Published online Nov 10, 2015. doi: 10.4239/wjd.v6.i15.1285
Peer-review started: May 21, 2015
First decision: September 17, 2015
Revised: September 25, 2015
Accepted: October 20, 2015
Article in press: October 27, 2015
Published online: November 10, 2015
Processing time: 174 Days and 11 Hours
The applicability of stable gut hormones for the treatment of obesity-related diabetes is now undisputable. This is based predominantly on prominent and sustained glucose-lowering actions, plus evidence that these peptides can augment insulin secretion and pancreatic islet function over time. This review highlights the therapeutic potential of glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), oxyntomodulin (OXM) and cholecystokinin (CCK) for obesity-related diabetes. Stable GLP-1 mimetics have already been successfully adopted into the diabetic clinic, whereas GIP, CCK and OXM molecules offer promise as potential new classes of antidiabetic drugs. Moreover, recent studies have shown improved therapeutic effects following simultaneous modulation of multiple receptor signalling pathways by combination therapy or use of dual/triple agonist peptides. However, timing and composition of injections may be important to permit interludes of beta-cell rest. The review also addresses the possible perils of incretin based drugs for treatment of prediabetes. Finally, the unanticipated utility of stable gut peptides as effective treatments for complications of diabetes, bone disorders, cognitive impairment and cardiovascular dysfunction is considered.
Core tip: Stable gut hormones have well defined therapeutic actions for type 2 diabetes mellitus. In addition, simultaneous modulation of gut hormone receptors could increase therapeutic efficacy, but timing and receptor activation profile may be important. Finally, gut-derived peptides could possess benefits for bone disorders, cognitive impairment and cardiovascular dysfunction.
- Citation: Irwin N, Flatt PR. New perspectives on exploitation of incretin peptides for the treatment of diabetes and related disorders. World J Diabetes 2015; 6(15): 1285-1295
- URL: https://www.wjgnet.com/1948-9358/full/v6/i15/1285.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i15.1285
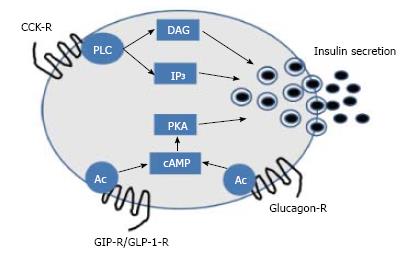
The human gastrointestinal tract (GIT) comprises the stomach, as well as the small (duodenum, jejunum and ileum) and large (caecum, colon and rectum) intestines. Aside from nutrient digestion, absorption and assimilation, the GIT also has significant endocrine functions[1]. To date, the most important endocrine function of the gut relates to evidence that intestinal derived peptides are fundamentally involved in postprandial insulin release[2]. This action is termed the “incretin effect”, and relates to the direct beta-cell insulin secretory effect of two hormones, namely glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) that are secreted from L- and K-cells, respectively (Figure 1)[3]. A number of other enteric peptide hormones released in response to feeding also have a role in energy regulation and possibly insulin secretion, including cholecystokinin (CCK) and oxyntomodulin (OXM) (Figure 1)[4,5]. However, only GLP-1 and GIP fulfil the criteria of a true incretin hormone that stimulates glucose-induced insulin secretion at physiological circulating concentrations[3]. Despite the obvious potential of incretin and incretin-like peptides for the treatment of conditions such as diabetes and obesity, the extremely short biological half-life of these peptides, due to efficient enzymatic degradation and subsequent renal filtration, severely limits therapeutic applicability[4,5]. However, interest in gut peptides has increased in recent years with knowledge that modified versions of these compounds, with vastly improved pharmacokinetic properties, have sustained beneficial physiological effects[6].
The biological actions of GLP-1 are largely preserved in type 2 diabetes and pharmacological doses of the peptide evoke robust insulin-releasing and antihyperglycaemic effects[7]. GLP-1 exerts its beta-cell effects through interaction with specific surface receptors that activate signal transduction pathways including the stimulation of intracellular cAMP mediated events[8]. GLP-1 also promotes beta-cell proliferation and islet cell neogenesis as well as inhibiting beta-cell apoptosis and alpha-cell glucagon secretion[8]. Notably, both GLP-1 and GIP expression and secretion has been described in islet alpha cells[9,10]. Indeed, it is feasible that intra-islet, rather than gut derived, GLP-1 and GIP make a significant contribution to these direct beneficial islet effects[11-13]. However, it should be noted that positive direct islets effects are still noted in rodents following prolonged exogenous delivery of stable GLP-1 mimetics[8].
GLP-1 not only targets pancreatic islet cells, but imparts positive actions in terms of inhibition of gastric emptying, suppression of appetite and weight loss[8]. Given this advantageous biological action profile, there are now several GLP-1 related enzyme-resistant, long-acting analogues available for clinical use in diabetes (Table 1), ranging from regimens that require twice daily injection to those that necessitate only once weekly administration[14]. Development of new GLP-1 mimetics, such as those conjugated to an antithrombin III-binding pentasaccharide, are also in the pipeline[15]. Interestingly, a recent commentary highlights that differences in the structure and pharmacokinetics of currently available GLP-1 mimetics could significantly alter immunogenicity, CNS signalling and overall therapeutic effect[16]. Thus, physicians may need to re-evaluate the most appropriate GLP-1 treatment strategy for each patient. Encouragingly however, GLP-1-R agonists demonstrate an efficacy approaching that of insulin treatment, but unlike insulin have the added benefits of promoting weight loss with minimal risk of hypoglycaemia[17].
| Drug name | Primary mechanism of action | EMA approval date |
| Exenatide | GLP-1 receptor agonist | Nov-06 |
| Sitagliptin | DPP-4 inhibitor | Mar-07 |
| Vildagliptin | DPP-4 inhibitor | Sep-07 |
| Liraglutide | GLP-1 receptor agonist | Jun-09 |
| Saxagliptin | DPP-4 inhibitor | Oct-09 |
| Exenatide-LAR | GLP-1 receptor agonist | Jun-11 |
| Linagliptin | DPP-4 inhibitor | Aug-11 |
| Lixisenatide | GLP-1 receptor agonist | Feb-13 |
| Alogliptin | DPP-4 inhibitor | Sep-13 |
| Dulaglutide | GLP-1 receptor agonist | Jan-15 |
Despite the widespread use of GLP-1 mimetics (Table 1), there have been recent safety concerns regarding the ability of sustained GLP-1-R activation to cause pancreatitis, pancreatic and thyroid cancer, as well as glucagon-producing neuroendocrine tumours in man[18,19]. As such, it is well recognised that pancreatitis is a risk factor for pancreatic cancer[20]. However, a recent meta-analysis did not support increased risk of pancreatitis or cancer associated with GLP-1 therapy[21]. Indeed, issues with poorly matched patient groups treated with incretin-based vs non-incretin-based medications and problems with specifically identifying glucagon-producing cells also calls into question the validity of these safety concerns[22]. Thyroid cancer fears appear to stem largely from rodent studies[23], and reduced expression of the GLP-1 receptors in human, as opposed to rodent, thyroid cells is the likely explanation for this[24]. The most frequently reported side effect of GLP-1 therapy is dose-dependent and transient mild to moderate nausea, vomiting and diarrhoea[16]. Thus, taken together the safety profile of GLP-1 based therapeutics is largely reassuring. However, pharmacovigilance with GLP-1 drugs is still required, especially in relation to patients with a history, or increased risk, of pancreatitis or thyroid cancer.
Although initially thought to play a role in impeding histamine induced gastric acid secretion[25], the primary physiological role of GIP is now considered to be stimulation of postprandial insulin secretion[13], The insulinotropic action of GIP, mediated by specific receptors on the surface of pancreatic beta-cells, is initiated largely by intracellular cAMP generation (Figure 1) and subsequent Ca2+ ion influx leading to insulin granule exocytosis[13]. An additional beneficial action of GIP involves enhanced survival of beta-cells, which is also mediated through cAMP dependent cell signaling pathways[26,27]. GIP also acts as beta-cell growth factor by stimulating mitogen-activated protein kinase pathways[28] and modulating KATP channel expression[29]. Given this impressive bioactive profile at the level of the beta-cell, there has been significant interest in the potential for GIP-based pharmaceuticals as antidiabetic drugs. However, like GLP-1 the pharmacokinetic profile GIP is severely hindered due to rapid plasma degradation by the ubiquitous enzyme dipeptidyl peptidase 4 (DPP-4), and clearance cleared from the body by efficient renal filtration[30]. In addition to this, the biological effects of GIP appear to be markedly reduced in patients with type 2 diabetes when compared to normal individuals[7].
The first of these barriers has been conquered, as with GLP-1 mimetics, through generation of N-terminally modified enzyme-resistant, long-acting GIP molecules, and these molecules has been reviewed extensively elsewhere[31,32]. However, the issue of reduced GIP responsiveness in type 2 diabetes still remains, and is thought to be linked to GIP receptor (GIP-R) down-regulation or desensitisation[7]. However, it is highly likely that that GIP desensitisation is a pathophysiological consequence as opposed to an aetiological factor of type 2 diabetes. In keeping with this, studies correcting hyperglycaemia using insulin or sulphonylureas indicate that GIP sensitivity can be restored[33,34]. It has also been demonstrated that a K-cell derived peptide co-secreted from the intestine with GIP, xenin-25, can potentiate the insulinotropic action of GIP[35,36]. As such, a novel long-acting palmitate-derivatised analogue of xenin-25 was shown to significantly augment GIP action in vitro and in vivo[37]. Moreover, sustained administration of this acylated xenin peptide exerted a spectrum of beneficial metabolic effects in high-fat-fed mice[38]. This presumably relates to restoration of GIP action in these diabetic mice[38]. In harmony with this, a recent study indicates that the impaired insulinotropic response to GIP under diabetic milieu involves mechanisms beyond simple expression of the GIP-R[39], further highlighting a potential role for xenin. Therefore, there still appears to be significant, as yet untapped, therapeutic potential for GIP-based compounds, especially in combination with molecules that can enhance GIP sensitivity directly or counter hyperglycaemia through other actions.
Similar to GLP-1, OXM is an L-cell derived proglucagon gene product secreted in response to feeding[40]. To date a specific OXM receptor has not been described, and the biological actions of OXM are attributed to binding and activation of GLP-1 and glucagon receptors (Figure 1), albeit with reduced potency compared to the native ligands[41]. In vitro and in vivo rodent studies suggest that through glucagon receptor agonism, OXM induces catabolic effects that favour weight loss and subsequent improved metabolic control, while glucose homeostasis and insulin resistance are improved through activation of GLP-1 receptors[5]. Promisingly, data from small clinical studies implies that beneficial effects on energy intake and weight loss also occur in humans[42,43]. However, as is this case for the incretin hormones, the therapeutic potential of OXM-based molecules is hindered by rapid cleavage of the first two N-terminal amino acids of OXM by DPP-4 in plasma, rendering the peptide inactive[44]. Nonetheless, structure-function studies show that N-terminal modification can protect against DPP-4 degradation without disproportionately affecting bioactivity of the molecule[44,45]. Indeed, a recent study of six novel OXM analogues has revealed that Oxm-based peptides with specific N-terminal position 2 modifications are stable and show particular promise for the treatment of diabetes[46]. These data suggest that further exploration of dual agonism of the GLP-1 and glucagon receptor is required for human diabetes. It is notable that co-administration of GLP-1 and glucagon in humans can replicate the beneficial actions of OXM[47], although this approach may be more cumbersome in clinical practice.
CCK is an intestinal I-cell derived gut hormone secreted in response to meal ingestion[48]. CCK binds to specific CCK1 receptors present on gastric mucosa and vagal afferent neurons which collectively leads to gallbladder secretions, release of pancreatic digestive juices, satiety and slowing of gut motility[1]. CCK2 receptors are mainly confined to the gastrointestinal tract and brain and may have a role in regulating anxiety and locomotion[49]. Importantly, CCK has also been shown to stimulate insulin secretion in rodents and man (Figure 1)[50,51], and act as a growth and anti-apoptotic factor for pancreatic beta-cells[52]. Thus, CCK agonists could have noteworthy potential for diabetes therapy, since their biological action profile is similar to the incretin hormones. However, native CCK is rapidly degraded by serum aminopeptidases upon secretion into the bloodstream[53], which hinders therapeutic potential. However, early studies have clearly shown that both N-terminal modification through glycation, or PEGylation, can prevent enzymatic degradation of CCK and extend biological action and therapeutic potential[53,54]. Following on from this, a more recently developed enzymatically stable, N-terminally modified, CCK analogue, namely (pGlu-Gln)-CCK-8, has been shown to have an improved pharmacodynamic profile, and to both alleviate and protect against obesity-related diabetes in animal models[51,55], with an encouraging safety profile[56]. The mechanism of action of (pGlu-Gln)-CCK-8 likely revolves around prominent and sustained reductions of energy intake, possibly related to modulation of central neuropeptide Y and melanocortin related pathways, and enhanced insulin release[57]. Encouragingly, a PEGylated version of (pGlu-Gln)-CCK-8 has now been fully characterised, that would be resistant to kidney filtration, and suitable for possible once daily dosing in man[58]. Further investigations relating to translation of beneficial effects to human type 2 diabetes together with safety evaluation are still required, but initial observations with specific and stable CCK1 receptor agonists are encouraging.
Given the beneficial effects of OXM-based peptides, it follows that design of hybrid peptides capable of modulating more than one receptor pathway could have distinct therapeutic benefits for the treatment of obesity-related diabetes. By utilising the correct ratio of receptor pathway interactions, efficacy should be enhanced with the potential for administration of lower doses, thereby reducing, or removing, adverse side effects. The most logical starting point for design of a synthetic dual acting hybrid peptide would inevitably involve a modified incretin hormones capable of activating both GIP and GLP-1 receptors. As such, GIP/GLP-1 chimeric peptides were characterised almost 20 years ago, and the structural requirements for specific ligand-receptor interactions well defined[59]. Combined administration of individual long-acting GIP and GLP-1 mimetics has been considered in preclinical studies, with some success[60]. However, issues of separate drug formulation and dosing still remain, although these may not be insurmountable as indicated by recent introduction of IDegLira for combined insulin and GLP-1 therapy in type 1 diabetes[61]. In terms of a single hybrid peptide that can directly activate both GIP and GLP-1 receptors, only MAR701, Marcadia Biotech (now Roche) has progressed to the evaluation of beneficial effects in man. However, since the clinical benefits of DPP-4 inhibitors clearly involves increased circulating levels of both incretin peptides[62], concomitant activation of GIP and GLP-1 receptors does appear to have promise for the treatment of type 2 diabetes (Table 1).
Further studies have investigated the effects of GLP-1 receptor agonism combined with either glucagon receptor agonism or antagonism[63,64]. Although somewhat contradictory in nature, these contrasting regimens both utilise the beneficial glucose-lowering effects of GLP-1, combined with either inhibition of glucagon-mediated gluconeogenesis and glycogenolysis[65], or activation of glucagon pathways involved in energy turnover and weight loss[64], as is this case for OXM. Other modified hybrid peptides for dual activation of regulatory peptide receptors include, ZP3022, a combined GLP-1-gastrin agonist[66]. Through activation of GLP-1 and CCK2 receptors, this peptide improved glycaemic control in db/db mice via enhancement of beta-cell mass[66]. However, perhaps more appealing is the potential for combined and sustained activation of GLP-1 and CCK1 receptors. As such, two independent studies have clearly shown pronounced synergistic metabolic benefits with combined administration of long-acting GLP-1 and CCK1 receptor agonists in rodent models of type 2 diabetes[67,68]. These extremely positive effects are believed to occur through activation of complementary pathways that lead to significant weight loss and dramatically improved metabolic control[67,68]. Furthermore, a novel CCK/GLP-1 hybrid peptide, based on the chemical structures of (pGlu-Gln)-CCK-8 and exenatide, has recently been described and shown to have significant therapeutic potential in high-fat fed mice[69]. This molecule clearly warrants further study as a potential new treatment option for type 2 diabetes.
Considering the evident therapeutic efficacy offered by dual peptide receptor interactions, single compounds with the ability to concurrently activate three or more regulatory peptide receptors could deliver even greater beneficial effects. Moreover, the celebrated success of bariatric surgery for restoring metabolic control in type 2 diabetic patients, independent of weight loss[70], results from a culmination of reduced energy intake and modulation of the secretion and biological action of numerous gut-derived peptides[71]. Thus, there is now significant enthusiasm arising from designer modified peptides with the ability to concurrently modulate GIP, GLP-1 and glucagon receptor signalling[72,73]. These triple-acting peptides have resulted in dramatic improvements in glucose homeostasis and overall metabolic control in high fat fed mice[72,73]. Despite their obvious potential, issues regarding the ratio of GIP, GLP-1 and glucagon receptor activation still need to be addressed, As such, a subsequent study has reported the distinct beneficial effects of a balanced glucagon, GLP-1 and GIP receptor tri-agonist to correct obesity and diabetes in high fat fed mice[74]. Taken together, there is a clear and attractive rationale for further testing of combinatorial hormone therapies for the treatment of obesity and diabetes in humans.
Although the future trend for peptide-based antidiabetic drugs seems to be development dual or triple agonists, treatment modalities that incorporate periods of beta-cell rest could be important for glycaemic control[75]. Thus, antidiabetic drugs that induce direct beta-cell stimulatory effects can erode beta-cell mass over time[76]. As such, intermittent periods of beta-cell rest may be useful to preserve long-term beta-cell function and lasting glycaemic control[75]. In contrast to sulphonylureas and meglitinides, incretin based drugs stimulate insulin secretion in a glucose-dependent fashion that should help preserve beta-cell mass and function[8]. Nonetheless, adequate periods of rest might still allow chronically stimulated pancreatic beta-cells to replenish both cell surface receptors and the immediately secretable insulin granule pool[77]. Such effects, together with the positive actions of incretins on beta-cell stimulus-secretion coupling, survival and growth, could be highly beneficial. Accordingly, the timing of injections of dual or triple acting therapies, as well as the profile of receptor pathways activated, could be of valuable clinical relevance. In relation to this, inhibition of GIP-R signalling has been shown to improve metabolic control and glycaemic status in animal models of obesity-related diabetes by enhancing insulin action and diminishing insulin secretion[78,79]. Thus a key aspect underlying the beneficial effects could be related to the induction of pancreatic beta-cell rest. Consistent with this, combination of morning injection of liraglutide, with stable GIP antagonist peptide in the evening, greatly improved glycaemic control in db/db mice compared with reciprocal administration or twice daily injection of liraglutide[80]. Further investigation of this potentially important treatment paradigm, in combination with other agents that stimulate and/or relieve beta cell insulin release, is required to fully explore therapeutic relevance and applicability.
Prediabetes describes to a situation where blood sugar is high, but not elevated sufficiently to classify as overt type 2 diabetes. However, the condition represents a high risk state for future development of diabetes, most likely linked to progressive beta-cell decline[81]. Thus, it follows that the positive effects of incretin mimetics on beta-cell function, including possible benefits for beta-cell proliferation and survival, plus additional weight-lowering and extrapancreatic actions[8], could hold significant promise for prediabetic patients. Moreover, patients with prediabetes have been shown to have an impaired incretin effect in response to oral nutrient delivery[82].
To date, there have been several tentative clinical studies conducted on the potential beneficial effects of incretin-based drugs for prediabetes. Studies with DPP-4 inhibitors (Table 1), which prevent incretin peptide degradation and increase active circulating levels of GIP and GLP-1, reported modest positive effects[83-85]. However, treatment with the stable incretin mimetics, exenatide or liraglutide, generated more positive outcomes[86,87]. This included significant reductions in the prevalence of prediabetes with reversion to normal glucose tolerance[86,87]. The inconsistency between DPP-4 inhibitors and GLP-1 mimetics most likely relates to differences in the circulating levels of active hormones achieved. However, issues of oral vs injectable delivery of DPP-4 inhibitors and GLP-1 mimetics, respectively, could significantly affect compliance in this patient subgroup. In addition, the potential adverse side-effect profile of incretin based therapies, as discussed above, would also have to be fully considered. Finally, the cost of therapy with DPP-4 inhibitors and particularly GLP-1 mimetics is greater when compared to other glucose-lowering agents[88]. Thus, given the limited experience to date regarding the effect of incretin therapies in prediabetes, future clinical trials would be recommended. In terms of GIP, CCK and OXM therapies, clinical effectiveness in type 2 diabetes would need to be fully established before beneficial actions in prediabetic patients could be considered.
Although incretin hormones have been studied extensively for therapeutic effectiveness in diabetes, research has uncovered unexpected benefits in various other tissues. For instance, a role for gastrointestinal derived hormones in bone remodeling is suspected since serum levels of bone biomarkers rapidly alter after a meal[89]. Indeed, functional GIP receptors have been evidenced on the surface of bone cells[90]. Notably, GIP has been shown to inhibit bone resorption in humans under both euglycaemic and hyperglycaemic states[91]. Thus, the beneficial effects of GIP on bone could be independent of feeding state. Indeed, exogenous prolonged administration of an N-terminally modified stable GIP receptor agonist imparted various beneficial effects on tissue-level bone material properties of rats[92]. In terms of GLP-1 effects on bone, the picture is less clear. This mostly relates to data from animal models being clouded by the fact that GLP-1 receptors are highly expressed on rodent thyroid cells, resulting alterations of circulating calcitonin levels[93]. Nonetheless, GLP-1 receptors have been found on the surface of human osteoblast-like cells[94]. Moreover, very recent data suggest that liraglutide has anabolic effects on bone in diabetic rats[95]. In keeping with this, a study in double incretin receptor knockout mice[89], reported a combination of detrimental bone abnormalities that mimicked observations from both GIP[96,97] and GLP-1[98] receptor knockout mice. Despite these observations in rodents, a preliminary meta-analysis suggests that GLP-1 mimetics do not modify the increased bone fracture risk in humans with type 2 diabetes[99], or could even potentially increase fracture risk in this population[100]. In keeping with this, a retrospective population based cohort study has suggested that DPP- 4 inhibition is not associated with reduced fracture risk in humans[101], whereas bone loss and strength where significantly improved by sitaglitpin therapy in diabetic rats[102]. Care is required therefore when extrapolating data on the effects of incretin-like drugs on bone from rodents to man, particularly in the case of GLP-1. However, actions of GIP are particularly promising and further research is required to determine if incretin hormones can be useful to treat abnormalities of bone encountered in diabetes and osteoporosis.
In terms of the central nervous system, expression of functional GIP and GLP-1 receptors has been demonstrated in several brain regions[103]. Much of the therapeutic interest for incretin-like molecules in the CNS revolves around neuroprotective effects for the treatment of Alzheimer’s and Parkinson’s diseases, as well as cognitive impairments in diabetes[3,104]. Accordingly, GIP receptor knockout mice exhibit impaired memory learning, synaptic plasticity, and neurogenesis[105]. In agreement, transgenic mice that over-express GIP exhibit enhanced sensorimotor coordination and memory recognition[106]. Earlier studies have already shown that stable forms of GIP can beneficially modulate synaptic transmission and enhance the induction of long-term potentiation, an important physiological cellular means of monitoring learning processes[107]. In addition, prolonged GIP receptor activation improved cognitive function, hippocampal synaptic plasticity and glucose homeostasis in obese-diabetic high-fat fed mice[108]. In agreement with this, GLP-1 receptor knockout mice display an impairment of synaptic plasticity and memory formation[109]. Furthermore, sustained treatment with long-acting GLP-1 agonists improves memory and learning in various rodent models of neurodegeneration and diabetes[108,110,111]. Moreover, liraglutide treatment has recently been shown to restore cerebral and systemic microvascular architecture in a rodent model of genetically-induced cognitive dysfunction[112]. Based on the positive neuroprotective effects of incretin compounds, there are several ongoing clinical trials with these drugs that should reveal encouraging effects for the potential treatment of Alzheimer’s and Parkinson’s diseases[104]. Finally, in harmony with the positive effects of incretin molecules on brain function, sitaglitpin treatment was recently shown to improve recognition memory, oxidative stress and hippocampal neurogenesis in diabetic mice[113]. Collectively, these observations strengthen the possibility that incretin peptides play a direct role in modulating aspects of brain function and could possess key clinical pharmacological benefits for patients with diabetes and neurodegenerative disorders.
The GLP-1 receptor has been demonstrated in the heart[114]. Although some controversy still exists as to the exact location of the receptor within the heart, various studies confirm the presence of GLP-1 receptor mRNA transcripts in rodent and human cardiac tissue[115]. In cardiomyocytes GLP-1 receptor signalling induced elevations in cAMP levels, but surprisingly this was not coupled to an increase in intracellular Ca2+ concentrations and cardiomyocyte contractility[116]. Indeed, there could be a paradoxical reduction in cardiomyocyte contractility despite elevated cAMP levels[116]. Moreover, GLP-1 receptor knockout mice present with decreased ventricular contractile function[117]. As such, the exact mechanism of action and physiological relevance of GLP-1 receptor signalling in the heart requires further detailed investigation. Despite this, and similar to the situation in pancreatic beta-cells, GLP-1 appears to have anti-apoptotic effects in cardiomyocytes and improves overall outcomes in mice after myocardial infarction[118]. Further to this, GLP-1 receptor protein has also been detected in human coronary artery endothelial cells and encouragingly, activation is believed to improve endothelial cell function in diabetic patients[119]. Thus, prospective clinical trials are ongoing to assess the cardiovascular safety profile of GLP-1 based peptides, and initial observations in humans with diabetes are positive[120]. Whilst the GIP receptor is believed to be present in the heart and on vasculature[103], there is a paucity of knowledge in relation to GIP effects on these tissues. Stimulation of GIP receptors may induce conflicting effects in different vascular beds[121], and this could explain for its unaccounted physiological effects in these tissues. In keeping with this, the overall effect of DPP-4 inhibition on cardiovascular function is still not clear[122].
Stable gut hormones have considerable potential for the treatment of obesity-related diabetes, and possibly other related pathologies. Whilst disorders of bone, cognitive function and the cardiovascular system can be considered as complications of diabetes, they are also standalone distinct illnesses in their own right. Thus, the therapeutic outlook of incretin mimetics may stretch well beyond diabetes. However, to date only GLP-1 based drugs are clinically available, exclusively for the treatment of type 2 diabetes and associated obesity. Concerns regarding the safety of GLP-1 analogues in man appear to have been allayed, but pharmacovigilance is still required. The potential promise of incretin based drugs such as GLP-1 mimetics for the treatment of prediabetes still requires detailed investigation. Stable forms of GIP, OXM and CCK also appear to offer distinct therapeutic possibilities for the treatment of type 2 diabetes based on data from animal models and preliminary human studies. Given this, and the multifactorial pathological nature of diabetes, it is not unexpected that concurrent activation of more than one regulatory peptide receptor signalling pathway appears to have promise for the future treatment of diabetes. This may be achieved through the development of double or triple acting agonists or use of a cocktail of existing peptidergic drugs. However, note should be taken of emerging evidence suggesting the utility of sequential peptide exposures to facilitate essential periods of beta-cell rest. Taken together, future advances in our understanding of gut peptide biology, coupled with therapeutic application, should lead to an expansion of clinically available gut peptide-based drugs with far-reaching benefits to the patient.
The authors work on incretin peptides has been supported over many years by Diabetes United Kingdom, European Foundation for the Study of Diabetes, Invest Northern Ireland, Irish Endocrine Society, SAAD Trading and Contracting Company, Department of Education and Learning Northern Ireland, Diabetes Research Wellness Foundation and University of Ulster strategic research funding.
P- Reviewer: Ali O, Collino M S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Rehfeld JF. Gastrointestinal hormones and their targets. Adv Exp Med Biol. 2014;817:157-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967;46:1954-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 620] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Irwin N, Flatt PR. Enteroendocrine hormone mimetics for the treatment of obesity and diabetes. Curr Opin Pharmacol. 2013;13:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012;19:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Pocai A. Unraveling oxyntomodulin, GLP1’s enigmatic brother. J Endocrinol. 2012;215:335-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Gribble FM. The gut endocrine system as a coordinator of postprandial nutrient homoeostasis. Proc Nutr Soc. 2012;71:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1148] [Cited by in RCA: 1203] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 8. | Drucker DJ. Deciphering metabolic messages from the gut drives therapeutic innovation: the 2014 Banting Lecture. Diabetes. 2015;64:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Fujita Y, Wideman RD, Asadi A, Yang GK, Baker R, Webber T, Zhang T, Wang R, Ao Z, Warnock GL. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet alpha-cells and promotes insulin secretion. Gastroenterology. 2010;138:1966-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Whalley NM, Pritchard LE, Smith DM, White A. Processing of proglucagon to GLP-1 in pancreatic α-cells: is this a paracrine mechanism enabling GLP-1 to act on β-cells? J Endocrinol. 2011;211:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Vasu S, Moffett RC, Thorens B, Flatt PR. Role of endogenous GLP-1 and GIP in beta cell compensatory responses to insulin resistance and cellular stress. PLoS One. 2014;9:e101005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Moffett RC, Vasu S, Thorens B, Drucker DJ, Flatt PR. Incretin receptor null mice reveal key role of GLP-1 but not GIP in pancreatic beta cell adaptation to pregnancy. PLoS One. 2014;9:e96863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Moffett RC, Vasu S, Flatt PR. Functional GIP receptors play a major role in islet compensatory response to high fat feeding in mice. Biochim Biophys Acta. 2015;1850:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Scott DA, Boye KS, Timlin L, Clark JF, Best JH. A network meta-analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo. Diabetes Obes Metab. 2013;15:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Patterson S, de Kort M, Irwin N, Moffett RC, Dokter WH, Bos ES, Miltenburg AM, Flatt PR. Pharmacological characterisation and antidiabetic activity of a long-acting GLP-1 analogue conjugated to an ATIII-binding pentasaccharide. Diabetes Obes Metab. 2015;17:760-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur J Intern Med. 2014;25:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Balena R, Hensley IE, Miller S, Barnett AH. Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab. 2013;15:485-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab. 2012;97:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595-2604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 20. | Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe? Diabetes Care. 2013;36:2118-2125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 21. | Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98:271-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 22. | Nauck MA, Meier JJ. Studying pancreatic risks caused by incretin-based therapies: is it a game? It’s not a game! J Diabetes Sci Technol. 2014;8:895-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 448] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 24. | Nauck MA, Friedrich N. Do GLP-1-based therapies increase cancer risk? Diabetes Care. 2013;36 Suppl 2:S245-S252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Ross SA, Dupre J. Effects of ingestion of triglyceride or galactose on secretion of gastric inhibitory polypeptide and on responses to intravenous glucose in normal and diabetic subjects. Diabetes. 1978;27:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Ehses JA, Casilla VR, Doty T, Pospisilik JA, Winter KD, Demuth HU, Pederson RA, McIntosh CH. Glucose-dependent insulinotropic polypeptide promotes beta-(INS-1) cell survival via cyclic adenosine monophosphate-mediated caspase-3 inhibition and regulation of p38 mitogen-activated protein kinase. Endocrinology. 2003;144:4433-4445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Renner S, Fehlings C, Herbach N, Hofmann A, von Waldthausen DC, Kessler B, Ulrichs K, Chodnevskaja I, Moskalenko V, Amselgruber W. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes. 2010;59:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Kubota A, Yamada Y, Yasuda K, Someya Y, Ihara Y, Kagimoto S, Watanabe R, Kuroe A, Ishida H, Seino Y. Gastric inhibitory polypeptide activates MAP kinase through the wortmannin-sensitive and -insensitive pathways. Biochem Biophys Res Commun. 1997;235:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Kim SJ, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem. 2005;280:22297-22307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Deacon CF, Ahrén B. Physiology of incretins in health and disease. Rev Diabet Stud. 2011;8:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Gault VA, O’Harte FP, Flatt PR. Glucose-dependent insulinotropic polypeptide (GIP): anti-diabetic and anti-obesity potential? Neuropeptides. 2003;37:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Aaboe K, Knop FK, Vilsboll T, Vølund A, Simonsen U, Deacon CF, Madsbad S, Holst JJ, Krarup T. KATP channel closure ameliorates the impaired insulinotropic effect of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Irwin N, Gault V, Flatt PR. Therapeutic potential of the original incretin hormone glucose-dependent insulinotropic polypeptide: diabetes, obesity, osteoporosis and Alzheimer’s disease? Expert Opin Investig Drugs. 2010;19:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Højberg PV, Vilsbøll T, Rabøl R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 328] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 35. | Taylor AI, Irwin N, McKillop AM, Patterson S, Flatt PR, Gault VA. Evaluation of the degradation and metabolic effects of the gut peptide xenin on insulin secretion, glycaemic control and satiety. J Endocrinol. 2010;207:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Wice BM, Wang S, Crimmins DL, Diggs-Andrews KA, Althage MC, Ford EL, Tran H, Ohlendorf M, Griest TA, Wang Q. Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J Biol Chem. 2010;285:19842-19853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Martin CM, Gault VA, McClean S, Flatt PR, Irwin N. Degradation, insulin secretion, glucose-lowering and GIP additive actions of a palmitate-derivatised analogue of xenin-25. Biochem Pharmacol. 2012;84:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Gault VA, Martin CM, Flatt PR, Parthsarathy V, Irwin N. Xenin-25[Lys13PAL]: a novel long-acting acylated analogue of xenin-25 with promising antidiabetic potential. Acta Diabetol. 2015;52:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Pathak V, Vasu S, Flatt PR, Irwin N. Effects of chronic exposure of clonal β-cells to elevated glucose and free fatty acids on incretin receptor gene expression and secretory responses to GIP and GLP-1. Diabetes Obes Metab. 2014;16:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Karra E, Batterham RL. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol Cell Endocrinol. 2010;316:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88:4696-4701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 43. | Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54:2390-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 294] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 44. | Druce MR, Minnion JS, Field BC, Patel SR, Shillito JC, Tilby M, Beale KE, Murphy KG, Ghatei MA, Bloom SR. Investigation of structure-activity relationships of Oxyntomodulin (Oxm) using Oxm analogs. Endocrinology. 2009;150:1712-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Kerr BD, Flatt PR, Gault VA. (D-Ser2)Oxm[mPEG-PAL]: a novel chemically modified analogue of oxyntomodulin with antihyperglycaemic, insulinotropic and anorexigenic actions. Biochem Pharmacol. 2010;80:1727-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Lynch AM, Pathak N, Flatt YE, Gault VA, O’Harte FP, Irwin N, Flatt PR. Comparison of stability, cellular, glucose-lowering and appetite supressing effects of oxyntomodulin analogues modified at the N-terminus. Eur J Pharmacol. 2014;743:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Cegla J, Troke RC, Jones B, Tharakan G, Kenkre J, McCullough KA, Lim CT, Parvizi N, Hussein M, Chambers ES. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63:3711-3720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 48. | Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 790] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 49. | Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269:G628-G646. [PubMed] |
| 50. | Ahrén B, Pettersson M, Uvnäs-Moberg K, Gutniak M, Efendic S. Effects of cholecystokinin (CCK)-8, CCK-33, and gastric inhibitory polypeptide (GIP) on basal and meal-stimulated pancreatic hormone secretion in man. Diabetes Res Clin Pract. 1991;13:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Irwin N, Frizelle P, Montgomery IA, Moffett RC, O’Harte FP, Flatt PR. Beneficial effects of the novel cholecystokinin agonist (pGlu-Gln)-CCK-8 in mouse models of obesity/diabetes. Diabetologia. 2012;55:2747-2758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Lavine JA, Raess PW, Stapleton DS, Rabaglia ME, Suhonen JI, Schueler KL, Koltes JE, Dawson JA, Yandell BS, Samuelson LC. Cholecystokinin is up-regulated in obese mouse islets and expands beta-cell mass by increasing beta-cell survival. Endocrinology. 2010;151:3577-3588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | O’Harte FP, Mooney MH, Kelly CM, Flatt PR. Glycated cholecystokinin-8 has an enhanced satiating activity and is protected against enzymatic degradation. Diabetes. 1998;47:1619-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Verbaeys I, León-Tamariz F, Buyse J, Decuypere E, Pottel H, Cokelaere M. Lack of tolerance development with long-term administration of PEGylated cholecystokinin. Peptides. 2009;30:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Irwin N, Montgomery IA, Moffett RC, Flatt PR. Chemical cholecystokinin receptor activation protects against obesity-diabetes in high fat fed mice and has sustainable beneficial effects in genetic ob/ob mice. Biochem Pharmacol. 2013;85:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Irwin N, Frizelle P, O’Harte FP, Flatt PR. Metabolic effects of activation of CCK receptor signaling pathways by twice-daily administration of the enzyme-resistant CCK-8 analog, (pGlu-Gln)-CCK-8, in normal mice. J Endocrinol. 2013;216:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Montgomery IA, Irwin N, Flatt PR. Beneficial effects of (pGlu-Gln)-CCK-8 on energy intake and metabolism in high fat fed mice are associated with alterations of hypothalamic gene expression. Horm Metab Res. 2013;45:471-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Irwin N, Frizelle P, O’Harte FP, Flatt PR. (pGlu-Gln)-CCK-8[mPEG]: a novel, long-acting, mini-PEGylated cholecystokinin (CCK) agonist that improves metabolic status in dietary-induced diabetes. Biochim Biophys Acta. 2013;1830:4009-4016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Gallwitz B, Witt M, Morys-Wortmann C, Fölsch UR, Schmidt WE. GLP-1/GIP chimeric peptides define the structural requirements for specific ligand-receptor interaction of GLP-1. Regul Pept. 1996;63:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Irwin N, Flatt PR. Evidence for beneficial effects of compromised gastric inhibitory polypeptide action in obesity-related diabetes and possible therapeutic implications. Diabetologia. 2009;52:1724-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Gough SC, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, Damgaard LH, Buse JB; NN9068-3697 (DUAL-I) trial investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885-893. [RCA] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 62. | Gallwitz B. Emerging DPP-4 inhibitors: focus on linagliptin for type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Pan CQ, Buxton JM, Yung SL, Tom I, Yang L, Chen H, MacDougall M, Bell A, Claus TH, Clairmont KB. Design of a long acting peptide functioning as both a glucagon-like peptide-1 receptor agonist and a glucagon receptor antagonist. J Biol Chem. 2006;281:12506-12515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, Du X, Petrov A, Lassman ME, Jiang G. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258-2266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 65. | Sinclair EM, Drucker DJ. Proglucagon-derived peptides: mechanisms of action and therapeutic potential. Physiology (Bethesda). 2005;20:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Fosgerau K, Jessen L, Lind Tolborg J, Østerlund T, Schæffer Larsen K, Rolsted K, Brorson M, Jelsing J, Skovlund Ryge Neerup T. The novel GLP-1-gastrin dual agonist, ZP3022, increases β-cell mass and prevents diabetes in db/db mice. Diabetes Obes Metab. 2013;15:62-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Irwin N, Hunter K, Montgomery IA, Flatt PR. Comparison of independent and combined metabolic effects of chronic treatment with (pGlu-Gln)-CCK-8 and long-acting GLP-1 and GIP mimetics in high fat-fed mice. Diabetes Obes Metab. 2013;15:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Trevaskis JL, Sun C, Athanacio J, D’Souza L, Samant M, Tatarkiewicz K, Griffin PS, Wittmer C, Wang Y, Teng CH. Synergistic metabolic benefits of an exenatide analogue and cholecystokinin in diet-induced obese and leptin-deficient rodents. Diabetes Obes Metab. 2015;17:61-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 69. | Irwin N, Pathak V, Flatt PR. A novel CCK-8/GLP-1 hybrid peptide exhibiting prominent insulinotropic, glucose-lowering and satiety actions with significant therapeutic potential in high-fat fed mice. Diabetes. 2015;64:2996-3009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Flatt PR. Dorothy Hodgkin Lecture 2008. Gastric inhibitory polypeptide (GIP) revisited: a new therapeutic target for obesity-diabetes? Diabet Med. 2008;25:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Knop FK, Taylor R. Mechanism of metabolic advantages after bariatric surgery: it’s all gastrointestinal factors versus it’s all food restriction. Diabetes Care. 2013;36 Suppl 2:S287-S291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Bhat VK, Kerr BD, Flatt PR, Gault VA. A novel GIP-oxyntomodulin hybrid peptide acting through GIP, glucagon and GLP-1 receptors exhibits weight reducing and anti-diabetic properties. Biochem Pharmacol. 2013;85:1655-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Bhat VK, Kerr BD, Vasu S, Flatt PR, Gault VA. A DPP-IV-resistant triple-acting agonist of GIP, GLP-1 and glucagon receptors with potent glucose-lowering and insulinotropic actions in high-fat-fed mice. Diabetologia. 2013;56:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, Chabenne J, Zhang L, Habegger KM, Fischer K. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 480] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 75. | Brown RJ, Rother KI. Effects of beta-cell rest on beta-cell function: a review of clinical and preclinical data. Pediatr Diabetes. 2008;9:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005;90:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 77. | Ritzel RA, Hansen JB, Veldhuis JD, Butler PC. Induction of beta-cell rest by a Kir6.2/SUR1-selective K(ATP)-channel opener preserves beta-cell insulin stores and insulin secretion in human islets cultured at high (11 mM) glucose. J Clin Endocrinol Metab. 2004;89:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Irwin N, Hunter K, Frizzell N, Flatt PR. Antidiabetic effects of sub-chronic activation of the GIP receptor alone and in combination with background exendin-4 therapy in high fat fed mice. Regul Pept. 2009;153:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Nasteska D, Harada N, Suzuki K, Yamane S, Hamasaki A, Joo E, Iwasaki K, Shibue K, Harada T, Inagaki N. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes. 2014;63:2332-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 80. | Pathak V, Vasu S, Gault VA, Flatt PR, Irwin N. Sequential induction of beta cell rest and stimulation using stable GIP inhibitor and GLP-1 mimetic peptides improves metabolic control in C57BL/KsJ db/db mice. Diabetologia. 2015;58:2144-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Ahmadieh H, Azar ST. The role of incretin-based therapies in prediabetes: a review. Prim Care Diabetes. 2014;8:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vänttinen M, Stancáková A, Jansson PA, Pellmé F, Holst JJ, Kuulasmaa T. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia. 2008;51:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Utzschneider KM, Tong J, Montgomery B, Udayasankar J, Gerchman F, Marcovina SM, Watson CE, Ligueros-Saylan MA, Foley JE, Holst JJ. The dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care. 2008;31:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 84. | Rosenstock J, Foley JE, Rendell M, Landin-Olsson M, Holst JJ, Deacon CF, Rochotte E, Baron MA. Effects of the dipeptidyl peptidase-IV inhibitor vildagliptin on incretin hormones, islet function, and postprandial glycemia in subjects with impaired glucose tolerance. Diabetes Care. 2008;31:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Bock G, Dalla Man C, Micheletto F, Basu R, Giesler PD, Laugen J, Deacon CF, Holst JJ, Toffolo G, Cobelli C. The effect of DPP-4 inhibition with sitagliptin on incretin secretion and on fasting and postprandial glucose turnover in subjects with impaired fasting glucose. Clin Endocrinol (Oxf). 2010;73:189-196. [PubMed] |
| 86. | Rosenstock J, Klaff LJ, Schwartz S, Northrup J, Holcombe JH, Wilhelm K, Trautmann M. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33:1173-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 87. | Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 791] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 88. | Aroda VR, Ratner R. The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: a review. Diabetes Metab Res Rev. 2011;27:528-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 89. | Mieczkowska A, Mansur S, Bouvard B, Flatt PR, Thorens B, Irwin N, Chappard D, Mabilleau G. Double incretin receptor knock-out (DIRKO) mice present with alterations of trabecular and cortical micromorphology and bone strength. Osteoporos Int. 2015;26:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Bollag RJ, Zhong Q, Phillips P, Min L, Zhong L, Cameron R, Mulloy AL, Rasmussen H, Qin F, Ding KH. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141:1228-35. [DOI] [Full Text] |
| 91. | Nissen A, Christensen M, Knop FK, Vilsbøll T, Holst JJ, Hartmann B. Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J Clin Endocrinol Metab. 2014;99:E2325-E2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 92. | Mabilleau G, Mieczkowska A, Irwin N, Simon Y, Audran M, Flatt PR, Chappard D. Beneficial effects of a N-terminally modified GIP agonist on tissue-level bone material properties. Bone. 2014;63:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y, Inagaki N. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 94. | Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011;11:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 95. | Sun HX, Lu N, Luo X, Zhao L, Liu JM. Liraglutide, the glucagon-like peptide-1 receptor agonist, has anabolic bone effects in diabetic Goto-Kakizaki rats. J Diabetes. 2015;7:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | Mieczkowska A, Irwin N, Flatt PR, Chappard D, Mabilleau G. Glucose-dependent insulinotropic polypeptide (GIP) receptor deletion leads to reduced bone strength and quality. Bone. 2013;56:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 97. | Gaudin-Audrain C, Irwin N, Mansur S, Flatt PR, Thorens B, Baslé M, Chappard D, Mabilleau G. Glucose-dependent insulinotropic polypeptide receptor deficiency leads to modifications of trabecular bone volume and quality in mice. Bone. 2013;53:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 98. | Mabilleau G, Mieczkowska A, Irwin N, Flatt PR, Chappard D. Optimal bone mechanical and material properties require a functional glucagon-like peptide-1 receptor. J Endocrinol. 2013;219:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 99. | Mabilleau G, Mieczkowska A, Chappard D. Use of glucagon-like peptide-1 receptor agonists and bone fractures: a meta-analysis of randomized clinical trials. J Diabetes. 2014;6:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 100. | Su B, Sheng H, Zhang M, Bu L, Yang P, Li L, Li F, Sheng C, Han Y, Qu S. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists’ treatment: a meta-analysis of randomized controlled trials. Endocrine. 2015;48:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 101. | Driessen JH, van Onzenoort HA, Henry RM, Lalmohamed A, van den Bergh JP, Neef C, Leufkens HG, de Vries F. Use of dipeptidyl peptidase-4 inhibitors for type 2 diabetes mellitus and risk of fracture. Bone. 2014;68:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Glorie L, Behets GJ, Baerts L, De Meester I, D’Haese PC, Verhulst A. DPP IV inhibitor treatment attenuates bone loss and improves mechanical bone strength in male diabetic rats. Am J Physiol Endocrinol Metab. 2014;307:E447-E455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 103. | Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133:2861-2870. [PubMed] |
| 104. | Hölscher C. New drug treatments show neuroprotective effects in Alzheimer’s and Parkinson’s diseases. Neural Regen Res. 2014;9:1870-1873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Faivre E, Gault VA, Thorens B, Hölscher C. Glucose-dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesis. J Neurophysiol. 2011;105:1574-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 106. | Ding KH, Zhong Q, Xie D, Chen HX, Della-Fera MA, Bollag RJ, Bollag WB, Gujral R, Kang B, Sridhar S. Effects of glucose-dependent insulinotropic peptide on behavior. Peptides. 2006;27:2750-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 107. | Gault VA, Hölscher C. Protease-resistant glucose-dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. J Neurophysiol. 2008;99:1590-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 108. | Porter DW, Irwin N, Flatt PR, Hölscher C, Gault VA. Prolonged GIP receptor activation improves cognitive function, hippocampal synaptic plasticity and glucose homeostasis in high-fat fed mice. Eur J Pharmacol. 2011;650:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 109. | Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res. 2009;205:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 110. | McClean PL, Hölscher C. Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology. 2014;86:241-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 111. | McGovern SF, Hunter K, Hölscher C. Effects of the glucagon-like polypeptide-1 analogue (Val8)GLP-1 on learning, progenitor cell proliferation and neurogenesis in the C57B/16 mouse brain. Brain Res. 2012;1473:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Kelly P, McClean PL, Ackermann M, Konerding MA, Hölscher C, Mitchell CA. Restoration of cerebral and systemic microvascular architecture in APP/PS1 transgenic mice following treatment with Liraglutide™. Microcirculation. 2015;22:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | Gault VA, Lennox R, Flatt PR. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab. 2015;17:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 114. | Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968-2978. [PubMed] |
| 115. | Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 1055] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 116. | Vila Petroff MG, Egan JM, Wang X, Sollott SJ. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res. 2001;89:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 117. | Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, Parker TG, Huang Q, Drucker DJ, Husain M. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 118. | Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 454] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 119. | Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, Sjöholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209-E1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 506] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 120. | Avogaro A, Vigili de Kreutzenberg S, Fadini GP. Cardiovascular actions of GLP-1 and incretin-based pharmacotherapy. Curr Diab Rep. 2014;14:483. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 121. | Ding KH, Zhong Q, Xu J, Isales CM. Glucose-dependent insulinotropic peptide: differential effects on hepatic artery vs. portal vein endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E773-E779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 122. | Wu S, Hopper I, Skiba M, Krum H. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: meta-analysis of randomized clinical trials with 55,141 participants. Cardiovasc Ther. 2014;32:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |