Published online Feb 27, 2021. doi: 10.4240/wjgs.v13.i2.96
Peer-review started: October 17, 2020
First decision: December 1, 2020
Revised: December 21, 2020
Accepted: December 29, 2020
Article in press: December 29, 2020
Published online: February 27, 2021
Processing time: 109 Days and 22.3 Hours
For a long time, colorectal cancer (CRC) has been ranked among the top cancer-related mortality rates, threatening human health. As a significant post-translational modification, O-GlcNAcylation plays an essential role in complex life activities. Related studies have found that the occurrence, development, and metastasis of CRC are all related to abnormal O-GlcNAcylation and participate in many critical biological processes, such as gene transcription, signal transduction, cell growth, and differentiation. Recently, nucleotide sugar analogs, tumor-specific carbohydrate vaccine, SIRT1 longevity gene, dendritic cells as targets, and NOTCH gene have become effective methods to induce antitumor therapy. Not long ago, checkpoint kinase 1 and checkpoint kinase 2 were used as therapeutic targets for CRC, but there are still many problems to be solved. With an in-depth study of protein chip, mass spectrometry, chromatography, and other technologies, O-GlcNAcylation research will accelerate rapidly, which may provide new ideas for the research and development of antitumor drugs and the discovery of new CRC diagnostic markers.
Core Tip: This article mainly reviews the occurrence and development of O-GlcNAcylation in colorectal cancer and the corresponding therapeutic targets. After a thorough review of the literature, we analyze and summarize O-GlcNAcylation research and predict that investigations will accelerate rapidly, which may provide new ideas for the development of antitumor drugs and promote the discovery of new colorectal cancer diagnostic markers.
- Citation: Liu Y, Peng FX. Research progress on O-GlcNAcylation in the occurrence, development, and treatment of colorectal cancer. World J Gastrointest Surg 2021; 13(2): 96-115
- URL: https://www.wjgnet.com/1948-9366/full/v13/i2/96.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i2.96
Colorectal cancer (CRC) is a common highly malignant digestive tract tumor worldwide, and it is also the third leading cause of cancer-related death in the world[1]. Once distant metastasis occurs, especially liver metastasis, cancer-related mortality increases significantly[2]. So far, more than 400 post-translational modification types and more than 80000 protein-specific post-translational modification sites have been reported, including glycosylation, phosphorylation, acetylation, methylation, and ubiquitination[3]. O-GlcNAcylation is a critical glycosylation modification. Studies have shown that the change of O-GlcNAcylation is a marker of CRC, and the inactivation of functional molecules involved in O-GlcNAcylation hinders the biosynthesis of normal glycosylation structures, thus promoting the progression and metastasis of CRC[4]. Table 1 shows the summary of studies on O-GlcNAcylation and CRC in the last 5 years. Table 2 summarizes the gene targets associated with O-GlcNAcylation in CRC. This article mainly reviews the occurrence and development of O-GlcNAcylation in CRC and the corresponding therapeutic targets.
| Ref. | Year | Clinical specimens | Cell source | Methods | Conclusion |
| Madunić et al[21] | 2020 | Human CRC cell lines. | (1) Department of Surgery of the LUMC, Leiden, The Netherlands. And (2) Department of Pathology of the VUMC, Amsterdam, The Netherlands | (1) OGlycan release and analysis. And (2) Glycan structure analysis and relative quantification | Further untargeted screening of cell line O-GlcNAcylation paves the way for further exploration of the role of glycosylation in CRC development and drug response, thus identifying new anticancer antibody development targets |
| Gao et al[93] | 2020 | LS174T Tn (+), LS174T Tn (-) and LSC cells | Professor Tongzhong Ju, Emory University School of Medicine, Atlanta, United States | (1) Vector construction and cell transfection. (2) Exosome isolation and purification. (3) RNA extraction and qRT-PCR. (4) Protein extraction, deglycosylated preparation, and Western blotting (WB). And (5) Flow cytometry analysis. | CD44 in exosomes may be a potential biomarker for abnormal O-GlcNAcylation. This is the first study to show that abnormal O-GlcNAcylation can affect the expression or delivery of O-glycoproteins through exosomes, providing a new perspective for our study of treatment strategies for human colon cancer |
| Gao et al[61] | 2020 | (1) The tumor tissues were freshly acquired by surgical resection. And (2) Normal colorectal mucosa were taken at biopsy from individuals without colorectal malignancies | Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China | (1) RNA extraction and qRT-PCR. (2) WB. (3) Lentivirus-mediated COSMC transfection. (4) Transwell migration and invasion assays. And (5) Flow cytometry analysis | The increased expression of COSMC in human CRC may be caused by endoplasmic reticulum stress, which further enhances malignant tumors by activating EMT without dependence on abnormal O-GlcNAcylation |
| Kvorjak et al[54] | 2020 | (1) SW480 (ATCC® CCL-228) and HT-29 (ATCC® HTB-38) cell Lines. And (2) Archived paraffin sections of colonic biopsies and those with colitis associated colon cancer | (1)American Type Culture Collection. And (2) Department of Gastroenterology, University of Pittsburgh, United States | (1) WB. (2) Immunofluorescence confocal microscopy. (3) IHC. (4) Migration and invasion assay. (5) Peripheral blood monocyte isolation and macrophage differentiation. (6) Indirect co-culture assay. Cytokine and chemokine expression detection. (7) Gene expression profiling. Chromatin IP assay. (8) qRT-PCR. And (9) Computational modeling and simulation | To construct a computational model of a signal pathway and detect the inhibitory effect of IL-13 as a possible therapeutic method. Our findings reveal a new cell crossover between colon cells and macrophages in the inflammatory and malignant colon, which contributes to the pathogenesis of colitis-associated CRC |
| Liu et al[65] | 2019 | (1) The human CRC cell lines HCT116 and SW480. (2) The human embryonic kidney cells HEK293T. And (3) The human CRC cells LS174T (Tn-positive) | (1) American Type Culture Collection (ATCC). And (2) Dr. Tongzhong Ju of the Emory University School of Medicine, Atlanta, United States | (1) CRISPR/Cas9-mediated knockout of COSMC chaperone. (2) Flow cytometry. (3) Cell migration and invasion assays. (4) Establishment of transplantable metastatic murine models. (5) IHC. (6) Knockdown of H-Ras with shRNA. (7) Re-expression of COSMC in LS174T cells. (8) RNA extraction and qRT-PCR. (9) WB and antibodies. And (10) TCGA colon cancer dataset | Tn antigen expression (a marker of abnormal O-GlcNAcylation) may promote EMT activation by upregulation of h-RAS, possibly leading to CRC metastasis. It also suggests that anti-Tn antigen has a great prospect in tumor immunotherapy |
| Biwi et al[22] | 2019 | Human fetal colon CCD841CoN, colon adenocarcinoma HT29, and colon carcinoma HCT116 cells | LUMC, Centre for Proteomics and Metabolomics 2333ZA Leiden, Netherlands | (1) Transcriptomic. (2) IP and WB. (3) Lectin labeling and flow cytometry analysis. (4) Indirect IF and confocal microscopy. (5) Cell harvest for MS. (6) N-glycan release from cell lysates and MS analysis. (7) O-glycan release and MS analysis. And (8) Glycosphingolipid analysis by MS | OGT silencing in HT29 cells upregulates E-cadherin (the main role of epithelial to mesenchymal transition) and changes its glycosylation. Alternatively, OGT silencing interferes with glycophosphatidylcholine biosynthesis, decreasing gangliosides and the increase of globular glycosides. In conclusion, these results provide new insight into the selective regulation of complex glycosylation of O-GlcNAcylation in CRC cells |
| Zhu et al[27] | 2019 | (1) The human colon cancer tissue microarray analysis (TMA). (2) Colon cancer tissues and adjacent normal colon tissues. And (3) The CRC cell lines SW480, HCT116, LoVo, COLO205, HT29, CaCo-2 and colonic epithelial cell line NCM-460 | (1) Shanghai Tenth People’s Hospital. And (2) Cell Bank of the Chinese Academy of Sciences. Shanghai, China | (1) IHC, IF, and WB. (2) qRT-PCR. (3) Cell proliferation, Caspase 3/7 activity and soft agar colony formation assay. (4) Co-IP. (5) Chromatin IP. (6) Protein ligation assay (PLA). (7) In vitro O-GlcNAcylation of YY1. (8) Enzymatic labelling of O-GlcNAc sites. (9) Mice experiments. And (10) Bioinformatics analysis | The O-GlcNAcylation of YY1 by SLC22A15 and AANAT provoked oncogenesis of CRC cells, indicating that YY1 O-GlcNAcylation might be a potential effective target for the treatment of CRC |
| Yu et al[48] | 2019 | CRC tumor tissue | CHINA-JAPAN Union Hospital of Jilin University | (1) IHC. (2) Cell culture and treatment. (3) Lentivirus obtainment and stable cell lines establishment. (4) qRT-PCR and WB. (5) Cell counting Kit-8 (CCK-8) assay. (6) Flow cytometry assay and IP. (7) In vitro O-GlcNAcylation of ITGA5. (8) Enzymatic labelling of shi'yO-GlcNAc sites. And (9) Xenotransplantation | ITGA5 was highly expressed in CRC tissues and cells, and with increased OGlcNAcylation, its stability was higher, thereby promoting cell proliferation and tumor formation, and reducing apoptosis |
| Jiang et al[52] | 2019 | (1) CRC tissue microarrays (HCol-Ade180Sur-09). And (2) Primary CRC tissues and paired adjacent normal tissues samples | (1) Shanghai Outdo Biotech. And (2) Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi an, China | (1) Cell culture. (2) Virus packaging. (3) The construction of human full-length OGT (NM_181672). And (4) Dual-luciferase reporter assay | In CRC cells, miR-101/O-GlcNAcylation/ EZH2 signaling forms a feedback loop that promotes metastasis, providing a new insight into the basic theory of tumor metastasis and treatment strategies |
| Wu et al[93] | 2019 | Human CRC cell lines HT29, HCT116, SW480, SW620, and normal intestinal epithelial cells NCM460 | Chinese Academy of Sciences Cell Bank, China | (1) RNA extraction, reverse transcription, and qRT-PCR. (2) Cycloheximide or Thiamet-G treatment. (3) WB and Co-IP. (4) Cell viability assay. (5) Colony formation assay. (6) ICH and IF. (7) Cell migration assay. (8) mTOR agonist and inhibitor treatment. (9) Lentivirus production and infection. And (10) Tumorgenicity assay in nude mice | By strengthening the stability of RNA helicase P68 (DDX5) and the activation of AKT/mTOR signaling pathway, the elevation of O-GlcNAcylation significantly promoted the proliferation and metastasis of CRC cells, and manifest a poor prognosis |
| Ubillos et al[72] | 2018 | (1) Human CRC cell lines HT29 (ATCC HTB38™), SW480 [SW-480] (ATCC CCL228™) and SW620. [SW620] (ATCC CCL227™). (2) Adenomas with different degrees of dysplastic lesions. And (3) Normal colon tissues from distal or proximal resection margin | Department of Pathology, Maciel Hospital, Montevideo | (1) qRT-PCR. (2) IF microscopy. (3) Analysis of GalNAc-T6 expression on cancer cell lines by flow cytometry. And (4) IHC | The molecular mechanism by which GalNAc-T6 expression predicts improved prognosis in CRC patients with reduced invasiveness in CRC cells expressing GalNAc-T6 is unclear |
| Sun et al[15] | 2018 | (1) Paraffin-embedded tissue sections. And (2) Frozen tissues | Emory University School of Medicine, Atlanta, United States | (1) IF. (2) Flow cytometry. (3) FACS. (4) IHC. (5) WB. (6) T-synthase activity assay. (7) Genomic DNA preparation. (8) LOH and mutation analyses. And (9) Total RNA extraction and qRT-PCR | The loss of T-synthetase/COSMC due to genetic and epigenetic inactivation of COSMC may be responsible for the expression of Tn in human CRC cell lines and pancreatic cancer. Simultaneously, there are other mechanisms in Tn positive CRC |
| Jiang et al[14] | 2018 | (1) The human primary/metastatic CRC and adjacent normal tissues. And (2) The human CRC LS174T cell line (Tn-positive) | (1) Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China. And (2) Provided by Dr. Tongzhong Ju of Emory University School of Medicine in Atlanta, United States | (1) Immunohistochemical staining of Tn antigen. (2) Exome sequencing. (3) Analysis of DNA methylation by MALDI-TOF mass spectrometry. (4) T-synthase activity assay. (5) RNA extraction and qRT-PCR. (6) WB. (7) Lentiviral-mediated COSMC transfection. (8) Flow cytometry analysis. (9) Cell proliferation, migration, and apoptosis. And (10) Multiplex IHC staining | The expression of MUC2, which plays an essential role in intestinal function, was decreased in CRC and LS174T cells. Abnormal O-GlcNAcylation contributes to the development of CRC by directly inducing the carcinogenic characteristics of cancer cells |
| Fernández et al[94] | 2018 | CRC and HEK-293T cells | ATCC | (1) Antibodies, WB, and IF. (2) Gene expression analysis. (3) Proliferation, cell viability, and invasion assays. (4) OCR and extracellular acidification rate (ECAR). And (5) IP and proteomic assay | GCNT3 can be used in stratified CRC patients with a high risk of recurrence and as a biomarker for monitoring the treatment response. The drugs that induce the expression of GCNT3 may be potential antitumor drugs for CRC. The purpose is to reduce adverse events and overcome drug resistance, which is a necessary demand for current patients and the health system |
| Harosh-Davidovich et al[50] | 2018 | CT26 murine colon carcinoma cells and NIH-3T3 murine fibroblasts | ATCC | (1) Protein extraction. β-Catenin IP. (2) Affinity purification of β-catenin with Wheat Germ Agglutinin (WGA). (3) WB. (4) Cell motility assay. (5) OGA and OGT silencing. (6) Luciferase reporter assays. (7) qRT-PCR. And (8) In vivo orthotopic mouse model of CRC | O-GlcNAcylation may enhance the proliferation and metastasis of CRC by regulating the expression of catenin and E-cadherin, which proves the influence of O-GlcNAcylation on the poor prognosis of CRC patients |
| Venkatakrishnan et al[95] | 2017 | Midsection samples of the spiral colon of the five infected pigs and healthy controls | (1) Life Technologies, Carlsbad, CA, United States. And (2) Department of Medical Chemistry and Cell Biology University of Gothenburg | (1) MUC2 and MUC5AC IF. (2) Fluorescence in situ hybridization of formalin-fixed tissue sections. (3) Mucin isolation and purification. (4) Analysis of mucin fractions. (5) Mucin sample preparation and concentration estimation. (6) Release of O-glycans from pig colon mucins. (7) PGC-LC-MS/MS characterization of O-glycans. And (8) qRT-PCR of core enzyme expression | This study provides a platform for the study of B. hyodysenteriae and its effect on the O-GlcNAcylation of mucopolysaccharide. Polysaccharides that change with infection are candidate structures that may affect the adhesion, growth, virulence gene expression, and chemotaxis |
| Guo et al[60] | 2017 | Human colon cancer cell lines LS180, HT-29, Caco-2, LS-174, SW480, and SW620 | American Type Culture Collection (Manassas, VA, United States) | (1) Regulation of colon cancer stem cells and colon tumorigenesis by expression levels of O-GlcNAc. (2) Identification of O-GlcNAc-bound genes in HT-29 cells. (3) Gene expression profiling regulated by O-GlcNAc. (4) Tumor-suppressive functions of transcription factor MYBL1. O-GlcNAc epigenetically regulated. And (5) MYBL1 | An epigenetic mechanism may be involved in the regulation of CCSC population and colon tumor progression through the O-GlcNAcylation level. MYBL1, a transcription activator, as a downstream target, is likely to regulate CRC progression by altering O-GlcNAcylation |
| Arike et al[56] | 2017 | (1) Mucus was scraped from the small and large intestine of ConvR and GF C57BL/6 mice. And (2) The insoluble Muc2 mucin was partially purified from duodenum, mid-jejunum, ileum, proximal colon, middle colon and distal colon by repeated 6 M guanidinium hydrochloride (GuHCl) extraction | Department of Medical Biochemistry, University of Gothenburg, Sweden | (1) Partial purification of the Muc2 mucin and its oligosaccharide analysis. And (2) Proteomics analysis of epithelial cells | There was a good correlation between the abundance of OGT and muc2-O-glycan pattern along the intestine. GF mice tend to have shorter glycans and fewer enzymes involved in glycan elongation. Compared with the mice colonized with symbiotic bacteria, the demand for glycan in GF was lower. Glycan is necessary to prevent mucin degradation, but it can also be used as a nutrient source for bacteria. However, the basic mechanism and signaling pathway of host recognizing and adapting intestinal bacteria by changing the expression of glycosyltransferase is still unclear |
| Lin et al[96] | 2016 | Surgical samples of stage III CRC patients resected | Chang Gung Memorial Hospital, Taiwan | (1) Two oxaliplatin-based regimens, mFOLFOX6 and XELOX were given postoperative treatment. And (2) GALNT14 genotyping | The GALNT14 TT genotype was associated with the T4 stage and with radical resection and adjuvant oxaliplatin chemotherapy in patients with stage III CRC. In the T4 stage, CEA > 5 ng/mL or mucus histopathology subgroup, the treatment effect was poor |
| Steenackers et al[19] | 2016 | Human CRC cell lines HT29, HCT116 and CCD841CoN | - | (1) SDS-PAGE, WB, and Antibody Staining. (2) Cell adhesion assay. (3) Proliferation assays. (4) In vitro cell Survival Assays. (5) Cell migration analysis. And (6) Confocal microscopy | The increase in O-GlcNAcylation of CRC cells gave rise to proliferation and migration of CRC cells. But the potential role and mechanism of O-GlcNAcylation in CRC transfer remain unclear |
| Fuell et al[97] | 2015 | (1) C57BL/6J WT and C57BL/6 TCRδ−/− mice. And (2) Mouse small intestine and colon tissue | C57BL/6J WT (WT; acquired from Harlan Labs) and C57BL/6 TCRδ−/− (B6.129P2-Tcrdtm1Mom/J acquired from JAX Laboratories) mice were bred and maintained as specific-pathogen free (SPF) in a conventional animal facility at the University of East Anglia | (1) RNA extraction. (2) qRT-PCR. (3) Sialic acid colorimetric assay. (4) O-glycan colorimetric assay. (5) Isolation of intestinal glycans. (6) Glycan derivatization. And (7) Analysis by MALDI-LIFT-ToF/ToF MS | The role of glycosylated proteins in the regulation of epithelial cells to limit the penetration of intestinal bacteria into the mucosa during microbial community composition changes and/or the acquisition of new organisms from the environment. It is essential to understand whether intestinal O-GlcNAcylation changes through changes in microbial communities or by signaling directly to epithelial cells |
| Category | Action site | Type | Pathway | Influence |
| Tumor suppressor gene | APC[99] | CPC; Apc cell | APC/Wnt/β-catenin signaling pathway | Affect apoptosis and growth |
| GT41 | OGT[100] | HT29 cell | OGT silencing | Accelerate invasion and metastasis |
| OGA[101] | SW480 cell | (1) OGA silencing. And (2) p53 signaling canonical Pathway | (1) Upregulate PPAR, HMG-CoA synthase, and reductase. And (2) Downregulate genes of the Akt1 substrate 1, CPT1A, AIF1, AIF2, and p53 | |
| Polypeptide N- GALNT12 | T491M[102] | Germ line cell | Genetic mutations | Lead to CRC susceptibility |
| T491M[102] | ||||
| R373H[102] | ||||
| R382H[102] | ||||
| Programmed Cell Death Protein 1 (PDCD1) | PD-L1[81] | CD8+ cell | β-catenin/STT3 signaling pathway | Accelerate immune evasion and reduce apoptosis |
| GalNAc-transferases (GalNAc-Ts) | GalNAc-T3[103] | Primary cell | - | Promote differentiation and invasion |
| GalNAc-T6[23] | Wild Type (WT) LS174T cell | Cell signaling pathway | Be a potential key regulator of the malignant phenotype of CRC | |
| Tumor Necrosis Factor Receptor (TNFR) | Death Receptor (DR)-4 and DR-5[97,104] | GALNT14 genotypes and stage III Cell | (1) Apoptotic signaling pathway. And (2) DR-mediated signaling pathway | Trigger the mechanism of apoptosis. |
| G Protein-Coupled Receptors (GPCRs) | CXCR4 [105] | CXCR4+/− ApcMin/+ cell | (1) lncRNA XIST/ miR-133a-3p/RhoA signaling pathway. (2) JAK2-STAT3 inflammatory signaling pathway. And (3) CXCL12/CXCR4 axis | (1) Increase IL-1, IL-6, and TNF levels. (2) Invasion and Metastasis. (3) Recruit immune suppressive cells. And (4) Regulate RhoA expression by sponging miR-133a-3p |
| Transforming Growth Factor (TGF) | TGF-β[106] | CD44+ cell | TGF-beta signaling pathway | Enhance stem cell properties |
| TWIST1[106] | CD44+ cell | TGF-beta signaling pathway | Enhance stem cell properties | |
| TGFβR2[107] | IBD-associated cell | p21-mediated/ TGF-β signaling pathway | Cause reduced p21 activation and reduced apoptosis | |
| Rho | RhoA[108] | CXCR4-overexpressing HCT116 cell | RhoA/ROCK signaling pathway | (1) Regulate RhoA expression by sponging miR-133a-3p. And (2) Promote the formation of actin stress fiber and actin contractile force |
| Transmembrane mucins | MUC1[109] | MUC1 (+) cell | PD1/PDL1 signaling pathway. | Recruit inflammatory cytokines and evade immune surveillance. |
| MUC2[110] | Muc2/Apc and ApcMin/+ cell | (1) Wnt signaling pathway. And (2) Genetic Inactivation | Contribute to the risk of developing CRC by changes in their levels | |
| MUC4[111] | early stage (stage I and II) cell | β-catenin signaling pathway | Predict poor survival among patients | |
| MUC13[112] | colitis-associated cell | β-catenin signaling pathway | Increase nuclear translocation of β-catenin and drive the development, progression, invasion and immunosuppression |
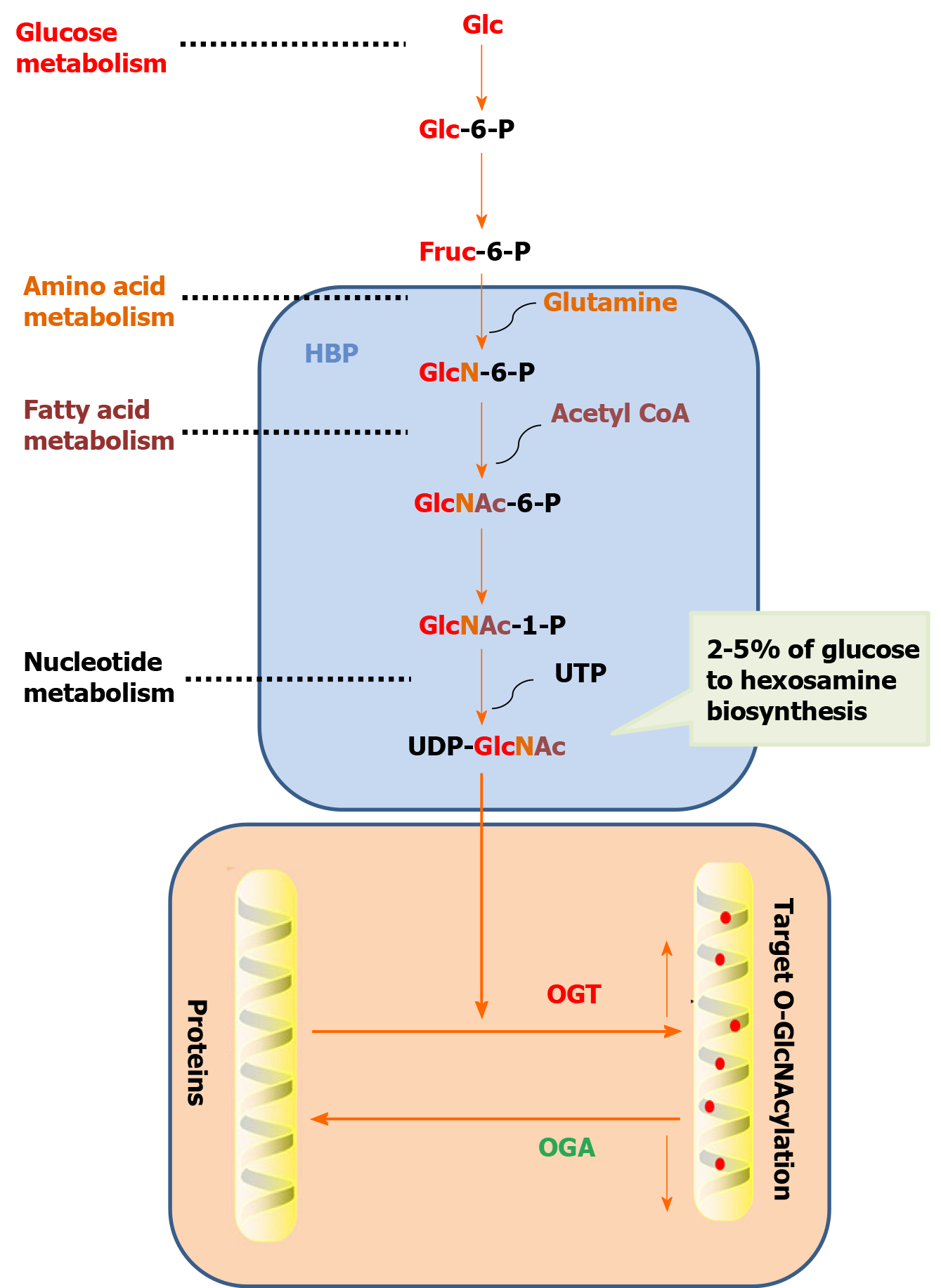
O-GlcNAcylation refers to a single N-acetylglucosamine (GlcNAc) with O-glycoside bond to a protein serine or threonine hydroxyl oxygen atom to modify the protein in the nucleus and cytoplasm[5]. O-GlcNAcylation precursors include Tn, sialic acid Tn (sTn), core1, core2, core3, core4, and N-acetyllactosamine (Table 3). O-GlcNAcylation is a dynamic protein modification process. The addition and removal of modification groups are accomplished by O-linked N-acetylglucosamine transferase (OGT) and N-acetylglucosaminidase (OGA), respectively. Uracil-5 '-diphosphate-N-GlcNAc (UDP-GlcNAc) is the only donor for the O-GlcNAcylation of protein[6]. When the content of glucose, glucosamine, or free fatty acid increase under hypoxia, heat shock, radiation, and other stress conditions or when the key enzyme in the hexosamine pathway is overexpressed, glucose is transformed into glucose-6-phosphate and then fructose-6-phosphate under the action of hexokinase. Most fructose-6-phosphate enters the glycolysis pathway, and approximately 2%-5% fructose-6-phosphate enters the hexosamine pathway (Figure 1). The end product UDP-GlcNAc is synthesized with the participation of glutamine, which raises the overall O-GlcNAcylation level of intracellular protein[7].
| Basic structural unit | Chemical formula |
| Tn antigen | GalNAcα-O-Ser/Thr |
| Stn antigen | Siaα2-6GalNAcα-O-Ser/Thr |
| Core 1 (T antigen) | Galβ1-3GalNAcα-O-Ser/Thr |
| Core 2 | GlcNAcβ1-6(Galβ1-3)GalNAcα-O-Ser/Thr |
| Core 3 | GlcNAcβ1-3GalNAcα-O-Ser/Thr |
| Core 4 | GlcNAcβ1-6(GlcNAcβ1-3)GalNAcα-O-Ser/Thr |
| LacNAc | Galβ1-3GlcNAc(Type1) or Galβ1-4GlcNAc(Type 2) |
| H-antigen | Fucα1-2Gal |
Recent studies have shown that O-GlcNAcylation is related to tumorigenesis and the development and initiation of malignant biological phenotypes[8]. It can regulate various physiological and pathological processes by affecting the function, location, conformation, and degradation of target proteins[9], and it is involved in many processes, such as cell signal transduction, gene transcription, cell division, metabolism, and cytoskeleton regulation[10]. More importantly, the change of O-GlcNAcylation is related to cell development, mitosis, proliferation and survival, and tumor metastasis[11]. O-GlcNAcylation can enhance the stability and transcriptional activity of some circadian clock proteins and affect the phosphorylation and cellular localization of other clock proteins. Inhibition of the O-GlcNAcylation of circadian clock proteins leads to the decline of cell rhythms and the downregulation of multiple circadian genes. O-GlcNAcylation can regulate the cycle length and amplitude of circadian rhythm[12].
O-GlcNAcylation is closely related to cell growth and development[13]. Abnormal changes of O-GlcNAcylation may lead to tumor transformation of CRC cells[14]. In addition to genetic, metabolic, and proteomic characteristics, O-GlcNAcylation is a major factor in colon differentiation and CRC occurrence[15]. Studies have shown that the malignant transformation changes the O-GlcNAcylation mechanism of cells and affects the function of carcinogenic receptors involved in the control of cell proliferation and differentiation[16]. In CRC cells, the activation of Thr58 of proto-oncogene c-Myc may increase the level of O-GlcNAcylation, and the increase in O-GlcNAcylation level can inhibit the phosphorylation modification at the same site, thus blocking the degradation of c-Myc, maintaining the high-level expression of c-Myc, and promoting the proliferation and differentiation of CRC cells[17]. The O-GlcNAcylation of tumor suppressor gene p53 at the Ser149 site also promotes the growth of tumor cells[18]. Since glucose is the precursor substrate in the reaction leading to UDP-GlcNAc, the level of O-GlcNAcylation is also increased along with the increase in glucose level in CRC cells, which is accompanied by a synchronous increase of glutamine fructose aminotransferase and OGT[19]. In vitro studies showed that OGT could be repositioned to the deoxyribonucleic acid (DNA) damage site and catalyze the O-GlcNAcylation reaction of histone H2AX, the mediator of DNA damage checkpoint 1 and histone H2A. O-GlcNAcylation enhances double-strand break repair in vivo and in vitro and promotes the proliferation of cancer cells[20]. OGT knockout is lethal in embryonic stem cells, embryonic fibroblasts, or tissues[21]. Therefore, in the process of tumorigenesis, O-GlcNAcylation is likely to promote cell proliferation by regulating the cell cycle, leading to carcinogenesis[22]. The fluctuation of O-GlcNAcylation has been correlated with the cell cycle—the lowest in the M phase, and the highest in G1/S and G2/M phases[23]. Peng et al[24] reported that OGT-mediated O-GlcNAcylation plays an essential role in the activation of the Hippo Yap pathway and found a new mechanism of extracellular nutrition signal regulating the Hippo pathway and tumor growth[24].
The transcription factor Yin Yang 1 (YY1) plays an important role in different biological processes such as embryogenesis, cell proliferation, and cancer progression[25]. It has been proved to be a target for various post-translational modifications, including ubiquitination, acetylation, O-GlcNAcylation, S-nitrosation, etc. Ample data show that YY1 expression is not regulated in many cancers, and most of them are related to the clinical behavior of different types of tumors[26]. Zhu et al[27] Have shown that YY1 stimulates the occurrence of CRC and increases the protein stability of YY1 through O-GlcNAcylation, which helps it play a proto oncogenic role in CRC[27].
The health of the body is regulated by several molecules and biochemical networks responsible for maintaining homeostasis in cells and tissues. However, an imbalance of homeostasis often occurs in response to various endogenous and environmental pressures, resulting in the accumulation of damage and increased sensitivity to diseases[28]. O-GlcNAcylation can dynamically regulate intracellular metabolism and signaling pathways, thus enhancing the resistance of CRC cells to various stimuli from the environment and itself. O-GlcNAcylation signal is considered essential stress and metabolic sensor. Increasing OGT expression or inhibiting OGA expression can enhance the stress tolerance of CRC cells; knockout of OGT or blocking of the hexosamine biosynthetic pathway will lead to apoptosis[29]. Excessive nutritional intake and hyperglycemia were thought to be the sources of the hexosamine biosynthetic pathway and contribute to the abnormally elevated O-GlcNAcylation level in CRC cells[30]. Therefore, the generally elevated O-GlcNAcylation level in CRC was considered to be a key factor in the occurrence and development of CRC[31]. Han et al[32] found that under pressure stimulation of genotoxicity, oxidation, and metabolism, the O-GlcNAcylation of SIRT1 is significantly increased[32]. The circulatory imbalance of O-GlcNAcylation on tumor-related targets, including DNA damage repair-related proteins and stress-related pathways, may contribute to tumorigenesis.
As a molecular chaperone of the endoplasmic reticulum, core 1 β3-galactosyltransferase (COSMC) may be induced by endoplasmic reticulum stress in cancer cells. It is associated with many aspects of the endoplasmic reticulum. Under endoplasmic reticulum stress, many chaperones are upregulated in cancer, including heat shock proteins and lectin-like chaperones[33]. Related studies have shown that Tn and sTn antigens are expressed in many types of cancer, including CRC, lung cancer, bladder cancer, cervical cancer, ovarian cancer, thyroid cancer, and breast cancer[34]. The findings in CRC cell lines and specimens suggest a more complex mechanism of Tn expression. The increase in the Tn antigen may also regulate the production of T-synthase/COSMC through a feedback loop[35]. In addition, the expression of the Tn antigen may be caused by the maladjustment of other enzymes, such as acetylgalactosaminyl-O-glycosyl-glycoprotein beta-1,3-N-acetylglucosaminyltrans-ferase. This enzyme transforms Tn antigens parallel to the T-synthase/COSMC complex in the gastrointestinal tract[36]. It inhibits metastatic potential and is downregulated in CRC cancer[37]. Mice lacking core-3-derived O-glycans were more susceptible to CRC[38].
O-GlcNAcylation regulates glycolysis of cancer cells through hypoxia-inducible factor-1 (HIF-1a) and its transcription target GLUT1. The reduction of O-GlcNAcylation increased a-ketoglutarate, hydroxylated HIF-1, and changed the levels of the von Hippel Lindau protein, resulting in the degradation of HIF-1a. In CRC cells, hypoxia can also upregulate UGT-1 transcription[39]. UGT-1 transports UDP-Gal and UDP-GalNAc, suggesting that hypoxia regulates O-GlcNAcylation by affecting the nucleotide sugar pool[40]. Reducing the expression of O-GlcNAcylation in cancer cells can induce the activation of endoplasmic reticulum stress and apoptosis of cancer cells through CCAAT/enhancer binding protein homologous protein. Cancer cells may use the O-GlcNAcylation pathway of a nutritional sensor to regulate critical metabolic factors, such as HIF-1a and c-Myc, which is necessary for maintaining glycolysis flux, rapid cell growth, and survival[41].
Uncontrolled cell division, extensive cell survival, and promotion of angiogenesis are characteristics of tumorigenesis[42]. O-GlcNAcylation affects the proliferation, recurrence, metastasis, and malignant transformation of tumor cells[43]. The expression of the sTn antigen in m/z691 of recurrent tumors was significantly higher than in non-recurrent tumors, i.e. overexpression of Tn and sTn antigens was a characteristic feature of advanced and poorly differentiated CRC[44]. The overexpression of the sTn antigen is associated with the upregulation of ST6GALNAC1 and the decrease of core 1 synthase gene (C1GALT1) in the mucinous CRC cell line LS174T[45]. Analysis of mucin glycosylation of recurrent tumors showed that the proportion of core-3 O-glycan was higher than in non-recurrent tumors, while the expression of core-1 O-glycan was weaker. ST6GalNAc II controlled the sialic acidolysis of the T antigen. Overexpression of T antigen messenger ribonucleic acid was associated with a low survival rate of CRC patients with lymph node metastasis[46]. The changes of O-GlcNAcylation in CRC include a decrease of the number and length of apolipoprotein carbohydrate side chains, loss of normal expression antigen, the reappearance of new antigen, and expression of blood group incompatible antigen[47]. In RKO and SW620 cells, integrin α5 (ITGA5) protein can be degraded by O-GlcNAcylation, and the increase of O-GlcNAcylation significantly slows down the degradation of ITGA5 protein, while the decrease of O-GlcNAcylation accelerates the degradation of ITGA5 protein, which indicates that the O-GlcNAcylation of ITGA5 endows ITGA5 enhancer with the ability to promote the progression of CRC[48].
Cancer cell invasion and metastasis require many steps, including epithelial-mesenchymal transition (EMT)[49]. This phenomenon is usually caused by the activation of β-catenin/Snail 1, which is in turn caused by abnormal signals, and then affects the downstream protein transcription. Studies have shown that O-GlcNAcylation reduces the expression of E-cadherin on the cell surface by directly modifying β-catenin, thus promoting tumor metastasis[50]. In two CRC cell lines (HCT116 and SW480) with stable and high expression of COSMC, ZO-1, another epithelial marker, was proved to be significantly reduced and the expression of several mesenchymal cell markers was increased, which fully reflects the characteristics of EMT[51]. The elevated level of O-GlcNAcylation in metastatic CRC tissues and cell lines may contribute to EMT by enhancing the stability and function of enhancer of zeste homolog 2 protein, demonstrating strong lymph node metastasis potential and a lower overall survival rate. The O-GlcNAcylation and H3K27me3 modification in the miR-101 promoter region inhibited the transcription of miR-101, leading to the upregulation of OGT and enhancer of zeste homolog 2 in metastatic CRC, thus forming a vicious circle[52]. The initiation of mucin O-GlcNAcylation in the endoplasmic reticulum promotes the invasiveness of cancer cells. Fibroblast growth factor receptor 2 (FGFR2) and its subtypes are highly expressed in CRC. C1GALT1 can regulate the O-GlcNAcylation of FGFR2 and its downstream signal molecule ERK, and promote tumor growth, metastasis, and angiogenesis[53]. Recent studies have shown that CCL17 and interleukin-13 (IL-13) produced by the Co-culture of M2 macrophages and colon cells in CRC could activate the AKT and STAT6 oncogenic pathways, among others. CCL17 and IL-13 also induced abnormal overexpression of ST6GALNAC1 glycosyltransferase, which resulted in increased expression of the tumor glycoside form mucin 1 (MUC1)-sTn[54]. After inhibition of MUC1, O-GlcNAcylation by C1GALT1, E-cadherin, and Fas in MUC1 positive cells was significantly increased. After the inhibition of C1GALT1, the activation of caspase-8 in MUC1 positive cells induced by exogenous Fas-L was also considerably increased. Therefore, the extensive O-GlcNAcylation of the MUC1 extracellular domain contributes to antianoikis by preventing the activation of cell surface loss initiation molecules and emphasizes the importance of O-GlcNAcylation in CRC progression and metastasis[55].
With the in-depth study of protein chip, mass spectrometry, chromatography, and other technologies, the treatment related to O-GlcNAcylation has made great progress. Table 4 summarizes the therapeutic targets associated with O-GlcNAcylation and CRC.
| Target/Drug | Binding site/Pathway | Result |
| Ac-5SGlcNAc[58] | HBP | Form UDP-5SGlcNAc, and greatly decrease O-GlcNAc levels in CRC |
| BGJ398[59] | FGF/FGFR2 signaling pathway | Block the effect of C1GALT1 on the malignant behavior of CRC cells. C1GALT1 could be a target for treating CRC |
| MYBL1[60] | - | Be involved in the suppression of gene expression in CRC |
| sTn antigen[61] | Core 1-mediated O-glycans biosynthesis pathway | Synthesize more truncated CD44 proteins |
| Tn antigen[47,63] | T-synthase/COSMC pathway | Play an essential part of the design of therapeutic carbohydrate conjugate antitumor vaccine |
| mAbs B72.3 and CC49[113] | TAG-72 | To help develop anti-Tn targeting vaccines |
| VVA and PNA lectins[68] | FGFR2 | Targeting C1GALT1 is a promising strategy to reduce the number of CRC tumor stem cells |
| SIRT1[71] | GAPDH-C150 | The binding of SNO-GAPDH to SIRT1 was selectively prevented in vitro |
| GalNAc-T6[23] | HLA class II histocompatibility antigen chain | Block the interaction between CD74 and MHC class II molecules |
| NHEJ inhibitors[73] | (1) HBP; (2) NHEJ | Regulate DNA repair |
| mAbs[74] | (1) ADCC; (2) Complement activation | Enhance the function of effector and remove certain glycan. |
| DC-SIGN[75] | MUC1 and CEA proteins | Improves tumor-specific T cell response and long-term tumor regression |
| β- catenin inhibitor KYA1797K[81] | β-catenin/STT3 signaling pathway | Reduce the stability of PD-L1, inhibit immune evasion, induce the apoptosis of Cancer stem cells, and promote the development of immunotherapy for CRC |
| Sunitinib[88] | NOTCH 1 signaling pathway/NOTCH 1 signaling pathway | Inhibit the expression of SW480. However, the specific regulatory mechanism needs to be further studied |
| Baicalin and Curcumin[88] | NOTCH 1 signaling pathway | Inhibit the proliferation and promote the apoptosis of CRC SW480 cells |
| CHK1/2 inhibitors | DNA damage response pathway | Undermine OGT stability and suppress replication, mitosis and cytokinesis of CRC cell |
| DR-5 and DR-4[97] | (1) DR-mediated signaling pathway; (2) Tenovin-6 | Enhance the cytotoxic effect of oxaliplatin in CRC by up-regulating |
Many studies have shown that O-GlcNAcylation and OGT may be potential targets for treating cancer[56]. These include analogs of the UDP-GlcNAc pathway and OGT inhibitors[57]. Research has identified the biosynthetic Ac-5SGlcNAc, which can act as nucleotide sugar analogs in cells and thus block the function of OGT[58]. O-GlcNAcylation may also be used as a biomarker with diagnostic or prognostic value in clinical practice. Therefore, OGT and O-GlcNAcylation in urine or blood may be helpful in the diagnosis or evaluation of treatment response. Further research on O-GlcNAcylation will continue to expand our understanding of cancer and other diseases.
OGT inhibitors may also provide new and effective drugs for treating O-GlcNAcylation. The evidence shows that BGJ398 can significantly block the effect of C1GALT1 on the malignant behavior of CRC cells and suggest that the FGF/FGFR2 signaling pathway is involved in the phenotype changes mediated by C1GALT1. Therefore, it is reasonable to speculate that other molecules may cooperate with FGFR2 to mediate the effect of C1GALT1. The identification of other receptor substrates of C1GALT1 by glycoproteomics will help reveal the detailed mechanism and behavior of C1GALT1 in regulating cancer[59]. MYB proto-oncogene like 1, a transcription activator, as a downstream target, is likely to regulate CRC progression by altering O-GlcNAcylation. Related studies reveal the molecular mechanism of O-GlcNAcylation in the occurrence and progression of CRC and supports OGT as a promising target for CRC therapy[60]. These findings provide new insights into the role of O-GlcNAcylation in CRC and suggest that C1GALT1 could be a target for treating CRC.
Core 1-mediated destruction of O-GlcNAcylation depends on the exosome secretion of glycoproteins, which conversely regulates the expression of CD44 in human CRC cells and tumor-derived exosomes. Therefore, a high proportion of CD44 positive exons can be used as biomarkers to indicate abnormal O-GlcNAcylation[61]. Recently, Tn antigen has attracted extensive attention in the field of tumor biology and clinical oncology. It may be a useful diagnostic marker because it is rarely found in normal tissues, but it is widely expressed in various adenocarcinoma and some malignant hematopoietic cells[62]. In the first investigation of the expression of T-synthase/COSMC pathway and enzyme activity in human CRC specimens, the expression of the Tn antigen was measured. Tn antigen increased in the transmembrane region and tumor. This not only supports the report that Tn and sTn antigens are prevalent in CRC samples but also suggests that the occurrence of intracellular Tn and sTn is an early event of CRC. Other studies have also confirmed the existence of Tn antigen in gastrointestinal nonmalignant lesions such as polyps and abnormal crypt lesions[63]. Therefore, Tn and sTn antigens may become potential biomarkers for prediction or early detection of human CRC, which can lead to an effective antitumor treatment response. The Tn antigen is related to the metastasis of tumor cells, and the invasiveness of the tumor is directly related to the density of antigens, such as the degree of tissue diffusion and vascular invasion[64]. Therefore, this antigen is an essential part of the design of therapeutic carbohydrate conjugate antitumor vaccine for selective tumor eradication[65].
Complex carbohydrate modifications occur mainly on proteins on the surface of cells, which can be directly contacted by antibody-based therapies, leading to endogenous immune effector cells to tumor lesions. Evidence shows that tumor growth ability exists in a small subset of cells called cancer initiation cells or cancer stem-like cells[66]. These cells are more resistant to chemotherapy and radiotherapy[67]. Therefore, eliminating cancer stem cells is the key to effective treatment. Inhibition of the expression of C1GALT1 can reduce the ability of CRC cells to form spheres, which indicates that targeting C1GALT1 is a promising strategy to reduce the number of cancer stem cells in CRC. FGFR2 can be inhibited by Vicia villosa and peanut agglutinin lectins. The removal of sialic acid enhances the binding of FGFR2 to FGFR2, which indicates that FGFR2 carries short O-glycans such as Tn and sTn in colon cancer cells. In addition, the expression of C1GALT1 can affect the binding of Vicia villosa to FGFR2 and regulate the phosphorylation of FGFR2, which further proves that FGFR2 is O-GlcNAcylated. The NETOGLYC4.0 server predicted four potential O-GlcNAcylation sites in the extracellular domain of FGFR2[68]. The results showed for the first time that FGFR2 is O-GlcNAcylated, and O-GlcNAcylation can regulate the activity of FGFR2 in CRC cells.
Short O-glycans are usually found in CRC. These carbohydrates are associated with tumor progression and have been developed as vaccines for cancer treatment[69]. Similarly, antibody-based therapies designed explicitly for tumor-associated O-GlcNAcylation will significantly increase the effective concentration of conjugated toxic antitumor drugs, thus enhancing the specificity of chemotherapy methods. In addition, vaccine methods using tumor-specific carbohydrates have the same ability to induce anticarbohydrate antibodies that promote immune memory against common tumor O-GlcNAcylation, thereby enhancing immune monitoring[70]. Although vaccination may indeed induce significant immunity to cancer-related carbohydrate antigens, such immunity may create selective pressures to develop tumor lesions independently of specific carbohydrate antigens.
Although the relevance of SIRT1 as a longevity gene has always been controversial, silent mating type information regulation 2 homolog (SIRT1) promotes cells to protect cells in the stress response by regulating the activities of their target enzymes and transcription factors, thus affecting their lifespan[71]. Small molecule SIRT1 activation can physically delay aging and prevent age-related diseases. Regulating SIRT1 O-GlcNAcylation is a potential new drug to prevent aging diseases and prolong a healthy life span. Lavrsen et al[23] provided comprehensive information on cellular, transcriptional, and glycoproteomic changes caused by the expression of a single GalNAcT[23]. It is important to investigate whether GalNAcT6 affects the structure of secretory organs (through its effect on melanoma inhibitory activity member 3) and glycan structure, which is deemed a factor in cell proliferation and invasion in other cell culture systems[72]. To be more precise, this study highlights the role of O-GlcNAcylation in health and CRC, and much remains to be found.
Cancer metabolic reprogramming associated with increased glucose and glutamine metabolic characteristics in advanced cancer is usually attributed to a higher demand for metabolic intermediates required for the rapid growth of tumor cells. Glucose and glutamine metabolites also act as substitutes for chromatin modifiers and cofactors of other protein post-translational modifying enzymes in cancer cells. In addition to the epigenetic mechanisms that regulate gene expression, many chromatin-modifying factors also regulate DNA repair. O-GlcNAcylation is a common way to increase glucose and glutamine metabolism, drive double-strand break repair, and resistance to treatment-induced cancer cell senescence[73]. Proteomic analysis showed that the DNA damage response pathway was abolished in O-GlcNAcylation-altered CRC cells. Promoting O-GlcNAcylation by targeting O-GlcNAc or simply treating animals with GlcNAc can protect CRC xenografts against radiation. In turn, the inhibition of O-GlcNAcylation by blocking the O-GlcNAc transferase activity resulted in delayed double-strand break repair, decreased CRC cell proliferation, and increased cell senescence[74]. Therefore, the critical link between cancer metabolic reprogramming, DNA damage response, and aging provides a basis for evaluating targeted O-GlcNAcylation drugs to restore CRC sensitivity to radiotherapy. Considering the complexity of glycosylation sites and the complexity of glycosylation sites, the complete characterization of tumor glycans and glycoproteomics is a challenge.
The inclusion of lectins in a microarray can be used for high-throughput analysis of glycoconjugates, and their application in clinical samples can be used as a new diagnostic tool for cancer. Lectins can be used not only to analyze cancer-related markers in serum but also to monitor O-GlcNAcylation changes in blood cells or to conduct histochemical staining in tissue biopsy. The use of these tools for immunohistochemistry and cell counting will enhance the systematic analysis of sugar code in CRC patients and reveal the immune status of the tumor microenvironment[75]. Glycobioinformatics is essential for revealing the sugar coding, which can better understand the polysaccharide lectin interaction between tumor and immune system, which may lead to the design of improved antitumor immunotherapy. For example, this information can be used to develop new tumor carbohydrate-specific antibodies or to improve future combination therapies. The development of new strategies for tumor glycocoding may ultimately benefit patients who do not respond to current immunotherapy regimens. Therapeutic modifications encoded by glucose, such as blocking sialic acid with metabolic mimics or glycosidases attached to tumor-targeted antibodies, inhibit tumor growth[76]. This inhibition is due to the enhanced antitumor response mediated by T cells and the increased activity of natural killer cells, which may be due to the reduction of SIGLECs triggering on these cells. The specific blocking antibody against lectin receptor or tumor-specific polysaccharides can selectively inhibit the interaction between leptin and polysaccharide, which can be used locally as a new immunotherapy.
As a target, dendritic cells (DCs) have recently become an interesting method to induce antitumor immunity. Targeting DCs with glycan-modified tumor antigens can improve the tumor-specific T cell response and long-term tumor regression. The power of cancer vaccines is demonstrated when combined with immune checkpoint blocking[77]. Some studies have shown that changes in O-GlcNAcylation during tumorigenesis can help deceive the immune system. For example, CRC cells express a large number of MUC1 and carcinoembryonic antigen (CEA) proteins that exhibit abnormal O-GlcNAcylation, are recognized by C-type lectin receptors expressed on DCs, and regulate the innate and adaptive tumor immune responses. Therefore, the interaction between macrophage galactose lectin (MGL) and Tn epitope on MUC1 indicates that DCs drive the type 2 helper T cells-mediated response and are not involved in tumor eradication compared with Th1 effector cells[78]. DC-dependent uptake of MGL containing Tn epitope antigen enhanced major histocompatibility complex II and I expression and triggered T cell responses[79]. Similar to MUC1/MGL interaction, tumor-specific CEA and CEACAM1 proteins that specifically express Lewis antigens promote their recognition through dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin, thus impairing DC maturation and increasing the secretion of the immunosuppressive cytokine IL-10. In the tumor microenvironment, both membrane-bound and soluble forms of CEA were expressed, which showed O-GlcNAcylation changes. Since CEA can be detected in the sera of patients with CRC, it can be speculated that this glycoprotein will damage the function of DCs far away from the tumor. O-GlcNAcylation of malignant tumors can also inhibit the function of natural killer cells, cytotoxic T cells, and macrophages from escaping their responses[80]. In the future, the individualized carbohydrate code of the tumor may destroy the immune state of the tumor by changing the O-GlcNAcylation of CRC and become the target of interference of glycan checkpoint. Alternatively, these new checkpoint blockers can be combined with DC-targeted vaccination strategies to achieve the best success of future immunotherapy regimens.
Tumor growth is accompanied by tumor escape of the immune system, which is promoted by immune checkpoint molecules such as programmed cell death protein 1 (PD-1)[81]. PD-1 evades antitumor immunity by inhibiting T cell activation signals. The PD-1/PD-ligand 1 blockade enhanced the induction, expansion, and survival of antitumor T cells[82]. However, although abnormal tumor O-GlcNAcylation changes the way the immune system perceives tumors and can induce immunosuppressive signals through glucose-binding receptors, the role of tumor O-GlcNAcylation in immune evasion is largely ignored. Therefore, the specific polysaccharide markers found on tumor cells can be considered a new type of immune checkpoint. Most cancers have O-GlcNAcylation. The glycosylation chain becomes shorter, which is characterized by the increased expression of Tn antigen and the loss of Core-2 structure[83]. O-GlcNAcylation changes the interaction between O-glycans and carbohydrate-binding proteins and leads to the malignant phenotype, metastasis, and immune escape[84]. Simultaneously, O-GlcNAcylation of tumor protein produces new antigens, which can be used as targets of tumor-specific T cells[85]. Obviously, abnormal O-GlcNAcylation adds another layer of complexity to the new antigenicity of the tumor, and the properties of tumor-infiltrating T cells need further study. Future experiments aimed at characterizing glycopeptide major histocompatibility complex I complexes expressed by tumor cells may identify new tumor-specific epitopes that can be used as a target for glycopeptide-specific T cells[86]. Epigenetic mechanism may be involved in the regulation of CRC progression through O-GlcNAcylation.
Studies have shown that NOTCH signaling plays an essential and diverse role in the regulation of CRC proliferation, and there may be a potential regulatory mechanism[87]. It was shown that baicalin and curcumin induced apoptosis of CRC cell line SW480 by inhibiting the NOTCH 1 signaling pathway[88]. Chu et al[89] demonstrated that overexpression of NOTCH 4 could inhibit the proliferation, migration, and invasion of CRC cells and induce apoptosis[89]. NOTCH signaling can regulate the apoptosis of CRC through O-GlcNAcylation and is an indicator to predict the overall survival rate of CRC patients[90]. Shutinib is a multi-target antitumor signal transduction pathway. The results showed that with the increase of sunitinib concentration, the expression of NOTCH 1 protein in SW480 cells was significantly decreased. The expression levels of NOTCH 4, p53, N-MYC downstream regulated gene 4, and B cell lymphoma-associated X were significantly increased, suggesting that sunitinib inhibited SW480 by inhibiting the expression of NOTCH 1 protein and promoting the expression of NOTCH 4, p53, N-MYC downstream regulated gene 4, and B cell lymphoma-associated X. However, the specific regulatory mechanism needs to be further studied[87]. Although great progress has been made since the first sequencing of the NOTCH gene, some aspects of the NOTCH signaling pathway, although widely studied so far, remain to be fully understood[91]. O-GlcNAcylation is an essential modification of NOTCH activation and regulation. The effect of O-GlcNAcylation on NOTCH indicates the direct effect of O-GlcNAcylation on protein function. The ability to manipulate these glycans to regulate the activity of NOTCH may provide a new research tool or potential treatment of NOTCH-related diseases[92].
CHK1 and CHK2 are still critical therapeutic targets. Targeting CHK1 and CHK2 destroys the stability of OGT and contributes to the therapeutic potential of targeting these kinases. O-GlcNAc acts as a critical regulator of intestinal stem cells/enteroblasts homeostasis by regulating DNA damage response. In particular, OGT can induce and respond to the DNA damage response pathway. Stress first causes DNA damage, induces a DNA damage response pathway, and then increases O-GlcNAc. However, there is only one gene mutation in O-GlcNAcase, the DNA damage response is induced, and O-GlcNAc is necessary for stress-mediated DNA damage response[43].
As a post-translational modification, O-GlcNAcylation plays a complex regulatory role in CRC[93-112]. Although the research on the relationship between O-GlcNAcylation and CRC has made progress, it is obvious that only the tip of the iceberg has been revealed. However, the close relationship between O-GlcNAcylation and the abnormal metabolism of CRC is undeniable. Therefore, an in-depth study of O-GlcNAcylation is imperative. Future research will provide new ideas and directions for the development of antitumor drugs and the discovery of new diagnostic markers and therapeutic targets of CRC.
Thanks to all the teachers of North Sichuan Medical College and Sichuan Mianyang 404 Hospital. We thank LetPub (http://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Sabelnikova EA, Sipos F S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
| 1. | Weinberg BA, Marshall JL, Salem ME. The Growing Challenge of Young Adults with Colorectal Cancer. Oncology (Williston Park). 2017;31:381-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3250] [Article Influence: 650.0] [Reference Citation Analysis (2)] |
| 3. | Cattaneo A, Chirichella M. Targeting the Post-translational Proteome with Intrabodies. Trends Biotechnol. 2019;37:578-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Nguyen AT, Chia J, Ros M, Hui KM, Saltel F, Bard F. Organelle Specific O-Glycosylation Drives MMP14 Activation, Tumor Growth, and Metastasis. Cancer Cell 2017; 32: 639-653. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Hanover JA, Chen W, Bond MR. O-GlcNAc in cancer: An Oncometabolism-fueled vicious cycle. J Bioenerg Biomembr. 2018;50:155-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011;469:564-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 7. | Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, van Aalten DM, Cantrell DA. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat Immunol. 2016;17:712-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 272] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 8. | Hann SR. Role of post-translational modifications in regulating c-Myc proteolysis, transcriptional activity and biological function. Semin Cancer Biol. 2006;16:288-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Shukla HD, Vaitiekunas P, Cotter RJ. Advances in membrane proteomics and cancer biomarker discovery: current status and future perspective. Proteomics. 2012;12:3085-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Lewis BA, Hanover JA. O-GlcNAc and the epigenetic regulation of gene expression. J Biol Chem. 2014;289:34440-34448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol. 2015;208:869-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 458] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 12. | Ma YT, Luo HJ, Jin Q, Jin Q, Zhang SJ, Li JD. Roles of O-GlcNAcylation on the regulation of circadian rhythms. Shengming Kexue. 2015;27:1403-1408. [DOI] [Full Text] |
| 13. | Berg KCG, Eide PW, Eilertsen IA, Johannessen B, Bruun J, Danielsen SA, Bjørnslett M, Meza-Zepeda LA, Eknæs M, Lind GE, Myklebost O, Skotheim RI, Sveen A, Lothe RA. Multi-omics of 34 colorectal cancer cell lines - a resource for biomedical studies. Mol Cancer. 2017;16:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 14. | Jiang Y, Liu Z, Xu F, Dong X, Cheng Y, Hu Y, Gao T, Liu J, Yang L, Jia X, Qian H, Wen T, An G. Aberrant O-glycosylation contributes to tumorigenesis in human colorectal cancer. J Cell Mol Med. 2018;22:4875-4885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Sun X, Ju T, Cummings RD. Differential expression of Cosmc, T-synthase and mucins in Tn-positive colorectal cancers. BMC Cancer. 2018;18:827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 2177] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 17. | Zhang X, Ma L, Qi J, Shan H, Yu W, Gu Y. MAPK/ERK signaling pathway-induced hyper-O-GlcNAcylation enhances cancer malignancy. Mol Cell Biochem. 2015;410:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | de Queiroz RM, Madan R, Chien J, Dias WB, Slawson C. Changes in O-Linked N-Acetylglucosamine (O-GlcNAc) Homeostasis Activate the p53 Pathway in Ovarian Cancer Cells. J Biol Chem. 2016;291:18897-18914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Steenackers A, Olivier-Van Stichelen S, Baldini SF, Dehennaut V, Toillon RA, Le Bourhis X, El Yazidi-Belkoura I, Lefebvre T. Silencing the Nucleocytoplasmic O-GlcNAc Transferase Reduces Proliferation, Adhesion, and Migration of Cancer and Fetal Human Colon Cell Lines. Front Endocrinol (Lausanne). 2016;7:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Efimova EV, Appelbe OK, Ricco N, Lee SS, Liu Y, Wolfgeher DJ, Collins TN, Flor AC, Ramamurthy A, Warrington S, Bindokas VP, Kron SJ. O-GlcNAcylation Enhances Double-Strand Break Repair, Promotes Cancer Cell Proliferation, and Prevents Therapy-Induced Senescence in Irradiated Tumors. Mol Cancer Res. 2019;17:1338-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Madunić K, Zhang T, Mayboroda OA, Holst S, Stavenhagen K, Jin C, Karlsson NG, Lageveen-Kammeijer GSM, Wuhrer M. Colorectal cancer cell lines show striking diversity of their O-glycome reflecting the cellular differentiation phenotype. Cell Mol Life Sci. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Biwi J, Clarisse C, Biot C, Kozak RP, Madunic K, Mortuaire M, Wuhrer M, Spencer DIR, Schulz C, Guerardel Y, Lefebvre T, Vercoutter-Edouart AS. OGT Controls the Expression and the Glycosylation of E-cadherin, and Affects Glycosphingolipid Structures in Human Colon Cell Lines. Proteomics. 2019;19:e1800452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Lavrsen K, Dabelsteen S, Vakhrushev SY, Levann AMR, Haue AD, Dylander A, Mandel U, Hansen L, Frödin M, Bennett EP, Wandall HH. De novo expression of human polypeptide N-acetylgalactosaminyltransferase 6 (GalNAc-T6) in colon adenocarcinoma inhibits the differentiation of colonic epithelium. J Biol Chem. 2018;293:1298-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Peng C, Zhu Y, Zhang W, Liao Q, Chen Y, Zhao X, Guo Q, Shen P, Zhen B, Qian X, Yang D, Zhang JS, Xiao D, Qin W, Pei H. Regulation of the Hippo-YAP Pathway by Glucose Sensor O-GlcNAcylation. Mol Cell 2017; 68: 591-604. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 25. | Castellano G, Torrisi E, Ligresti G, Malaponte G, Militello L, Russo AE, McCubrey JA, Canevari S, Libra M. The involvement of the transcription factor Yin Yang 1 in cancer development and progression. Cell Cycle. 2009;8:1367-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Chen YH, Chung CC, Liu YC, Lai WC, Lin ZS, Chen TM, Li LY, Hung MC. YY1 and HDAC9c transcriptionally regulate p38-mediated mesenchymal stem cell differentiation into osteoblasts. Am J Cancer Res. 2018;8:514-525. [PubMed] |
| 27. | Zhu G, Qian M, Lu L, Chen Y, Zhang X, Wu Q, Liu Y, Bian Z, Yang Y, Guo S, Wang J, Pan Q, Sun F. O-GlcNAcylation of YY1 stimulates tumorigenesis in colorectal cancer cells by targeting SLC22A15 and AANAT. Carcinogenesis. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Riera CE, Merkwirth C, De Magalhaes Filho CD, Dillin A. Signaling Networks Determining Life Span. Annu Rev Biochem. 2016;85:35-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 29. | Martinez MR, Dias TB, Natov PS, Zachara NE. Stress-induced O-GlcNAcylation: an adaptive process of injured cells. Biochem Soc Trans. 2017;45:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Hart GW. Three Decades of Research on O-GlcNAcylation - A Major Nutrient Sensor That Regulates Signaling, Transcription and Cellular Metabolism. Front Endocrinol (Lausanne). 2014;5:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Burén S, Gomes AL, Teijeiro A, Fawal MA, Yilmaz M, Tummala KS, Perez M, Rodriguez-Justo M, Campos-Olivas R, Megías D, Djouder N. Regulation of OGT by URI in Response to Glucose Confers c-MYC-Dependent Survival Mechanisms. Cancer Cell. 2016;30:290-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Han C, Gu Y, Shan H, Mi W, Sun J, Shi M, Zhang X, Lu X, Han F, Gong Q, Yu W. O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat Commun. 2017;8:1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Wang C, Zhang Y, Guo K, Wang N, Jin H, Liu Y, Qin W. Heat shock proteins in hepatocellular carcinoma: Molecular mechanism and therapeutic potential. Int J Cancer. 2016;138:1824-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Krzeslak A, Pomorski L, Lipinska A. Elevation of nucleocytoplasmic beta-N-acetylglucosaminidase (O-GlcNAcase) activity in thyroid cancers. Int J Mol Med. 2010;25:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Iwai T, Kudo T, Kawamoto R, Kubota T, Togayachi A, Hiruma T, Okada T, Kawamoto T, Morozumi K, Narimatsu H. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc Natl Acad Sci. 2005;102:4572-4577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Iwai T, Inaba N, Naundorf A, Zhang Y, Gotoh M, Iwasaki H, Kudo T, Togayachi A, Ishizuka Y, Nakanishi H, Narimatsu H. Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N-acetylglucosaminyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J Biol Chem. 2002;277:12802-12809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Hassinen A, Pujol FM, Kokkonen N, Pieters C, Kihlström M, Korhonen K, Kellokumpu S. Functional organization of Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J Biol Chem. 2011;286:38329-38340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417-1429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep. 2015;42:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 403] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 40. | Li LX, Ashikov A, Liu H, Griffith CL, Bakker H, Doering TL. Cryptococcus neoformans UGT1 encodes a UDP-Galactose/UDP-GalNAc transporter. Glycobiology. 2017;27:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN, Reginato MJ. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014;54:820-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 42. | Méndez-Huergo SP, Blidner AG, Rabinovich GA. Galectins: emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr Opin Immunol. 2017;45:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 43. | Na HJ, Akan I, Abramowitz LK, Hanover JA. Nutrient-Driven O-GlcNAcylation Controls DNA Damage Repair Signaling and Stem/Progenitor Cell Homeostasis. Cell Rep. 2020;31:107632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Giuffrè G, Vitarelli E, Tuccari G, Ponz de Leon M, Barresi G. Detection of Tn, sialosyl-Tn and T antigens in hereditary nonpolyposis colorectal cancer. Virchows Arch. 1996;429:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Chik JH, Zhou J, Moh ES, Christopherson R, Clarke SJ, Molloy MP, Packer NH. Comprehensive glycomics comparison between colon cancer cell cultures and tumours: implications for biomarker studies. J Proteomics. 2014;108:146-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Schneider F, Kemmner W, Haensch W, Franke G, Gretschel S, Karsten U, Schlag PM. Overexpression of sialyltransferase CMP-sialic acid:Galbeta1,3GalNAc-R alpha6-Sialyltransferase is related to poor patient survival in human colorectal carcinomas. Cancer Res. 2001;61:4605-4611. [PubMed] |
| 47. | Kudelka MR, Ju T, Heimburg-Molinaro J, Cummings RD. Simple sugars to complex disease--mucin-type O-glycans in cancer. Adv Cancer Res. 2015;126:53-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 370] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 48. | Yu M, Chu S, Fei B, Fang X, Liu Z. O-GlcNAcylation of ITGA5 facilitates the occurrence and development of colorectal cancer. Exp Cell Res. 2019;382:111464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Carvalho-Cruz P, Alisson-Silva F, Todeschini AR, Dias WB. Cellular glycosylation senses metabolic changes and modulates cell plasticity during epithelial to mesenchymal transition. Dev Dyn. 2018;247:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 50. | Harosh-Davidovich SB, Khalaila I. O-GlcNAcylation affects β-catenin and E-cadherin expression, cell motility and tumorigenicity of colorectal cancer. Exp Cell Res. 2018;364:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1104] [Cited by in RCA: 1454] [Article Influence: 207.7] [Reference Citation Analysis (0)] |
| 52. | Jiang M, Xu B, Li X, Shang Y, Chu Y, Wang W, Chen D, Wu N, Hu S, Zhang S, Li M, Wu K, Yang X, Liang J, Nie Y, Fan D. O-GlcNAcylation promotes colorectal cancer metastasis via the miR-101-O-GlcNAc/EZH2 regulatory feedback circuit. Oncogene. 2019;38:301-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 53. | Matsuda Y, Ueda J, Ishiwata T. Fibroblast growth factor receptor 2: expression, roles, and potential as a novel molecular target for colorectal cancer. Patholog Res Int. 2012;2012:574768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Kvorjak M, Ahmed Y, Miller ML, Sriram R, Coronnello C, Hashash JG, Hartman DJ, Telmer CA, Miskov-Zivanov N, Finn OJ, Cascio S. Cross-talk between Colon Cells and Macrophages Increases ST6GALNAC1 and MUC1-sTn Expression in Ulcerative Colitis and Colitis-Associated Colon Cancer. Cancer Immunol Res. 2020;8:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 55. | Krishn SR, Kaur S, Smith LM, Johansson SL, Jain M, Patel A, Gautam SK, Hollingsworth MA, Mandel U, Clausen H, Lo WC, Fan WT, Manne U, Batra SK. Mucins and associated glycan signatures in colon adenoma-carcinoma sequence: Prospective pathological implication(s) for early diagnosis of colon cancer. Cancer Lett. 2016;374:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 56. | Arike L, Holmén-Larsson J, Hansson GC. Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology. 2017;27:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 57. | Ortiz-Meoz RF, Jiang J, Lazarus MB, Orman M, Janetzko J, Fan C, Duveau DY, Tan ZW, Thomas CJ, Walker S. A small molecule that inhibits OGT activity in cells. ACS Chem Biol. 2015;10:1392-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 58. | Gloster TM, Zandberg WF, Heinonen JE, Shen DL, Deng L, Vocadlo DJ. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol. 2011;7:174-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 59. | Bergstrom KS, Xia L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology. 2013;23:1026-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 60. | Guo H, Zhang B, Nairn AV, Nagy T, Moremen KW, Buckhaults P, Pierce M. O-Linked N-Acetylglucosamine (O-GlcNAc) Expression Levels Epigenetically Regulate Colon Cancer Tumorigenesis by Affecting the Cancer Stem Cell Compartment via Modulating Expression of Transcriptional Factor MYBL1. J Biol Chem. 2017;292:4123-4137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Gao T, Wen T, Ge Y, Liu J, Yang L, Jiang Y, Dong X, Liu H, Yao J, An G. Disruption of Core 1-mediated O-glycosylation oppositely regulates CD44 expression in human colon cancer cells and tumor-derived exosomes. Biochem Biophys Res Commun. 2020;521:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Mazal D, Lo-Man R, Bay S, Pritsch O, Dériaud E, Ganneau C, Medeiros A, Ubillos L, Obal G, Berois N, Bollati-Fogolin M, Leclerc C, Osinaga E. Monoclonal antibodies toward different Tn-amino acid backbones display distinct recognition patterns on human cancer cells. Implications for effective immuno-targeting of cancer. Cancer Immunol Immunother. 2013;62:1107-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Wargovich MJ, Chang P, Velasco M, Sinicrope F, Eisenbrodt E, Sellin J. Expression of cellular adhesion proteins and abnormal glycoproteins in human aberrant crypt foci. Appl Immunohistochem Mol Morphol. 2004;12:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl. 2011;50:1770-1791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 65. | Liu Z, Liu J, Dong X, Hu X, Jiang Y, Li L, Du T, Yang L, Wen T, An G, Feng G. Tn antigen promotes human colorectal cancer metastasis via H-Ras mediated epithelial-mesenchymal transition activation. J Cell Mol Med. 2019;23:2083-2092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6910] [Article Influence: 287.9] [Reference Citation Analysis (0)] |
| 67. | Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 651] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 68. | Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Bennett EP, Mandel U, Brunak S, Wandall HH, Levery SB, Clausen H. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1106] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 69. | Heimburg-Molinaro J, Lum M, Vijay G, Jain M, Almogren A, Rittenhouse-Olson K. Cancer vaccines and carbohydrate epitopes. Vaccine. 2011;29:8802-8826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 70. | Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 341] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 71. | Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM, Law L, Hester LD, Snyder SH. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 324] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 72. | Ubillos L, Berriel E, Mazal D, Victoria S, Barrios E, Osinaga E, Berois N. Polypeptide-GalNAc-T6 expression predicts better overall survival in patients with colon cancer. Oncol Lett. 2018;16:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Efimova EV, Takahashi S, Shamsi NA, Wu D, Labay E, Ulanovskaya OA, Weichselbaum RR, Kozmin SA, Kron SJ. Linking Cancer Metabolism to DNA Repair and Accelerated Senescence. Mol Cancer Res. 2016;14:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Kang KA, Piao MJ, Ryu YS, Kang HK, Chang WY, Keum YS, Hyun JW. Interaction of DNA demethylase and histone methyltransferase upregulates Nrf2 in 5-fluorouracil-resistant colon cancer cells. Oncotarget. 2016;7:40594-40620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 75. | RodrÍguez E, Schetters STT, van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 2018;18:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 314] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 76. | Xiao H, Woods EC, Vukojicic P, Bertozzi CR. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci. 2016;113:10304-10309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 318] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 77. | Unger WW, Mayer CT, Engels S, Hesse C, Perdicchio M, Puttur F, Streng-Ouwehand I, Litjens M, Kalay H, Berod L, Sparwasser T, van Kooyk Y. Antigen targeting to dendritic cells combined with transient regulatory T cell inhibition results in long-term tumor regression. Oncoimmunology. 2015;4:e970462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Saeland E, Belo AI, Mongera S, van Die I, Meijer GA, van Kooyk Y. Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients. Int J Cancer. 2012;131:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 79. | Singh SK, Streng-Ouwehand I, Litjens M, Kalay H, Saeland E, van Kooyk Y. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int J Cancer. 2011;128:1371-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Démoulins T, Schneider C, Wehrli M, Hunger RE, Baerlocher GM, Simon HU, Romero P, Münz C, von Gunten S. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest. 2014;124:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 340] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 81. | Ruan Z, Liang M, Lai M, Shang L, Deng X, Su X. KYA1797K down-regulates PD-L1 in colon cancer stem cells to block immune evasion by suppressing the β-catenin/STT3 signaling pathway. Int Immunopharmacol. 2020;78:106003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Chamoto K, Al-Habsi M, Honjo T. Role of PD-1 in Immunity and Diseases. Curr Top Microbiol Immunol. 2017;410:75-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 83. | Brockhausen I, Stanley P, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH. O-GalNAc Glycans 2015. [PubMed] [DOI] [Full Text] |
| 84. | Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 543] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 85. | Sutoh Yoneyama M, Tobisawa Y, Hatakeyama S, Sato M, Tone K, Tatara Y, Kakizaki I, Funyu T, Fukuda M, Hoshi S, Ohyama C, Tsuboi S. A mechanism for evasion of CTL immunity by altered O-glycosylation of HLA class I. J Biochem. 2017;161:479-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Li G, Chu DK. Effects of sunitinib on Notch signaling pathway and apoptosis in colon cancer cell line. Shanxi Yike Daxue Xueban. 2020;51:301-306. [DOI] [Full Text] |
| 87. | Liang L, Yan MG, Yang QM, Lin CL, Xi R, Zhao YY. Effect of baicalin on proliferation and apoptosis of human colorectal cancer SW480 cells via Notch pathway. Xiandai Zhongliu Yixue. 2019;27:2648-2472. [DOI] [Full Text] |
| 88. | Zhang Z, Bu X, Yang J, Zhu S, He S, Zheng J, Wang W, Chu D. NOTCH4 regulates colorectal cancer proliferation, invasiveness, and determines clinical outcome of patients. J Cell Physiol. 2018;233:6975-6985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Chu D, Zhang Z, Zhou Y, Li Y, Zhu S, Zhang J, Zhao Q, Ji G, Wang W, Zheng J. NDRG4, a novel candidate tumor suppressor, is a predictor of overall survival of colorectal cancer patients. Oncotarget. 2015;6:7584-7596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Müller J, Rana NA, Serth K, Kakuda S, Haltiwanger RS, Gossler A. O-fucosylation of the notch ligand mDLL1 by POFUT1 is dispensable for ligand function. PLoS One. 2014;9:e88571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 91. | Harvey BM, Haltiwanger RS. Regulation of Notch Function by O-Glycosylation. Adv Exp Med Biol. 2018;1066:59-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 92. | Gao T, Du T, Hu X, Dong X, Li L, Wang Y, Liu J, Liu L, Gu T, Wen T. Cosmc overexpression enhances malignancies in human colon cancer. J Cell Mol Med. 2020;24:362-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Wu N, Jiang M, Han Y, Liu H, Chu Y, Liu H, Cao J, Hou Q, Zhao Y, Xu B, Xie X. O-GlcNAcylation promotes colorectal cancer progression by regulating protein stability and potential catcinogenic function of DDX5. J Cell Mol Med. 2019;23:1354-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 94. | Fernández LP, Sánchez-Martínez R, Vargas T, Herranz J, Martín-Hernández R, Mendiola M, Hardisson D, Reglero G, Feliu J, Redondo A, Ramírez de Molina A. The role of glycosyltransferase enzyme GCNT3 in colon and ovarian cancer prognosis and chemoresistance. Sci Rep. 2018;8:8485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 95. | Venkatakrishnan V, Quintana-Hayashi MP, Mahu M, Haesebrouck F, Pasmans F, Lindén SK. Brachyspira hyodysenteriae Infection Regulates Mucin Glycosylation Synthesis Inducing an Increased Expression of Core-2 O-Glycans in Porcine Colon. J Proteome Res. 2017;16:1728-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Lin WR, Chiang JM, Liang KH, Lim SN, Lai MW, Tsou YK, Hsieh TY, Hsu CK, Yeh CT. GALNT14 Genotype Predicts Postoperative Outcome of Stage III Colorectal Cancer With Oxaliplatin as Adjuvant Chemotherapy. Medicine (Baltimore). 2016;95:e3487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Fuell C, Kober OI, Hautefort I, Juge N. Mice deficient in intestinal γδ intraepithelial lymphocytes display an altered intestinal O-glycan profile compared with wild-type littermates. Glycobiology. 2015;25:42-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Morin PJ, Vogelstein B, Kinzler KW. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci. 1996;93:7950-7954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 358] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 99. | Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, Cong Q, Yu W. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta. 2011;1812:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 100. | Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I. O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-β-D-glucosaminidase silencing on cell phenotype and transcriptome. J Biol Chem. 2012;287:28755-28769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 101. | Guda K, Moinova H, He J, Jamison O, Ravi L, Natale L, Lutterbaugh J, Lawrence E, Lewis S, Willson JK, Lowe JB, Wiesner GL, Parmigiani G, Barnholtz-Sloan J, Dawson DW, Velculescu VE, Kinzler KW, Papadopoulos N, Vogelstein B, Willis J, Gerken TA, Markowitz SD. Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers. Proc Natl Acad Sci. 2009;106:12921-12925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 102. | Shibao K, Izumi H, Nakayama Y, Ohta R, Nagata N, Nomoto M, Matsuo K, Yamada Y, Kitazato K, Itoh H, Kohno K. Expression of UDP-N-acetyl-alpha-D-galactosamine-polypeptide galNAc N-acetylgalactosaminyl transferase-3 in relation to differentiation and prognosis in patients with colorectal carcinoma. Cancer. 2002;94:1939-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 103. | Ueno T, Endo S, Saito R, Hirose M, Hirai S, Suzuki H, Yamato K, Hyodo I. The sirtuin inhibitor tenovin-6 upregulates death receptor 5 and enhances cytotoxic effects of 5-fluorouracil and oxaliplatin in colon cancer cells. Oncol Res. 2013;21:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Yu X, Wang D, Wang X, Sun S, Zhang Y, Wang S, Miao R, Xu X, Qu X. CXCL12/CXCR4 promotes inflammation-driven colorectal cancer progression through activation of RhoA signaling by sponging miR-133a-3p. J Exp Clin Cancer Res. 2019;38:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 105. | Nakano M, Kikushige Y, Miyawaki K, Kunisaki Y, Mizuno S, Takenaka K, Tamura S, Okumura Y, Ito M, Ariyama H, Kusaba H, Nakamura M, Maeda T, Baba E, Akashi K. Dedifferentiation process driven by TGF-beta signaling enhances stem cell properties in human colorectal cancer. Oncogene. 2019;38:780-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 106. | Rojas A, Padidam M, Cress D, Grady WM. TGF-beta receptor levels regulate the specificity of signaling pathway activation and biological effects of TGF-beta. Biochim Biophys Acta. 2009;1793:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 107. | Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5:e29846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 405] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 108. | Zhang Y, Dong X, Bai L, Shang X, Zeng Y. MUC1-induced immunosuppression in colon cancer can be reversed by blocking the PD1/PDL1 signaling pathway. Oncol Lett. 2020;20:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Yang K, Popova NV, Yang WC, Lozonschi I, Tadesse S, Kent S, Bancroft L, Matise I, Cormier RT, Scherer SJ, Edelmann W, Lipkin M, Augenlicht L, Velcich A. Interaction of Muc2 and Apc on Wnt signaling and in intestinal tumorigenesis: potential role of chronic inflammation. Cancer Res. 2008;68:7313-7322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 110. | Lu S, Catalano C, Huhn S, Pardini B, Partu L, Vymetalkova V, Vodickova L, Levy M, Buchler T, Hemminki K, Vodicka P, Försti A. Single nucleotide polymorphisms within MUC4 are associated with colorectal cancer survival. PLoS One. 2019;14:e0216666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 111. | Sheng YH, Wong KY, Seim I, Wang R, He Y, Wu A, Patrick M, Lourie R, Schreiber V, Giri R, Ng CP, Popat A, Hooper J, Kijanka G, Florin TH, Begun J, Radford KJ, Hasnain S, McGuckin MA. MUC13 promotes the development of colitis-associated colorectal tumors via β-catenin activity. Oncogene. 2019;38:7294-7310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | Dong X, Jiang Y, Liu J, Liu Z, Gao T, An G, Wen T. T-Synthase Deficiency Enhances Oncogenic Features in Human Colorectal Cancer Cells via Activation of Epithelial-Mesenchymal Transition. Biomed Res Int. 2018;2018:9532389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |