Published online Jul 10, 2015. doi: 10.4239/wjd.v6.i7.936
Peer-review started: October 21, 2014
First decision: January 20, 2015
Revised: February 23, 2015
Accepted: March 30, 2015
Article in press: April 2, 2015
Published online: July 10, 2015
Processing time: 263 Days and 14.6 Hours
Polycystic ovary syndrome (PCOS) is a common endocrine disorder that affects up to 6.8% of reproductive age women. Experimental research and clinical observations suggest that PCOS may originate in the very early stages of development, possibly even during intrauterine life. This suggests that PCOS is either genetically-transmitted or is due to epigenetic alterations that develop in the intrauterine microenvironment. Although familial cases support the role of genetic factors, no specific genetic pattern has been defined in PCOS. Several candidate genes have been implicated in its pathogenesis, but none can specifically be implicated in PCOS development. Hypotheses based on the impact of the intrauterine environment on PCOS development can be grouped into two categories. The first is the “thrifty” phenotype hypothesis, which states that intrauterine nutritional restriction in fetuses causes decreased insulin secretion and, as a compensatory mechanism, insulin resistance. Additionally, an impaired nutritional environment can affect the methylation of some specific genes, which can also trigger PCOS. The second hypothesis postulates that fetal exposure to excess androgen can induce changes in differentiating tissues, causing the PCOS phenotype to develop in adult life. This review aimed to examine the role of fetal programming in development of PCOS.
Core tip: Polycystic ovary syndrome (PCOS) is a highly complex and heterogeneous disorder that is significantly influenced by genetic and environmental factors. There is some evidence that the development of PCOS may begin during the intrauterine period. Fetuses exposed to intrauterine nutritional restriction often have lowered insulin secretion and, as a compensatory mechanism, insulin resistance, which is known as the “thrifty” phenotype. Additionally, an impaired intrauterine nutritional environment can affect the methylation of some specific genes, which can trigger PCOS. The other hypothesis postulates that fetal exposure to excess androgen can induce changes in differentiating tissues, causing the PCOS phenotype and related disorders to develop in adult life.
- Citation: Gur EB, Karadeniz M, Turan GA. Fetal programming of polycystic ovary syndrome. World J Diabetes 2015; 6(7): 936-942
- URL: https://www.wjgnet.com/1948-9358/full/v6/i7/936.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i7.936
Polycystic ovary syndrome (PCOS) is a complex disorder characterized by defects in primary cellular control mechanisms that can result in hyperandrogenemia, hyperinsulinemia, insulin resistance, and chronic anovulation. PCOS is the most common endocrinologic disorder among women of reproductive age. Its prevalence typically ranges between 4% and 8% in diverse populations, but it has been reported to be as high as 25%[1]. The variations in the reported prevalence of PCOS have been attributed to the use of different diagnostic criteria. Three main diagnostic criteria systems that are currently accepted for PCOS are those from the National Institutes of Health (NIH, 1990), Rotterdam (ASRM/ESHRE, 2003), and Androgen Excess Society (AES, 2006). According to the Rotterdam criteria, the diagnosis of PCOS is based on the presence of at least two of the following three clinical features: polycystic ovarian morphology, oligo/amenorrhea and hyperandrogenism. However, the NIH criteria require only oligo/amenorrhea and hyperandrogenism for a diagnosis, while the AES criteria require a combination of biochemical or clinical hyperandrogenism along with chronic anovulation or polycystic ovarian morphology[1,2]. Although it is considered to be a disorder of reproductive age women (based on its classical symptoms of amenorrhea, hirsutism, and infertility), it can affect a woman any time during her life. Affected persons have a lifetime risk of disorders, including glucose metabolism, cardiovascular diseases, endometrial hyperplasia and/or cancer[2].
The underlying causes of PCOS are not known. However, its signs and symptoms typically appear during or close to the onset of puberty. Signs of precocious pubarche and adolescent hyperandrogenemia with or without insulin resistance may indicate the early stages of PCOS[3]. Further, epidemiologic studies have shown that adolescents with the aforementioned signs of PCOS had lower birth weights than those of controls[4]. These results suggest the hypothesis that PCOS is a continuum of a process that begins during intrauterine life.
PCOS is also believed to be caused by several genetic and environmental factors. The prevalence of PCOS has risen in populations where the gene pool has been relatively constant, which indicates that environmental factors may be playing a more important role in its development[5]. Further, obesity has been linked to the development of PCOS in susceptible individuals. A recent study revealed that, when compared with matched controls, non-obese women with PCOS had higher levels of glycotoxins, hyperandrogenemia, and advanced glycation end products, which were positively correlated with insulin resistance indices[6]. Some recent animal studies and observational human studies have suggested that impaired nutrition and steroidal environment during intrauterine life may play an important role in the development of PCOS[7-9].
Although case reports indicate that PCOS clusters within families, genetic studies have been inconclusive[10]. Twin studies have shown a heritability of 79% for PCOS with a correlation of 0.71 between monozygotic twins and 0.38 between dizygotic twins[11]. The clinical presentation of PCOS varies widely and there is currently no consensus on its diagnostic criteria[12,13]. Studies aimed at determining a genetic model of PCOS have produced different results when varying diagnostic criteria were used. For instance, some studies accepted hirsutism and ovaries with a polycystic appearance as diagnostic criteria (Rotterdam criteria) for the disease; these studies suggested that PCOS may have an autosomal dominant or X-linked simple Mendelian trait. Other studies using oligomenorrhea and hirsutism as the diagnostic criteria (NIH criteria) have reported lower genetic penetration rates[14-17]. On the other hand, some other recent studies have shown that the genetic aspect of insulin resistance is more prominent than that of hyperandrogenism in PCOS patients[18]. In conclusion, there is not yet a clearly established genetic model of PCOS. This is due to the diversity of both the diagnostic criteria and the clinical presentations of the disease, differences in its prevalence among various ethnic populations, and the limitations of some prior studies with respect to the number of subjects and statistical analyses used[10].
While the etiology of PCOS remains unclear, intrinsic abnormalities in the synthesis and secretion of androgens, insulin and gonadotropins provide a plausible basis for the syndrome. Therefore, it has been suggested that specific primary enzyme abnormalities in these steroidogenic pathways may be an important cause of PCOS. Many different genes encoding these enzymes have been studied to determine the etiology of PCOS; these genes have altered expression, suggesting that the genetic abnormalities in PCOS affects signal transduction pathways controlling steroidogenesis, steroid hormone action, gonadotropin action and regulation, insulin action and secretion, energy homeostasis, chronic inflammation, and more (Table 1)[19-27]. These genes may each contribute separately, or they might act collectively. Moreover, different variation in the same gene (allelic heterogeneity) and possible gene-environment interactions may have different effect on gene function. Data suggests that as of yet, there are no gene defects considered to be responsible for the etiology of PCOS; however, several studies have looked at many candidate genes and have suggested that alterations in these genes may contribute to the development of PCOS. Nevertheless, future studies are needed to determine which genes are the most appropriate PCOS biomarkers. In addition, more recent genetic approaches, namely genome-wide association studies, may begin a new era in PCOS research[28].
| The genes associated with PCOS | Genetic mutation (specific enzyme, protein or receptor) |
| Genes involved in ovarian and adrenal steroidogenesis | CYP11A (P450 cytochrome) |
| CYP21 (21-hydroxylase) | |
| CYP17 (17α-hydroxylase and 17,20-lyase) | |
| CYP19 (the enzyme complex aromatase: cytochrome P450 aromatase, the NADPH cytochrome P450 reductase 30, and P450 arom) | |
| Genes involved in steroid hormone actions | AR-VNTR polymorphism (the androgen receptors) |
| 4-kb gene - A pentanucleotide repeat polymorphism (SHBG) | |
| Genes involved in gonadotropin action and regulation | Trp8Arg and Ilg15Thr (the β-subunit of LH) |
| Genes involved in insulin action and secretion | INS -VNTR (insulin) |
| INSR-SNP (insulin receptor) | |
| Gly972Arg for IRS1, Gly1057Asp for IRS2 (insulin receptor substrates) | |
| 112/121 haplotype of CAPN10 (calpain-10) | |
| ApaI; rs680-SNP (IGF-1, IGF-2) | |
| Genes involved in energy homeostasis | T45G in exon 2 and G276T in intron 2 (adipocytokines) |
| Genes involved in chronic inflammation | Mutation 308 A alleles (TNF-α) |
| TNFR2, IL-6 signal transducer gp 130, IL-6 receptor genes (type-2 TNF receptor, IL-6) | |
| Polymorphism 4G/5G (PAI-1) |
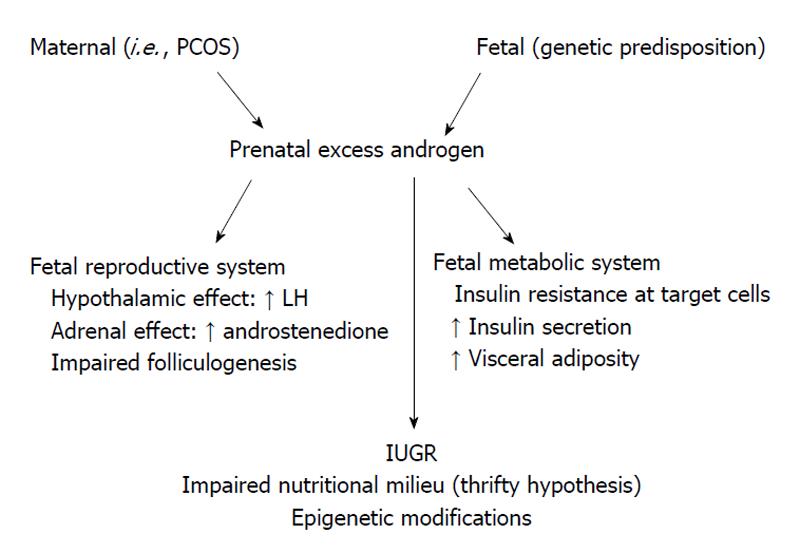
Experimental evidence supports the hypothesis that the phenotypic expression of PCOS is strongly influenced by the intrauterine environment. Initial studies on rats have shown that elevated testosterone (T) levels early in intrauterine life are related to anovulatory sterility and ovarian polycystic changes in offspring[29-31]. Prenatally T-treated monkeys and sheep serve as good models of PCOS because follicular differentiation in these species is similar to that in humans[32]. The results of studies on prenatally androgenized rhesus monkeys are summarized as follows: (1) Following cessation of maternal testosterone treatment in the early period (beginning on days 40-44 of gestation; term 165 ± 10 d), fetuses of these mothers had irregular ovulatory menstrual cycles, ovarian hyperandrogenism, enlarged polyfollicular ovaries and luteinizing hormone (LH) hypersecretion, insulin resistance, diminished insulin secretion, increased incidence of type 2 diabetes, visceral adiposity, and hyperlipidemia. These conditions may be related to an increase in Gonadotropin-releasing hormone (GnRH) secretion, a reduced negative feedback effect of steroids on LH release and/or increased gonadotropin response to GnRH[33,34]. Normally, healthy fetuses undergo a “critical hypothalamic hormonal period” during sexual differentiation. During this period, a sufficient androgenic stimulus in the brain allows for tonic gonadotropin release and contributes to male-type development; on the other hand, an insufficient androgenic stimulation level promotes the development of a female-type synaptology that is characterized by cyclic GnRH release[35]. An increased frequency and amplitude of GnRH release increases LH levels and impairs folliculogenesis, resulting in the anovulatory clinical picture that is characteristic of PCOS. Female offspring of monkeys that were androgenized during fetal life and have high LH levels have hormonal profiles similar to those of the normal male-type hormonal profile; (2) female monkeys similarly exposed to androgen excess during late gestation (100-110 gestation days) also exhibit an adult PCOS-like phenotype, but they do not have obvious abnormalities in LH and insulin secretion or in insulin action[33]; (3) prenatally androgenized monkeys have high blood levels of androstenedione at birth and their androgens of adrenal origin continue to increase for a period of 4-25 mo after birth, suggesting that prenatal androgen exposure may permanently alter adrenal androgen production[36]; (4) fetuses that are androgenized during the prenatal period have an increased number of primary, growing preantral, and small antral follicles and an accelerated proliferation of granulosa cells. In addition, an excess of prenatal androgen increases the mRNA expression of follicle-stimulating hormone receptor, insulin-like growth factor I (IGF-I) and the IGF-I receptor in granulosa cells. These morphological changes are similar to the increased follicular development from the primordial follicle pool that is seen in PCOS patients. Furthermore, prenatally androgenized fetuses have increased 5α-reductase and decreased aromatase activities, which are similar to mechanisms involved in the impaired follicular maturation of PCOS patients[37,38]; (5) prenatally androgenized female monkeys exhibit enhanced insulin secretion in both the fetal and infant zona reticularis. Therefore, an excess of fetal androgen may induce relative insulin hypersecretion in exposed female fetuses and infants, which in turn programs adrenal hyperandrogenism. In addition, the amelioration of impaired insulin action has beneficial glucoregulatory effects in both PCOS patients and in prenatally androgenized female monkeys. Treatment with Pioglitazone (a thiazolidinedione-based insulin sensitizer) in prenatally androgenized female monkeys diminishes the aspects of adrenal androgen excess and normalizes menstrual cyclicity[34]; (6) the hypothesis that metabolic disorders are programmed during the fetal stage is supported by the finding that, despite normal T levels after birth, prenatally androgenized male fetuses have insulin resistance and pancreatic beta-cell defects similar to those observed in females[39]; and (7) T excess, when introduced prenatally, decreases birth weight in rodent and sheep offspring. In addition, in humans, impaired placental aromatization is accompanied by diminished uteroplacental perfusion and low infant birth weight[40-44]. It has been suggested that maternal T excess may reduce fetal growth and birth weight via impaired placental function (Figure 1).
There is some evidence that female fetuses exposed to high androgen levels during the intrauterine period develop the clinical features of PCOS later in life. In humans, it is not possible to perform controlled studies to observe the fetal consequences of maternal androgens; this is left to animal research. However, some observations have been made in humans to support the validity of this hypothesis. Female fetuses having a congenital virilizing tumor or congenital adrenal hyperplasia due to 21-hydroxylase deficiency have been shown to display features of PCOS later in life, even after eliminating the hyperandrogenemia with postnatal therapies[45]. Similarly, it has been reported that female fetuses of women with defects in the p-450 aromatase gene and sex hormone-binding globulin gene, which are rare conditions that cause androgenization, also develop PCOS later in life[46]. Furthermore, it has been shown that exposure to androgen-like chemicals (e.g., Bisphenol A) can lead to PCOS[47,48].
Another study showed that the maternal androgen level is significantly higher in pregnant mothers with PCOS compared to that in healthy pregnant women[49].
While the reason for elevated androgen levels in pregnant women with PCOS has not yet been clarified, it is hypothesized that it may be due to hCG-stimulated androgen production in maternal theca cells or the placenta. Under normal conditions, maternal androgens or fetal adrenal androgens are rapidly converted to estrogens by the activity of the placental enzyme aromatase. However, when the activity of this enzyme is inhibited, the availability of androgens may increase. Insulin has been shown to inhibit aromatase activity in human cytotrophoblasts and can stimulate 3-hydroxysteroid dehydrogenase activity[50]. Therefore, hyperinsulinemia appears to coincide with elevated maternal androgen levels in the development of PCOS in offspring of pregnant women with the same disease. Furthermore, this hypothesis may be supported by the observation that the fetuses of diabetic mothers using insulin have increased levels of macrosomia and fetal pancreatic β-cell hyperplasia, as well as hirsutism, ovarian theca-lutein cysts, ovarian theca cell hyperplasia, and high T and hCG levels in the amniotic fluid[32].
In addition to having increased androgen levels during pregnancy, women with PCOS may also deliver small-for-gestational age newborns at a higher prevalence than do normal control mothers[51]. It is hypothesized that prenatal exposure to androgens in the offspring of women with PCOS may cause the development of the PCOS phenotype later in life, and it may also be the reason for low birth weight during the intrauterine period. With this in mind, recent studies in girls have shown that low birth weight is related to the development of premature pubarche followed by functional hyperandrogenism, insulin resistance with hyperinsulinism, and dyslipidemia during adolescence. It has been suggested that these manifestations may have a common early origin[3,52]. A study in which the authors followed pregnant women during their entire pregnancies reported that pregnant women with PCOS had a progressive increase in both maternal androgens (testosterone and androstenedione) and insulin resistance during their pregnancies, and that these women were exposed to adverse pregnancy-related events significantly more often than those in the control group with a similar body mass index[49]. Fetuses of mothers with PCOS can have developmental delay, which may be related to an elevated T level and insulin resistance. It has been shown that increased insulin resistance during pregnancy is related to adverse pregnancy outcomes including gestational diabetes, preeclampsia, preterm labor, and intrauterine growth restriction (IUGR)[53,54]. This hypothesis is also supported by the fact that male children of mothers with PCOS also have increased prevalence of impaired glucose tolerance, insulin resistance, type-2 diabetes, dyslipidemia and pancreatic beta-cell defects later in life[55].
Another possible mechanism related to the fetal programming of PCOS involves an impaired intrauterine environment. Independent of elevated androgen levels, intrauterine nutritional insufficiency for any reason may lower insulin secretion and insulin resistance in target tissues as an adaptive mechanism (the thrifty hypothesis). The development of insulin resistance is believed to be directly related to the body “predicting” a life of starvation for the developing fetus. This fetus or infant will have retarded growth and will likely develop PCOS when exposed to nutritional surplus later in life. Epidemiologic studies have demonstrated that babies born with IUGR have an increased prevalence of metabolic syndrome, type-2 diabetes, and hypertension later in life[48]. A recent study showed that urine from neonatal infants with IUGR contained significantly increased levels of metabolic syndrome-associated markers[56]. Although conclusive evidence is lacking, it has been suggested that an impaired intrauterine nutritional environment causes epigenetic changes that trigger metabolic disorders in adult life. The best evidence for this is that there is hypomethylation in the 11p15 imprinting center region that is responsible for the etiology of Silver-Russell syndrome, which is characterized by severe IUGR, lack of catch-up after birth, and specific dysmorphisms[57].
In conclusion, PCOS is a highly complex and heterogeneous disorder that is significantly influenced by both genetic and environmental factors. Environmental factors may play a role in the early stages of human development by helping to convert a predisposed genotype to the phenotypic expression of PCOS. In this review, the possible roles of intrauterine environmental factors in PCOS were summarized. Experimental animal studies suggest that maternal hyperandrogenism at a critical stage of fetal development may cause permanent changes in fetal physiology that can trigger PCOS development later in adult life. In humans, it is not possible to perform controlled studies to observe the fetal consequences of maternal androgens; however, some observations have been made in humans to support the validity of this hypothesis. In addition to having increased androgen levels during pregnancy may also deliver small-for-gestational age newborns at a higher prevalence that do normal control mothers. Furthermore, an insufficient intrauterine nutritional environment may also affect PCOS development by affecting cellular metabolism in target tissues or by causing epigenetic alterations to specific genes.
Mechanisms triggering PCOS may be eliminated by making improvements to the maternal hormonal environment and to the intrauterine nutritional environment. Future studies are necessary in order to determine whether insulin-sensitizing treatment of pregnant women with PCOS, or prenatally androgenized animals, will prevent postnatal PCOS in their daughters/female offspring. Results from such studies may help to identify a specific programming mechanism for PCOS.
P- Reviewer: Hutz RJ, Zhang QX, Zafrakas M S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 862] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 2. | Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1311] [Cited by in RCA: 1220] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 3. | Ibáñez L, Potau N, Francois I, de Zegher F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab. 1998;83:3558-3562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 238] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, Recabarren S, Cassorla F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Diamanti-Kandarakis E, Piperi C, Spina J, Argyrakopoulou G, Papanastasiou L, Bergiele A, Panidis D. Polycystic ovary syndrome: the influence of environmental and genetic factors. Hormones (Athens). 2006;5:17-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Diamanti-Kandarakis E, Christakou C, Marinakis E. Phenotypes and enviromental factors: their influence in PCOS. Curr Pharm Des. 2012;18:270-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2005;62:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab. 2006;91:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Dumesic DA, Schramm RD, Abbott DH. Early origins of polycystic ovary syndrome. Reprod Fertil Dev. 2005;17:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Menke MN, Strauss JF. Genetic approaches to polycystic ovarian syndrome. Curr Opin Obstet Gynecol. 2007;19:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91:2100-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 399] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 12. | Urbanek M, Wu X, Vickery KR, Kao LC, Christenson LK, Schneyer A, Legro RS, Driscoll DA, Strauss JF, Dunaif A. Allelic variants of the follistatin gene in polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:4455-4461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Urbanek M, Du Y, Silander K, Collins FS, Steppan CM, Strauss JF, Dunaif A, Spielman RS, Legro RS. Variation in resistin gene promoter not associated with polycystic ovary syndrome. Diabetes. 2003;52:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Hague WM, Adams J, Reeders ST, Peto TE, Jacobs HS. Familial polycystic ovaries: a genetic disease? Clin Endocrinol (Oxf). 1988;29:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 113] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Givens JR. Ovarian hyperthecosis. N Engl J Med. 1971;285:691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Wilroy RS, Givens JR, Wiser WL, Coleman SA, Andersen RN, Summitt RL. Hyperthecosis: an inheritable form of polycystic ovarian disease. Birth Defects Orig Artic Ser. 1975;11:81-85. [PubMed] |
| 17. | Lunde O, Magnus P, Sandvik L, Høglo S. Familial clustering in the polycystic ovarian syndrome. Gynecol Obstet Invest. 1989;28:23-30. [PubMed] |
| 18. | Diamanti-Kandarakis E, Alexandraki K, Bergiele A, Kandarakis H, Mastorakos G, Aessopos A. Presence of metabolic risk factors in non-obese PCOS sisters: evidence of heritability of insulin resistance. J Endocrinol Invest. 2004;27:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Franks S, McCarthy MI, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl. 2006;29:278-285; discussion 286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Shah NA, Antoine HJ, Pall M, Taylor KD, Azziz R, Goodarzi MO. Association of androgen receptor CAG repeat polymorphism and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:1939-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Guo R, Zheng Y, Yang J, Zheng N. Association of TNF-alpha, IL-6 and IL-1beta gene polymorphisms with polycystic ovary syndrome: a meta-analysis. BMC Genet. 2015;16:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Kommoju UJ, Maruda J, Kadarkarai S, Irgam K, Kotla JP, Velaga L, Mohan Reddy B. No detectable association of IGF2BP2 and SLC30A8 genes with type 2 diabetes in the population of Hyderabad, India. Meta Gene. 2013;1:15-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Zhang TT, Yuan L, Yang YM, Ren Y. The -675 4G/5G polymorphism in the PAI-1 gene may not contribute to the risk of PCOS. Eur Rev Med Pharmacol Sci. 2014;18:2326-2331. [PubMed] |
| 24. | Song do K, Lee H, Oh JY, Hong YS, Sung YA. FTO Gene Variants Are Associated with PCOS Susceptibility and Hyperandrogenemia in Young Korean Women. Diabetes Metab J. 2014;38:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | T Mutib M, B Hamdan F, R Al-Salihi A. INSR gene variation is associated with decreased insulin sensitivity in Iraqi women with PCOs. Iran J Reprod Med. 2014;12:499-506. [PubMed] |
| 26. | Ramezani Tehrani F, Daneshpour M, Hashemi S, Zarkesh M, Azizi F. Relationship between polymorphism of insulin receptor gene, and adiponectin gene with PCOS. Iran J Reprod Med. 2013;11:185-194. [PubMed] |
| 27. | Dowling AR, Nedorezov LB, Qiu X, Marino JS, Hill JW. Genetic factors modulate the impact of pubertal androgen excess on insulin sensitivity and fertility. PLoS One. 2013;8:e79849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Urbanek M. The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2211] [Cited by in RCA: 1997] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 30. | Franks S, Gharani N, McCarthy M. Candidate genes in polycystic ovary syndrome. Hum Reprod Update. 2001;7:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72:1475-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 364] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 34. | Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DH. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reprod Toxicol. 2007;23:438-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Matalliotakis I, Kourtis A, Koukoura O, Panidis D. Polycystic ovary syndrome: etiology and pathogenesis. Arch Gynecol Obstet. 2006;274:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Plant TM, Zorub DS. A study of the role of the adrenal glands in the initiation of the hiatus in gonadotropin secretion during prepubertal development in the male rhesus monkey (Macaca mulatta). Endocrinology. 1984;114:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83:2479-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 207] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Dumesic DA, Schramm RD, Bird IM, Peterson E, Paprocki AM, Zhou R, Abbott DH. Reduced intrafollicular androstenedione and estradiol levels in early-treated prenatally androgenized female rhesus monkeys receiving follicle-stimulating hormone therapy for in vitro fertilization. Biol Reprod. 2003;69:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, Abbott DH. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab. 2004;89:6218-6223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol. 2006;246:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | McGivern RF. Low birthweight in rats induced by prenatal exposure to testosterone combined with alcohol, pair-feeding, or stress. Teratology. 1989;40:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 43. | Tanguy G, Thoumsin HJ, Zorn JR, Cedard L. DHEA-S-loading test in cases of intrauterine growth retardation: relationship between the pattern of the maternal plasma metabolites and the fetoplacental dysfunction. Gynecol Obstet Invest. 1981;12:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Thoumsin HJ, Alsat E, Cedard L. In vitro aromatization of androgens into estrogens in placental insufficiency. Gynecol Obstet Invest. 1982;13:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Hague WM, Adams J, Rodda C, Brook CG, de Bruyn R, Grant DB, Jacobs HS. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf). 1990;33:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80:3689-3698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 213] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Fernández M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118:1217-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 48. | Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, Palimeri S, Panidis D, Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96:E480-E484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 49. | Mehrabian F, Kelishadi R. Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. J Res Med Sci. 2012;17:207-211. [PubMed] |
| 50. | Nestler JE. Modulation of aromatase and P450 cholesterol side-chain cleavage enzyme activities of human placental cytotrophoblasts by insulin and insulin-like growth factor I. Endocrinology. 1987;121:1845-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Melo AS, Vieira CS, Barbieri MA, Rosa-E-Silva AC, Silva AA, Cardoso VC, Reis RM, Ferriani RA, Silva-de-Sá MF, Bettiol H. High prevalence of polycystic ovary syndrome in women born small for gestational age. Hum Reprod. 2010;25:2124-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Ibañez L, Hall JE, Potau N, Carrascosa A, Prat N, Taylor AE. Ovarian 17-hydroxyprogesterone hyperresponsiveness to gonadotropin-releasing hormone (GnRH) agonist challenge in women with polycystic ovary syndrome is not mediated by luteinizing hormone hypersecretion: evidence from GnRH agonist and human chorionic gonadotropin stimulation testing. J Clin Endocrinol Metab. 1996;81:4103-4107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Hauth JC, Clifton RG, Roberts JM, Myatt L, Spong CY, Leveno KJ, Varner MW, Wapner RJ, Thorp JM Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Tolosa JE, Saade G, Sorokin Y, Anderson GD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol. 2011;204:327.e1-327.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Mastrogiannis DS, Spiliopoulos M, Mulla W, Homko CJ. Insulin resistance: the possible link between gestational diabetes mellitus and hypertensive disorders of pregnancy. Curr Diab Rep. 2009;9:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Hunter A, Vimplis S, Sharma A, Eid N, Atiomo W. To determine whether first-degree male relatives of women with polycystic ovary syndrome are at higher risk of developing cardiovascular disease and type II diabetes mellitus. J Obstet Gynaecol. 2007;27:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Dessì A, Atzori L, Noto A, Visser GH, Gazzolo D, Zanardo V, Barberini L, Puddu M, Ottonello G, Atzei A. Metabolomics in newborns with intrauterine growth retardation (IUGR): urine reveals markers of metabolic syndrome. J Matern Fetal Neonatal Med. 2011;24 Suppl 2:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Eggermann T, Gonzalez D, Spengler S, Arslan-Kirchner M, Binder G, Schönherr N. Broad clinical spectrum in Silver-Russell syndrome and consequences for genetic testing in growth retardation. Pediatrics. 2009;123:e929-e931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |