Published online Apr 15, 2015. doi: 10.4239/wjd.v6.i3.380
Peer-review started: September 11, 2014
First decision: November 14, 2014
Revised: November 26, 2014
Accepted: January 9, 2015
Article in press: January 12, 2015
Published online: April 15, 2015
Processing time: 221 Days and 2.4 Hours
Type 1 diabetes (T1D) is an autoimmune disease characterized by loss of insulin producing beta cells and reliance on exogenous insulin for survival. T1D is one of the most common chronic diseases in childhood and the incidence is increasing, especially in children less than 5 years of age. In individuals with a genetic predisposition, an unidentified trigger initiates an abnormal immune response and the development of islet autoantibodies directed against proteins in insulin producing beta cells. There are currently four biochemical islet autoantibodies measured in the serum directed against insulin, glutamic decarboxylase, islet antigen 2, and zinc transporter 8. Development of islet autoantibodies occurs before clinical diagnosis of T1D, making T1D a predictable disease in an individual with 2 or more autoantibodies. Screening for islet autoantibodies is still predominantly done through research studies, but efforts are underway to screen the general population. The benefits of screening for islet autoantibodies include decreasing the incidence of diabetic ketoacidosis that can be life threatening, initiating insulin therapy sooner in the disease process, and evaluating safe and specific therapies in large randomized clinical intervention trials to delay or prevent progression to diabetes onset.
Core tip: Type 1 diabetes (T1D), the immune mediated form of diabetes, is now a predictable disease with the measurement of islet autoantibodies. The presence of two or more antibodies defines preclinical disease as nearly everyone with multiple antibodies progresses to clinical diabetes. With improved platforms to measure islet autoantibodies, screening the general population is now a goal. Early identification of preclinical diabetes allows for less diabetic ketoacidosis, early initiation of insulin therapy, and the potential to delay or prevent diabetes onset. Clinical trials using safe and specific therapies to block disease specific immune cells are underway in T1D.
- Citation: Simmons KM, Michels AW. Type 1 diabetes: A predictable disease. World J Diabetes 2015; 6(3): 380-390
- URL: https://www.wjgnet.com/1948-9358/full/v6/i3/380.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i3.380
Type 1 diabetes (T1D) is a chronic disease caused by immune-mediated destruction of insulin producing beta cells in the pancreas[1]. The destruction of beta cells results in insulin insufficiency, and patients develop life-threatening hyperglycemia that clinically manifests with weight loss, polyuria, and polydipsia. The majority of patients who develop T1D have high-risk human leukocyte antigen (HLA) genes. Islet autoantibodies can be measured in the serum of these high-risk individuals years before the onset of any clinical symptoms, making T1D a predictable disease. Multiple prevention trials in patients with high-risk HLA genes or in patients who have measureable autoantibodies have been completed. To date, no trial has prevented the onset of T1D, but data indicates that the disease process may be delayed by administering oral insulin to induce insulin specific regulatory T-cells in the gut, resulting in decreased inflammation in the pancreas. This review summarizes the epidemiology, risk factors and pathogenesis of T1D. The review also examines the goal of screening the general population for T1D risk and preventing disease onset in individuals with preclinical disease.
T1D is one of the most common chronic diseases in childhood and is diagnosed at an increasing rate in adults. The incidence rate varies significantly by geographical region. Sweden, Finland, Norway, United Kingdom, and Sardinia have the highest incidence of T1D at an age-adjusted rate of > 20/100000 patient years. For comparison, the United States has an incidence rate of 17.8/100000 patient years in a predominantly Caucasian population. China and South America have the lowest incidence of T1D, reported as < 1/100000 patient years[2-5]. The rate of T1D diagnosis is increasing in most countries, with rates dramatically increasing in children less than 5 years of age[6]. The annual incidence of T1D is increasing globally by 2.3% per year and is estimated to be increasing by 2.7%-2.8% in non-Hispanic white youth in the United States[7]. Large registries in both Europe and the United States show that the incidence of T1D peaks between 5 to 7 years of age and again when children enter puberty[8]. Unlike most autoimmune diseases, T1D is more common in males than females. The risk of T1D development in the general population is 1:300[9]. In children who have a genetically related sibling, the risk is increased to 1:7 and is greatest in children under 5 years of age[10,11]. Offspring of mothers with T1D carry approximately 3% risk and offspring of fathers with T1D carry approximately 5% risk[12]. Genetics confer risk for development of T1D, as does seasonal variation and birth month suggesting an environmental influence on disease pathogenesis. Children born in the spring tend to be at a greater risk for developing T1D, while diagnosis is increased during climatically cold seasons[13-16]. This is an epidemiological association that requires further investigation.
T1D is a polygenic disorder with many genes contributing varying amounts of genetic risk for disease development. The genes conferring risk for diabetes are generally classified as HLA and non-HLA genes. Large genome wide association studies show that over 40 genes increase susceptibility to T1D[17,18]. The major determinant of genetic susceptibility to T1D, contributing greater than 50% of the genetic risk, is conferred by genes in the HLA complex located on chromosome 6[9]. The HLA complex is divided into 3 regions: classes I, II, and III. Alleles of the class II genes, DQ and DR (and to a lesser extent DP), are the most important determinants of T1D. These class II molecules are expressed on antigen-presenting cells (macrophages, dendritic cells, and B cells) and present antigens to CD4 T lymphocytes. DQ and DR genes are in close linkage disequilibrium on chromosome 6 with specific DQ and DR genes inherited together. The presence of the DR4/DQ8 haplotype increases the odds ratio for T1D development to approximately 11, indicating an individual with this haplotype is 11 times more likely to develop T1D than those without. Approximately 90% of all individuals with T1D have either or both the DR4/DQ8 or DR3/DQ2 haplotypes. Interestingly, HLA genes also confer protection from T1D development. Individuals who have the specific DQ6 allele (DQB1*06:02) are dominantly protected from T1D, with an odds ratio of 0.03 for disease development[19].
Of the non-HLA genes, insulin and protein tyrosine phosphatase non-receptor type 22 (PTPN22) confer risk for T1D development but to lesser degrees than HLA genes[20]. Similar to HLA class II genes, insulin gene polymorphisms can confer both susceptibility to and protection from T1D development. At the 5’ end of the insulin gene, there are variable numbers of tandem repeats. Having more repeats correlates to more insulin message being expressed in the thymus. The thymus responds by developing central tolerance to insulin. In individuals with fewer repeats, autoreactive T-cells can persist, and the risk for T1D development is increased[21]. PTPN22 helps regulate antigen receptor signaling and T cell activation, and a single nucleotide polymorphism (arginine to tryptophan at position 620) has been associated with a number of autoimmune disorders including T1D. A gain of function polymorphism decreases T cell receptor signaling which confers diabetes risk. It is unknown why decreased T cell activation leads to T1D risk, but it can be hypothesized that deficient negative selection of thymic cells may be involved[22,23].
Genetics alone does not lead to T1D; the environment also plays a pivotal role. This is evidenced by the fact that not all individuals with high-risk genes develop T1D. In fact, the majority of individuals with high-risk HLA class II genes (DR4/DQ8 and DR3/DQ2) do not develop T1D. There are likely one or more environmental factors that trigger and perpetuate the autoimmune disease process prior to hyperglycemia and a clinical diagnosis of hyperglycemia and T1D. Large natural history studies indicate that the development of islet autoantibodies (the first laboratory evidence of beta cell autoimmunity) in high-risk individuals often occurs between 9 mo and 2 years of age[24]. This suggests that an environmental trigger is present early in life, possibly in utero.
One of the most extensively evaluated environmental triggers is viral infection. Many viruses are implicated in the development of T1D including enteroviruses such as coxsackie B virus, cytomegalovirus, congenital rubella syndrome, and rotavirus[25-32]. Enterovirus is the leading candidate for contributing to T1D development. Epidemiologic studies in Finland show that the development of beta cell autoimmunity parallels the seasonal pattern of enterovirus infection and clinical symptoms of enteroviral infection[33,34]. Enterovirus infection was strongly associated with the development of autoantibodies in the Diabetes Autoimmunity Study in the Young (DAISY) cohort[35]. Laboratory evidence of enterovirus infection is reproducibly present in individuals with new onset T1D, pregnant women whose children develop T1D, and donor pancreata of individuals with T1D[36,37]. The exact mechanism of how viruses induce autoimmunity is not clear. The molecular mimicry hypothesis proposes that because the P2-C protein sequence of enterovirus is similar to glutamic decarboxylase (GAD), which is expressed in islet cells, the immune system erroneously targets destruction of beta cells[38]. The other leading hypothesis is that viral infection activates autoreactive T cells. As evidence, Cytomegalovirus B4 has tropism for pancreatic tissue and infection results in release of beta cell antigens that are phagocytized by macrophages and presented to autoreactive T cells[39].
Another potential environmental influence relates to the north-south division of diabetes development in the world, with a higher incidence of T1D in northern climates compared to southern. The north-south hypothesis implicates that a lack of vitamin A and/or D exposure early in life predisposes individuals to the development of autoimmune diseases including T1D. Offspring of mothers supplemented with vitamin D during pregnancy and young children supplemented with vitamin D have shown a reduced risk of T1D development that may be dose responsive[40,41]. However, an analysis from the DAISY Study found that vitamin D intake and 25(OH) vitamin D levels throughout childhood were not associated with the development of islet autoantibodies or T1D development[42].
Early introduction of cow’s milk and gluten have also been extensively studied. The introduction of gluten into an infant’s diet prior to three months and after 7 mo has been associated with increased autoantibody development[24,43]. Some studies also indicate that breastfeeding or using elemental formula may be protective against T1D. Other environmental factors that continue to be explored include nitrosamine compounds, maternal age, pre-eclampsia, and childhood obesity. There is no evidence to suggest that vaccines increase the risk of T1D development[44]. To date, there are no causal environmental factors that trigger the development of islet autoantibodies or increase the risk of progression to clinical T1D development. However, there is a large international prospective longitudinal study, The Environmental Determinants of Diabetes in the Young, currently underway to evaluate potential environmental factors in T1D[45].
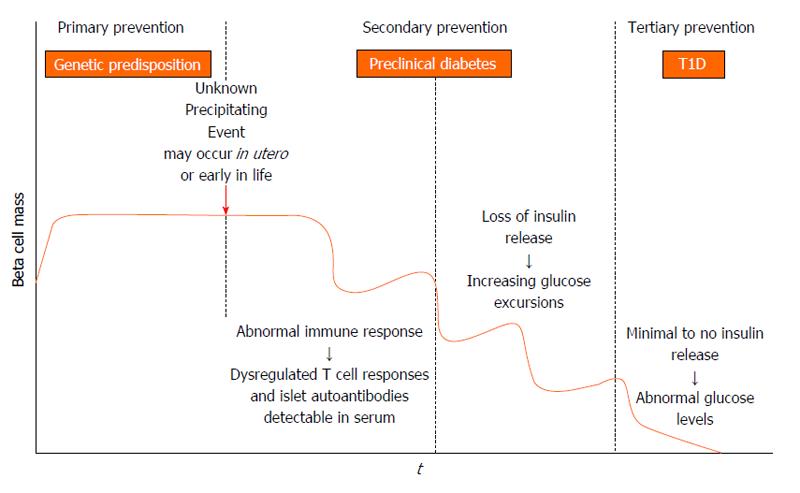
Three decades ago, it was hypothesized that T1D is a chronic autoimmune disorder that develops in stages, and the model remains valid today (Figure 1). In genetically predisposed individuals (those with DR4/DQ8 and/or DR3/DQ2 haplotypes) there is an environmental trigger that leads to a break in immunologic tolerance and loss of beta cell mass. Over a period of time, usually years, there is autoimmune destruction of insulin producing beta cells that is marked by the presence of serum islet autoantibodies (Figure 2). As the process continues, very likely in a relapsing and remitting manner, there is a loss of glucose stimulated insulin release, and eventually insulin deficiency such that overt hyperglycemia results and clinical T1D is diagnosed[4].
How an inciting event leads to an aberrant immune response is not completely understood. Most hypotheses focus on immunologic abnormalities in antigen presentation by HLA molecules to T cells in the thymus and peripheral lymph organs. T cells are educated in the thymus to self-antigens, such as insulin, and if there are dysregulated immune processes, self-reactive T cells can escape central tolerance and exist in the periphery[46,47]. Once these cells encounter their target antigen or peptide in peripheral lymph organs, they become activated to target beta cells. Other hypotheses focus on environmental triggers leading to immune activation and targeting of beta cells. The molecular mimicry theory proposes that a viral or bacterial protein shares amino acid sequence homology with beta cells and induces immune system activation through targeting beta cell antigen that is molecularly similar to a foreign antigen[38]. Finally an infectious triggering event may allow beta cells to become more sensitive to cytokine and free radical induced inflammation[48].
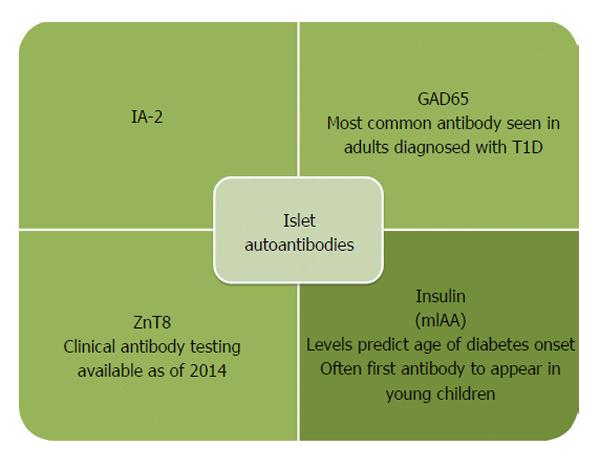
Recently the network for pancreatic organ donors has been established to study the pancreata of deceased donors with islet autoantibodies (preclinical disease) or established T1D[49]. The goal is to understand mechanisms of disease pathogenesis and interactions between beta cells and the immune system[50]. What we have gleaned from the initial efforts is that islet infiltrates (insulitis) are present in a lobular pattern in the pancreas, and there is a predominance of CD8 and CD4 T cells, B-lymphocytes, and macrophages[51,52]. Pancreata from established T1D patients also show an overall decrease in weight compared to age matched controls, potentially related to atrophy of the exocrine pancreas with the loss of beta cells[52]. The first serological evidence of an autoimmune response to beta cells is the appearance of autoantibodies to insulin (IAA), GAD, islet antigen 2, and zinc transporter 8[53]. Placental antibodies are no longer present after approximately 6 mo, so any antibodies in serum after that time reflect endogenous antibodies. If an individual develops two or more of these antibodies, they will eventually progress to clinical onset of T1D[54]. Approximately 90% of individuals have two or more islet cell autoantibodies at diagnosis, and it is likely that the remaining 10% of individuals (islet autoantibody negative) have autoantibodies against antigens that have yet to be discovered. In children, IAA is usually the first antibody to develop, and the progression to T1D is 100% in children with a persistently high level of IAA[55,56]. This is in contrast to adults who tend to have higher levels of GAD at diagnosis. Islet autoantibodies can be easily measured in the serum, with the gold standard method for detecting antibodies being fluid phase radioimmunoassays (RIA)[57]. More recently, islet autoantibodies are now able to be measured from smaller volumes of serum and without the use of radioactivity using electrochemiluminescense as a detection method while maintaining similar sensitivity and specificity to RIA[58,59]. The rate at which individuals with positive islet autoantibodies progress to clinical T1D is dependent upon the age of appearance, insulin autoantibody level, and the number of autoantibodies present[55]. Hemoglobin A1c rises 1 to 1.5 years prior to diagnosis. Therefore, reduced insulin secretion and resultant hyperglycemia occur before T1D is clinically diagnosed[60,61]. Once T1D is clinically diagnosed, individuals must commit to lifelong blood glucose monitoring and intensive insulin administration via multiple daily injections or an insulin pump to achieve good glycemic control. With improved diabetes management, the risk for long-term complications such as renal failure, myocardial infarctions, stroke, and lower extremity amputations has decreased over the last two decades[62]. However despite the decreasing prevalence of complications in diabetes, the need still exists to understand the underlying pathogenesis of complications such as diabetic cardiomyopathy and novel approaches for treating complications such as neovascularization in diabetic foot disease[63,64].
The American Diabetes Association recently adapted their guidelines to recommend screening for islet autoantibodies in high-risk individuals[65]. Highly sensitive serological assays are not widely available, and all screening is recommended to be done in the setting of a clinical research study. To date, general population screening has been done through large clinical trial networks such as the National Institutes of Health sponsored TrialNet, which enroll and screen first or second degree family members of individuals with T1D. By identifying individuals with positive islet autoantibodies, the rate of diabetic ketoacidosis (DKA) at diagnosis is reduced[66]. Preventing DKA is important as altered mental status, coma, and even death can occur[67]. In fact, DKA is the most common cause of death in children with T1D[68]. Without screening, DKA at diagnosis is relatively common[69]. In the EURODIAB study, 42% of children presented in DKA (pH < 7.3) at the time of diagnosis with T1D[3]. By identifying individuals with positive autoantibodies, insulin therapy can be initiated early, and these children can enroll in studies aimed at preserving beta cell mass. In adults, maintaining endogenous insulin secretion reduces hemoglobin A1C, reduces the risk of severe hypoglycemia, decreases reliance on exogenous insulin, and decreases the rate of long-term complications[70-77]. In children, there has been very little data collected regarding residual beta cell mass beyond the first year after diagnosis[78-82]. A case-control study did show that children without severe hypoglycemia had increased residual beta cell mass compared to those children with severe hypoglycemia[83]. An effective method of preserving beta cell mass is not yet available, and the benefit of increased residual beta cell mass in children remains to be confirmed.
According to the World Health Organization’s principles of early disease detection, T1D is a condition that meets criteria for the establishment of a screening program. These principles include the condition is an important health problem, there is a recognizable latent stage of the disease, the natural history of the disease is understood, there is an adequate and accepted laboratory screening test, providers agree on who should receive treatment and there is a treatment available, there are adequate resources for diagnosis and treatment, and the cost of overall medical care would not increase[84]. Islet autoantibodies can be reliably measured in serum, with each antibody assay having a specificity of 99% when measured by radioimmunoassay in tertiary referral centers such as the Barbara Davis Center for Diabetes. The sensitivity for each autoantibody assay ranges from 70%-80%. We view these radioimmunoassays as a confirmatory test for T1D. A desired screening test needs to be reliable with high sensitivity, cost effective, and technically feasible, likely as a multiplex assay in which all four autoantibodies are measured in a single well of an assay plate. Currently, to measure islet autoantibodies a blood draw is required with subsequent shipping of venous or capillary blood samples to a reference laboratory. This is not feasible for population wide screening due to technical requirements of sample collection and high cost. Screening large populations of infants for metabolic diseases and other congenital disorders has been successfully done using dried blood spots[85]. To establish an accepted screening program for T1D, the sensitivity and specificity of islet autoantibodies, specifically insulin autoantibody, needs to be established using a feasible collection method such as dried blood spots on filter paper, which would be a simplified collection method and more cost effective. Overall, T1D would not be over diagnosed with general population screening as diagnosis of the disorder requires both the presence of islet autoantibodies and metabolic abnormalities.
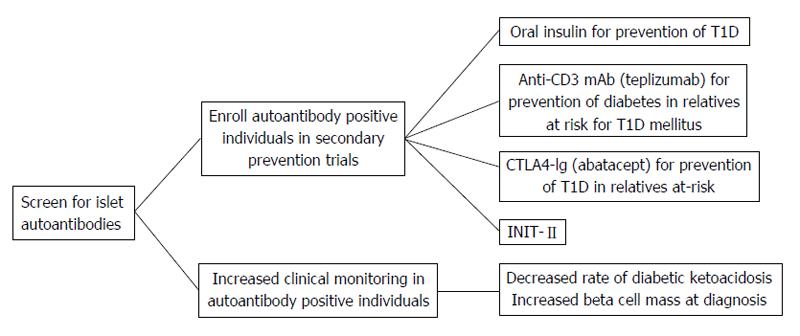
Ideally, individuals who screen positive for islet autoantibodies can be offered a treatment to prevent or delay the progression to T1D. Many secondary prevention trials have been completed with more currently underway[86]. As population based screening may be feasible in the near future, it is important to continue secondary prevention trials with the goal of delaying or preventing progression to T1D in islet autoantibody positive individuals. Patients enrolled in clinical intervention trials benefit from close follow up by medical professionals, early diagnosis of T1D, decreased incidence of DKA, and early initiation of insulin therapy (Figure 3).
As T1D is a predictable disease with the measurement of islet autoantibodies, it logically follows that the disease should be preventable. To date, the majority of secondary prevention trials (enrolled individuals with preclinical disease) have administered different preparations of insulin to autoantibody positive individuals in an attempt to slow the progression to T1D onset[87]. The first such trial was the diabetes prevention trial-type 1 in which at risk patients were either administered subcutaneous insulin or oral insulin in randomized, double-blinded, placebo controlled trials. Oral insulin has no metabolic effect; however, orally administered insulin does encounter mucosal gut-associated lymphoid tissue. The role of this lymphoid tissue is to provide protection from orally acquired pathogens and to keep individuals from developing reactions to ingested proteins. By administering low doses of oral insulin, insulin-specific T-regulatory cells are produced which may release cytokines that inhibit the inflammatory cascade that leads to β-cell destruction[88-90]. Relatives of patients with T1D who were 3 to 45 years of age and had high-risk HLA genes and one or more positive autoantibodies were evaluated for abnormal glucose metabolism. Those individuals who had abnormal glucose tolerance (n = 339) were administered 0.25 units/kg per day of Ultralente insulin twice daily and received an intravenous insulin infusion for four days at the beginning of the study and then annually. There was no effect of low-dose subcutaneous insulin on delaying the progression to T1D[91]. Participants with normal glucose tolerance received 7.5 mg/kg per day of oral insulin (n = 372). An oral glucose tolerance test was completed every 6 mo during a 6-year follow up, and there was not a delay in progression to T1D. Of interest, a post-hoc analysis showed in participants with persistently high levels of IAA (≥ 80 nU/mL) there was a delay in disease onset of approximately five years[92]. Also, the rate of progression to T1D onset after stopping insulin was more rapid[93]. A follow-up oral insulin trial through TrialNet is currently enrolling participants in order to determine if oral insulin can delay the progression to T1D in individuals with high IAA levels (ClinicalTrials.gov Identifier: NCT00419562).
Another insulin intervention trial from the Belgian T1D Registry identified study participants who were insulin autoantibody positive and did not have a HLA haplotype conferring protection (DQB*0602). Study participants were given two subcutaneous injections of insulin daily for 3 years (n = 25) or observed and prospectively followed (n = 25). The participants who were treated with insulin and those who refused treatment or agreed to observation developed T1D at the same rate[94].
Many preclinical studies have suggested that administration of intranasal insulin may delay T1D development through mucosal tolerance, in which mucosal antigens have been shown to impact regulatory T cell development[95]. To translate these findings to humans, individuals with high-risk HLA haplotypes and one or more islet autoantibodies were enrolled in the Intranasal Insulin Trial (INIT-I) (n = 38). This randomized, double-blinded, crossover pilot study suggested that intranasal insulin protects against the development of T1D by increasing antibody formation and decreasing T cell responsiveness[96]. The INIT-II, a randomized, double-blinded, placebo controlled trial, is now enrolling individuals to determine if intranasal insulin can delay or prevent the progression to T1D (ClinicalTrials.gov Identifier: NCT 00336674). However, a large study in Finland, the T1D Prediction and Prevention Trial enrolled and followed siblings of children with T1D or infants of mothers with T1D who had high-risk HLA genes for islet autoantibody development. Once two or more autoantibodies were detected (n = 264), they were randomized to intranasal insulin (n = 137) or placebo (n = 127). Interim analyses showed no benefit of intranasal insulin in delaying the onset of T1D[97]. This indicates that intranasal insulin may not be effective at delaying diabetes onset at the administered dose and timing in the disease process. Potentially insulin antigen specific therapies may need to be administered earlier in the disease course to have an impact on delaying progression to T1D.
Several trials using non-antigen specific therapies including bacille calmette-guerin injections, Ketotifen (histamine antagonist), oral cyclosporine, and nicotinamide (B6) have been completed. No study has prevented or delayed T1D development[98-104].
Clinical trials with drugs aimed at modulating the immune response and preserving endogenous insulin secretion in patients with new-onset T1D are termed tertiary prevention trials[51]. Only recently have these drugs expanded to prevention trials in islet autoantibody positive individuals (Figure 3). The CTLA4-Ig antibody (Abatacept) for Prevention of Abnormal Glucose Tolerance and Diabetes in Relatives At-Risk (ClinicalTrials.gov Identifier: NCT 01773707) and Anti-CD3 monoclonal antibody (Teplizumab) for Prevention Of Diabetes In Relatives At Risk For T1D mellitus (ClinicalTrials.gov Identifier: NCT01030861) are both TrialNet studies currently enrolling participants. Abatacept is a fusion antibody that binds to antigen presenting cells and blocks co-stimulation to T cells. Anti-CD3 monoclonal antibodies bind the CD3 molecule which is present on CD8 and CD4 T cells, thereby inhibiting T cell activation[105]. Both of these drugs have shown some degree of success when used in new-onset trials[106-111]. DIAPREV-IT is an antigen-based treatment currently enrolling individuals who are positive for GAD and one or more additional autoantibodies (ClinicalTrials.gov Identifier: NCT 01122446). A GAD/alum vaccine is given at enrollment and 1 mo later. Although GAD vaccination is safe and easily administered, new-onset intervention trials have not shown long-term preservation of endogenous insulin secretion[108,112,113].
Currently, insulin is the only medication approved by the United States Food and Drug Administration for the treatment of T1D. Despite T1D being a predictable chronic autoimmune disorder, there are not any therapies to preserve endogenous insulin production. As mentioned above, many large clinical intervention trials have not slowed the progression or prevented disease onset. We believe T1D will be preventable and that safe and specific therapies targeting the immune system are needed. One such approach is to target the trimolecular complex, which consists of a self-reactive CD4 T cell, insulin, and HLA molecule[114]. It is well established that specific HLA alleles, namely HLA DQ8 which is present in approximately 60% of all T1D patients, confer significant disease risk. DQ8 is a molecular target for diabetes intervention by using small “drug-like” molecules to block antigen presentation, thereby inhibiting specific T cell activation. Preclinical studies have shown this to be a potential pathway for diabetes intervention[115]. This concept has been advanced from bench to bedside as a clinical trial in which methyldopa (Aldomet), a clinically well-established antihypertensive drug, is being investigated to block DQ8 antigen presentation. The phase 1b dose escalation trial is using personalized medicine as methyldopa is being administered to recent onset adult T1D patients with the presence of the DQ8 gene (ClinicalTrials.gov Identifier: NCT01883804). Methyldopa is orally administered, safe as it has been used clinically for the last 50 years, and currently indicated for the treatment of pregnancy induced hypertension. Furthermore, all individuals have three class II molecules (DQ, DR, and DP), and by blocking a single class II molecule, there are two others to permit normal immune system function.
Other approaches have targeted components of the insulin trimolecular complex including antibodies that specifically bind to an insulin peptide in the HLA molecule. Preclinical studies in an animal model of spontaneous autoimmune diabetes indicate that this approach can delay diabetes onset[116]. Efforts are currently being made to make a human antibody, which again is a very specific immune therapy for diabetes intervention. Finally, insulin antigen specific therapy has the potential to evolve with recent advances in the field of immunology. A peptide from the insulin B chain amino acids 9-23 (B:9-23) has been extensively studied in animal models and human T1D[117,118]. It is now appreciated that insulin B:9-23 is a key autoantigen in the disease process of both mice and humans, sharing an identical amino acid sequence in both species[119,120]. A mutated insulin B:9-23 peptide, but not the native peptide sequence, induced protective immune responses (regulatory T cells) and prevented diabetes onset in preclinical animal models[121]. With a deeper understanding of how the insulin peptide binds to HLA molecules and activates T cells, an insulin vaccine again holds promise for diabetes prevention.
In conclusion, T1D is now a predicable disease with the measurement of islet autoantibodies and prevention will naturally follow. To prevent T1D, general population screening for islet autoantibodies is needed along with a safe and specific therapy for disease intervention. The genes that confer diabetes risk are now molecular targets, and tailoring therapies to specific HLA genes is personalized medicine. The future holds promise for delaying the progression and ultimately preventing diabetes.
P- Reviewer: Ciccone MM, Faienza MF, Haidara M, Ilangumaran S, Gray SG
S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1462] [Cited by in RCA: 1651] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 2. | Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie RS, Hamman RF, Klingensmith G, Eisenbarth GS. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia. 1996;39:807-812. [PubMed] |
| 3. | Lévy-Marchal C, Patterson CC, Green A; EURODIAB ACE Study Group. Europe and Diabetes. Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. European and Dibetes. Diabetologia. 2001;44 Suppl 3:B75-B80. [PubMed] |
| 4. | Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1105] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 5. | DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 822] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 6. | Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1186] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 7. | Lawrence JM, Imperatore G, Dabelea D, Mayer-Davis EJ, Linder B, Saydah S, Klingensmith GJ, Dolan L, Standiford DA, Pihoker C. Trends in incidence of type 1 diabetes among non-Hispanic white youth in the U.S., 2002-2009. Diabetes. 2014;63:3938-3945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 442] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 9. | Redondo MJ, Eisenbarth GS. Genetic control of autoimmunity in Type I diabetes and associated disorders. Diabetologia. 2002;45:605-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Nokoff N, Rewers M. Pathogenesis of type 1 diabetes: lessons from natural history studies of high-risk individuals. Ann N Y Acad Sci. 2013;1281:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Gillespie KM, Gale EA, Bingley PJ. High familial risk and genetic susceptibility in early onset childhood diabetes. Diabetes. 2002;51:210-214. [PubMed] |
| 12. | Hämäläinen AM, Knip M. Autoimmunity and familial risk of type 1 diabetes. Curr Diab Rep. 2002;2:347-353. [PubMed] |
| 13. | Rothwell PM, Gutnikov SA, McKinney PA, Schober E, Ionescu-Tirgoviste C, Neu A. Seasonality of birth in children with diabetes in Europe: multicentre cohort study. European Diabetes Study Group. BMJ. 1999;319:887-888. [PubMed] |
| 14. | Vaiserman AM, Carstensen B, Voitenko VP, Tronko MD, Kravchenko VI, Khalangot MD, Mechova LV. Seasonality of birth in children and young adults (0-29 years) with type 1 diabetes in Ukraine. Diabetologia. 2007;50:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Ostman J, Lönnberg G, Arnqvist HJ, Blohmé G, Bolinder J, Ekbom Schnell A, Eriksson JW, Gudbjörnsdottir S, Sundkvist G, Nyström L. Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983-2002. J Intern Med. 2008;263:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Kordonouri O, Shuga N, Lewy H, Ashkenazi I, Laron Z. Seasonality of month of birth of children and adolescents with type 1 diabetes mellitus in Berlin differs from the general population. Eur J Pediatr. 2002;161:291-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1360] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 18. | Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet. 1996;59:1134-1148. [PubMed] |
| 19. | Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 589] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 20. | Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet. 2011;12:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Pugliese A, Zeller M, Fernandez A, Zalcberg LJ, Bartlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 658] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 22. | Bradfield JP, Qu HQ, Wang K, Zhang H, Sleiman PM, Kim CE, Mentch FD, Qiu H, Glessner JT, Thomas KA. A genome-wide meta-analysis of six type 1 diabetes cohorts identifies multiple associated loci. PLoS Genet. 2011;7:e1002293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 23. | Steck AK, Baschal EE, Jasinski JM, Boehm BO, Bottini N, Concannon P, Julier C, Morahan G, Noble JA, Polychronakos C. rs2476601 T allele (R620W) defines high-risk PTPN22 type I diabetes-associated haplotypes with preliminary evidence for an additional protective haplotype. Genes Immun. 2009;10 Suppl 1:S21-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Snell-Bergeon JK, Smith J, Dong F, Barón AE, Barriga K, Norris JM, Rewers M. Early childhood infections and the risk of islet autoimmunity: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetes Care. 2012;35:2553-2558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Hyöty H, Leinikki P, Reunanen A, Ilonen J, Surcel HM, Rilva A, Käär ML, Huupponen T, Hakulinen A, Mäkelä AL. Mumps infections in the etiology of type 1 (insulin-dependent) diabetes. Diabetes Res. 1988;9:111-116. [PubMed] |
| 26. | Hyöty H, Taylor KW. The role of viruses in human diabetes. Diabetologia. 2002;45:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Honeyman MC, Coulson BS, Stone NL, Gellert SA, Goldwater PN, Steele CE, Couper JJ, Tait BD, Colman PG, Harrison LC. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes. 2000;49:1319-1324. [PubMed] |
| 28. | Honeyman MC, Stone NL, Harrison LC. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: potential for mimicry with rotavirus and other environmental agents. Mol Med. 1998;4:231-239. [PubMed] |
| 29. | Pak CY, Eun HM, McArthur RG, Yoon JW. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;2:1-4. [PubMed] |
| 30. | Forrest JM, Menser MA, Burgess JA. High frequency of diabetes mellitus in young adults with congenital rubella. Lancet. 1971;2:332-334. [PubMed] |
| 31. | Menser MA, Forrest JM, Bransby RD. Rubella infection and diabetes mellitus. Lancet. 1978;1:57-60. [PubMed] |
| 32. | Devendra D, Liu E, Eisenbarth GS. Type 1 diabetes: recent developments. BMJ. 2004;328:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (2)] |
| 33. | Kimpimäki T, Kupila A, Hämäläinen AM, Kukko M, Kulmala P, Savola K, Simell T, Keskinen P, Ilonen J, Simell O. The first signs of beta-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab. 2001;86:4782-4788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Lönnrot M, Korpela K, Knip M, Ilonen J, Simell O, Korhonen S, Savola K, Muona P, Simell T, Koskela P. Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes. 2000;49:1314-1318. [PubMed] |
| 35. | Stene LC, Oikarinen S, Hyöty H, Barriga KJ, Norris JM, Klingensmith G, Hutton JC, Erlich HA, Eisenbarth GS, Rewers M. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY). Diabetes. 2010;59:3174-3180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Hyöty H, Hiltunen M, Knip M, Laakkonen M, Vähäsalo P, Karjalainen J, Koskela P, Roivainen M, Leinikki P, Hovi T. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44:652-657. [PubMed] |
| 37. | Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 38. | Kaufman DL, Erlander MG, Clare-Salzler M, Atkinson MA, Maclaren NK, Tobin AJ. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 288] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Horwitz MS, Ilic A, Fine C, Balasa B, Sarvetnick N. Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin Immunol. 2004;110:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 41. | Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1156] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 42. | Simpson M, Brady H, Yin X, Seifert J, Barriga K, Hoffman M, Bugawan T, Barón AE, Sokol RJ, Eisenbarth G. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia. 2011;54:2779-2788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Hummel S, Pflüger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34:1301-1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 44. | Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005;23:3876-3886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Lee HS, Burkhardt BR, McLeod W, Smith S, Eberhard C, Lynch K, Hadley D, Rewers M, Simell O, She JX. Biomarker discovery study design for type 1 diabetes in The Environmental Determinants of Diabetes in the Young (TEDDY) study. Diabetes Metab Res Rev. 2014;30:424-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat Rev Endocrinol. 2010;6:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Michels AW, Nakayama M. The anti-insulin trimolecular complex in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci. 2013;1281:16-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 49. | Campbell-Thompson M, Wasserfall C, Kaddis J, Albanese-O’Neill A, Staeva T, Nierras C, Moraski J, Rowe P, Gianani R, Eisenbarth G. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 50. | Pugliese A, Yang M, Kusmarteva I, Heiple T, Vendrame F, Wasserfall C, Rowe P, Moraski JM, Ball S, Jebson L. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes. 2014;15:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 51. | Michels AW, Eisenbarth GS. Immune intervention in type 1 diabetes. Semin Immunol. 2011;23:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Spencer J, Peakman M. Post-mortem analysis of islet pathology in type 1 diabetes illuminates the life and death of the beta cell. Clin Exp Immunol. 2009;155:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Watkins RA, Evans-Molina C, Blum JS, DiMeglio LA. Established and emerging biomarkers for the prediction of type 1 diabetes: a systematic review. Transl Res. 2014;164:110-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 867] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 55. | Steck AK, Johnson K, Barriga KJ, Miao D, Yu L, Hutton JC, Eisenbarth GS, Rewers MJ. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;34:1397-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 56. | Parikka V, Näntö-Salonen K, Saarinen M, Simell T, Ilonen J, Hyöty H, Veijola R, Knip M, Simell O. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia. 2012;55:1926-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 57. | Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 1996;81:4264-4267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Yu L, Miao D, Scrimgeour L, Johnson K, Rewers M, Eisenbarth GS. Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes. 2012;61:179-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Miao D, Guyer KM, Dong F, Jiang L, Steck AK, Rewers M, Eisenbarth GS, Yu L. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes. 2013;62:4174-4178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 60. | Sosenko JM, Skyler JS, Krischer JP, Greenbaum CJ, Mahon J, Rafkin LE, Cuthbertson D, Cowie C, Herold K, Eisenbarth G, Palmer JP; Diabetes Prevention Trial-Type 1 Study Group. Glucose excursions between states of glycemia with progression to type 1 diabetes in the diabetes prevention trial-type 1 (DPT-1). Diabetes. 2010;59:2386-2389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS. Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes. 2010;59:679-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 62. | Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370:1514-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1224] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 63. | Ciccone MM, Scicchitano P, Cameli M, Cecere A, Cortese F, Dentamaro I, Gentile F, Gesualdo M, Maiello M, Modesti PA. Endothelial Function in Pre-diabetes, Diabetes and Diabetic Cardiomyopathy: A Review. J Diabetes Metab. 2014;5:364. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Ciccone MM, Marchese A, Generali A, Loiodice C, Cortese F, Carbonara R, Scicchitano P, Laviola L, Giorgino F. Interventional therapy in diabetic foot: risk factors, clinical events and prognosis at one year follow-up (a study of 103 cases). Pak J Biol Sci. 2012;15:789-794. [PubMed] |
| 65. | American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2830] [Cited by in RCA: 3016] [Article Influence: 274.2] [Reference Citation Analysis (0)] |
| 66. | Elding Larsson H, Vehik K, Gesualdo P, Akolkar B, Hagopian W, Krischer J, Lernmark Å, Rewers M, Simell O, She JX. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes. 2014;15:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Rewers A. Current concepts and controversies in prevention and treatment of diabetic ketoacidosis in children. Curr Diab Rep. 2012;12:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990-96. Arch Dis Child. 1999;81:318-323. [PubMed] |
| 69. | Dabelea D, Rewers A, Stafford JM, Standiford DA, Lawrence JM, Saydah S, Imperatore G, D’Agostino RB, Mayer-Davis EJ, Pihoker C; SEARCH for Diabetes in Youth Study Group. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014;133:e938-e945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 265] [Article Influence: 24.1] [Reference Citation Analysis (2)] |
| 70. | Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. J Clin Endocrinol Metab. 1987;65:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 187] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832-836. [PubMed] |
| 72. | Sjöberg S, Gjötterberg M, Berglund L, Möller E, Ostman J. Residual C-peptide excretion is associated with a better long-term glycemic control and slower progress of retinopathy in type I (insulin-dependent) diabetes mellitus. J Diabet Complications. 1991;5:18-22. [PubMed] |
| 73. | Nakanishi K, Kobayashi T, Miyashita H, Ohkubo M, Sugimoto T, Murase T, Kosaka K, Inouye K, Kono M. Relationships among islet cell antibodies, residual beta-cell function, and metabolic control in patients with insulin-dependent diabetes mellitus of long duration: use of a sensitive C-peptide radioimmunoassay. Metabolism. 1990;39:925-930. [PubMed] |
| 74. | Nakanishi K, Watanabe C. Rate of beta-cell destruction in type 1 diabetes influences the development of diabetic retinopathy: protective effect of residual beta-cell function for more than 10 years. J Clin Endocrinol Metab. 2008;93:4759-4766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Panero F, Novelli G, Zucco C, Fornengo P, Perotto M, Segre O, Grassi G, Cavallo-Perin P, Bruno G. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care. 2009;32:301-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Mühlhauser I, Overmann H, Bender R, Bott U, Berger M. Risk factors of severe hypoglycaemia in adult patients with Type I diabetes--a prospective population based study. Diabetologia. 1998;41:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998;128:517-523. [PubMed] |
| 78. | Mortensen HB, Swift PG, Holl RW, Hougaard P, Hansen L, Bjoerndalen H, de Beaufort CE, Knip M. Multinational study in children and adolescents with newly diagnosed type 1 diabetes: association of age, ketoacidosis, HLA status, and autoantibodies on residual beta-cell function and glycemic control 12 months after diagnosis. Pediatr Diabetes. 2010;11:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Picardi A, Visalli N, Lauria A, Suraci C, Buzzetti R, Merola MK, Manfrini S, Guglielmi C, Gentilucci UV, Pitocco D. Metabolic factors affecting residual beta cell function assessed by C-peptide secretion in patients with newly diagnosed type 1 diabetes. Horm Metab Res. 2006;38:668-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Böber E, Dündar B, Büyükgebiz A. Partial remission phase and metabolic control in type 1 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab. 2001;14:435-441. [PubMed] |
| 81. | Bonfanti R, Bognetti E, Meschi F, Brunelli A, Riva MC, Pastore MR, Calori G, Chiumello G. Residual beta-cell function and spontaneous clinical remission in type 1 diabetes mellitus: the role of puberty. Acta Diabetol. 1998;35:91-95. [PubMed] |
| 82. | Komulainen J, Knip M, Lounamaa R, Vähäsalo P, Karjalainen J, Sabbah E, Akerblom HK. Poor beta-cell function after the clinical manifestation of type 1 diabetes in children initially positive for islet cell specific autoantibodies. The Childhood Diabetes in Finland Study Group. Diabet Med. 1997;14:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 83. | Egger M, Gschwend S, Smith GD, Zuppinger K. Increasing incidence of hypoglycemic coma in children with IDDM. Diabetes Care. 1991;14:1001-1005. [PubMed] |
| 84. | Strong K, Wald N, Miller A, Alwan A. Current concepts in screening for noncommunicable disease: World Health Organization Consultation Group Report on methodology of noncommunicable disease screening. J Med Screen. 2005;12:12-19. [PubMed] |
| 85. | Clague A, Thomas A. Neonatal biochemical screening for disease. Clin Chim Acta. 2002;315:99-110. [PubMed] |
| 86. | Skyler JS. Primary and secondary prevention of Type 1 diabetes. Diabet Med. 2013;30:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Michels AW, von Herrath M. 2011 Update: antigen-specific therapy in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2011;18:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 88. | Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004;4:407-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 89. | Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 126] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Weiner HL, Mayer LF. Oral tolerance: mechanisms and applications. Introduction. Ann N Y Acad Sci. 1996;778:xiii-xviii. [PubMed] |
| 91. | Diabetes Prevention Trial--Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 571] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 92. | Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005;28:1068-1076. [PubMed] |
| 93. | Vehik K, Cuthbertson D, Ruhlig H, Schatz DA, Peakman M, Krischer JP; DPT-1 and TrialNet Study Groups. Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial. Diabetes Care. 2011;34:1585-1590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Vandemeulebroucke E, Gorus FK, Decochez K, Weets I, Keymeulen B, De Block C, Tits J, Pipeleers DG, Mathieu C; Belgian Diabetes Registry. Insulin treatment in IA-2A-positive relatives of type 1 diabetic patients. Diabetes Metab. 2009;35:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 95. | Martinez NR, Augstein P, Moustakas AK, Papadopoulos GK, Gregori S, Adorini L, Jackson DC, Harrison LC. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J Clin Invest. 2003;111:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 96. | Harrison LC, Honeyman MC, Steele CE, Stone NL, Sarugeri E, Bonifacio E, Couper JJ, Colman PG. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27:2348-2355. [PubMed] |
| 97. | Näntö-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipilä JI, Haavisto L. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 98. | Böhmer KP, Kolb H, Kuglin B, Zielasek J, Hübinger A, Lampeter EF, Weber B, Kolb-Bachofen V, Jastram HU, Bertrams J. Linear loss of insulin secretory capacity during the last six months preceding IDDM. No effect of antiedematous therapy with ketotifen. Diabetes Care. 1994;17:138-141. [PubMed] |
| 99. | Carel JC, Boltard C, Eisenbarth G, Bach JF, Bougneres PF. Cyclosporine Delays but does not Prevent Clinical Onset in Glucose Intolerant Pre-type 1 Diabetic Children. J Autoimmun. 1996;9:739-745. |
| 100. | Huppmann M, Baumgarten A, Ziegler AG, Bonifacio E. Neonatal Bacille Calmette-Guerin vaccination and type 1 diabetes. Diabetes Care. 2005;28:1204-1206. [PubMed] |
| 101. | Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 308] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 102. | Dulin WE, Wyse BM. Reversal of streptozotocin diabetes with nicotinamide. Proc Soc Exp Biol Med. 1969;130:992-994. [PubMed] |
| 103. | Yamada K, Nonaka K, Hanafusa T, Miyazaki A, Toyoshima H, Tarui S. Preventive and therapeutic effects of large-dose nicotinamide injections on diabetes associated with insulitis. An observation in nonobese diabetic (NOD) mice. Diabetes. 1982;31:749-753. [PubMed] |
| 105. | Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 283] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 106. | Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 889] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 107. | Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763-1769. [PubMed] |
| 108. | Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 109. | Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766-3774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 110. | Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Bode B, Aronoff S, Holland C, Carlin D. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 376] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 111. | Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 112. | Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 366] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 113. | Ludvigsson J, Krisky D, Casas R, Battelino T, Castaño L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 114. | Michels AW. Targeting the trimolecular complex. Clin Immunol. 2013;149:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Michels AW, Ostrov DA, Zhang L, Nakayama M, Fuse M, McDaniel K, Roep BO, Gottlieb PA, Atkinson MA, Eisenbarth GS. Structure-based selection of small molecules to alter allele-specific MHC class II antigen presentation. J Immunol. 2011;187:5921-5930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 116. | Zhang L, Crawford F, Yu L, Michels A, Nakayama M, Davidson HW, Kappler JW, Eisenbarth GS. Monoclonal antibody blocking the recognition of an insulin peptide-MHC complex modulates type 1 diabetes. Proc Natl Acad Sci USA. 2014;111:2656-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 117. | Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 589] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 118. | Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, Conlon PJ, Gottlieb PA, Putnam AL, Gaur A. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 119. | Crawford F, Stadinski B, Jin N, Michels A, Nakayama M, Pratt P, Marrack P, Eisenbarth G, Kappler JW. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci USA. 2011;108:16729-16734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 120. | Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA. 2010;107:10978-10983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 121. | Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med. 2011;208:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |