Published online Jul 15, 2017. doi: 10.4239/wjd.v8.i7.317
Peer-review started: November 10, 2016
First decision: February 17, 2017
Revised: March 22, 2017
Accepted: June 6, 2017
Article in press: June 8, 2017
Published online: July 15, 2017
Processing time: 235 Days and 14.5 Hours
Diabetic retinopathy (DR) is the most feared ocular manifestation of diabetes. DR is characterized by progressive retinal damage that may eventually result in blindness. Clinically, this blindness is caused by progressive damage to the retinal microvasculature, which leads to ischemia, retinal swelling, and neovascularization. Retinopathy is associated with both type 1 and type 2 diabetes, with DR being the leading cause of new onset blindness in United States adults. Despite this strong association with diabetes, it must be noted that the development of retinopathy lesions is multifactorial and may occur in individuals without an established history of diabetes. Metabolic syndrome is a multifactorial condition of central obesity, hypertriglyceridemia, dyslipidemia, hypertension, fasting hyperglycemia, and insulin resistance. Although several studies examined the individual components observed in the metabolic syndrome in relation to the development of DR, there is conflicting data as to the association of the metabolic syndrome with the development of retinopathy lesions in non-diabetic subjects. This review will summarize the current literature on the evidence of the metabolic syndrome on retinopathy in subjects with and without an established history of diabetes. This review will also discuss some of the mechanisms through which metabolic syndrome can contribute to the development of retinopathy.
Core tip: Metabolic syndrome is a multifactorial condition of central obesity, hypertriglyceridemia, dyslipidemia, hypertension, and fasting hyperglycemia and insulin resistance. Although several studies examined the individual components of the metabolic syndrome in relation to development of diabetic retinopathy, there is conflicting data as to the association of the metabolic syndrome with the development of retinopathy lesions in non-diabetic subjects. This review examined the current literature on the prevalence and impact of the components of the metabolic syndrome on the development of retinopathy in subjects with and without an established history of diabetes.
- Citation: Mbata O, Abo El-Magd NF, El-Remessy AB. Obesity, metabolic syndrome and diabetic retinopathy: Beyond hyperglycemia. World J Diabetes 2017; 8(7): 317-329
- URL: https://www.wjgnet.com/1948-9358/full/v8/i7/317.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i7.317
Diabetes mellitus (DM) is a pathologic condition affecting approximately 29 million or 9% of people in the United States[1]. DM is a condition classified by metabolic disorder caused by chronic hyperglycemia that results in a number of pathologies including microvascular and macrovascular complications such as retinopathy, neuropathy, nephropathy, ischemic heart disease, cerebrovascular disease and peripheral vascular diseases[2,3]. Classically, DM has two etiologies that are classified as either type 1 or type 2. The hyperglycemia in type 1 DM is a direct result of destruction of the pancreatic beta cells; whereas, the hyperglycemia seen in type 2 DM is a result of insulin resistance and subsequent pancreatic beta cell dysfunction[4-6].
DM may occur due to other endocrine disorders such as pituitary, adrenal and/or thyroid diseases (reviewed in[7]). These endocrine disorders are associated with sustained release of hormones that are antagonistic to insulin action including growth hormone, glucocorticoids, catecholamines or glucagon. Therefore, patients with acromegaly, Cushing syndrome, pheochromocytoma, primary hyperaldosteronism, hyperthyroidism, or glucagonoma are at high risk to develop secondary diabetes[8,9]. Chronic alcoholic pancreatitis can also result in secondary DM, where diabetes develops mainly due to deficiency in insulin[10]. A recent study identified incidence of slight to moderate diabetic retinopathy in 12.5% patients with acromegaly[11]. Nevertheless, there are no large or comprehensive studies on development of microvascular complication in general or diabetic retinopathy in particular in patients with secondary-DM.
Although the ADA guidelines for a clinical diagnosis of type 2 diabetes require a random blood sugar greater than 200 mg/dL or fasting blood sugar greater than 125 mg/dL, current evidence suggests that the body undergoes significant metabolic changes prior to the development of frank diabetes[1,12]. These changes include the following: Insulin resistance and the associated hyperinsulinemia and hyperglycemia, vascular endothelial dysfunction partially due to inappropriate release of cytokines from adipose tissue[13-15]. Clinically, the metabolic syndrome has several definitions but is generally diagnosed in individuals presenting with 3 of the criteria listed on Table 1: Central obesity, hypertriglyceridemia, dyslipidemia, hypertension, and fasting hyperglycemia[15-17]. Current population studies have found that the metabolic syndrome affects a large number of individuals. One study in particular found its prevalence to be roughly 22% of adults in the United States, more significantly, an age-dependent increase in prevalence was also found[18]. Furthermore, a more recent study found that prevalence increased to roughly 34.5%[19]. Of note, the presence of the metabolic syndrome increases an individual’s risk of developing type 2 diabetes, cardiovascular disease, and all-cause mortality from cardiovascular disease[20-22].
| ≥ 3 of the following | |
| Fasting glucose | ≥ 6.1 mmol/L (110 mg/dL) |
| HDL cholesterol | Male: < 1.0 mmol/L (40 mg/dL) |
| Female: < 1.3 mmol/L (50 mg/dL) | |
| Triglycerides | ≥ 1.7 mmol/L (150 mg/dL) |
| Abdominal obesity | Male waist circumference: ≥ 102 cm |
| Female waist circumference: ≥ 88 cm | |
| Blood pressure | ≥ 130/85 mmHg |
Retinopathy has been defined in different studies to include microaneurysms, retinal hemorrhages, hard exudates, cotton wool spots, retinal venular abnormalities (venous beading and tortuosity), intraretinal microvascular abnormalities, and new blood vessels[23,24]. Although, hyperglycemia and hypertension are strongly associated with incident retinopathy, there are other etiologies including ocular and systemic causes. Ocular etiologies include central or branch retinal vein occlusion, retinal telangiectasia (“spider veins”), and retinal macroaneurysms[24]. Systemic causes range from the hypertension, carotid atherosclerotic disease, previous head radiotherapy, severe forms of all anemias, and other blood abnormalities such as sickle cell. Systemic diseases such as lupus, toxoplasmosis, and acquired immune deficiency syndrome have also been associated with the development of retinopathy lesions in patients with no history of diabetes[24,25]. There are several studies that examined the association of the components of metabolic syndrome with the development of retinopathy lesions in non-diabetic subjects. With this in mind, the focus of this review will primarily be the impact of metabolic syndrome on the development of retinopathy lesions in patients with established history of primary-DM or without history of diabetes. This review will also discuss some of the mechanisms through which metabolic syndrome can contribute to the development of retinopathy.
Among the microvascular complications of diabetes, diabetic retinopathy (DR) is among the most feared one. Retinopathy has traditionally been viewed as a product of ischemic insult; however, this topic is well documented in other reviews[26,27]. DR is broadly classified into two stages: Nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). Classification is determined by the presence of neovascularization in the retina[27]. NPDR typically precedes PDR and is divided into the following stages: Mild, moderate, severe, and very severe. These stages are based on the likelihood that the retinopathy will progress to PDR. Clinically, a patient with NPDR presents with microvascular abnormalities such as microaneurysms and hemorrhage, affecting the macula and posterior retina. Vascular abnormalities, such as an increased permeability of the retinal vasculature and serum leakage, contribute to capillary loss and subsequent ischemia[27]. PDR is defined by the presence of neovascularization and is divided into the following stages: Early, high risk, and severe neovascularization[27]. In response to retinal hypoperfusion, an increase in local production of vasoproliferative factors such as vascular endothelial growth factor (VEGF)[27] and platelet-derived growth factor (PDGF)[28,29] occurs as a maladaptive protection mechanism. Increased levels of VEGF are traditionally correlated with stabilization of the transcription factor hypoxia-inducible factor-1 (HIF-1) levels under hypoxic conditions[30]. Both VEGF and PDGF are strongly associated with neovascularization via induction of new vascular development typically from optic disc or retinal vessels[31,32]. This neovascularization further compounds the damage by contributing to the development of preretinal and vitreous hemorrhage, fibrosis, potential retinal detachment, and blindness[33-35].
The mainstay standard of care for DR is the laser treatment, a highly effective procedure to slowdown visual loss in patients with PDR. The laser-mediated photocoagulation seals leaking blood vessels directly or by eliminating abnormal newly formed blood vessels in the periphery of the retina that is thought to be involved in VEGF production[36]. With VEGF being a common product strongly associated with the progression of DR, current pharmacologic treatment strategies have been based on its local inhibition within the retina[37]. Anti-angiogenic therapy was developed in attempt to improve vision in patients with diabetic macular edema (DME) as well as PDR. Indeed, monthly injections of ranibizumab, an anti-VEGF improved vision, reduced the risk of further vision loss. These results were observed after 2-years and were sustained for 3-years[38]. Anti-VEGF treatment improved macular edema in diabetic patients as well as when it was used in combination with panretinal photocoagulation in patients with PDR. The reported side effects of ranibizumab in “as - needed” treatment regimen over a 5-year.
Researchers have proposed several mechanisms for the development of insulin resistance and the metabolic syndrome. These include: Genetic defects in proteins involved in the insulin action cascade, increased levels of visceral adiposity, free fatty acid levels (FFA), and chronic inflammation[39,40]. Insulin resistance in adipose tissue, regardless its molecular or environmental basis, causes decrease in FFA uptake by fat cells and/ or increase in FFA release from fat cells. Under the insulin resistant state, there is impaired glucose handling by skeletal muscle and adipose tissue. This impaired glucose intake is a significant contributor to the hyperglycemia and associated vascular endothelial damage observed in insulin resistant individuals[41,42]. Additionally, insulin is important in the signaling for nitric oxide release from vascular endothelial cells, resulting in vasodilation and reduced vascular resistance, which reduces blood pressure[43,44]. Thus, there is a strong associations between the presence and extent of insulin resistance with hypertension due to increased vascular resistance and impaired glucose regulation[45,46].
Hypertension, affecting 29.8% of United States adults[47] represents the best known systemic condition associated with non-diabetic retinopathy. Hypertension is an established risk factor for the development of several cardiovascular complications including retinopathy, atherosclerosis, and aneurysms[48,49]. Poorly controlled systemic hypertension causes worsening of microvascular disease of the eye like DR[50]. Hypertensive retinopathy shared the pathophysiology of damaged retinal vascular endothelium similar to DR[51,52]. In contrast to the metabolic damage in DR, this vascular endothelial damage is mechanically induced by increased blood flow[53,54]. Despite the relationship between retinopathy and hypertension in patients without history of diabetes, one study, “the Hoorn”, identified retinopathy 8 of the 17 individuals without history of diabetes who developed retinopathy did not have hypertension. In addition, HbA1c level and waste to hip ratio (WHR) were risk factors in the nondiabetic individuals[23]. These finding suggest that retinal pathologies begin to develop prior to a clinical diagnosis of hypertension that eventually result in retinopathy.
In the absence of a clinical diagnosis of diabetes, associations have already been found between the metabolic syndrome and macro- or microvascular pathologies such as atherosclerosis, arteriosclerosis, and endothelial dysfunction[55,56]. Several studies examined the associations between the independent components of the metabolic syndrome with the development of retinal vascular injury, by measuring the mean retinal artery and venous caliber[31]. In this study, components of the metabolic syndrome including large waist circumference, lower HDL cholesterol levels, and higher BP were independently associated with reduced mean retinal arterial caliber in non-diabetic persons. Individuals with hypertriglyceridemia were significantly more likely to have arteriovenous nicking and later develop retinopathy[31]. These finding clearly show an association between the MS and retinal vascular dysfunction.
Following the earlier notion that dyslipidemia plays a critical role in DR, several studies examined the individual components observed in the metabolic syndrome in relation to DR[57-60]. Similar to the impact of dyslipidemia, there is conflicting data as to the association of the metabolic syndrome with the development of retinopathy lesions in non-diabetic subjects. One study of obese individuals older than the age of 40, found no significant correlation between the metabolic syndrome and retinopathy once diabetes and hypertension were controlled for[61]. Another population study found no significant association in the incidence of retinopathy and the metabolic syndrome in the non-diabetic population, but there was a significant association between hypertension and retinopathy[57].
In contrast, studies focusing on specific patient populations found differing results. A recent study in a Chinese population identified a positive correlation between the metabolic syndrome and retinopathy in the examined non-diabetic subjects[62,63]. In a study of Japanese adults, the metabolic syndrome was found to be associated with retinopathy; a larger waist circumference was associated with wider venular diameter and retinopathy lesions; a higher blood pressure level was associated with focal arteriolar narrowing, arteriovenous nicking, enhanced arteriolar wall reflex and narrower arteriolar diameter; and a higher triglyceride level was associated with enhanced arteriolar wall reflex[64]. In the Hoorn study, in the Netherlands, there was significant correlation of retinopathy with the combination of high waste-to-hip ratio (WHR), HbA1c level, and hypertension in non-diabetics and in glucose-impaired subjects, supporting a role for insulin resistance in the pathogenesis of retinopathy[23].
Interestingly, there was no significant correlation between incidence of retinopathy with serum levels of triglycerides, and total cholesterol or body mass index (BMI)[23]. Despite generalized obesity indicated by high BMI not being associated with retinopathy, a high-body-fat percentage indicated by WHR has been shown to be significantly associated with development of retinopathy in patients with type-2 diabetes[65]. Similar to the Hoorn study, the WHR was also an independent risk factor in the diabetic patients in the EURODIAB study[66]. These discrepancies speak to a number of possible factors including the inability of the BMI calculation to accurately estimate body composition[67,68] while the WHR is an indicator for central obesity and is associated with insulin resistance[69]. In addition, differences in measurement methods and quantification for incidence and/or rate of progression of retinopathy[57,70,71]. The other factor is that dysfunction of adipose tissue has been shown to increase oxidative stress and subsequent cytokine, contributing to the pathogenesis of retinopathy[72]. Given that hyperglycemia and hypertension are the strongest risk factors for the development of retinopathy lesions, and that these two conditions are contributors to the diagnosis of the metabolic syndrome, it may be beneficial to modify the clinical approach to individuals with the metabolic syndrome, namely those with hypertension and hyperglycemia coupled with obesity, calculated by WHR, in order to prevent or slow the development of hyperglycemia, hypertension, and hypertriglyceridemia, which in turn could possibly delay the onset of retinopathy lesions and visual impairment in subjects with these comorbidities.
Although insulin resistance is a key pathogenic factor in both non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome, few studies examined the relationship between NAFLD and retinopathy in the presence or absence of diabetes. Central adiposity and visceral fat are important source of triglycerides leading to steatosis and NAFLD[73]. The prevalence increases in subjects with impaired glucose tolerance (43%) and in subjects with newly diagnosed DM[74]. The NHANES III was conducted by the Centers for Disease Control and Prevention using a nationwide probability sample of the United States non-institutionalized civilian population from 1988 to 1994. While a strong association between diabetes and retinopathy was observed, NAFLD was not associated with retinopathy in the non-diabetic population[75]. No significant relationship between NAFLD and incident retinopathy was observed in either diabetic or non-diabetic after adjusting for the confounders such as age, gender, ethnicity, and metabolic components. In addition, this same study found no significant increase in DR prevalence in individuals with both DM and NAFLD[75]. In contrast, prior studies observed a positive association between pediatric NAFLD participants and the degree of retinopathy signs[76]. Additionally, NAFLD was associated with increased rates of chronic kidney disease and proliferative diabetic retinopathy in individuals with type 2 diabetes in Italy[77]. Of note, NAFLD was not significantly correlated with the incidence of retinopathy in patients with NPDR after adjusting for multiple factors[77].
Traditionally, the development of DR in patients with type 1 or type 2 diabetics has been linked to the associated hyperglycemia[78]. Whether the existence of metabolic syndrome in these patients can accelerate or aggravate the incidence of DR is not clear. For example, the findings of the landmark studies, Land mark clinical trials including United Kingdom Prospective Diabetes Study (UKPDS) in patients with type-2 diabetes[79,80], the Diabetic Control and Complications Trial (DCCT), in patients with type-1 diabetes[81] and its follow-up, the Epidemiology of Diabetes and Interventions and Complications (EDIC)[82,83] were traditionally interpreted that tight glycemic control significantly delayed development of DR. However, the level of reduction was significantly lower in patients with type 2 diabetes (25%) and 76% in patients with type 1 diabetes, suggesting that factors outside of hyperglycemia associated with type 2 diabetes may play a role in the pathology of the microvascular complications such as retinopathy[82,83]. Also, the tight metabolic control, individually and coupled with other interventions, has been shown to significantly decrease the incidence of retinopathy, while also increasing the quality and duration of life in these patients[82]. With this population beginning to live longer, the rates and incidence of comorbid metabolic syndrome and type 1 diabetes has begun to increase as this population begins to be more representative of the general United States population[84].
Another study in patients with type 1 diabetes found that tight glycemic control had threshold effectiveness at reducing the incidence of retinopathy. When looking for other associations, they found that once the duration of hyperglycemia was controlled for, increased WHR and fasting triglyceride levels were the only other factors strongly associated with the incidence of retinopathy in these patients[85]. Interestingly, a study in Belgium found that patients with type 1 diabetes who are overweight and had higher BMI had more retinopathy than normal-weight diabetic patients. Patients with retinopathy were older and had a longer diabetes duration and higher A1C than individuals without retinopathy[86]. However, one study took a different approach to studying this relationship by estimating the prevalence of DR in individuals with the metabolic syndrome depending on the number of MS components these individuals had[65]. This study focused on diabetic patients but normalized for several associated parameters including HbA1C. This study found a linear relationship between the number of MS components and the prevalence of DR[65]. These findings support the relationship between the metabolic syndrome, namely the obesity and hypertriglyceridemia, and the development and/or progression of DR. Given that these conditions are strongly associated with type 2 diabetes, and are components of the metabolic syndrome, it would be logical to look into their contribution to the incidence of retinopathy in this population[87]. Other studies found positive correlations between the comorbid metabolic syndrome and type 2 diabetes with all cardiovascular complications including DR[88,89]. Furthermore, other studies found that the presence of hyperinsulinemia and dyslipidemia in type 2 diabetics was associated with the onset of microvascular complications[90,91]. A case-controlled study, with data obtained from 2551 Chinese participants found that the trend to develop DR with metabolic syndrome was significantly higher than that without metabolic syndrome. Metabolic syndrome was an independent statistical indicator of the presence of DR after adjusting for age and sex as well as HbA1c and duration of diabetes[65]. Additively these findings bolster the claim that in addition to hyperglycemia and hypertension, the hypertriglyceridemia seen in several individuals in this population may very well play a significant role in the pathogenesis of DR in this population.
Diabetic dyslipidemia is traditionally characterized by high plasma triglycerides and low-density lipoprotein cholesterol (LDL) and reduced levels of high density lipoprotein cholesterol (HDL). While early studies have suggested positive correlation between dyslipidemia and hard exudates, longitudinal studies in patients with type-1 diabetes found modest impact of increased total cholesterol and HDL on the incidence of DR[92]. Of note, serum triglyceride levels were not considered in that study. As discussed in the review by Sabanayagam et al[93] 2016, changes in the circulating levels of lipids are not always associated with DR progression. A recent meta-analysis revealed that the triglycerides, total cholesterol and LDL cholesterol were significantly elevated in persons with diabetic macular edema (DME) when compared to those with DM without DME[94]. In the Madrid Diabetes Study, higher LDL cholesterol level increased the 4-year risk for DR by 8-fold in type 2 diabetes[95]. In contrast, higher levels of total and LDL cholesterol were found to be protective of any retinopathy in a Singapore study as well as in a multi-ethnic United States population study[96,97]. In a follow-up analysis of the DCCT-EDIC from type-1 diabetic patients, the severity of retinopathy was positively associated with triglycerides (combined cohort) and negatively associated with HDL cholesterol in men from combined cohort[98]. Advanced lipoprotein profiling identified positive association of retinopathy with small and medium VLDL and negatively with VLDL sizes. No associations were found with apolipoprotein-A1, Lipoprotein(a), or susceptibility of LDL to oxidation in type-1 diabetic patients.
The early belief that dyslipidemia plays a critical role in DR, initiated several studies that examined the impact of lipid lowering drugs on DR. As discussed in the review by Modjtahedi et al[99] 2016, overall there are variable results but the majority of the studies support a protective role of some lipid lowering agents such as fenofibrate in mitigating hard exudates and DR. The beneficial effects of lipid lowering agents are not fully attributed to correcting dyslipidemia and may be attributed to anti-inflammatory, antioxidants and anti-apoptotic effects. Finally, two major randomized clinical trials showed that fenofibrate, a drug that is used to reduce cholesterol significantly inhibited DR progression in diabetic patients[100,101]. Lastly, a multinational case-control study suggested that conventional serum lipids profiles are unlikely to show clear and dependent effects on the development of DR[102]. The published literature suggested an association between diabetic dyslipidemia and DR; however, a more detailed and specific subtype of lipids or lipoproteins may have a clear pathogenic role rather than traditional lipid profile. In addition, alternative hypothesis suggests that initial damage to the retina barrier facilitate leakage of lipids and its oxidized metabolite to exert local adverse effects that are not necessarily mirrored by significant alteration in serum lipid profile[99].
DR is classically perceived as microvascular disease with initial vascular endothelial damage as a direct result of hyperglycemia. Given the known pathophysiology of the components of the metabolic syndrome, as well as its association with type 2 diabetes[46,103], we will discuss the common major mechanisms of pathology in both the metabolic syndrome and diabetes.
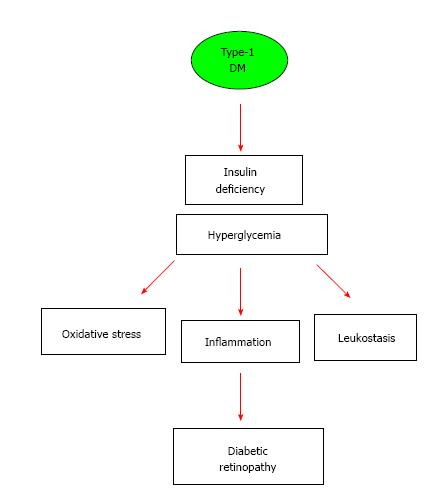
For retinopathy in patients with an established history of diabetes, hyperglycemia has been identified as primary factor evident by the strong correlation between an individual’s HbA1c and the development of DR[79]. Results from the clinical trial UKPDS in patients with type 2 diabetes[79,80] and the DCCT in patients with type 1 diabetes[81] established that intensive glycemic control significantly reduced the incidence of retinopathy. More specifically, risk reduction of DR was found to be 76% in patients with type 1 diabetes and 25% in type 2 diabetics. Several studies examined mechanisms involved in hyperglycemic damage include non-enzymatic glycosylation of vascular basement membrane, advanced glycation end products and osmotic damage due to the conversion of circulating sugars to sorbitol by aldose reductase[78,104,105]. Studies have found that diabetes mellitus and the metabolic syndrome both, increase reactive oxygen species (ROS) production and decrease the antioxidant capacity. This is associated with oxidative damage of cell components such as proteins, lipids, and nucleic acids can trigger a chronic inflammatory response[106,107]. Impact and sources of hyperglycemia-derived oxidative stress and proinflammatory cytokines in the diabetic retina are well-documented in the literature[108,109]. As depicted in Figure 1, the aforementioned mechanisms result in vascular endothelial cell dysfunction and an increase in local immune cell activity resulting in a leukocyte oxidative burst and the associated increased leukostasis, vascular permeability[110,111]. Inflammation-mediated leukostasis has been linked to pericyte and endothelial cell death, retinal ischemia, and neovascularization, which contribute to vision loss in DR[109].
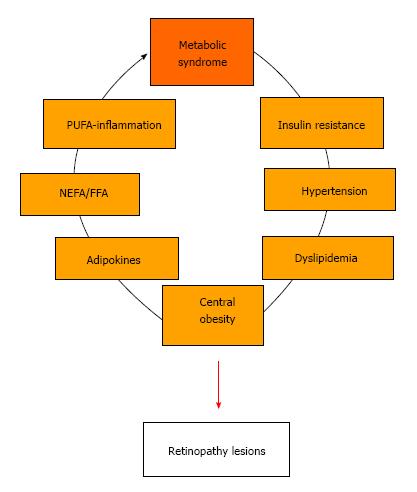
In contrast, identifying mechanisms involved in retinopathy associated with the metabolic syndrome is not a straightforward task. The metabolic syndrome is a combination of several criteria (Table 1) including central obesity, hypertriglyceridemia, insulin resistance, dyslipidemia, hypertension, and fasting hyperglycemia. Furthermore, central obesity and the excess adipose tissue observed in obese individuals partially contribute to the development of the insulin resistance syndrome and cardiovascular disease. As depicted in Figure 2, metabolic syndrome and endocrine dysfunction of adipose tissue can be very important in the development of retinopathy lesions and progression of the DR.
In healthy adults, adipose tissue secretes a number of factors such as resistin and pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin-6 (IL-6), which participate in the activation of macrophages that further perpetuate this inflammatory cascade. When adipose tissue is accumulated, as it is in obesity due to increased adipose tissue, it begins to operate in a dysfunctional manner. Additionally, in the obese state, adipose tissue secretes reduced amounts of the anti-inflammatory adipokine adiponectin, an adipokine that normally acts to increase insulin sensitivity by inhibiting hepatic gluconeogenesis and augmenting skeletal muscle glucose uptake[14,112]. In addition to these factors, it appears that excess adipose tissue leads to dysfunctional release of several other factors including retinol binding protein-1 and leptin[9,14]. The abnormal release of these factors contribute to insulin resistance by further dysregulating glucose homeostasis and augmenting hyperphagia and hyperglycemia induced damage[14].
Recently, the endocrine function of adipose tissue has become the focus of several studies. These functions include the secretion of adiponectin and resistin, both of which are involved in insulin sensitization, and subsequent increased glucose uptake[14,113]. Adiponectin functions to increase insulin sensitivity and is secreted at high levels in lean individuals with low levels of adipose tissue. However, as adipose tissue increases, the level of adiponectin secreted by adipose tissue decreases partially due to the increased secretion of pro-inflammatory markers and their associated induction of oxidative stress[14,113,114]. Another significant adipokine, resistin, is abnormally increased in obese individuals and is a contributor to the development and progression of the insulin resistance[14,115,116]. Dysfunctions in these processes, independent of causing direct damage to vascular endothelium, can contribute to insulin resistance and hyperglycemia. One study looking at the progression from non-proliferative to proliferative, found a strong association between the progression of retinopathy and increased c-reactive protein levels and increased serum resistin, a protein secreted by several tissues including white adipose tissue, nonfat cells of adipose tissue, bone marrow, and lung. This study found that increased serum resistin was associated with obesity and was highest in obese individuals with diabetes. Furthermore, increased serum resistin was associated with increased triglycerides and the progression of retinopathy[117,118]. These findings further bolster the association between DR and hypertriglyceridemia particularly due to resistin’s association with obesity and increased triglycerides. Furthermore, resistin’s association with retinopathy is made more plausible given its association with type 2 DM, insulin resistance, and inflammation. All of these factors have demonstrated associations with DR; however, the specific role of resistin in inflammation has not yet been elucidated[117,119]. Given that c-reactive protein is a known inflammatory biomarker related to vascular endothelial dysfunction and atherogenesis, its association with increased serum resistin levels provides additional support for adipose tissue dysfunction in the obese state contributing to the pathologies associated with diabetes including DR[117,118].
Adipose tissue can contribute to insulin resistance by regulating the level of the FFAs or the non-esterified fatty acids (NEFAs) depending on the body’s energy status[120,121]. Circulating NEFAs/FFAs reduce adipocyte and muscle glucose uptake, and also promote hepatic glucose output. Because lipolysis in adipocytes is depressed by insulin, insulin resistance from any cause can lead to NEFA elevation, which, in turn, induces additional insulin resistance as part of a vicious cycle that eventually contribute to diabetic complication including DR[120,121]. Furthermore, a prior study found that a long-acting antilipolytic drug could lower FFA levels in 9-lean control subjects, 13-obese nondiabetic subjects, 10-obese subjects with impaired glucose tolerance, and 11 patients with type 2 diabetes[122]. The results showed FFA lowering drugs improved oral glucose tolerance in both lean and obese nondiabetic subjects and in obese patients with type 2 diabetes, supporting the notion that adipose tissue can contribute to the pathologies associated with insulin resistance[122].
The involvement of the inflammatory pathway in retinopathy associated with metabolic syndrome is critical in the light of the observation that obesity, hypertension, hyperlipidemias, and insulin resistance are considered low-grade systemic inflammatory conditions[123]. A major contributor to this inflammation is the release of pro-inflammatory cytokines by immune cells such as macrophages, lymphocytes, and leukocytes after they infiltrate the adipose tissue[14,124,125]. The pro-inflammatory cytokines secreted by adipose tissue include interleukins (ILs), notably IL-1β, IL-6, and tumor necrosis factor alpha[126]. These cytokines contribute to the impairment of glucose homeostasis, insulin signaling and development of insulin resistance and cardiovascular complications such as DR[127-130].
The retina is rich in n-3 polyunsaturated fatty acids (PUFAs) and upon activation of phospholipase A2, release of the metabolites arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in response to various stimuli including growth factors, cytokines and free radicals[131]. The AA, EPA and DHA are metabolized by cyclooxygenases (COXs), lipoxygenases (LOXs), and cytochrome P450 (CYP450) enzymes[123]. The AA forms a precursor to pro-inflammatory prostaglandins, thromboxanes and leukotrienes in general. It is noteworthy that AA can also give rise to lipoxins, which are potent anti-inflammatory molecules. Similarly, EPA gives rise to resolvins and DHA to protectins, which possess significant anti-inflammatory properties. As such, these molecules may have a role in modulating the chronic inflammatory state observed in DR and metabolic syndrome[131].
Inflammation in DR has also been linked to induction of the cyclooxygenase-2 (COX-2) pathway. Induction of the COX-2 pathway results in a dysfunctional increase in the production of prostaglandins and thromboxane[132]. This dysfunctional production contributes to the chronic inflammatory pathologies by augmenting the vascular permeability noted in these patients, increasing platelet aggregation and leukostasis, which results in the increased local production of pro-inflammatory cytokines[32]. Furthermore, the increased products of the COX-2 pathway in the diabetic patients, has been associated with the increased local secretion of VEGF in the retina, a major contributor to the progression of DR[133,134]. Prior studies have shown that PUFAs, especially EPA and DHA, inhibit the production of both IL-6 and TNF-α and suppress the expression of VEGF[135]. Leukotrienes have been found to be increased in the retinas of diabetic mice. These inflammatory factors contribute to several of the dysfunctions noted chronic retinal inflammation including the increased vascular permeability and increased production of free radicals[131].
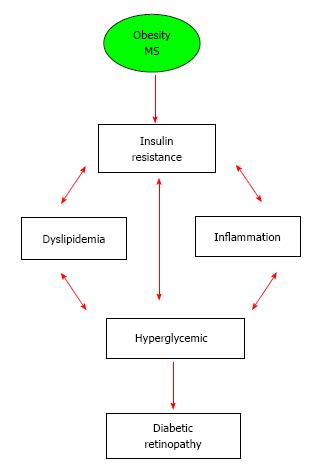
The prevalence of DM and its associated pathologies have significantly increased as a result of increasing prevalence of obesity and insulin resistance. For retinopathy in patients with established history of DM, hyperglycemia remains the most consistent risk factor for DR in type 1 diabetes across different studies and populations (Figure 1). While blood pressure is an important risk factor for DR in type 2 diabetes, correlation with serum levels of lipids are not consistent. Meanwhile, considerable evidence showed that central obesity, insulin resistance and dyslipidemia are associated with retinopathy lesion in non-diabetic and diabetic patients. As depicted in Figure 3, the cascade of events involved in development of DR is complex and interrelated and not entirely driven by hyperglycemia in patients with metabolic syndrome. Moreover, diabetic macular edema rather than PDR is the increasingly common cause of visual impairment. Patients with type 2 diabetes with dyslipidemia show higher tendency for DME and can benefit from cholesterol reducing agents such as fenfibrate in addition to antidiabetic agents. Although the current anti-VEGF therapies are beneficial in some patients with DR, about 50% of other patients do not respond adequately. This calls for additional studies to find better treatment and management strategies to prevent its currently incurable complications, such as DR, should be of high priority. Therefore, targeting components of the metabolic syndrome could be a beneficial preventative/dilatory intervention. Specifically, understanding the pathways involved in dysfunction of adipose tissue and the associated alteration of PUFA, FFA and adipokines such as adiponectin and resistin contribute to inflammation and cardiovascular complications including retinopathy lesions in both diabetics and non-diabetics. Novel strategies to suppress systemic and local inflammation seen in DR should be further explored in order to prevent and/or delay DR.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Malfitano C, Raghow RS, Riutta AA, Tarantino G, Zhao J S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Herman WH. Diabetes epidemiology: guiding clinical and public health practice: the Kelly West Award Lecture, 2006. Diabetes Care. 2007;30:1912-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (3)] |
| 2. | Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853-876. [PubMed] |
| 3. | Mahler RJ, Adler ML. Clinical review 102: Type 2 diabetes mellitus: update on diagnosis, pathophysiology, and treatment. J Clin Endocrinol Metab. 1999;84:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Van den Driessche A, Eenkhoorn V, Van Gaal L, De Block C. Type 1 diabetes and autoimmune polyglandular syndrome: a clinical review. Neth J Med. 2009;67:376-387. [PubMed] |
| 5. | Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 662] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 6. | Resmini E, Minuto F, Colao A, Ferone D. Secondary diabetes associated with principal endocrinopathies: the impact of new treatment modalities. Acta Diabetol. 2009;46:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Reddy R, Hope S, Wass J. Acromegaly. BMJ. 2010;341:c4189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Arnaldi G, Mancini T, Polenta B, Boscaro M. Cardiovascular risk in Cushing’s syndrome. Pituitary. 2004;7:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Larsen S. Diabetes mellitus secondary to chronic pancreatitis. Dan Med Bull. 1993;40:153-162. [PubMed] |
| 10. | Azzoug S, Chentli F. Diabetic retinopathy in acromegaly. Indian J Endocrinol Metab. 2014;18:407-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Classification and Diagnosis of Diabetes. Diabetes Care. 2015;S13. |
| 12. | Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 538] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 13. | Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3103] [Cited by in RCA: 3168] [Article Influence: 226.3] [Reference Citation Analysis (0)] |
| 14. | Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. [PubMed] |
| 15. | Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20476] [Cited by in RCA: 20694] [Article Influence: 862.3] [Reference Citation Analysis (2)] |
| 16. | Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005;149:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 17. | Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 998] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 18. | Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 789] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 19. | Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002;51:3120-3127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 412] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 20. | Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, Ford I, Forouhi NG, Freeman DJ, Jukema JW. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371:1927-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 21. | Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31:1898-1904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 22. | van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Polak BC. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. 2003;121:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Venkatramani J, Mitchell P. Ocular and systemic causes of retinopathy in patients without diabetes mellitus. BMJ. 2004;328:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Brown GC, Shields JA, Sanborn G, Augsburger JJ, Savino PJ, Schatz NJ. Radiation retinopathy. Ophthalmology. 1982;89:1494-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 212] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 513] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 26. | Aiello LM. Perspectives on diabetic retinopathy. Am J Ophthalmol. 2003;136:122-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Cox OT, Simpson DA, Stitt AW, Gardiner TA. Sources of PDGF expression in murine retina and the effect of short-term diabetes. Mol Vis. 2003;9:665-672. [PubMed] |
| 28. | Mori K, Gehlbach P, Ando A, Dyer G, Lipinsky E, Chaudhry AG, Hackett SF, Campochiaro PA. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy. Invest Ophthalmol Vis Sci. 2002;43:2001-2006. [PubMed] |
| 29. | Lin M, Chen Y, Jin J, Hu Y, Zhou KK, Zhu M, Le YZ, Ge J, Johnson RS, Ma JX. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Müller cells. Diabetologia. 2011;54:1554-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest Ophthalmol Vis Sci. 2012;53:5906-5911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Wong-Riley MT. Energy metabolism of the visual system. Eye Brain. 2010;2:99-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 319] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 33. | Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. [PubMed] |
| 34. | Deschler EK, Sun JK, Silva PS. Side-effects and complications of laser treatment in diabetic retinal disease. Semin Ophthalmol. 2014;29:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Waisbourd M, Goldstein M, Loewenstein A. Treatment of diabetic retinopathy with anti-VEGF drugs. Acta Ophthalmol. 2011;89:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 637] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 37. | Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;2:82-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4273] [Cited by in RCA: 4504] [Article Influence: 225.2] [Reference Citation Analysis (0)] |
| 39. | Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:12587-12594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 518] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 40. | Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 352] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 41. | Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 595] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 42. | Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 43. | Akande TO, Adeleye JO, Kadiri S. Insulin resistance in Nigerians with essential hypertension. Afr Health Sci. 2013;13:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Rao G. Insulin resistance syndrome. Am Fam Physician. 2001;63:1159-1163. [PubMed] |
| 45. | Prevention CfDCa. Prevalence of Hypertension and Controlled Hypertension - United States, 2007-2010. MMWR. 2013;144. |
| 46. | Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 393] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 47. | Kumar J. Epidemiology of hypertension. Clinical Queries. Nephrology. 2013;56. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Chatterjee S, Chattopadhyay S, Hope-Ross M, Lip PL. Hypertension and the eye: changing perspectives. J Hum Hypertens. 2002;16:667-675. [PubMed] |
| 49. | Klein R, Klein BE, Moss SE, Wang Q. Hypertension and retinopathy, arteriolar narrowing, and arteriovenous nicking in a population. Arch Ophthalmol. 1994;112:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Yu T, Mitchell P, Berry G, Li W, Wang JJ. Retinopathy in older persons without diabetes and its relationship to hypertension. Arch Ophthalmol. 1998;116:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Bhargava M, Ikram MK, Wong TY. How does hypertension affect your eyes? J Hum Hypertens. 2012;26:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Garner A, Ashton N. Pathogenesis of hypertensive retinopathy: a review. J R Soc Med. 1979;72:362-365. [PubMed] |
| 53. | Golden SH, Folsom AR, Coresh J, Sharrett AR, Szklo M, Brancati F. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: the atherosclerosis risk in communities study. Diabetes. 2002;51:3069-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 775] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 55. | Keenan JD, Fan AZ, Klein R. Retinopathy in nondiabetic persons with the metabolic syndrome: findings from the Third National Health and Nutrition Examination Survey. Am J Ophthalmol. 2009;147:934-44, 944.e1-2. [PubMed] |
| 56. | Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006;104:98-107. [PubMed] |
| 57. | Engerman RL. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38:1203-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 58. | Klein R, Myers CE, Lee KE, Klein BE. 15-year cumulative incidence and associated risk factors for retinopathy in nondiabetic persons. Arch Ophthalmol. 2010;128:1568-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Wong TY, Duncan BB, Golden SH, Klein R, Couper DJ, Klein BE, Hubbard LD, Sharrett AR, Schmidt MI. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk In Communities study. Invest Ophthalmol Vis Sci. 2004;45:2949-2954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 60. | Liu L, Yue S, Wu J, Zhang J, Lian J, Teng W, Huang D, Chen L. Prevalence and risk factors of retinopathy in patients with or without metabolic syndrome: a population-based study in Shenyang. BMJ Open. 2015;5:e008855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Peng XY, Wang FH, Liang YB, Wang JJ, Sun LP, Peng Y, Friedman DS, Liew G, Wang NL, Wong TY. Retinopathy in persons without diabetes: the Handan Eye Study. Ophthalmology. 2010;117:531-537. [PubMed] |
| 62. | Kawasaki R, Tielsch JM, Wang JJ, Wong TY, Mitchell P, Tano Y, Tominaga M, Oizumi T, Daimon M, Kato T. The metabolic syndrome and retinal microvascular signs in a Japanese population: the Funagata study. Br J Ophthalmol. 2008;92:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Gao L, Xin Z, Yuan MX, Cao X, Feng JP, Shi J, Zhu XR, Yang JK. High Prevalence of Diabetic Retinopathy in Diabetic Patients Concomitant with Metabolic Syndrome. PLoS One. 2016;11:e0145293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 659] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 65. | Liu P, Ma F, Lou H, Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health. 2013;13:629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 66. | Etchison WC, Bloodgood EA, Minton CP, Thompson NJ, Collins MA, Hunter SC, Dai H. Body mass index and percentage of body fat as indicators for obesity in an adolescent athletic population. Sports Health. 2011;3:249-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 67. | Widgren BR, Urbanavicius V, Attvall S, Persson B. Insulin sensitivity is more related to fat distribution than to heredity for hypertension in normotensive men. Metabolism. 1994;43:883-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 993] [Cited by in RCA: 962] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 69. | Bhargava M, Cheung CY, Sabanayagam C, Huang L, Lamoureux EL, Wang JJ, Tai ES, Heng CK, Ikram MK, Mitchell P. Prevalence and risk factors for retinopathy in persons without diabetes: the Singapore Indian Eye Study. Acta Ophthalmol. 2014;92:e602-e609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Blüher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci (Lond). 2016;130:1603-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 71. | Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 892] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 72. | Ortiz-Lopez C, Lomonaco R, Orsak B, Finch J, Chang Z, Kochunov VG, Hardies J, Cusi K. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care. 2012;35:873-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 73. | Lin TY, Chen YJ, Chen WL, Peng TC. The Relationship between Nonalcoholic Fatty Liver Disease and Retinopathy in NHANES III. PLoS One. 2016;11:e0165970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Liccardo D, Mosca A, Petroni S, Valente P, Giordano U, Mico’ AG, Pescosolido S, Buzzonetti L, Nobili V. The association between retinal microvascular changes, metabolic risk factors, and liver histology in pediatric patients with non-alcoholic fatty liver disease (NAFLD). J Gastroenterol. 2015;50:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 76. | Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 762] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 77. | Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5819] [Cited by in RCA: 5965] [Article Influence: 238.6] [Reference Citation Analysis (0)] |
| 78. | King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 383] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 79. | Diabetes Control and Complications Trial (DCCT): results of feasibility study. The DCCT Research Group. Diabetes Care. 1987;10:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 338] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 80. | Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care. 2016;39:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 451] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 81. | Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 866] [Cited by in RCA: 1021] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 82. | Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, Wadén J, Rönnback M, Rosengård-Bärlund M, Björkesten CG. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care. 2005;28:2019-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 83. | Chaturvedi N, Sjoelie AK, Porta M, Aldington SJ, Fuller JH, Songini M, Kohner EM. Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care. 2001;24:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 84. | De Block CE, De Leeuw IH, Van Gaal LF. Impact of overweight on chronic microvascular complications in type 1 diabetic patients. Diabetes Care. 2005;28:1649-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Anan F, Takayuki M, Takahashi N, Nakagawa M, Eshima N, Saikawa T, Yoshimatsu H. Diabetic retinopathy is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients. Hypertens Res. 2009;32:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 86. | Bonadonna R, Cucinotta D, Fedele D, Riccardi G, Tiengo A. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care. 2006;29:2701-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 87. | Abdul-Ghani M, Nawaf G, Nawaf F, Itzhak B, Minuchin O, Vardi P. Increased prevalence of microvascular complications in type 2 diabetes patients with the metabolic syndrome. Isr Med Assoc J. 2006;8:378-382. [PubMed] |
| 88. | Mavrakanas T, Frachebois C, Soualah A, Aloui F, Julier I, Bastide D. C-peptide and chronic complications in patients with type-2 diabetes and the metabolic syndrome. Presse Med. 2009;38:1399-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Ahamed A, Shantha GP, Agarwal G, Senthil N, Paunikar N, Sudhakar MK. Prevalence of microvascular complications in metabolic syndrome in comparison to type 2 diabetes mellitus. J Indian Med Assoc. 2008;106:583-584, 586, 588. [PubMed] |
| 90. | Klein BE, Myers CE, Howard KP, Klein R. Serum Lipids and Proliferative Diabetic Retinopathy and Macular Edema in Persons With Long-term Type 1 Diabetes Mellitus: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. JAMA Ophthalmol. 2015;133:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 91. | Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Ten Emerging Trends in the Epidemiology of Diabetic Retinopathy. Ophthalmic Epidemiol. 2016;23:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 92. | Das R, Kerr R, Chakravarthy U, Hogg RE. Dyslipidemia and Diabetic Macular Edema: A Systematic Review and Meta-Analysis. Ophthalmology. 2015;122:1820-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Salinero-Fort MÁ, San Andrés-Rebollo FJ, de Burgos-Lunar C, Arrieta-Blanco FJ, Gómez-Campelo P. Four-year incidence of diabetic retinopathy in a Spanish cohort: the MADIABETES study. PLoS One. 2013;8:e76417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 94. | Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, Lim SC, Tai ES, Mitchell P. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115:1869-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 282] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 95. | Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141:446-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 456] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 96. | Lyons TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT, Klein RL. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 97. | Modjtahedi BS, Bose N, Papakostas TD, Morse L, Vavvas DG, Kishan AU. Lipids and Diabetic Retinopathy. Semin Ophthalmol. 2016;31:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 98. | Morgan CL, Owens DR, Aubonnet P, Carr ES, Jenkins-Jones S, Poole CD, Currie CJ. Primary prevention of diabetic retinopathy with fibrates: a retrospective, matched cohort study. BMJ Open. 2013;3:e004025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2540] [Cited by in RCA: 2220] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 100. | Sacks FM, Hermans MP, Fioretto P, Valensi P, Davis T, Horton E, Wanner C, Al-Rubeaan K, Aronson R, Barzon I. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation. 2014;129:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 101. | Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1182] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 102. | Yeh PT, Yang CM, Huang JS, Chien CT, Yang CH, Chiang YH, Shih YF. Vitreous levels of reactive oxygen species in proliferative diabetic retinopathy. Ophthalmology. 2008;115:734-737.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994;43:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 419] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 104. | Kim JH, Baik HW, Yoon YS, Joung HJ, Park JS, Park SJ, Jang EJ, Park SW, Kim SJ, Kim MJ. Measurement of antioxidant capacity using the biological antioxidant potential test and its role as a predictive marker of metabolic syndrome. Korean J Intern Med. 2014;29:31-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 507] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 106. | Coucha M, Elshaer SL, Eldahshan WS, Mysona BA, El-Remessy AB. Molecular mechanisms of diabetic retinopathy: potential therapeutic targets. Middle East Afr J Ophthalmol. 2015;22:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 107. | Roy S, Kern TS, Song B, Stuebe C. Mechanistic Insights into Pathological Changes in the Diabetic Retina: Implications for Targeting Diabetic Retinopathy. Am J Pathol. 2017;187:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 108. | Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. Clinical Diabetes. 2008;77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1057] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 109. | Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1256] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 110. | Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 764] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 111. | Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1517] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 112. | Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, Nagai M, Matsuzawa Y, Funahashi T. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 2004;68:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 560] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 113. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3205] [Cited by in RCA: 3212] [Article Influence: 133.8] [Reference Citation Analysis (1)] |
| 114. | Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 115. | Zaidi SI, Shirwany TA. Relationship of serum resistin with insulin resistance and obesity. J Ayub Med Coll Abbottabad. 2015;27:552-555. [PubMed] |
| 116. | Azab N, Abdel-Aziz T, Ahmed A, El-deen IM. Correlation of serum resistin level with insulin resistance and severity of retinopathy in type 2 diabetes mellitus. J of Saudi Chem Soci. 2016;272. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 117. | Tokuyama Y, Osawa H, Ishizuka T, Onuma H, Matsui K, Egashira T, Makino H, Kanatsuka A. Serum resistin level is associated with insulin sensitivity in Japanese patients with type 2 diabetes mellitus. Metabolism. 2007;56:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 118. | Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1730] [Cited by in RCA: 1594] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 119. | Kalderon B, Mayorek N, Berry E, Zevit N, Bar-Tana J. Fatty acid cycling in the fasting rat. Am J Physiol Endocrinol Metab. 2000;279:E221-E227. [PubMed] |
| 120. | Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 308] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 121. | Shen J, Bi YL, Das UN. Potential role of polyunsaturated fatty acids in diabetic retinopathy. Arch Med Sci. 2014;10:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 122. | Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013;14:10497-10538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 336] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 123. | Vendrell J, Chacón MR. TWEAK: A New Player in Obesity and Diabetes. Front Immunol. 2013;4:488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 124. | Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 687] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 125. | Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88:1343-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 126. | El-Asrar AM. Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr J Ophthalmol. 2012;19:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 127. | Liu Y, Biarnés Costa M, Gerhardinger C. IL-1β is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1β autostimulation. PLoS One. 2012;7:e36949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 128. | Lyons CL, Kennedy EB, Roche HM. Metabolic Inflammation-Differential Modulation by Dietary Constituents. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 129. | Behl T, Kaur I, Kotwani A. Role of leukotrienes in diabetic retinopathy. Prostaglandins Other Lipid Mediat. 2016;122:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 130. | El-Asrar AM, Missotten L, Geboes K. Expression of cyclo-oxygenase-2 and downstream enzymes in diabetic fibrovascular epiretinal membranes. Br J Ophthalmol. 2008;92:1534-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | Du Y, Sarthy VP, Kern TS. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R735-R741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 132. | Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur J Pharmacol. 2003;458:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 133. | Zhuang W, Wang G, Li L, Lin G, Deng Z. Omega-3 polyunsaturated fatty acids reduce vascular endothelial growth factor production and suppress endothelial wound repair. J Cardiovasc Transl Res. 2013;6:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 134. | National Institutes of Health NHLaBI. ATP III Guidelines At-A-Glance: Quick Desk Reference. Services UDoHaH, editor. National Cholesterol Education Program: Public Health Service 2001; . |