Published online Aug 25, 2016. doi: 10.4239/wjd.v7.i16.321
Peer-review started: February 20, 2016
First decision: March 25, 2016
Revised: April 8, 2016
Accepted: June 1, 2016
Article in press: June 3, 2016
Published online: August 25, 2016
Processing time: 187 Days and 10.6 Hours
The global prevalence of diabetes mellitus is increasing; arguably as a consequence of changes in diet, lifestyle and the trend towards urbanization. Unsurprisingly, the incidence of both micro and macrovascular complications of diabetes mirrors this increasing prevalence. Amongst the complications with the highest symptom burden, yet frequently under-diagnosed and sub-optimally treated, is diabetic autonomic neuropathy, itself potentially resulting in cardiovascular autonomic neuropathy and gastrointestinal (GI) tract dysmotility. The aims of this review are fourfold. Firstly to provide an overview of the pathophysiological processes that cause diabetic autonomic neuropathy. Secondly, to discuss both the established and emerging cardiometric methods for evaluating autonomic nervous system function in vivo. Thirdly, to examine the tools for assessing pan-GI and segmental motility and finally, we will provide the reader with a summary of putative non-invasive biomarkers that provide a pathophysiological link between low-grade neuro inflammation and diabetes, which may allow earlier diagnosis and intervention, which in future may improve patient outcomes.
Core tip: Autonomic complications are common and bothersome long-term sequelae of diabetes. However, they are frequently under-diagnosed and sub-optimally treated. Arguably this is as a consequence of a lack of appreciation of the various testing options that are available, particularly for end organ dysfunction such as within the cardiovascular and gastrointestinal systems. Our review aims to provide a succinct review of the current investigational armamentarium that are available and also provide the reader with a summary of the cutting edge techniques that have the potential to influence clinical practice in the future.
- Citation: Brock C, Brock B, Pedersen AG, Drewes AM, Jessen N, Farmer AD. Assessment of the cardiovascular and gastrointestinal autonomic complications of diabetes. World J Diabetes 2016; 7(16): 321-332
- URL: https://www.wjgnet.com/1948-9358/full/v7/i16/321.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i16.321
The world prevalence of diabetes among adults will be 7.7%, affecting 439 million adults by 2030. Between 2010 and 2030, there will be a 69% increase in numbers of adults with diabetes in developing countries and a 20% increase in developed countries[1]. This epidemic is potentially a consequence of changes in diet, lifestyle and the trend towards urbanization. Diabetes is associated with significant economic burden with healthcare costs estimated to be in the order of $132 billion annually in the United States and £10 billion in the United Kingdom[2,3]. Unsurprisingly, the prevalence of complications of diabetes reflects the increases in prevalence. Arguably amongst the most burdensome from a symptomatic point of view, yet frequently under-diagnosed, is the neuropathy that causes dysfunction of the autonomic nervous system (ANS), referred to as diabetic autonomic neuropathy (DAN), itself potentially leading to myriad of complications frequently manifest in the cardiovascular system and gastrointestinal (GI) tract. In addition to the bothersome nature of symptoms, Ewing et al[4] reported that in those with DAN, the survival rate at 5 years following diagnosis is as low as 47%.
The ANS is a bi-directional hierarchically controlled brain body interface that serves to integrate and modulate the internal milieu in response to the external environment thereby serving to maintain homeostasis. The ANS consists of the enteric nervous system and two broadly opposing branches referred to as the sympathetic (SNS) and parasympathetic nervous systems (PNS), having ubiquitous innervation throughout the body. The overall aim of this paper is to provide the reader with a contemporaneous and succinct review of the assessment of the autonomic complications of diabetes and discuss potential future biomarkers.
One of the major microvascular complications of diabetes is development of neuropathy, defined as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes”, which may deleteriously affect sensory, motor and autonomic nerve fibers[5].
Diabetes induced sensory and motor neuropathies affects C-fibers first and then progressively symmetrical affection of thick (Aβ) and thin (Aδ) - fiber neuropathy influencing axons of the distal lower extremities in a “glove and stocking” distribution. Interestingly, that despite comparable traditional risk factors, Asians with diabetes have substantially less large and small fiber neuropathy in comparison to matched Europeans[6]. Whilst clinicians would readily recognize that such symptoms represent a sensory neuropathy, symptoms related to DAN are often under appreciated, recognized and investigated. Diabetic sensorimotor polyneuropathies can be categorized according to the Toronto classification (Table 1)[5]. The traditional view of the pathogenesis od DAN is considered to be sequelae of vascular compromise, it has been more recently proposed that such complications represent the progression of systemic capillary dysfunction, which frequently are already present at diagnosis in those with type 2 diabetes[7]. Moreover, distinct differences in haemodynamic properties within the epineurium of e.g., the sural nerve, have also been proposed to play an important role in the pathogenesis of painful diabetic neuropathy[8]. However, the order of these factors in the aetiopathogenesis of diabetic neuropathy has been challenged by Danish researchers, as changes in endoneurial capillary morphology and vascular reactivity apparently may predate the development of diabetic neuropathy in humans[7].
| Definition of minimal criteria for diabetic sensorimotor polyneuropathy | Clinical features |
| Possible | Reduced sensation, positive neuropathic sensory symptoms (burning pain in the distal lower extremities), symmetrical reduction in distal sensation and/unequivocally decreased or absent ankle reflexes |
| Probable | A combination of two or more of the following: Neuropathic symptoms, decreased distal sensation, or unequivocally decreased or absent ankle reflexes |
| Confirmed | Decreased nerve conduction on objective testing with signs and symptoms as above |
| Subclinical | Decreased nerve conduction on objective testing in the absence of signs or symptoms |
Considering that the pathophysiology is largely similar, DAN can be regarded as an entity not dissimilar to the aforementioned peripheral neuropathy[5]. DAN can be usefully regarded as both a structural and/or a metabolic disorder, and the clinical manifestation of which can be present with or without the presence of large fiber neuropathy. DAN may affect cardiovascular, GI sensorimotor, urogenital systems, and sudomotor function. The presence of DAN confers a heightened risk of mortality in diabetes and frequently co-exists with other peripheral polyneuropathies[9]. Evidence suggests that subclinical DAN can occur within the first year of onset of type 2 diabetes (T2DM), and within two years in type 1 diabetes (T1DM), although often unrecognized for a number of years after their onset[10].
Nevertheless, the formal diagnosis of DAN is frequently delayed, the causes of which are most certainly multifactorial but arguably includes the non-specificity of presenting symptoms, the lack of clinician appreciation and the limited availability of specialized diagnostic services. Cardiovascular autonomic neuropathy is frequent, which can results in life threatening complications such as arrhythmias, silent myocardial ischemia and sudden death. However, DAN can potentially affect any portion of the ANS, and should therefore be considered a systemic disorder[11]. Evidence suggests that up to 10% of those with diabetes are at risk of developing DAN which may manifest as a variety of troublesome symptoms including orthostatic hypotension, aberrant GI motility and erectile dysfunction all of which can lead to a diminution in quality of life[5].
Hitherto, the focus of assessment for DAN has been derived from measures such as heart rate variability (HRV) and sudomotor function. However, over the recent past there have been considerable advances in measuring the “downstream” effects of DAN on both the cardiovascular system and the GI tract.
After 20 years of diabetes, neuropathy can be objectively demonstrated in up to 40%-50%[12]. The pathophysiology of neuropathy is multifactorial with structural and metabolic alterations having been described within axons, Schwann cells, and microvascular elements within the endoneurium and extracellular matrices[13]. Newer findings suggest that changes in the endoneurial capillary morphology and vascular reactivity are present before development of diabetic neuropathy in humans[7]. In addition, the authors found an association between the level of endoneurial hypoxia and reductions in nerve conduction velocity, in diabetes patients with manifest neuropathy.
Using experimental models of diabetes, reduced levels of neurotrophic support, including nerve growth factor and insulin like growth factor, have been implicated in reducing endoneurial blood flow thereby leading to neuronal damage[14]. In addition, such impairments in blood flow also result in alterations in Na+/K+ ATP-ase activity and nitric oxide metabolism. Animal studies suggest that altered Na+/K+ pump function may occur due to C-peptide deficiency, resulting in the shunting of glucose through the polyol pathway, thereby leading to increased levels of sorbitol and alterations of the nerve excitability recovery cycle which further contribute to neuronal damage[15,16].
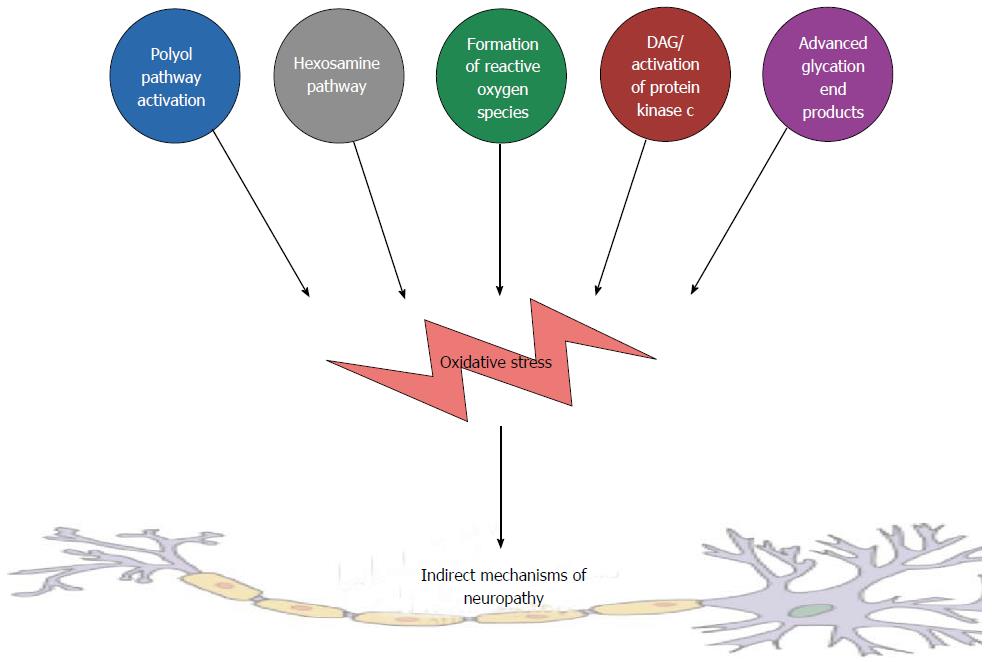
Peripheral and autonomic neurons, as well as their interconnections, are particularly vulnerable to hyperglycemia[17]. The mechanisms that underlie this vulnerability can be considered to both direct, as a consequence of heightened influx of extracellular glucose and indirectly through a plethora of other biochemical pathways. Examples of such indirect metabolic pathway are summarized in Figure 1 and include, but are not limited to, the following.
Polyol pathway: In the polyol pathway intracellular glucose is converted to sorbitol by the rate limiting enzyme aldose reductase, in an energy dependent manner via nicotinamide adenine dinucleotide phosphate[18]. The activation of this pathway may result in osmotic damage and diminution of Na+/K+ -ATPase activity[19]. These processes lead to increased intracellular oxidative stress[20].
Hexosamine pathway: The hexosamine biosynthesis pathway is a minor branch of glycolysis, where fructose-6-phosphate is converted to glucosamine-6-phosphate, catalyzed by the rate-limiting enzyme: Glutamine: Fructose-6-P-amidotransferase.
Formation of reactive oxygen species: In diabetes, reactive oxygen species (ROS) play an important role in the development of cardiovascular diseases, through excessive formation of oxidants, decreased bioavailability of nitric oxide, and decreased antioxidant capacity in the vasculature and kidneys[21]. These processes are initiated and amplified during chronic hyperglycemic conditions[22].
Increased diacylglycerol and protein kinase C pathways: Increased activation of the polyol pathway may cause a decrease in the activity of (Na+/K+) ATPase, and studies have suggested that this drop may activate diacylglycerol and protein kinase C (PKC) pathways[23]. Activation of PKC pathways increase cytosolic phospholipase A2 activity and produces a pro-inflammatory mediators such as prostaglandin E2, which inhibits cellular (Na+/K+) ATPase[24].
Formation of advanced glycation end products: Hyperglycaemia results in the formation of advanced glycation end products, comprising of proteins or lipids that become glycated after prolonged exposure to sugars[25]. This results in a diminished redox capacity of the neuron leading to enhanced vulnerability to ROS.
Cumulatively, these biochemical pathways, in conjunction with activation of the complement system[26], coalesce to form a cumulative indirect cascade that can initiate and summate neuro-inflammation, as is observed in DAN.
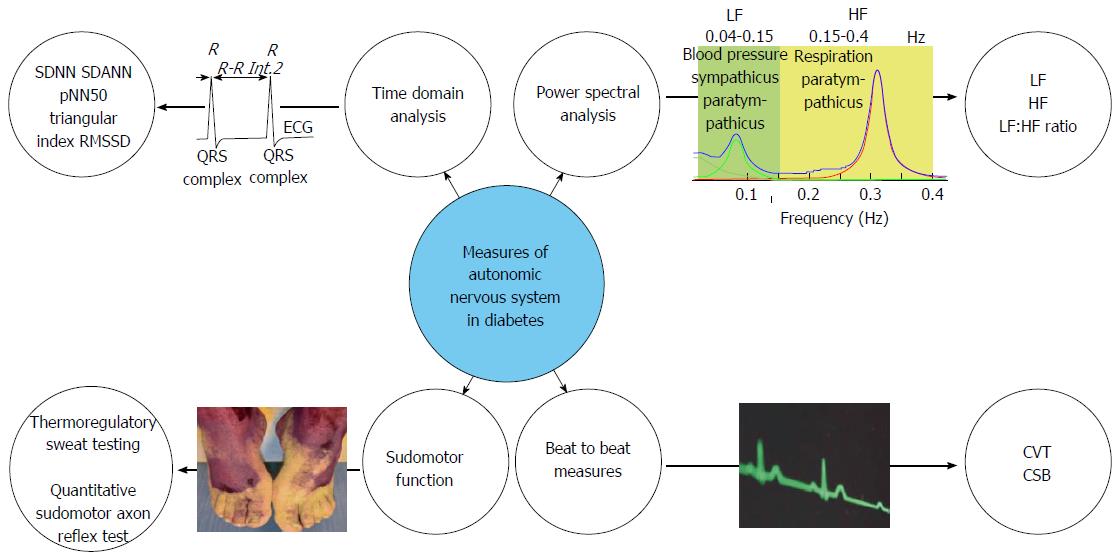
The last three decades have witnessed the increasing recognition of the pivotal role of the ANS in the pathophysiology of a number of disorders including diabetes. Although ANS function can be measured directly, using a needle recording of the peroneal nerve for instance, such methods are invasive and time consuming. Therefore, indirect, or proxy measures of ANS function have been developed, the most popular and widely utilised being HRV and are summarised in Figure 2.
The clinical relevance of HRV was first appreciated in 1965 when Lee et al[27] demonstrated that foetal distress was preceded by alterations in the inter-beat intervals between successive R waves in the electrocardiogram (ECG), before any appreciable changes occurred in heart rate (HR) per se. This epiphenomenon in the oscillations in the interval between successive heartbeats is known as “heart rate variability”. In deriving physiologically salient measures from HRV, there are three broad methods, time domain, power spectral analysis and beat-to-beat measures.
Since HR is controlled within a negative feedback loop influenced by both the SNS and PNS, the examination of beat-to-beat periodicities can provide an insight into their relative influences. Such variations in HR may be examined using time domain analysis. In a continuous ECG recording, the interval between consecutive normal QRS complexes on the ECG is known as the normal-to-normal (NN) interval. From the NN interval, statistical time domain measures can be derived and are divided into two classes, firstly those derived from the direct measurement of NN intervals and secondly those derived from the difference between NN intervals. The simplest variable is the standard deviation of normal-to-normal (SDNN RR intervals), which reflects the cyclic components of variability within the recording. Other commonly used measures are detailed in Table 2. The major disadvantage of these methods is the limited statistical power for the evaluation of short-term recordings of less than five minutes. Time domain analysis has been widely used to characterize autonomic neuropathy in diabetes, and has shown to be associated with the degree of sensorimotor neuropathy and also influences symptom generation peripherally within the GI tract[28]. Finally, reduced HRV also were associated with altered central processing within the operculum-insular network, underlining the systemic influence of diabetic neuropathy[29].
| Variable (units) | Description | Physiological relevance |
| SDNN (ms) | Standard deviation of the normal RR (NN) interval reflecting all of the cyclic components responsible for variability in the period of recording | An overall estimate of HRV, but does not indicate the contribution of any particular influence |
| SDANN (ms) | Standard deviation of the averages of NN intervals calculated over a short period of time, usually less than five minutes | Reflects the influence of circadian rhythms on autonomic function |
| pNN50 (%) | The proportion of NN intervals having a difference of > 50 ms | Reflects predominant vagal influence on variability |
| Triangular index (ms) | The integration of the density distribution of all the NN intervals as a function of the maximum density | Overall estimate of HRV similar to SDNN |
| RMSSD (m/s) | The square root of the means squared differences in successive NN intervals | Estimate of the short-term components of HRV |
The ANS activity that influences HRV is periodic in its nature, with sympathetic and parasympathetic components oscillating at distinct frequencies. The purpose of the frequency domain analysis of HRV (spectral analysis) is to dissect HRV into its specific frequency components, which defines the energy per unit time, which is often referred to as “power”, contained in each frequency component. Power spectral analysis has become the prevailing model for exploring HRV, and therefore autonomic function, within the literature. When considering short-term recordings obtained in resting conditions, the HRV spectrum is characterised by three major components at high (HF), low (LF) and very low frequency. The HF band represents respiratory sinus arrhythmia, as this is generally considered to represent vagal output to the heart, and this is termed cardiac vagal control, whereas the LF band is considered to represent sympathetic activity. Thus by examining the ratio between LF and HF power, sympathovagal balance can be derived. However, there are a number of methodological challenges of using HRV, notwithstanding assumptions concerning a relative constant respiratory rate and depth, referred to as respiratory stationarity, and limited temporal resolution such that these measures are not validated for time epochs of less than five minutes[30,31]. Such a shift in the sympathovagal balance has been proposed to be the underlying mechanism of symptom improvement in patients suffering from gastroparesis, who were treated with gastric electrical stimulation.
In attempting to overcome these methodological challenges, beat-to-beat measures of ANS “tone” have been recently developed and validated such as cardiac vagal tone and cardiac sensitivity to the baroreflex which measure efferent and afferent vagal tone respectively. In a preliminary study, we have demonstrated in 14 T1DM patients that lower cardiac vagal tone and cardiac sensitivity to the baroreflex were associated with disease duration, which was independent of glycaemic control and age[32]. Therefore, such novel autonomic indices may offer a longitudinal biomarker, which may aid in the prediction of autonomic neuropathy. Given their relative ease of use, and the lack of need for expert interpretation, these parameters could be useful as near patient screening tools in the future.
Patients with diabetic neuropathy typically have decreased sweating in the feet, which is associated with dry skin, itching and foot ulceration. Sweat glands are innervated by the sudomotor, postganglionic, unmyelinated cholinergic sympathetic C-fibers. Several methods have been developed to assess sudomotor function and contribute to the detection of autonomic dysfunction in diabetic peripheral neuropathy. The thermoregulatory Sweat testing (TST) evaluates the integrity of central and peripheral sympathetic sudomotor pathways[33]. The core body temperature is artificially raised to 38 °C, by increasing the ambient room temperature within a chamber, and a maximal sweat response is detected by a change in an indicator dye colour. Abnormal sweating patterns can therefore be recorded and provides a general index of severity of the autonomic failure. Nevertheless TST is limited by the fact that it cannot differentiate pre- from post-ganglionic lesions, is time consuming, requires special equipment, research facilities and patient preparation[33]. The quantitative sudomotor axon reflex test (QSART) evaluates postganglionic sympathetic cholinergic sudomotor function. Sweat glands are stimulated with a cholinergic agent and the sweat production is measured as an increase of humidity through a hygrometer. QSART is capable of detecting neuropathy with a sensitivity of > 75%[5]. However, QSART is unable to detect preganglionic lesions, requires special equipment and is not widely available[5]. By combining QSART with TST sensitivity is improved to 98% and it furthermore provides the clinician with the possibility to localize the lesion[34]. A relatively novel non-invasive rapid screening test, the Sudoscan, has been introduced, which provides sensitivity of 65% and specificity of 80% in correct classification of DAN. Furthermore, the test showed strong association between foot and hand electric skin conductance and nerve conduction tests[35]. Another user-friendly technique is the visual indicator test, referred to as the Neuropad. The Neuropad has high sensitivity but moderate specificity against large fibre neuropathy assessments. However the receiver operator characteristics of Neuropad is significantly improved, when used in combination with corneal nerve fibre length (< 14 mm/mm2) with a sensitivity and specificity of 83% and 80%, respectively[36].
GI symptoms, maybe divided into those arising in the foregut, including the oesophagus and stomach, and those limited to the mid and hindgut. Although intuitively, considering that diabetes is a systemic disorder, a considerable degree of overlap between these three distinct anatomical areas would be expected. Up to 50% of patients with diabetes have experienced disabling GI symptoms, including nausea, vomiting, bloating, early satiety, and abdominal pain and are thought to be sequelae of GI dysmotility[37]. GI dysmotility includes delayed gastric emptying, gastroparesis, rapid gastric emptying and other motor dysfunctions, such as impaired distention within the gastric fundus.
Gastroparesis, i.e., the pathological delay in the emptying of contents from the stomach into the small bowel, is one of the most frequently encountered GI complications. However, the degree of gastric emptying and symptom burden is often poorly correlated[38]. Up to 12% of patients with diabetes report symptoms consistent with GI dysmotility and such symptoms may result in nutritional compromise, diminished quality of life and poor glycaemic control as a result of impaired nutritional delivery into the small bowel[39].
GI motility is regulated and coordinated in a complex bidirectional interaction between the central nervous system, the ANS, the enteric nervous system and various endocrine and hormonal pathways. As its name suggests, the vagus nerve, which innervates the entire GI tract, apart from the distal third of the colon, has a stimulatory effect on the enteric nervous system and thus enhances GI motility, an effect that is broadly antagonized by sympathetic fibres. The interplay between these and changes in cellular level is largely unknown, but the pathophysiological mechanisms leading to gastroparesis are multifactorial in nature. However, the ANS is likely to be of critical importance. For instance, similar GI symptoms to those reported by patients with diabetes are seen in non-diabetics following truncal vagotomy, a previously frequently used surgical intervention for peptic ulcer disease in the pre-proton pump inhibitor era. These observations gave rise to the initial assumption that gastric dysmotility reflects irreversible damage to the vagal nerve. Currently, as there is a paucity of investigations to directly assess GI autonomic function directly, vide infra, the evaluation of cardiometrically derived autonomic function is often used as surrogate marker of the function of the abdominal vagus. However, the reported correlations to date have been relative weak, and in other studies, no relationship between gastric emptying and autonomic function has been shown[40]. The prevalence GI dysmotility is likely to be associated with the duration of diabetes, and thus attributable to an increased prevalence of autonomic neuropathy. The prevalence of GI dysmotility, and specifically gastroparesis, also appears to be higher in females than in males for uncertain reasons, but potentially suggesting a hormonal effect on the disease process.
As mentioned, GI symptoms per se whilst occurring frequently in diabetes are not strongly predictive of physiological abnormalities on objective testing. Therefore, the use of patient reported tools is insufficient to establish a formal diagnosis but are useful in establishing symptom severity. There are a number of methods for objectively evaluating GI motility and these are summarised in Table 3.
| Technique | Area of the GI tract evaluated | Length of stay required in clinic/office | Acceptability to the patient | Radiation exposure | Physiological conditions of measurement | Standardization of test | Measurement of propagating contractions | Availability/expense of test | Ease of interpretation of the result |
| Gastric emptying scintigraphy | Stomach | c.5 h | High | Yes | Yes | No | No | Widely/moderately expensive | Moderate |
| Whole gut scintigraphy | Pan-GI | c.8 h | High | Yes | Yes | Yes | No | Very limited/very expensive | Difficult |
| Radio - opaque marker study | Stomach colon | 30 min to | High | Yes | Yes | No | No | Widely/inexpensive | Easy |
| 13C octanoic acid breath test | Stomach | c.4 h | High | No | Yes | Yes | No | Very limited/inexpensive | Relatively easy |
| Wireless motility capsule | Pan-GI | c.30 min | High | None | Yes | Yes | No | Limited/currently moderately expensive | Relatively easy |
The current gold standard for the diagnosis of diabetic gastroparesis is the scintigraphic evaluation of gastric emptying[38]. It is generally recommended that prior to testing patient should (1) have serum glucose levels that are stable; (2) avoid medications that influences gastric emptying for 48-72 h prior to testing; and (3) avoid nicotine exposure during the test period as these factors can confound interpretation of the test results. Patients are fed a standardized meal of 99mTc-sulfur colloid-containing eggs, following which serial imaging over 4 h is undertaken using a gamma camera. Gastroparesis is considered to be present if > 60% of the isotope activity remains in the stomach 2 h after the test ingestion - or if at least 10% of the initial activity is still detected after 4 h[37].
Small bowel and colonic transit can be measured in a similar manner to gastric emptying, although serial imaging is prolonged. Delayed small bowel transit is diagnosed if < 40% of total small bowel of the isotope activity has accumulated in the terminal ileum-cecum at 6 h. To assess colonic transit, images of the colon are acquired at 24, 48, and 72 h after ingestion of the radiolabelled meal, with subsequent calculation of a metric referred to as the geometric centre. The geometric centre is an average of the intra colonic, weighted by segment colonic region, and intra-faecal distribution of the isotope.
However, scintigraphy is relatively expensive, associated with radiation exposure and still not standardized across centres. Moreover, due to is significant radiation burden, scintigraphy limits its application in children, women of child bearing potential, and subjects undergoing repetitive measurements of gastric emptying in a short period of time.
An alternative method for measuring GI motility is to use indigestible radio-opaque markers (ROMs) coupled with standard radiography/fluoroscopy.
ROM is given together with a standard meal. Emptying of ROM is followed with fluoroscopy every hour until all ROMs are emptied or for a maximum of 6 h. When compared to scintigraphic method, the ROM method in diabetic patients has comparable specificity albeit with less sensitivity[41]. In on other words, this means a normal ROM test does not exclude delayed gastric emptying, and if the clinical suspicion of gastroparesis remains, scintigraphy should be performed. However, the ROM method may represent a reasonable “screening test” for delayed gastric emptying as it is inexpensive and a widely available.
Colonic transit can be measured using a ROM technique, although there is a current lack of standardization, for instance more than 10 different testing protocols have been published. In broad terms, a patient’s ingests a known quantity of ROM and then subsequent has a plain abdominal radiograph undertaken at a defined time point, usually a number of days post ingestion, to define whether the transit is normal or delayed.
Gastric emptying tests uses non-radioactive forms of carbon incorporated in safely ingestible food or liquid products. The substrate is 13C-octanoic acid, which is labelled to a standardized meal, which is absorbed in the small intestine and metabolized to 13CO2, which is then expelled from the lungs during respiration. The rate-limiting step in this conversion is gastric emptying. The breath test correlates well with scintigraphic findings, it has been proposed as a non-invasive, reliable test for measuring gastric emptying, without recourse to the use of ionizing radiation[42]. Additionally, the breath test is less expensive and easier to perform than scintigraphy and offers the added advantage of being able to be undertaken in the office environment and shipped to a laboratory for analysis[42].
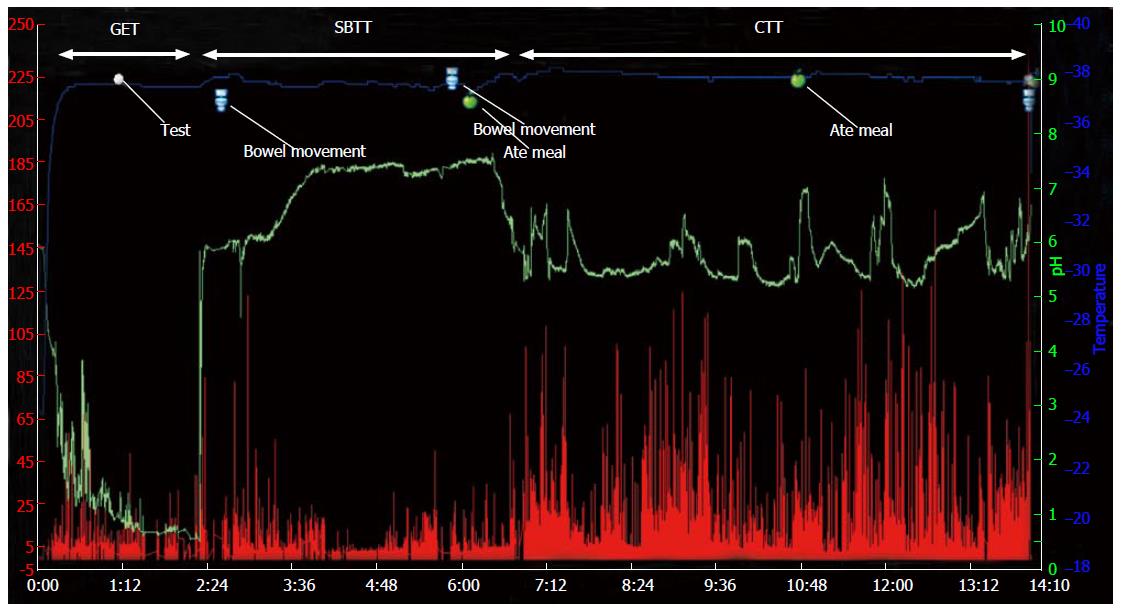
The wireless motility capsule (WMC) is an indigestible single-use capsule which provides a further option in which gastric emptying, small bowel transit and colonic transit times can be concurrently measured[43]. The WMC consists of a wireless transmitting capsule, a portable receiver worn by the patient for the duration of the test as well as analysis software. Following an overnight fast, the patient consumes a standardized meal of known fat and calorific content, which initiates postprandial motility patterns. Immediately after the meal, the patient ingests the WMC after which they are free to leave the clinical setting.
The WMC records pH, pressure and temperature as it transverses the GI tract. Gastric emptying time is reflected by an abrupt change in pH as the capsule moves from the acidic environment of the stomach to the alkaline environment of the duodenum. Small bowel transit time is the time from exit of the stomach to an abrupt pH drop of at least 1 pH unit around the ileocaecal junction. Colonic transit time is defined as time between caecal entry of capsule and its exit from the body. The whole gut transit time is the combined transit time of gastric emptying time, small bowel transit time and colonic transit time and is defined as delayed when greater than 73 h (Figure 3)[44]. Thus the WMC offers a minimally invasive alternative to the measurement of regional and whole gut transit. The capsule does not require any radiation, is standardized and can be carried out in most clinical settings. However, the WMC measures gastric emptying indirectly through the use of a physiologic meal. The pressure profiles are based on non-stationary, single point pressure measurements throughout the GI tract, which limits it utility in comparison to traditional manometric testing[45,46].
Ultrasonography represents a simple non-invasive technique to evaluate gastric function. Although operator dependent, ultrasonography provides information on gastric emptying, with a high correlation with scintigraphic techniques.
Magnetic resonance-techniques offer a potentially exciting non-invasive method for evaluating segmental and global motility within the GI tract, although protocols are currently limited to the research sphere. Nevertheless, given the widespread distribution of magnetic resonance imaging scanners across many clinical centres, it is likely that this method of imaging may become the method of choice in the future. However, further work is needed to standardize protocols and testing conditions.
Since the measurement of nerve conduction velocity per se is resource intensive, both in terms of equipment and specialist neurophysiological interpretation, the development and validation of non-invasive biomarkers remains an important priority. Considering the putative pathophysiological link between low-grade inflammation and diabetes[47], we shall highlight some novel biochemical biomarkers, which have the potential to complement detailed neurophysiological testing in the future.
There is increasing evidence that the immune system may play a role in the genesis and maintenance of diabetic neuropathy. Therefore, autoantibodies directed towards neuronal structures have received considerable attention, although reports are conflicting. For instance, Zanone et al[48] reported an association between autoantibodies directed against sympathetic ganglia, vagal afferents and the adrenal medulla in T1DM patients with symptomatic autonomic neuropathy.
In contrast however, Husebye et al[49] did not demonstrate any quantitative differences in autoantibodies binding to adrenal medulla in T1DM or T2DM in comparison to health controls. Nevertheless, the objective demonstration of a causal pathway where the identification of antigens directed towards specific end organ neuronal targets, which can be reversed with neutralising antibodies, remains a prerequisite step.
Oxidative stress is considered a central facet in the development of diabetes and associated micro- and macrovascular complications. DNA and RNA oxidation have been linked to several diseases including diabetes. Whilst tissue specific levels of oxidation represent a single time point within a certain organ or cell system, urine excretion of 8-Oxo-2’-deoxyguanosine and 8-hydroxyguanine gives a more global measure of oxidative stress. Not unsurprisingly therefore, it has been argued that in multi-system disorders, such as diabetes, such measures of global oxidative stress are of more pertinence. While an association between increased excretion of 8-Oxo-2’-deoxyguanosine in both diabetic retinopathy and nephropathy has been shown, there remains a paucity of data concerning in those patients with neuropathy. However, increased levels of 8-Oxo-2’-deoxyguanosine have been demonstrated in neurodegenerative disorders, such as Alzheimer’s disease, and therefore such markers warrant further objective evaluation in patients with diabetic neuropathy.
The influential cytokine theory of disease posits that a number of cytokines are involved in the maintenance of health and homeostasis within the peripheral, central and autonomic nervous systems. Cytokines are produced by cells from the immune system including mast cells, Schwann cells, fibroblasts and sensory neurons. Tumour necrosis factor-alpha (TNF-α) is a potent systemic pro-inflammatory cytokine and is a central component of the inflammatory response, in various immune mediated inflammatory diseases. It is a pathophysiological feature of such disorders, such as rheumatoid arthritis, which are characterised by chronic inflammation. TNF-α is produced in Schwann cells and has a role in peripheral nerve regeneration and regulation of apoptosis. Elevated concentrations of TNF-α and heightened disease activity in immune mediated inflammatory disorders is well described, however, more recently a similar association has been reported with neuropathy in diabetes T1DM and T2DM[49]. As such, TNF-α in diabetes may play a role in the pathogenesis and development of diabetic neuropathy and therefore could represent a candidate biomarker for the presence, severity and progression of diabetic neuropathy. Recent evidence provides further support for this proposition, as it has been showed that T2DM patients with neuropathy had higher levels of TNF-α in comparison to patients without neuropathy and healthy controls[50]. In addition, an animal model of painful diabetic neuropathy showed that treatment with anti-TNF-α monoclonal antibody exhibited a neuroprotective effect[51]. Finally, Yamakawa et al[52] demonstrated that a single dose of anti-TNF-α attenuated the electrophysiological and biochemical deficits associated with diabetic neuropathy for at least 1 mo. To the best of our knowledge there are no reports in the literature of anti-TNF therapy being utilised in patients with established diabetic neuropathy, although it is plausible that it would benefit clinical symptoms in selected patient groups, although single case reports exists, which describes mixed sensorimotor neuropathies as a consequence of anti-TNF therapy.
CD163 is an endocytotic receptor for haptoglobin-haemoglobin complexes, which is expressed exclusively in macrophages and monocytes. The extracellular portion of CD163 is soluble (sCD163) and circulates in the peripheral blood. Although the absolute function of sCD163 is incompletely understood, an association is observed between increased circulating levels of and chronic inflammatory states[53]. Interestingly, increased levels of sCD163 have been reproducibly demonstrated in diabetes[54]. Furthermore, sCD163 has been shown to be associated with insulin resistance in T2DM; an association that is independent of TNF-α[55]. In addition, a trend towards increased levels of sCD163 has very recently been demonstrated in cerebrospinal fluid in a preliminary study of patients with T2DM with established diabetic polyneuropathy as compared to matched controls without neuropathy[56]. Taken together, these data provide an interesting rationale for the further evaluation in larger prospective studies of sCD163 as a candidate biomarker, particularly as it links both inflammatory and neuropathic processes.
Although there is a current paucity of non-invasive diagnostic and prognostic biomarkers for diabetic neuropathy, there are a number of promising candidates. Whilst singularly each has their respective limitations, in combination a higher clinical utility may be derived in the future.
DAN remains an under-recognized complication, yet its symptomatic sequelae are troublesome and combine to reduce the quality of life and worsen prognosis in patients with diabetes. Although biomarkers for early identification of DAN and testing for ANS dysfunction and its specific end-organ complications, such as in the GI tract, remain in their infancy, further objective evaluation is warranted to improve detection rates and diagnostic accuracy, which may potentially lead to improved patient outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Charoenphandhu N, Georgescu A, Kusmic C, Traub M, Tziomalos K S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4375] [Article Influence: 291.7] [Reference Citation Analysis (4)] |
| 2. | Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care. 2006;29:2114-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 434] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | FACTS and STATS Diabetes UK, november 2015. Available from: https://www.diabetes.org.uk/Documents. |
| 4. | Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980;49:95-108. [PubMed] |
| 5. | Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1948] [Cited by in RCA: 1744] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 6. | Abbott CA, Chaturvedi N, Malik RA, Salgami E, Yates AP, Pemberton PW, Boulton AJ. Explanations for the lower rates of diabetic neuropathy in Indian Asians versus Europeans. Diabetes Care. 2010;33:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Østergaard L, Finnerup NB, Terkelsen AJ, Olesen RA, Drasbek KR, Knudsen L, Jespersen SN, Frystyk J, Charles M, Thomsen RW. The effects of capillary dysfunction on oxygen and glucose extraction in diabetic neuropathy. Diabetologia. 2015;58:666-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Eaton SE, Harris ND, Ibrahim S, Patel KA, Selmi F, Radatz M, Ward JD, Tesfaye S. Increased sural nerve epineurial blood flow in human subjects with painful diabetic neuropathy. Diabetologia. 2003;46:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Maser RE, Pfeifer MA, Dorman JS, Kuller LH, Becker DJ, Orchard TJ. Diabetic autonomic neuropathy and cardiovascular risk. Pittsburgh Epidemiology of Diabetes Complications Study III. Arch Intern Med. 1990;150:1218-1222. [PubMed] |
| 10. | Trotta D, Verrotti A, Salladini C, Chiarelli F. Diabetic neuropathy in children and adolescents. Pediatr Diabetes. 2004;5:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Verrotti A, Prezioso G, Scattoni R, Chiarelli F. Autonomic neuropathy in diabetes mellitus. Front Endocrinol (Lausanne). 2014;5:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol. 2014;2014:674987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 13. | Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43:957-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Ekberg K, Johansson BL. Effect of C-peptide on diabetic neuropathy in patients with type 1 diabetes. Exp Diabetes Res. 2008;2008:457912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wahren J, Ekberg K, Johansson J, Henriksson M, Pramanik A, Johansson BL, Rigler R, Jörnvall H. Role of C-peptide in human physiology. Am J Physiol Endocrinol Metab. 2000;278:E759-E768. [PubMed] |
| 16. | Krishnan AV, Kiernan MC. Altered nerve excitability properties in established diabetic neuropathy. Brain. 2005;128:1178-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Rudchenko A, Akude E, Cooper E. Synapses on sympathetic neurons and parasympathetic neurons differ in their vulnerability to diabetes. J Neurosci. 2014;34:8865-8874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kitada M, Zhang Z, Mima A, King GL. Molecular mechanisms of diabetic vascular complications. J Diabetes Investig. 2010;1:77-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, Nyengaard JR, van den Enden M, Kilo C, Tilton RG. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 573] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 20. | Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14:S233-S236. [PubMed] |
| 21. | Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31 Suppl 2:S170-S180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 501] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 22. | Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 228] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Cameron NE, Cotter MA, Jack AM, Basso MD, Hohman TC. Protein kinase C effects on nerve function, perfusion, Na(+), K(+)-ATPase activity and glutathione content in diabetic rats. Diabetologia. 1999;42:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 852] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 25. | Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1600] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 26. | Flyvbjerg A. Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily. Nat Rev Endocrinol. 2010;6:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Lee ST, Hon EH. The fetal electrocardiogram. iv. unusual variations in the QRS complex during labor. Am J Obstet Gynecol. 1965;92:1140-1148. [PubMed] |
| 28. | Brock C, Søfteland E, Gunterberg V, Frøkjær JB, Lelic D, Brock B, Dimcevski G, Gregersen H, Simrén M, Drewes AM. Diabetic autonomic neuropathy affects symptom generation and brain-gut axis. Diabetes Care. 2013;36:3698-3705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Lelic D, Brock C, Simrén M, Frøkjaer JB, Søfteland E, Dimcevski G, Gregersen H, Drewes AM. The brain networks encoding visceral sensation in patients with gastrointestinal symptoms due to diabetic neuropathy. Neurogastroenterol Motil. 2014;26:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Farmer AD, Coen SJ, Kano M, Weltens N, Ly HG, Botha C, Paine PA, Oudenhove LV, Aziz Q. Normal values and reproducibility of the real-time index of vagal tone in healthy humans: a multi-center study. Ann Gastroenterol. 2014;27:362-368. [PubMed] |
| 31. | Tak LM, Riese H, de Bock GH, Manoharan A, Kok IC, Rosmalen JG. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biol Psychol. 2009;82:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Brock C, Brock B, Pedersen AG. Novel Parameters of Parasympathetic Nervous System Tone-Noninvasive Biomarkers for Diabetic Autonomic Neuropathy? Diabetes. 2015;64:2441. |
| 33. | Illigens BM, Gibbons CH. Sweat testing to evaluate autonomic function. Clin Auton Res. 2009;19:79-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Selvarajah D, Cash T, Davies J, Sankar A, Rao G, Grieg M, Pallai S, Gandhi R, Wilkinson ID, Tesfaye S. SUDOSCAN: A Simple, Rapid, and Objective Method with Potential for Screening for Diabetic Peripheral Neuropathy. PLoS One. 2015;10:e0138224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Ponirakis G, Petropoulos IN, Fadavi H, Alam U, Asghar O, Marshall A, Tavakoli M, Malik RA. The diagnostic accuracy of Neuropad for assessing large and small fibre diabetic neuropathy. Diabet Med. 2014;31:1673-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Horváth VJ, Izbéki F, Lengyel C, Kempler P, Várkonyi T. Diabetic gastroparesis: functional/morphologic background, diagnosis, and treatment options. Curr Diab Rep. 2014;14:527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 460] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 39. | Talley SJ, Bytzer P, Hammer J, Young L, Jones M, Horowitz M. Psychological distress is linked to gastrointestinal symptoms in diabetes mellitus. Am J Gastroenterol. 2001;96:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Revicki DA, Rentz AM, Tack J, Stanghellini V, Talley NJ, Kahrilas P, De La Loge C, Trudeau E, Dubois D. Responsiveness and interpretation of a symptom severity index specific to upper gastrointestinal disorders. Clin Gastroenterol Hepatol. 2004;2:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Chang CS, Chen GH, Kao CH, Wang SJ, Peng SN, Poon SK, Huang CK. Gastric clearance of radiopaque markers in non-ulcer dyspepsia patients. Scand J Gastroenterol. 1996;31:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 508] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 43. | Farmer AD, Scott SM, Hobson AR. Gastrointestinal motility revisited: The wireless motility capsule. United European Gastroenterol J. 2013;1:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Lee YY, Erdogan A, Rao SS. How to assess regional and whole gut transit time with wireless motility capsule. J Neurogastroenterol Motil. 2014;20:265-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 45. | Tran K, Brun R, Kuo B. Evaluation of regional and whole gut motility using the wireless motility capsule: relevance in clinical practice. Therap Adv Gastroenterol. 2012;5:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Cassilly D, Kantor S, Knight LC, Maurer AH, Fisher RS, Semler J, Parkman HP. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 47. | Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799-1805. [PubMed] |
| 48. | Zanone MM, Peakman M, Purewal T, Watkins PJ, Vergani D. Autoantibodies to nervous tissue structures are associated with autonomic neuropathy in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Husebye ES, Winqvist O, Sundkvist G, Kämpe O, Karlsson FA. Autoantibodies against adrenal medulla in type 1 and type 2 diabetes mellitus: no evidence for an association with autonomic neuropathy. J Intern Med. 1996;239:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Zhu T, Meng Q, Ji J, Lou X, Zhang L. Toll-like receptor 4 and tumor necrosis factor-alpha as diagnostic biomarkers for diabetic peripheral neuropathy. Neurosci Lett. 2015;585:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Dogrul A, Gul H, Yesilyurt O, Ulas UH, Yildiz O. Systemic and spinal administration of etanercept, a tumor necrosis factor alpha inhibitor, blocks tactile allodynia in diabetic mice. Acta Diabetol. 2011;48:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Yamakawa I, Kojima H, Terashima T, Katagi M, Oi J, Urabe H, Sanada M, Kawai H, Chan L, Yasuda H. Inactivation of TNF-α ameliorates diabetic neuropathy in mice. Am J Physiol Endocrinol Metab. 2011;301:E844-E852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 53. | Møller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 54. | Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013;18:2352-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 431] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 55. | Llauradó G, González-Clemente JM, Maymó-Masip E, Subías D, Vendrell J, Chacón MR. Serum levels of TWEAK and scavenger receptor CD163 in type 1 diabetes mellitus: relationship with cardiovascular risk factors. a case-control study. PLoS One. 2012;7:e43919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Parkner T, Sørensen LP, Nielsen AR, Fischer CP, Bibby BM, Nielsen S, Pedersen BK, Møller HJ. Soluble CD163: a biomarker linking macrophages and insulin resistance. Diabetologia. 2012;55:1856-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |