Published online Feb 27, 2022. doi: 10.4240/wjgs.v14.i2.161
Peer-review started: July 15, 2021
First decision: November 8, 2021
Revised: December 13, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: February 27, 2022
Processing time: 222 Days and 8.6 Hours
Laparoscopic total gastrectomy (LTG) has drawn increasing attention over the years. Although LTG has shown surgical benefits compared to open TG (OTG) in early stage gastric cancer (GC), little is known about the surgical and oncological outcomes of LTG for advanced GC following neoadjuvant therapy (NAT).
To compare the long- and short-term outcomes of advanced GC patients who underwent LTG vs OTG following NAT.
Advanced GC patients who underwent TG following NAT between April 2011 and May 2018 at the Cancer Hospital of the Chinese Academy of Medical Sciences were enrolled and stratified into two groups: LTG and OTG. Propensity score matching analysis was performed at a 1:1 ratio to overcome possible bias.
In total, 185 patients were enrolled (LTG: 78; OTG: 109). Of these, 138 were paired after propensity score matching. After adjustment for propensity score matching, baseline parameters were similar between the two groups. Compared to OTG, LTG was associated with a significantly shorter length of hospital stay (P = 0.012). The rates of R0 resection, lymph node harvest, and postoperative morbidity did not significantly differ between the two groups. Overall survival (OS) outcomes were comparable between the two groups. Pathological T and N stages were found to be independent risk factors for OS.
LTG can be a feasible method for advanced GC patients following NAT, as it appears to be associated with better short- and comparable long-term outcomes compared to OTG.
Core Tip: Laparoscopic total gastrectomy (LTG) is known to have better short-term outcomes and prognosis than open TG (OTG) in early gastric cancer (GC). However, its application in advanced GC remains controversial. In this study, we evaluated both long- and short-term outcomes of LTG compared to those of OTG in 185 patients with advanced GC who had received neoadjuvant therapy (NAT). Our results indicate that LTG is associated with better short-term and comparable long-term outcomes compared to the traditional OTG surgery. Therefore, it can be a feasible surgical treatment for advanced GC patients following NAT.
- Citation: Hu HT, Ma FH, Xiong JP, Li Y, Jin P, Liu H, Ma S, Kang WZ, Tian YT. Laparoscopic vs open total gastrectomy for advanced gastric cancer following neoadjuvant therapy: A propensity score matching analysis. World J Gastrointest Surg 2022; 14(2): 161-173
- URL: https://www.wjgnet.com/1948-9366/full/v14/i2/161.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i2.161
According to the latest data from the Global Cancer Statistics 2020 report, gastric cancer (GC) is the fifth most common cancer and the fifth leading cause of cancer-related deaths worldwide[1]. Despite a slight drop in mortality rates, a considerable number of patients with GC have locally advanced disease at first diagnosis. Since the MAGIC trial[2], neoadjuvant therapy (NAT) has played a significant role in the comprehensive treatment of advanced GC (AGC). Numerous prospective studies have been carried out in Western and Eastern Asian countries, and although the efficacy of NAT has been validated, chemotherapy regimens are quite different between Western and Eastern Asian countries.
After NAT, patients generally undergo D2 gastrectomy with curative intent. Laparoscopic gastrectomy (LG) has gained popularity in the management of early GC (EGC) because of its minimal invasiveness and similar long-term outcomes compared to those of conventional open gastrectomy (OG)[3]. Although its use is still under debate, the application of LG in AGC has drawn increasing attention over the years. The available evidence from the CLASS-01 and KLASS-02 trials suggests that laparoscopy-assisted distal gastrectomy is safe and provides faster postoperative recovery than open distal gastrectomy (ODG) does for patients with AGC[4]. Moreover, the CLASS-01 trial demonstrated that laparoscopic distal gastrectomy (LDG) did not lead to inferior disease-free survival at 3 years compared to ODG for patients with AGC[5].
Since there has been a recent increase in the prevalence of adenocarcinoma of the esophagogastric junction (AEG), total gastrectomy (TG) constitutes an increasing proportion of all gastric operations[6]. Laparoscopic TG (LTG) has been confirmed to have better short-term outcomes and prognosis than those of open TG (OTG) in EGC; however, its application in AGC remains controversial[7,8]. Some retrospective studies and meta-analyses have shown that LTG has lower rates of complications and amount of blood loss; however, there is still a need for high-volume research to validate its efficacy and safety compared to those of OTG[9,10].
Chemotherapy-induced tissue fibrotic changes and edema provide new technical challenges for LG, and the effect of NAT on LG compared to that on OG remains unclear. A randomized controlled trial conducted by Li et al[11] (2019) reported the safety and efficacy of LDG with D2 lymphadenectomy following neoadjuvant chemotherapy (NAC) for AGC. The STOMACH trial also published preliminary results for LTG after NAC, showing that LTG is not inferior to OTG in short-term outcomes[12]. However, the rate of D2 lymphadenectomy was quite low in both groups-49% for OTG and 36.2% for LTG-and it is still doubtful whether LTG is safe in clinical oncology practice. To the best of our knowledge, only two studies with small sample sizes have investigated the long-term survival of LG following NAC, and no previous study has examined the long-term survival of patients who received LTG[13,14].
Therefore, we conducted this study to evaluate the long- and short-term outcomes of LTG for AGC following NAT and to determine the surgical and oncological safety of LTG as an acceptable alternative to OTG.
We retrospectively screened our database of patients with GC and identified those with preoperative and pathological diagnoses of AGC who received LTG or OTG with lymphadenectomy after NAT from April 2011 to May 2018 at the Cancer Hospital of the Chinese Academy of Medical Sciences. The inclusion criteria were as follows: (1) Gastric adenocarcinoma; (2) Clinical stages cT2-4a, N-/+, and M0; and (3) Received chemotherapy or chemoradiotherapy before surgery. The exclusion criteria were as follows: (1) Remnant GC; (2) Siewert type I AEG; (3) Emergent gastrectomy; (4) Other simultaneous malignant diseases; and (5) Missing clinical data. In total, 185 patients were included, of whom 107 had undergone LTG, and 78 had undergone OTG. This study was approved by the Ethics Committee of the Cancer Hospital of the Chinese Academy of Medical Sciences and the requirement was waived.
NAC regimens were divided into three categories: (1) Platinum-based doublets (SOX, XELOX, CS, FOLFOX, and TP); (2) Epirubicin-based triplets (ECF); or (3) Taxane-based triplets (DCF, DCX). As neoadjuvant chemoradiotherapy (NCRT), patients received concurrent chemoradiotherapy with tegafur/gimeracil/oteracil (S-1). The planned dose of total radiotherapy was 45 Gy with a daily fraction of 1.8 Gy for 5 wk. S-1 was administered orally twice daily when receiving radiotherapy. After evaluation by experienced oncologists and surgeons, surgery was performed approximately 4-6 wk after the completion of NAT.
Approximately 2-4 wk after the end of NAT, patients underwent TG with standard D2 lymphadenectomy following the Japanese Gastric Cancer Treatment Guidelines[15]. A total of 5 trocars were used in the LTG surgery. The resection margins were examined intraoperatively in the frozen sections. Reconstruction of the gastrointestinal passage is typically accomplished using the Roux-en-Y gastric bypass. All operations were performed by a lead surgeon who had performed at least 60 OG or LG operations and two or three assistants. Intraoperative and postoperative complications and corresponding outcomes were documented.
Clinical and pathological data were collected from medical records. Clinical staging was assessed using the 8th American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) classification through biopsy, endoscopic ultrasonography, and computed tomography (CT) data. Enlarged lymph nodes > 8 mm along their longest axis or those with necrosis were classified as cN+. Postoperative complications included pancreatic fistula, abdominal bleeding, anastomotic leakage, wound infection, lymphorrhagia, intestinal obstruction, abdominal infection, duodenal fistula, and gastroparesis. These were considered surgical and other medical complications and graded according to the Clavien-Dindo system[16]. The response to NAT was evaluated using the Mandard tumor regression grading (TRG) system[17]. Pathological T status, N status, and ypTNM stage were also determined using the 8th AJCC/UICC staging system. Overall survival (OS) was measured from the day of surgery.
In the first 2 years, patients were followed-up every 3 mo, then every 6 mo for the next 3 years, and yearly thereafter. Any loss to follow-up was censored. The final follow-up was completed in October 2020.
We performed propensity score matching (PSM) to minimize bias between the baseline of the two groups. Propensity scores were calculated using a logistic regression model and the following variables: Sex, age, American Society of Anesthesiologists physical status classification (ASA), body mass index (BMI), tumor size, histological differentiation, ypT, ypN, and ypTNM status. Patients were then individually matched using the 1:1 nearest neighbor matching method with a caliper width of 0.05. This method randomly ordered the case (LTG) and control (OTG) subjects based on the propensity score and matched the control subject with the closest comparison from the first case subject[18].
Categorical values are presented as percentages and continuous values are presented as mean ± SEM. Clinical and pathological variables were analyzed using the chi-squared test, Fisher’s exact test and Student’s t-test, depending on the distribution of the parameters. We used the Kaplan-Meier survival analysis and the log-rank test to estimate OS and compare the survival distributions. Multivariate Cox regression analysis was used to adjust for confounding factors and non-balanced between-group variables in univariate analysis. Statistical significance was set at P < 0.05. All analyses were performed using SPSS statistical software (version 26.0; IBM Corp., Armonk, New York, United States).
Table 1 shows the clinical data, clinical staging, tumor status, and pathological staging of the patients before PSM (n = 185) and after PSM (n = 138). Before PSM, there was a significant difference between the two groups in terms of BMI (P = 0.028). Compared to the OTG group, the average age was younger (P = 0.120), tumor size was smaller (P = 0.126), and occurrence of yN stage (P = 0.168) was lower in the LTG group; however, the differences were not statistically significant. Distant metastasis was confirmed by operative pathological examination in all 11 patients (LTG: 6, OTG: 5). In the LTG and OTG groups, distant metastasis occurred in the peritoneum of five and four patients and in the liver of one and one patients, respectively. After PSM, all clinicopathological characteristics were comparable between the LTG and OTG groups.
| Variable | All patients | P value | Matched patients | P value | ||
| LTG (n = 78) | OTG (n = 107) | LTG (n = 69) | OTG (n = 69) | |||
| Age (yr) | 52.7 ± 16.1 | 56.0 ± 12.0 | 0.120 | 53.42 ± 13.4 | 53.9 ± 12.7 | 0.828 |
| Gender n (%) | ||||||
| Male | 61 (78.2) | 78 (72.9) | 0.409 | 53 (76.8) | 52 (75.4) | 0.842 |
| Female | 17 (21.8) | 29 (27.1) | 16 (23.2) | 17 (24.6) | ||
| BMI (kg/m2) | 22.6 ± 3.1 | 23.7 ± 3.7 | 0.028 | 22.6 ± 3.1 | 22.8 ± 3.3 | 0.750 |
| ASA n (%) | ||||||
| 1-2 | 74 (94.9) | 99 (92.5) | 0.522 | 65 (94.2) | 64 (92.8) | 1.000 |
| 3 | 4 (5.1) | 8 (7.5) | 4 (5.8) | 5 (7.2) | ||
| The history of abdominalsurgery n (%) | ||||||
| Yes | 10 (12.8) | 19 (17.8) | 0.362 | 8 (11.6) | 13 (18.8) | 0.236 |
| No | 68 (87.2) | 88 (82.2) | 61 (88.4) | 56 (81.2) | ||
| Tumor location n (%) | 0.775 | 0.698 | ||||
| Upper | 30 (38.5) | 35 (37.6) | 28 (25.0) | 22 (25.0) | ||
| Middle | 25 (32.1) | 42 (39.3) | 23 (33.3) | 26 (37.7) | ||
| Lower | 9 (11.5) | 12 (11.2) | 7 (10.1) | 10 (14.5) | ||
| More than two position or total | 14 (17.9) | 18 (16.8) | 11 (15.9) | 11 (15.9) | ||
| Clinical T stage n (%) | 0.402 | 0.784 | ||||
| 2 | 3 (3.8) | 1 (0.9) | 3 (4.3) | 1 (1.4) | ||
| 3 | 19 (24.4) | 26 (24.3) | 17 (24.6) | 18 (26.1) | ||
| 4 | 56 (71.8) | 80 (74.8) | 49 (71.0) | 50 (72.5) | ||
| Clinical N stage n (%) | 0.404 | 0.619 | ||||
| 0 | 1 (1.3) | 5 (4.7) | 1 (1.4) | 3 (4.3) | ||
| 1-3 | 77 (98.7) | 102 (95.3) | 68 (98.6) | 66 (95.7) | ||
| Clinical TNM stage n (%) | 0.966 | 1.000 | ||||
| II | 4 (5.1) | 6 (5.6) | 4 (5.8) | 4 (5.8) | ||
| III | 73 (93.6) | 100 (93.5) | 64 (92.8) | 65 (94.2) | ||
| IVA | 1 (1.3) | 1 (0.9) | 1 (1.4) | 0 (0) | ||
| Tumor size (cm) | 5.2 ± 3.1 | 6.0 ± 3.4 | 0.126 | 5.4 ± 3.3 | 5.4 ± 3.1 | 0.953 |
| Nerve invasion n (%) | 1.000 | 0.394 | ||||
| Yes | 43 (55.1) | 59 (55.1) | 38 (55.1) | 33 (47.8) | ||
| No | 35 (44.9) | 48 (44.9) | 31 (44.9) | 36 (52.2) | ||
| Lymph-vascular invasion n (%) | 0.410 | 1.000 | ||||
| Yes | 43 (55.1) | 59 (55.1) | 23 (33.3) | 23 (33.3) | ||
| No | 35 (44.9) | 48 (44.9) | 46 (66.7) | 46 (66.7) | ||
| Differentiation n (%) | 0.360 | 0.780 | ||||
| Well | 4 (5.1) | 3 (2.8) | 1 (1.4) | 2 (2.9) | ||
| Moderate | 24 (30.8) | 25 (23.4) | 22 (31.9) | 19 (27.5) | ||
| Poor | 50 (64.1) | 79 (73.8) | 46 (66.7) | 48 (69.6) | ||
| Pathological T stage n (%) | 0.254 | 0.282 | ||||
| ypT0-1 | 8 (10.3) | 8 (7.5) | 6 (8.7) | 7 (10.1) | ||
| ypT2 | 11 (14.1) | 7 (6.5) | 11 (15.9) | 4 (5.8) | ||
| ypT3 | 23 (29.5) | 31 (29.0) | 17 (24.6) | 21 (30.4) | ||
| ypT4a/4b | 36 (46.2) | 61 (57.0) | 35 (50.7) | 37 (53.6) | ||
| Pathological N stage n (%) | 0.168 | 0.443 | ||||
| ypN0 | 26 (33.3) | 26 (24.3) | 23 (33.3) | 18 (26.1) | ||
| ypN1 | 12 (15.4) | 23 (21.5) | 11 (15.9) | 18 (26.1) | ||
| ypN2 | 16 (20.5) | 14 (13.1) | 14 (20.3) | 11 (15.9) | ||
| ypN3 | 24 (30.8) | 44 (41.1) | 21 (30.4) | 22 (31.9) | ||
| Distant metastasis n (%) | 0.531 | 1.000 | ||||
| Yes | 6 (7.7) | 5 (4.7) | 4 (5.8) | 3 (4.3) | ||
| No | 72 (92.3) | 102 (95.3) | 65 (94.2) | 66 (95.7) | ||
| Pathological TNM stage n (%) | 0.576 | 0.781 | ||||
| IIA | 12 (15.4) | 13 (12.1) | 10 (14.5) | 9 (13.0) | ||
| IIB | 17 (55.1) | 20 (64.5) | 17 (24.6) | 13 (18.8) | ||
| III | 43 (55.1) | 69 (64.5) | 38 (55.1) | 44 (63.8) | ||
| IV | 6 (7.7) | 5 (4.7) | 4 (5.8) | 3 (4.3) | ||
| Adjuvant chemotherapy n (%) | 0.824 | 0.848 | ||||
| Yes | 58 (74.4) | 78 (72.9) | 50 (72.5) | 51 (73.9) | ||
| No | 20 (25.6) | 29 (27.1) | 19 (27.5) | 18 (26.1) | ||
There was no significant difference in the type of NAT between the two groups neither before nor after PSM. A total of 17 patients received NCRT, and the remaining received NAC. For NAC regimens, there was no significant difference between the groups with respect to the use of platinum-based doublets or epirubicin/taxane-based triplets, although the former was more common. The mean cycles of the groups after PSM were not statistically significantly different (3.3 vs 3.6, P = 0.300). There was no significant difference between the two groups in terms of clinical response and TRG scores before and after PSM (Table 2).
| Variable | All patients | P value | Matched patients | P value | ||
| LTG (n = 78) | OTG (n = 107) | LTG (n = 69) | OTG (n = 69) | |||
| Type n (%) | 0.345 | 0.784 | ||||
| NAC | 69 (88.5) | 99 (92.5) | 61 (88.4) | 62 (89.9) | ||
| NCRT | 9 (11.5) | 8 (7.5) | 8 (11.6) | 7 (10.1) | ||
| NAC regimens n (%) | 0.491 | 0.659 | ||||
| Platinum-based doublets | 41 (59.4) | 64 (64.6) | 36 (59.0) | 39 (62.9) | ||
| Epirubicin/taxane-based triplets | 28 (40.6) | 35 (35.4) | 25 (41.0) | 23 (37.1) | ||
| Cycles | 3.3 ± 1.3 | 3.8 ± 1.8 | 0.086 | 3.3 ± 1.3 | 3.6 ± 1.6 | 0.300 |
| Clinical response n (%) | 0.939 | 0.859 | ||||
| PR | 50 (64.1) | 68 (63.6) | 44 (63.8) | 45 (65.2) | ||
| SD | 28 (35.9) | 39 (36.4) | 25 (36.2) | 24 (34.8) | ||
| Mandard TRG score n (%) | 0.316 | 0.654 | ||||
| 1 | 26 (33.3) | 52 (48.6) | 22 (31.9) | 29 (42.0) | ||
| 2 | 4 (5.1) | 4 (3.7) | 4 (5.8) | 2 (2.9) | ||
| 3 | 30 (38.5) | 34 (31.8) | 26 (37.7) | 25 (36.2) | ||
| 4 | 5 (6.4) | 5 (4.7) | 5 (7.2) | 5 (7.2) | ||
| 5 | 13 (16.7) | 12 (11.2) | 12 (17.4) | 8 (11.6) | ||
In total, 4 patients in the OTG group and none in the LTG group underwent combined resection. Before and after PSM, the LTG group showed significant differences in the following characteristics: Postoperative hospital days (11.5 ± 7.1 vs 16.0 ± 12.8 d, P = 0.012), time to removal of gastric tube (5.1 ± 2.0 vs 6.8 ± 5.2, P = 0.013), and length of incision (10.4 ± 4.6 vs 21.9 ± 3.8, P < 0.001). Although the difference was not statistically significant, we found that blood loss during surgery in the LTG group was less than that in the OTG group (200.6 ± 162.0 vs 237.1 ± 194.9, P = 0.116). The R0 resection rates of the LTG and OTG groups were 95.7% and 97.1%, respectively, and the numbers of dissected lymph nodes were 37.3 ± 14.2 and 35.5 ± 15.9, respectively, which were not significantly different (Table 3).
| Variable | All patients | P value | Matched patients | P value | ||
| LTG (n = 78) | OTG (n = 107) | LTG (n = 69) | OTG (n = 69) | |||
| Operation time (min) | 207.6 ± 49.3 | 205.2 ± 52.1 | 0.744 | 204.0 ± 45.8 | 207.1 ± 53.1 | 0.713 |
| Blood loss (mL) | 197.2 ± 162.4 | 228.1 ± 193.4 | 0.252 | 200.6 ± 162.0 | 237.1 ± 194.9 | 0.116 |
| Combined resection n (%) | 0.139 | 0.245 | ||||
| Yes | 0 (0) | 4 (3.7) | 0 (0) | 3 (4.3) | ||
| No | 78 (100) | 107 (96.3) | 69 (100) | 66 (95.7) | ||
| Resection n (%) | 0.651 | 1.000 | ||||
| R0 | 75 (96.2) | 105 (98.1) | 66 (95.7) | 67 (97.1) | ||
| R1/R2 | 3 (3.8) | 2 (1.9) | 3 (4.3) | 2 (2.9) | ||
| Blood transfusion n (%) | 0.608 | 0.507 | ||||
| Yes | 13 (16.7) | 21 (80.4) | 11 (15.9) | 14 (20.3) | ||
| No | 65 (83.3) | 86 (19.6) | 58 (84.1) | 55 (79.7) | ||
| Length of incision (cm) | 10.29 ± 4.4 | 21.6 ± 3.8 | < 0.001 | 10.4 ± 4.6 | 21.9 ± 3.8 | < 0.001 |
| Postoperative hospital stay (d) | 11.6 ± 7.0 | 15.1 ± 10.9 | 0.015 | 11.5 ± 7.1 | 16.0 ± 12.8 | 0.012 |
| Dissected lymph nodes | 37.7 ± 14.5 | 37.8 ± 17.6 | 0.950 | 37.3 ± 14.2 | 35.5 ± 15.9 | 0.465 |
| Time to ambulation (d) | 3.0 ± 1.2 | 3.4 ± 2.4 | 0.130 | 3.0 ± 1.3 | 3.4 ± 2.3 | 0.229 |
| Time to first flatus (d) | 4.8 ± 1.7 | 5.2 ± 2.3 | 0.235 | 4.9 ± 1.7 | 5.1 ± 1.8 | 0.381 |
| Time to first liquid intake (d) | 9.2 ± 5.6 | 10.1 ± 7.8 | 0.404 | 9.1 ± 5.6 | 10.7 ± 8.7 | 0.201 |
| Time to removal of gastric tube (d) | 5.0 ± 2.0 | 6.5 ± 5.0 | 0.008 | 5.1 ± 2.0 | 6.8 ± 5.2 | 0.013 |
| Time to removal of all drainage tubes | 9.7 ± 10.1 | 11.1 ± 11.1 | 0.391 | 9.7 ± 10.5 | 10.9 ± 10.3 | 0.488 |
The overall postoperative complication rates of the LTG and OTG groups were 19.2% and 29.9%, respectively, before PSM, and 20.3% and 29.0%, respectively, after PSM. The overall postoperative complications had no significant difference between the two groups before and after PSM. The most common surgical complications after LTG include abdominal infection, anastomotic leakage and wound infection. For OTG, the most common surgical complications include wound infection, anastomotic leakage, abdominal infection, and gastroparesis. Notably, 8 patients in the OTG group developed medical complications, including pulmonary infection, arterial catheter-related infection, and renal failure, whereas none in the LTG group did. There were no significant differences in terms of minor complications (Grades I-II according to the Clavien-Dindo classification) and severe complications (Grade III-V) between the two groups before and after PSM (Table 4). None of the patients in either group died within the first 30 d after surgery.
| Variable | All patients | P value | Matched patients | P value | ||
| LTG (n = 78) | OTG (n = 107) | LTG (n = 69) | OTG (n = 69) | |||
| Complications, n (%) | ||||||
| Overall | 0.100 | 0.236 | ||||
| Yes | 15 (19.2) | 32 (29.9) | 14 (20.3) | 20 (29.0) | ||
| No | 63 (80.8) | 75 (70.1) | 55 (79.7) | 49 (71.0) | ||
| Surgical complications | ||||||
| Pancreatic fistula | 0 (0) | 1 (0.9) | 1.000 | 0 (0) | 1 (1.4) | 1.000 |
| Abdominal bleeding | 1 (1.3) | 0 (0) | 0.422 | 1 (1.4) | 0 (0) | 1.000 |
| Anastomotic leakage | 5 (6.4) | 6 (5.6) | 1.000 | 4 (5.8) | 3 (4.3) | 1.000 |
| Wound infection | 4 (5.1) | 5 (4.7) | 1.000 | 4 (5.8) | 4 (5.8) | 1.000 |
| Lymphorrhagia | 1 (1.3) | 0 (0) | 0.422 | 1 (1.4) | 0 (0) | 1.000 |
| Intestinal obstruction | 0 (0) | 2 (1.9) | 0.510 | 0 (0) | 1 (1.4) | 1.000 |
| Abdominal infection | 5 (6.4) | 9 (8.4) | 0.611 | 5 (7.2) | 2 (2.9) | 0.441 |
| Duodenal fistula | 0 (0) | 1 (0.9) | 1.000 | 0 (0) | 0 (0) | NA |
| Gastroparesis | 0 (0) | 3 (2.8) | 0.264 | 0 (0) | 3 (4.3) | 0.245 |
| Medical complications | ||||||
| Pulmonary infection | 0 (0) | 6 (5.6%) | 0.04 | 0 | 5 (7.2) | 0.058 |
| Arterial catheter-related infection | 0 (0) | 1 (0.9) | 1.000 | 0 (0) | 1 (1.4) | 1.000 |
| Renal failure | 0 (0) | 1 (0.9) | 1.000 | 0 (0) | 1 (1.4) | 1.000 |
| Clavien-Dindo classification n (%) | 0.331 | 1.000 | ||||
| Grade I-II | 12 (80.0) | 20 (64.5) | 11 (78.6) | 14 (73.7) | ||
| Grade III-V | 3 (20.0) | 11 (35.5) | 3 (21.4) | 5 (26.3) | ||
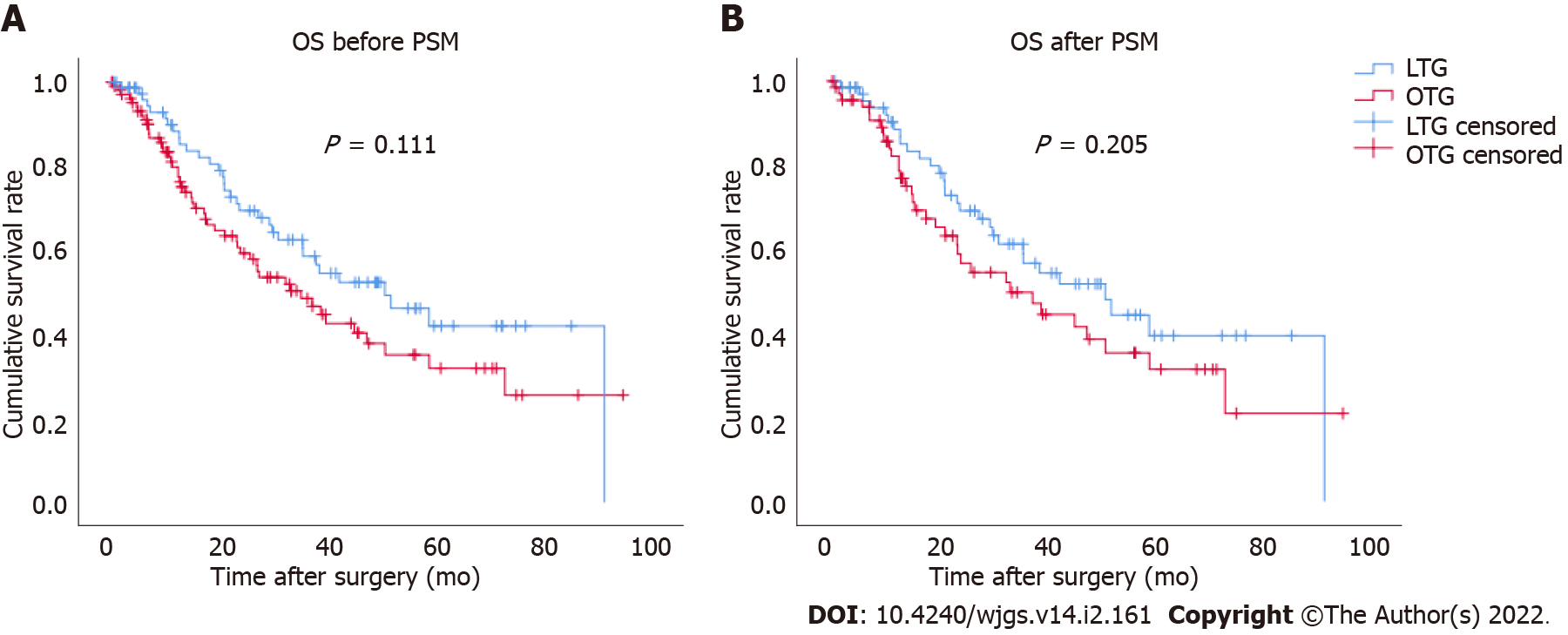
The Kaplan-Meier survival curve for OS between the LTG and OTG groups was plotted (Figure 1). The median follow-up period was 45 mo (range, 3-94 mo). There were no significant differences between the two groups before (P = 0.111) and after PSM (P = 0.205). After PSM, the calculated 5-year cumulative survival rates of the LTG and OTG groups were 39.4% and 31.4%, respectively.
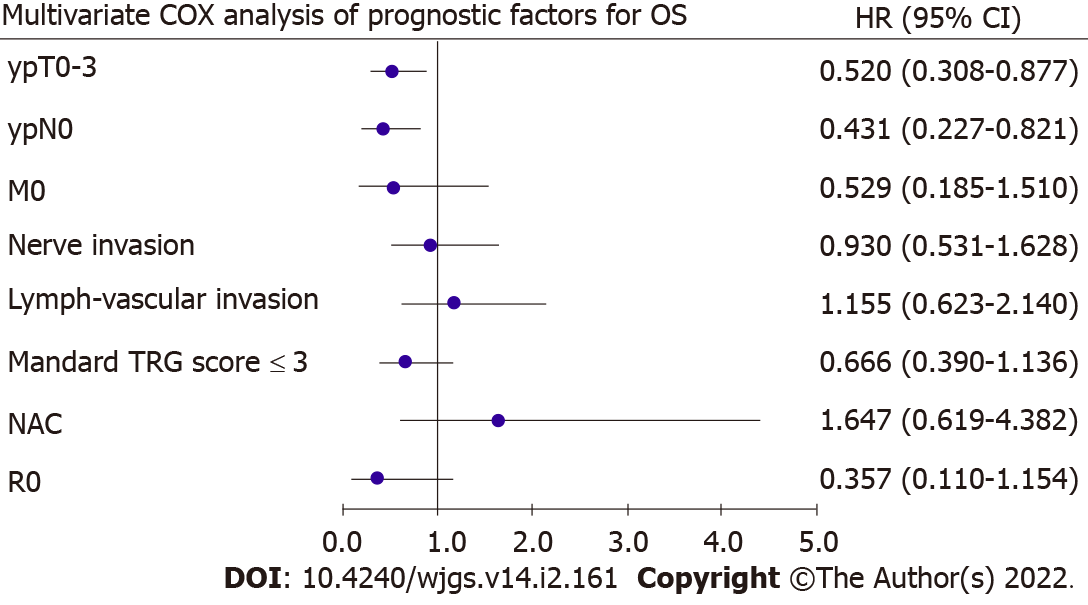
To identify prognostic factors, univariate and multivariate Cox regression analyses were performed after PSM (Table 5). In the univariate analysis, ypT (P = 0.002), ypN (P = 0.004), metastasis (P = 0.103), nerve invasion (P = 0.064), lymph-vascular invasion (P = 0.005), Mandard TRG scores (P = 0.007), type of NAT (P = 0.083), and R0 (P = 0.109) were closely associated with OS. These variables were entered into the multivariate analysis and revealed that ypT0–3 (P = 0.014) and ypN0 (P = 0.010) were indepen-dently associated with OS (Figure 2).
| Variables | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | |
| Age (yr): < 60 vs ≥ 60 | 0.806 | 0.491-1.323 | 0.393 | |||
| Sex: Female vs Male | 1.244 | 0.711-2.177 | 0.444 | |||
| ASA: 1-2 vs 3 | 0.978 | 0.355-2.696 | 0.965 | |||
| Surgery: LTG vs OTG | 0.729 | 0.446-1.192 | 0.207 | |||
| BMI: < 28 vs ≥ 28 | 1.608 | 0.504-5.133 | 0.422 | |||
| Differentiation: Well/moderate vs Poor | 0.713 | 0.416-1.224 | 0.220 | |||
| ypT stage: T0-3 vs T4 | 0.446 | 0.267-0.746 | 0.002 | 0.520 | 0.308-0.877 | 0.014 |
| ypN stage: N0 vs N1-3 | 0.401 | 0.217-0.741 | 0.004 | 0.431 | 0.227-0.821 | 0.010 |
| Metastasis: M0 vs M1 | 0.425 | 0.152-1.188 | 0.103 | 0.529 | 0.185-1.510 | 0.234 |
| Nerve invasion: Yes vs No | 1.601 | 0.973-2.635 | 0.064 | 0.930 | 0.531-1.628 | 0.799 |
| Lymph-vascular invasion: Yes vs No | 2.046 | 1.236-3.388 | 0.005 | 1.155 | 0.623-2.140 | 0.647 |
| Mandard TRG: ≤ 3 vs > 3 | 0.510 | 0.312-0.833 | 0.007 | 0.666 | 0.390-1.136 | 0.136 |
| Postoperative complication: Yes vs No | 0.635 | 0.338-1.193 | 0.158 | |||
| Type of NAT: NAC vs NCRT | 2.248 | 0.900-5.619 | 0.083 | 1.647 | 0.619-4.382 | 0.317 |
| Resection: R0 vs R1/R2 | 0.385 | 0.120-1.237 | 0.109 | 0.357 | 0.110-1.154 | 0.085 |
Recently, LTG has been widely performed in many high-volume hospitals and has gradually expanded the indications for surgery from EGC to AGC[19,20]. However, only one study to date has confirmed the non-inferiority of LTG compared to OTG after NAC in short-term outcomes[12]. To the best of our knowledge, our study is the first to report the long- and short-term outcomes of LTG. Moreover, we found that LTG offered significant advantages in terms of shorter postoperative hospital days and earlier gastric tube removal and had similar postoperative complication rates and OS to those of OTG for patients with GC treated with NAT.
Although NAT is regarded as a key step in the comprehensive treatment of GC, the difference in NAC regimens between Western and Eastern Asian countries should be considered. Three or four-drug NAC regimens have been proved effective in AGC[2,21-24]; however, NAC clinical trials based on two-drug regimens have been exten-sively undertaken in Eastern Asian countries, including JCOG 0210[25], JCOG 0405[26], JCOG 0501[27] in Japan, the NEO-CLASSIC study[28] and the RESOLVE trial (NCT01534546) in China. The optimal NAC regimen for treating AGC remains controversial worldwide, and the differences between Eastern and Western treatment regimens in GC cannot be neglected[29]. In our study, over 60% of all patients received platinum-based doublets, and the overall response rate was more than 60%. Over 80% of all cases were TRG 1-3, which was proved to be an independent prognostic factor[30].
Previous studies have confirmed the oncological and surgical safety of LDG after NAC. Studies by Li et al[11] demonstrated that compared to open surgery, LDG has an advantage in postoperative rehabilitation and complications. A number of meta-analyses and retrospective studies have shown that although there is no significant difference between LTG and OTG in the number of lymph node dissections and the rate of radical surgery, LTG has a lower amount of intraoperative bleeding, lower rate of postoperative complications, and faster postoperative rehabilitation[9,10,31-33]. However, none of these studies specifically focused on the influence of NAT on TG. In our study, we found that in addition to the advantage in incision length, the LTG group had a faster postoperative recovery than that of the OTG group after NAT, which was mainly reflected in the postoperative hospital stay. Compared to a previous study[19], the mean postoperative hospital stay in the LTG group (11.5 d) was slightly longer, which was possibly attributed to the NAT. The number of dissected lymph nodes can be considered an indicator to evaluate the quality of gastrectomy, and is positively correlated with the prognosis of GC[34-36]. The number of dissected lymph nodes between the LTG and OTG groups was not significantly different, and the mean number in LTG (37.3 ± 14.2) was similar to that observed in a previous study[37].
Whether NAT will negatively influence the incidence of postoperative morbidities is of great concern to oncologists and surgeons. A few prospective studies have indicated that NAT does not significantly increase postoperative morbidity in patients with GC[2,22,38]. In the present study, morbidity rates were in accordance with those observed in previous studies, which ranged from 9.6% to 23.8% in LTG, and from 15.6% to 68% in OTG[10,39-41]. To fully elucidate the influence of NAT, large-sample multicenter studies are needed. As for the specific complications, we noticed that both groups had comparable numbers of cases of anastomotic leakage. Moreover, pulmonary infection occurred in 6 patients in the OTG group and none in the LTG group, which was in accordance with a previous study[10]. This rather intriguing finding might be a result of minimally invasive techniques which avoid unnecessary trauma while detaching the cardia region[42].
Whether LTG can achieve the same oncologic outcomes as those of OTG is still debatable. Although LTG is minimally invasive and offers quicker rehabilitation, it also allows a limited visual field and poses challenges to prognosis. Current guidelines only recommend attempting LTG with caution[15,43]. Several retrospective studies showed that there is no significant difference between LTG and OTG in oncological results[44]; however, none of these studies focused on the prognosis of patients treated with NAT. In our study, we found a comparable OS between the LTG and OTG groups, which showed that LTG is non-inferior to OTG after NAT in long-term oncologic outcomes. By using a univariate and multivariate Cox regression analysis, we further found that pathological T stage and N stage were independent risk factors for OS and that the type of TG did not influence the prognosis. With the development of the concept of comprehensive treatment for GC, patients are expected to have a better prognosis.
The major limitation of our study is that it was a single retrospective study. To reduce sample bias and balance the baseline, PSM was performed, which decreased the sample size. In our study, we excluded the missing data instead of multiple imputation, which may bring less statistical power and bias. Therefore, further high-volume, prospective, and multi-center clinical trials are required to evaluate the surgical and oncological outcomes of LTG after NAT.
In conclusion, LTG is considered advantageous in the postoperative rehabilitation of AGC patients treated with NAT and can achieve similar long-term outcomes compared to those of OTG.
Laparoscopic total gastrectomy (LTG) has been widely used these days. Its surgical and oncological outcomes following neoadjuvant therapy (NAT) is still unkown.
To compare the long- and short-term outcomes between LTG and open TG (OTG) following NAT.
Advanced gastric cancer (GC) patients who underwent TG following NAT.
Patients were divided into two groups: LTG and OTG. Propensity score matching analysis was performed to minimize possible bias.
LTG had advantages in short-term outcomes, such as shorter length of hospital stay (P = 0.012), and the oncological outcomes were close to OTG. Overall survival (OS) outcomes were comparable between the two groups. Pathological T and N stages were independent risk factors for OS.
LTG can be a safe and effective method for advanced GC patients following NAT.
Further high-volume, prospective, and multi-center clinical trials are required to evaluate the surgical and oncological outcomes of LTG.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kawabata H, Masaki S, Taira K S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64684] [Article Influence: 16171.0] [Reference Citation Analysis (177)] |
| 2. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 3. | Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Hyung WJ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival Among Patients With Stage I Gastric Cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncol. 2019;5:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 379] [Article Influence: 75.8] [Reference Citation Analysis (1)] |
| 4. | Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 5. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 527] [Article Influence: 87.8] [Reference Citation Analysis (1)] |
| 6. | Liu K, Yang K, Zhang W, Chen X, Zhang B, Chen Z, Chen J, Zhao Y, Zhou Z, Chen L, Hu J. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China. Ann Surg. 2016;263:88-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, Ito S, Takagi M, Takagane A, Teshima S, Koeda K, Nunobe S, Yoshikawa T, Terashima M, Sasako M. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 8. | Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, Yu P, Li Y, Suo J, Zhao N, Zhang W, Li H, He H, Sun Y; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6:1590-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 9. | Straatman J, van der Wielen N, Cuesta MA, de Lange-de Klerk ES, Jansma EP, van der Peet DL. Minimally Invasive Versus Open Total Gastrectomy for Gastric Cancer: A Systematic Review and Meta-analysis of Short-Term Outcomes and Completeness of Resection : Surgical Techniques in Gastric Cancer. World J Surg. 2016;40:148-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:1509-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Li Z, Shan F, Ying X, Zhang Y, E JY, Wang Y, Ren H, Su X, Ji J. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2019;154:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 12. | van der Wielen N, Straatman J, Daams F, Rosati R, Parise P, Weitz J, Reissfelder C, Diez Del Val I, Loureiro C, Parada-González P, Pintos-Martínez E, Mateo Vallejo F, Medina Achirica C, Sánchez-Pernaute A, Ruano Campos A, Bonavina L, Asti ELG, Alonso Poza A, Gilsanz C, Nilsson M, Lindblad M, Gisbertz SS, van Berge Henegouwen MI, Fumagalli Romario U, De Pascale S, Akhtar K, Jaap Bonjer H, Cuesta MA, van der Peet DL. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer. 2021;24:258-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 13. | Fujisaki M, Mitsumori N, Shinohara T, Takahashi N, Aoki H, Nyumura Y, Kitazawa S, Yanaga K. Short- and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc. 2021;35:1682-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Wang N, Zhou A, Jin J, Huang H, Zhang Y, Chen Y, Zhao D. Open vs. laparoscopic surgery for locally advanced gastric cancer after neoadjuvant therapy: Short-term and long-term survival outcomes. Oncol Lett. 2020;20:861-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (2)] |
| 16. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24849] [Article Influence: 1183.3] [Reference Citation Analysis (0)] |
| 17. | Langer R, Becker K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch. 2018;472:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 19. | Huang CJ, Zhang RC, Mou YP, Zhou YC, Wang YY, Lu C, Xu XW. Short and long-term outcomes of laparoscopic total gastrectomy for gastric cancer: A single-center experience (retrospective cohort study). Int J Surg. 2018;51:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Komatsu S, Kosuga T, Kubota T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Ichikawa D, Otsuji E. Comparison of short- and long-term outcomes following laparoscopy and open total gastrectomy for gastric cancer: a propensity score-matched analysis. Am J Transl Res. 2020;12:2225-2233. [PubMed] |
| 21. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1504] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 22. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1648] [Article Influence: 274.7] [Reference Citation Analysis (0)] |
| 23. | Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H, Probst S, Koenigsmann M, Egger M, Prasnikar N, Caca K, Trojan J, Martens UM, Block A, Fischbach W, Mahlberg R, Clemens M, Illerhaus G, Zirlik K, Behringer DM, Schmiegel W, Pohl M, Heike M, Ronellenfitsch U, Schuler M, Bechstein WO, Königsrainer A, Gaiser T, Schirmacher P, Hozaeel W, Reichart A, Goetze TO, Sievert M, Jäger E, Mönig S, Tannapfel A. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 511] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 24. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1693] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 25. | Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, Tsujinaka T, Nashimoto A, Fukushima N, Tsuburaya A; Gastric Cancer Surgical Study Group of Japan Clinical Oncology Group. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol. 2013;107:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 27. | Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito Y, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Boku N, Sano T, Sasako M; Stomach Cancer Study Group, Japan Clinical Oncology Group. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. 2019;22:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Yu Y, Fang Y, Shen Z, Wang Y, Yan M, Cao H, Liu Y, Wang X, Cui Y, Liu F, Chen W, Li W, Li Q, Jiang H, Sun Y, Liu T. Oxaliplatin plus Capecitabine in the Perioperative Treatment of Locally Advanced Gastric Adenocarcinoma in Combination with D2 Gastrectomy: NEO-CLASSIC Study. Oncologist. 2019;24:1311-e989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Chan WL, Lam KO, Lee VHF, Davidson M, So TH, Li JS, Chau I, Kwong DLW. Gastric Cancer - From Aetiology to Management: Differences Between the East and the West. Clin Oncol (R Coll Radiol). 2019;31:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 31. | Kawamura H, Yokota R, Homma S, Kondo Y. Comparison of invasiveness between laparoscopy-assisted total gastrectomy and open total gastrectomy. World J Surg. 2009;33:2389-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Kim MG, Kim BS, Kim TH, Kim KC, Yook JH. The effects of laparoscopic assisted total gastrectomy on surgical outcomes in the treatment of gastric cancer. J Korean Surg Soc. 2011;80:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Shim JH, Oh SI, Yoo HM, Jeon HM, Park CH, Song KY. Short-term outcomes of laparoscopic versus open total gastrectomy: a matched-cohort study. Am J Surg. 2013;206:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Yamashita K, Hosoda K, Ema A, Watanabe M. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer. Eur J Surg Oncol. 2016;42:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Fujiwara Y, Fukuda S, Tsujie M, Ishikawa H, Kitani K, Inoue K, Yukawa M, Inoue M. Effects of age on survival and morbidity in gastric cancer patients undergoing gastrectomy. World J Gastrointest Oncol. 2017;9:257-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G, De Santis F, Baiocchi L, Coniglio A, Nitti D; Italian Research Group for Gastric Cancer (IRGGC). The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 37. | Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Jun L, Chen QY, Lin M, Tu R. Evaluation of laparoscopic total gastrectomy for advanced gastric cancer: results of a comparison with laparoscopic distal gastrectomy. Surg Endosc. 2016;30:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Téoule P, Trojan J, Bechstein W, Woeste G. Impact of Neoadjuvant Chemotherapy on Postoperative Morbidity after Gastrectomy for Gastric Cancer. Dig Surg. 2015;32:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Li SS, Costantino CL, Mullen JT. Morbidity and Mortality of Total Gastrectomy: a Comprehensive Analysis of 90-Day Outcomes. J Gastrointest Surg. 2019;23:1340-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Selby LV, Vertosick EA, Sjoberg DD, Schattner MA, Janjigian YY, Brennan MF, Coit DG, Strong VE. Morbidity after Total Gastrectomy: Analysis of 238 Patients. J Am Coll Surg. 2015;220:863-871.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Jeong O, Jung MR, Kim GY, Kim HS, Ryu SY, Park YK. Comparison of short-term surgical outcomes between laparoscopic and open total gastrectomy for gastric carcinoma: case-control study using propensity score matching method. J Am Coll Surg. 2013;216:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Cho H, Tsuchida K, Iwasaki K, Maezawa Y. Risk factors of post-operative pneumonia in elderly patients with gastric cancer: a retrospective cohort study. Jpn J Clin Oncol. 2021;51:1044-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1118] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 44. | Tsumura T, Kuroda S, Nishizaki M, Kikuchi S, Kakiuchi Y, Takata N, Ito A, Watanabe M, Kuwada K, Kagawa S, Fujiwara T. Short-term and long-term comparisons of laparoscopy-assisted proximal gastrectomy with esophagogastrostomy by the double-flap technique and laparoscopy-assisted total gastrectomy for proximal gastric cancer. PLoS One. 2020;15:e0242223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |