Published online Feb 27, 2021. doi: 10.4240/wjgs.v13.i2.127
Peer-review started: August 27, 2020
First decision: November 4, 2020
Revised: November 27, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: February 27, 2021
Processing time: 161 Days and 4.8 Hours
Post-hepatectomy liver failure (PHLF) increases morbidity and mortality after liver resection for patients with advanced liver fibrosis and cirrhosis. Preoperative liver stiffness using two-dimensional shear wave elastography (2D-SWE) is widely used to evaluate the degree of fibrosis. However, the 2D-SWE results were not accurate. A durometer measures hardness by quantifying the ability of a material to locally resist the intrusion of hard objects into its surface. However, the durometer score can only be obtained during surgery.
To measure correlations among 2D-SWE, palpation by surgeons, and durometer-measured objective liver hardness and to construct a liver hardness regression model.
We enrolled 74 hepatectomy patients with liver hardness in a derivation cohort. Tactile-based liver hardness scores (0-100) were determined through palpation of the liver tissue by surgeons. Additionally, liver hardness was measured using a durometer. Correlation coefficients for durometer-measured hardness and preoperative parameters were calculated. Multiple linear regression models were constructed to select the best predictive durometer scale. Receiver operating characteristic (ROC) curves and univariate and multivariate analyses were used to calculate the best model’s prediction of PHLF and risk factors for PHLF, respectively. A separate validation cohort (n = 162) was used to evaluate the model.
The stiffness measured using 2D-SWE and palpation scale had good linear correlation with durometer-measured hardness (Pearson rank correlation coefficient 0.704 and 0.729, respectively, P < 0.001). The best model for the durometer scale (hardness scale model) was based on stiffness, hepatitis B virus surface antigen, and albumin level and had an R2 value of 0.580. The area under the ROC for the durometer and hardness scale for PHLF prediction were 0.807 (P = 0.002) and 0.785 (P = 0.005), respectively. The optimal cutoff value of the durometer and hardness scale was 27.38 (sensitivity = 0.900, specificity = 0.660) and 27.87 (sensitivity = 0.700, specificity = 0.787), respectively. Patients with a hardness scale score of > 27.87 were at a significantly higher risk of PHLF with hazard ratios of 7.835 (P = 0.015). The model’s PHLF predictive ability was confirmed in the validation cohort.
Liver stiffness assessed by 2D-SWE and palpation correlated well with durometer hardness values. The multiple linear regression model predicted durometer hardness values and PHLF.
Core Tip: In this study, we developed a linear regression model to predict liver hardness and found that surgeons’ subjective palpation scores were comparable with durometer measures of liver hardness. The hardness model had a good ability to predict post-hepatectomy liver failure.
- Citation: Ju BJ, Jin M, Tian Y, Zhen X, Kong DX, Wang WL, Yan S. Model for liver hardness using two-dimensional shear wave elastography, durometer, and preoperative biomarkers. World J Gastrointest Surg 2021; 13(2): 127-140
- URL: https://www.wjgnet.com/1948-9366/full/v13/i2/127.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i2.127
Post-hepatectomy liver failure (PHLF) represents postoperative dysfunction in the liver’s synthesis, excretory, and detoxification functions. Morbidity and mortality after liver resection increase, particularly in patients with advanced liver fibrosis and cirrhosis[1]. Cirrhosis is not only related to the occurrence of PHLF but also causes a coagulation disorder that increases the risk of increased blood loss and the need for blood transfusion during surgery[2,3]. In China, more than 80% of hepatocellular carcinoma (HCC) cases are related to hepatitis B virus (HBV) infections that can cause liver dysfunction and chronic fibrosis[4]. In preoperative evaluations for hepatectomy, liver function and the degree of liver fibrosis are commonly considered.
Liver fibrosis biomarkers such as serum biomarkers and fibrosis models are used alone or in combination to determine the degree of liver fibrosis[5-9] and predict PHLF[10-12]. Currently, liver stiffness measurements (LSM) using imaging methods, including transient elastography and magnetic resonance elastography (MRE), have been reported as sufficient tools to diagnose liver fibrosis[13-18] and to predict the occurrence of PHLF[19-21]. Two-dimensional shear wave elastography (2D-SWE) reportedly improves fibrosis diagnostic accuracy compared to real-time tissue elastography[22]. However, the predictive accuracy of 2D-SWE for PHLF has not yet been discussed.
In clinical work, we found that the LSM results of some patients were inconsistent with the findings of palpation by surgeons. When we reviewed the stiffness measured by 2D-SWE and fibrosis pathology in hepatectomy patients at our center, we found that a group of patients had high-grade fibrosis but a low level of stiffness. This led to situations in which cases required open surgery after visualization under laparoscopy. For patients with a high degree of stiffness but a low fibrosis grade, open surgery may be avoided. The results of SWE can be influenced by age, inflammation, and obesity[22-24].
Liver hardness measured by the durometer is correlated with the degree of liver fibrosis and is a potential parameter for predicting PHLF[25,26]. However, these studies did not analyze the predictive accuracy of hardness for PHLF. Furthermore, the correlation between liver hardness measured by the durometer and liver stiffness measured by 2D-SWE has not been discussed yet. Thus, the aim of this study was to measure correlations between 2D-SWE and durometer-measured objective liver hardness and to improve 2D-SWE's ability to accurately predict liver hardness by constructing a preoperative liver hardness linear regression model.
We screened 279 patients who were scheduled to undergo liver resection for liver masses or intrahepatic bile duct stones at the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China, from March 2019 to December 2019 for inclusion in this study.
Forty-three patients were excluded because of the following criteria: Preoperative condition precluding surgery (n = 30), no preoperative LSM (n = 7), unsuccessful LSM (n = 1), and history of hepatectomy (n = 5). To develop the new hardness scale model, a derivation cohort that included 74 patients with liver hardness, measured by the durometer, between March 2019 and June 2019 was created. The validation cohort included the remaining 162 patients between July 2019 and December 2019. All surgeries were performed by three medical teams. Liver resection of more than and less than three segments was defined as major resection and minor resection, respectively[27]. The study protocol was approved by the institution’s ethics committee of each hospital and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The requirement for informed consent was waived.
Patients underwent a thorough medical history enquiry, routine preoperative laboratory measurements, and HBV and fibrosis serologic marker testing. A biomarker model associated with liver fibrosis, fibrosis-4 (FIB-4), was calculated as previously described by Sterling et al[28]: [age (year)] × [AST (U/L)]/[platelet count (109/L)] × [ALT (U/L)1⁄2] (AST, aspartate aminotransferase; ALT, alanine aminotransferase). Preoperative imaging examinations including ultrasonography, computed tomography, and magnetic resonance were performed to evaluate the tumors. Some patients underwent the indocyanine green retention rate after 15 min test to ensure minimal residual liver volume. The resection range was determined by the size and location of the lesion, and minimum residual liver volume. Depending on the condition of the patients before and during the operation, the patients were transferred to the intensive care unit for treatment, as necessary. According to the criterion of the International Study Group of Liver Surgery[1], PHLF was defined as an increased international normalized ratio and concomitant hyperbilirubinemia on or after postoperative day 5.
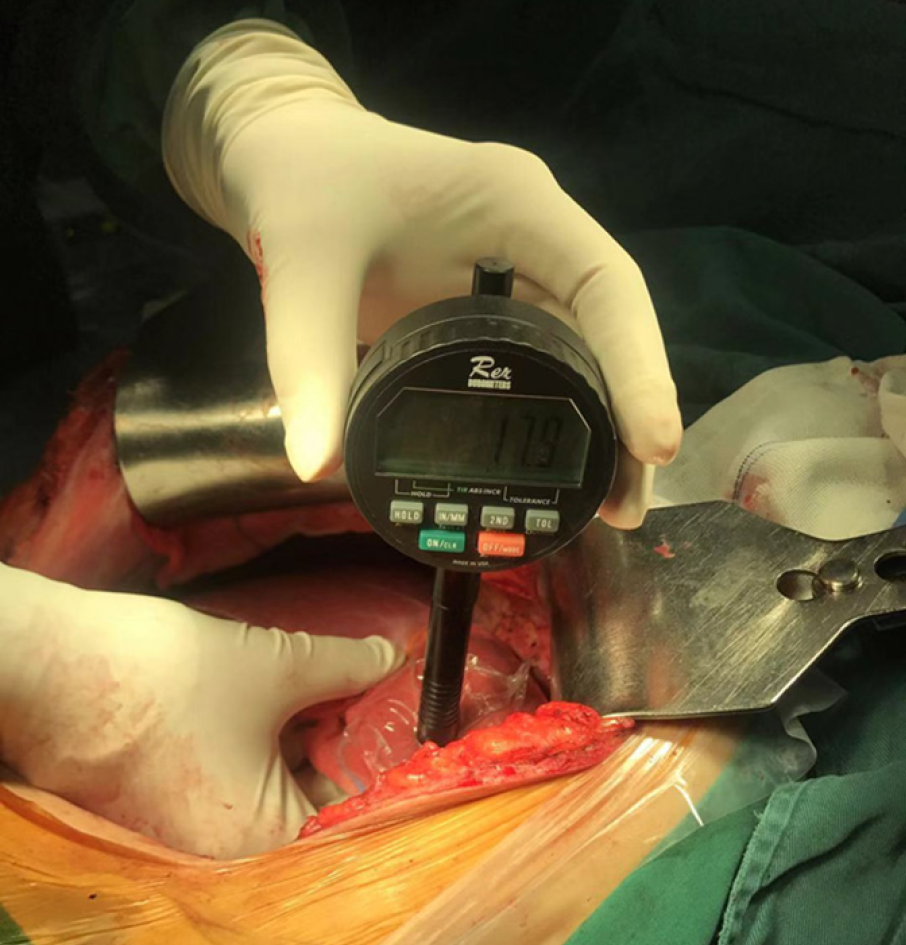
Liver stiffness was measured using 2D-SWE (SuperSonic Imagine, Aix-en-Provence, France), operated by sonographers, 3 d before surgery. The measurement, as recommended by the World Federation of Ultrasound in Medicine and Biology guidelines, of the intercostal space on the right lobe of the patient’s liver was recorded[29]. The mean stiffness was used for further statistical analysis. A hand-held durometer (Rex Gauge, Buffalo Grove, IL, United States) was placed perpendicularly to the liver surface to obtain an objective measurement of liver hardness during surgery (Figure 1). The area of liver tissue tested was more than 1 cm thick, and without large blood vessels or tumors[25]. Ten readings were taken at different sites of the liver tissue. The durometer measurements were scored using a 0 to 100 scale in durometer units (DU). Three surgeons who had performed over 50 cases of open hepatectomy each, performed palpation of the same part of each liver, independently, and gave a score on a scale from 1 to 100. A palpation score of 50 represented a normal liver while a higher score indicated a correspondingly harder liver.
The statistical methods used in this study were reviewed by Professor Dexing Kong from the School of Mathematical Sciences, Zhejiang University. All statistical analyses were performed using SPSS software (version 20.0; IBM Corp., Armonk, NY, United States). The chi-square test, Student’s t test, and Mann-Whitney test were used to compare the patients’ clinical features and postoperative outcomes. The medians and quartiles of parameters were used in the analysis. The means of durometric readings and palpation score of each sample were calculated. The Pearson rank correlation coefficient R and the Spearman rank correlation coefficient R were calculated to assess the independent variables associated with the durometer measurement. Only significant parameters associated with the durometer measurement in the correlation analysis were used for multiple linear regression analysis, and the hardness scale model was determined by mixed stepwise regression. The receiver operating characteristic (ROC) and corresponding area under the ROC (AUROC) curves were used to assess the predictive ability of the hardness scale for PHLF. The optimal cutoff value was set as the value maximizing Youden index, which was defined as sensitivity-specificity+1. Univariate analysis and multivariate analysis were performed by logistic regression to identify predictive factors for PHLF. Odds ratios with 95% confidence intervals derived from logistic regression were calculated. P values were two-tailed and considered statistically significant if less than 0.05.
Overall, 58 males and 16 females were included in the derivation cohort. Seventy of the patients underwent surgery for liver masses (malignant tumors n = 62; benign masses n = 8). The remaining four patients underwent surgery for intrahepatic bile duct stones. There were 57 patients who had HBV infections defined as hepatitis B surface antigens (HBsAg)-pos/neg +anti-HBc-pos[30]. One death occurred within 1 mo after the operation due to postoperative bleeding.
The validation cohort included 126 males and 36 females. The mean age was 56.30 ± 11.20 (range: 15-85) years. Further, 148 patients underwent surgery for malignant liver tumors, and 14 for benign liver diseases. There were 148 patients who had HBV infections. Patient characteristics of the two cohorts are shown in Table 1.
| Parameters | Derivation cohort, n = 74 | Validation cohort, n = 162 | P value |
| Age in yr, median (IQR) | 61 (52-66) | 57 (20-63) | 0.063 |
| INR, median (IQR) | 1 (0.95-1.07) | 1.05 (1-1.1) | < 0.001 |
| PT in s, median (IQR) | 12.5 (11.8-13) | 11.7 (11.2-12.5) | < 0.001 |
| TT in s, median (IQR) | 18.5 (16.8-19.5) | 18.4 (17.2-19.1) | 0.426 |
| Platelet as 109/L, median (IQR) | 165 (125-232) | 156 (116-214) | 0.547 |
| Albumin in g/L, median (IQR) | 43 (39.4-45.7) | 44.3 (40.3-47.2) | 0.103 |
| ALT in U/L, median (IQR) | 25 (18-38.8) | 26 (20-37) | 0.644 |
| AST in U/L, median (IQR) | 24 (21-37) | 27 (22-37) | 0.301 |
| TB in µmol/L, median (IQR) | 13.2 (9.7-19.5) | 12 (9-15) | 0.017 |
| Cholinesterase in U/L, median (IQR) | 7310 (5844-8030) | 6968 (5981-8298) | 0.213 |
| GGT in U/L, median (IQR) | 44 (29-99) | 46 (27-92) | 0.136 |
| Creatinine in μmol/L, median (IQR) | 70 (62-79) | 72 (64-83) | 0.223 |
| HBV–DNA in U/mL, median (IQR) | 8 (0-1800) | 0 (0-1074) | 0.143 |
| HBsAg in IU/mL, median (IQR) | 24.9 (0-171.31) | 250 (0-254) | 0.823 |
| HA in μg/L, median (IQR) | 72 (47-113) | 77 (44-213) | 0.462 |
| Stiffness in kPa, median (IQR) | 9.3 (6.8-11.35) | 10.1 (8.0-17) | 0.022 |
| Palpation scale, median (IQR) | 57.5 (53.3-62.8) | - | - |
| Duration of surgery in min, median (IQR) | 258 (193-318) | 244 (147-328) | 0.382 |
| Hospital stay in d, median (IQR) | 12 (7-19) | 7 (6-10) | < 0.001 |
| Child Pugh score, n (%) | 0.080 | ||
| A | 67 (90.5) | 157 (96.9) | |
| B | 7 (9.5) | 5 (3.1) | |
| Open surgery, n (%) | 74 (100) | 67 (41.4) | - |
| LH, n (%) | - | 91 (56.2) | - |
| Open convert LH, n (%) | - | 4 (2.4) | - |
| Major liver resection, n (%) | 11 (14.9) | 35 (21.6) | 0.225 |
| PHLF, n (%) | 14 (18.9) | 19 (11.7) | 0.139 |
| Grade A, n (%) | 12 (16.1) | 17 (10.5) | |
| Grade B, n (%) | 1 (1.4) | 2 (1.2) | |
| Grade C, n (%) | 1 (1.4) | - |
The characteristics, blood tests, plasma biochemical parameters, HBV tests, and liver fibrosis biomarkers of all patients were determined. The results of the correlation analysis between the patients’ clinical parameters and durometer measurements are shown in Table 2.
| Parameters | Correlation coefficient R with subjective scale | |||
| Pearson r (95%CI) | P value | Spearman r (95%CI) | P value | |
| Age in yr | 0.150 (-0.144-0.377) | 0.201 | 0.024 (-0.211-0.273) | 0.839 |
| INR | 0.097 (-0.125-0.306) | 0.416 | 0.138 (0.127-0.350) | 0.249 |
| PT in s | 0.197 (-0.122-0.318) | 0.093 | 0.139 (-0.172-0.353) | 0.239 |
| TT in s | 0.232 (-0.086-0.376) | 0.046 | 0.219 (-0.055-0.406) | 0.061 |
| Platelet as 109/L | –0.244 (-0.405-0.043) | 0.039 | -0.313 (-0.475-0.071) | 0.008 |
| Albumin in g/L | –0.382 (-0.522-0.073) | 0.005 | -0.359 (-0.518-0.037) | 0.002 |
| ALT in U/L | 0.144 (-0.003-0.327) | 0.227 | 0.299 (-0.088-0.441) | 0.011 |
| AST in U/L | 0.192 (0.034-0.417) | 0.106 | 0.340 (-0.071-0.469) | 0.004 |
| TB in µmol/L | 0.015 (-0.165-0.151) | 0.901 | -0.049 (-0.278-0.241) | 0.683 |
| Cholinesterase in U/L | –0.298 (-0.479-0.104) | 0.026 | –0.263 (-0.476-0.000) | 0.05 |
| GGT in U/L | 0.053 (-0.121-0.149) | 0.662 | 0.043 (-0.257-0.244) | 0.719 |
| Creatinine in μmol/L | 0.152 (-0.067-0.280) | 0.205 | 0.298 (-0.144-0.342) | 0.012 |
| HBV–DNA in U/mL | -0.043 (-0.012-0.185) | 0.749 | 0.110 (-0.070-0.538) | 0.415 |
| HBsAg in IU/mL | 0.269 (-0.345-0.390) | 0.023 | 0.358 (0.018-0.612) | 0.002 |
| HA in μg/L | 0.547 (0.176-0.661) | < 0.001 | 0.578 (0.246-0.723) | < 0.001 |
| Preoperative FIB–4 | 0.128 (-0.085-0.474) | 0.284 | 0.378 (0.084-0.633) | 0.001 |
| Stiffness in kPa | 0.704 (0.607-0.789) | < 0.001 | 0.769 (0.639-0.850) | < 0.001 |
| Palpation scale | 0.729 (0.524-0.861) | < 0.001 | 0.729 (0.509-0.859) | < 0.001 |
| Hospital stay in d | 0.261 (0.059-0.420) | 0.042 | 0.352 (0.123-0.561) | 0.005 |
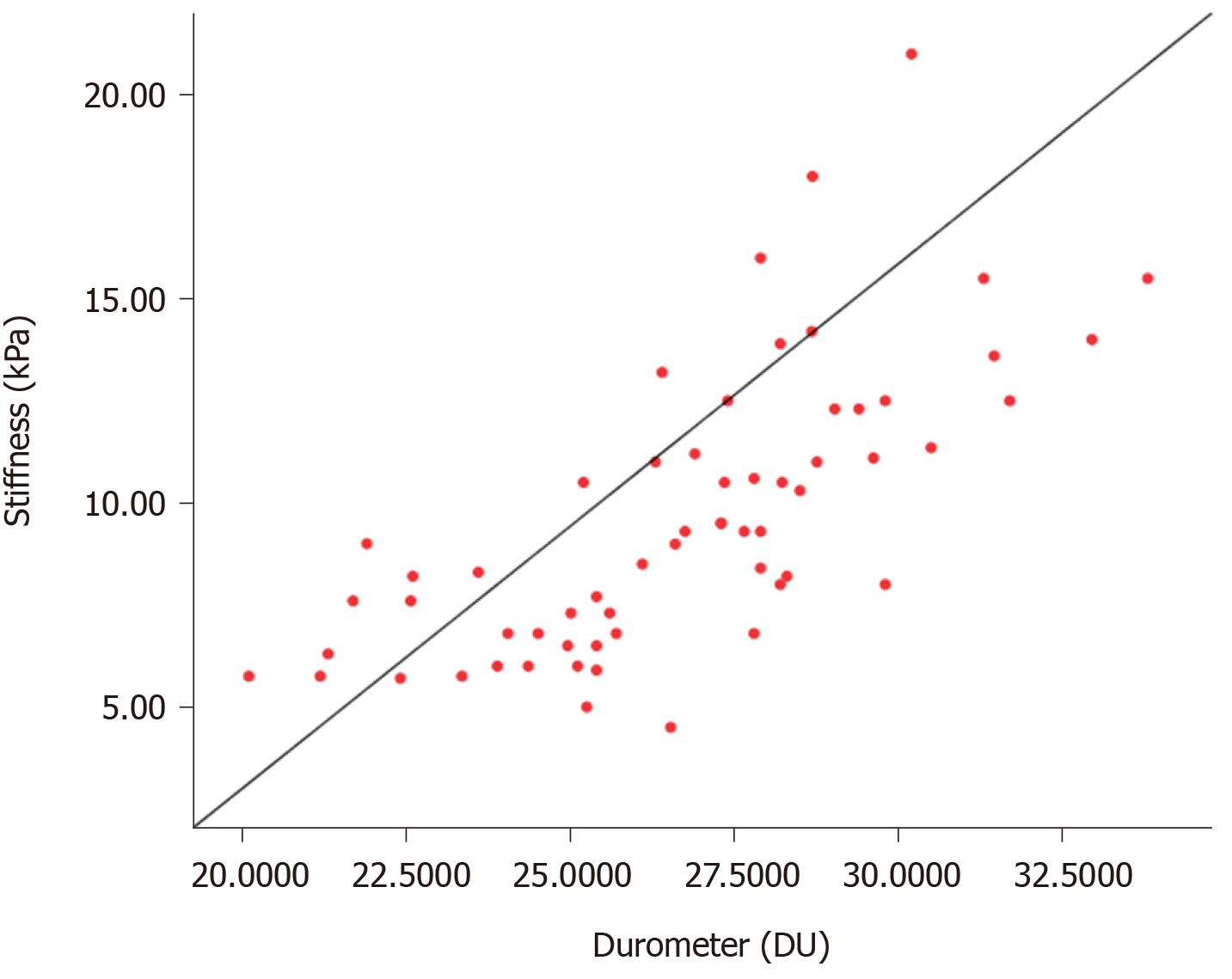
The Pearson and Spearman rank correlation coefficients between the durometer measurement and SWE stiffness were 0.704 (P < 0.001) and 0.769 (P < 0.001), respectively. A scatterplot representing the correlation is shown in Figure 2.
Seven preoperative parameters were associated significantly with the durometer measurement. Among these parameters, four (thrombin time [TT], HBV surface antigen, hyaluronic acid [HA] and SWE stiffness) and three (platelet, albumin and cholinesterase) parameters had a positive and negative linear correlation (P < 0.05) with the durometer measurement, respectively. Preoperative FIB-4 as a chronic disease score also had a positive correlation with liver hardness (r = 0.378, P = 0.001). These results showed that coagulation, HBV infection, liver function, and the degree of fibrosis were factors indicating liver hardness and consistent with signs of chronic liver diseases.
The parameters mentioned above were used to construct the model to determine the liver hardness scale. Multiple linear regression (R2, goodness-of-fit measure) models were developed to explore the multiple linear regression relationships between these parameters and the durometer measurement. The results of parameter estimates were obtained by mixed stepwise regression analysis (Table 3). The best multiple linear regression model for liver hardness scale was:
| Term | Estimate | SD | T ratio | Sig |
| Intercept | 27.172 | 2.173 | 12.506 | < 0.001 |
| Stiffness | 0.414 | 0.076 | 5.417 | < 0.001 |
| Albumin | -0.103 | 0.042 | -2.490 | 0.017 |
| HBsAg | 0.001 | 0.000 | 2.181 | 0.035 |
Hardness scale = 27.172 + 0.414 × SWE + 0.001 × HBsAg - 0.103 × albumin (P < 0.001).
Hardness scale represented the predicted value of durometer measurement. The R2 and adjusted R2 values of this model were 0.580 and 0.552, respectively. They were higher than the univariate linear regression model built with SWE: Hardness scale = 20.707 + 0.616 × SWE (P < 0.001). The R2 and adjusted R2 values of the second model were 0.527 and 0.519, respectively.
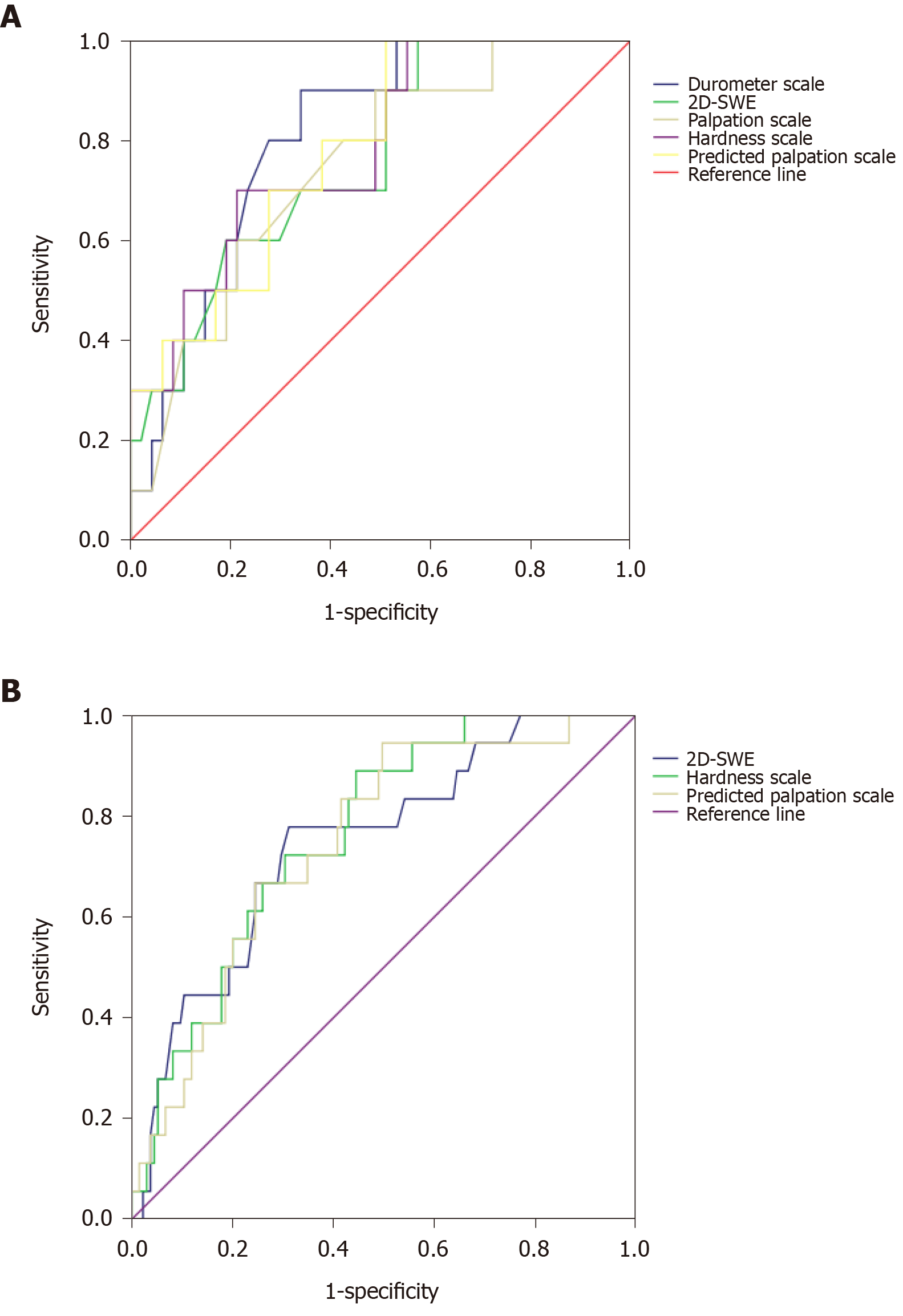
Depending on the ROC curve, the durometer measurement and hardness scale could be used to predict PHLF in the derivation cohort. The AUROC of the durometer measurement and the hardness scale were 0.807 (P = 0.002) and 0.785 (P = 0.005), respectively. The optimal cutoff values of the durometer measurement and hardness scale were 27.38 DU (sensitivity = 0.900, specificity = 0.660) and 27.87, respectively. The maximizing Youden index of the hardness scale was 0.487 (sensitivity = 0.700, specificity = 0.787). The ROC curve is shown in Figure 3A. To eliminate the effect of a small remnant liver volume on PHLF after major resection, we evaluated the efficacy of the hardness scale model in predicting the risk of PHLF in patients who underwent minor hepatectomy. The AUROC was 0.780 (P = 0.019).
The prognostic accuracy of the hardness scale model was also evaluated in the validation cohort. The derivation and validation cohorts exhibited similar baseline characteristics (Table 1). The model appeared to significantly predict PHLF in the validation cohort (Figure 3B), with an AUROC of 0.765 (P < 0.001). The AUROC was 0.768 (P = 0.001) in patients who underwent minor hepatectomy.
Similar prognostic accuracy for 2D-SWE was observed between the two cohorts. The AUROC in the derivation and validation cohorts was 0.762 (P = 0.010) and 0.747 (P = 0.001), respectively.
The patients were divided into two groups based on the hardness scale cutoff value: The low scale group comprising 52 patients (hardness scale ≤ 27.87) and the high scale group comprising 22 patients (hardness scale > 27.87). Patient characteristics of the two groups were compared as indicated in Table 4. The differences between the high and low hardness scale groups were noted for age, coagulation, liver synthesis function, and fibrosis. Preoperative platelet counts, levels of albumin, and cholinesterase were significantly lower among the high hardness scale group than among the low hardness scale group. The incidence of PHLF in the high hardness scale group was higher than that in the low scale group (36.4% vs 11.5%; P < 0.05). Four patients in the high hardness scale group developed massive pleural effusion and required drainage. The difference between the two groups in postoperative hospital stays indicated that patients with high hardness scale required a longer time to recover.
| Characteristics | Median (IQR) | ||
| High, n = 22 | Low, n = 52 | P value | |
| Age in yr | 64 (60-69) | 56 (51-65) | 0.042 |
| INR | 1.04 (0.97-1.07) | 1.00 (0.96-1.05) | 0.350 |
| PT in s | 12.5 (11.8-13.0) | 12.5 (11.8-13.9) | 0.0.578 |
| Platelet as 109/L | 126 (80-162) | 184 (136-244) | 0.026 |
| Albumin in g/L | 40 (37-42) | 45 (43-47) | < 0.001 |
| ALT in U/L | 23.0 (16.0-49.0) | 27.0 (17.5-34.5) | 0.966 |
| AST in U/L | 24.0 (21.0-28.0) | 23.0 (18.0-42.0) | 0.608 |
| TB in µmol/L | 11.8 (8.3-14.2) | 13.5 (9.4-20.6) | 0.365 |
| Cholinesterase in U/L | 4960 (4600-7329) | 7523 (6845-8254) | 0.004 |
| Creatinine in μmol/L | 77.0 (60.0-85.0) | 67.0 (60.5-75.0) | 0.625 |
| HA in μg/L | 142.0 (76.5-184.5) | 58.2 (42.5-82.1) | < 0.001 |
| HBsAg in IU/mL | 170.1 (1.7-591.0) | 24.9 (0.0-131.3) | 0.026 |
| Stiffness in kPa | 13.6 (12.3-15.5) | 7.9 (6.4-9.3) | < 0.001 |
| Durometer in DU | 28.6 (27.8-31.4) | 25.3 (23.7-27.6) | < 0.001 |
| Palpation scale | 64.0 (60.0-70.0) | 55.0 (50.4-60) | < 0.001 |
| Major resection, % | 6 (27.3%) | 5 (9.6%) | 0.138 |
| PHLF, % | 8 (36.4%) | 6 (11.5%) | 0.004 |
| Duration of surgery in min | 275 (175-310) | 265 (185-343) | 0.340 |
| Postoperative hospital stays in d | 15 (11-22) | 9 (6-16) | 0.047 |
To identify the predictive factors for PHLF, univariate and multivariate analyses was performed for factors of patients with and without liver insufficiency. The results are shown in Table 5. The hardness scale (> 27.87) and major liver resection were identified as independent predictive factors of PHLF.
| Factors | Univariate analysis | Multivariate analysis | ||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Age > 60 yr | 2.339 (0.660-8.288) | 0.188 | - | - |
| INR ≥ 1.1 | 2.200 (0.344-14.079) | 0.405 | - | - |
| Platelet < 150 × 109/L | 4.316 (1.156-16.119) | 0.030 | 3.156 (0.600-16.607) | 0.175 |
| Albumin ≤ 42 g/L | 4.022 (1.128-14.335) | 0.032 | - | - |
| TB > 17.1 μmol/L | 1.195 (0.367-3.890) | 0.768 | - | - |
| ALT ≥ 44 U/L | 5.000 (1.336-18.710) | 0.017 | 5.002 (0.926-27.022) | 0.061- |
| Durometer > 27.38 DU | 5.667 (1.278-25.114) | 0.022 | - | - |
| Stiffness ≥ 12 kPa | 5.467 (1.363-21.924) | 0.017 | - | - |
| Hardness scale > 27.87 | 8.867 (1.937-40.595) | 0.005 | 7.835 (1.486-41.306) | 0.015 |
| Major liver resection | 5.100 (1.331-19.538) | 0.017 | 4.616 (1.149-18.553) | 0.031 |
A model to transform the hardness scale to the palpation scale was designed as follows: Predicted value of palpation scale = 2.451 × hardness scale - 0.023 × platelet (P < 0.001). The R2 and adjusted R2 values were 0.647 and 0.634, respectively. The ability of the palpation scale to predict PHLF was similar to the durometer measurement (AUROC 0.781 vs 0.807, P < 0.05) (Figure 3A).
LSM is a good technique to evaluate intraoperative and postoperative outcomes[31-33]; however, it is not an accurate indicator of liver hardness when used alone because it can be impacted by age, gender, fibrosis, obesity, increased parietal wall thickness and, inflammation[22,23,34]. For patients with a high stiffness score but a soft liver, open surgery may be avoided.
Given the establishment of enhanced recovery after surgery (ERAS)[35] and the motivation to avoid wasting medical resources, this study evaluated liver hardness measurements using a durometer as an objective indicator. The durometer is placed directly on the liver surface, which eliminates interference factors such as obesity and abdominal wall thickness. The positive correlation between durometer readings and liver fibrosis has been shown previously[25,26]. However, the durometer score can only be obtained during surgery. Therefore, we sought to construct a model to predict the durometer score based on 2D-SWE and preoperative laboratory parameters.
Patient selection in this study was not limited to HCC because the resection range was primarily determined by the location of the lesion, liver tolerance, and whether an R0 resection could be performed[36]. The surgical processing of malignant tumors and benign masses was similar despite the fact that these cannot be identified before surgery.
The preoperative parameters that were correlated with the durometer measurement in the present study may be divided into four categories: Coagulation, liver function, HBV infection, and fibrosis. Recent studies have demonstrated that platelets have a positive role in promoting liver regeneration and protecting hepatocytes[37]. A low platelet count caused by liver disease increases the risk of intraoperative bleeding. Operative blood loss has been reported as an independent predictor of both perioperative morbidity and mortality[36,38]. Preoperative albumin and cholinesterase were associated with liver hardness. A decrease in these two parameters indicates impaired liver synthetic function, which is consistent with the symptoms of liver fibrosis[39]. Decreased serum cholinesterase is an excellent biomarker of cirrhosis[40]. The presence of HBsAg is a hallmark of HBV infection[41], and titers of HBsAg are useful for discriminating different chronic hepatitis B phases and stages of chronic liver disease[42]. HA is one of the liver fibrosis serum biomarkers, and its level increases with advanced fibrosis. Hence, the level of HA has been used as an independent predictor of liver-related mortality in patients with liver disease[43]. Hansen et al[5] reported that a combination of multiple biomarkers improved diagnostic accuracy of high grades of liver fibrosis.
To select parameters for a linear regression model, we performed correlation analysis of preoperative parameters and durometer scores. The levels of seven parameters (TT, HBsAg, HA, stiffness, platelet count, albumin, cholinesterase) were significantly associated with the durometer measurement (Table 2). Using the plasma biochemical parameters mentioned above, we developed several possible multiple linear regression models. Model one that included three parameters (albumin, SWE, and HBsAg) had the best linear fit for durometer measurement. The R2 value for model one was 0.580, which was higher than the R2 value for the model based on stiffness measured by SWE alone. These results indicated that model one, which included serum parameters and liver stiffness, improved preoperative clinical assessment for liver hardness as opposed to stiffness alone.
Based on the ROC curve, durometer measurements, hardness scale, and palpation scale had a predictive ability for postoperative liver dysfunction. The predictive accuracy of 2D-SWE for all grades of PHLF in the derivation cohort was lower than that of the durometer measurement and palpation scale and the hardness scale in both cohorts. The AUROC of the validation cohort (0.747, P < 0.001) was higher than the results of 1D-SWE of HCC reported by Chong et al[19].
In comparing the characteristics of the hardness scale ≤ 27.87 and > 27.87 groups, age, platelet count, HA, and cholinesterase were significantly different between the two groups. The > 27.87 hardness scale group had a higher incidence of PHLF, which might have prolonged postoperative hospital stays.
Preoperative platelet < 150 × 109 U/L, albumin ≤ 42 g/L, ALT ≥ 44 U/L, major resection, and hardness-related parameters (stiffness, > 12 kPa, durometer scale, > 27.38 DU, hardness scale, > 27.87) were identified by univariate analyses as risk factors for PHLF. In the present study, the main preoperative factors that predicted PHLF were the presence of impaired preoperative liver function, chronic liver disease, and major liver resection, which are consistent with the conclusions drawn in previous studies[11,12,19].
Quantifying the hardness of liver tissue may aid surgeons in surgical planning and postoperative management. Liver hardness provides vital information regarding the liver. It indicates difficulty of hemostasis and possible postoperative liver dysfunction. Determining liver hardness before surgery using a hardness scale model may prompt the surgeon to prepare for intraoperative blood transfusion, low central venous pressure during liver parenchymal transection, and preventive administration of liver protective drugs. For cancer patients with a high-grade fibrosis but low hardness scale scores, appropriate expansion of the resection range while ensuring the minimum residual liver volume can be considered to ensure R0 resection. Therefore, liver hardness is important in daily surgical practice.
While the durometer is a useful tool, it remains difficult for surgeons to use durometer values in clinical decision-making. The quantified palpation method is intuitive and easier to apply than the durometer measurement. Palpation by surgeons has been shown to be correlated with hardness obtained by a durometer in the pancreas[44,45]. In the present study, we found that the palpation scale was correlated with the durometer measurement and was a reliable predictive factor for PHLF. Although the palpation scale is not a precise method for liver hardness, it provides surgeons with an intuitive method. The present study has several limitations. This study was restricted to the Chinese population. In China, HBV infection is the major cause of chronic fibrosis and cirrhosis[4]. Thus, the parameter reflecting the grades of HBV infections was included in the hardness scale model. In locations with other major causes of chronic fibrosis beside HBV infections, the parameters correlated with liver hardness may be different. The derivation cohort was derived from patients with durometer-measured-hardness. However, this resulted in a small cohort that did not allow us to allot some of these patients to a verification cohort. Although the predictive accuracy of the hardness scale model for PHLF has been validated, the predictive accuracy for durometer-measured hardness of the new model requires further validation for a larger cohort. In this cohort, the lack of patients with grade C PHLF led to the failure of 2D-SWE’s predictive efficiency for high-grade PHLF. The accuracy of palpation of different surgeons might be related to their surgical expertise. Therefore, the palpation scale should be verified by more surgeons with a range of surgical expertise.
In subsequent studies, additional surgical groups and patients should be included to improve the efficiency and accuracy of the hardness scale model. Moreover, the significance of liver hardness in perioperative management and long-time postoperative outcomes should continue to be explored. Considering that the degree of fibrosis may not be uniform in the liver, efforts to combine durometer readings and imaging LSM to establish a liver stiffness contour system must be useful. The ultimate goal should be to combine 3D printing technology with guide surgical planning and drill surgery procedures, which may in turn improve the accuracy of medicine and ERAS.
In this study, 2D-SWE showed a good correlation with durometer-measured liver hardness. The hardness scale model based on liver stiffness, HBsAg and albumin could predict liver hardness before surgery. Additionally, the model and liver hardness demonstrated PHLF predictive ability.
Preoperative noninvasive measurement of liver stiffness using shear wave elastography (SWE) is widely used to evaluate the degree of fibrosis. The SWE measurement involves applying a time-varying force to the tissue, and liver tissue with increased stiffness is considered harder, indicating severe fibrosis. Some studies reported that SWE predicted high-grade post-hepatectomy liver failure (PHF).
The results of the two-dimensional SWE are not a good representation of the actual hardness of the liver. Predicting liver hardness before surgery can prompt the surgeon to prepare for surgical practice.
This study aimed to construct a preoperative liver hardness model.
Correlation coefficients for durometer-measured hardness and preoperative parameters were calculated. Multiple linear regression models were constructed to select the best predictive durometer scale.
In the present study, we developed a linear regression model to predict liver hardness and found that surgeons’ subjective palpation scores were comparable with durometer measures of liver hardness. The hardness model had a good ability to predict PHF.
Liver stiffness assessed by two-dimensional shear wave elastography correlated well with durometer hardness values. The multiple linear regression model predicted durometer hardness values and post-hepatectomy liver failure.
The ultimate goal should be to combine three-dimensional printing technology to build a model that works in both touch and vision to guide surgical planning and drill surgery procedure with the spirit of accuracy medicine and enhanced recovery after surgery.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jamali R S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH
| 1. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1729] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 2. | Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35:2073-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Hackl C, Schlitt HJ, Renner P, Lang SA. Liver surgery in cirrhosis and portal hypertension. World J Gastroenterol. 2016;22:2725-2735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Zhang S, Wang F, Zhang Z. Current advances in the elimination of hepatitis B in China by 2030. Front Med. 2017;11:490-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Hansen JF, Christiansen KM, Staugaard B, Moessner BK, Lillevang S, Krag A, Christensen PB. Combining liver stiffness with hyaluronic acid provides superior prognostic performance in chronic hepatitis C. PLoS One. 2019;14:e0212036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Mosca A, Comparcola D, Romito I, Mantovani A, Nobili V, Byrne CD, Alisi A, Targher G. Plasma N-terminal propeptide of type III procollagen accurately predicts liver fibrosis severity in children with non-alcoholic fatty liver disease. Liver Int. 2019;39:2317-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Okanoue T, Ebise H, Kai T, Mizuno M, Shima T, Ichihara J, Aoki M. A simple scoring system using type IV collagen 7S and aspartate aminotransferase for diagnosing nonalcoholic steatohepatitis and related fibrosis. J Gastroenterol. 2018;53:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Dong H, Xu C, Zhou W, Liao Y, Cao J, Li Z, Hu B. The combination of 5 serum markers compared to FibroScan to predict significant liver fibrosis in patients with chronic hepatitis B virus. Clin Chim Acta. 2018;483:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Ogawa Y, Honda Y, Kessoku T, Tomeno W, Imajo K, Yoneda M, Kawanaka M, Kirikoshi H, Ono M, Taguri M, Saito S, Yamanaka T, Wada K, Nakajima A. Wisteria floribunda agglutinin-positive Mac-2-binding protein and type 4 collagen 7S: useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2018;33:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Mai RY, Ye JZ, Long ZR, Shi XM, Bai T, Chen J, Li LQ, Wu GB, Wu FX. Preoperative aspartate aminotransferase-to-platelet-ratio index as a predictor of posthepatectomy liver failure for resectable hepatocellular carcinoma. Cancer Manag Res. 2019;11:1401-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Mai RY, Wang YY, Bai T, Chen J, Xiang BD, Wu GB, Wu FX, Li LQ, Ye JZ. Combination Of ALBI And APRI To Predict Post-Hepatectomy Liver Failure After Liver Resection For HBV-Related HCC Patients. Cancer Manag Res. 2019;11:8799-8806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Wang H, Li L, Bo W, Liu A, Feng X, Hu Y, Tian L, Zhang H, Tang X, Zhang L, Zhang M. Immediate postoperative Fibrosis-4 predicts postoperative liver failure for patients with hepatocellular carcinoma undergoing curative surgery. Dig Liver Dis. 2018;50:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Petitclerc L, Sebastiani G, Gilbert G, Cloutier G, Tang A. Liver fibrosis: Review of current imaging and MRI quantification techniques. J Magn Reson Imaging. 2017;45:1276-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 607] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 15. | Xia S, Ren X, Ni Z, Zhan W. A Noninvasive Method-Shear-Wave Elastography Compared With Transient Elastography in Evaluation of Liver Fibrosis in Patients With Chronic Hepatitis B. Ultrasound Q. 2019;35:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Jian ZC, Long JF, Liu YJ, Hu XD, Liu JB, Shi XQ, Li WS, Qian LX. Diagnostic value of two dimensional shear wave elastography combined with texture analysis in early liver fibrosis. World J Clin Cases. 2019;7:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017; 152: 598-607. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 543] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 18. | Chou CT, Chen RC, Wu WP, Lin PY, Chen YL. Prospective Comparison of the Diagnostic Performance of Magnetic Resonance Elastography with Acoustic Radiation Force Impulse Elastography for Pre-operative Staging of Hepatic Fibrosis in Patients with Hepatocellular Carcinoma. Ultrasound Med Biol. 2017;43:2783-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Chong CC, Wong GL, Chan AW, Wong VW, Fong AK, Cheung YS, Wong J, Lee KF, Chan SL, Lai PB, Chan HL. Liver stiffness measurement predicts high-grade post-hepatectomy liver failure: A prospective cohort study. J Gastroenterol Hepatol. 2017;32:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Procopet B, Fischer P, Horhat A, Mois E, Stefanescu H, Comsa M, Graur F, Bartos A, Lupsor-Platon M, Badea R, Grigorescu M, Tantau M, Sparchez Z, Al Hajjar N. Good performance of liver stiffness measurement in the prediction of postoperative hepatic decompensation in patients with cirrhosis complicated with hepatocellular carcinoma. Med Ultrason. 2018;20:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Abe H, Midorikawa Y, Mitsuka Y, Aramaki O, Higaki T, Matsumoto N, Moriyama M, Haradome H, Abe O, Sugitani M, Tsuji S, Takayama T. Predicting postoperative outcomes of liver resection by magnetic resonance elastography. Surgery. 2017;162:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Wu T, Wang P, Zhang T, Zheng J, Li S, Zeng J, Kudo M, Zheng R. Comparison of Two-Dimensional Shear Wave Elastography and Real-Time Tissue Elastography for Assessing Liver Fibrosis in Chronic Hepatitis B. Dig Dis. 2016;34:640-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Dong Y, Sirli R, Ferraioli G, Sporea I, Chiorean L, Cui X, Fan M, Wang WP, Gilja OH, Sidhu PS, Dietrich CF. Shear wave elastography of the liver - review on normal values. Z Gastroenterol. 2017;55:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 391] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 25. | Yoon YC, Lee JS, Park SU, Kwon JH, Hong TH, Kim DG. Quantitative assessment of liver fibrosis using shore durometer. Ann Surg Treat Res. 2017;93:300-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Suzuki S, Watanabe Y, Yazawa T, Ishigame T, Sassa M, Monma T, Takawa T, Kumamoto K, Nakamura I, Ohoki S, Hatakeyama Y, Sakuma H, Ono T, Omata S, Takenoshita S. Tactile sensor is useful for estimating liver hardness and liver fibrosis compared with ultrasonography and computed tomography. Fukushima J Med Sci. 2014;60:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Alkozai EM, Nijsten MW, de Jong KP, de Boer MT, Peeters PM, Slooff MJ, Porte RJ, Lisman T. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (3)] |
| 28. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3566] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 29. | Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, Cosgrove D, Dietrich CF, Amy D, Bamber JC, Barr R, Chou YH, Ding H, Farrokh A, Friedrich-Rust M, Hall TJ, Nakashima K, Nightingale KR, Palmeri ML, Schafer F, Shiina T, Suzuki S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 482] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 30. | Wang M, Wang Y, Feng X, Wang R, Wang Y, Zeng H, Qi J, Zhao H, Li N, Cai J, Qu C. Contribution of hepatitis B virus and hepatitis C virus to liver cancer in China north areas: Experience of the Chinese National Cancer Center. Int J Infect Dis. 2017;65:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Nanashima A, Sakamoto A, Sakamoto I, Hayashi H, Abo T, Wakata K, Murakami G, Arai J, Wada H, Takagi K, Takeshita H, Hidaka S, To K, Nagayasu T. Usefulness of evaluating hepatic elasticity using artificial acoustic radiation force ultrasonography before hepatectomy. Hepatol Res. 2014;44:1308-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Ragazzo TG, Paranagua-Vezozzo D, Lima FR, de Campos Mazo DF, Pessoa MG, Oliveira CP, Alves VAF, Carrilho FJ. Accuracy of transient elastography-FibroScan®, acoustic radiation force impulse (ARFI) imaging, the enhanced liver fibrosis (ELF) test, APRI, and the FIB-4 index compared with liver biopsy in patients with chronic hepatitis C. Clinics (Sao Paulo). 2017;72:516-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Huang Z, Zheng W, Zhang YJ, Xu L, Chen JB, Chen JC, Chen MS, Zhou Z. Assessing Hepatic Fibrosis Using 2-D Shear Wave Elastography in Patients with Liver Tumors: A Prospective Single-Center Study. Ultrasound Med Biol. 2017;43:2522-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Lee MS, Bae JM, Joo SK, Woo H, Lee DH, Jung YJ, Kim BG, Lee KL, Kim W. Prospective comparison among transient elastography, supersonic shear imaging, and ARFI imaging for predicting fibrosis in nonalcoholic fatty liver disease. PLoS One. 2017;12:e0188321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1748] [Article Influence: 62.4] [Reference Citation Analysis (2)] |
| 36. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406. [PubMed] |
| 37. | Takahashi K, Liang C, Oda T, Ohkohchi N. Platelet and liver regeneration after liver surgery. Surg Today. 2020;50:974-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 38. | Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002;22:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 39. | Spinella R, Sawhney R, Jalan R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int. 2016;10:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 40. | Ramachandran J, Sajith KG, Priya S, Dutta AK, Balasubramanian KA. Serum cholinesterase is an excellent biomarker of liver cirrhosis. Trop Gastroenterol. 2014;35:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Song JE, Kim DY. Diagnosis of hepatitis B. Ann Transl Med. 2016;4:338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Chung KH, Kim W, Kim BG, Lee HY, Jin E, Cho Y, Seo JY, Kim HY, Jung YJ, Kim JW, Jeong JB, Lee KL. Hepatitis B Surface Antigen Quantification across Different Phases of Chronic Hepatitis B Virus Infection Using an Immunoradiometric Assay. Gut Liver. 2015;9:657-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Plevris N, Sinha R, Hay AW, McDonald N, Plevris JN, Hayes PC. Index serum hyaluronic acid independently and accurately predicts mortality in patients with liver disease. Aliment Pharmacol Ther. 2018;48:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Hong TH, Choi JI, Park MY, Rha SE, Lee YJ, You YK, Choi MH. Pancreatic hardness: Correlation of surgeon's palpation, durometer measurement and preoperative magnetic resonance imaging features. World J Gastroenterol. 2017;23:2044-2051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 45. | Belyaev O, Herden H, Meier JJ, Muller CA, Seelig MH, Herzog T, Tannapfel A, Schmidt WE, Uhl W. Assessment of pancreatic hardness-surgeon versus durometer. J Surg Res. 2010;158:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |