Published online Nov 15, 2018. doi: 10.4239/wjd.v9.i11.180
Peer-review started: July 23, 2018
First decision: August 3, 2018
Revised: August 12, 2018
Accepted: October 11, 2018
Article in press: October 11, 2018
Published online: November 15, 2018
Processing time: 114 Days and 1.9 Hours
The worldwide rise in the prevalence of obesity supports the need for an increased interaction between ongoing clinical research in the allied fields of gastrointestinal medicine/surgery and diabetes mellitus. There have been a number of clinically-relevant advances in diabetes, obesity, and metabolic syndrome emanating from gastroenterological research. Gastric emptying is a significant factor in the development of upper gastrointestinal symptoms. However, it is not the only mechanism whereby such symptoms occur in patients with diabetes. Disorders of intrinsic pacing are involved in the control of stomach motility in patients with gastroparesis; on the other hand, there is limited impact of glycemic control on gastric emptying in patients with established diabetic gastroparesis. Upper gastrointestinal functions related to emptying and satiations are significantly associated with weight gain in obesity. Medications used in the treatment of diabetes or metabolic syndrome, particularly those related to pancreatic hormones and incretins affect upper gastrointestinal tract function and reduce hyperglycemia and facilitate weight loss. The degree of gastric emptying delay is significantly correlated with the weight loss in response to liraglutide, a glucagon-like peptide-1 analog. Network meta-analysis shows that liraglutide is one of the two most efficacious medical treatments of obesity, the other being the combination treatment phentermine-topiramate. Interventional therapies for the joint management of obesity and diabetes mellitus include newer endoscopic procedures, which require long-term follow-up and bariatric surgical procedure for which long-term follow up shows advantages for individuals with diabetes. Newer bariatric procedures are presently undergoing clinical evaluation. On the horizon, combination therapies, in part directed at gastrointestinal functions, appear promising for these indications. Ongoing and future gastroenterological research when translated to care of individuals with diabetes mellitus should provide additional options to improve their clinical outcomes.
Core tip: The worldwide prevalence of obesity continues to rise. Delayed gastric emptying and impaired gastric accommodation result in upper gastrointestinal symptoms, through intrinsic nerve and pacemaker dysfunction. Glycemic control has a limited effect on gastric emptying in diabetic gastroparesis. Treatment of diabetes with pancreatic hormones and incretins inhibits gastric emptying, reduces hyperglycemia, and facilitates weight loss. Meta-analysis shows that glucagon-like peptide-1 analog, liraglutide, is one of the two most efficacious treatments of obesity. Bariatric surgery and endoscopic interventions are efficacious in diabetes and obesity, but long term follow-up is required for endoscopic interventions as well as for newer bariatric procedures. On the horizon, combination therapies directed at gastrointestinal function appear promising for these indications.
- Citation: Koch TR, Shope TR, Camilleri M. Current and future impact of clinical gastrointestinal research on patient care in diabetes mellitus. World J Diabetes 2018; 9(11): 180-189
- URL: https://www.wjgnet.com/1948-9358/full/v9/i11/180.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i11.180
In a recent international study of 195 countries, the prevalence of obesity doubled in more than 70 countries since 1980[1]. This worldwide rise in the prevalence of obesity supports the need for an increased interaction between ongoing clinical research in fields of gastrointestinal medicine/surgery and diabetes mellitus. There have been a number of clinically-relevant advances in diabetes, obesity and metabolic syndrome, emanating from gastrointestinal research. These advances include newer information in pharmacological or medical care, endoscopic procedures, and bariatric surgical procedures.
The most exciting gastrointestinal research areas relevant to diabetes are focused on the stomach and on weight loss, with the goals of resolution of hyperglycemia and/or prevention of secondary complications of diabetes mellitus. Ongoing studies have focused on the stomach because patients with diabetes develop upper gastrointestinal symptoms, including the syndrome of gastroparesis. In addition, pharmacological treatments and bariatric procedures directed to the stomach have been the most efficacious treatments of obesity. Some of these clinical gastrointestinal research observations are considered likely to impact patient care in diabetes mellitus and/or obesity and may thus lead to improved patient outcomes.
Based on a systematic review of the literature, including 92 gastric emptying studies (26 breath test, 62 scintigraphy, 1 ultrasound, and 3 wireless motility capsule) there is an association between optimally measured delayed gastric emptying and upper gastrointestinal symptoms[2]. Twenty-five of these studies provided quantitative data for meta-analysis (15 scintigraphy studies enrolling 4056 participants and 10 breath test studies enrolling 2231 participants). Evaluating the studies that used optimal gastric emptying test methodology, there were significant associations between gastric emptying and nausea, vomiting, abdominal pain, and early satiety/fullness in patients with upper gastrointestinal symptoms; gastric emptying and early satiety/fullness in patients with diabetes; and gastric emptying and nausea in patients with gastroparesis.
Among 108 adult patients with diabetes mellitus (60.2% females; median age 49.0 years; 71.3% with type 2 diabetes mellitus; one-third insulin dependent with median hemoglobin A1C 6.7%) presenting with upper gastrointestinal symptoms, the manifestations of diabetic triopathy (peripheral neuropathy, nephropathy, and retinopathy) were uncommon at the time of presentation[3]. Nausea was the most common symptom (80.6%). Gastric emptying was rapid in 37% and slow in 19%. Gastric accommodation was abnormal in 39%. There was normal gastric accommodation and gastric emptying in 28% and 40.3% of the patients with type 2 diabetes mellitus had accelerated gastric emptying at one hour. These observations emphasize the importance of measuring these functions in patients with upper gastrointestinal symptoms in order to individualize treatment, such as with a dopamine D2 antagonist or a 5-hydroxytryptamine receptor (5-HT4) agonist for patients with delayed gastric emptying and a 5-HT1A agonist in patients with impaired gastric accommodation.
In different morphological studies based on light microscopy examination of full-thickness gastric biopsies and immunofluorescence, there is evidence of reduction in the pacemaker cell repertoire (interstitial cells of Cajal[4,5] and fibroblast-like cells positive for platelet-derived growth factor alpha[6]), reduced numbers of neurons expressing nNOS[4], and reduced numbers of M2 macrophages, which normally express the mannose receptors (CD206) and heme oxygenase-1, mediate cell repair, and have anti-inflammatory roles[5]. Other studies show increase in CD68 immunocytes, suggesting immune-mediated damage to these pacing mechanisms[6], and this may be aggravated in the presence of vagal denervation, a common sequel of longstanding type 1 diabetes mellitus. Normally, the efferent vagus nerve signals release of norepinephrine from splenic nerves, activating the β2-adrenergic receptor expressed on T cells, and macrophages and other immune cells, suppressing the release of pro-inflammatory cytokines[7]. In summary, the interplay of vagal neuropathy, intrinsic neuropathy and immune modulation are considered combination factors leading to the gastric motility disorder.
The role of hyperglycemia in diabetic gastroparesis is unclear. On the one hand, there is epidemiological evidence of association of glycemia with upper gastrointestinal symptoms[8], documentation of poor glycemic control in 36% patients admitted to the hospital for exacerbations of diabetic gastroparesis[9], kidney and pancreas transplants improve gastric emptying and associated gastrointestinal symptoms[10]. Conversely, hemoglobin A1C was not a statistically significant predictor of abnormal (compared to normal) gastric emptying of solids in a study of 129 patients[11], and long-term blood glucose control had no significant effect on gastric emptying in type 2 diabetes mellitus[12].
The available literature suggests that the stomach emptying does have an impact on glycemic control, and not only in patients with gastroparesis. Some published reports confirm the notion that gastroparesis impacts glycemic control: (1) patients with gastroparesis as documented in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort, gastroparesis was associated with relatively worse glycemic control, as assessed by glycosylated hemoglobin[13]; (2) there is poor glycemic control in 36% patients admitted to the hospital for exacerbations of diabetic gastroparesis[9]; and (3) in insulin-treated patients with gastroparesis, delayed gastric emptying may increase the potential for a mismatch the timing of exogenous, preprandial, insulin and the actual delivery of nutrients such as glucose from the stomach to be absorbed from in the small intestine. In a study involving 11 type 1 patients, less insulin was required to achieve euglycemia during the first 120 min after a meal in the 5 with gastroparesis, and more between 180-240 min[14]. In addition, there is also evidence that the rate of gastric emptying has a major impact on the glycemic response to carbohydrate-containing meals in health and diabetes, particularly the initial postprandial increment[15]. Therefore, it is now appreciated that postprandial glycemic excursions make a major contribution to “overall” glycemic control as assessed by hemoglobin A1C. Delayed gastric emptying in type 1 diabetes has recently been reported to be associated with an overall increase in blood glucose during the day; this may reflect the mismatch between the preprandial insulin and the later absorption of food due to the delayed gastric emptying[16].
Liraglutide, a long-acting glucagon-like peptide-1 (GLP-1) receptor agonist, is approved for treatment of obesity; however, the mechanisms of action of liraglutide are incompletely understood and include increase in satiety, increase in resting energy expenditure, and direct effects on appetite centers in the brain[17]. In a randomized, double-blind, placebo-controlled trial of subcutaneous liraglutide (3.0 mg) in 40 patients at Mayo Clinic, liraglutide delayed gastric emptying of solids at 5 wk and 16 wk, and there was significantly greater weight loss and lower volume of a nutrient drink to reach the maximum tolerated volume in the liraglutide group than in the placebo group. The effects of liraglutide on weight loss are associated with delay in gastric emptying of solids, and the measurement of gastric emptying (e.g., at 5 wk of treatment) may be a biomarker of responsiveness and may help to select individuals for prolonged treatment with this class of drug[18].
The effect of GLP-1 receptor agonist on weight loss does not appear to be impacted by the presence of metabolic derangements such as type 2 diabetes. However, there is evidence of a significant correlation in the weight loss induced by liraglutide and delay of gastric emptying[18].
Incretin and pancreatic hormones [e.g., amylin, glucagon, glucose-stimulated insulinotropic peptide (GIP), GLP-1 and peptide tyrosine tyrosine (PYY)] generally inhibit upper gastrointestinal motor function[19] or secretion (e.g., oxyntomodulin). Moreover, many of these hormones also exert central effects that reduce appetite[20], and some (e.g., GLP-1 analogs or GLP-1 receptor agonists) are efficacious in the treatment of obesity[21].
Several combined incretin hormones have been tested in the context of obesity. Co-administration of GLP-1 with glucagon in humans increased energy expenditure and reduced food intake[22,23]. A unimolecular dual incretin consisting of PEGylated GLP-1 and GIP co-agonist maximized metabolic benefits in rodents, monkeys, and humans[24].
The combination of GLP-1 and PYY3-36 also exerts synergistic effects with a reduction of 30.4% of food intake compared to placebo and more than the sum of each hormone independently, suggesting a synergistic effect[25]. Acute, continuous, subcutaneous infusion for 10.5 h/d of GLP-1, PYY, and oxyntomodulin (summarized as GOP) was administered at doses that replicate postprandial levels observed after Roux-en-Y gastric bypass in a placebo-controlled, crossover study. GOP reduced food intake with a mean reduction of 32% without significantly altering resting energy expenditure[26].
One study compared the effects of an intragastric balloon in 64 patients compared to a combination of balloon plus liraglutide, up to 1.8 mg/d, in 44 patients matched for body mass index (BMI) at baseline[27]. The mean weight loss after balloon removal was 8.3 kg greater in the balloon plus liraglutide group than in the balloon alone group, and the advantage persisted 6 mo post-balloon removal in the group receiving liraglutide[27].
The development of endoscopic interventions for treatment of obesity and diabetes mellitus has focused on two areas (Table 1), the placement of intraluminal devices and intraluminal suturing[28,29].
The first intragastric balloon, the Garren-Edwards bubble, was approved by the United States Food and Drug Administration in 1985. The United States Food and Drug Administration has now approved 3 separate intragastric balloon systems in the past 3 years: The Orbera balloon, the ReShape balloon, and the Obalon balloon. Delayed gastric emptying has been identified as a mechanism for weight loss in individuals who have undergone insertion of a fluid-filled intragastric balloon[29], which raises the question of their utility in individuals with diabetic gut autonomic neuropathy. After the intraluminal balloons are removed, individuals required a maintenance program (which has not yet been standardized) to prevent weight regain. Our previous concern[29] that specialized training is needed for the use of these devices appears to be supported by three warnings (in February 2017, August 2017, and June 2018) from the United States Food and Drug Administration with regards to issues related to intragastric balloons including multiple deaths related to intragastric balloons. Two major reviews in the past two years examined the 30+ year experience with intragastric balloons[30,31]. Brethauer et al[30] concluded that more study was required in patients with type 2 diabetes mellitus. Popov et al[31] concluded that intragastric balloons were more effective that diet alone for an initial improvement of metabolic risk factors, but that their conclusions are limited by the small number of participants and the lack of long-term follow-up data.
Some countries have the availability of a duodenojejunal bypass sleeve, termed the EndoBarrier. This impermeable fluoropolymer sleeve with a nitinol anchor is deployed from the duodenal bulb and into the jejunum under fluoroscopic and endoscopic guidance. A clinical trial of this device in the United States in individuals with diabetes mellitus was halted early due to the development of liver infections. Three studies involving the EndoBarrier in individuals with type 2 diabetes mellitus have been published in the past one year. Betzel et al[32] were able to implant the device in 185 out of 198 participants. Sixty-nine percent of the participants were able to complete a one year program prior to removal of the device. Hemoglobin A1C levels declined by a mean of 9%, but no long term data was available. Forner et al[33] reported their findings in 114 individuals who maintained an EndoBarrier for a mean of 51.1 wk after its placement; the authors reported that mean Hemoglobin A1C was not significantly improved but that 6 subjects developed device obstructions, 5 individuals had gastrointestinal hemorrhage, 2 individuals developed liver abscesses, and 1 individual developed acute pancreatitis. Patel et al[34] reported a multicenter trial involved EndoBarrier Placement in 45 individuals with type 2 diabetes. Thirty-one individuals (69%) completed the 12 mo study. The mean hemoglobin A1C reduction at 12 mo was 0.8% below baseline. After explant, these subjects were only followed for an additional 6 mo.
The AspireAssist (Aspire Bariatrics, King of Prussia, Pennsylvania, United States) was approved by the United States Food and Drug Administration in 2016. A specialized aspiration tube (with both an intragastric portion with holes to permit aspiration as well as a skin port) is placed percutaneously at upper endoscopy into an individual’s stomach. Stomach contents are then aspirated 20 min after a meal containing more than 200 kcal. A recent European trial examining the aspiration tube described a decrease in Hemoglobin A1C from 7.8% at baseline to 6.8% at only 1 year post-placement and mean percent weight loss of 19.2% at 4 years post-placement[35].
As described above, long-term weight loss and metabolic results are not available for these endoscopic devices. Therefore their role in the treatment of obese individuals with diabetes mellitus remains to be defined.
Formation of an endoscopic sleeve gastroplasty or a transoral gastroplasty has been described by using intraluminal suturing devices during upper endoscopy. The Mayo Clinic, Rochester, Minnesota United States reported a method in 2013 for an endoscopic sleeve gastroplasty. This procedure involves the use of a commercially available suturing device (OverStitch, Apollo Endosurgery, Austin, Texas United States), and the endoscopic sleeve gastrectomy is the predominant intraluminal technique presently described in the literature. Development of an endoscopic procedure to mimic the surgical vertical sleeve gastrectomy could reduce the risks of a gastric leak or perforation and of general anesthesia. However, results from the surgical literature support the importance of obtaining long-term weight loss data. When the Mayo Clinic, Rochester, Minnesota, United States reported their results from the surgical non-banded vertical gastroplasty, only 31% of patients were judged to have persistent excess weight lost after 4 years[36]. A proposed mechanism for weight loss after the endoscopic sleeve gastrectomy is slowing of gastric emptying, which raises the question of its utility in individuals with diabetic gut autonomic neuropathy. Further research should better define the potential long-term role of intraluminal suturing in weight loss and the treatment of obese individuals with diabetes mellitus.
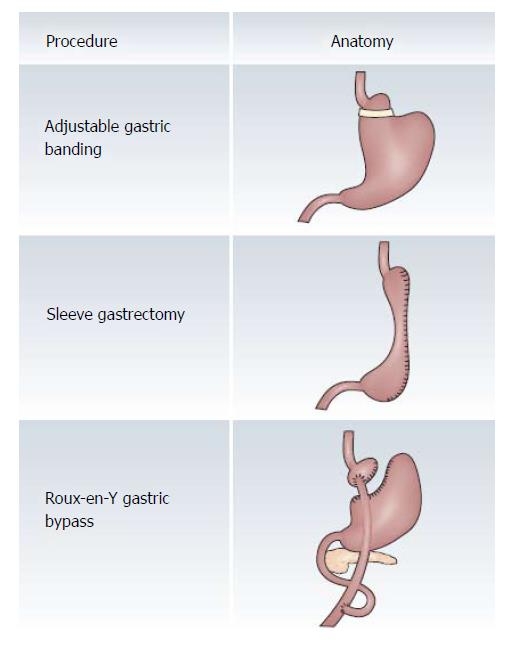
The well described and worldwide utilized bariatric surgical procedures[37] include the adjustable gastric band, the vertical sleeve gastrectomy, and the Roux-en-Y gastric bypass (Figure 1). By 2014, there were 579000 yearly bariatric surgical procedures of which 45.9% were the vertical sleeve gastrectomy, 39.6% were the Roux-en-Y gastric bypass, and 7.4% were the adjustable gastric band[38]. The adjustable gastric band systems now use a soft, silicone ring which is placed around the upper part of the stomach approximately 4 cm below the gastroesophageal junction and is connected to an access port by tubing to adjust the band volume. Restriction of the proximal stomach is altered by addition or removal of sterile saline through the access port and there is no cutting or stapling of the stomach or bypass of small intestine. The vertical sleeve gastrectomy or gastric sleeve resection can be completed with a single step restrictive operation. By resection of 60% to 80% of the stomach along the greater curvature, multiple staplers can produce a tubular gastric pouch. Weight loss after vertical sleeve gastrectomy appears to involve several potential mechanisms in addition to restriction in the size of meal portions[39]. In the Roux-en-Y gastric bypass, there is complete division of native stomach with production of a gastric pouch of less than 30 mL. The surgeon divides the jejunum 30 to 70 cm distal to the junction of the duodenum with the jejunum. The location of the jejuno-enteric anastomosis determines the lengths of the Roux limb (e.g., the gastric pouch to the jejuno-enteric anastomosis) and the common channel (e.g., the jejuno-enteric anastomosis to the ileocecal valve). A common channel that is shorter than 120 cm can induce a severe malabsorptive disorder. The mechanisms of weight loss after gastric bypass are complex and can include upper gut bacterial overgrowth, a common intestinal disorder in individuals with diabetes mellitus, as well as glucose malabsorption[40]. Studies of glucose malabsorption after gastric bypass are of interest because jejunal administration of glucose appears to suppress plasma levels of the orexigenic hormone, acyl ghrelin[41]. Further studies of the mechanisms of weight loss after bariatric surgery are clearly important since a proportion of individuals have poor long-term weight loss[42].
The potential importance of bariatric surgery in individuals with diabetes mellitus was well publicized following a 2007 report that after a mean follow up of 7.1 years, individuals who underwent gastric bypass surgery had a 40% decrease in their adjusted long-term mortality (but by 92% for diabetes) compared to the control group[43]. This landmark study has been supported by a report of decreased mortality compared to usual care at 16 years in the Swedish Obese Subjects trial[44]. In a national study from Israel, bariatric surgery at a mean follow up of 4.5 years was shown to lower all-cause mortality compared to usual care obesity management[45]. Finally, in a recent examination of the American National Health and Nutrition Examination Survey, it was reported that bariatric surgery can result in a relevant reduction of mortality in the United States obese population[46].
Evaluation of the results reported for the different major bariatric surgical procedures may vary dependent upon whether single center data or multicenter data is examined. The largest discrepancy appears in reports concerning the adjustable gastric band, which may in part explain its decreased worldwide utilization. Several major reports in the past one year have supported major weaknesses of the adjustable band. In a single center report from Switzerland, after over 10 years of follow up, 71% of patients had lost their gastric band and only 15% of patients had good to excellent results[47]. In a French national study of 52868 patients up to 7 years after adjustable gastric banding, the band removal rate was about 6% per year[48]. In a study from the state of New York, among 16444 patients who underwent adjustable gastric banding, with at least four years of follow up the rate of revisions/conversions was 26.0%[49]. An early meta-analysis reported that bariatric surgery does result in a weight loss of 20 to 30 kg, which is maintained for up to 10 years[50]. A follow up meta-analyses in 2013 by Gloy and associates reported in a shorter follow up that individuals allocated to bariatric surgery lost a mean of 26 kg more body weight[51]. Representative reports of long-term weight loss after bariatric surgery are summarized in Table 2. The most effective bariatric surgical procedure for weight loss at up to 15 years of post-operative follow up is the Roux-en-Y gastric bypass surgery[44,52]. The least effective major bariatric surgical procedure in long term studies of weight loss is the adjustable gastric band[44,52,56].
| Ref. | Studya | Type of surgeryb | Follow up | Result (%)1 |
| [52] | MA | AGB | ≥ 10 yr | EWL: 47.4 |
| [52] | MA | VSG | ≥ 5 yr | EWL: 53.2 |
| [52] | MA | RYGB | ≥ 10 yr | EWL: 63.5 |
| [44] | MCS | AGB | 15 yr | MWL: 13.0 |
| [44] | MCS | RYGB | 15 yr | MWL: 27.0 |
| [53] | SCS | VSG | 8 yr | EWL: 67.0 |
| [54] | SCS | VSG | 8 yr | EWL: 51.1 |
| [55] | MCS | VSG | 10 yr | EWL: 70.5 |
| [56] | SR | AGB | 3-5 yr | EWL: 45.0 |
| [56] | SR | VSG | 3-5 yr | EWL: 64.5 |
| [56] | SR | RYGB | 3-5 yr | EWL: 65.7 |
A joint statement by international diabetes organizations supports consideration of bariatric surgery in individuals with diabetes mellitus and: BMI ≥ 40 kg/m2, BMI 35-39.9 kg/m2 and inadequate control of hyperglycemia with optimal medical therapy, or BMI 30-34.9 kg/m2 and inadequate control of hyperglycemia with oral or injectable medications[59]. In the United States, individuals with diabetes mellitus considering bariatric surgery are evaluated if they fulfill National Institutes of Health criteria, which is a BMI of ≥ 35 kg/m2, while in other countries individuals with diabetes and a BMI as low as 25 kg/m2 may be considered for Roux-en-Y gastric bypass. Specific bariatric surgical procedures such as the vertical sleeve gastrectomy may not be effective for treatment of individuals with type 1 diabetes mellitus[60]. A meta-analysis comparing non-surgical treatment for obesity with bariatric surgery concluded that individuals allocated to bariatric surgery had a higher remission rate of type 2 diabetes[51]. A second meta-analysis with 5 years of follow-up confirmed a significant decline in the relative risk of diabetes after bariatric surgery[61]. Representative reports of long-term control of diabetes mellitus after bariatric surgery are summarized in Table 3. The most effective bariatric surgical procedure for remission of diabetes mellitus at up to 6 years of post-operative follow up is the Roux-en-Y gastric bypass surgery[56,62]. The least effective major bariatric surgical procedure in long term studies of remission of diabetes mellitus is the adjustable gastric band[56,62]. The importance of remission of diabetes is supported by a report of decreased incidence of microvascular and macrovascular complications in post-operative bariatric patients compared to controls[58]. These published results do support the importance of ongoing development of more effective bariatric surgical procedures for the treatment of individuals with obesity and type 2 diabetes mellitus.
| Ref. | Type of studya | Surgeryb | Follow up | Result1 |
| [53] | SCS | VSG | 8 yr | NoRMRxDM: 43.4% |
| [54] | SCS | VSG | 8 yr | NoRMRxDM: 37% |
| [56] | SR | AGB | 3-5 yr | NoRMRxDM: 28.6% |
| [56] | SR | RYGB | 3-5 yr | NoRMRxDM: 66.7% |
| [57] | SCS | RYGB | 9 yr | NoRMRxDM: 73% |
| [58] | MCS | AGB | 15 yr | NoRMRxDM: 38% |
| [58] | MCS | RYGB | 15 yr | NoRMRxDM: 35% |
| [62] | NPBCS | AGB | 6 yr | NoRMRxDM: 32% |
| [62] | NPBCS | VSG | 6 yr | NoRMRxDM: 41% |
| [62] | NPBCS | RYGB | 6 yr | NoRMRxDM: 58% |
| [63] | SCS | AGB | 10 yr | NoRMRxDM: 18% |
Gastrointestinal surgeons who specialize in bariatrics have seen an improvement in weight related comorbidities for decades. Encouraged by mounting evidence of resolution or substantial improvement in diseases such as diabetes mellitus, hyperlipidemia, and hypertension, leaders in the field created a paradigm shift by renaming the American Society of Bariatric Surgery as the American Society of Metabolic and Bariatric Surgery in 2007 (ASMBS.org; accessed on July 15, 2018). Gastrointestinal surgeons continue to manipulate the gastrointestinal tract in an effort to maximize the physiologic benefit to the individual patient. Restrictive procedures (i.e., vertical sleeve gastrectomy, adjustable gastric banding, and the largely abandoned vertical banded gastroplasty) provide a benefit usually in proportion to the absolute weight loss achieved. Newer endoscopic and surgical procedures including the intragastric balloon, vBloc (described below), and aspirational therapy have more modest results.
Procedures that combine restriction with malabsorption by bypassing a portion of the foregut and midgut do provide measurable changes in comorbid conditions out of proportion to absolute weight loss. The traditional “gold standard” of surgical weight loss procedures, the Roux-en-Y gastric bypass, can provide rapid glucose control for patients. This procedure may take advantage of the “foregut hypothesis” that bypassing the foregut reduces or suppresses the secretion of anti-incretin hormones, which in turn leads to improvement of blood glucose control[64]. Proponents of the “hindgut hypothesis” feel this improvement is more likely secondary to rapid delivery of nutrients to the distal small intestine, which facilitates the release of hormones such as GLP-1[64,65]. Procedures which take advantage of this scenario include the biliopancreatic diversion/duodenal switch. Based upon these previous observations and notions, more recent modifications to this malabsorption procedure have shown favorable results in treatment of metabolic diseases, with up to 4 years of follow up[66,67]. Further refinements in these procedures will likely yield more promising results which may be able to be individualized for specific patient needs.
Another area of ongoing, active clinical research has been vagal nerve stimulation, based on the important role the vagus nerve plays in regulation pathways involving short-term regulation of dietary intake. The therapy termed vBloc for Vagal BLOCking therapy (ReShape Lifesciences, San Clemente, California United States) uses intermittent intra-abdominal high-frequency electrical currents for vagal blocking. At laparoscopy, electrodes are placed on the two vagal trunks near the gastroesophageal junction. There is no anatomical modification and an external controller is used to program the device. Vagal nerve stimulation with vBloc in a 2 year study has shown promise for weight loss (mean of 21% of excess weight loss), but with only an marginal impact on diabetes (only a 0.3% decline in hemoglobin A1C)[68]. Further evaluation is therefore required to determine which patients with obesity and diabetes mellitus may benefit from this bariatric procedure.
The worldwide prevalence of obesity continues to rise. This rise increases the incidence of type 2 diabetes mellitus with subsequent requirements for additional health care in countries across the world. This supports the need for an increased interaction between ongoing clinical research in the allied fields of gastrointestinal medicine/surgery and diabetes mellitus. Among the clinically-relevant advances in diabetes, obesity, and metabolic syndrome emanating from gastroenterological research, delayed gastric emptying and impaired gastric accommodation result in upper gastrointestinal symptoms, through intrinsic nerve and pacemaker dysfunction. Glycemic control has a limited effect on gastric emptying in diabetic gastroparesis. Treatment of diabetes with pancreatic hormones and incretins inhibits gastric emptying, reduces hyperglycemia, and facilitates weight loss. The GLP-1 analog, liraglutide, is one of the two most efficacious treatments of obesity. New bariatric endoscopic procedures have been developed for weight loss in individuals with obesity, but long term follow-up with regards to maintenance of weight loss and control of hyperglycemia in individuals with diabetes is required prior to mass introduction of these endoscopic interventions. Bariatric surgical procedures are efficacious in diabetes and obesity, but a proportion of individuals have poor long-term weight loss after bariatric surgery. On the horizon, combination therapies directed at gastrointestinal function and newer bariatric surgical procedures appear promising for individuals with obesity and type 2 diabetes mellitus.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Reggiani GM, Tziomalos K S- Editor: Dou Y L- Editor: A E- Editor: Bian YN
| 1. | GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5048] [Article Influence: 631.0] [Reference Citation Analysis (2)] |
| 2. | Vijayvargiya P, Jameie-Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 3. | Chedid V, Brandler J, Vijayvargiya P, Park SY, Szarka LA, Camilleri M. Characterization of Upper Gastrointestinal Symptoms, Gastric Motor Functions, and Associations in Patients with Diabetes at a Referral Center. Am J Gastroenterol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575-85.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 5. | Bernard CE, Gibbons SJ, Mann IS, Froschauer L, Parkman HP, Harbison S, Abell TL, Snape WJ, Hasler WL, McCallum RW, Sarosiek I, Nguyen LA, Koch KL, Tonascia J, Hamilton FA, Kendrick ML, Shen KR, Pasricha PJ, Farrugia G; NIDDK Gastroparesis Clinical Research Consortium (GpCRC). Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol Motil. 2014;26:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Herring BP, Hoggatt AM, Gupta A, Griffith S, Nakeeb A, Choi JN, Idrees MT, Nowak T, Morris DL, Wo JM. Idiopathic gastroparesis is associated with specific transcriptional changes in the gastric muscularis externa. Neurogastroenterol Motil. 2018;30:e13230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Han B, Li X, Hao J. The cholinergic anti-inflammatory pathway: An innovative treatment strategy for neurological diseases. Neurosci Biobehav Rev. 2017;77:358-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Uppalapati SS, Ramzan Z, Fisher RS, Parkman HP. Factors contributing to hospitalization for gastroparesis exacerbations. Dig Dis Sci. 2009;54:2404-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Gaber AO, Oxley D, Karas J, Cardoso S, Hathaway D, Shokouh-Amiri MH, Jensen SL, Abell TL. Changes in gastric emptying in recipients of successful combined pancreas-kidney transplants. Dig Dis. 1991;9:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Bharucha AE, Camilleri M, Forstrom LA, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf). 2009;70:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Holzäpfel A, Festa A, Stacher-Janotta G, Bergmann H, Shnawa N, Brannath W, Schernthaner G, Stacher G. Gastric emptying in Type II (non-insulin-dependent) diabetes mellitus before and after therapy readjustment: no influence of actual blood glucose concentration. Diabetologia. 1999;42:1410-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, Driscoll M, Harth J, Larkin M, Christofi M. Delayed Gastric Emptying Is Associated With Early and Long-term Hyperglycemia in Type 1 Diabetes Mellitus. Gastroenterology. 2015;149:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Ishii M, Nakamura T, Kasai F, Onuma T, Baba T, Takebe K. Altered postprandial insulin requirement in IDDM patients with gastroparesis. Diabetes Care. 1994;17:901-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Parthasarathy G, Kudva YC, Low PA, Camilleri M, Basu A, Bharucha AE. Relationship Between Gastric Emptying and Diurnal Glycemic Control in Type 1 Diabetes Mellitus: A Randomized Trial. J Clin Endocrinol Metab. 2017;102:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Horowitz M, Flint A, Jones KL, Hindsberger C, Rasmussen MF, Kapitza C, Doran S, Jax T, Zdravkovic M, Chapman IM. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2012;97:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Halawi H, Khemani D, Eckert D, O’Neill J, Kadouh H, Grothe K, Clark MM, Burton DD, Vella A, Acosta A. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2:890-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 19. | Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Zanchi D, Depoorter A, Egloff L, Haller S, Mählmann L, Lang UE, Drewe J, Beglinger C, Schmidt A, Borgwardt S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci Biobehav Rev. 2017;80:457-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 21. | Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA. 2016;315:2424-2434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 556] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 22. | Tan TM, Field BC, McCullough KA, Troke RC, Chambers ES, Salem V, Gonzalez Maffe J, Baynes KC, De Silva A, Viardot A. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Cegla J, Troke RC, Jones B, Tharakan G, Kenkre J, McCullough KA, Lim CT, Parvizi N, Hussein M, Chambers ES. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63:3711-3720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Finan B, Ma T, Ottaway N, Müller TD, Habegger KM, Heppner KM, Kirchner H, Holland J, Hembree J, Raver C. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 492] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 25. | Schmidt JB, Gregersen NT, Pedersen SD, Arentoft JL, Ritz C, Schwartz TW, Holst JJ, Astrup A, Sjödin A. Effects of PYY3-36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am J Physiol Endocrinol Metab. 2014;306:E1248-E1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Tan T, Behary P, Tharakan G, Minnion J, Al-Najim W, Albrechtsen NJW, Holst JJ, Bloom SR. The Effect of a Subcutaneous Infusion of GLP-1, OXM, and PYY on Energy Intake and Expenditure in Obese Volunteers. J Clin Endocrinol Metab. 2017;102:2364-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Mosli MM, Elyas M. Does combining liraglutide with intragastric balloon insertion improve sustained weight reduction? Saudi J Gastroenterol. 2017;23:117-122. [PubMed] |
| 28. | Rashti F, Gupta E, Ebrahimi S, Shope TR, Koch TR, Gostout CJ. Development of minimally invasive techniques for management of medically-complicated obesity. World J Gastroenterol. 2014;20:13424-13445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Koch TR, Shope TR, Gostout CJ. Organization of future training in bariatric gastroenterology. World J Gastroenterol. 2017;23:6371-6378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Brethauer SA, Chang J, Galvao Neto M, Greve JW. Gastrointestinal devices for the treatment of type 2 diabetes. Surg Obes Relat Dis. 2016;12:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Popov VB, Ou A, Schulman AR, Thompson CC. The Impact of Intragastric Balloons on Obesity-Related Co-Morbidities: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Betzel B, Homan J, Aarts EO, Janssen IMC, de Boer H, Wahab PJ, Groenen MJM, Berends FJ. Weight reduction and improvement in diabetes by the duodenal-jejunal bypass liner: a 198 patient cohort study. Surg Endosc. 2017;31:2881-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Forner PM, Ramacciotti T, Farey JE, Lord RV. Safety and Effectiveness of an Endoscopically Placed Duodenal-Jejunal Bypass Device (EndoBarrier®): Outcomes in 114 Patients. Obes Surg. 2017;27:3306-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Patel N, Mohanaruban A, Ashrafian H, Le Roux C, Byrne J, Mason J, Hopkins J, Kelly J, Teare J. EndoBarrier®: a Safe and Effective Novel Treatment for Obesity and Type 2 Diabetes? Obes Surg. 2018;28:1980-1989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Nyström M, Machytka E, Norén E, Testoni PA, Janssen I, Turró Homedes J, Espinos Perez JC, Turro Arau R. Aspiration Therapy As a Tool to Treat Obesity: 1- to 4-Year Results in a 201-Patient Multi-Center Post-Market European Registry Study. Obes Surg. 2018;28:1860-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Hocking MP, Kelly KA, Callaway CW. Vertical gastroplasty for morbid obesity: clinical experience. Mayo Clin Proc. 1986;61:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8:544-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 38. | Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27:2279-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 580] [Cited by in RCA: 549] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 39. | Sharbaugh ME, Shope TR, Koch TR. Upper gut bacterial overgrowth is a potential mechanism for glucose malabsorption after vertical sleeve gastrectomy. New Insights Obes Gent Beyond. 2017;1:30-35. [DOI] [Full Text] |
| 40. | Andalib I, Shah H, Bal BS, Shope TR, Finelli FC, Koch TR. Breath Hydrogen as a Biomarker for Glucose Malabsorption after Roux-en-Y Gastric Bypass Surgery. Dis Markers. 2015;2015:102760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Tamboli RA, Sidani RM, Garcia AE, Antoun J, Isbell JM, Albaugh VL, Abumrad NN. Jejunal administration of glucose enhances acyl ghrelin suppression in obese humans. Am J Physiol Endocrinol Metab. 2016;311:E252-E259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Pucci A, Batterham RL. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J Endocrinol Invest. 2018;1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 43. | Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1882] [Cited by in RCA: 1682] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 44. | Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1260] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 45. | Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer I, Rasmussen-Torvik LJ, Balicer RD. Association of Bariatric Surgery Using Laparoscopic Banding, Roux-en-Y Gastric Bypass, or Laparoscopic Sleeve Gastrectomy vs Usual Care Obesity Management With All-Cause Mortality. JAMA. 2018;319:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 46. | Gaeta M, Rausa E, Malavazos AE, Bonavina L, Smuts CM, Ricci C. Bariatric Surgery to Reduce Mortality in US Adults. A Public Health Perspective from the Analysis of the American National Health and Nutrition Examination Survey Linked to the US Mortality Register. Obes Surg. 2018;28:900-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Vinzens F, Kilchenmann A, Zumstein V, Slawik M, Gebhart M, Peterli R. Long-term outcome of laparoscopic adjustable gastric banding (LAGB): results of a Swiss single-center study of 405 patients with up to 18 years’ follow-up. Surg Obes Relat Dis. 2017;13:1313-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Lazzati A, De Antonio M, Paolino L, Martini F, Azoulay D, Iannelli A, Katsahian S. Natural History of Adjustable Gastric Banding: Lifespan and Revisional Rate: A Nationwide Study on Administrative Data on 53,000 Patients. Ann Surg. 2017;265:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Altieri MS, Yang J, Nie L, Blackstone R, Spaniolas K, Pryor A. Rate of revisions or conversion after bariatric surgery over 10 years in the state of New York. Surg Obes Relat Dis. 2018;14:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 944] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 51. | Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 938] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 52. | Golzarand M, Toolabi K, Farid R. The bariatric surgery and weight losing: a meta-analysis in the long- and very long-term effects of laparoscopic adjustable gastric banding, laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy on weight loss in adults. Surg Endosc. 2017;31:4331-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 53. | Noel P, Nedelcu M, Eddbali I, Manos T, Gagner M. What are the long-term results 8 years after sleeve gastrectomy? Surg Obes Relat Dis. 2017;13:1110-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Kowalewski PK, Olszewski R, Walędziak MS, Janik MR, Kwiatkowski A, Gałązka-Świderek N, Cichoń K, Brągoszewski J, Paśnik K. Long-Term Outcomes of Laparoscopic Sleeve Gastrectomy-a Single-Center, Retrospective Study. Obes Surg. 2018;28:130-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 55. | Chang DM, Lee WJ, Chen JC, Ser KH, Tsai PL, Lee YC. Thirteen-Year Experience of Laparoscopic Sleeve Gastrectomy: Surgical Risk, Weight Loss, and Revision Procedures. Obes Surg. 2018;28:2991-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Puzziferri N, Roshek TB 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312:934-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 678] [Cited by in RCA: 599] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 57. | MacDonald KG Jr, Long SD, Swanson MS, Brown BM, Morris P, Dohm GL, Pories WJ. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg. 1997;1:213-220; discussion 220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 298] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 58. | Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, Bouchard C, Carlsson B, Karason K, Lönroth H, Näslund I, Sjöström E, Taube M, Wedel H, Svensson PA, Sjöholm K, Carlsson LM. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 741] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 59. | Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: a Joint Statement by International Diabetes Organizations. Obes Surg. 2017;27:2-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 60. | Al Sabah S, Al Haddad E, Muzaffar TH, Almulla A. Laparoscopic Sleeve Gastrectomy for the Management of Type 1 Diabetes Mellitus. Obes Surg. 2017;27:3187-3193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Ricci C, Gaeta M, Rausa E, Asti E, Bandera F, Bonavina L. Long-term effects of bariatric surgery on type II diabetes, hypertension and hyperlipidemia: a meta-analysis and meta-regression study with 5-year follow-up. Obes Surg. 2015;25:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 62. | Thereaux J, Lesuffleur T, Czernichow S, Basdevant A, Msika S, Nocca D, Millat B, Fagot-Campagna A. Association Between Bariatric Surgery and Rates of Continuation, Discontinuation, or Initiation of Antidiabetes Treatment 6 Years Later. JAMA Surg. 2018;153:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 63. | Wentworth JM, Cheng C, Laurie C, Skinner S, Burton PR, Brown WA, O’Brien PE. Diabetes Outcomes More than a Decade Following Sustained Weight Loss After Laparoscopic Adjustable Gastric Band Surgery. Obes Surg. 2018;28:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab. 2009;35:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 65. | Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447-E453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 66. | Cottam A, Cottam D, Zaveri H, Cottam S, Surve A, Medlin W, Richards C. An Analysis of Mid-Term Complications, Weight Loss, and Type 2 Diabetes Resolution of Stomach Intestinal Pylorus-Sparing Surgery (SIPS) Versus Roux-En-Y Gastric Bypass (RYGB) with Three-Year Follow-Up. Obes Surg. 2018;28:2894-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 67. | Zaveri H, Surve A, Cottam D, Cottam A, Medlin W, Richards C, Belnap L, Cottam S, Horsley B. Mid-term 4-Year Outcomes with Single Anastomosis Duodenal-Ileal Bypass with Sleeve Gastrectomy Surgery at a Single US Center. Obes Surg. 2018;28:3062-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 68. | Apovian CM, Shah SN, Wolfe BM, Ikramuddin S, Miller CJ, Tweden KS, Billington CJ, Shikora SA. Two-Year Outcomes of Vagal Nerve Blocking (vBloc) for the Treatment of Obesity in the ReCharge Trial. Obes Surg. 2017;27:169-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |