Published online Nov 27, 2022. doi: 10.4240/wjgs.v14.i11.1204
Peer-review started: July 4, 2022
First decision: July 31, 2022
Revised: August 27, 2022
Accepted: October 12, 2022
Article in press: October 12, 2022
Published online: November 27, 2022
Processing time: 143 Days and 17.9 Hours
As the lymph-node metastasis rate and sites vary among pancreatic head carcinomas (PHCs) of different T stages, selective extended lymphadenectomy (ELD) performance may improve the prognosis of patients with PHC.
To investigate the effect of ELD on the long-term prognosis of patients with PHC of different T stages.
We analyzed data from 216 patients with PHC who underwent surgery at our hospital between January 2011 and December 2021. The patients were divided into extended and standard lymphadenectomy (SLD) groups according to extent of lymphadenectomy and into T1, T2, and T3 groups according to the 8th edition of the American Joint Committee on Cancer’s staging system. Perioperative data and prognoses were compared among groups. Risk factors associated with prognoses were identified through univariate and multivariate analyses.
The 1-, 2- and 3-year overall survival (OS) rates in the extended and SLD groups were 69.0%, 39.5%, and 26.8% and 55.1%, 32.6%, and 22.1%, respectively (P = 0.073). The 1-, 2- and 3-year disease-free survival rates in the extended and SLD groups of patients with stage-T3 PHC were 50.3%, 25.1%, and 15.1% and 22.1%, 1.7%, and 0%, respectively (P = 0.025); the corresponding OS rates were 65.3%, 38.1%, and 21.8% and 36.1%, 7.5%, and 0%, respectively (P = 0.073). Multivariate analysis indicated that portal vein invasion and lymphadenectomy extent were risk factors for prognosis in patients with stage-T3 PHC.
ELD may improve the prognosis of patients with stage-T3 PHC and may be of benefit if performed selectively.
Core Tip: Since the lymph node metastasis rate and site differ in pancreatic head carcinoma(PHC) patients at different T stage, we hypothesized that selectively performing extended lymphadenectomy (ELD) can improve the outcome of surgical treatment in PHC patients. The result confirmed that proceeding ELD in T3 stage PHC patients can increase long-term prognosis, providing a new idea to optimized the surgical procedure of PHC. Therefore we concluded that it may be beneficial to perform ELD in PHC patients at T3 stage and potentially increase the clinical outcome of these patients.
- Citation: Lyu SC, Wang HX, Liu ZP, Wang J, Huang JC, He Q, Lang R. Clinical value of extended lymphadenectomy in radical surgery for pancreatic head carcinoma at different T stages. World J Gastrointest Surg 2022; 14(11): 1204-1218
- URL: https://www.wjgnet.com/1948-9366/full/v14/i11/1204.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i11.1204
Pancreatic carcinoma, a common digestive system pathology, is a highly malignant cancer and the third leading cause of cancer-related death, according to the American Cancer Society[1]. Its morbidity rate has increased recently[2-4]. Pancreatic head carcinoma (PHC) is located at the head and uncinate process of the pancreas, and radical surgery is currently the only potential curative therapy for it[5]. However, the postoperative long-term prognosis of patients with PHC is unsatisfactory due to local and distant recurrence in the early postoperative stage.
Lymph-node metastasis is an important PHC transfer pathway, and radical lymph-node dissection is mandatory following anti-tumor treatment[6]. Fortner[7] first proposed extended lymphadenectomy (ELD) in 1973, and this technique has been adopted increasingly widely with its improvement. However, randomized controlled trials have shown that although this procedure increases the lymph-node count, it does not improve the metastatic lymph-node count or long-term prognosis, and thus is of limited clinical value[8]. Variations in the lymph-node metastasis rate and sites among PHC stages may explain this phenomenon. Song et al[9] reported that patients with higher T-stage gastric cancer tend to have higher lymph-node metastasis rates, and confirmed the potential beneficial effect of extensive station-7 lymph-node resection. Considering the positive correlation between the lymph-node metastasis rate and tumor size in patients with PHC, as well as the tendency for distant lymph-node metastasis in advanced PHC[10,11], the selective performance of ELD in patients with PHC of higher T stages may improve the PHC prognosis. In this study, we evaluated the effect of ELD on the long-term prognoses of patients with PHC of different T stages, and the potential clinical value of this procedure.
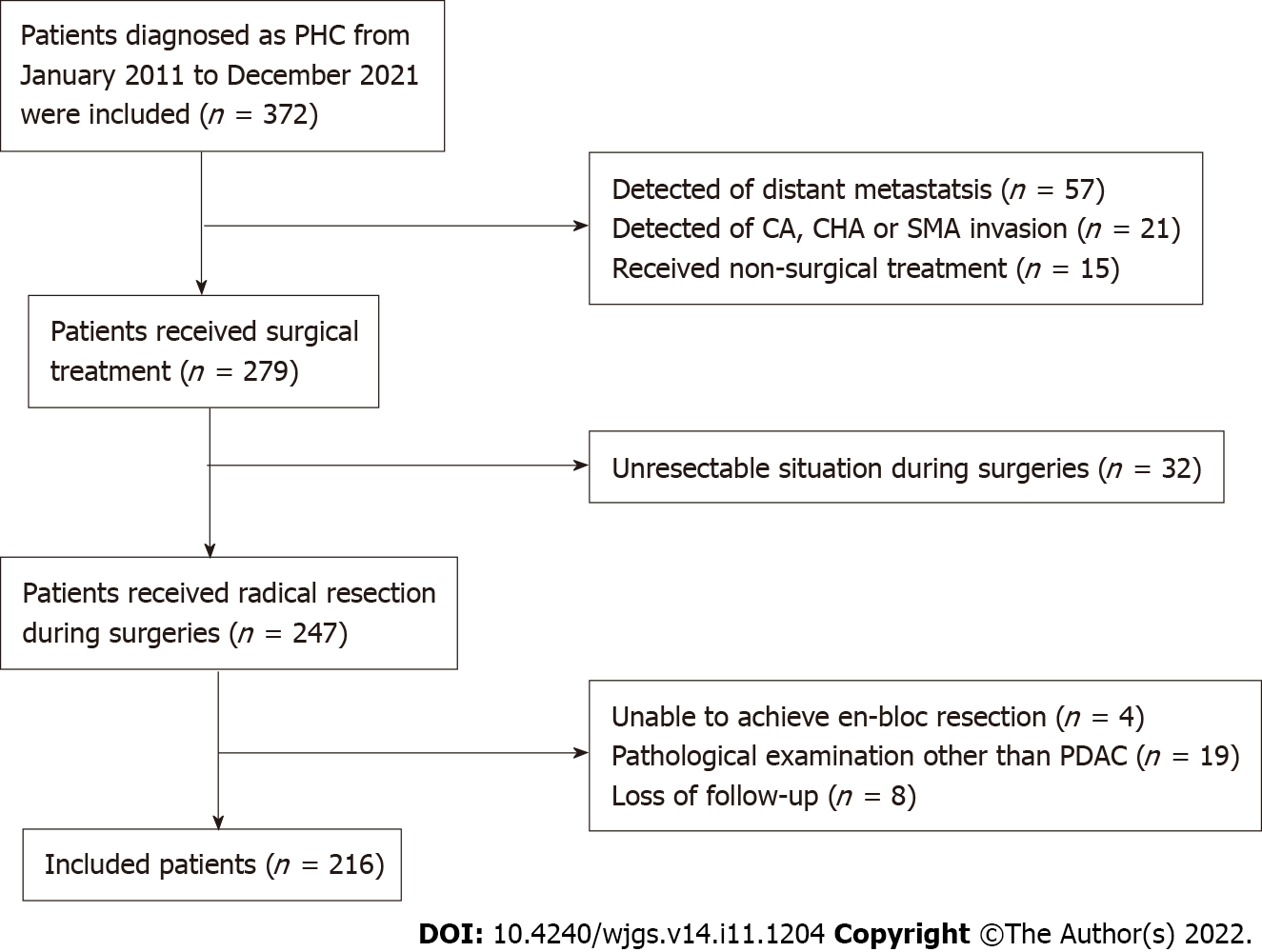
We retrospectively analyzed data from patients with PHC who received surgical treatment in the Hepatobiliary Surgery Department of Beijing Chaoyang Hospital between January 2011 and December 2021. The application of the inclusion and exclusion criteria yielded a sample of 216 patients as shown in Figure 1. The inclusion criteria were: (1) Age 20-85 years; (2) No distant metastasis on preoperative evaluation; (3) No celiac axis, common hepatic artery, or superior mesenteric artery invasion on preoperative evaluation; (4) Surgical treatment including successful en-bloc resection; (5) Postoperative pathological confirmation of the diagnosis of pancreatic ductal adenocarcinoma; and (6) Completeness of clinical and follow-up information. The exclusion criterion was postoperative loss to follow-up.
All surgical procedures and treatment strategies examined in this study were performed with the informed consent of the patients and their family members. The Ethics Committee of Beijing Chaoyang Hospital approved the study and granted access to the patients’ clinical information (No. 2020-D.-302).
The sample of 216 patients comprised 124 males and 92 females (male:female ratio, 1.3:1) with a mean age of 63.6 ± 10.4 (range: 29-84) years. The patients’ initial symptoms included jaundice (n = 110), abdominal pain (n = 78), and atypical gastrointestinal symptoms (n = 9); PHC was detected by physical examination in 19 patients. Sixty-eight (34.5%) patients had diabetes. Sixty-one of the patients exhibiting jaundice received preoperative jaundice-reducing treatment, consisting of endoscopic retrograde cholangiopancreatography (n = 10) and percutaneous transhepatic biliary drainage (n = 51).
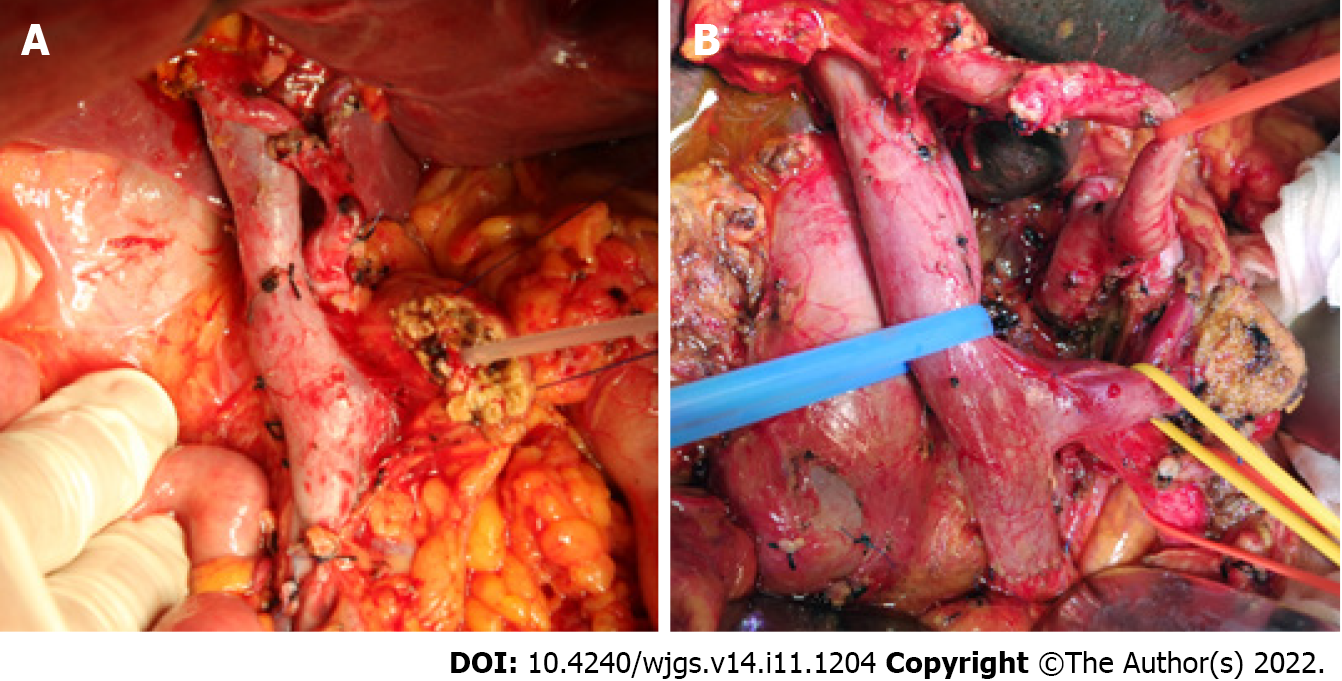
The patients were divided according to T stage, based on the 8th edition of the American Joint Committee on Cancer manual, into T1 (tumor diameter ≤ 2 cm, n = 44), T2 (2 cm < tumor diameter ≤ 4 cm, n = 127), and T3 (tumor diameter > 4 cm, n = 45) groups. They were divided into standard and ELD groups according to the extent of lymphadenectomy intraoperatively as shown in Table 1, with lymph-node stations designated using the Japan Pancreas Society’s nomenclature for peripancreatic lymph nodes[12]. The standard lymphadenectomy (SLD) group (Figure 2A) consisted of cases in which station-5 (suprapyloric), station-6 (infrapyloric), station-8a (anterosuperior along the common hepatic artery), station-12b and c (along the bile duct and around the cystic duct), station-13a and p (on the posterior aspect of the superior and inferior portions of pancreas head), and station-17a and p (on the anterior surface of the superior and inferior portions of the pancreas head) lymph nodes were removed. The ELD group (Figure 2B) consisted of cases not only involving the above-mentioned lymph nodes, but also in which station-8p (posterior along the common hepatic artery), station-9 (around the celiac artery), station-12a and p (along the proper hepatic artery and posterior to the portal vein), station-14a and b (on the right side of the superior mesenteric artery), station-14c and d (on the left side of the superior mesenteric artery), and station-16 (around the abdominal aorta) lymph nodes were removed.
| Location | Standard lymphadenectomy | Extended lymphadenectomy |
| Superior pyloric (No.5) | O | O |
| Inferior pyloric (No.6) | O | O |
| Anterior CHA (No.8a) | O | O |
| Posterior CHA (No.8p) | X | O |
| Celiac axis (No.9) | X | O |
| Proper hepatic artery (No.12a) | X | O |
| Bile duct (No.12b) | O | O |
| Cystic duct (No.12c) | O | O |
| Portal vein (No.12p) | X | O |
| Posterior pancreaticoduodenal (No.13a-b) | O | O |
| Origin and right side of SMA (No.14a-b) | X | O |
| Left side of SMA(No.14c-d) | X | O |
| Celiac axis to IMA (No.16a2, No.16b1) | X | O |
| Anterior pancreaticoduodenal (No.17a-b) | O | O |
Portal vein invasion was categorized as type I (≤ 1/4 of the superior mesenteric-portal vein circumference), type II (> 1/4 of the superior mesenteric-portal vein circumference), type III (superior mesenteric/splenic vein junction), and type IV (superior mesenteric-portal vein including the portal vein trunk and superior mesenteric vein branches), according to the Chaoyang vascular classification proposed by our center[13]: Patients with type I invasion underwent partial venous excision and direct closure, those with type II invasion underwent direct end-to-end anastomosis or allogenic vein reconstruction after segmental venous excision, those with type III invasion underwent allogenic vein reconstruction after segmental venous excision, and those with type IV invasion underwent phleboplasty of the superior mesenteric vein branch ends and allogenic vein reconstruction after segmental venous excision.
General preoperative data and intraoperative and postoperative recovery data were obtained from the patients’ medical records. The perioperative data were compared among different groups. The patients underwent follow-up evaluations in the first and third months after surgery, and then every 3 mo until 2 years postoperatively and every 6 mo thereafter. The follow-up evaluations consisted of blood testing [routine bloodwork, blood biochemistry, and carbohydrate antigen (CA)19-9 level measurement], imaging examinations (pulmonary and enhanced abdominal computed tomography), postoperative treatment, and the assessment of tumor recurrence and survival. Tumor recurrence and death were follow-up visit endpoints. The long-term prognoses of patients in different groups were analyzed and compared.
The data are presented as means ± standard errors of the mean. Nominal and continuous data were compared using the chi-squared and student’s t tests, respectively. Survival outcomes were calculated using the Kaplan-Meier method and compared using the log-rank test. Variables that were significant in univariate analysis were included in a multivariate Cox proportional-hazards regression model. All statistical analyses were performed using SPSS (version 24.0; IBM Corporation, Armonk, NY, United States), with two-sided P values < 0.05 considered to be significant.
All surgeries were successful, and no intraoperative death occurred. Seven patients died in the perioperative period, of abdominal hemorrhage secondary to pancreatic fistula, pulmonary infection (n = 2 each), abdominal infection, renal failure, and heart failure (n = 1 each); the perioperative mortality rate was 3.2%. The SLD group consisted of 88 patients and the ELD group consisted of 128 patients. Portal vein invasion was observed in 116 patients; 83 of these patients underwent allogenic vascular replacement, 27 underwent end-to-end anastomosis after vascular resection, while 6 patients underwent direct suturing after wedge vascular resection. The average volume of intraoperative blood loss was 500 mL (400, 800), and 103 (47.7%) patients received blood transfusions. The average operative time was 11.0 ± 2.9 h (range: 6-20 h).
Postoperative complications were observed in 69 (31.9%) cases, comprising 26 (12.0%) cases of postoperative diarrhea, 24 (11.1%) cases of gastric emptying disturbance, 22 (10.2%) cases of abdominal infection, 9 (4.2%) cases of biochemical fistula, 7 (3.2%) cases of abdominal hemorrhage, 6 (2.8%) cases of level-C pancreatic fistula, 5 (2.3%) cases of pulmonary infection, 4 (2.8%) cases of level-B pancreatic fistula, 4 (1.9%) cases of biliary fistula, 4 (1.9%) cases of gastrointestinal hemorrhage, 4 (1.9%) cases of lymphorrhagia, 3 (1.4%) cases of wound infection, 2 (0.9%) cases of intestinal fistula, 1 (0.5%) case of portal vein thrombosis, 1 (0.5%) case of renal failure, and 1 (0.5%) case of heart failure.
All patients were diagnosed with pancreatic ductal adenocarcinoma, confirmed by postoperative pathological examination. The numbers of cases of highly, moderately, and poorly differentiated adenocarcinoma were 18 (8.3%), 126 (58.3%), and 72 (33.3%), respectively. The average tumor diameter was 3.5 ± 1.5 cm. Postoperative pathological examination led to the detection of an average of 24.2 ± 13.5 lymph nodes per patient and 145 metastatic lymph nodes overall; the lymph-node metastasis rate was 67.1%. Radical resection (R0) was achieved in 201 (93.1%) cases, and R1 resection was achieved in the remaining cases [5 (2.3%) cases each with positive pancreatic and peripancreatic excision margins, 3 (1.4%) cases with positive portal-vein excision margins, and 2 (0.9%) cases with positive uncinate-process excision margins].
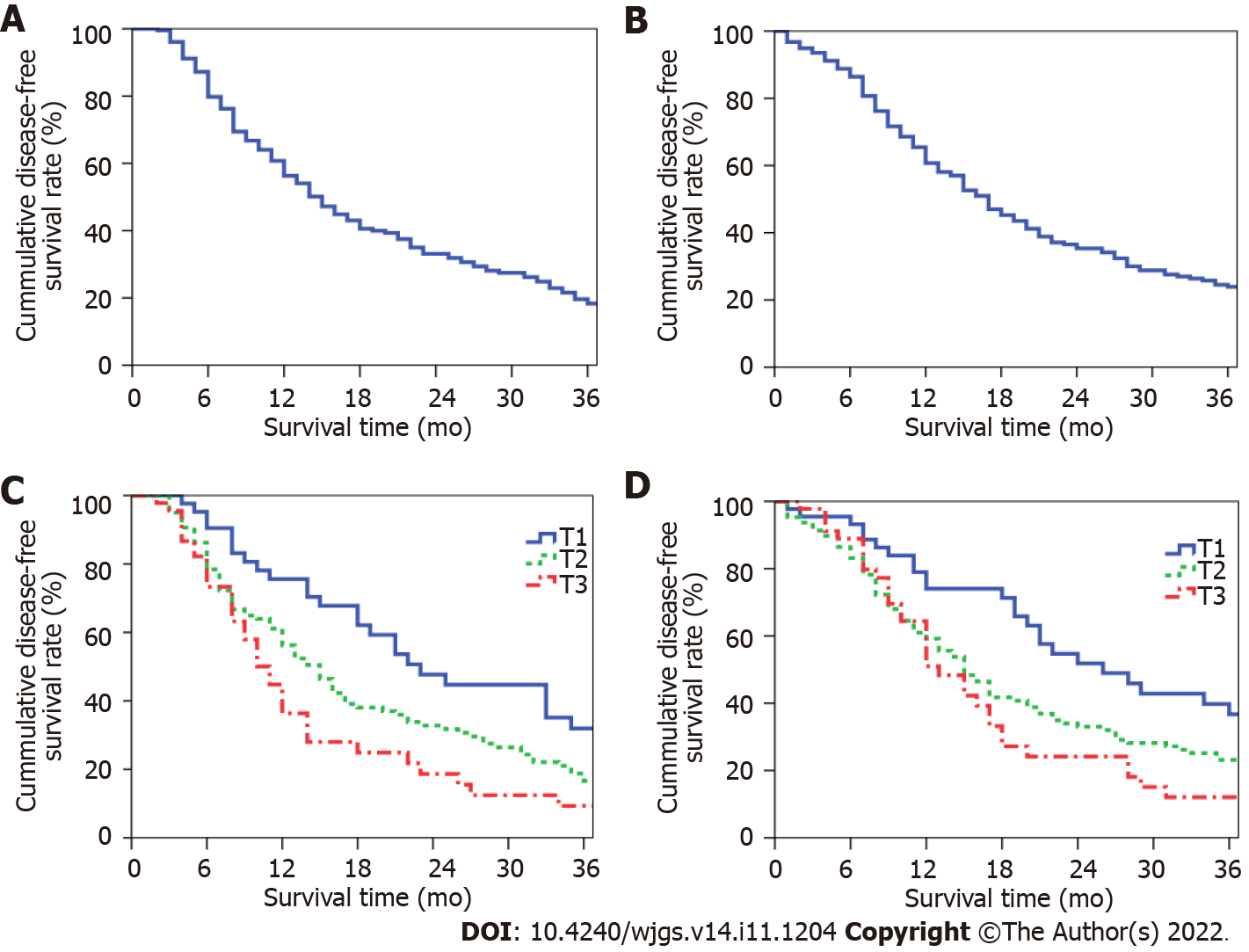
The study follow-up period ended in March 2022. During this period, 109 (50.5%) patients received 1-12 cycles of postoperative adjuvant chemotherapy. The median disease-free survival (DFS) period in the total sample was 15 mo, and the 1-, 2-, and 3-year postoperative DFS rates were 56.3%, 33.1%, and 18.3%, respectively (Figure 3A). The median overall survival (OS) period was 17 mo, and the 1-, 2-, and 3-year postoperative OS rates were 60.7%, 35.3%, and 23.9%, respectively (Figure 3B). The median DFS periods for patients with stage-T1-, -T2, and -T3 PHC were 23, 15, and 11 mo, respectively; the 1-, 2-, and 3-year postoperative DFS rates for these patients were 75.6%, 47.7%, and 31.9%; 56.3%, 32.8%, and 16.6%; and 36.4%, 18.7%, and 9.3%, respectively (P = 0.002, Figure 3C). The median OS periods for patients with stage-T1-, -T2, and -T3 PHC were 26, 15, and 13 mo, respectively; the 1-, 2-, and 3-year postoperative OS rates for these patients were 74.0%, 51.8%, and 36.7%; 59.2%, 33.0%, and 23.1%; and 51.0%, 24.1%, and 12.1%, respectively (P = 0.005, Figure 3D).
More lymph nodes were detected postoperatively in the extended than in the SLD group (P < 0.05; Table 2). The incidence rates of postoperative complications and the mortality rate did not differ between the extended and SLD groups, except that more patients in the former had postoperative diarrhea (P < 0.05; Table 3).
| Variables | ELD group (n = 88) | SLD group (n = 128) | P value |
| Gender (male/female) | 51/37 | 73/55 | 0.893 |
| Age (yr) | 62.1 ± 11.0 | 64.6 ± 10.0 | 0.080 |
| TB (μmol/L) | 62.6 (15.3, 144.6) | 57.7 (12.7, 143.4) | 0.679 |
| CA19-9 (U/ml) | 161.8 (38.5, 544.9) | 202.1 (44.9, 773.2) | 0.342 |
| Intraoperative blood loss (mL) | 500 (400, 800) | 600 (400, 800) | 0.332 |
| Operation time (h) | 11.1 ± 2.8 | 11.0 ± 2.9 | 0.693 |
| Tumor size (cm) | 3.5 ± 1.4 | 3.5 ± 1.7 | 0.790 |
| Tumor differentiation (poorly/ modrately& highly) | 25/63 | 47/81 | 0.203 |
| Portal vein invasion (yes/no) | 47/41 | 69/59 | 0.943 |
| Lymph node metastasis (yes/no) | 54/34 | 91/37 | 0.135 |
| Retrieved lymph node count | 25 (18, 35) | 19 (14, 28) | 0.001 |
| Positive lymph node count | 1 (0, 4) | 2 (0, 3) | 0.614 |
| Resection margin (R0/R1) | 83/5 | 118/10 | 0.545 |
| Postoperative chemotherapy (yes/no) | 48/40 | 61/67 | 0.320 |
| Variables | ELD (n = 88) | SLD (n = 128) | P value |
| Perioperative death | 2 | 5 | 0.783 |
| Postoperative complications | 27 | 42 | 0.741 |
| Biochemical fistula | 3 | 6 | 0.908 |
| Pancreatic fistula (grade B/C) | 4 | 6 | 0.779 |
| DGE | 9 | 15 | 0.732 |
| Diarrhea | 22 | 4 | < 0.001 |
| Abdominal infection | 9 | 13 | 0.987 |
| Abdominal hemorrhage | 3 | 4 | 0.783 |
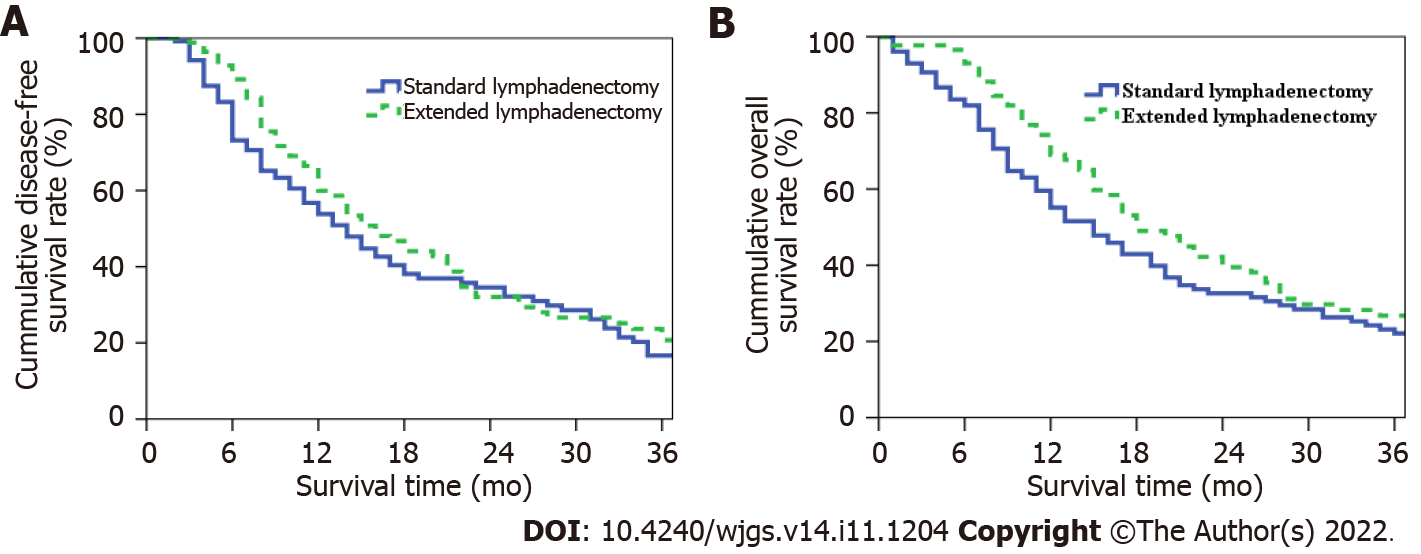
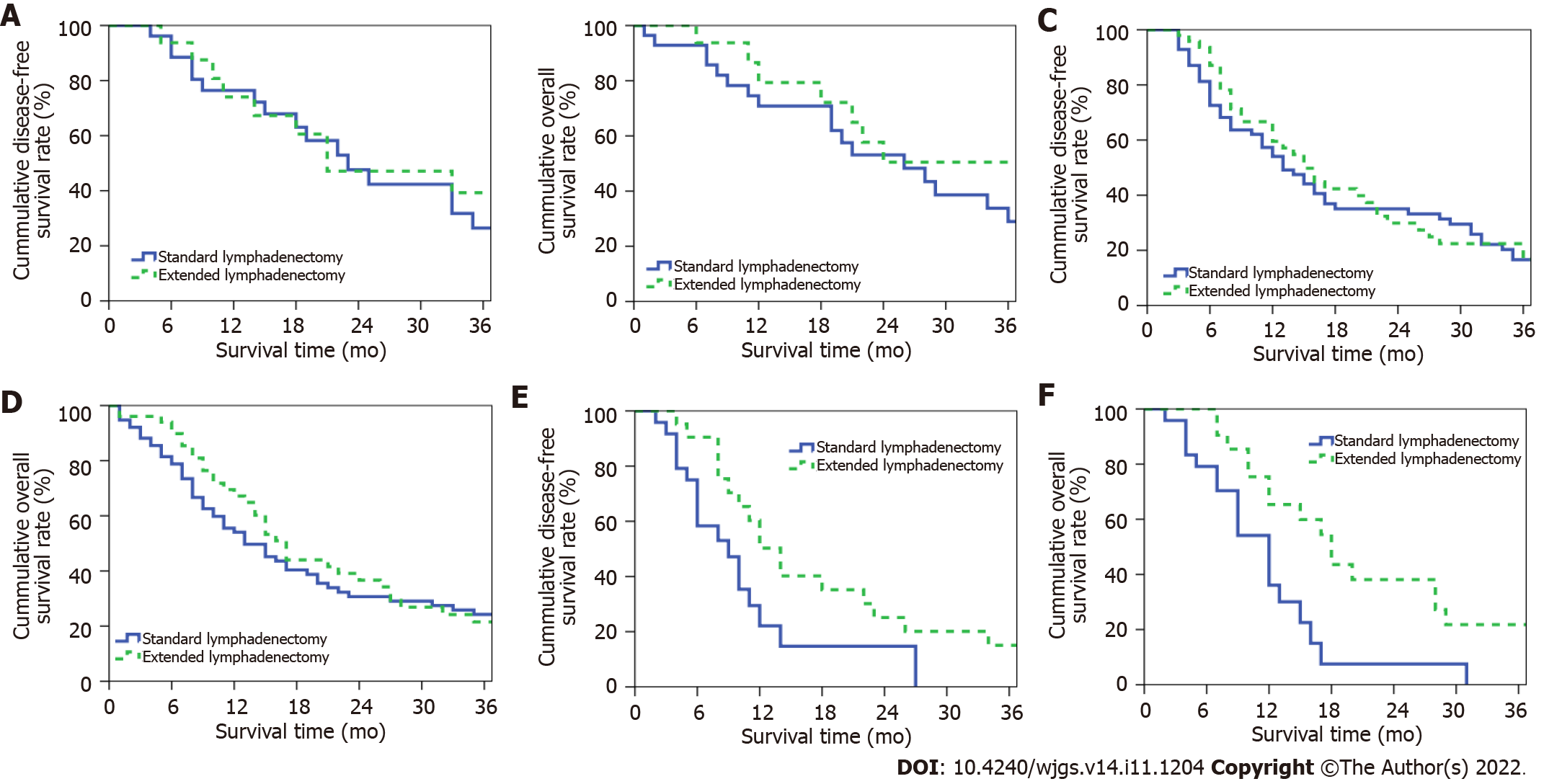
The median DFS periods for patients in the extended and SLD groups were 16 and 14 mo, respectively; the 1-, 2-, and 3-year postoperative DFS rates in these groups were 59.9%, 32.1%, and 20.7% and 53.8%, 34.6%, and 16.7%, respectively (P = 0.227, Figure 4A). The median OS periods for patients in the extended and SLD groups were 18 and 15 mo, respectively; the 1-, 2-, and 3-year postoperative OS rates in these groups were 69.0%, 39.5% and 26.8% and 55.1%, 32.6%, and 22.1%, respectively (P = 0.073, Figure 4B).
ELD increased the numbers of lymph nodes detected in patients with stage-T1- and -T3 disease (P < 0.05; Table 4). Patients in the ELD group were younger than those in the SLD group (P < 0.05). ELD increased the incidence rate of postoperative diarrhea in patients with stage-T2- and -T3 disease (P < 0.05) without affecting the incidence rates of other perioperative complications or the mortality rate (Table 5).
| Variables | T1 stage | T2 stage | T3 stage | ||||||
| ELD group (n = 16) | SLD group (n = 28) | P value | ELD group (n = 51) | SLD group (n = 76) | P value | ELD group (n = 21) | SLD group (n = 24) | P value | |
| Gender (male/female) | 10/6 | 14/14 | 0.423 | 32/19 | 46/30 | 0.801 | 9/12 | 13/11 | 0.449 |
| Age (yr) | 62.8 ± 12.4 | 64.1 ± 9.7 | 0.696 | 64.2 ± 9.8 | 64.7 ± 10.3 | 0.801 | 56.2 ± 10.9 | 64.8 ± 9.7 | 0.001 |
| TB (μmol/L) | 58.1 (16.8, 107.5) | 72.3 (28.1, 149.0) | 0.742 | 80.8 (14.6, 149.3) | 60.6 (12.7, 168.7) | 0.885 | 44.4 (13.0, 137.7) | 29.4 (10.3, 96.8) | 0.285 |
| CA19-9 (U/mL) | 115.9 (24.0, 262.8) | 92.5 (38.7, 312.5) | 0.817 | 152.7 (53.7, 545.9) | 207.0 (43.5, 1058.9) | 0.507 | 180.2 (39.6, 556.4) | 424.5 (77.8, 1285.6) | 0.270 |
| Intraoperative blood loss (mL) | 500 (400, 600) | 500 (400, 650) | 0.788 | 500 (400, 800) | 600 (400, 800) | 0.310 | 500 (400, 1000) | 550 (400, 1000) | 0.741 |
| Operation time (h) | 10.8 ± 3.5 | 9.5 ± 2.9 | 0.194 | 10.9 ± 2.5 | 11.3 ± 2.7 | 0.404 | 11.9 ± 3.0 | 11.6 ± 3.2 | 0.746 |
| Tumor size (cm) | 1.7 ± 0.4 | 1.8 ± 0.3 | 0.274 | 3.2 ± 0.5 | 3.4 ± 0.5 | 0.193 | 5.4 ± 1.0 | 6.1 ± 2.0 | 0.155 |
| Tumor differentiation (poorly/moderately-highly) | 2/14 | 11/17 | 0.126 | 14/37 | 24/52 | 0.619 | 9/12 | 12/12 | 0.632 |
| Portal vein invasion (yes/no) | 4/12 | 6/22 | 0.919 | 28/23 | 45/31 | 0.630 | 15/6 | 18/6 | 0.787 |
| Lymph node metastasis (yes/no) | 10/6 | 15/13 | 0.565 | 31/20 | 55/21 | 0.171 | 15/6 | 23/5 | 0.587 |
| Retrieved lymph node count | 21 (18, 32) | 15 (12, 19) | 0.004 | 26 (21, 33) | 23 (16, 31) | 0.509 | 25 (15, 40) | 20 (15, 30) | 0.030 |
| Positive lymph node count | 2 (0, 2) | 1 (0, 2) | 0.373 | 1 (0, 4) | 2 (0, 4) | 0.513 | 1 (0, 3) | 4 (1, 5) | 0.022 |
| Resection margin (R0/R1) | 16/0 | 28/0 | - | 48/3 | 68/8 | 0.555 | 19/2 | 22/2 | 0.700 |
| Postoperative chemotherapy (yes/no) | 6/10 | 15/13 | 0.305 | 29/22 | 36/40 | 0.294 | 13/8 | 10/14 | 0.175 |
| Variable | T1 stage | T2 stage | T3 stage | ||||||
| ELD group (n = 16) | SLD group (n = 28) | P value | ELD group (n = 51) | SLD group (n = 76) | P value | ELD group (n = 21) | SLD group (n = 24) | P value | |
| Perioperative death | 0 | 1 | 1.000 | 2 | 4 | 0.938 | 0 | 0 | - |
| Postoperative complications | 4 | 12 | 0.391 | 14 | 25 | 0.514 | 9 | 5 | 0.111 |
| Biochemical fistula | 1 | 3 | 0.961 | 1 | 3 | 0.912 | 1 | 0 | 0.467 |
| Pancreatic fistula (grade B/C) | 0 | 3 | 0.463 | 4 | 3 | 0.585 | 0 | 0 | - |
| DGE | 2 | 4 | 0.771 | 4 | 10 | 0.349 | 3 | 1 | 0.506 |
| Diarrhea | 3 | 1 | 0.254 | 12 | 2 | < 0.001 | 7 | 1 | 0.031 |
| Abdominal infection | 1 | 3 | 0.961 | 5 | 8 | 0.895 | 3 | 2 | 0.874 |
| Abdominal hemorrhage | 0 | 1 | 1.000 | 3 | 3 | 0.938 | 0 | 0 | - |
The median DFS periods for patients with stage-T1 PHC in the extended (n = 16) and standard (n = 28) lymphadenectomy groups were 21 and 23 mo, respectively; the 1-, 2-, and 3-year DFS rates in these groups were 74.0%, 47.1%, and 39.3% and 76.4%, 47.6%, and 26.5%, respectively (P = 0.797, Figure 5A). The OS periods for patients with stage-T1 disease in the extended and SLD groups were 41 and 26 mo, respectively; the 1-, 2-, and 3-year OS rates in these groups were 79.3%, 50.5% and 50.5% and 70.8%, 53.1%, and 29.0%, respectively (P = 0.322, Figure 5B).
The median DFS periods for patients with stage-T2 PHC in the extended (n = 51) and standard (n = 76) lymphadenectomy groups were 15 and 13 mo, respectively; the 1-, 2-, and 3-year DFS rates in these groups were 59.5%, 29.9%, and 16.8% and 54.0%, 35.0%, and 16.6%, respectively (P = 0.549, Figure 5C). The OS periods for patients with stage-T2 disease in the extended and SLD groups were 17 and 13 mo, respectively; the 1-, 2-, and 3-year OS rates in these groups were 67.2%, 36.7%, and 21.5% and 54.1%, 30.7%, and 24.2%, respectively (P = 0.411, Figure 5D).
The median DFS periods for patients with stage-T3 PHC in the extended (n = 21) and standard (n = 24) lymphadenectomy groups were 14 and 9 mo, respectively; the 1-, 2-, and 3-year DFS rates in these groups were 50.3%, 25.1%, and 15.1% and 22.1%, 1.7%, and 0%, respectively (P = 0.025, Figure 5E). The OS periods for patients with stage-T3 disease in the extended and SLD groups were 18 and 12 mo, respectively; the 1-, 2-, and 3-year OS rates in these groups were 65.3%, 38.1%, and 21.8% and 36.1%, 7.5%, and 0%, respectively (P = 0.005, Figure 5F).
In the univariate analysis, the postoperative long-term prognosis served as the dependent variable and preoperative data (sex, age, CA19-9 level), intraoperative data (operation time, blood loss), pathological data (tumor differentiation, lymph-node metastasis, metastatic lymph node count, portal vein invasion, excision margin condition, lymphadenectomy extent), and postoperative adjuvant therapy data served as independent variables. The univariate analysis results are shown in Table 6. Lymph-node metastasis, portal vein invasion, and lymphadenectomy extent were significant risk factors in the univariate analysis and were included in the Cox proportional-hazard model. Portal vein invasion [relative risk (RR) = 2.471, 95% confidence interval (CI): 1.028-5.942] and the extent of lymphadenectomy (RR = 2.395, 95%CI: 1.065-5.383) were independent risk factors associated with the long-term prognosis of patients with stage-T3 PHC (Table 7). Among these patients, those with no portal vein invasion who underwent ELD tended to have better long-term prognoses.
| Variables | Number (n = 45) | yr OS (%) | 3-yr OS (%) | χ2 | P value |
| Gender | 0.004 | 0.949 | |||
| Male | 22 | 46.8 | 13.7 | ||
| Female | 23 | 54.1 | 10.8 | ||
| Age (yr) | 2.192 | 0.139 | |||
| ≤ 60 | 22 | 60.2 | 20.1 | ||
| > 60 | 23 | 43.6 | 5.5 | ||
| CA19-9 (U/mL) | 1.504 | 0.220 | |||
| ≤ 37 | 9 | 59.3 | 29.6 | ||
| > 37 | 36 | 48.9 | 7.5 | ||
| Operation time (h) | 2.647 | 0.104 | |||
| ≤ 10 | 18 | 63.2 | 19.0 | ||
| > 10 | 27 | 42.5 | 6.1 | ||
| Intraoperative blood loss (mL) | 0.253 | 0.615 | |||
| ≤ 800 | 30 | 49.2 | 16.5 | ||
| > 800 | 15 | 55.9 | 0 | ||
| Tumor differentiation | 0.996 | 0.318 | |||
| Poorly | 21 | 39.3 | 8.2 | ||
| Moderately-highly | 24 | 59.9 | 15.0 | ||
| Lymph node metastasis | 5.542 | 0.019 | |||
| Yes | 34 | 42.9 | 7.9 | ||
| No | 11 | 77.8 | 25.9 | ||
| Positive lymph node count | 0.569 | 0.451 | |||
| ≤ 3 | 33 | 52.9 | 8.2 | ||
| > 3 | 12 | 46.3 | 23.1 | ||
| Portal vein invasion | 4.141 | 0.042 | |||
| Yes | 33 | 42.3 | 7.7 | ||
| No | 12 | 72.7 | 24.2 | ||
| Resection margin | 0.035 | 0.852 | |||
| R0 | 41 | 48.1 | 13.9 | ||
| R1 | 4 | 75.0 | 0 | ||
| Extent of lymphadenectomy | 7.843 | 0.005 | |||
| ELD | 21 | 65.3 | 21.8 | ||
| SLD | 24 | 36.1 | 0 | ||
| Postoperative chemotherapy | 0.027 | 0.869 | |||
| Yes | 23 | 41.5 | 11.9 | ||
| No | 22 | 61.2 | 12.4 |
| Variables | RR | 95%CI | P value |
| Lymph node metastasis | 1.915 | 0.724-5.063 | 0.190 |
| Portal vein system invasion | 2.471 | 1.028-5.942 | 0.043 |
| Extent of lymphadenectomy | 2.395 | 1.065-5.383 | 0.035 |
Pancreatic carcinoma is a highly malignant cancer originating from the pancreatic ductal epithelial cells. It is usually characterized by early local invasion and distant metastasis, leading to poor long-term prognosis[14]. Although radical surgery remains the only potential curative therapy for PHC[5,15], the long-term postoperative prognosis remains unsatisfactory, emphasizing the importance and necessity of optimizing surgical procedures for PHC, especially that of advanced T stages.
Lymph-node metastasis is an important pancreatic carcinoma transfer pathway; it is confirmed by postoperative pathological examination in about 60% of patients[16]. It has also been recognized as an independent predictor of postoperative recurrence[17-19] and a factor affecting the long-term prognosis of patients with pancreatic carcinoma[20]. The International Study Group on Pancreatic Surgery has published recommendations for the extent and minimum number of retrieved lymph nodes for SLD[21]. However, Nakao et al[22] observed in resected PHC specimens lymph-node metastasis rates of 23% and 26% at stations 14 and 16, reflecting incomplete removal of involved lymph nodes by SLD. Imamura et al[23] found that the lymph-node recurrence rate was as high as 21% and that recurrence was seen most commonly at stations 14 and 16, contributing to 11% and 10% of all recurrence, in patients. Thus, expansion of the lymphadenectomy extent may be beneficial[24].
According to the 2021 Chinese guidelines for the diagnosis and treatment of pancreatic cancer[25], ELD in patients who have undergone pancreaticoduodenectomy for PHC should involve the excision of station-8p, -9, -12a, -12p, -14p, -14d, -16a2, and -16b1 lymph nodes in addition to those excised in SLD. However, recent research has shown that ELD not only prolongs the operation time, but increases intraoperative blood loss, the incidence rate of perioperative complications, and the perioperative mortality rate[8,26,27], Thus, the safety of ELD remains controversial. In contrast to these findings, the operation time, intraoperative blood loss, perioperative mortality rate, and incidence rates of perioperative complications except postoperative diarrhea did not differ between the extended and SLD groups in this study. The circumferential dissection of lymphatic and connective tissue around the root of the superior mesenteric artery in ELD may explain the higher incidence of postoperative diarrhea in patients who have undergone this procedure[26,27]. Farnell et al[28] reported that the incidence rates of postoperative diarrhea at 4, 8, and 14 mo postoperatively in patients with PHC who underwent extended and SLD were 42%, 11%, and 15% and 8%, 11%, and 0%, respectively, with no difference between groups at 8 and 14 mo. Nimura et al[27] found that the influence of diarrhea on the quality of life of patients with PHC who had undergone ELD gradually decreased, with no significant difference from patients who had undergone SLD at 1 year postoperatively. Thus, postoperative diarrhea secondary to ELD is a controllable and temporary complication with no long-term patient effect. Considering that ELD did not increase the incidence rate of postoperative complications or the perioperative mortality rate, we believe that it can be performed feasibly and safely.
Radical surgery that reduces the tumor load via complete removal of the tumor and lymph nodes is currently considered to be a precondition for a promising prognosis for patients with PHC and to lay a foundation for postoperative adjuvant chemoradiotherapy[6]. ELD, which enables the removal of potentially invaded lymph nodes, can be used to achieve radical resection and, theoretically, improve the prognosis of patients with PHC[29]. However, recent research indicates that although this procedure increases the number of lymph nodes retrieved for postoperative pathological examination, it does not increase the positive lymph-node count or improve the long-term PHC prognosis[8,28,30-33]. Notably, little attention has been paid in this research to differences in the lymph-node metastasis rate and sites according to the PHC stage or the clinical value of the selective performance of ELD in patients with PHC at certain stages. Our previous study confirmed that ELD improved the OS and DFS rates in patients with borderline resectable PHC[34], emphasizing the potential clinical value of the selective performance of ELD in patients at greater risk of lymph-node metastasis and local invasion (in whom radical resection may not be achieved with SLD). Muralidhar et al[35] reported that lymph-node metastasis was more likely to occur in patients with larger pancreatic tumors at advanced T stages, illustrating the potential correlation between the T stage and lymph-node metastasis. Pu et al[10] found that the lymph-node metastasis rate reached a plateau of 70%-80% in patients with pancreatic tumors of > 40 mm diameter, and that about 50% of patients with stage-T3 pancreatic carcinoma and lymph-node metastasis were categorized as stage N2. After researching the mode of lymph node metastasis in pancreatic carcinoma patients, Kanda et al[11] reported that distant lymph-node metastasis was seen only in stage-T3- and -T4 pancreatic carcinoma, with station-16 metastasis observed in 10.7% and 33.3% of cases, respectively. These findings shows that patients with advanced T-stage pancreatic carcinoma tend to have higher lymph-node metastasis rates and distant lymph-node metastasis, and thus that SLD is insufficient to achieve radical resection in these patients. Hence, we hypothesized that the selective performance of ELD in patients with PHC of advanced T stages would improve these patients’ long-term prognosis. Our results showed that ELD increased the retrieved and positive lymph node counts and improved the long-term prognosis of patients with stage-T3 PHC, supporting our hypothesis.
PHC usually invades the peri-pancreatic plexus and vessels, and the perivascular region and lymph nodes are the most common sites of local recurrence after surgical treatment[26]. Kovač et al[36] reported that ELD with the achievement of R0 resection reduced the local recurrence rate in patients with PHC. The peripancreatic connective tissue and nerve plexus are excised during ELD, constituting the radical removal of potential invasion and recurrence sites, which may explain the ability of this procedure to improve the prognosis of patients with stage-T3 PHC. In our study, the positive lymph node count and long-term prognosis after ELD were not improved in patients with stage-T1 and -T2 disease. Radical resection can be achieved with SLD in these patients due to the relatively low lymph-node metastasis rate and absence of distant lymph-node metastasis[10,11], which may explain the limited benefit of ELD in these cases. The clinical value of ELD in patients with stage-T1 and -T2 PHC needs to be analyzed further.
As ELD inevitably causes complications such as diarrhea, delayed gastric emptying, and malnutrition[27,37], surgeons must balance the pros and cons of performing it[6]. Due to technical limitations, the N stage of pancreatic carcinoma cannot be determined precisely[38], the T stage is the only accessible preoperative index. The selective performance of ELD based on the T stage can help surgeons not only to make reasonable surgical plans and radically excise potentially invaded lymph nodes, but also to avoid severe postoperative complications secondary to extensive surgical excision. Thus, our results have certain clinical value.
With rapid progress in medical technology, the treatment of PHC is becoming more comprehensive and surgically focused. Perioperative chemotherapy, especially preoperative neoadjuvant chemotherapy, has gained popularity as a part of PHC treatment due to its ability to improve the R0 resection rate[39,40]. Currently, neoadjuvant chemotherapy is considered to be the first-line treatment for patients with borderline resectable pancreatic carcinoma, according to the National Comprehensive Cancer Network’s guidelines. Postoperative chemotherapy, most commonly mFORFILRINOX, has been widely adopted in PHC treatment[41]. Molecular targeting agents are currently suitable only for patients confirmed to have related gene mutations. Despite the progress in perioperative adjuvant chemotherapy, surgery remains the focus of PHC treatment, and radical surgery with comprehensive perioperative chemotherapy is understood to improve long-term patient survival. Thus, determination of the relationships between ELD and perioperative chemotherapeutic parameters is of clinical value. Only a few patients who received neoadjuvant chemotherapy were included in this retrospective study, making the statistical assessment of such relationships difficult. Whether patients benefit from ELD combined with perioperative chemotherapy remains unknown. With the popularity of perioperative therapy, our department began to perform ELD with postoperative neoadjuvant chemotherapy and additional follow-up chemotherapy for patients with PHC. The accumulation of data on such cases and cooperation among departments and medical centers are needed to further explore the clinical value of ELD in comprehensive PHC treatment.
Our study has several limitations. First, it had a single-center retrospective design. Second, the ELD group was younger than the SLD group, which may have confounded the results due to selection bias. However, as age has not been identified as an independent prognostic factor for the postoperative prognosis of patients with PHC, any such bias effect was likely slight. A multicenter prospective study is needed to verify our findings. Third, as we found that ELD increases the retrieved and positive lymph-node counts, it may enable more accurate postoperative N staging. The selective provision of postoperative chemoradiotherapy based on the postoperative N and tumor stages may be of benefit to patients with PHC; additional research on this possibility is needed.
ELD can be performed in patients with PHC feasibly and safely. Its performance may improve the long-term prognosis of patients with stage-T3 PHC through the expansion of the lymphadenectomy extent and elimination of potentially invaded lymph nodes.
Pancreatic head carcinoma (PHC) is a highly malignant tumor, and radical surgery is the only potential curative treatment. However, the long-term postoperative prognosis remains unsatisfactory. As lymph-node metastasis is commonly seen in patients with PHC and has been identified as an independent prognostic factor for postoperative prognosis, extended lymphadenectomy (ELD) has been proposed for the resection of potentially invaded lymph nodes and improvement of the surgical outcome. However, no such improvement in prognosis has been observed. The PHC lymph-node metastasis rate correlates with the T stage, and selective ELD performance for advanced T-stage cases may improve the long-term prognosis.
Given the increases in the lymph-node metastasis rate and sites in patients with PHC, particularly that of advanced T stage, selective ELD performance for patients with advanced T-stage PHC may enable the elimination of more potentially invaded lymph nodes and improvement of the postoperative prognosis.
The objective of this study was to assess the therapeutic effect of ELD in patients with PHC of different T stages.
We retrospectively analyzed data from 216 patients diagnosed with pancreatic ductal adenocarcinoma who underwent surgical treatment at Beijing Chaoyang Hospital between January 2011 and December 2021. The patients were allocated to T1, T2, and T3 groups according to the 8th edition of the American Joint Committee on Cancer’s staging manual and divided into ELD and standard lymphadenectomy (SLD) groups according to the intraoperative extent of lymphadenectomy. Perioperative data and prognoses were compared between the ELD and SLD groups at the T1, T2, and T3 stages, and univariate and multivariate analyses were performed to identify risk factors.
The 1-, 2-, and 3-year disease-free survival (DFS) rates in the ELD and SLD groups were 59.9%, 32.1%, and 20.7% and 53.8%, 34.6%, and 16.7%, respectively (P = 0.227); corresponding overall survival (OS) rates were 69.0%, 39.5%, and 26.8% and 55.1%, 32.6%, and 22.1%, respectively (P = 0.073). The 1-, 2-, and 3-year DFS rates for patients with stage-T3 PHC in the ELD and SLD groups were 50.3%, 25.1%, and 15.1% and 22.1%, 1.7%, and 0%, respectively (P = 0.025); corresponding OS rates were 65.3%, 38.1%, and 21.8% and 36.1%, 7.5%, and 0%, respectively (P = 0.005). Multivariate analysis indicated that portal vein invasion and lymphadenectomy extent were risk factors affecting the prognosis of patients with stage-T3 PHC.
Our research confirmed that ELD can be performed safely for PHC. Although ELD may not improve the overall prognosis of patients with PHC, its selective performance in patients with stage-T3 PHC may improve the long-term postoperative prognosis.
Several limitations of this study must be recognized. First, it was a single-center retrospective study; our findings need to be verified in multicenter prospective studies. Second, the stage-T3 SLD and ELD groups differed in age, which may have confounded our results; further research with more balanced samples is needed. As ELD increases the retrieved land positive lymph node counts, it may enable more accurate N staging, which may aid decision making about postoperative adjuvant therapy; further research on this possibility is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Byeon H, South Korea; de Melo FF, Brazil; Tsujinaka S, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11419] [Article Influence: 3806.3] [Reference Citation Analysis (4)] |
| 2. | Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, Zhang T, Dai M, Zhao Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 3. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 691] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 4. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1261] [Article Influence: 180.1] [Reference Citation Analysis (39)] |
| 5. | Donahue TR, Reber HA. Surgical management of pancreatic cancer--pancreaticoduodenectomy. Semin Oncol. 2015;42:98-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 603] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 8. | Jang JY, Kang MJ, Heo JS, Choi SH, Choi DW, Park SJ, Han SS, Yoon DS, Yu HC, Kang KJ, Kim SG, Kim SW. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 9. | Song W, He Y, Wang S, He W, Xu J. Significance of the lymph nodes in the 7th station in rational dissection for metastasis of distal gastric cancer with different T categories. Chin J Cancer Res. 2014;26:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Pu N, Chen Q, Gan W, Shen Y, Gao S, Habib JR, Yin H, Zhang J, Kinny-Köster B, Cui M, Li J, Dong Y, Nagai M, Liu L, Yu J, Wu W, Lou W. Lymph Node Metastatic Patterns and Survival Predictors Based on Tumor Size in Pancreatic Ductal Adenocarcinoma. Adv Ther. 2021;38:4258-4270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Kanda M, Fujii T, Nagai S, Kodera Y, Kanzaki A, Sahin TT, Hayashi M, Yamada S, Sugimoto H, Nomoto S, Takeda S, Morita S, Nakao A. Pattern of lymph node metastasis spread in pancreatic cancer. Pancreas. 2011;40:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Kawarada Y. [New classification of pancreatic carcinoma--Japan Pancreas Society]. Nihon Shokakibyo Gakkai Zasshi. 2003;100:974-980. [PubMed] |
| 13. | Zhu J, Li X, Kou J, Ma J, Li L, Fan H, Lang R, He Q. Proposed Chaoyang vascular classification for superior mesenteric-portal vein invasion, resection, and reconstruction in patients with pancreatic head cancer during pancreaticoduodenectomy - A retrospective cohort study. Int J Surg. 2018;53:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Chari ST, Kelly K, Hollingsworth MA, Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto M, Cleeter DF, Firpo MA, Gambhir SS, Go VL, Hines OJ, Kenner BJ, Klimstra DS, Lerch MM, Levy MJ, Maitra A, Mulvihill SJ, Petersen GM, Rhim AD, Simeone DM, Srivastava S, Tanaka M, Vinik AI, Wong D. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 2015;44:693-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 15. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1709] [Article Influence: 155.4] [Reference Citation Analysis (0)] |
| 16. | Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Yoo HJ, You MW, Han DY, Hwang JH, Park SJ. Tumor conspicuity significantly correlates with postoperative recurrence in patients with pancreatic cancer: a retrospective observational study. Cancer Imaging. 2020;20:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Feng Q, Li C, Zhang S, Tan CL, Mai G, Liu XB, Chen YH. Recurrence and survival after surgery for pancreatic cancer with or without acute pancreatitis. World J Gastroenterol. 2019;25:6006-6015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Kayahara M, Nagakawa T, Ueno K, Ohta T, Takeda T, Miyazaki I. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Nagakawa T, Konishi I, Higashino Y, Ueno K, Ohta T, Kayahara M, Ueda N, Maeda K, Miyazaki I. The spread and prognosis of carcinoma in the region of the pancreatic head. Jpn J Surg. 1989;19:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, Andrén-Sandberg A, Asbun HJ, Bockhorn M, Büchler MW, Conlon KC, Fernández-Cruz L, Fingerhut A, Friess H, Hartwig W, Izbicki JR, Lillemoe KD, Milicevic MN, Neoptolemos JP, Shrikhande SV, Vollmer CM, Yeo CJ, Charnley RM; International Study Group on Pancreatic Surgery. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156:591-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 472] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 22. | Nakao A, Harada A, Nonami T, Kaneko T, Murakami H, Inoue S, Takeuchi Y, Takagi H. Lymph node metastases in carcinoma of the head of the pancreas region. Br J Surg. 1995;82:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Imamura T, Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, Ohgi K, Uesaka K. Reconsidering the Optimal Regional Lymph Node Station According to Tumor Location for Pancreatic Cancer. Ann Surg Oncol. 2021;28:1602-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Michalski CW, Kleeff J, Wente MN, Diener MK, Büchler MW, Friess H. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 25. | Chinese Pancreatic Surgery Association; Chinese Society of Surgery; Chinese Medical Association. [Guidelines for the diagnosis and treatment of pancreatic cancer in China(2021)]. Zhonghua Wai Ke Za Zhi. 2021;59:561-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Iacono C, Accordini S, Bortolasi L, Facci E, Zamboni G, Montresor E, Marinello PD, Serio G. Results of pancreaticoduodenectomy for pancreatic cancer: extended versus standard procedure. World J Surg. 2002;26:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, Miyagawa S, Yamaguchi A, Ishiyama S, Takeda Y, Sakoda K, Kinoshita T, Yasui K, Shimada H, Katoh H. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 28. | Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, Foster N, Sargent DJ; Pancreas Cancer Working Group. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618-28; discussion 628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 372] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 29. | Manabe T, Ohshio G, Baba N, Miyashita T, Asano N, Tamura K, Yamaki K, Nonaka A, Tobe T. Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer. 1989;64:1132-1137. [PubMed] [DOI] [Full Text] |
| 30. | Ignjatovic I, Knezevic S, Knezevic D, Dugalic V, Micev M, Matic S, Ostojic S, Bogdanovic M, Pavlovic I, Jurisic V. Standard versus extended lymphadenectomy in radical surgical treatment for pancreatic head carcinoma. J BUON. 2017;22:232-238. [PubMed] |
| 31. | Wang Z, Ke N, Wang X, Chen Y, Chen H, Liu J, He D, Tian B, Li A, Hu W, Li K, Liu X. Optimal extent of lymphadenectomy for radical surgery of pancreatic head adenocarcinoma: 2-year survival rate results of single-center, prospective, randomized controlled study. Medicine (Baltimore). 2021;100:e26918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Capussotti L, Massucco P, Ribero D, Viganò L, Muratore A, Calgaro M. Extended lymphadenectomy and vein resection for pancreatic head cancer: outcomes and implications for therapy. Arch Surg. 2003;138:1316-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Henne-Bruns D, Vogel I, Lüttges J, Klöppel G, Kremer B. Surgery for ductal adenocarcinoma of the pancreatic head: staging, complications, and survival after regional versus extended lymphadenectomy. World J Surg. 2000;24:595-601; discussion 601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Wang J, Lyu SC, Zhu JQ, Li XL, Lang R, He Q. Extended lymphadenectomy benefits patients with borderline resectable pancreatic head cancer-a single-center retrospective study. Gland Surg. 2021;10:2910-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 35. | Muralidhar V, Nipp RD, Mamon HJ, Punglia RS, Hong TS, Ferrone C, Fernandez-Del Castillo C, Parikh A, Nguyen PL, Wo JY. Association Between Very Small Tumor Size and Decreased Overall Survival in Node-Positive Pancreatic Cancer. Ann Surg Oncol. 2018;25:4027-4034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Kovač JD, Mayer P, Hackert T, Klauss M. The Time to and Type of Pancreatic Cancer Recurrence after Surgical Resection: Is Prediction Possible? Acad Radiol. 2019;26:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Dasari BV, Pasquali S, Vohra RS, Smith AM, Taylor MA, Sutcliffe RP, Muiesan P, Roberts KJ, Isaac J, Mirza DF. Extended Versus Standard Lymphadenectomy for Pancreatic Head Cancer: Meta-Analysis of Randomized Controlled Trials. J Gastrointest Surg. 2015;19:1725-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Tran Cao HS, Zhang Q, Sada YH, Silberfein EJ, Hsu C, Van Buren G 2nd, Chai C, Katz MHG, Fisher WE, Massarweh NN. Value of lymph node positivity in treatment planning for early stage pancreatic cancer. Surgery. 2017;162:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Barnes CA, Chavez MI, Tsai S, Aldakkak M, George B, Ritch PS, Dua K, Clarke CN, Tolat P, Hagen C, Hall WA, Erickson BA, Evans DB, Christians KK. Survival of patients with borderline resectable pancreatic cancer who received neoadjuvant therapy and surgery. Surgery. 2019;166:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, van Eijck CHJ, Groot Koerkamp B, Rasch CRN, van Tienhoven G; Dutch Pancreatic Cancer Group. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105:946-958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 387] [Article Influence: 55.3] [Reference Citation Analysis (1)] |
| 41. | Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |