Published online Jul 15, 2017. doi: 10.4239/wjd.v8.i7.374
Peer-review started: November 14, 2016
First decision: December 1, 2016
Revised: February 5, 2017
Accepted: June 6, 2017
Article in press: June 7, 2017
Published online: July 15, 2017
Processing time: 230 Days and 23.2 Hours
To investigate changes in adiposity and cardio-metabolic risk profile following Roux-en-Y gastric bypass in patients of Middle Eastern ethnicity with severe obesity.
This prospective cohort study involved 92 patients who met the indications of bariatric surgery. Post-procedure markers of obesity and cardiometabolic profile were monitored regularly for a year.
Mean body mass index decreased by 29.5% from 41.9 to 29.5 kg/m2 between baseline and 12-mo follow-up, while mean fat mass decreased by 45.9% from 64.2 kg to 34.7 kg. An improvement was also observed in the gluco-metabolic profile with both fasting glucose and HbA1c substantially decreasing (P < 0.001).
The present study shows the short to medium term (1 year) health benefits of bariatric surgery for patients of Middle Eastern ethnicity.
Core tip: The present study obviously shows the health benefits of Roux-en-Y gastric bypass bariatric surgery for the patients of Middle Eastern ethnicity, particularly during the first twelve months of follow-up.
- Citation: Mazidi M, Rezaie P, Jangjoo A, Tavassoli A, Rajabi MT, Kengne AP, Nematy M. Effect of bariatric surgery on adiposity and metabolic profiles: A prospective cohort study in Middle-Eastern patients. World J Diabetes 2017; 8(7): 374-380
- URL: https://www.wjgnet.com/1948-9358/full/v8/i7/374.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i7.374
Obesity and related complications pose increasing health challenges worldwide[1]. Obesity is associated with the development of various comorbidities including type 2 diabetes mellitus, hypertension, and dyslipidemia, which are well-documented risk factors for cardiovascular disease (CVD), as well as musculoskeletal disorders[2-5].
Dietary intervention, lifestyles modification and prescription of pharmaceuticals are the main methods of obesity prevention and control. However, nowadays there is growing attention to metabolic surgery/bariatric surgery, as a promising method to treat obesity. Roux-en-Y gastric bypass (RYGB) is a practical bariatric surgical procedure that has been shown to induce considerable weight loss in obese patients through restriction and malabsorption[6]. Improvement in insulin secretion and sensitivity; and consequently improvement in type 2 diabetes mellitus control after bariatric surgery have been reported in the clinical investigations[7,8]. Randomized controlled trials and observational studies support the medium-to-long term efficacy of RYGB in reducing body weight and fatness and controlling the major metabolic comorbidities of obesity[9,10]. But there still remain some uncertainties about the effects of bariatric surgery on cardiometabolic profiles of obese individuals especially in the Middle East countries and available studies on the efficacy of bariatric surgery mostly originate from the West and predominantly involve Caucasian or African American patients, while a growing number of people with severe obesity is increasingly found in developing countries[11]. Asian subjects have a different relationship between obesity and diabetes risk to Caucasians and hence the impacts of bariatric surgery in Asian populations may also differ from the effects reported previously in Caucasians. For example, Capella et al[12] reported when they looked at patient sub-populations, they found that Afro-Americans lost significantly less weight than Hispanic Americans or White Americans. In addition, Hispanic American women lost less weight than White American women.
To the best of our knowledge, there is no study to investigate the effect of bariatric surgery in Iranian and Middle Eastern ethnicity, hence the aim of the current study was to investigate the impact of the bariatric surgery on adiposity and metabolic profiles in the patients with Middle Eastern ethnicity.
This prospective observational cohort study was conducted between 2011 and 2015 at the Qaem and Imam Reza hospitals of Mashhad, Iran. A total of 92 participants (35 women, 38.0%) who had a body mass index (BMI) greater than 40 kg/m2 (or more than 35.40 kg/m2 with severe comorbidities due to obesity), aged between 25 and 65 years took part in the study. They all met the criteria for performance of bariatric surgery[13]. Pregnant or breast feeding women, patients with known malignancies and those with any condition precluding surgery or general anesthesia were excluded from the study. None of the patients had previously undergone bariatric surgery.
Height (to the nearest 0.1 cm) was measured using a portable stadiometer (OTM, Tehran, Iran) in the upright position, without shoes, with the subject stretching to the maximum height and the head positioned in the Frankfurt plane. The weight and body composition were measured by a bio-impedance analyzer (BIA) (Tanita BC-418 MA, Tanita Corp., Japan) and participants were dressed in light clothing (i.e., no shoes, sweaters or jackets, with 0.1 kg accuracy, frequency range 50-60 Hertz)[14]. The BIA was calibrated according to the manufacturer’s guidelines before each testing and the participants were informed in advance not to use any substance affecting their body composition (e.g., alcohol and coffee) 24 h before the test[14].
Body composition (weight, fat mass, free fat mass) was determined by bioelectric impedance using a Tanita Body Composition Analyzer (Tanita Corporation, Tokyo, Japan). Blood pressure was measured from the dominant arm, with subjects in a sitting position, after 10 min of rest. Measurements were repeated 3 times at 2-min intervals and the means of the 3 measurements were recorded.
For each participant, blood samples were drawn into serum-separating tubes after an overnight fast. Part of the sample was used immediately to measure fasting plasma glucose by the glucose-oxidase, GOD-PAP method[15] and plasma lipids [cholesterol and triglyceride, high-density lipoprotein (HDL) and low-density lipoprotein (LDL)] were measured using enzymatic methods. Aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were analyzed using standardized methods [spectrophotometry corresponding commercial kits (Pars Azmoon, Iran)][16]. Total bilirubin and HbA1c were measured by commercially provided kits (Pars Azmun, Iran).
All 92 participants underwent a RYGB procedure following the same surgical technique performed by a single surgeon[17]. Patients were NPO (Nil per OS) for three days after the surgery; thereafter, liquid diet was carefully started after a swallow test with gastrografin, and was maintained for two weeks. Upon discharge, patients were followed at the outpatient clinics of Qaem and Imam Reza hospitals, Mashhad, each 3 mo for 12 mo with repetition of the assessments performed at baseline under the supervision of nutritionist and medical group.
All patients were completely informed about the surgical procedure offered including potential advantages, probable complications and cost-benefit ratio and completed a written informed consent to participate in the study. The study protocol was approved by the Mashhad University of Medical Sciences’ Ethics Committee.
SPSS software (version 11.5, Chicago, IL, United States) was used for statistical analysis. Kolomogrov-Smirnov tests were used to evaluate the normality of data. Values are expressed as mean ± SD for normally distributed variables. For normally distributed variables paired t-test was used to compare the before and after surgery and equivalent test was used for the skewed variables. Change in adiposity markers and cardio-metabolic profiles during follow-up were investigated using the analysis of variance (ANOVA) and Kruskal-Wallis tests for repeated measures. P-value ≤ 0.05 was considered significant.
The mean BMI was 41.9 ± 4.5 kg/m2 at baseline and steadily decreased down to 29.5 ± 3.8 kg/m2 at 12 mo, giving a relative change of -29.5%. The relative change in BMI from baseline was -11.4% at 3 mo and -18.8% at 6 mo. The mean fat mass was 64.2 ± 11.0 kg at baseline and decreased by 45.9% down to 34.7 ± 8.2 kg at 12 mo. The relative change in fat mass was -21.0% at 3 mo and -37.5% at 6 mo (Table 1). In analyses stratified by gender, the patterns were very similar in men and women. For instance, the mean BMI decreased from 42.4 ± 5.2 kg/m2 at baseline to 29.4 ± 4.5 kg/m2 at twelve months in men, and from 41.1 ± 6.1 kg/m2 to 29.6 ± 3.3kg/m2 in women.
| Factors | Baseline (91) | 3 mo (86) | 6 mo (83) | 12 mo (80) | P value |
| BMI (kg/m2) | 41.9 ± 4.5 | 37.1 ± 5.0 | 34.0 ± 4.6 | 29.5 ± 3.8 | < 0.001 |
| FM | 64.2 ± 11.4 | 50.7 ± 9.2 | 40.1 ± 8.7 | 34.7 ± 8.2 | < 0.001 |
| FFM | 68.3 ± 15.2 | 64.1 ± 15.8 | 60.7 ± 16.3 | 58.0 ± 16.6 | < 0.001 |
| LDL (mg/dL) | 162.2 ± 9.7 | 147.9 ± 11.3 | 139.7 ± 13.2 | 122.8 ± 19.5 | < 0.001 |
| HDL (mg/dL) | 35.7 ± 3.0 | 37.1 ± 3.4 | 36.9 ± 3.7 | 39.4 ± 5.3 | < 0.001 |
| TG (mg/dL) | 232.6 ± 26.1 | 208.5 ± 31.3 | 168.2 ± 28.7 | 132.6 ± 25.8 | < 0.001 |
| TC (mg/dL) | 244.1 ± 20.1 | 224.9 ± 24.8 | 198.2 ± 28.4 | 180.2 ± 42.7 | < 0.001 |
| FBG (mg/dL) | 142.5 ± 18.1 | 115.1 ± 15.8 | 102.5 ± 10.1 | 97.1 ± 8.2 | < 0.001 |
| HbA1c (%) | 6.8 ± 1.1 | 5.7 ± 0.92 | 5.5 ± 1.0 | 5.4 ± 1.2 | < 0.001 |
| ALT (IU/L) | 44.8 ± 8.5 | 33.0 ± 9.6 | 30.1 ± 9.4 | 25.1 ± 8.3 | < 0.001 |
| AST (IU/L) | 35.7 ± 7.0 | 28.1 ± 5.5 | 24.5 ± 5.2 | 23.5 ± 4.8 | < 0.001 |
| Total Bilirubin (mg/dL) | 5.0 (3.2-6.1) | 6.0 (4.1-8.5) | 8.0 (5.5-9.5) | 9.0 (6.1-11.8) | < 0.001 |
| Hs-CRP (mg/dL) | 24.0 (19.0-26.0) | 20.0 (14.5-27.2) | 18.1 (12.1-26.6) | 7.7 (4.6-10.8) | < 0.001 |
HDL increased (P < 0.001) while other cardiovascular risk factors including total cholesterol, LDL cholesterol, triglycerides and Hs-CRP levels steadily decreased (P < 0.001) between baseline and 12-mo follow-up, indicating an improvement of the cardiovascular risk profile (Table 1). An improvement was also observed in the gluco-metabolic profile with both fasting glucose and HbA1c substantially decreasing (P < 0.001) (Table 1). Both ALT and AST steadily decreased while total bilirubin increased during the first twelve months of follow-up (P < 0.001). Again, patterns were very similar in men and women taken separately.
For the first time in a population of Middle East ethnicity, this study evaluated the effect of bariatric surgery on adiposity indices and cardio-metabolic profiles in severely obesity subjects. We found that surgical intervention had a marked effect on adiposity which was substantiated by the significant decrease in BMI and fat mass during the first twelve months of follow-up. This was paralleled by significant and gradual improvement of the cardio-metabolic profiles over the same time period.
Consistent with our results, several studies have previously reported the beneficial effects of bariatric surgery on adiposity and cardio-metabolic profiles[18-20]. A prospective study conducted on 1156 severely obese participants in Utah reported that patients lost 27.7% of their initial body weight six years after RYGB surgery[21]. They also found that 94% of patients receiving RYGB surgery maintained at least 20% weight loss two years after surgery[21]. Observed weight loss in the Utah study was similar to the results of the Longitudinal Assessment of Bariatric Surgery (LABS) study[22]. Furthermore, in line with our findings, clinical studies with different age groups showed clinically meaningful weight loss and improvement in key health conditions among the participants who underwent bariatric surgery[23,24].
Considerable improvement of all lipid sub-fractions was observed during follow-up in our study, in line with other investigations[20,25,26]. The Swedish obese subject (SOS) study indicated that the incidence rate of hypertriglyceridemia was significantly lower in the surgically treated group than in the control group after two years[27]. In addition, significant post-operative improvement of gluco-metabolic profiles was observed during follow-up in our study. Improvement of HbA1C without medications has been reported in other studies[1,21,28].
In line with our findings, several pieces of evidence from clinical trials suggest that RYGB is associated with marked improvement in nonalcoholic fatty liver disease[29-31]. The long-term effect of bariatric surgery on liver enzymes in the Swedish Obese Subjects (SOS) study[32] indicated that bariatric surgery was related to lower serum ALT and AST levels at 2- and 10- year follow-up. In addition, analysis of the relation between changes in transaminase levels and changes in body weight indicated that weight gain was related to a substantial increase in transaminase levels.
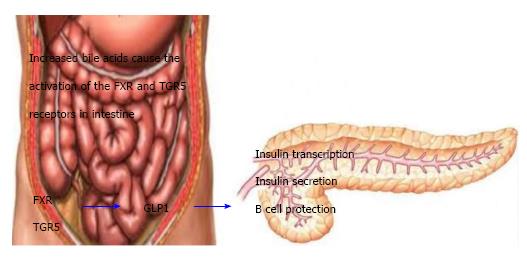
In this study we have found that total bilirubin increased after the surgery, which is in line with other studies[33-42]. Very recently, Mazidi et al[43] have reviewed role of bile acids its subtractions on weight loss and glycaemic control after the bariatric surgeries. They have elaborated that there is a correlation between the concentration of the total bile acid and improvements in several key metabolic parameters after bariatric surgery[43]. It has been reported that there was an inverse correlation between bile acid concentrations and postprandial glucose and triglycerides, and a positive correlation with adiponectin and peak GLP-1 levels following a mixed meal test[33,44]. Moreover, augmented bile acid concentration (It has been suggested that changed upper intestinal tract structure after surgery might have an impact on the enterohepatic circulation of bile acids) could contribute to enhancements in insulin sensitivity, incretin secretion, lipid metabolism and postprandial glycemia after surgery[33,45]. As mentioned above, the release of GLP-1 is correlated with bile acids[43] (Figure 1). Therefore bile acid-dependent increases in postprandial GLP-1 concentrations may be somewhat responsible for the achievements of bariatric surgery in terms of both weight loss and glycaemic control[46].
This study has strengths. The study is sufficiently powered to test the associations. We have repeated investigations of a range of adiposity and cardio-metabolic markers at baseline and during follow-up, which allowed us to carefully characterize their trajectories up to 12 mo after bariatric surgery. Moreover, it is one of the biggest studies which have done in Middle East population. The findings from our study have to be considered in the context of some study limitations as well. We didn’t collect data on the lifestyle of participants and are therefore unable to determine their contribution to some of the observed effects. Moreover, for evaluating the body composition dual-energy X-ray absorptiometry would be a better choice which we did not use it.
The present study suggests health benefits of RYGB bariatric surgery for the patients of Middle Eastern ethnicity, particularly during the first twelve months of follow-up. Our findings suggest that RYGB bariatric surgery has favorable short and medium term effects on adiposity and cardio-metabolic profiles in this population.
Roux-en-Y gastric bypass (RYGB) is a type of weight-loss surgery, bariatric surgery. It’s often done as a laparoscopic surgery, with small incisions in the abdomen.
This is the first and biggest study in middle-east subjects.
In this study they follow the subjects for 12 mo and through time, for each three months they have explore their adiposity and cardiometabolic factors.
Practical applications of the finding is that it can shed light on the post-operative side effcts and changes after the bariatrics surgery for such a novel population.
The article “Effect of bariatric surgery on adiposity and metabolic profiles. A prospective cohort study in middle-eastern patients”, by Mohsen Nematy et al is a clinical prospective study on a cohort of middle eastern obese patients treated by RYGB. The alleged main difference between this and other similar studies is that the population was of Middle Eastern ethnicity.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akusoba I, Fogli L, Li X, Zhao JB S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Fallahi-Shahabad S, Mazidi M, Tavasoli A, Rezaie P, Rohani F, Habibzadeh S, Darchini-Maragheh E, Sefidi ZS, Safarian M, Mobarhan MG. Metabolic improvement of morbid obese patients following Roux-en-Y gastric bypass surgery: A prospective study in Mashhad, Iran. Indian J Gastroenterol. 2016;35:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2015;56:369-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 771] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 3. | Feingold KR, Grunfeld C. Obesity and Dyslipidemia. SourceEndotext [Internet]. South Dartmouth (MA): MDText.com, Inc 2000; . [PubMed] |
| 4. | Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 323] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 5. | Leonetti F, Capoccia D, Coccia F, Casella G, Baglio G, Paradiso F, Abbatini F, Iossa A, Soricelli E, Basso N. Obesity, type 2 diabetes mellitus, and other comorbidities: a prospective cohort study of laparoscopic sleeve gastrectomy vs medical treatment. Arch Surg. 2012;147:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25:1822-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1165] [Article Influence: 116.5] [Reference Citation Analysis (1)] |
| 7. | Chen Y, Corsino L, Shantavasinkul PC, Grant J, Portenier D, Ding L, Torquati A. Gastric Bypass Surgery Leads to Long-term Remission or Improvement of Type 2 Diabetes and Significant Decrease of Microvascular and Macrovascular Complications. Ann Surg. 2016;263:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Singh RP, Gans R, Kashyap SR, Bedi R, Wolski K, Brethauer SA, Nissen SE, Bhatt DL, Schauer P. Effect of bariatric surgery versus intensive medical management on diabetic ophthalmic outcomes. Diabetes Care. 2015;38:e32-e33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Arble DM, Sandoval DA, Seeley RJ. Mechanisms underlying weight loss and metabolic improvements in rodent models of bariatric surgery. Diabetologia. 2015;58:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 10. | Ikramuddin S, Korner J, Lee WJ, Connett JE, Inabnet WB, Billington CJ, Thomas AJ, Leslie DB, Chong K, Jeffery RW. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 547] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 11. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7951] [Cited by in RCA: 8006] [Article Influence: 727.8] [Reference Citation Analysis (0)] |
| 12. | Capella RF, Capella JF. Ethnicity, Type of Obesity Surgery and Weight Loss. Obes Surg. 1993;3:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Furuya CK, de Oliveira CP, de Mello ES, Faintuch J, Raskovski A, Matsuda M, Vezozzo DC, Halpern A, Garrido AB, Alves VA. Effects of bariatric surgery on nonalcoholic fatty liver disease: preliminary findings after 2 years. J Gastroenterol Hepatol. 2007;22:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Mazidi M, Rezaie P, Norouzy A, Saeb MH, Mehdizadeh Hakkak A, Balali S, Mohsen N. Investigating the relation between macronutrients intake and anthropometric indices. Med J Nutr Metab. 2015;8:131-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Burtis CA, Ashwood ER, DE B. Tietz Textbook of Clinical Chemistry AND Molecular Diagnostics. Philadelphia: Elsevier Saunders 2001; . |
| 16. | Henley KS. IFCC method for alanine aminotransferase. Clin Chimica Acta. 1980;105:155-166. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 17. | Ikramuddin S, Kendrick ML, Kellogg TA, Sarr MG. Open and laparoscopic Roux-en-Y gastric bypass: our techniques. J Gastrointest Surg. 2007;11:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Beamish AJ, Olbers T, Kelly AS, Inge TH. Cardiovascular effects of bariatric surgery. Nat Rev Cardiol. 2016;13:730-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Kirwan JP, Aminian A, Kashyap SR, Burguera B, Brethauer SA, Schauer PR. Bariatric Surgery in Obese Patients With Type 1 Diabetes. Diabetes Care. 2016;39:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Sanchis P, Frances C, Nicolau J, Rivera R, Fortuny R, Julian X, Pascual S, Gomez LA, Rodriguez I, Olivares J. Cardiovascular risk profile in Mediterranean patients submitted to bariatric surgery and intensive lifestyle intervention: impact of both interventions after 1 year of follow-up. Obes Surg. 2015;25:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, Strong MB, Vinik R, Wanner NA, Hopkins PN. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 474] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 22. | Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 23. | Batsis JA, Miranda WR, Prasad C, Collazo-Clavell ML, Sarr MG, Somers VK, Lopez-Jimenez F. Effect of bariatric surgery on cardiometabolic risk in elderly patients: A population-based study. Geriatr Gerontol Int. 2016;16:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Inge TH, Krebs NF, Garcia VF, Skelton JA, Guice KS, Strauss RS, Albanese CT, Brandt ML, Hammer LD, Harmon CM. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 334] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Brandão I, Ramalho S, Pinto-Bastos A, Arrojado F, Faria G, Calhau C, Coelho R, Conceição E. Metabolic profile and psychological variables after bariatric surgery: association with weight outcomes. Eat Weight Disord. 2015;20:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Strain GW, Saif T, Ebel F, Dakin GF, Gagner M, Costa R, Chiu YL, Pomp A. Lipid profile changes in the severely obese after laparoscopic sleeve gastrectomy (LSG), 1, 3, and 5 years after surgery. Obes Surg. 2015;25:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3301] [Cited by in RCA: 3032] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 28. | Aminian A, Brethauer SA, Daigle CR, Kirwan JP, Burguera B, Kashyap SR, Schauer PR. Outcomes of bariatric surgery in type 2 diabetic patients with diminished pancreatic secretory reserve. Acta Diabetol. 2014;51:1077-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Cazzo E, Jimenez LS, Pareja JC, Chaim EA. Effect of Roux-en-Y gastric bypass on nonalcoholic fatty liver disease evaluated through NAFLD fibrosis score: a prospective study. Obes Surg. 2015;25:982-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Froylich D, Corcelles R, Daigle C, Boules M, Brethauer S, Schauer P. Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on nonalcoholic fatty liver disease: a comparative study. Surg Obes Relat Dis. 2016;12:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Loy JJ, Youn HA, Schwack B, Kurian M, Ren Fielding C, Fielding GA. Improvement in nonalcoholic fatty liver disease and metabolic syndrome in adolescents undergoing bariatric surgery. Surg Obes Relat Dis. 2015;11:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Burza MA, Romeo S, Kotronen A, Svensson PA, Sjöholm K, Torgerson JS, Lindroos AK, Sjöström L, Carlsson LM, Peltonen M. Long-term effect of bariatric surgery on liver enzymes in the Swedish Obese Subjects (SOS) study. PLoS One. 2013;8:e60495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17:1671-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 455] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 34. | Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63:891-902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 35. | Werling M, Vincent RP, Cross GF, Marschall HU, Fändriks L, Lönroth H, Taylor DR, Alaghband-Zadeh J, Olbers T, Le Roux CW. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scand J Gastroenterol. 2013;48:1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708-E712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 37. | Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond). 2013;37:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Ashrafian H, Li JV, Spagou K, Harling L, Masson P, Darzi A, Nicholson JK, Holmes E, Athanasiou T. Bariatric surgery modulates circulating and cardiac metabolites. J Proteome Res. 2014;13:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, Strodel WE, Still CD, Argyropoulos G. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859-1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 40. | Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J, Schaap FG. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Käkelä P, Pääkkönen M, Hallikainen M, Kolehmainen M, Uusitupa M, Moilanen L. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22:1473-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, Jacobsen SH, Clausen TR, Worm D, Hartmann B, Rehfeld JF, Damgaard M. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes (Lond). 2013;37:1452-1459. [PubMed] [DOI] [Full Text] |
| 43. | Mazidi M, de Caravatto PP, Speakman JR, Cohen RV. Mechanisms of Action of Surgical Interventions on Weight-Related Diseases: the Potential Role of Bile Acids. Obes Surg. 2017;27:826-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Mazidi M, Karimi E, Rezaie P, Ferns GA. Treatment with GLP1 receptor agonists reduce serum CRP concentrations in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J Diabetes Complications. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Mazidi M, Rezaie P, Karimi E, Kengne AP. The effects of bile acid sequestrants on lipid profile and blood glucose concentrations: A systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2017;227:850-857. [PubMed] |
| 46. | Roberts RE, Alaghband-Zadeh J, Le Roux CW. The Role of Bile Acids in Gut-Hormone-Induced Weight Loss After Bariatric Surgery: Implications for Appetite Control and Diabetes. Handbook of Behavior, Food and Nutrition: Springer 2011; 1317-1330. |