, Lv Y. Sutureless intestinal anastomosis with a novel device of magnetic compression anastomosis. Chin Med Sci J. 2011;26:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Schietroma M, Cecilia EM, Carlei F, Sista F, De Santis G, Piccione F, Amicucci G. Prevention of anastomotic leakage after total gastrectomy with perioperative supplemental oxygen administration: a prospective randomized, double-blind, controlled, single-center trial. Ann Surg Oncol. 2013;20:1584-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Deguchi Y, Fukagawa T, Morita S, Ohashi M, Saka M, Katai H. Identification of risk factors for esophagojejunal anastomotic leakage after gastric surgery. World J Surg. 2012;36:1617-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |