Published online Oct 27, 2022. doi: 10.4240/wjgs.v14.i10.1107
Peer-review started: February 21, 2022
First decision: April 19, 2022
Revised: May 15, 2022
Accepted: July 19, 2022
Article in press: July 19, 2022
Published online: October 27, 2022
Processing time: 246 Days and 3.9 Hours
Pylorus and vagus nerve-preserving gastrectomy (PPG) is a function-preserving surgery for early gastric cancer (GC) that has gained considerable interest in the recent years. The operative technique performed using the Da Vinci Xi robot system is considered ideal for open and laparoscopic surgery.
To introduce Da Vinci Xi robot-assisted PPG (RAPPG)-based operative procedure and technical points as well as report the initial experience based on the clinical pathology data of eight cases of early GC.
Da Vinci Xi robot-assisted pylorus and vagus nerve-preserving gastrectomy (RAPPG) was performed for 11 consecutive patients with middle GC from December 2020 to July 2021. Outcome measures were postoperative morbidity, operative time, blood loss, number of lymph nodes harvested, postoperative hospital stay, time to first flatus, time to diet, and resection margins.
Eight of the 11 patients who were pathologically diagnosed with early GC were enrolled in a retrospective study to assess the feasibility and safety of RAPPG. The mean operative time, mean blood loss, mean number of lymph nodes harvested, length of preserved pylorus canal, distal margin, and proximal margin were 330.63 ± 47.24 min, 57.50 ± 37.70 mL, 18.63 ± 10.57, 3.63 ± 0.88 cm, 3.50 ± 1.31 cm, and 3.63 ± 1.19 cm, respectively. None of the cases required conversion to laparotomy. Postoperative complications occurred in two (25.0%) patients. Post
The core technique in the Da Vinci Xi RAPPG is lymph node dissection and the anatomic method of the nerve. Robotic surgical procedures are feasible and safe. With the progress of surgical technology, optimization of medical insurance structure, and emergence of evidence-based medicine, automated surgery systems will have a broad application in clinical treatment.
Core Tip: The robotic surgery system is widely used in the surgical field. Pylorus and vagus nerve-preserving gastrectomy is a function-preserving surgery for early gastric cancer (GC). We introduced an robot-assisted pylorus and vagus nerve-preserving gastrectomy-based operative procedure and technical points as well as report the initial experience. We analyzed the the mean operative time, mean blood loss, mean number of lymph nodes harvested, length of preserved pylorus canal, distal margin, proximal margin, and postoperative complications of 8 patients with early GC. None of the cases required con
- Citation: Zhang C, Wei MH, Cao L, Liu YF, Liang P, Hu X. Performing robot-assisted pylorus and vagus nerve-preserving gastrectomy for early gastric cancer: A case series of initial experience. World J Gastrointest Surg 2022; 14(10): 1107-1119
- URL: https://www.wjgnet.com/1948-9366/full/v14/i10/1107.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i10.1107
Gastric cancer (GC) is the most frequent neoplastic diagnosis and the second most common cause of cancer-related deaths worldwide[1]. The incidence of early GC is increasing annually. Function-preserving surgery for GC has been gaining attention in recent years[2]. Pylorus and vagus nerve-preserving gastrectomy (PPG) as a function-preserving surgical treatment has gained gradual acceptance and promotion. Clinical studies have shown that PPG is a safer option with a better oncological prognosis than distal gastrectomy for managing early GC[3,4].
Moreover, PPG can reduce the incidence of cholelithiasis, diarrhea, and dumping syndrome. It is conducive to the recovery of nutritional indicators and body weight, reducing insulin secretion disorders[5]. Although laparoscopic techniques are improving, the “chopstick” effect caused by the parallel arrangement of the instruments in the umbilicus is considered an obstacle in delicate operations. The tremor filter, scale motions, three-dimensional imaging, and dexterous arm of the Da Vinci robot have advantages in localizing the anatomy of the nerves, vessels, and lymph nodes for clearly demarcated dissections. A meta-analysis evaluated the advantages of robotic gastrectomy (RG) vs laparoscopic gastrectomy (LG) for GC. The results showed that the operative time of RG was significantly shorter and the cost was relatively higher, but RG had advantages in increasing the number of retrieved lymph nodes and controlling intraoperative blood loss. Although there was no significant difference in overall complications, complications with Clavien–Dindo classification greater than grade 3 in RG were significantly lower than those in LG. Distal and proximal resection margin distance, conversion rate to open surgery, mortality rate, and recurrence rate were not significantly different between them[6]. Han et al[7] from South Korea first compared perioperative efficacy and oncologic safety between robot-assisted and laparoscopy-assisted pylorus-preserving gastrectomy in the treatment of middle-third early GC. The operative time of the robot-assisted pylorus-preserving gastrectomy was longer, but there was no significant difference in complications and the number of examined lymph nodes[7].
Experience showed that reasonable surgical process, close cooperation of the surgical team, rational use of energy equipment, and avoidance of surgical risks are key factors to ensure surgical quality. The purpose of this study was to introduce an robot-assisted pylorus and vagus nerve-preserving gastrectomy (RAPPG)-based operative procedure and technical points as well as report the initial experience based on the clinical pathology data of eight cases.
After introducing the Da Vinci Xi robot system, RAPPG was performed for 11 consecutive patients with middle GC from December 2020 to July 2021. All patients were diagnosed with GC with gastroscopy and histological examination before surgery. Gastroscopy and upper gastrointestinal radiography were performed to locate the lesion. Complemented with computed tomography (CT) examination, nine patients with early middle GC with preoperative stage cT1N0 were treated with PPG according to the Japanese GC Treatment Guidelines 2018 (5th edition). One patient was preoperatively diagnosed with cT2N0 without enlarged lymph nodes in the superior pyloric region on CT. PPG was correspondingly performed upon indication due to the clinical assessment of tumor enlargement. Another patient was preoperatively diagnosed with cT4aN2M0. This patient’s case was complicated with chronic obstructive pulmonary disease, and the patient had dyspnea after activity; ASA grade was 3. PPG was performed by a multi-disciplinary team as an extended indication. All patients’ treatment protocols were formulated by preoperative discussion without ethical committee involvement. Before surgery, the procedure details were explained to all patients, and appropriate informed consent was obtained.
The inclusion criteria were as follows: (1) ECOG score ≤ 2 points; (2) Histologically confirmed adenocarcinoma (papillary adenocarcinoma, tubular adenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma, poorly differentiated adenocarcinoma) with gastroscopic pathological biopsy before operation; (3) No group 1 and 5 lymph node metastasis on abdominal CT; (4) A distance of ≥4 cm from the distal end of the tumor to the pylorus on gastroscopy, abdominal CT, and upper gas
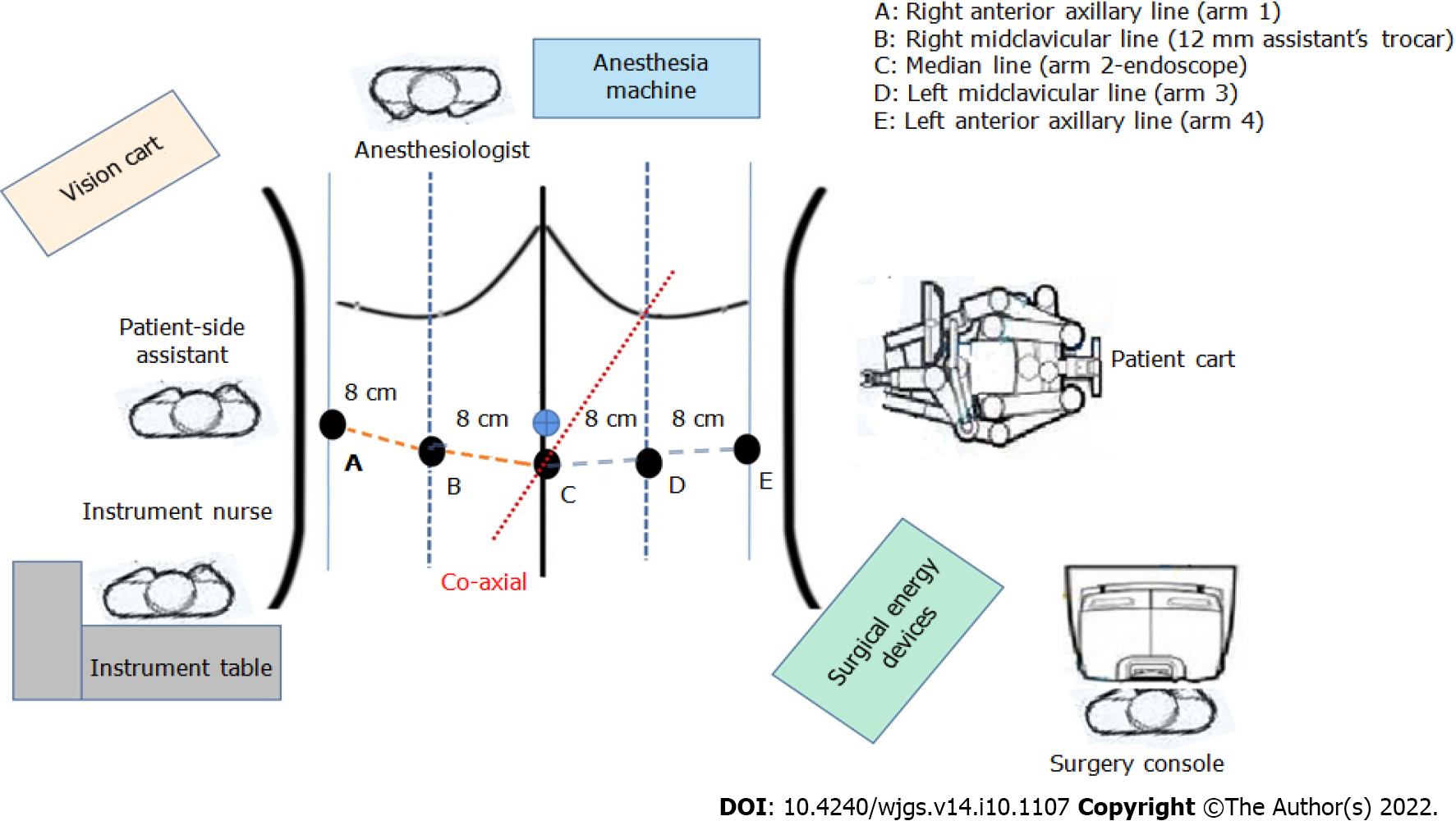
Patient and robot position and port placement: The patient’s position, setting of the trocar puncture sheath, position of the assistant, and choice of the surgical approach play a role in surgical difficulty. R-PPG operation position: the patient was placed in the supine position, the head is held high at 15°, feet are maintained low at 15°, and the assistant is on the right side of the patient. The “Smile” layout was used for the punch card setting (Figure 1).
The coaxial axis was set as the line connecting the umbilicus to the splenic hilum. Arm 3 was used as the central operation hole, and the Maryland bipolar coagulation forceps, ultrasonic scalpel, and Hem-O-lock applier were used. Arm 1 could be inserted with proGrasp forceps and fenestrated bipolar coagulation forceps, whereas Arm 4 could only be inserted with proGrasp forceps. Arm 2 could be used as the endoscope hole (8 mm, 30 ° endoscope). The assistant used the right B hole (12 mm) to assist the operator in exposing the operation field using a Hem-O-lock, aspirator, electrocoagulation rod, and cutting closure device.
Exploration: The pneumoperitoneum was established, abdominal pressure was maintained at 12 mmHg, and the liver was suspended. Tumor location was determined and marked preoperatively and confirmed again by gastroscopy during the procedure.
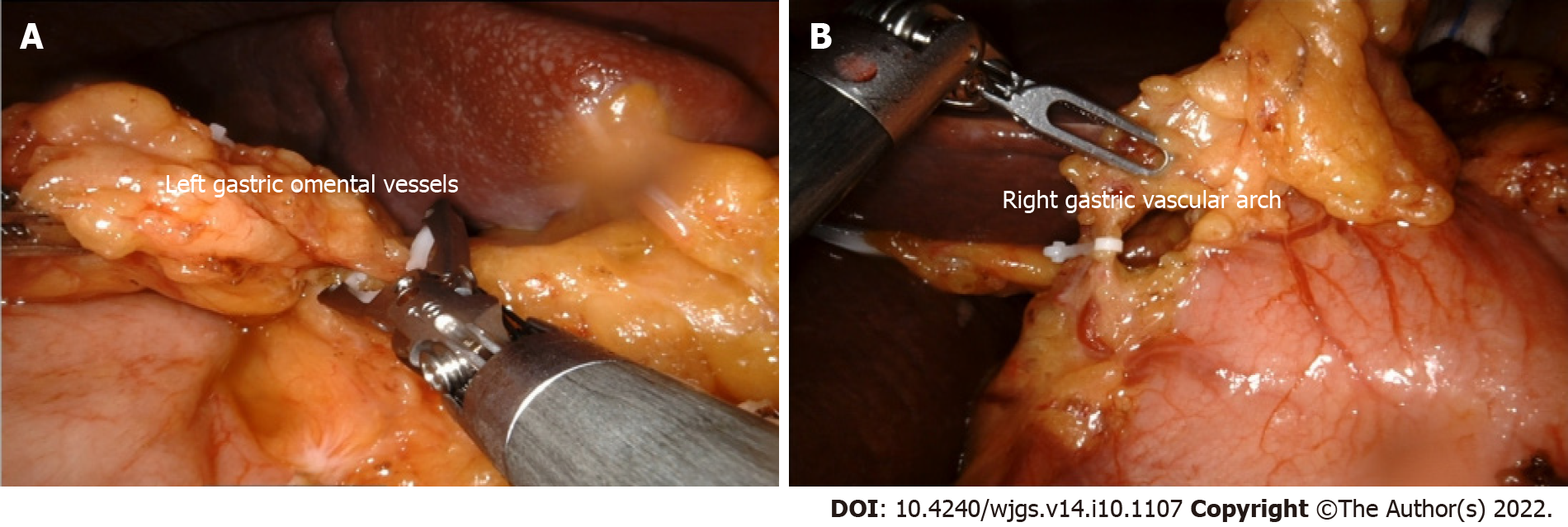
In the middle of the stomach, Arm 4 used proGrasp forceps to lift the vascular arch of the greater curvature of the stomach and pull it to the ventral wall and cephalic side. Arm 1 used proGrasp forceps to expand the gastrocolic ligament from the right side. The assistant pulled the greater omentum to the right and foot sides to expand the gastrocolic ligament in a bullfight towel style. Focus should be on observing the distribution of the transverse colon and omental branch blood vessels. The transverse colon should not be damaged during the operation. Hemostasis of omental branch blood vessels should be reliable, and the operation field should be kept clean. Arm 3 used an ultrasonic scalpel or Maryland bipolar electrocoagulation to open the gastrocolic ligament and enter the omental sac. The gastrocolic ligament was cut at the center of the resultant force and clamped directly to the inferior pole of the spleen. The pancreatic tail was used as a landmark to expose the left gastroepiploic vessels from the ventral and dorsal sides. At the same time, group 4 Lymph nodes were cleared, the medium-large clip was placed in Arm 3, and the left gastric omental vessels were clamped using an applier (Figure 2A) and cut off using an ultrasonic scalpel. The repair of the greater curvature of the stomach and preparation for gastric disconnection and anastomosis were correspondingly facilitated.
Arm 4 used proGrasp forceps to pull the omentum of the greater curvature of the gastric antrum to the left and abdominal wall side, and the assistant pulled the liver region of the transverse colon to the middle and foot side. The duodenum and pancreatic head were transferred to the abdomen’s central part by traction, and the descending duodenum and pancreatic head were fully exposed. Arm 1 used proGrasp forceps to assist exposure and lifting, whereas Arm 3 used Maryland bipolar electrocoagulation. First, the omentum was opened along with the descending duodenum. The transverse mesocolon was dissected along the front of the pancreatic head to nearly reach the horizontal part of the descending duodenum. Part of the hepatocolic ligament was opened outside the duodenum to facilitate traction of the colonic liver region and dissociation of the transverse mesocolon. The accessory right colonic vein, superior anterior pancreaticoduodenal vein, and right gastroepiploic vein were exposed on the right side. The operative field was turned to the middle part of the stomach. The gastrocolic ligament incision in the greater curvature of the stomach was dissociated along the transverse colon to the right.
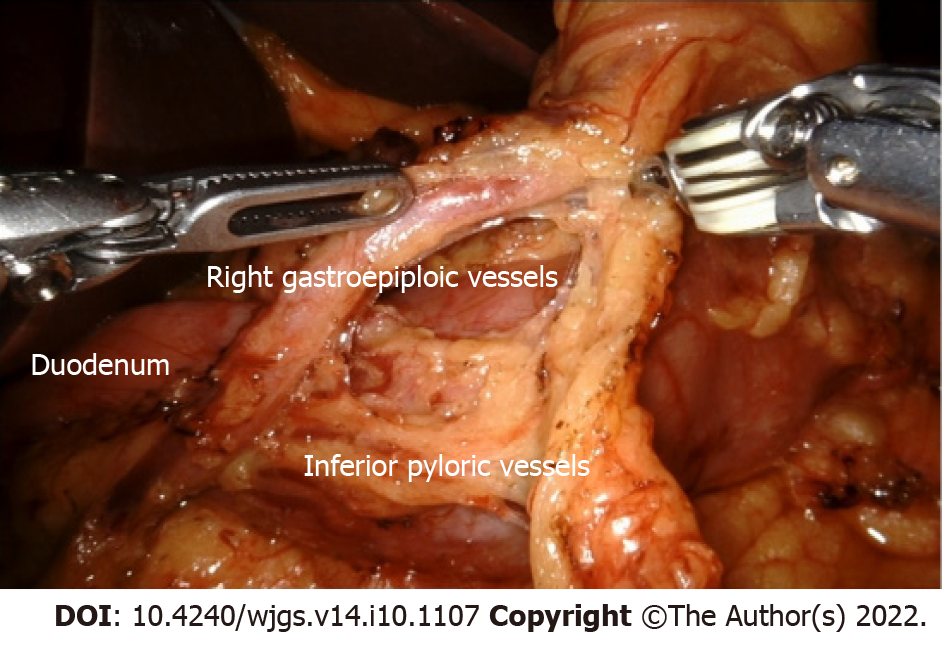
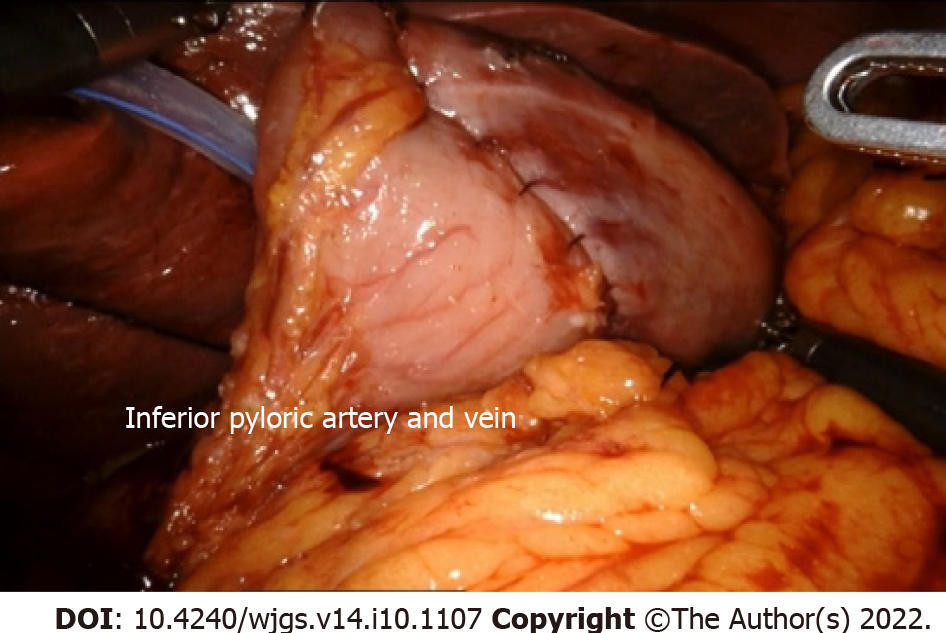
After communicating with the free plane of the descending duodenum, the transverse mesocolon was dissociated from the lower edge of the pancreas to reach the right gastroepiploic vein. The antrum, pylorus, and posterior wall of the duodenum were dissociated to expose the gastroduodenal artery. At this time, the operative field of the area under the pylorus was fully expanded from the right, left, and lower sides. It is safe to dissect the right gastroepiploic vessels and blood vessels under the pylorus and clean the lymph nodes of group 6. First, the lymph nodes in the inferior pylorus region were dissected from the right side along the front of the pancreatic head. The omentum was opened in the avascular area between the inferior pylorus vessel and the first branch of the right gastroepiploic vessel to communicate with the left free plane. The right gastroepiploic vessel branches were cut off one by one along the gastric wall, and the gastric wall of the great curvature of the gastric antrum was exposed by 4–5 cm. The lymph nodes were dissected from the pylorus and duodenum to the bifurcation of the inferior pylorus vessels and right gastroepiploic vessels. Lymph node dissection was performed from the bottom along the root of the right gastroepiploic vein to the top of the bifurcation. Finally, the lymph nodes were dissected from the left side of the pancreas along the blood vessels to reach the bifurcation. The right gastroepiploic vessels were circumscribed 4–5 cm to complete the lymph node dissection in the lower pylorus region (Figure 3). The inferior pyloric artery and veins were preserved. The right gastroepiploic artery was clamped and severed using a Hem-O-lock near the bifurcation.
Arm 4 Lifted the lesser omentum to the oral, left, and abdominal sides, and the assistant pulled the greater curvature of the stomach (the part to be excised) to the left side and under the left side of Arm 3 to fully expose the lesser curvature of the upper pylorus. There was no need to clean group 5 Lymph nodes in the upper pylorus area, and the first to second right gastric vascular branches were preserved. The distance of 4 cm from the lesser curvature to the pylorus was measured as the precut line. Arm 4 Lifted the right gastric artery near the precut line with proGrasp forceps. Arm 3 used an ultrasonic scalpel or Maryland bipolar electrocoagulation. The precut line was close to the gastric wall, and the right gastric vascular arch was circumscribed. Hem-O-lock was used to clamp and disconnect the right gastric vascular arch (Figure 2B). The vascular branches of the gastric wall were cut off one by one along the anterior and posterior wall of the gastric wall to the oral side along the lesser curvature, and the naked gastric wall reached 1 cm distal to the lesion.
Arm 4 used proGrasp forceps to lift the descending branch of the left gastric artery and omental adipose tissue together and pull to the abdominal wall, shifting the operation field to the left and right sides to facilitate better exposure. The assistant can carry a piece of gauze to hide the tip of the forceps, press the middle and lower one-third of the pancreatic body, pull the pancreas to the foot side, turn the superior margin of the pancreas outward, and pull the pancreas to the left and right sides with the change in the operative field. Assistant forceps are typically located in the field of operation. Do not use brute force to avoid injury to the pancreas, mesenteric blood vessels, superior mesenteric blood vessels, and intestine. Arm 3 used Maryland bipolar electrocoagulation, which could be operated from a multi-dimensional angle and was convenient for lymph node dissection and nerve exposure at the superior margin of the pancreas. Arm 1 was pulled and exposed with proGrasp forceps.
Arm 4 pulled the stomach to the abdominal wall and right side, while the assistant pulled the pancreas to the foot and right side. The left retroperitoneal approach was performed by double-click electrocoagulation to open the gastropancreatic fold on the upper edge of the pancreas, expose the left edge of the left gastric artery, and continue to expand the gastropancreatic fold up to the main trunk of the left gastric artery to the bifurcation of the descending branch. The left serosa is opened to the posterior wall of the lesser curvature of the stomach and determines the medial edge of the left approach.
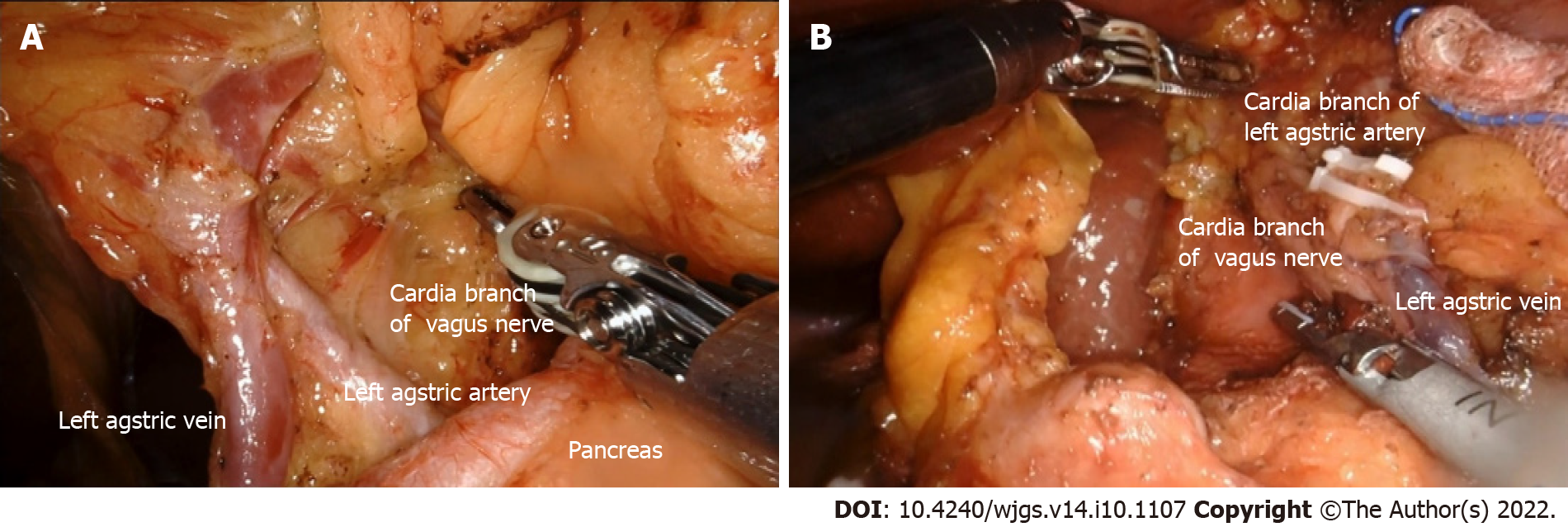
The dorsal membrane of the pancreas was opened along the superior margin of the pancreas, and the superficial nerve of the splenic artery was used to clean the lymph nodes of group 11p, which directly contacted the posterior gastric artery. The lower edge of the left approach was determined. An L-shaped section is formed, and along this section, the nerve is dissociated to the direction of the esophageal hiatus to the posterior wall of the lesser curvature of the stomach. The celiac branch of the vagus nerve can be seen behind the left gastric artery (Figure 4A). The nerve is dissociated into the superficial layer without damage.
Arm 4 pulled the stomach to the abdominal wall and left side, while the assistant pulled the pancreas to the foot and left side. The right branch of the diaphragmatic foot was exposed and dissociated along with the superficial layer of the nerve bundle on the surface of the common hepatic artery. The portal vein bounded the right side, and the left gastric artery bound the left side. The lymph nodes of groups 8a and 9 were dissected carefully towards the diaphragmatic foot.
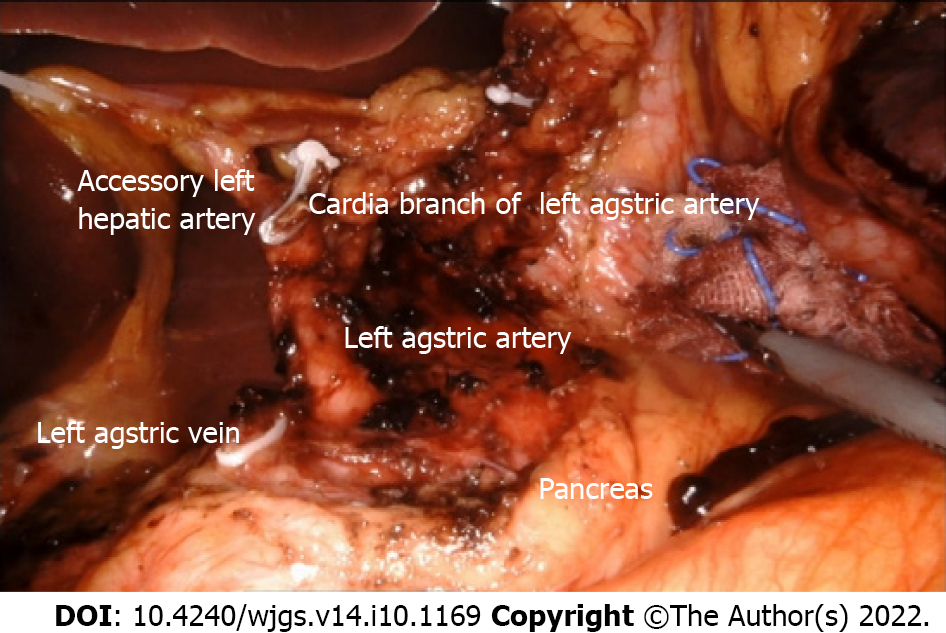
The celiac ganglion was not damaged on the left side. The lymphatic vessels in this area were abundant and should be carefully coagulated using the Maryland bipolar coagulation. The serous membrane was opened on the surface of the right branch of the diaphragm crus to reach the cardia from above. From the right surface of the main left gastric artery, the left gastric artery was dissociated to the bifurcation of the cardia branch and descending branch, forming an L-shaped free plane with the right branch of the foot of the diaphragm (Figure 4B). Along this plane, the left gastric artery was pushed along the cardia branch to the lower part of the cardia, and the abdominal branch of the vagus nerve was exposed from the right side.
The transection of the left gastric artery was performed by preserving the abdominal branch of the vagus nerve and the cardia branch of the left gastric artery via the esophageal approach.
It is vital to maintain the right surgical field, expose the anterior wall of the lesser curvature of the stomach below the cardia, and determine the cardia branch of the left gastric artery, which should be retained. At the distal end of this branch, the group 1 and 3 Lymph nodes were cleared along the lesser curvature of the gastric wall, and the right branch of the foot of the diaphragm. The distal end of the stomach was dissociated from the lower part of the cardia. The left gastric artery was exposed throughout the entire process, and the esophageal cardia branch went directly to the bifurcation of the descending branch of the left gastric artery. The left approach can be connected to the descending branch along the bifurcation ring. The descending branch of the left gastric artery can also be seen from the left approach, communicating with the right approach, retaining the abdominal branch of the vagus nerve and the cardia branch of the left gastric artery, and cutting off the left gastric artery (Figure 5).
The gastric wall was repaired, and the stomach was cut 2 cm from the distal and proximal ends of the tumor. The specimens were removed through a small incision in the upper abdomen. Intraoperative pathology confirmed that the cutting edge was negative. Correspondingly, gastrostomy was performed through a small abdominal incision. The length of the pylorus tube was 3–4 cm. No pyloroplasty was performed (Figure 6).
Data in the text and tables are presented as mean ± SD. Statistical analysis was performed using the SPSS software ver. 20.0 for Windows.
The clinical data of the 11 patients are shown in Table 1. The postoperative pathological diagnosis results of patients 1, 5, and 8 showed that the depth of tumor infiltration exceeded the submucosa, which represents advanced GC and thus did not meet the inclusion criteria of this study. Therefore, these three patients were excluded. Finally, eight patients remained in this study.
| No. | Sex | Year | Body mass index (kg/m2) | Operative time (min) | Tumor size (cm) | pT | pN | Histology | Number of resected lymph nodes | Number of metastatic lymph nodes |
| 11 | M | 70 | 24.20 | 300 | 7 | 3 | 2 | Poorly | 29 | 3 |
| 2 | M | 62 | 21.70 | 390 | 2 | 1b | 0 | Well | 19 | 0 |
| 3 | F | 64 | 20.70 | 330 | 3 | 1a | 0 | Medium | 32 | 0 |
| 4 | M | 56 | 27.10 | 315 | 3 | 1a | 0 | Signet ring cell | 8 | 0 |
| 51 | M | 65 | 29.50 | 325 | 4 | 4a | 3 | Poorly | 51 | 13 |
| 6 | F | 72 | 26.20 | 410 | 1.5 | 1a | 0 | Well | 9 | 0 |
| 7 | M | 70 | 26.42 | 330 | 2.5 | 1a | 0 | Poorly | 21 | 0 |
| 81 | M | 79 | 28.73 | 240 | 3 | 2 | 0 | Poorly | 12 | 0 |
| 9 | M | 52 | 28.02 | 270 | 3 | 1a | 0 | Medium | 13 | 0 |
| 10 | M | 66 | 23.95 | 300 | 2 | 1a | 0 | Well | 11 | 0 |
| 11 | F | 66 | 25.08 | 300 | 4 | 1b | 2 | Poorly | 36 | 3 |
The eight patients had an average BMI of 24.90 ± 2.60 kg/m2 and successfully underwent RAPPG. Patient characteristics are summarized in Table 2.
| Variables | mean ± SD, n = 8 |
| Age (yr) | 63.50 ± 6.74 (52.0-72.0) |
| Sex (male/female) | 5/3 |
| Body mass index (kg/m2) | 24.90 ± 2.60 (20.70-28.02) |
| ASA status | |
| I | 7 |
| II | 1 |
| Comorbidity | |
| Chronic obstructive pulmonary dysfunction | 0 |
| Diabetes | 0 |
| Valvular heart disease | 0 |
| Chronic atrial fibrillation | 0 |
| Hypertension | 1 |
| Occlusive vascular disease | 0 |
| History of appendectomy | 1 |
There were no laparoscopic conversions or intraoperative complications. The mean intraoperative blood loss was 57.50 ± 37.70 mL, no transfusions were required, and the mean operative time was 330.63 ± 47.24 min (Table 3). Lymph node dissection was D1 + 8a, 9, 11p. Postoperative complications occurred in two patients. The incidence of complications was 25.0%. One patient had gastric stasis and hyperamylasemia postoperatively. The Clavien–Dindo classification of complications was grade 2. The patient had first flatus on day 9, liquid diet on day 11, and semi-liquid diet on day 13 after the operation. On day 1 after the surgery, the blood amylase level increased above 500 U/dL. After the application of somatostatin, the blood amylase level returned to normal. No abdominal infection occurred, and the patient was discharged on day 18 after the operation. The other patient had incision infection about grade 2 of Clavien–Dindo classification.
| Variables | mean ± SD, n = 8 |
| Operative time (min) | 330.63 ± 47.24 (270.0-410.0) |
| Estimated blood loss (mL) | 57.50 ± 37.70 (10.0-100.0) |
| Postoperative hospital stay (d) | 10.13 ± 4.55 (6.0-18.0) |
| Time to first flatus (d) | 3.75 ± 2.49 (2.0-9.0) |
| Time to diet (d) | |
| Liquid | 5.38 ± 2.56 (3.0-11.0) |
| Solid | 7.63 ± 2.67 (5.0-13.0) |
| Morbidity | |
| Stomach stasis | 1 |
| Atelectasis | 0 |
| Incision infection | 1 |
| Anastomotic leakage | 0 |
| Hyperamylasemia | 1 |
| Valvular heart disease | 0 |
| Ascites | 0 |
| Trocar bleeding | 0 |
| Ileus | 0 |
The pathological data are listed in Table 4. Among the eight patients, one had early GC invading the submucosa; however, three metastatic lymph nodes were found [groups 4d (1/7) and 6 (2/8)]. Pathological diagnosis showed protuberant lesions, invasion of the submucosa, low adhesion carcinoma, and poorly differentiated carcinoma. Immunohistochemistry showed HER-2 (0), Ki67 (+60%), MLH-1 (loss of expression), MSH-2 (expression), MSH-6 (expression), PMS-2 (loss of expression), and EGFR (-). The mean number of resected lymph nodes was 18 in the eight early GC patients.
| Variables | mean ± SD, n = 8 |
| T | |
| T1a | 6 |
| T1b | 2 |
| N | |
| N0 | 7 |
| N2 | 1 |
| Stage (8th AJCC TNM staging system for gastric cancer) | |
| IA | 7 |
| IIA | 1 |
| Histology | |
| Well | 3 |
| Medium | 2 |
| Poorly | 2 |
| Signet ring cell | 1 |
| Size of tumor (cm) | 2.66 ± 0.82 (1.5-4.0) |
| Distance between anastomosis and pylorus (cm) | 3.63 ± 0.88 (2.5-5.0) |
| Resection margins (cm) | |
| Proximal | 3.63 ± 1.19 (2.0-5.0) |
| Distal | 3.50 ± 1.31 (2.0-5.0) |
| Mean resected Lymph nodes | 18.63 ± 10.57 (8.0-36.0) |
| Number of metastatic lymph nodes | 0.38 ± 1.06 (0-3) |
PPG was first proposed by Maki in the 1960s to treat peptic ulcers. At the beginning of the 1990s, lymph node dissection and the applied PPG technology became popular for early GC treatment in Japan. With the increasing incidence of early GC, this technology is widely used in Asian countries, mainly in China, Japan, and South Korea. The 3rd edition of the Japanese guidelines for the treatment of GC (2010) stipulates the indications for PPG. For early middle GC, the distance from the distal part of the tumor to the pylorus was > 4 cm. Group 5 Lymph nodes above the pylorus were not removed, and the hepatic branches and celiac branches of the vagus nerve were preserved. Clinical studies have found that compared with distal gastrectomy, vagus-preserving gastrectomy can reduce postoperative cho
The Da Vinci robotic surgery system is widely used in the surgical field because of its advantages of high definition, an enlarged 3D field of vision, good stability, and flexibility. In 2002, Hashizume et al[13] reported the first Da Vinci robot-assisted radical gastrectomy. A meta-analysis published in 2019 included 8413 patients with GC from 24 non-randomized studies. A total of 2741 cases were treated with RG, and 5672 cases were treated with LG. The results showed that the operative time in the RG group was longer than that in the LG group, but the number of lymph nodes was higher. Complications such as delayed gastric emptying, intestinal obstruction, abdominal infection, incision infection, anastomotic leakage, and pancreatic complications were not significantly different. There were no significant differences in the 3-year and 5-year overall survival rates[14]. Uyama et al[15] reported a multicenter, single-arm, prospective study of robot-assisted distal gastrectomy in 253 patients with stage I/II GC. The results showed that the average operative time of robot-assisted distal gastrectomy was 313 min, and blood loss was 20 mL. No 30 d mortality occurred, the incidence of complications was 2.45%, and incidence of complications was lower than that of laparoscopic distal gastrectomy (6.4%). Wang et al[16] compared the incidence of complications between the RG and LG groups.
The results showed that the overall incidence of complications and severe complications in the robotic gastric surgery group was 18.8% and 8.9%, respectively, lower than 24.5% and 17.5% in the laparoscopic group. The robot system is safe and feasible for the surgical treatment of GC. The latest Da Vinci robot system is the Da Vinci Xi. A Korean study compared the short-term effects of the Da Vinci Xi System and the Da Vinci Si System on gastrectomy for GC. Early and advanced GCs were included in this study. Surgical methods included distal gastrectomy, total gastrectomy, and proximal gastrectomy. The results showed no significant difference in operative time, intraoperative blood loss, first postoperative exhaust time, hospital stay, and complications between the two groups[17]. At present, there is no evidence-based medicine such as an RCT comparing robotic GC surgery with laparoscopy and laparotomy. Ojima et al[18] carried out an RCT on robot-assisted laparoscopic radical gastrectomy in 2018 and planned to include 240 patients with GC of clinical stages I–III. The primary endpoint was to assess the incidence of postoperative complications of intra-abdominal infection, including pancreatic fistula, intra-abdominal abscess, and anastomotic fistula. Secondary endpoints included the incidence of any complications, surgical outcomes, postoperative course of the disease, and oncological outcomes.
The fundamental techniques of PPG are (1) Group 6 Lymph node dissection with preservation of the inferior pylorus vessels and (2) Treatment of the upper edge of the pancreas with preservation of the abdominal branch of the vagus nerve. Kiyokawa et al[19] proposed that the incidence of gastric stasis after PPG with preservation and disconnection of inferior pyloric vein was 5.4% and 23.4% respectively. Based on the concept of structure-determining function, preserving the blood vessels around the pylorus can maintain the basic shape of the pylorus and has minimal effect on the function of the pylorus after PPG. The inferior pylorus artery and vein were preserved during PPG. The lymph nodes in the inferior pylorus region were dissected and exposed from the upper, lower, right, and left directions and from the ventral and dorsal sides by taking the bifurcation of the right gastroepiploic artery and the inferior pylorus artery as the center. Upper part: duodenal bulb, pylorus, significant curvature of the gastric antrum; lower part: root of the right gastroepiploic vein; right side: medial edge of the descending duodenum; left side: right edge of the first branch of the right gastroepiploic vessel; ventral side: the anterior wall of the stomach; dorsal side: the posterior wall of the stomach. The dissociation order can be as follows: right ventral border, lower dorsal upper left border, right ventral border, and upper-lower dorsal left border.
Moreover, preservation of the esophageal branch of the cardia plays a vital role in maintaining the shape and function of the cardia. The right diaphragmatic foot approach was combined with the left retroperitoneal approach to determine the distribution of the vagus nerve. Lymph node dissection outside the nerve fiber membrane is vital to this technique. In addition, Maryland bipolar electrocoagulation is better than ultrasonic scalpel in treating Arm 3 of the superior margin of the pancreas.
The major limitation of this single center is the retrospective design and small sample size. This study aimed to highlight the surgical process, technical details, technical points, and precautions of RAPPG and retrospectively analyze the short-term prognosis of early GC cases. More cases should be accu
Overall, these study results are preliminary, and on establishing a standard surgical treatment, large-sample, multi-center, and prospective clinical trial should be conducted.
Laparoscopic PPG for GC management has advanced, but the chopstick effect of laparoscopic surgery limits its delicate operation. The robot system functions as a high-degree-of-freedom simulation operation instrument, with a high-definition magnified 3D field of vision and tremor elimination, which significantly improves the safety, flexibility, and stability of a more effective operation platform for PPG operation. However, the application of robot systems remains limited due to its bulky volume and high cost, resulting in decreased operation cost and efficiency. In addition, evidence-based medicine is essential to confirm the safety and feasibility of the Da Vinci surgical system in the treatment of GC. However, with the continuous improvement and upgrading of robot systems, advancement of surgical technology, optimization of medical insurance structure, and accumulation of research samples, the robot system will occupy an important position in the minimally invasive treatment of GC in the future.
Pylorus and vagus nerve-preserving gastrectomy (PPG) as a function-preserving surgical treatment has gained gradual acceptance and promotion. Although laparoscopic techniques are improving, the “chopstick” effect caused by the parallel arrangement of the instruments in the umbilicus is considered an obstacle in delicate operations. The results of study showed that operative time of the robot-assisted pylorus-preserving gastrectomy (RAPPG) was longer, but there was no significant difference in complications and the number of examined lymph nodes compared with laparoscopy-assisted pylorus-preserving gastrectomy (LAPPG).
In order to formulate the reasonable surgical process and technical standards for RAPPG.
To introduce Da Vinci Xi RAPPG-based operative procedure and technical points as well as report theinitial experienc.
This retrospective analysis of clinical and pathological data of 8 early middle gastric cancer (GC) cases who have performed RAPPG.The fundamental techniques of RAPPG are (1) The inferior pylorus artery and vein were preserved during operation; and (2) The right diaphragmatic foot approach was combined with the left retroperitoneal approach to determine the distribution of the vagus nerve.
There were no laparoscopic conversions or intraoperative complications. The mean intraoperative blood loss was 57.50 ± 37.70 mL; the mean operative time was 330.63 ± 47.24 min .The incidence of complications was 25.0%.
The core technique in the RAPPG is lymph node dissection and the anatomic method of the nerve. Robotic surgical procedures are feasible and safe. Reasonable surgical process, close cooperation of the surgical team, rational use of energy equipment, and avoidance of surgical risks are key factors to ensure surgical quality.
This study aimed to highlight the surgical process, technical details, technical points, and precautions of RAPPG and retrospectively analyze the short-term prognosis of early GC cases. More cases should be accumulated, long-term follow-up should be conducted, and data should be compared with data for LAPPG to gather more data for RAPPG in the treatment of patients with early GC.
We would like to thank the editors and the reviewers for their useful remarks that improved this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shah OJ, India; Tanabe H, Japan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1086] [Article Influence: 271.5] [Reference Citation Analysis (0)] |
| 2. | Tsujiura M, Nunobe S. Functional and nutritional outcomes after gastric cancer surgery. Transl Gastroenterol Hepatol. 2020;5:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Aizawa M, Honda M, Hiki N, Kinoshita T, Yabusaki H, Nunobe S, Shibasaki H, Matsuki A, Watanabe M, Abe T. Oncological outcomes of function-preserving gastrectomy for early gastric cancer: a multicenter propensity score matched cohort analysis comparing pylorus-preserving gastrectomy versus conventional distal gastrectomy. Gastric Cancer. 2017;20:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 4. | Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 2008;95:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 5. | Kojima K, Yamada H, Inokuchi M, Kawano T, Sugihara K. Functional evaluation after vagus-nerve-sparing laparoscopically assisted distal gastrectomy. Surg Endosc. 2008;22:2003-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 6. | Guerrini GP, Esposito G, Magistri P, Serra V, Guidetti C, Olivieri T, Catellani B, Assirati G, Ballarin R, Di Sandro S, Di Benedetto F. Robotic versus laparoscopic gastrectomy for gastric cancer: The largest meta-analysis. Int J Surg. 2020;82:210-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 7. | Han DS, Suh YS, Ahn HS, Kong SH, Lee HJ, Kim WH, Yang HK. Comparison of Surgical Outcomes of Robot-Assisted and Laparoscopy-Assisted Pylorus-Preserving Gastrectomy for Gastric Cancer: A Propensity Score Matching Analysis. Ann Surg Oncol. 2015;22:2323-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Eom BW, Park B, Yoon HM, Ryu KW, Kim YW. Laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer: A retrospective study of long-term functional outcomes and quality of life. World J Gastroenterol. 2019;25:5494-5504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 9. | Isozaki H, Okajima K, Momura E, Ichinona T, Fujii K, Izumi N, Takeda Y. Postoperative evaluation of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 1996;83:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 10. | Tsujiura M, Hiki N, Ohashi M, Nunobe S, Kumagai K, Ida S, Hayami M, Sano T, Yamaguchi T. Excellent Long-Term Prognosis and Favorable Postoperative Nutritional Status After Laparoscopic Pylorus-Preserving Gastrectomy. Ann Surg Oncol. 2017;24:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 11. | Oh SY, Lee HJ, Yang HK. Pylorus-Preserving Gastrectomy for Gastric Cancer. J Gastric Cancer. 2016;16:63-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 12. | Takahashi R, Ohashi M, Hiki N, Makuuchi R, Ida S, Kumagai K, Sano T, Nunobe S. Risk factors and prognosis of gastric stasis, a crucial problem after laparoscopic pylorus-preserving gastrectomy for early middle-third gastric cancer. Gastric Cancer. 2020;23:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 13. | Hashizume M, Shimada M, Tomikawa M, Ikeda Y, Takahashi I, Abe R, Koga F, Gotoh N, Konishi K, Maehara S, Sugimachi K. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc. 2002;16:1187-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Qiu H, Ai JH, Shi J, Shan RF, Yu DJ. Effectiveness and safety of robotic versus traditional laparoscopic gastrectomy for gastric cancer: An updated systematic review and meta-analysis. J Cancer Res Ther. 2019;15:1450-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Uyama I, Suda K, Nakauchi M, Kinoshita T, Noshiro H, Takiguchi S, Ehara K, Obama K, Kuwabara S, Okabe H, Terashima M. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi-institutional prospective single-arm study. Gastric Cancer. 2019;22:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 16. | Wang WJ, Li HT, Yu JP, Su L, Guo CA, Chen P, Yan L, Li K, Ma YW, Wang L, Hu W, Li YM, Liu HB. Severity and incidence of complications assessed by the Clavien-Dindo classification following robotic and laparoscopic gastrectomy for advanced gastric cancer: a retrospective and propensity score-matched study. Surg Endosc. 2019;33:3341-3354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Alhossaini RM, Altamran AA, Choi S, Roh CK, Seo WJ, Cho M, Son T, Kim HI, Hyung WJ. Similar Operative Outcomes between the da Vinci Xi® and da Vinci Si® Systems in Robotic Gastrectomy for Gastric Cancer. J Gastric Cancer. 2019;19:165-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Ojima T, Nakamura M, Nakamori M, Hayata K, Katsuda M, Kitadani J, Maruoka S, Shimokawa T, Yamaue H. Robotic versus laparoscopic gastrectomy with lymph node dissection for gastric cancer: study protocol for a randomized controlled trial. Trials. 2018;19:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Kiyokawa T, Hiki N, Nunobe S, Honda M, Ohashi M, Sano T. Preserving infrapyloric vein reduces postoperative gastric stasis after laparoscopic pylorus-preserving gastrectomy. Langenbecks Arch Surg. 2017;402:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (1)] |