INTRODUCTION
Jaundice manifests as a yellowish pigmentation of the skin and sclera, which is caused by interrupted or impaired excretion of bilirubin and biliverdin. Obstructive jaundice (OJ), i.e., obstructed bile outflow, can be either intrahepatic or extrahepatic. Intrahepatic OJ can be subdivided into non-obstructive intrahepatic cholestasis and obstructive intrahepatic obstructive cholestasis. Non-obstructive intrahepatic cholestasis may result from viral hepatitis, drug-related cholestasis (e.g., caused by chlorpromazine, methandrostenolone, and birth control pills), primary biliary cirrhosis, or intrahepatic cholestasis during pregnancy[1]. In contrast, obstructive intrahepatic cholestasis occurs due to intrahepatic sediment-like stones, cancerous emboli, or parasitic infections (e.g., Toxoplasma gondii infection)[2-4]. Extrahepatic cholestasis can be caused by obstruction of the common bile duct by stones, strictures, inflammatory edema, biliary atresia, bile duct injury, tumors, pancreatic tuberculosis and roundworms[5-7]. The occurrence of jaundice is also related to genetic factors[8]. Common bile duct stones are undoubtedly the leading cause of extrahepatic biliary obstruction, and bile duct cancer, malignancies such as periampullary and pancreatic cancer, and benign strictures including chronic pancreatitis have become increasingly common[9-11].
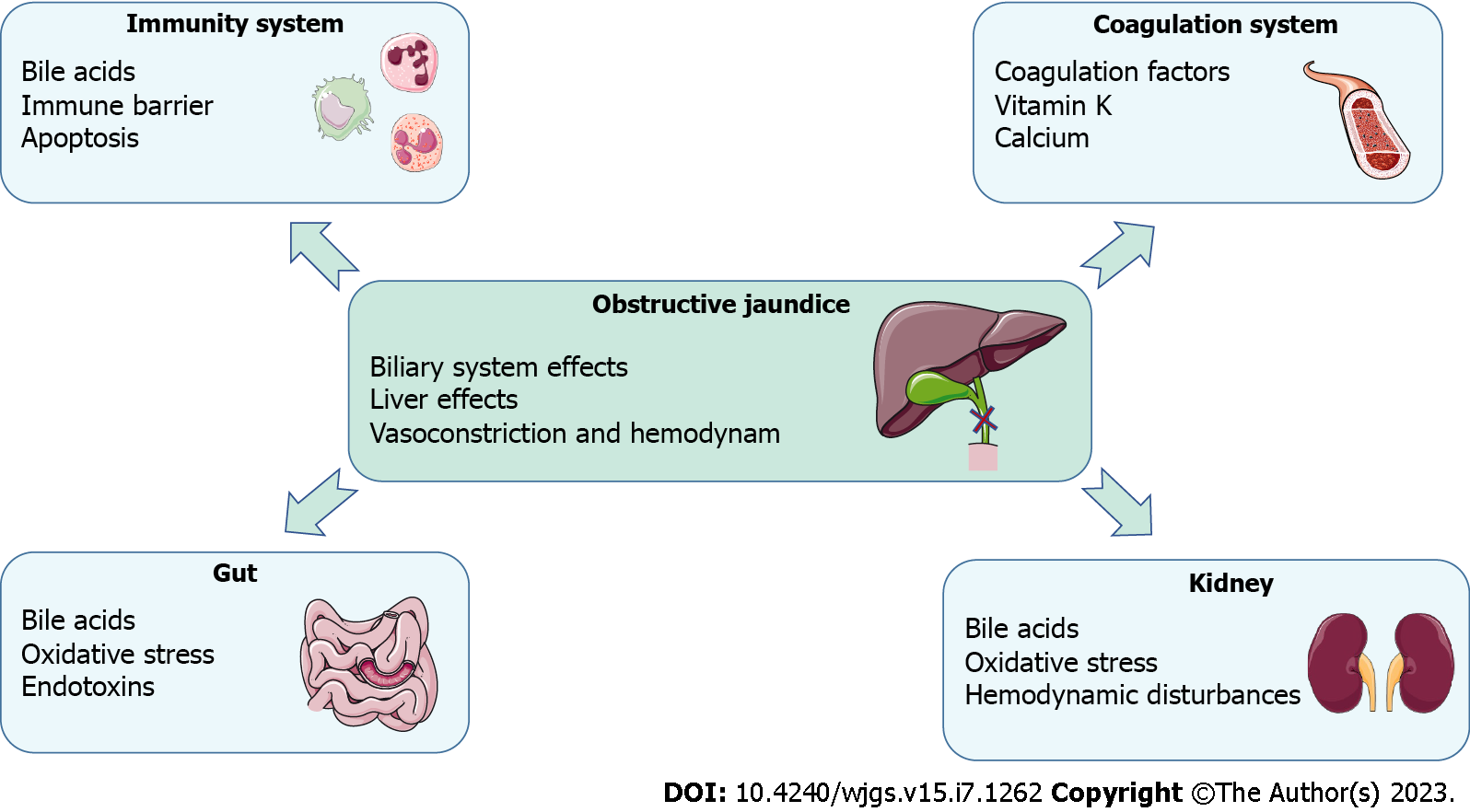
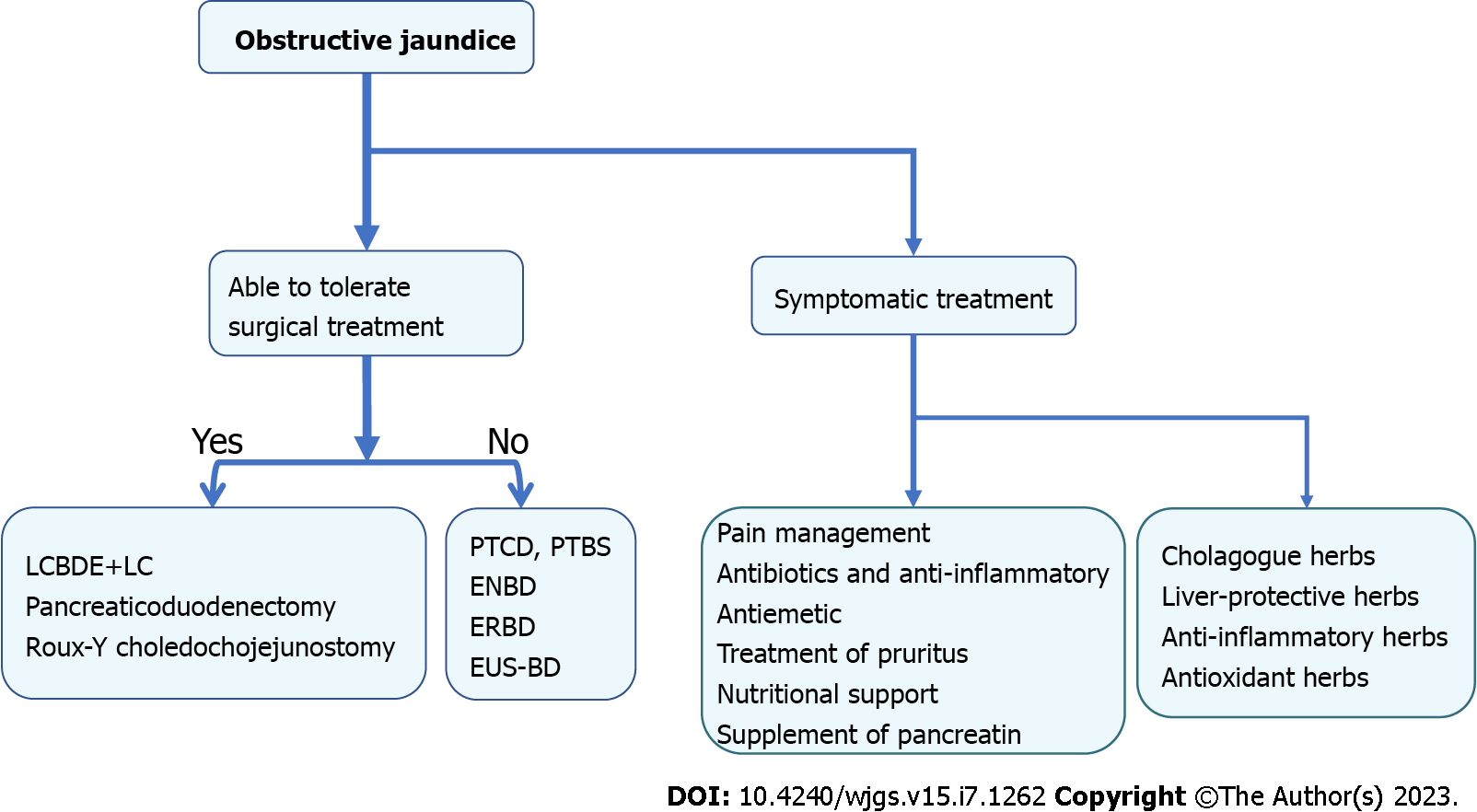
OJ can result in various pathophysiological consequences, including local effects on the hepatic parenchyma, biliary tree, and systemic manifestations[12]. Consequently, patients with jaundice are at a high risk of developing liver dysfunction, renal failure, nutritional deficiencies, bleeding tendencies, compromised immunity, infections, and increased morbidity and mortality. A comprehensive understanding of the pathophysiology in patients with jaundice (Figure 1) is crucial to implementing optimal preventive measures and improving the outcomes[13]. Currently, surgical intervention remains the predominant approach for treating OJ[14]. However, pre-operative jaundice typically results in liver cell damage, liver dysfunction, biliary tract infections, skin itching, and pain, which requires comprehensive management (Figure 2). Traditional Chinese medicine (TCM) offers important complementary and alternative therapy approaches to relieve these symptoms[15]. Therefore, TCM can constitute an adjunctive treatment modality. This review provides a detailed analysis of the pathophysiological manifestations and various treatment strategies for bile stasis resulting from extrahepatic OJ. The article aims to offer a comprehensive understanding of this medical condition and the potential solutions available for managing it.
Figure 1
Pathophysiological consequences for obstructive jaundice.
Figure 2 The treatment for obstructive jaundice.
PTCD: Percutaneous transhepatic cholangiodrainage; PTBS: Percutaneous transhepatic biliary stenting; ENBD: Endoscopic nasociliary drainage; ERBD: Endoscopic retrograde biliary drainage; EUS-BD: Endoscopic ultrasound-guided biliary drainage; LCBDE+LC: Laparoscopic common bile duct exploration with cholecystectomy.
PATHOPHYSIOLOGICAL CONSEQUENCES
Effects on the biliary system
Under physiological conditions, the pressure in the biliary tract ranges from 5 to 10 cmH2O. Any obstruction of bile transport and excretion results in increased pressure in the bile duct, and when the pressure exceeds 10-15 cmH2O, the liver no longer excretes bile[16].
Bile sludge facilitates the growth and proliferation of microorganisms in the bile, whereas under normal conditions, bile is sterile. The duodenal microflora ascends retrogradely along the bile duct. During biliary obstruction, the pressure in the bile duct above the obstruction increases, and the bile duct dilates, causing rupture of the small and capillary bile ducts, and bilirubin in the bile flows back into the blood. As the pressure in the biliary tree increases, its barrier function is compromised, leading to increased permeability and bile reflux into the hepatic sinusoids; consequently, this reflux into the blood leads to the entry of microorganisms and their degradation products in the body circulation system. Cholangitis occurs mainly during acute obstruction. Elevated biliary pressure pushes bacteria into the bile ducts, hepatic veins, and perihepatic lymphatics, which results in bacteremia[17,18]. Increased pressure in the bile ducts during acute cholangitis frequently renders the ducts more permeable to bacteria and toxins, leading to severe infection and sepsis[19]. In addition, bile reflux leads to inflammatory infiltration of polymorphonuclear neutrophilic leukocytes into the portal sinus and increases fibrin deposition.
Effects on the structure of the liver
Jorge et al[20] used experiments to observe that neutrogranular inflammatory cytocytosis in rat liver tissue with OJ showed liver contour changes, liver fibrosis, ductal hyperplasia, inflammatory portal vein infiltration, regenerated nodules, port-portal septum, and necrotic foci, with severe cases developing cirrhosis. Margaritis et al[21] found that in rats with jaundice through bile duct ligation (BDL), bilirubin values increased significantly, liver tissue bacterial cultures were positive, and endotoxin values collected from the portal vein and aorta were significantly higher than those in the controls. Liver biopsy revealed tubular cholestasis and portal vein changes, portal vein dilation, ductal hyperplasia, and neutrophil inflammation.
Ozozan et al[22] created an OJ model in rats by ligating the bile duct. In their experiments on liver cell damage, they observed large lipid particles were observed in liver tissue indicating oxidative damage to the liver, but increases in indium tin oxide particles, lipid particles, and normal mitochondria were observed after removal of the obstruction, reflecting the regenerative function of liver cell structure and suggesting that liver damage after obstruction may be reversible. The liver biopsy study of Vij et al[5] revealed that the primary pathologic characteristic of OJ caused by biliary atresia was the dilation of the portal vein bundle, showing edema fibrosis with biliary tract hyperplasia, typically involving fibroblasts and different inflammatory cells. Portal vein bundles may also show hematopoietic components, especially myelopoiesis. Luo et al[23] conducted a study using rat experiments to investigate the effects of OJ on the liver. They stained liver sections of rats with OJ with hematoxylin-eosin, revealing bile duct epithelial lesions including fuller epithelial cells in the bile duct, disordered cell morphology, thickening of the basement membrane, edema in the interstitium of the common bile duct, increased thickness of small bile ducts, and dilation of the bile duct. Additionally, irregular masses were observed around liver cells, and over time, the fibrous membrane thickened, leading to an increase in fiber spacing. Zhang et al[24] found that rats with BDL formed jaundice lesions, and compared with the controls, the treated rats showed hepatocyte apoptosis, endotoxemia, reduced plasma glutathione, and rapidly increased oxidized glutathione levels; further, the tight junctions of hepatocyte structures were destroyed, and oral administration of Lactobacillus plantarum helped restore the barrier function of liver activity.
Effects on hepatic vasoconstriction and hemodynamics
OJ damages the endothelial cells of the hepatic sinuses, rendering the liver more susceptible to ischemia-reperfusion, and reduces vasoconstrictor tension and lowers vasoreactivity. Reactive oxygen species (ROS) play a major role in the pathogenesis of jaundice as they reduce the bioavailability of nitric oxide, thereby impairing vasodilation and endothelial cell growth, causing oxidative damage which may lead to atherosclerosis[25,26], Color Doppler flow imaging is an ideal non-invasive method for monitoring hepatic artery and portal blood flow. If there is a more significant increase in hepatic arterial blood flow compared to the decrease in portal blood flow, it indicates a smoother postoperative recovery of liver function and a better prognosis[27].
The presence of portosystemic venous shunts in OJ exacerbates endotoxin entry into systemic circulation. Endotoxins are components of the cell walls of gram-negative bacteria, termed lipopolysaccharides (LPS)[28]. Small amounts of endotoxins are produced in the gut under normal conditions, and the liver is a central immunological organ that is particularly enriched in innate immune cells and constantly exposed to circulating nutrients and endotoxins derived from the gut microbiota[29]. Endotoxins may occur due to damage to Kupffer cells, which allows endotoxins to enter systemic circulation. During OJ, insufficient bile flow and other factors that predispose to portal endotoxin absorption and impaired reticuloendothelial function allow the development of systemic endotoxemia. Obstruction of the intestine after bile enters the intestine leads to abnormal proliferation of the normal flora, resulting in damage to the intestinal mucosal barrier of the intestinal mucosa, bacterial translocation, and ultimately, increased endotoxin absorption[30]. Endotoxins in the blood circulation cause organ damage by stimulating various cells such as monocytes, macrophages, granulocytes, and endothelial cells to produce cytokines such as tumor necrosis factor (TNF), platelet-activating factor, interleukin (IL), oxygen radicals, prostaglandins (PG), and procoagulants. This may explain why patients with OJ frequently experience complications such as infection, gastrointestinal bleeding, renal failure, wound nonunion, and even multi-organ failure after surgery[31,32].
Effects on the kidney
In 1990, a retrospective study revealed that 49% of patients with hepatoportal cholangiocarcinoma (HCCA)-associated OJ (130 cases) had developed acute renal failure[33]. The incidence of this complication ranges from 5% to 16%, and patient mortality rates were approximately 70%-80%[34,35].
OJ kidney injury (OJKI) is a form of kidney injury that occurs due to an obstruction of the bile ducts, which causes accumulation of bile acids (BAs) in the body. The kidneys are responsible for removing excess BAs from the body, and when the natural excretory route for BAs is blocked, the kidneys become the primary organ for excretion. BA accumulation in the body can lead to renal tubular epithelial injury, which can cause OJKI. In animal models of OJ, feeding hydrophilic nordemethyldeoxycholic acid in advance can inhibit renal tubular epithelial injury, indicating that toxic BAs excreted through the urine are a key trigger factor of OJKI[36]. This indicates that toxic BAs excreted through the urine are a key trigger factor of bile cast nephropathy, which causes renal tubular epithelial injury in mice with BDL[37]. In addition, OJKI may also be caused by gut-derived endotoxemia, which can affect various bodily functions, including the clotting system, hemodynamic balance, inflammation, and oxidative damage. For example, Houdijk et al[38] observed that renal blood flow in rats with BDL was significantly lower than in sham-operated rats, and limiting gut endotoxins prevented the decrease of renal blood flow.
In cases of OJKI, hemodynamic disturbances can occur as a result of liver dysfunction and kidney injury. The liver plays a key role in maintaining normal blood flow and pressure, and damage to the liver can lead to changes in systemic blood flow and pressure. Based on renal hypoperfusion, disturbances and changes in renal hemodynamics are the basic mechanisms underlying OJKI. Hypotension resulting from hemodynamic disturbance further aggravates the ischemic state of the kidney[39] which is highly sensitive to ischemia and prone to renal insufficiency[40]. The renin-angiotensin-aldosterone system (RAAS) is a key player in the progression of renal diseases. Inhibiting renin and aldosterone, which blocks the RAAS, is an effective way to prevent OJKI[41]. Naranjo et al[42] conducted a study on rats with OJKI and found that urinary prostaglandin E2 (PGE2) secretion increased in OJ rats. Rats with creatinine clearance lower than the average excreted less PGE2 into the urine. This indicates that PGE2 may play a protective role in the kidney and prevent the deterioration of renal functioning.
Oxidative stress (OS) is caused by an imbalance between the oxidation and antioxidation systems when the body is exposed to accumulation of numerous harmful factors, such as drugs, toxic metabolites, cholestasis, and alcohol metabolism products. In a prospective study on patients with OJ, the level of lipid peroxide in the blood was a predictor of OJKI and was closely related to age; further, renal impairment was more frequent in OJ patients than in healthy individuals[43]. The levels of cholesterol and malondialdehyde in the mitochondrial membrane of rats increased with the time of common bile duct obstruction and the degree of OJKI[44]. At the obstruction stage, the level of superoxide dismutase in rat kidneys decreased and was negatively correlated with renal apoptosis and endotoxin. This suggests that free oxygen radicals participate in the process of OJKI in rats.
Effects on the gut and intestinal barrier
Under normal physiological conditions, bile flows freely through the bile ducts, which helps remove bacteria and prevents their migration from the small intestine to the bile duct by the action of the sphincter of Oddi[45]. Bile also has anti-inflammatory functions. However, when the bile duct is obstructed, cholestasis occurs, and bacteria can multiply due to the absence of bile in the intestine and the accumulation of BAs in the systemic circulation. This, in turn, leads to OS injury of the intestinal mucosal epithelium, destruction of the intestinal tight junctions, and down-regulation of small intestinal tight junction protein expression[31]. Ultimately, these changes result in the destruction of the intestinal tissue structure and disintegration of the intestinal mucosal barrier function, which then allows for intestinal endotoxins and bacteria to enter systemic circulation.
Effects on the immune system
Bile affects the homing and distribution of T lymphocytes in intestine-associated lymphoid tissue. Thus, the absence of bile in the intestinal lumen leads to a decrease in the abundance of CD4+ and CD8+ T lymphocytes and mucosal cell adhesion molecule 1-expressing cells in the lamina propria[46]. Bile also affects the size and abundance of B lymphocytes in Peyer's patches, and the absence of bile in the intestinal lumen induces apoptosis of B lymphocytes in Peyer's patches. Bile contains immunoglobulin A, which enhances mucosal defenses or binds to bacteria and viruses. This suggests that bile is essential for maintaining local immune barrier functioning in the intestine[47].
Immune dysfunction leads to higher susceptibility to the translocation of intestinal bacteria, and immune dysfunction in patients with jaundice can also lead to a systemic inflammatory response[48]. Activated macrophages are highly sensitive to high concentrations of total BAs. Therefore, accumulation of hepatic and circulating total BAs may kill or severely damage activated macrophages (or Kupffer cells), thereby impairing hepatic and systemic immune functions and accelerating the development of sepsis in patients with OJ[49].
Effects on the coagulation system
It is widely accepted that patients with OJ are at a higher risk of developing thromboembolism. Research into procoagulant activity, specifically by measuring coagulation time, fibrin formation, and purified coagulation complexes, has revealed that the presence of neutrophil extracellular traps (NETs) in patients with OJ is significantly higher than in healthy individuals. This suggests that targeting NET values could be a promising approach to improving coagulation disorders in OJ patients[50]. Cakir et al[51] conducted a study that observed hypercoagulability in more than half of patients with OJ. The coagulation index was found to be associated with an increase in direct bilirubin concentration, but the hypercoagulability trend appeared to be independent of prothrombin time (PTT), possibly due to increased activity of fibrin polymers on platelet membranes.
Prentice et al[52] found that OJ may lead to acquired coagulation disorders, insufficient bile salt secretion leading to vitamin K malabsorption, insufficient coagulation factor synthesis after hepatocyte failure, inability to clear coagulation and fibrillin activation products, and disseminated intravascular coagulation. Deficiency in vitamin K due to reduced absorption can result in bleeding diathesis, even with normal laboratory indices, such as PTT and international normalized ratio (INR). Furthermore, reduced absorption of other fat-soluble vitamins, such as vitamin D, and lipids can lead to their deficiency, resulting in a reduction in calcium levels[53,54].
THE TREATMENT OF OJ: MEDICATION AND SURGERY
Medical treatment
OJ may lead to bilirubin accumulation in the bloodstream, and infection can occur when there is an obstruction in the bile ducts. In addition to jaundice, patients with OJ may experience symptoms including fever, abdominal pain, nausea and vomiting, dark urine, pale stools, pruritus, fatigue, lack of appetite, and nutritional deficiencies. These symptoms can be managed with medications and nutritional support, respectively. Of note, the symptoms of OJ may vary depending on the severity and underlying cause of the obstruction.
Treatment of pain: Depending on the degree and type of pain, several medication options are available for patients with abdominal pain. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used for mild to moderate pain relief[55,56]. These include medications such as aspirin, ibuprofen, and naproxen. Acetaminophen (APAP) is a pain reliever and fever reducer that is used for mild to moderate pain relief. While NSAIDs can be effective for managing pain and inflammation, they come with potential risks. One of the main concerns is that they can cause upper gastrointestinal bleeding, congestive heart failure, and renal failure. Kidney damage is a particular risk, as NSAIDs can reduce renal blood flow and glomerular filtration rate, cause sodium and water retention, and lead to hyperkalemia and water poisoning. Patients with cirrhosis or asthma should avoid using NSAIDs altogether, and caution is necessary when administering them to patients taking anticoagulants prior to surgery. Adequate hydration and drug testing before using NSAIDs can help prevent or reduce the risk of acute kidney injury[57,58]. Opioids are strong pain relievers for moderate to severe pain relief. Examples of opioids include morphine, codeine, and hydrocodone[59].
Antibiotics and anti-inflammatory treatment: When OJ is complicated by an infection, it should be treated with appropriate antibiotics. Most infections are caused by gram-negative bacteria. The local microbial epidemiology should be considered in the selection of antimicrobial agents for initial empirical therapy. The appropriate anti-infective regimen is selected after assessing the severity of the patient's disease and the risk of infection with drug-resistant pathogens. Initial treatments include quinolones (ofloxacin, ciprofloxacin, etc.), beta-lactam antibiotics (penicillin, carbapenems, cephalosporins, cephalosporins, etc.), and nitroimidazole antibiotics[60,61]. Commonly used drugs include ampicillin-sulbactam, piperacillin-tazobactam, ticarcillin-clavulanic acid, cephalosporins plus metronidazole, imipenem-cilastatin, and ciprofloxacin plus metronidazole[62,63]. Blood cultures are usually taken from a patient before they receive antibiotics to diagnose the infection. However, it is essential to note that these cultures yield positive results in less than 40% of cases[64]. Hence, clinicians must rely on empiric therapy with the local antibiogram's knowledge to manage patients with biliary sepsis. Bile cultures are obtained and drug sensitivity tests are performed in patients after biliary drainage. This is done to identify the presence of infections and to determine the type of pathogenic bacteria and the antibiotic to which they are sensitive. Pseudomonas aeruginosa infections are commonly treated with a combination of antibiotics such as β-lactams, aminoglycosides, and polymyxins. On the other hand, when patients develop fungal infections, antifungal drugs such as fluconazole, metronidazole, and echinocandins are usually prescribed[65]. Corticosteroids are anti-inflammatory medications that reduce inflammation in the body and which can alleviate pain and fever. Patients with fever may also benefit from physical cooling or NSAIDs. While antibiotics are an essential part of treating infections, they are not always sufficient on their own. Drainage or a definitive surgical procedure may also be necessary to fully address the issue, as stated in reference[64].
Antiemetic treatment: Patients with nausea and vomiting can take certain medications such as ondansetron, metoclopramide, or promethazine to relieve the discomfort. Metoclopramide is an empirically established drug for patients with vomiting. Central antiemetics such as selective serotonin type 3 (5HT3) antagonists (ondansetron and granisetron) for patients with malignancies treated with chemoradiotherapy. Vomiting can also be treated with neurokinin 1 receptor antagonists (fosaprepitant and aripitant) and synthetic cannabinoids (nabilone). Patients with vomiting are frequently treated with hydration and electrolytes to replace lost fluids. Clinical studies have shown that anti-dopamine agents (haloperidol, levocarnitine, or olanzapine) are very effective in stopping emetics[66].
Treatment of pruritus: During cholestasis, compounds that are typically eliminated in bile build up in the tissues[67]. Although the exact nature of pruritogens (substances that cause itching) is unknown, it is believed that they accumulate in the plasma due to cholestasis. Ursodeoxycholic acid, chenodeoxycholic acid, cholic acid, cholestyramine[68], the antibiotic rifampin[69], and the selective serotonin reuptake inhibitor sertraline[70] can be prescribed to help reduce itching in OJ patients. Opioid antagonists[71] such as naloxone, naltrexone, and nalmefene can reduce itching in various cases. In addition, treatment with ondansetron can decrease pruritus effectively[72]. Peroxisome proliferator-activated receptor (PPAR) is a fatty acid-activated transcription factor of the nuclear hormone receptor superfamily. Bezafibrate, a PPAR agonist, can significantly reduce the levels of alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin in the blood. Fibroblast growth factor 19 agonists and their synthetic analog aldafermin (NGM282) markedly improve liver function[72]. Furthermore, skincare and cooling compresses can also reduce itchy symptoms. Phototherapy can alleviate itching in some cases. Thirteen patients with pruritus symptoms due to different cholestatic liver diseases were examined in a previous study, and ultraviolet B phototherapy was performed three times per week until the visual analog scale scores of perception of itching did not improve further or the patient's itching symptoms improved by 80%; 77% of the patients reported > 60% reduction in perceived pruritus[73].
Nutritional support: Various treatment options for patients with nutritional deficiencies are available. Depending on the specific deficiencies, nutritional supplements may be prescribed to help replace missing nutrients. For example, fat-soluble vitamins, electrolytes such as sodium, potassium, and magnesium as well as water, protein, and fats may be supplemented[74,75]. In severe cases where oral nutrition is not possible, parenteral nutrition may be administered intravenously to provide essential nutrients[76].
Supplementation of pancreatic exocrine enzyme: In some cases, pancreatic exocrine insufficiency may occur in the presence of malignant biliary obstruction such as due to pancreatic cancer. After surgical resection of pancreatic cancer, patients may develop pancreatic exocrine insufficiency. Digestive enzyme replacement therapy may be used to help improve the digestion and absorption of nutrients[77]. A retrospective population-based observational cohort study was conducted by Roberts et al[78], with 1614 subjects, i.e., 807 matched pairs. Propensity-matched analysis showed significantly higher median survival in patients receiving pancreatic enzyme replacement therapy.
TCM treatment
While there are modern medical treatments for this condition, TCM has shown promise as a complementary therapy approach for its management. In TCM, several herbal remedies are used to treat OJ, as listed below.
Cholagogue herbs: These herbs help improve the flow of bile and reduce the buildup of bile in the blood. Examples of cholagogue herbs include Curcuma aromatica (Yu Jin), Curcuma zedoaria (E Zhu), and Lysimachia christinae (Jin Qiancao).
Curcuma aromatica is the dry root of Curcuma longa L. (turmeric) that can be used as a spice or traditional medicine. According to Maeda et al[79], oral administration of Curcuma aromatica powder to rats led to a significant increase in bile secretion. Moreover, Wang et al[80] found that supplementing a lithogenic diet with 0.5% curcumin for 10 wk reduced the incidence of gallstone formation in young male mice to 26%, compared to 100% in the group fed with the lithogenic diet alone. Further, Li et al[81] found that curcumin can prevent the formation of cholesterol gallstones induced by a high-fat diet in mice by reducing the expression of Niemann-Pick C1-like 1 and sterol regulatory element binding transcription factor 2 at the mRNA and protein levels. Combining curcumin with piperine can also enhance its effectiveness by increasing its bioavailability. Some chemical components of Curcuma zedoaria , such as curcumin and gingeric acid, can play a role in improving bile flow and excretion by regulating gallbladder contraction and relaxation of biliary smooth muscle[82]. This is beneficial to the prevention or treatment of OJ, cholecystitis, and other diseases[83]. Yang et al[84] suggested that Lysimachia christinae Hance extracts (LCHE) exert excellent anti-cholecystitis effects and can alleviate lesion severity in a lithocholic acid-induced adult guinea pig model. Their study also revealed that LCHE can positively affect bile secretion, and a high dose of LCHE significantly enhanced bile secretion and bile emptying.
Liver-protective herbs: Liver-protective herbs help protect the liver and improve liver function. Examples include Scutellaria baicalensis (Huang Qin) and Mori Fructus (Sang Shen).
According to the experimental results presented by Jang et al[85], baicalin, the principal constituent of Scutellaria baicalensis, may offer protection against APAP-induced liver damage by blocking the biological activity of APAP-induced hepatotoxicity. This protective effect is thought to arise from the ability of baicalin to inhibit the expression of P450 family 2, subfamily E, and polypeptide 1. Liao et al[86] found that baicalin administration can effectively reduce liver damage induced by APAP. This protective effect is believed to be due to the down-regulation of the ERK signaling pathway and the subsequent reduction of its downstream inflammatory markers and microscopic changes. Mori Fructus polysaccharides (MFP) are large molecules extracted from Mori Fructus, which exhibit biological activity in terms of anti-liver damage. In a study conducted by Bian et al[87], MFP was found to regulate the metabolism of linoleic acid, alpha-linolenic acid, and glycerol phospholipids, thereby ameliorating acute alcoholic liver injury.
Anti-inflammatory herbs: Anti-inflammatory herbs help reduce inflammation in the liver and improve liver function. Examples include Zingiber officinale (Sheng Jiang), Fructus Schisandrae Chinensis (Wu Weizi), and Semen Sojae Praepatum (Dan Douchi).
Grzanna et al[88] found that Zingiber officinale exerts its anti-inflammatory effects through multiple mechanisms. First, it suppresses prostaglandin synthesis by inhibiting both cyclooxygenase-1 and cyclooxygenase-2 enzymes. Second, ginger shares a similar property with NSAIDs in inhibiting prostaglandin synthesis. Additionally, some of the constituents present in ginger act as dual inhibitors of cyclooxygenase and lipoxygenase, which effectively reduces the biosynthesis of PG and leukotrienes. Li et al[89] found that Fructus Schisandrae Chinensis Fructus can significantly inhibit the expression of epidermal growth factor receptor (EGFR) in APAP-induced drug-induced liver injury (DILI). Moreover, stem cell factor also inhibits the expression of TNF-α, IL-6, and IL-1β, suggesting that it can regulate the inflammatory response to decelerate liver injury by modulating the EGFR signaling pathway. Fermented soya beans can affect the downstream targets of the NF-κB pathway. Fermented soy extract (FSE) can inhibit the expression of proinflammatory cytokines, including IL-1β, IL-6, IL-8, IL-12, TNF-α, and monocyte chemoattractant protein-1. This ultimately suppresses the exacerbation of inflammatory responses, as demonstrated in cell cultures or animal models challenged with various inflammation-promoting agents. Moreover, FSE can inhibit the binding of monocytes with endothelial cells after LPS induction, suggesting that FSE can prevent inflammatory cell-endothelial adherence and sentinel cell trafficking at the site of inflammation. Mouse models showed that FSE can limit the infiltration of immune cells, such as mast cells and macrophages, at the site of inflammation[90].
Antioxidant herbs: Antioxidant herbs help to reduce OS in the liver and protect liver cells from damage. Examples Fructus Ligustri Lucidi (Nv Zhenzi), Artemisiae Scopariae Herba (Yin Chen), Fructus Schisandra chinensis (Wu Weizi), Taraxacum platycarpum Dahlst. (Pu Gongying), and Rhizoma Chuanxiong (Chuan Xiong).
According to a previous study[91], Fructus Ligustri Lucidi treatment may prevent acute OS induced by BHT in rats. This was demonstrated by the reduction in concentrations of ALT, AST, ALP, and lactate dehydrogenase, as well as an increase in the levels of antioxidant enzymes in the liver, kidney, and lung. Yuan et al[92] proposed that Artemisiae Scopariae Herba has a potent hepatoprotective effect on APAP-induced liver injury in mice. The primary mechanisms underlying the protective effects of Artemisiae Scopariae Herba may include its alleviation of GSH depletion, inhibition of lipid peroxidation, and inactivation of caspase-8 and caspase-3 via inhibition of TNF-α, a pro-inflammatory mediator. Li et al[93] found that schisandrol A, which is the primary constituent of Fructu Schisandra chinensiss, can help treat DILI caused by APAP. This study suggested that the mechanism underlying this treatment is related to the activation of the TNF signaling pathway. Additionally, schisandrol A can play a significant role in treating DILI caused by APAP by reducing inflammation and OS while inhibiting the activities of cytochrome P450 enzymes. Qi et al[94] developed a procedure for rapid isolation of HO-1 inducers from Rhizoma Chuanxiong extract using bioactivity guidance. Their study revealed that senkyunolide-H and -I, two compounds isolated from the extract, induced HO-1 expression and inhibited lipid peroxidation and intracellular ROS formation. As a result, they enhanced cellular resistance to H2O2-induced cytotoxicity. A study conducted by Talapphet et al[95] found that Taraxacum platycarpum Dahlst., a commonly used drug in Korea, has the potential to promote Nrf2-mediated antioxidant activity by modulating the PI3K/Akt pathway. This natural remedy is often used to treat various ailments, such as mastitis, hepatitis, and jaundice. Moreover, the study revealed that the root extract of Taraxacum platycarpum Dahlst. can reduce alcohol-induced oxidative stress, while the leaf extract can alleviate fatty liver induced by a high-fat diet. Additionally, the flower extract of this plant was found to effectively remove ROS and prevent reactive oxygen damage. These findings suggest that Taraxacum platycarpum Dahlst. may have therapeutic potential in managing oxidative stress-related diseases. However, further research is needed to fully understand the mechanisms behind its antioxidant activity and its potential use as a treatment option.
Chinese herbal compound: Yinchenhao decoction (YCHD) is a TCM formula with a long history of use. It is derived from the Treatise on Exogenous Febrile Disease and is commonly used to treat damp-heat jaundice. The formula includes three main ingredients, Artemisiae Scopariae Herba (Yin Chen), Gardeniae Fructus (Zhi Zi), and Radix Rhei et Rhizoma (Da Huang). Through our previous research[96] using network pharmacology, we confirmed that YCHD can exert multi-component, multi-target, and multi-pathway functions. The respective mechanisms may be related to various biological processes, including anti-inflammatory, inhibition of liver fibrosis, antioxidant, and apoptosis. We demonstrated that YCHD can increase the expression of nuclear factor E2 related factor 2 (Nrf2), promote its translocation to the nucleus, reduce the overexpression of nitric oxide by regulating endothelin nitric oxide synthase and inducible nitric oxide synthase, and activate downstream GSH and NAD(P)H quinone dehydrogenase 1 expression, thereby protecting liver tissue from oxidative damage. Furthermore, YCHD can alleviate liver injury and oxidative damage, promote the translocation of Nrf2 to the nucleus, and upregulate the Nrf2 signaling pathway. Our research[97] revealed that OJ can lead to liver damage and apoptosis of hepatocytes. This is caused by the activation of the PERK-CHOP-GADD34 pathways and an increase in the Bax/Bcl-2 ratio. Further, we found that YCHD can improve liver functioning and reduce hepatocyte apoptosis through inhibiting the activation of the PERK-CHOP-GADD34 pathways and decreasing the Bax/Bcl-2 ratio. YCHD has been found to have a significant impact on various indicators, including sodium taurocholate cotransporting polypeptide, multidrug resistance protein 1, bile salt export pump, organic cation transporter 1, and organic anion transporter 1A2, leading to their increase. Furthermore, YCHD can regulate the main metabolic pathway of phase II, which inhibits the increase of naphthalene isothiocyanate content[98].
To sum up, herbal medicines may offer therapeutic benefits for OJ, especially when combined with modern medical treatments. Current evidence suggests that it can be a valuable complementary therapy in the management of this condition. Further research is needed to comprehensively assess the mechanisms of action and potential side effects of herbal medicines for this condition.
Interventional and endoscopic treatment
The surgical treatment of OJ depends on the cause, location, and severity of the obstruction. The causes of OJ include bile duct stones, tumors, and congenital anatomical abnormalities of the bile duct. Depending on the patient's condition, the obstruction can be relieved by biliary drainage, surgery, and endoscopic intervention.
Percutaneous transhepatic cholangiodrainage and percutaneous transhepatic biliary stenting: Percutaneous transhepatic cholangiodrainage (PTCD) is a common biliary drainage method used in clinical practice, mostly in patients with acute biliary obstruction that is more critical and needs to be treated as soon as possible, such as acute obstructive purulent cholangitis and acute obstructive cholecystitis. This method can rapidly alleviate the symptoms of biliary obstruction, allowing rapid reduction of jaundice and rapid recovery of liver functions. Mocan et al[99] studied 52 patients with HCCA, and the success rate of drainage in the PTCD-treated group was 88.46%, with significant recovery of liver function and fewer complications than before treatment. PTCD can also be used as preoperative preparation for surgical procedures to reduce intraoperative and postoperative complications. In patients with malignant cholestatic jaundice requiring surgery, the major adverse effects were markedly less frequent in the preoperative bile drainage group than in the direct surgery group[100]. If patients cannot undergo surgery due to their physical condition, or if the tumor is too large or has metastases that cannot be removed surgically, PTCD treatment can be used as a palliative treatment to relieve the symptoms of biliary obstruction. However, due to the risk of bleeding and hemobilia caused by jaundice and coagulopathy, PTCD procedures should be carefully monitored, and the tubes should be changed if they are left in place for an extended period of time. If they are not changed, there is a risk that they may fracture and leave foreign bodies inside the biliary tree, which can lead to the formation of a stone nidus or recurrent sepsis[101].
Percutaneous transhepatic biliary stenting (PTBS) is a minimally invasive procedure that is performed under local anesthesia, which greatly reduces the risk of complications, especially in high-risk patients[102]. Furthermore, this procedure has minimal side effects. The effectiveness of PTBS in managing both malignant and benign biliary strictures is remarkable, as it boasts a success rate of over 90%[103]. PTBS can be conducted as a standalone procedure to provide comprehensive biliary drainage or in conjunction with other treatment modalities such as chemotherapy or radiation[104]. According to a study, combining PTBS with 125I particles significantly lowers the odds of biliary re-obstruction and enhances survival rates when compared to PTBS alone, without raising the risk of postoperative complications[105]. Additionally, the findings of a multivariate analysis show that the combined treatment of PTBS and 125I particles is an independent significant factor for overall survival. The integration of these two treatments can reduce the likelihood of death by 74%[106]. Nevertheless, PTBS is technically challenging and requires the expertise of highly skilled interventional radiologists. Complications associated with PTBS include stent displacement, bleeding, and infection, which may necessitate further interventions. It is also worth mentioning that PTBS carries a greater risk of postoperative pancreatitis when compared to other biliary drainage methods[107,108].
Endoscopic nasobiliary drainage: With the continuous innovation of endoscopic technology, endoscopic nasobiliary drainage (ENBD) has been developed based on endoscopic retrograde cholangiopancreatography (ERCP). ENBD is a simple and easy approach for relieving biliary obstruction by reducing biliary pressure, thereby rapidly relieving the symptoms of biliary obstruction and controlling biliary infection[109,110]. However, it is important to note that the nasobiliary duct can irritate the throat, is prone to slippage or blockage, and has the disadvantage of removing abundant bile salts, which can further cause disorders of the water-electrolyte balance and digestive dysfunction in patients[111].
Endoscopic retrograde biliary drainage: Endoscopic retrograde biliary drainage has become a widespread method for the treatment of various benign and malignant biliary obstruction diseases. The conditions of patients with benign biliary strictures, such as primary sclerosing cholangitis, post-liver transplantation, post-cholecystectomy injury, chronic pancreatitis, and biliary immunoglobulin G4 involvement can be improved biliary stenting during ERCP. The purpose of placing the stents is to maintain the long-term patency of bile ducts[112]. Malignant biliary obstruction (MBO) can be classified as distal or hilar obstruction. In patients with malignant biliary obstruction and unresectable tumors, placement of a biliary stent can be considered palliative treatment. ERCP with stent placement is the most common treatment modality for unresectable distal MBO decompression, the treatment of which can significantly improve obstructive symptoms and improve the patient's quality of life[113]. Its role in the preoperative drainage of patients with surgically resectable tumors remains controversial. A retrospective analysis showed that preoperative biliary drainage reduced the risk of postoperative liver failure, but it failed to improve long-term survival[114]. Liu et al[115] concluded that preoperative drainage should not be routinely performed in patients with proximal bile duct cancer scheduled for surgical resection. The material of stents can be plastic or metal. Plastic stents must be replaced regularly every 12 months to keep the drainage open, however, they are less expensive. Placing a fully covered self-expanding metal stent is more expensive but it has a high success rate, easier insertion, and a high safety profile that can prevent tumor ingrowth[116]. A recent European guideline[117] strongly advocates the use of self-expandable metal stents (SEMS) over plastic stents for palliative drainage of distal MBO. This recommendation is due to the fact that SEMSs have been shown to lead to longer patient survival, lower risk of stent dysfunction/cholangitis, and reduced need for reinterventions. Additionally, the guideline suggests the use of uncovered SEMSs for palliative drainage of hilar MBO, which should ensure ≥ 50% drainage of liver volume. Several high-quality meta-analyses have explored the early selection of stent type (SEMS vs plastic)[118,119], and all have consistently demonstrated that SEMS placement is associated with a lower rate of re-intervention. The Archimedes stent is a novel biodegradable stent that shows promise for use in a range of surgical procedures, including liver transplantation and pancreaticoduodenectomy. Its use can help prevent bile leakage and improve patient outcomes[120,121]. A drug-eluting stent is a stent that has been coated with a medication that slowly releases over time. The coating on a drug-eluting stent can be made of various materials, such as polymers or metals, and the medication used can range from corticosteroids to anti-inflammatory drugs to chemotherapy agents. The medication helps to prevent scar tissue from forming around the stent, which can cause it to become blocked again[122].
Endoscopic ultrasound-guided biliary drainage: Some patients cannot undergo biliary stenting with ERCP because of anatomical difficulties arising from local infiltration of the malignancy. For example, when a tumor infiltrates the jugular abdomen and duodenal obstruction occurs, endoscopic ultrasound-guided biliary drainage (EUS-BD) may be an option as it allows better visualization of biliary obstruction and access to the bile duct through the gastrointestinal tract. EUS-BD techniques include EUS-guided rendezvous (RV), EUS-guided choledochoduodenostomy (CDS), and EUS-guided antegrade stenting (AS). Indications for EUS-RV include benign or potentially resectable malignant cases and unresectable malignant cases for which other EUS methods are not suited. A meta-analysis by Tsuchiya et al[123] showed that the overall success rate of EUS-RV was 81% and the complication rate was 10% in 382 EUS-RV cases. EUS-AS is frequently combined with hepatogastrostomy. EUS-AS includes EUS-guided antegrade stenting for malignant biliary obstruction and EUS-guided antegrade intervention for benign biliary diseases.
Operative treatment
The key aspect of OJ surgical treatment is to clarify the cause, location, and extent of the obstruction. The root cause of the obstruction must be identified as early as possible.
Laparoscopic common bile duct exploration with cholecystectomy: In cases of OJ associated with gallstones, different procedures are chosen depending on the site of the obstruction. Laparoscopic common bile duct exploration with cholecystectomy is widely regarded as an excellent surgical approach. The use of indocyanine green dye fluorescence cholangiogram is recommended as it allows for better exploration of the structure of the biliary system compared to traditional intraoperative cholangiogram[124,125]. Recent technological advancements, such as 3D technology and barbed sutures, have made laparoscopic common bile duct exploration safer and more feasible[126]. In the future, further technological development of cholangioscopy is crucial, particularly in improving the accuracy and visualization of cholangioscopy, as well as exploring the potential role of artificial intelligence in diagnosis[127].
Pancreaticoduodenectomy: Common surgical procedures for the treatment of OJ caused by malignant tumors of the bile ducts, duodeno-pancreas, and extrahepatic bile ducts invaded by hepatic peritonitis include pancreaticoduodenectomy, which can be done by open abdomen, laparoscopy or robotic surgery. Laparoscopic pancreaticoduodenectomy (LPD) has inherent advantages over open pancreaticoduodenectomy (OPD), including a clear visual field, lower bleeding, lower pain intensity, and faster recovery for individuals, particularly in the elderly. Patients with malignant tumors undergo surgery with the primary outcome measure being long-term survival, which serves as a fundamental purpose. Croome et al[128] compared overall survival and disease-free survival in two groups of patients with pancreatic ductal adenocarcinoma who underwent different surgical procedures. They found no significant differences in overall survival between the 108 cases in the LPD group and the 214 cases in the OPD group, but the LPD group had a higher disease-free survival rate. Similarly, Kantor et al[129] compared the overall survival of 828 LPD cases and 7385 OPD cases in the United States National Cancer Database from 2010 to 2013 and found no significant differences between the two groups (20.7 months compared to 20.9 mo). However, there is currently a lack of prospective multicenter randomized controlled trials studying the long-term survival of patients with malignant tumors treated with LPD and OPD.
Roux-Y choledochojejunostomy: At the time of initial diagnosis, some patients have already progressed to intermediate and advanced stages, thus having missed the optimal time for radical surgery, thus leaving palliative surgical treatment the proper option. When biliary lesions cause obstructive biliary strictures, the lesions can be surgically removed, and the bile ducts can be anastomosed to the jejunum to reconstruct the physiology of the bile duct and drain the bile. This procedure is termed Roux-Y choledochojejunostomy, which can better preserve the integrity of the intestinal canal and maintain the normal physiological function of the intestine, and the probability of recurrence of biliary stricture after surgery is lower[130]. External T-tube drainage and internal bile-intestinal drainage are suitable for patients of advanced age who cannot undergo prolonged surgery. It has the advantages of minimal surgical trauma, easy operation, and rapid postoperative recovery. However, this review excludes pediatric population issues.