Published online Sep 15, 2016. doi: 10.4239/wjd.v7.i17.406
Peer-review started: March 7, 2016
First decision: June 16, 2016
Revised: June 24, 2016
Accepted: July 14, 2016
Article in press: July 18, 2016
Published online: September 15, 2016
Processing time: 188 Days and 23.8 Hours
In vivo corneal confocal microscopy (IVCCM) is a novel, reproducible, easy and noninvasive technique that allows the study of the different layers of the cornea at a cellular level. As cornea is the most innervated organ of human body, several studies investigated the use of corneal confocal microscopy to detect diabetic neuropathies, which are invalidating and deadly complications of diabetes mellitus. Corneal nerve innervation has been shown impaired in subjects with diabetes and a close association between damages of peripheral nerves due to the diabetes and alterations in corneal sub-basal nerve plexus detected by IVCCM has been widely demonstrated. Interestingly, these alterations seem to precede the clinical onset of diabetic neuropathies, paving the path for prevention studies. However, some concerns still prevent the full implementation of this technique in clinical practice. In this review we summarize the most recent and relevant evidences about the use of IVCCM for the diagnosis of peripheral sensorimotor polyneuropathy and of autonomic neuropathy in diabetes. New perspectives and current limitations are also discussed.
Core tip: Diabetic neuropathies are common, invalidating and often undiagnosed complications affecting a huge number of subjects with diabetes. In vivo corneal confocal microscopy is a novel, reproducible, easy and noninvasive technique that has been widely studied as a useful tool for the diagnosis of neuropathy. Promising data suggest its implementation in clinical and research practice will help to face the current health emergency related to nerve damages in diabetes.
- Citation: Maddaloni E, Sabatino F. In vivo corneal confocal microscopy in diabetes: Where we are and where we can get. World J Diabetes 2016; 7(17): 406-411
- URL: https://www.wjgnet.com/1948-9358/full/v7/i17/406.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i17.406
Diabetic neuropathies are common and invalidating, but often undiagnosed, complications affecting up to 50% of subjects with diabetes[1,2]. Diabetic neuropathies encompass a wide spectrum of clinical and pathophysiological frameworks characterized by a progressive loss of nerve fibers, which may affect both somatic and autonomic nerves. While the rates of myocardial infarction, stroke, low-extremity amputations and end-stage renal disease due to diabetes are declining[3], this is not the case for trends in the incidence of neuropathies. An early diagnosis and correct staging of neuropathy is essential for risk stratification, therapeutic decisions and research purposes. To date screening and diagnosis of diabetic neuropathies mainly relies on symptoms questionnaires, clinical examination, quantitative sensory tests and reflex tests[4,5]. Nerve conduction studies should also be used to confirm the diagnosis and to assess the severity of the disease[2]. In vivo corneal confocal microscopy (IVCCM) is a non-invasive technique to visualize and analyze corneal anatomy at high magnification allowing the study of the different layers and cells of cornea, the most innervated organ of human body[6]. Several studies in the last decade investigated the use of IVCCM for the diagnosis of sensorimotor and, more recently, autonomic neuropathies[7]. In this review we aim to summarize the most recent and relevant evidences about IVCCM use in people with diabetes, focusing on strength and limitations future studies should overcome.
The eye has historically been considered as a pivotal organ for the study of diabetes-related complications. The transparency of the ocular structures has always been used as a diagnostic tool to investigate and actually see in vivo vascular changes in the retina. More recently, increasing attention has been paid to corneal nerve anatomy for the study of human neuropathies. The cornea is provided of the densest innervation within the body receiving nerve fibers from 50-450 sensory trigeminal neurons via the ophthalmic branch of trigeminal nerve[8,9]. These fibers travel above the choroid, reach the limbus where they organize into a nerve plexus[10]. Corneal stromal nerves derive from the limbal plexus and branch into fibers with smaller diameter that establish close connections with keratocytes and corneal epithelial cells[11]. The fibers become denser and smaller in diameter as they reach the corneal apex creating a sub-epithelial dense network in the epithelium, known as sub-basal corneal nerve plexus.
The introduction of IVCCM in ophthalmology (Figure 1) has represented a breakthrough for the study of ocular as well as systemic diseases since this diagnostic tool allows for a non-invasive and in vivo visualization of all the corneal layers, including nerves. There are currently three models of confocal microscope available, namely the slit-scanning, the tandem scanning and the laser scanning confocal microscope. They provide images with different resolution, contrast and magnification with high inter-device variability. However all of them result in high-resolution scans of the cornea. These features make IVCCM an ideal tool to investigate changes in corneal and ocular surface. It is used as an aid in the diagnosis and to monitor efficacy of therapies in different ocular diseases like, dry eyes, Acanthamoeba keratitis or keratoconus or following corneal surgery[12]. Several studies have also investigated the role of IVCCM as a diagnostic tool to be implemented for the assessment of several systemic diseases. Indeed, changes in the corneal sub-basal nerves have been shown to correlate with several neurodegenerative diseases, small fiber neuropathies, Fabry disease and other conditions causing peripheral neuropathy like diabetes, HIV-infection, genetic diseases, toxic drugs or autoimmune diseases[13].
Changes in corneal morphology including reduced thickness, thinner epithelium, irregular endothelium and reduction in corneal nerve bundles have been described in diabetes[14]. Therefore, qualitative and quantitative analysis of the sub-basal corneal nerve plexus by IVCCM has been hypothesized to be a good method for the evaluation and quantification of nerve damages in people affected by diabetes. Several studies have been conducted to test this hypothesis in both type 1 and type 2 diabetes[7,15]. Overall, corneal nerve innervation has been shown impaired in subjects with diabetes, independently by the presence of overt neuropathy[16]. Interestingly, IVCCM was able in identifying early neuropathy also in subjects with pre-diabetes. Asghar et al[17] assessed corneal innervation in thirty-seven subjects with impaired glucose tolerance showing that IVCCM, but not electrophysiology studies, detected signs of nerve damages which correlated with neuropathy symptoms, neurological deficits and intraepidermal nerve fiber density (IENFD).
A close association between damages of peripheral nerves due to the diabetes and alterations in corneal sub-basal nerve plexus detected with IVCCM has been widely demonstrated by a number of studies. A landmark small cross-sectional study by Malik et al[18] showed corneal nerve fiber density, length and branch density were reduced in eighteen diabetic subjects with different grade neuropathy vs healthy controls, with a gradual reduction of these parameters with increasing neuropathy severity. Similarly, corneal nerve tortuosity was found increased in diabetics and in those with more severe neuropathy[19]. Subsequently several studies by this and other groups confirmed the efficacy of IVCCM for the identification of diabetic neuropathy in larger populations of type 1[20-23], type 2 diabetes[24] or both[25-27]. In particular, baseline features of the population enrolled in the LANDMark study, the largest study testing IVCCM in 242 type 1 diabetes vs 154 controls, confirmed the reduced corneal never fiber length in those with neuropathy[22]. Longitudinal results of this study are not yet available.
As cornea is innervated by small Aδ and C fibers, IVCCM was tested with good results as a surrogate marker of small fiber neuropathy[28]. Before IVCCM, the evaluation of IENFD by skin biopsy was the gold standard method to quantitatively assess small fiber damages, with obvious limitations for a routine implementation in clinical practice due to its invasiveness. A comparable diagnostic efficiency between IVCCM and IENFD in type 1 diabetes has been recently shown in a study by Chen et al[29] where the area under the receiver operator curve for the identification of neuropathy did not significantly differ between the two techniques.
Among all the parameters of corneal innervation evaluated by IVCCM, the great majority of published studies agree about the validity and the overall good reproducibility of corneal nerve fiber density and length. In particular, the latter was shown to be the best predictor of diabetic sensorimotor polyneuropathy in 81 subjects affected by type 1 diabetes, with an optimized threshold for sensitivity and specificity at 14.0 mm/mm2[30]. This threshold, however, has to be applied taking into account the natural age-dependent variation in corneal nerve fiber length. On the contrary, corneal nerve beadings and tortuosity showed the highest inter and intra-individual variability, questioning the validity of these two measurements for the diagnosis of diabetic peripheral neuropathy[21]. However, the measurement of nerve tortuosity could be relevant to ameliorate the predictive value of fiber length, as tortuosity-standardized corneal nerve fiber length was better than non-standardized length in differentiating between individuals with and without neuropathy[31].
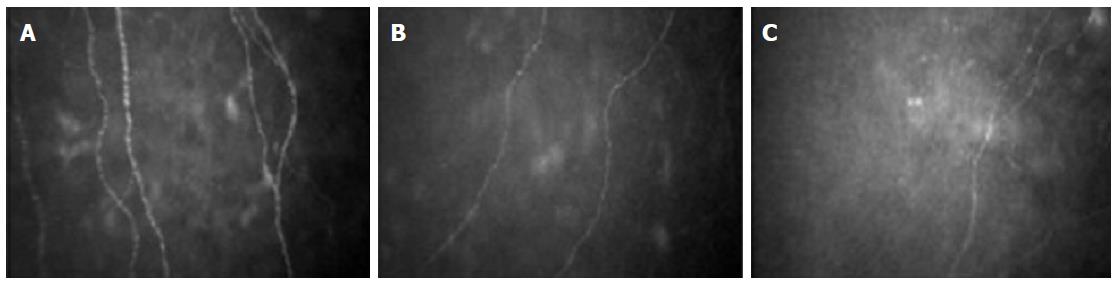
Diabetic autonomic neuropathy is a form of diabetic neuropathy that results from the damage of small (Aδ, B and C) nerve fibers. It represents one of the most overlooked but life-threatening complications of diabetes, associated with gastrointestinal, genitourinary, vasomotor and cardiac symptoms. In particular cardiac autonomic neuropathy affects up to 40% of diabetic patients and is associated with silent myocardial ischemia, stroke and increased mortality[5]. Because of the structural similarity between the corneal nerve fibers analyzed with IVCCM and the small fibers conducting autonomic signals, IVCCM has been recently tested as a diagnostic tool for autonomic neuropathies. We showed that subjects affected by type 1 diabetes with cardiac autonomic neuropathy, as evaluated by cardiovascular autonomic reflex tests (CARTs), had reduced corneal nerve fiber density and length when compared to peers without cardiac autonomic neuropathy and to healthy controls, independently of the presence of peripheral neuropathy[32] (Figure 2). Subsequently, Tavakoli et al[33] confirmed our observation in a population of both type 1 and type 2 diabetic subjects. Subjects were evaluated by the Composite Autonomic Symptom Scale (COMPASS), by CARTs, by sympathetic skin response, and by IVCCM. The Composite Autonomic Severity Score (CASS) was also calculated. Corneal nerve fiber density, length and branch density were significantly reduced in subjects with autonomic deficits than in those without. IVCCM showed moderate-to-strong correlations with COMPASS and CASS, with a good sensitivity and specificity for fiber length and fiber density for the diagnosis of diabetic autonomic neuropathy[33]. Similarly, corneal nerve innervation was found to be related to sudomotor function in subjects affected by type 2 diabetes[34]. Moreover, a significant correlation between corneal sensitivity and measures of cardiac autonomic function in subjects with type 1 diabetes was recently reported[35]. However, in the same study no significant relationship with sub-basal nerve density was found.
A growing literature supports IVCCM as an innovative technique helpful to face diabetic neuropathies, which are prevalent complications of diabetes and cause of high healthcare expenditures, reduced quality of life, high morbidity and mortality. In particular IVCCM could have possible implications for the prevention of diabetic neuropathies and for research studies about its pathophysiology and treatments.
Changes in the corneal sub-basal nerve plexus anticipate other clinical and electrophysiology signs of neuropathy[35,36]. Two longitudinal studies showed that lower corneal nerve fiber length predicts the onset of diabetic sensorimotor polyneuropathy in type 1 diabetic subjects followed-up for 3.5 and 4 years[37,38]. For the identification of new cases of neuropathy, the sensitivity and specificity of corneal nerve fiber length were 82% and 69%, respectively, with an optimal threshold of 14.9 mm/mm2 in one study[37], and 63% and 74% with an optimal threshold of 14.1 mm/mm2 in the other one[38]. This suggests IVCCM allows the identification of at-risk patients to implement preventive strategies such as tight glycemic control and multifactorial interventions. Indeed, a prospective cohort study in subjects with type 1 diabetes without overt neuropathy showed that modifications in the corneal sub-basal nerve plexus over 4 years of follow-up were related to clinical and metabolic factors such as age, HbA1c and HDL cholesterol. This highlights the capability of IVCCM for monitoring the efficacy of preventive strategies aimed to modify the natural history of diabetic neuropathy before symptoms and signs become measurable by classical screening tests[39].
Moreover, Petropoulos et al[40] showed that degeneration of corneal nerve fibers are detectable before other microvascular complications appear, questioning whether early detection of small fibers distress by IVCCM could also work as a precocious surrogate marker for vascular risk stratification as well as microalbuminuria or retinopathy. However, to date no studies have specifically investigated the predictive value of IVCCM with regards to the development of diabetic complications other than neuropathy.
Besides the clinical implications, IVCCM could be a useful research tool to investigate neuropathy pathophysiology. The promising data showing early degeneration of corneal nerve fibers in impaired glucose tolerance and new onset type 2 diabetes[17,24,41] support the hypothesis the pathophysiology of diabetic neuropathy starts very early in diabetes[42]. Overall the data discussed so far pose IVCCM as a research tool to investigate the first steps of neuropathy, where other methods such as nerve conduction studies are not enough sensitive to detect pathological changes. Moreover, even though corneal nerve fibers have sensitive but not autonomic function, the above reported results about the association of IVCCM and diabetic autonomic neuropathy overall suggest that molecular cascades eventually leading to the damage of the sub-basal corneal plexus could also occur to nerves with similar structure such as the autonomic ones, even if they have different function. They also suggest that IVCCM is a surrogate easy and non-invasive marker of autonomic dysfunction, which mostly remains undiagnosed because of scarce implementation of the recommended diagnostic tests. However, these promising findings have still to be tested for their usefulness for cardiovascular risk-stratification in larger and homogenous populations.
Interestingly, some reports show that IVCCM is also able in detecting regeneration of small nerve fiber after therapeutics. IVCCM showed significant improvements in nerve morphology after pancreas transplantation[43], after simultaneous kidney-pancreas transplantation[44], after improvements in risk factors for diabetic neuropathy[45], in subjects treated with continuous subcutaneous insulin infusion[46] and in phase 2 studies[47]. These studies suggest IVCCM is novel noninvasive tool to establish early nerve repair consequent to medical intervention that is missed by current assessment techniques.
Some pitfalls still exist about the use of IVCCM for the diagnosis of diabetic neuropathies. In particular, an age-related decline in corneal nerve fibers density and length occurs, claiming for age-standardized normative values. Moreover, there are uncertainties about racial differences in corneal nerve measures. IVCCM implementation and utility is also limited by the time and the expertise required for image analysis. Some arguments against IVCCM also claim a scarce reproducibility of corneal nerve measurements. In this regard, it has been recently shown preservation in the inter- and intra-observer reproducibility of fiber length measurements when using a fully automated analysis program, which also eliminates the need for trained analyst personnel and reduces the analysis time[25,48].
In conclusion, IVCCM currently represents a fascinating link between laboratory and clinical sciences through which diabetic neuropathies can be analyzed by assessing nerve density, tortuosity and length. We acknowledge our conclusions may be limited by the fact this manuscript is not a systematic review. However, to limit a possible selection bias, we carefully search throughout the literature for human studies testing IVCCM in people with diabetes and reported both positive and negative results, strength and limitations. As a result of our search, we reported that several evidences support the role of IVCCM as an easy and non-invasive clinical and research tool for the study of diabetic neuropathies, but some limitations including bias in image selection, reproducibility and the required expertise to perform the scan and read the images still need to be fully addressed.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ciccone MM, Hwu CM, Kesavadev J S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 936] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 2. | Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1948] [Cited by in RCA: 1748] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 3. | Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370:1514-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1224] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 4. | Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 542] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 5. | Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 608] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 6. | Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M. In vivo confocal microscopy of the human cornea. Br J Ophthalmol. 2003;87:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Papanas N, Ziegler D. Corneal confocal microscopy: Recent progress in the evaluation of diabetic neuropathy. J Diabetes Investig. 2015;6:381-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 855] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 9. | Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 10. | Al-Aqaba MA, Fares U, Suleman H, Lowe J, Dua HS. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010;94:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Müller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996;37:476-488. [PubMed] |
| 12. | Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Wang EF, Misra SL, Patel DV. In Vivo Confocal Microscopy of the Human Cornea in the Assessment of Peripheral Neuropathy and Systemic Diseases. Biomed Res Int. 2015;2015:951081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915-2921. [PubMed] |
| 15. | Papanas N, Ziegler D. Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Curr Diab Rep. 2013;13:488-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Mocan MC, Durukan I, Irkec M, Orhan M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006;25:769-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Asghar O, Petropoulos IN, Alam U, Jones W, Jeziorska M, Marshall A, Ponirakis G, Fadavi H, Boulton AJ, Tavakoli M. Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care. 2014;37:2643-2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, Boulton AJ. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 356] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Kallinikos P, Berhanu M, O’Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Dehghani C, Pritchard N, Edwards K, Vagenas D, Russell AW, Malik RA, Efron N. Natural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: development of a potential measure of diabetic peripheral neuropathy. Invest Ophthalmol Vis Sci. 2014;55:7982-7990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Sivaskandarajah GA, Halpern EM, Lovblom LE, Weisman A, Orlov S, Bril V, Perkins BA. Structure-function relationship between corneal nerves and conventional small-fiber tests in type 1 diabetes. Diabetes Care. 2013;36:2748-2755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Pritchard N, Edwards K, Dehghani C, Fadavi H, Jeziorska M, Marshall A, Petropoulos IN, Ponirakis G, Russell AW, Sampson GP. Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): study design and baseline characteristics. Diabetes Res Clin Pract. 2014;104:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Ishibashi F, Okino M, Ishibashi M, Kawasaki A, Endo N, Kosaka A, Uetake H. Corneal nerve fiber pathology in Japanese type 1 diabetic patients and its correlation with antecedent glycemic control and blood pressure. J Diabetes Investig. 2012;3:191-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, Brüggemann J, Strom A, Peschel S, Köhler B. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63:2454-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 25. | Petropoulos IN, Alam U, Fadavi H, Marshall A, Asghar O, Dabbah MA, Chen X, Graham J, Ponirakis G, Boulton AJ. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55:2071-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 26. | Petropoulos IN, Alam U, Fadavi H, Asghar O, Green P, Ponirakis G, Marshall A, Boulton AJ, Tavakoli M, Malik RA. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013;36:3646-3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Stem MS, Hussain M, Lentz SI, Raval N, Gardner TW, Pop-Busui R, Shtein RM. Differential reduction in corneal nerve fiber length in patients with type 1 or type 2 diabetes mellitus. J Diabetes Complications. 2014;28:658-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 379] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 29. | Chen X, Graham J, Dabbah MA, Petropoulos IN, Ponirakis G, Asghar O, Alam U, Marshall A, Fadavi H, Ferdousi M. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38:1138-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 30. | Ahmed A, Bril V, Orszag A, Paulson J, Yeung E, Ngo M, Orlov S, Perkins BA. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012;35:821-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Standardizing corneal nerve fibre length for nerve tortuosity increases its association with measures of diabetic neuropathy. Diabet Med. 2014;31:1205-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Maddaloni E, Sabatino F, Del Toro R, Crugliano S, Grande S, Lauria Pantano A, Maurizi AR, Palermo A, Bonini S, Pozzilli P. In vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetes. Diabet Med. 2015;32:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Tavakoli M, Begum P, McLaughlin J, Malik RA. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle Nerve. 2015;52:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Ishibashi F, Kojima R, Kawasaki A, Yamanaka E, Kosaka A, Uetake H. Correlation between sudomotor function, sweat gland duct size and corneal nerve fiber pathology in patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:588-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Misra SL, Craig JP, Patel DV, McGhee CN, Pradhan M, Ellyett K, Kilfoyle D, Braatvedt GD. In Vivo Confocal Microscopy of Corneal Nerves: An Ocular Biomarker for Peripheral and Cardiac Autonomic Neuropathy in Type 1 Diabetes Mellitus. Invest Ophthalmol Vis Sci. 2015;56:5060-5065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet. 2005;366:1340-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Lovblom LE, Halpern EM, Wu T, Kelly D, Ahmed A, Boulet G, Orszag A, Ng E, Ngo M, Bril V. In vivo corneal confocal microscopy and prediction of future-incident neuropathy in type 1 diabetes: a preliminary longitudinal analysis. Can J Diabetes. 2015;39:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. 2015;38:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Dehghani C, Pritchard N, Edwards K, Russell AW, Malik RA, Efron N. Risk Factors Associated With Corneal Nerve Alteration in Type 1 Diabetes in the Absence of Neuropathy: A Longitudinal In Vivo Corneal Confocal Microscopy Study. Cornea. 2016;35:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Petropoulos IN, Green P, Chan AW, Alam U, Fadavi H, Marshall A, Asghar O, Efron N, Tavakoli M, Malik RA. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PLoS One. 2015;10:e0123517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Azmi S, Ferdousi M, Petropoulos IN, Ponirakis G, Alam U, Fadavi H, Asghar O, Marshall A, Atkinson AJ, Jones W. Corneal Confocal Microscopy Identifies Small-Fiber Neuropathy in Subjects With Impaired Glucose Tolerance Who Develop Type 2 Diabetes. Diabetes Care. 2015;38:1502-1508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 42. | Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol. 2011;7:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 43. | Mehra S, Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30:2608-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 44. | Tavakoli M, Mitu-Pretorian M, Petropoulos IN, Fadavi H, Asghar O, Alam U, Ponirakis G, Jeziorska M, Marshall A, Efron N. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62:254-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 45. | Tavakoli M, Kallinikos P, Iqbal A, Herbert A, Fadavi H, Efron N, Boulton AJ, A Malik R. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med. 2011;28:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Azmi S, Ferdousi M, Petropoulos IN, Ponirakis G, Fadavi H, Tavakoli M, Alam U, Jones W, Marshall A, Jeziorska M. Corneal confocal microscopy shows an improvement in small-fiber neuropathy in subjects with type 1 diabetes on continuous subcutaneous insulin infusion compared with multiple daily injection. Diabetes Care. 2015;38:e3-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Brines M, Dunne AN, van Velzen M, Proto PL, Ostenson CG, Kirk RI, Petropoulos IN, Javed S, Malik RA, Cerami A. ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med. 2014;20:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 48. | Ostrovski I, Lovblom LE, Farooqi MA, Scarr D, Boulet G, Hertz P, Wu T, Halpern EM, Ngo M, Ng E. Reproducibility of In Vivo Corneal Confocal Microscopy Using an Automated Analysis Program for Detection of Diabetic Sensorimotor Polyneuropathy. PLoS One. 2015;10:e0142309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |