Published online May 15, 2017. doi: 10.4239/wjd.v8.i5.222
Peer-review started: November 2, 2016
First decision: December 1, 2016
Revised: December 20, 2016
Accepted: February 28, 2017
Article in press: March 2, 2017
Published online: May 15, 2017
Processing time: 198 Days and 5.8 Hours
To evaluate the influence of creatinine methodology on the performance of chronic kidney disease (CKD)-Epidemiology Collaboration Group-calculated estimated glomerular filtration rate (CKD-EPI-eGFR) for CKD diagnosis/staging in a large cohort of diabetic patients.
Fasting blood samples were taken from diabetic patients attending our clinic for their regular annual examination, including laboratory measurement of serum creatinine and eGFR.
Our results indicated an overall excellent agreement in CKD staging (kappa = 0.918) between the Jaffé serum creatinine- and enzymatic serum creatinine-based CKD-EPI-eGFR, with 9% of discordant cases. As compared to the enzymatic creatinine, the majority of discordances (8%) were positive, i.e., associated with the more advanced CKD stage re-classification, whereas only 1% of cases were negatively discordant if Jaffé creatinine was used for eGFR calculation. A minor proportion of the discordant cases (3.5%) were re-classified into clinically relevant CKD stage indicating mildly to moderately decreased kidney function (< 60 mL/min per 1.73 m2). Significant acute and chronic hyperglycaemia, assessed as plasma glucose and HbA1c levels far above the recommended glycaemic goals, was associated with positively discordant cases. Due to a very low frequency, positive discordance is not likely to present a great burden for the health-care providers, while intensified medical care may actually be beneficial for the small number of discordant patients. On the other hand, a very low proportion of negatively discordant cases (1%) at the 60 mL/min per 1.73 m2 eGFR level indicate a negligible possibility to miss the CKD diagnosis, which could be the most prominent clinical problem affecting patient care, considering high risk of CKD for adverse patient outcomes.
This study indicate that compensated Jaffé creatinine procedure, in spite of the glucose-dependent bias, is not inferior to enzymatic creatinine in CKD diagnosis/staging and therefore may provide a reliable and cost-effective tool for the renal function assessment in diabetic patients.
Core tip: Analytical performance of the serum creatinine assays is the critical determinant of estimated glomerular filtration rate (eGFR) accuracy. The most widely used compensated Jaffé creatinine assay suffers from a non-specific bias from pseudo-creatinine chromogens (glucose, ketones), which is not the case with the costly enzymatic assays. We evaluated the influence of creatinine methodology on the performance of chronic kidney disease (CKD)-Epidemiology-calculated eGFR for CKD diagnosis/staging in diabetic patients. Our results indicate that compensated Jaffé creatinine procedure, in spite of the glucose-dependent bias, is not inferior to enzymatic creatinine in CKD diagnosis/staging and therefore may provide a reliable and cost-effective tool for the renal function assessment in diabetic patients.
- Citation: Lovrenčić MV, Biljak VR, Blaslov K, Božičević S, Duvnjak LS. Impact of creatinine methodology on glomerular filtration rate estimation in diabetes. World J Diabetes 2017; 8(5): 222-229
- URL: https://www.wjgnet.com/1948-9358/full/v8/i5/222.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i5.222
Global prevalence of diabetes mellitus is rising progressively[1]. Chronic morbidity, associated with various debilitating complications, increased risk for adverse health-outcomes and significant impact regarding both the working ability and quality of life identify diabetes as one of the greatest health-care and socio-economic challenges worldwide. Appropriate strategies to tackle diabetes epidemic include education and lifestyle interventions, evidence-based clinical management as well as the screening for and monitoring of diabetes and/or diabetes-related complications using state-of-the art diagnostic tools.
Diabetic kidney disease (DKD) is one of the most prevalent chronic complications of diabetes and the most common single cause of end-stage renal failure[1,2]. It has been amply evidenced that appropriate interventions at an early stage of DKD can be efficient in preventing and/or delaying the progression of kidney disease and improving patient outcomes. Thus, the regular screening for DKD has became one of the cornerstones of diabetes care. Current clinical guidelines recommend at least an annual screening of DKD in patients with type 1 diabetes with a duration above 5 years, in all patients with type 2 diabetes and in all hypertensive diabetic patients[3]. Once detected, DKD is treated according to clinical guidelines and further monitored at regular intervals[2,3]. Two simple laboratory tests are used for both the screening and staging of CKD in diabetes: Urinary albumin excretion (UAE) and serum creatinine-based estimated glomerular filtration rate (SCr-eGFR).
Abnormal UAE has long been identified as a sensitive marker of the glomerular basal membrane damage, which is one of the early pathophysiological events in the development of DKD[4]. However, a significant decline in eGFR is a common finding in a notable proportion of diabetic patients with normal UAE, probably reflecting a diversity in the natural history of DKD[5]. Thus, the pathophysiology of DKD has shifted from the “albuminuric paradigm”[6], and the accumulated evidence implicating the progressive renal function decline as an equally relevant pathway identified reliable and accurate laboratory testing for serum creatinine and SCr-eGFR as a very important issue for the diagnosis, staging and monitoring of CKD in diabetic patients.
SCr has been used as a cost-effective and practical marker of kidney function for decades, despite severe limitations due to both biological and analytical variability[7]. A handful of biological factors such as age, gender, ethnicity and nutritional habits substantially influence serum creatinine levels, while partial tubular reabsorption and secretion of creatinine further compromise its use as the glomerular filtration marker[8,9]. Nevertheless, SCr-based estimation of GFR by the use of appropriate predictive equation remains the recommended surrogate marker for the assessment of kidney function, since the actual measurement of GFR, due to its complexity and high costs, is not available outside the specialized clinical settings. Current guidelines from the Kidney Disease Improving Global Guidelines (KDIGO) CKD Working Group recommend the use of the chronic kidney disease-Epidemiology Collaboration Group (CKD-EPI) equation[2]. CKD-EPI equation offers an improved reproducibility and accuracy at higher GFR levels (> 60 mL/min per 1.73 m2), which is the most prominent disadvantage of the previously recommended Modification of Diet in Renal Disease equation[10].
Analytical performance and specificity of SCr assay are critical determinants of the eGFR accuracy[11]. The relationship between SCr and GFR is exponential, therefore, errors in SCr measurements resulting from imprecision and bias could strongly impact eGFR results and result in misclassification of the patients regarding their kidney function[2]. Despite standardization and harmonization by the calibration traceable to isotope-dilution-mass spectrometry (IDMS), the non-specific bias from pseudo-creatinine chromogens (glucose, proteins, ketone bodies) is still affecting the most widely used compensated Jaffé alkaline picrate colorimetric creatinine assay[11,12]. Enzymatic creatinine methods are free from these interferences, but far more expensive and therefore not widely used. High-volume routine enzymatic creatinine testing may introduce a substantial financial challenge for the laboratories, even in the otherwise fairly resourced health-care systems[13]. Several analytical and clinical studies advocated the replacement of the compensated Jaffé with enzymatic creatinine assays in order to improve reliability of the eGFR, especially in the diabetic population, which is expected to have an increased amount of interfering substances in serum. However, recently published risk-analysis study, using both analytical and biological variability criteria, revealed a low risk for misclassification of CKD based on Jaffé-SCr-eGFR results in the general population[13], while the clinical impact in diabetic population remains unclear.
The aim of this study was to evaluate the influence of creatinine methodology on the performance of CKD-EPI-calculated eGFR for CKD evaluation and staging in a large cohort of diabetic patients.
Fasting blood samples were taken from diabetic patients attending our clinic for their regular annual examination, including laboratory measurement of SCr and eGFR. Samples from the patients with concomitant infection, limb-amputation and malignancies, as well as the pregnant patients and the patients with severe kidney disease (stage 5, according to KDIGO-2012 classification) were not included in the study. A subset of samples of the patients with severe hyperglycaemia were included in order to evaluate the interference of glucose on the CKD classification across various eGFR categories. Serum creatinine was measured by both IDMS-traceable compensated Jaffé (cJ-SCr) and enzymatic (e-SCr) (Beckman Coulter, Inc., Pasadena, California, United States) procedures with intra-assay imprecision (CV) of 1.58% and 1.39%, respectively. Hexokinase (Beckman Coulter, Inc., Pasadena, California, United States) and NGSP-traceable immunoturbidimetric assays (Tina Quant, Roche, F.Hoffmann-La Roche, Basle, Switzerland) were used for plasma glucose and HbA1c measurement.
Assay-specific SCr-eGFR was estimated by the 4-variable CKD-EPI equation using respective creatinine values[10]. UAE was measured by an automated immunoturbidimetric procedure (Beckman Coulter, Inc., Pasadena, California, United States) in fresh spot urine samples. Urinary creatinine was measured in the same samples and UAE results expressed as the urinary albumin/creatinine ratio.
Staging of albuminuria and CKD, as well as risk assessment for CKD progression was carried out according to KDIGO-2012 criteria (Table 1).
| Albuminuria categories [albumin/creatinine (mg/mmol)] | ||||
| A1 | A2 | A3 | ||
| < 3 | 3-30 | ≥ 30 | ||
| Normal to mildly increased | Moderately increased | Severely increased | ||
| eGFR categories (mL/min per 1.73 m2) | G1, ≥ 90 | Low risk | Moderately increased risk | High risk |
| Normal/high | ||||
| G2, 60-90 | Low risk | Moderately increased risk | High risk | |
| Mildly decreased | ||||
| G3a, 45-59 | Moderately increased risk | High risk | Very high risk | |
| Mildly to moderately decreased | ||||
| G3b, 30-44 | High risk | Very high risk | Very high risk | |
| Moderately to severely decreased | ||||
| G4, 15-29 | Very high risk | Very high risk | Very high risk | |
| Severely decreased | ||||
| G5, < 15 | Very high risk | Very high risk | Very high risk | |
| Kidney failure | ||||
The results were analyzed in the entire population and in sub-groups according to albuminuria (Table 2). Normality of distribution was tested by the Kolmogorov-Smirnov test and the significance of differences between the groups was assessed by the Kruskal-Wallis and Mann-Whitney test, as appropriate. Comparison between the creatinine methods in the study population was tested by Passing-Bablok regression analysis. Specific SCr-eGFR data were compared by Bland Altman analysis, and their agreement regarding clinical CKD staging was evaluated by inter-rater agreement (kappa-analysis). Statistical analyses were performed using MedCalc for Windows, version 9.4.2.0 (MedCalc Software, Ostend, Belgium). P < 0.05 was defined as the threshold of significance.
| Category of Albuminuria | |||
| A1 | A2 | A3 | |
| N (M/F) | 372 (212/198) | 166 (87/79) | 72 (38/34) |
| Age (yr) | 63 (19-88) | 68 (18-88) | 60a,b (29-85) |
| Glucose (mmol/L) | 9.0 (8.8-9.3) | 9.4 (8.9-10.0) | 7.4a,b (5.7-8.9) |
| HbA1c (%) | 7.4 (7.3-7.6) | 7.5 (7.3-7.7) | 7.8 (7.4-8.5) |
| HbA1c (mmol/mol) | 59 (57-61) | 59 (57-61) | 62 (58-71) |
| e-SCr (μmol/L) | 69b,d (66-72) | 75a,d (72-77) | 100a,b (88-137) |
| cJ-SCr (μmol/mol) | 70b,d (67-72) | 77a,d (72-81) | 108a,b (87-140) |
| e-SCr-eGFR (mL/min per 1.73 m2) | 91b,d (88-93) | 85a,d (80-88) | 60a,b (39-77) |
| cJ-SCr-eGFR (mL/min per 1.73 m2) | 90b,d (87-92) | 83a,d (76-86) | 55a,b (39-73) |
The study was approved by the Merkur University Hospital Ethics Committee. Due to the retrospective nature of the study, with the post-hoc selection of anonymized samples from the routine laboratory visits, patient’s informed consent was not obtained.
A total of 648 Caucasian diabetic patients (337 males) was included in this study. No gender-related differences were observed in the clinical and biochemical parameters, except significantly lower creatinine levels in females (P < 0.001, data not shown). There was a significant increase of SCr and a decrease of eGFR, as measured/estimated by both methods across the categories of albuminuria (Table 2). Fasting plasma glucose was significantly lower in the A3 subgroup only, while HbA1c levels showed no differences regarding albuminuria (Table 2). eGFR and creatinine results did not differ significantly depending on creatinine methodology in either category of albuminuria (P = 0.228, 0.2306 and 0.7553 for A1, A2 and A3 category, respectively; Mann-Whitney test).
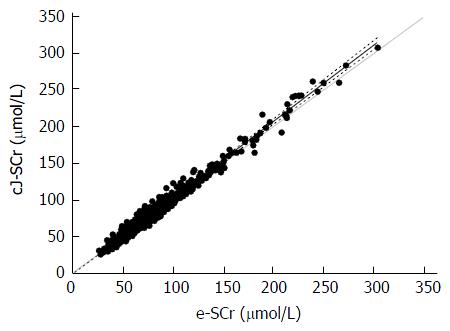
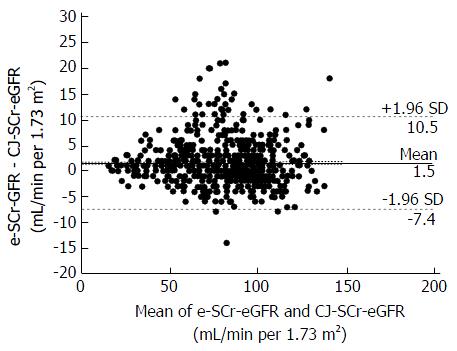
Passing-Bablok regression analysis revealed a small, but significant constant difference between the enzymatic and compensated Jaffé SCr assays [y = -2.8095 (95%CI: -3.8125 to -1.6066) + 1.0476 × (95%CI: 1.0328-1.0625)] across a wide range of creatinine values (Figure 1). This was accompanied by a minor, but significant creatinine assay-dependent difference in SCr-eGFR values [Bland Altman: y = 1.5154 (95%CI: 1.1635-1.8674; lower limit: -7.4276; upper limit: 10.4585) P < 0.001] (Figure 2). The severity of both acute and chronic hyperglycaemia was identified as the significant predictor of between-method SCr-eGFR bias (Spearman's rho = -0.363 and -0.369 for fasting plasma glucose and HbA1c, respectively, P < 0.001).
Inter-rater agreement analysis showed an excellent agreement (weighted kappa = 0.918; 95%CI: 0.894-0.94) between the method-specific SCr-eGFRs when classifying subjects into KDIGO-2012 CKD-stages. However, some cases were classified differently between CKD stages depending on the creatinine method used for eGFR calculation (Table 3). Compared to e-SCr-eGFR-based CKD classification, 58/648 (9%) patients were re-classified into a different CKD stage when cJ-SCr-based eGFR was used. The majority of these (54/648; 8%) were re-classified into a more advanced stage of CKD (positive discordance), with 23 (3.5%) cases re-classified into the clinically significant eGFR category indicating mildly to moderately decreased kidney function (< 60 mL/min per 1.72 m2). Among these, 7 cases (1%) had A1 stage of albuminuria, whereas the rest of clinically significant positive discordant cases had more advanced stages of albuminuria. On the other hand, 8/648 (1%) of patients were re-classified into a less-advanced CKD stage when compensated Jaffé-SCr-eGFR was used (negative discordance), with only 2 cases being re-classified between the 3A and 2 eGFR categories. A1 and A2 stage of albuminuria was detected in each one of these two cases.
There was a significant difference in fasting plasma glucose values regarding concordance of CKD staging, with higher glucose values for positive- and lower glucose values for negative discordant subjects, in comparison to concordant sub-group (11.2 ± 4.3 vs 7.5 ± 1.8 vs 8.9± 2.1 mmol/L, P < 0.001). HbA1c, indicating a chronic level of hyperglycaemia, showed an identical pattern (8.4 ± 2.3% ± /69 ± 25 mmol/mol vs 6.6 ± 0.7%/49 ± 7.3 mmol/mol vs 7.8 ± 1.7%/62 ± 19 mmol/mol; P < 0.01). We analyzed the frequency of discordances according to the level of hyperglycaemia, by using the fasting plasma glucose cut-off of 17.0 mmol/L, which was reported to significantly influence SCr results obtained by the colorimetric Jaffé procedure[7]. Positively discordant results were more prevalent in the sub-group of patients with fasting plasma glucose above (n = 59), than below (n = 589), 17.0 mmol/L glucose cut-off (20% vs 8%, χ2 = 11.968, P = 0.0025). However, in general, patients with eGFR < 60 mL/min per 1.73 m2 had lower fasting plasma glucose than those with eGFR > 60 mL/min per 1.73 m2 (7.3 ± 1.9 mmol/L vs 9.2 ± 2.0 mmol/L, P < 0.0001).
In this study, we attempted to evaluate the influence of creatinine methodology on the performance of CKD-EPI-calculated eGFR for CKD staging in a large cohort of diabetic patients. Our results indicate an overall excellent agreement in CKD staging (kappa = 0.918) between the Jaffé serum creatinine- and enzymatic serum creatinine-based CKD-EPI-eGFR, with 9% of discordant cases. As compared to the enzymatic creatinine, the majority of discordances (8%) were positive, i.e., associated with the more advanced CKD stage re-classification, whereas only 1% of cases were negatively discordant if Jaffé creatinine was used for eGFR calculation. Plasma glucose was identified as a significant determinant of between-method bias.
Kidney function is rather uniquely affected by diabetes. Elevated GFR, known as hyperfiltration, is a common finding in new-onset diabetes, probably as a consequence of hyperglycaemia and related metabolic and endocrine disturbances. Hyperfiltration, being considered as an early sign of DKD[14], is declining with the progression of diabetes and the intensive diabetes treatment was found to be effective in reducing the risk for the progression to DKD by delaying the GFR decline in both type 1 and type 2 diabetes[15,16]. Thus, specific features of DKD in diabetes indicate the need for an accurate and reliable method for GFR estimation over the wide range of GFR. Our previous study showed that CKD-EPI-eGFR, with improved accuracy in the GFR range above 60 mL/min per 1.73 m2, represented a superior surrogate marker of GFR in diabetic patients, particularly those with normoalbuminuria and hyperfiltration implicating its use as a reliable screening tool for an early renal impairment in diabetes[17]. Either compensated Jaffé or enzymatic creatinine assay, traceable to the reference IDMS procedure, is needed for eGFR-CKD-EPI calculation[2].
Analytical interference of the glucose and other reducing substances in the alkaline picrate Jaffé creatinine assay has long been identified[7]. Several method improvements, including modified spectral kinetics and standardization to the IDMS reference procedure with a mathematical adjustment of results to compensate for interferences (compensated Jaffé assays), have remarkably improved the accuracy of the method. Nevertheless, the non-specificity remained a matter of concern in selected patient subpopulations, such as subjects with diabetes[8]. Enzymatic creatinine assays offer improved specificity and several authors argued that Jaffé method should be entirely abandoned, particularly in the diabetic population. It was reported that CKD-EPI-eGFR showed better concordance to the measured GFR, empowering further the enzymatic method as a method of choice for serum creatinine measurement in diabetic patients[15]. However, evidence supporting this proposition is based either on cross-sectional method-comparison studies including a limited number of patients, or simulation studies using analytical bias extracted from inter-laboratory comparisons, with no data regarding the clinical outcome-associated risk[18-21]. Our results demonstrate a minor, but significant glucose-dependent positive bias between the serum creatinine levels measured by compensated Jaffé and enzymatic procedure, with a mirroring effect regarding respective eGFRs, but the key question of this study was the clinical relevance of the observed difference.
State-of-the-art strategies for laboratory test evaluation implicate not only analytical, but also clinical performance together with clinical- and cost-effectiveness as essential interactive components of the overall diagnostic test utility assessment[22]. In a recently published outcome-based study, Schmidt et al[13] reported on a very low risk for patient outcomes due to the miss-classification of CKD stages with Jaffé creatinine assay in the general population. Our study reveals that most of the discordant cases in diabetic subjects were positive, i.e., Jaffé method was likely to classify 8% of the patients into a more advanced CKD stage than the enzymatic method. Among these, 23/648 cases were classified into the clinically significant stage 3A, while only 7 positively discordant cases with normal UAE (1%) were re-classified between the low4 and moderately increased risk for the CKD progression, according to KDIGO guidelines (Table 1). The frequency of positively discordant cases was 2.5 times greater in the sub-group of patients with plasma glucose above the 17.7 mmol/L, previously reported as a threshold for a significant analytical interference[7]. It is important to emphasize that both fasting plasma glucose and HbA1c in positively discordant sub-group was far above the glycaemic recommendations for adults with diabetes, requiring immediate interventions to reduce the hyperglycaemia regardless of the kidney function[3], which is known to be afflicted by the renal hypoperfusion in acute hyperglycaemic episodes[23,24].
Apart from assessing frequency, our main goal was to evaluate the clinical consequences of the discordant Jaffé creatinine-dependent CKD staging. In general, clinical guidelines recommend the optimization of glycaemic control and blood pressure as the treatment strategy to prevent and/or delay the development/progression of CKD in diabetic patients[3]. Patients with stage 3a CKD (eGFR range 45-60 mL/min per 1.73 m2) should be referred to a nephrologist for further evaluation, their diet and medication adjusted and eGFR monitoring intensified to twice a year. The more advanced stages of CKD require further specialized care and intensified monitoring, and metformin should be discontinued in patients with stage 3b CKD (30-45 mL/min per 1.73 m2). However, KDIGO-2012 guidelines recommend repeated eGFR measurement within 3 mo for all subjects with eGFR < 60 mL/min per 1.73 m2, in order to confirm the classification[2]. Considering the recommended diabetes guidelines, our positively discordant group at the 60 mL/min per 1.73 m2 eGFR level (3.5%) would not experience any harm from being classified into more advanced CKD stage according to the Jaffé creatinine-based eGFR. On the contrary, clinical intervention to improve glycaemic control was identified as being immediately needed for all these patients, and, if KDIGO-2012-recommended confirmatory eGFR was to be done after 3 mo, presumably reached glycaemic goals would allow more accurate CKD classification not only by the Jaffé method. Limited design of our study did not allow the insight into actual follow-up data, but the well-established ameliorating effect of improved glycaemic control on renal function is likely to elicit the consequent beneficial effect on eGFR via biological mechanism(s)[23], regardless of the creatinine method used. Furthermore, the majority of the positively discordant cases had A2 and A3 stages of albuminuria, confirming an increased or high risk of CKD progression in these patients[2].
Significant improvements in CKD screening strategies were enabled by creatinine standardization and automated eGFR reporting by clinical laboratories[11,12]. However, recommended practice is still not implemented in many clinical settings, indicating a lack of appropriate clinical care with significant medical and financial consequences[25]. One of the reasons for the reluctance to implement the guidelines is the concern of increased costs of specific creatinine testing. Our results show that low-cost Jaffé creatinine assay can be safely used for the CKD screening in patients with diabetes, despite confirmed positive analytical bias in comparison to enzymatic assay, provided that compensated Jaffé assay, traceable to the reference IDMS procedure, and CKD-EPI-calculated eGFR are used. The frequency of positively discordant CKD classification is very low and associated with severe hyperglycaemia, where the appropriate interventions to attain glycaemic goals are warranted regardless of CKD. Concomitant presence of increased albuminuria in the majority of discordant cases further diminishes the clinical practice consequences, which may, in turn, be beneficial for the patients in terms of increasing frequency of eGFR monitoring and intensifying efforts to control hyperglycaemia and hypertension. Due to a very low frequency, positive discordance is not likely to present a great burden for the health-care providers. On the other hand, a very low proportion of negatively discordant cases (1%) at the 60 mL/min per 1.73 m2 eGFR level indicate a negligible possibility to miss the CKD diagnosis, which could be the most prominent clinical problem affecting patient outcomes. Namely, CKD, being a major risk factor for cardiovascular disease and overall mortality, should be detected as early as possible, since appropriate interventions can substantially reduce risks and improve patient outcomes[2].
Limitations of our study include cross-sectional design and singular ethnicity. A large cohort of diabetic patients stratified according to albuminuria and degree of hyperglycaemia, as well as clinical outcome-based, rather than method-comparison-based approach can be regarded as the study advantages.
In conclusion, results from our study indicate that compensated Jaffé creatinine procedure, in spite of the glucose-dependent bias, is not inferior to enzymatic creatinine in CKD diagnosis/staging and therefore may provide a reliable and cost-effective tool for the renal function assessment in diabetic patients.
Diabetic kidney disease (DKD) is one of the most prevalent chronic complications of diabetes and the most common single cause of end-stage renal failure. Current clinical guidelines recommend regular screening for and staging of DKD by the laboratory assessement of both urinary albumin excretion rate and estimation of glomerular filtration rate from serum creatinine (SCr-eGFR).
Analytical performance of the serum creatinine assays is the critical determinant of eGFR accuracy. The most widely used compensated Jaffé creatinine assay suffers from a non-specific bias from pseudo-creatinine chromogens (glucose, ketones), which is not the case with the costly enzymatic assays. Several studies advocated the replacement of the compensated Jaffé with enzymatic creatinine assays in order to improve reliability of the eGFR, especially in the diabetic population, which is expected to have an increased amount of interfering substances in serum. However, recently published risk-analysis study, using both analytical and biological variability criteria, revealed a low risk for misclassification of CKD based on Jaffé-SCr-eGFR results in the general population, while the clinical impact in the diabetic population remains unclear.
This study evaluated the influence of creatinine methodology on the performance of CKD-EPI-calculated eGFR for CKD diagnosis/staging in diabetic patients. The results indicate that compensated Jaffé creatinine procedure, in spite of the glucose-dependent bias, is not inferior to enzymatic creatinine in CKD diagnosis/staging and therefore may provide a reliable and cost-effective tool for the renal function assessment in diabetic patients.
Significant improvements in CKD screening strategies were enabled in the last decade by creatinine standardization and automated eGFR reporting by clinical laboratories. However, recommended practice is still not implemented in many clinical settings, indicating a lack of appropriate clinical care with significant medical and financial consequences. One of the reasons for the reluctance to implement the guidelines is the concern of increased costs of specific creatinine testing. This results show that low-cost Jaffé creatinine assay can be safely used for the CKD screening in patients with diabetes, despite confirmed positive analytical bias in comparison to enzymatic assay, provided that compensated Jaffé assays, traceable to the reference IDMS procedure, and CKD-EPI-calculated eGFR are used. This finding is particularly valuable for the primary health-care facilities, since available and cost-effective screening for DKD may substantially improve patient outcomes and reduce overall costs associated with more advanced complications of diabetes.
The paper is far from being a useful tool for the clinical management of CKD patients, it shows an interesting new approach to be validated in a prospective way and bigger sample size in order to clarify the potential use in specific clinical situations of CKD patients.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: D’Orazio P, Zhao JB S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | International Diabetes Federation. IDF Diabetes Atlas, 7th edition. Brussels: IDF 2015; . |
| 2. | National Kidney Foundation. KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150. |
| 3. | American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2830] [Cited by in RCA: 3016] [Article Influence: 274.2] [Reference Citation Analysis (0)] |
| 4. | Lin CH, Chang YC, Chuang LM. Early detection of diabetic kidney disease: Present limitations and future perspectives. World J Diabetes. 2016;7:290-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 5. | Tang SC, Chan GC, Lai KN. Recent advances in managing and understanding diabetic nephropathy. F1000Res. 2016;5:pii: F1000 Faculty Rev-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Krolewski AS. Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2015;38:954-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 7. | Husdan H, Rapoport A. Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin Chem. 1968;14:222-238. [PubMed] |
| 8. | Hoste L, Deiteren K, Pottel H, Callewaert N, Martens F. Routine serum creatinine measurements: how well do we perform? BMC Nephrol. 2015;16:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313:837-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 412] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 10. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [PubMed] |
| 11. | Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 851] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 12. | Greenberg N, Roberts WL, Bachmann LM, Wright EC, Dalton RN, Zakowski JJ, Miller WG. Specificity characteristics of 7 commercial creatinine measurement procedures by enzymatic and Jaffe method principles. Clin Chem. 2012;58:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Schmidt RL, Straseski JA, Raphael KL, Adams AH, Lehman CM. A Risk Assessment of the Jaffe vs Enzymatic Method for Creatinine Measurement in an Outpatient Population. PLoS One. 2015;10:e0143205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Jerums G, Premaratne E, Panagiotopoulos S, Clarke S, Power DA, MacIsaac RJ. New and old markers of progression of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82 Suppl 1:S30-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 419] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 16. | Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, MacMahon S, Cooper ME, Hamet P, Marre M. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 17. | Vučić Lovrenčić M, Radišić Biljak V, Božičević S, Prašek M, Pavković P, Knotek M. Estimating glomerular filtration rate (GFR) in diabetes: the performance of MDRD and CKD-EPI equations in patients with various degrees of albuminuria. Clin Biochem. 2012;45:1694-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Weykamp C, Kuypers A, Bakkeren D, Franck P, Loon Dv, Gunnewiek JK, Jonge Rd, Steigstra H, Cobbaert C. Creatinine, Jaffe, and glucose: another inconvenient truth. Clin Chem Lab Med. 2015;53:e347-e349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kuster N, Cristol JP, Cavalier E, Bargnoux AS, Halimi JM, Froissart M, Piéroni L, Delanaye P. Enzymatic creatinine assays allow estimation of glomerular filtration rate in stages 1 and 2 chronic kidney disease using CKD-EPI equation. Clin Chim Acta. 2014;428:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Cheuiche AV, Soares AA, Camargo EG, Weinert LS, Camargo JL, Silveiro SP. Comparison between IDMS-traceable Jaffe and enzymatic creatinine assays for estimation of glomerular filtration rate by the CKD-EPI equation in healthy and diabetic subjects. Clin Biochem. 2013;46:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Drion I, Cobbaert C, Groenier KH, Weykamp C, Bilo HJ, Wetzels JF, Kleefstra N. Clinical evaluation of analytical variations in serum creatinine measurements: why laboratories should abandon Jaffe techniques. BMC Nephrol. 2012;13:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Horvath AR, Lord SJ, StJohn A, Sandberg S, Cobbaert CM, Lorenz S, Monaghan PJ, Verhagen-Kamerbeek WD, Ebert C, Bossuyt PM. From biomarkers to medical tests: the changing landscape of test evaluation. Clin Chim Acta. 2014;427:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 23. | Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1090] [Cited by in RCA: 1188] [Article Influence: 74.3] [Reference Citation Analysis (3)] |
| 24. | Orban JC, Maizière EM, Ghaddab A, Van Obberghen E, Ichai C. Incidence and characteristics of acute kidney injury in severe diabetic ketoacidosis. PLoS One. 2014;9:e110925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Biljak VR, Honović L, Matica J, Knežević B, Vojak SŠ. Laboratory diagnostics of chronic kidney disease in Croatia: state of the art. Biochem Med (Zagreb). 2015;25:73-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |