Published online Oct 25, 2015. doi: 10.4239/wjd.v6.i14.1259
Peer-review started: June 11, 2015
First decision: August 16, 2015
Revised: August 25, 2015
Accepted: September 25, 2015
Article in press: September 28, 2015
Published online: October 25, 2015
Processing time: 137 Days and 11.9 Hours
Erythropoietin (EPO) is a 30.4 kDa growth factor and cytokine that governs cell proliferation, immune modulation, metabolic homeostasis, vascular function, and cytoprotection. EPO is under investigation for the treatment of variety of diseases, but appears especially suited for the treatment of disorders of metabolism that include diabetes mellitus (DM). DM and the complications of this disease impact a significant portion of the global population leading to disability and death with currently limited therapeutic options. In addition to its utility for the treatment of anemia, EPO can improve cardiac function, reduce fatigue, and improve cognition in patients with DM as well as regulate cellular energy metabolism, obesity, tissue repair and regeneration, apoptosis, and autophagy in experimental models of DM. Yet, EPO can have adverse effects that involve the vasculature system and unchecked cellular proliferation. Critical to the cytoprotective capacity and the potential for a positive clinical outcome with EPO are the control of signal transduction pathways that include protein kinase B, the mechanistic target of rapamycin, Wnt signaling, mammalian forkhead transcription factors of the O class, silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae), and AMP activated protein kinase. Therapeutic strategies that can specifically target and control EPO and its signaling pathways hold great promise for the development of new and effective clinical treatments for DM and the complications of this disorder.
Core tip: Erythropoietin and the downstream signaling pathways of this cytokine that include protein kinase B, mechanistic target of rapamycin, Wnt signaling, Factors of the O class proteins, silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae), and AMP activated protein kinase offer new avenues for the development of novel treatments for diabetes mellitus and the complications of this disease.
- Citation: Maiese K. Erythropoietin and diabetes mellitus. World J Diabetes 2015; 6(14): 1259-1273
- URL: https://www.wjgnet.com/1948-9358/full/v6/i14/1259.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i14.1259
The concept of circulating agents that travel throughout the body may have initially originated from Ernest Starling[1]. In 1905 at the Royal College of Surgeons, Sterling introduced the term “hormones”, a term with Greek origins meaning to “excite” or “arouse”, to depict the action of chemicals that are dispersed in the body and can target specific organs. Earlier work prior to the presentation by Sterling also described processes that could come under the description as being defined as “hormonal”. Claude Bernard described the chemical release of glucose that was processed from glycogen in the liver[2]. Arnold Adolphe Berthold, another pioneer, also described messenger signals that could communicate among the different bodily organs[3].
Interestingly, almost as a counterpart to the discussions provided by Starling, Carnot et al[4] in 1906 presented the agent “hemopoietine”. This agent was detected in the blood of rabbits after prompted by bleeding that led to the production of immature erythrocytes in untreated rabbits. Subsequent work by other investigators also showed that bled animals could result in prominent reticulocytosis in the plasma[5-7]. Later, the agent responsible for reticulocytosis was termed erythropoietin (EPO). EPO was linked to depressed oxygen levels and was shown to increase hemoglobin levels in parabiotic rat experiments when one of the two rats experienced hypoxia[8]. Subsequently, purification of the EPO protein in humans was achieved and cloning of the EPO gene fostered recombinant EPO (rhEPO) production for clinical treatments[9,10].
EPO is located on chromosome 7 and is a single copy in a 5.4 kb region of the genomic DNA[11]. The EPO gene encodes for a polypeptide chain that has initially 193 amino acids. A 27 amino acid hydrophobic secretory leader at the amino-terminal to result in a 166 amino acid peptide in the EPO protein is then cleaved[12]. Additional post-translational processing occurs with the removal of a carboxy-terminal arginine166 in the mature human and rhEPO to lead to a protein of 30.4 kDa with 165 amino acids[13-16].
EPO has four glycosylated chains that include three N-linked and one O-linked acidic oligosaccharide side chains[17]. The N-linked glycosylation sites are at aspartate24, aspartate38, and aspartate83 and the O-linked glycosylation site is at serine126. Both the production and secretion of the mature EPO protein is dependent upon N- and O-linked chain integrity[18]. Replacement of asparagine38 and asparagine83 by glutamate or the replacement of serine126 by glycine can impair EPO production and secretion[19].
Several factors determine the biological activity of EPO[20]. The two disulfide bonds formed between cysteine7 and cysteine160 as well as cysteine29 and cysteine33 control the function of EPO[21]. EPO biological activity is lost with reduction of these disulfide bonds and with alkylation of the sulfhydryl groups. Almost 85% of EPO biological activity is restored with re-oxidization of EPO after reduction by guanidine[22]. In addition, EPO biological activity is maintained by the by the glycosylated chains[23] and EPO stability is fostered by the carbohydrate chains[24]. Free radical degradation of EPO is limited by both the glycosylated chains[23] and the oligosaccharides[25].
Currently, erythropoiesis-stimulating agents including EPO are approved for the treatment of anemia that results from chronic kidney failure, chemotherapy, human immunodeficiency virus, and to limit the number of blood transfusions for surgery[21,26]. The principal source for the production and secretion of EPO are the kidney peritubular interstitial cells[27]. Other organs that include the brain, uterus, and liver are also responsible for EPO production and secretion[17,27-30]. Expression of EPO is controlled by changes in oxygen tension and not by the concentration of red blood cells[28,31,32]. Hypoxia-inducible factor 1 (HIF-1) can control EPO expression and the EPO receptor (EPOR) to increase the production of EPO[11,28,33,34]. EPO and EPOR gene transcription occurs following HIF-1 activation. This gene transcription is governed by the transcription enhancer region in the 3’-flanking region of the EPO gene that binds to HIF-1[11,14]. HIF-1 also can foster pathways that provide cellular protection against injury[35-37]. Of note, EPO also can be generated from stimuli that may not directly involve hypoxia. During maturation of the brain that may be exposed to various toxic elements, EPO blood levels may be elevated and associated with greater disability[38]. Elevated EPO serum concentrations have been reported following xenon anesthesia in cardiac surgery[39]. Agents that decrease inflammation in cerebral microglia have been recently shown to lead to the release of EPO[40] and infection with malaria can result in significant serum levels of EPO[41]. Under some conditions during chronic hyperglycemia in adults, EPO levels may be depressed[42]. Conversely, EPO in the amniotic fluid of diabetic patients can be elevated and be suggestive of perinatal complications[43]. Furthermore, trophic factors such as insulin can stimulate EPO production in specific cells such as astrocytes[44].
As a cytoprotective agent, EPO promotes cellular survival, at least in part, through the control of oxidative stress mediated cell injury[45,46]. Reactive oxygen species (ROS) are released during oxidative stress[47]. This in turn can cause mitochondrial injury, DNA damage, and protein misfolding[48-52].
Following the generation of ROS, cell death pathways of programmed cell death can ultimately determine cell survival[53-62]. Two particular pathways of programmed cell death involve autophagy[50,63-65] and apoptosis[15,55,57,66,67]. EPO prevents autophagic cell injury in glomerular mesangial cells during lipopolysaccharide exposure[68]. Administration of EPO also limits excessive autophagy that precedes apoptosis during experimental neonatal necrotizing enterocolitis[69]. During hyperoxia exposure and oxygen toxicity to the developing rodent brain, EPO has been shown to modify the activity of autophagy and limit neonatal brain damage[70].
In regards to apoptotic cell death, EPO prevents apoptotic injury during oxidative stress in endothelial progenitor cells[71] and attenuates neuroinflammation that can result in apoptosis[72]. EPO can assist with erythroid differentiation and prevent cellular apoptosis[73] as well as promote ventricular-subventricular zone neurogenesis and oligodendrogenesis[74]. Derivatives of EPO, such as glutaraldehyde-EPO, can protect renal cells from apoptosis during ischemia/re-perfusion injury and oxidative stress[75]. Administration of EPO also can block apoptotic cell death during neuronal kainate-induced oxidative stress[76], wound injury[77], vascular oxygen-glucose deprivation[78-80], loss of protective zinc finger transcription factors[81], anoxia[82-84], astroglial glutamate toxicity[85], beta-amyloid (Aβ) toxicity[86-90], renal adriamycin-induced nephropathy[91], ischemic brain injury[92], and multi-organ dysfunction induced by thermal injury[93]. In addition, EPO is protective against retinal disease[94], sepsis[95,96], advanced glycation endproducts (AGEs) exposure in Schwann cells[97], elevated glucose[78,98-102], free radicals[103-108], and toxins that lead to microglial injury[30,40,90,94,109].
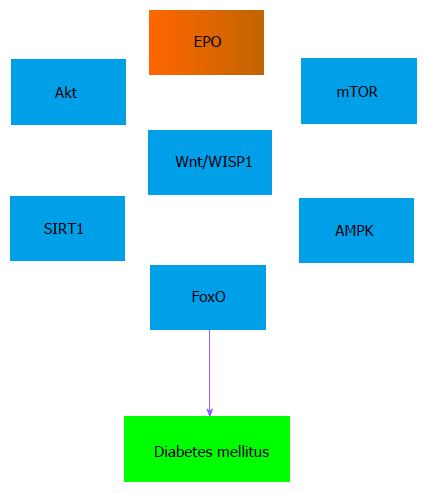
EPO cytoprotection is tied to a number of cell pathways[3]. In particular, phosphoinositide 3-kinase (PI 3-K) and protein kinase B (Akt) can lead to increased cellular survival with EPO (Figure 1). PI 3-K phosphorylates membrane lipids and controls Akt transition from the cytosol to the plasma membrane. Phosphorylation of Akt occurs at serine473 and threonine308 by phosphoinositide dependent kinase (PDK) PDK1 and PDK2[110-112]. EPO leads to Akt phosphorylation on serine473 to activate this kinase. EPO uses the Akt pathway to protect against autophagy and apoptosis injury in gastrointestinal disease[69], maintain vascular integrity and reduce inflammation[113], limit Aβ toxicity in microglia and neurons[90,114-116], reduce injury from sepsis[95,117], increase survival in cardiomyocytes during cardiac hypoxic/re-oxygenation injury[118], and block oxidative stress injury[78,82,104,105,119-122]. Akt in conjunction with EPO also improves the function of cells. For example, EPO activates Akt to increase the adhesive properties of endothelial cells and improve the vasculogenic potential of peripheral blood mononuclear cells[123].
The mechanistic target of rapamycin (mTOR) is closely linked to PI 3-K and Akt[124] (Figure 1). mTOR is a 289-kDa serine/threonine protein kinase that is encoded by a single gene FRAP1[124,125]. mTOR is important for the function of mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2)[126-129]. Neurons are protected against sepsis during exposure to EPO and activation of mTOR[95]. EPO prevents microglial cell injury through mTOR activation during oxidative stress[109] and Aβ toxicity[90]. During oxygen-glucose exposure in neurons, EPO affects multiple pathways of mTOR signaling[130] to include Akt and proline rich Akt substrate 40 kDa (PRAS40) to increase neuronal survival[79]. EPO and mTOR are required for the differentiation of neural precursor cells[131] and to control bone homeostasis with osteoblastogenesis and osteoclastogenesis[132]. EPO through mTOR can mediate resistance to hypoxia and oxidative stress in retinal progenitor cells[133] and also protect against increased activity of autophagy in epithelial cells[69]. Activation of mTOR prevents the induction of autophagy by phosphorylating autophagic related genes (Atg) and proteins that include Atg13 and ULKs to inhibit the UNC like kinase complex ULK-Atg13-FIP200[128]. Under some conditions, the concentration of EPO and activity of mTOR may be important for the degree of cellular protection that can be achieved. Elevated concentrations of EPO have been reported to lead to decreased phosphorylation and activity of mTOR with increased apoptotic cell death[134]. Increased mTOR activity also is tied to tumor cell growth[135-138].
Closely associated to the protective pathways of Akt and mTOR are the wingless pathways of Wnt proteins[139] (Figure 1). Crosstalk occurs among Wnt signaling pathways, Akt, and mTOR[140] to foster cellular survival during Aβ toxicity[141,142], reduce cerebral ischemia[143,144], promote progenitor cell activation during intestinal inflammation[145], prevent neuronal cell loss[146], limit 6-hydroxydopamine toxicity[147], enhance microglial and macrophage survival and function[148,149], and increase tissue fibrosis[150]. EPO employs the Wnt pathway to lead to cellular protection. During renal ischemia and reperfusion, EPO limits tubular cell apoptosis by increasing the expression of Wnt7b and β-catenin as well as by down-regulating specific micro-RNAs (miRNA)[151,152]. Through Wnt1, EPO protects against elevated glucose exposure in cerebral endothelial cells and maintains the expression of Wnt1[100]. In addition, EPO uses Wnt signaling to prevent immune cell loss during oxidative stress[109], prevent Aβ toxicity in microglia[90], limit the activity of forkhead transcription factors that result in apoptosis[99,153], and maintain the survival of mesenchymal stem cells[154]. Of note, both EPO and the pathways of Wnt signaling are proliferative in nature and have the potential to lead to tumorigenesis. For example, prolonged exposure of growth factors such as EPO that rely upon Wnt signaling can result in inflammation, blood-brain barrier injury[155], and tumor growth[156-158].
Cellular protection with EPO that relies upon Wnt signaling also can be associated with the modulation of mammalian forkhead transcription factors[159]. Mammalian FOXO proteins are assigned to the O class of the forkhead box class transcription factors[160,161] (Figure 1). These transcription factors consist of FOXO1, FOXO3, FOXO4, and FOXO6 and exist throughout the body[162]. FoxO proteins can impact cellular survival[163] and are homologous to DAuer Formation-16 (DAF-16), a transcription factor in Caenorhabditis elegans, that leads to lifespan extension and affects insulin signaling[164,165]. Under many circumstances, the activation of FoxO proteins results in apoptotic cell death[153]. FoxO3a expression increases in the hippocampus during cerebral ischemia[166] and FoxO3a may lead to cell cycle induction that can promote neuronal apoptotic cell death[167]. Loss of FoxO3a expression and prevention of nuclear shuttling of FoxO3a in microglial cells and neurons results in increased survival during oxidative stress[146,148]. Inhibitory phosphorylation of FoxO3a and the nuclear export of FoxO3a during periods of elevated glucose also protects vascular cells[80,99,168,169] and neuronal cells[170].
In endothelial cells, EPO uses Wnt1 to block FoxO3a activity and maintain cerebral endothelial survival during elevated glucose[99]. Without Wnt signaling, EPO also has been shown to phosphorylate FoxO3a and lead to its inactivation to block apoptosis in neuronal cells[73]. EPO can prevent endothelial cell injury during oxygen-glucose deprivation by preventing FoxO3a nuclear subcellular trafficking that would lead to “pro-apoptotic” protein transcription and translation[20,80]. EPO can oversee stem cell proliferation through FoxO protein regulation. Through the control of FoxO3a activity, EPO promotes the development of erythroid progenitor cells[57,73,171,172].
FoxO protein activity is controlled by post-translation protein modifications that involve phosphorylation, ubiquitylation, and acetylation[162,173]. In regards to acetylation, FoxO proteins are deacetylated by histone deacetylases that includes the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1)[54] (Figure 1). SIRT1 deacetylation of FoxO proteins can influence autophagic pathways such that glucose deprivation leads to increases in autophagic flux that maintain left ventricular function during periods of starvation[174]. SIRT1 may be required to promote cortical bone formation with osteoblast progenitors by deacetylation of FoxOs and preventing FoxO protein binding to β-catenin to inhibit Wnt signaling[175]. However, the degree of SIRT1 expression in relation to FoxO protein activity may be a significant determinant for cellular survival[160,161]. For example, during exercise a controlled up-regulation of FoxO3a and SIRT1 expression in cardiac tissue may be important to improve cell survival[176]. During oxidative stress, cell injury may be reduced with catalase expression regulated by FoxO1a expression and SIRT1 levels less than 7.5-fold. However, decreased cardiac function and apoptotic cell death in cardiomyocytes can ensue with elevated SIRT1 levels of 12.5-fold[177]. FoxO proteins, such as FoxO1, also can control SIRT1 transcription and increase SIRT1 expression[178]. Under some circumstances, SIRT1 and FoxO proteins may function synergistically to promote cell survival. Loss of the forkhead transcription factors FoxO1 and FoxO3 in combination with decreased SIRT1 activity during oxidative stress leads to a reduction in autophagy with chondrocyte cell death, demonstrating that SIRT1 with FoxO proteins may be required for cellular protection[179]. SIRT1 also has been shown to increase lifespan in higher organisms and offer protection against oxidative stress[180]. EPO relies upon SIRT1 activity to prevent cell injury during oxidative stress and elevated glucose[181]. EPO can raise cellular activity of SIRT1 and promote the subcellular trafficking of SIRT1 to the nucleus to protect endothelial cells during oxidative stress[80]. EPO is able to maintain adipose cell energy homeostasis and protect against metabolic disorders through SIRT1[101]. Pathways that involve Wnt signaling with the CCN family member Wnt1 inducible signaling pathway protein 1 (WISP1)[139] also require up-regulation of SIRT1 activity to block apoptotic pathways controlled by FoxO proteins[182] (Figure 1). WISP1 can increase neuronal survival by limiting FoxO3a activity and FoxO3a deacetylation, blocking caspase 1 and 3 activation, and promoting SIRT1 activity and trafficking to the cell nucleus[146].
Growth factors such as EPO offer potentially new treatment approaches for numerous disorders, but given the signal transduction pathways that are regulated by EPO, this agent provides exciting prospects for the treatment of diabetes mellitus (DM)[16,45]. DM affects at least 350 million individuals worldwide[182] and is increasing in incidence[183]. Of potentially greater concern are the numbers of undiagnosed individuals that just in the United States alone may exceed 8 million individuals who are believed to suffer from metabolic disorders[32,184,185]. DM can affect the entire body and involve the immune system[63,77,181,186-190], liver[55,191-196], musculoskeletal function[197-201], kidney[202-206], and cardiovascular system[163,188,207-213] to result in endothelial cell dysfunction[15,16,99,100,168,214,215] and atherosclerosis[45,67,199,216]. These disorders can easily affect other regions of the body such as the nervous system to lead to cognitive loss[14,217-219], visual deterioration[32,119,220,221], peripheral nerve disease[55], and ischemic disease of the brain[23,49,67,222-224].
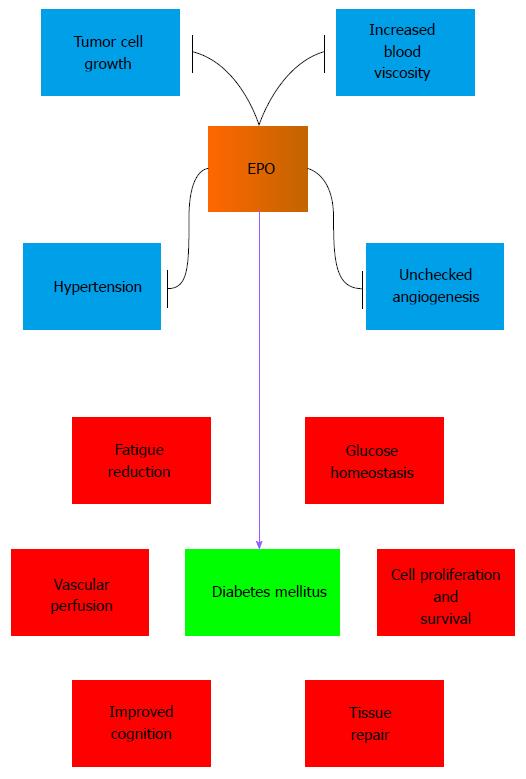
EPO as well as its downstream pathways have been shown to have a high potential to treat multiple complications of DM[32] (Figure 2). In earlier work that examined diabetics and non-diabetics with severe congestive heart failure, EPO increased left ventricular ejection fraction, reduced fatigue, and lessened duration of hospital stay[225]. In patients with Type 1 DM and cognitive impairment related to hypoglycemia, administration of EPO leads to improvement in complex reaction time task assessing associated with attention and working memory[226]. EPO also could provide a small improvement to treat fatigue in patients with Type 2 DM and chronic kidney disease[227].
In experimental models of DM, EPO can reduce blood glucose levels in animal models of DM and obesity[228], protect against the detrimental effects of obesity in animal models[16], treat diabetic peripheral neuropathy[229], and block apoptosis in Schwann cells mediated by AGEs[97]. EPO has been shown to limit high glucose-induced oxidative stress in renal tubular cells[230], control cellular mitochondrial function[76,80,103,109,118], and maintain energy metabolism[15]. Through anti-inflammatory mechanisms and the blockade of apoptosis, EPO can protect pancreatic islet cells in models of type 1 DM and Type 2 DM[98]. Intravitreal administration of EPO in rodent models of DM can normalize gene expression that can lead to apoptotic and inflammatory cell death[231]. EPO is cardioprotective in DM models with the inhibition of glycogen synthase kinase -3β (GSK-3β)[232] that can limit Wnt signaling pathways[233]. Through increased angiogenesis and decreased apoptotic cell death, EPO can improve wound healing and wound closure in diabetic mice[77,234]. In vascular disease, EPO has been reported to protect the neuroglialvascular unit in a model of retinal neurodegeneration and secondary vasoregression[119]. EPO can directly protect against endothelial cell apoptosis during elevated glucose through activation of Wnt1[100] and the inhibition of GSK-3β and FoxO3a[99]. Improvement in vascular perfusion by EPO[123] also may afford indirect protection to assist with cognitive repair[235] and decrease peripheral nerve injury during DM[102].
Not all studies demonstrate a beneficial effect with EPO during DM, suggesting that focus upon the downstream signaling pathways of EPO with mTOR, Wnt signaling, FoxO proteins, and SIRT1 may yield greater utility for some clinical populations with complications of DM. In patients with DM and renal disease, EPO administration results in a two-fold increase in stroke that is not attributed to any baseline characteristic or to blood pressure, hemoglobin, platelet count, or treatment dose of EPO[236]. In mice that overexpress EPO, blood viscosity has been reported to be increased with a reduction in cerebral blood flow[237]. As a result, EPO may increase the risk for stroke through increased blood viscosity. Although systemic administration of EPO may block retinopathy in animal models[94], elevated EPO concentrations in patients with DM also may lead to proliferative diabetic retinopathy[238] that could be associated with excessive vascular growth. EPO can increase vascular responsiveness[239] and may lead to hypertension[26,57,240]. Sustained erythrocytosis with agents such as EPO may result in the activation of inflammatory pathways and blood-brain barrier dysfunction[155]. As a proliferative agent, EPO also can lead to new tumor growth as well as foster the progression of existing tumors[156-158,241].
The potential adverse effects of EPO may be avoided by targeting more specific pathways controlled by EPO such as mTOR and AMP activated protein kinase (AMPK)[40,208] (Figure 2). AMPK oversees the activity of the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex that is an inhibitor of mTORC1[135]. Metformin, an agent that controls hyperglycemia in DM, can reduce cardiomyopathy in experimental models of DM through AMPK activation[242]. EPO as well may dependent upon AMPK to promote antioxidant gene expression[243]. Furthermore, other EPO signaling pathways play a role in controlling AMPK. AMPK can increase nicotinamide phosphoribosyltransferase levels during glucose limitation resulting in elevated nicotinamide adenine dinucleotide[244] and lower levels of the SIRT1 inhibitor nicotinamide[245]. SIRT1 and AMPK activation promotes autophagy that offers endothelial cell protection during exposure to oxidized low density lipoproteins that can lead to atherosclerosis[246]. WISP1, a component of Wnt signaling, also controls the post-translational phosphorylation of AMPK that is involved in glucose homeostasis[124,247-249]. WISP1 regulates AMPK activation by decreasing phosphorylation of TSC2 at serine1387, a target of AMPK, and increasing phosphorylation of TSC2 at threonine1462, a target of Akt[142]. The ability of WISP1 to modulate AMPK activity is vital for the regulation of cellular metabolism during DM[249]. AMPK activity is able to reduce insulin resistance and lessen oxidative stress through activation of autophagy[200]. AMPK can prevent myocardial ischemia in experimental models of DM[250], assist with proper metabolic function of cells[251], and limit adipocyte differentiation, lipid accumulation, and obesity[252]. Yet, similar to SIRT1, the degree of AMPK activity is a significant consideration in DM. AMPK activation can lead to apoptosis in pancreatic islet cells in some experimental models of Type 2 DM[253].
In the global population, DM is a significant cause of disability and death. Treatment options to limit the onset and progression of this disease are insufficient and warrant the development of novel treatments. EPO, as a cytoprotective agent that controls a broad array of signal transduction pathways offers exceptional promise for the treatment of DM and pathways of oxidative stress. EPO has been shown in diabetic patients to improve cardiac function, reduce fatigue, and improve cognition. In experimental models of DM, EPO can reduce blood glucose levels, limit peripheral neuropathy, maintain mitochondrial function and energy metabolism, and block programmed cell death in many cell types such as Schwann cells, endothelial cells, neurons, pancreatic islet cells, and cardiomyocytes.
However, several challenges exist to move EPO forward as an effective treatment for DM. EPO has been reported to increase the risk of stroke in patients with DM and renal disease and has been demonstrated to increase blood viscosity in animal studies. EPO may be contraindicated in hypertensive patients and may contribute to elevated mean arterial blood pressure. Elevated concentrations of EPO have been linked to proliferative diabetic retinopathy that may be associated with excessive microvascular angiogenesis. Finally, EPO, as a growth factor and proliferative agent, may lead to new tumor growth and also promote the growth of existing tumors, especially in the treatment of patients with cancer and anemia.
Further investigations that assess the protective capacity of EPO and limit any potential detrimental clinical outcomes are warranted. New work has been directed to improving the molecular stability, solubility, and immunogenicity of EPO for improved therapeutic strategies to treat the complications of DM. Glycoengineering, a method that introduces N-linked glycosylation consensus sequences into proteins to increase serum half-life and biological activity, has been examined for EPO[254]. Darbepoetin alpha is one such example of a hyperglycosylated EPO derivative. Darbepoetin alpha has an increased serum half-life when compared to recombinant EPO[255] and is considered more potent than recombinant EPO[256]. EPO mimetic proteins are other avenues being pursued that can be used to activate the EPOR, potentially increase treatment half-life and maintain potency when compared to EPO, and lessen immunogenicity[257,258]. For example, CNTO 530 has been shown to increase reticulocytes, red blood cells and total hemoglobin in β- thalassemic mice[259].
A promising investigative course also could target the downstream signaling pathways of EPO that include Akt, mTOR, Wnt signaling, FoxO proteins, SIRT1, and AMPK. EPO employs Akt and mTOR for stem cell maintenance and differentiation, resistance against oxidative stress, and the regulation of autophagy. In experimental models of DM, EPO relies upon Wnt signaling, β-catenin, and the inhibition of GSK-3β to block apoptotic cell death. EPO also governs FoxO proteins and SIRT1 to protect against DM apoptotic vascular injury, maintain adipose cell energy homeostasis, and modulate autophagic flux to improve cardiac function during metabolic disturbances. Pathways that involve EPO and AMPK also offer interesting targets to maximize clinical efficacy and minimize unwanted side effects. AMPK reduces insulin resistance and lessens oxidative stress through activation of autophagy, prevents myocardial ischemia in models of DM, and limits adipocyte lipid accumulation and obesity. WISP1 controls AMPK activity for the regulation of cellular metabolism during DM. In addition, SIRT1 and AMPK in conjunction with SIRT1 can increase autophagy activity to provide endothelial cell protection during exposure to oxidized low-density lipoproteins. However, it should be noted that consideration of these pathways may still require use of EPO or an EPO analogue since therapeutic success may be dependent on modulation of more than one of these down-stream pathways of EPO. In addition, one needs to emphasize that each of these pathways also can lead to undesirable biological outcomes under some circumstances such as tumorigenesis, pancreatic islet cell death, and cardiac dysfunction. Carefully targeting future investigations for EPO and its relevant signal transduction pathways for specific clinical disturbances of DM should offer the greatest promise for novel therapeutic strategies.
P- Reviewer: Chen XZ, Fan YX, Vlachopanos G, Zhang H S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Maiese K, Chong ZZ, Shang YC, Wang S. Erythropoietin: new directions for the nervous system. Int J Mol Sci. 2012;13:11102-11129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Bernard C; Remarques sur le sécrétion du sucre dans la foie, faitesàl’occasion de la communication de m lehman. Comptes rendus Academies de Sciences. 1855;40:589-592. |
| 3. | Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85:194-213. [PubMed] |
| 4. | Carnot P, DeFlandre C. Sur l’activite hemopoietique de serum au cours de la regeneration du sang. C R Acad Sci (Paris). 1906;143:384-386. |
| 5. | Erslev AJ. In vitro production of erythropoietin by kidneys perfused with a serum-free solution. Blood. 1974;44:77-85. [PubMed] |
| 6. | Gibelli C. Uber den wert des serums anamisch gemachten tiere bei der regeneration des blutes. Arch Exp Pathol Pharmacol. 1911;65:284-302. |
| 7. | Sandor G. Uber die blutbidende wirkung des serums von tieren, die in verdunnter luft gehalten wuren. Z Gesante Exp Med. 1932;82:633-646. |
| 8. | Reissmann KR. Studies on the mechanism of erythropoietic stimulation in parabiotic rats during hypoxia. Blood. 1950;5:372-380. [PubMed] |
| 9. | Jacobs K, Shoemaker C, Rudersdorf R, Neill SD, Kaufman RJ, Mufson A, Seehra J, Jones SS, Hewick R, Fritsch EF. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313:806-810. [PubMed] |
| 10. | Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, Chen KK, Fox GM, Martin F, Stabinsky Z. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci USA. 1985;82:7580-7584. [PubMed] |
| 11. | Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 12. | Imai N, Kawamura A, Higuchi M, Oh-eda M, Orita T, Kawaguchi T, Ochi N. Physicochemical and biological comparison of recombinant human erythropoietin with human urinary erythropoietin. J Biochem. 1990;107:352-359. [PubMed] |
| 13. | Castaneda-Arellano R, Beas-Zarate C, Feria-Velasco AI, Bitar-Alatorre EW, Rivera-Cervantes MC. From neurogenesis to neuroprotection in the epilepsy: signalling by erythropoietin. Front Biosci (Landmark Ed). 2014;19:1445-1455. [PubMed] |
| 14. | Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19:145-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Wang L, Di L, Noguchi CT. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci. 2014;10:921-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Wang L, Dey S, Alnaeeli M, Suresh S, Rogers H, Teng R, Noguchi CT. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci. 2014;15:10296-10333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577-583. [PubMed] |
| 19. | Dubé S, Fisher JW, Powell JS. Glycosylation at specific sites of erythropoietin is essential for biosynthesis, secretion, and biological function. J Biol Chem. 1988;263:17516-17521. [PubMed] |
| 20. | Maiese K, Hou J, Chong ZZ, Shang YC. Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology. ScientificWorldJournal. 2009;9:1072-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Li F, Chong ZZ, Maiese K. Erythropoietin on a tightrope: balancing neuronal and vascular protection between intrinsic and extrinsic pathways. Neurosignals. 2004;13:265-289. [PubMed] |
| 22. | Wang FF, Kung CK, Goldwasser E. Some chemical properties of human erythropoietin. Endocrinology. 1985;116:2286-2292. [PubMed] |
| 23. | Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5:125-142. [PubMed] |
| 24. | Toyoda T, Itai T, Arakawa T, Aoki KH, Yamaguchi H. Stabilization of human recombinant erythropoietin through interactions with the highly branched N-glycans. J Biochem. 2000;128:731-737. [PubMed] |
| 25. | Uchida E, Morimoto K, Kawasaki N, Izaki Y, Abdu Said A, Hayakawa T. Effect of active oxygen radicals on protein and carbohydrate moieties of recombinant human erythropoietin. Free Radic Res. 1997;27:311-323. [PubMed] |
| 26. | Palazzuoli A, Ruocco G, Pellegrini M, De Gori C, Del Castillo G, Giordano N, Nuti R. The role of erythropoietin stimulating agents in anemic patients with heart failure: solved and unresolved questions. Ther Clin Risk Manag. 2014;10:641-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Moore EM, Bellomo R, Nichol AD. Erythropoietin as a novel brain and kidney protective agent. Anaesth Intensive Care. 2011;39:356-372. [PubMed] |
| 28. | Caprara C, Grimm C. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 2012;31:89-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002;11:863-871. [PubMed] |
| 30. | Kato S, Aoyama M, Kakita H, Hida H, Kato I, Ito T, Goto T, Hussein MH, Sawamoto K, Togari H. Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury. J Neurosci Res. 2011;89:1566-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Maiese K. Triple play: promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62:218-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Maiese K. Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural Regen Res. 2015;10:518-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Guven Bagla A, Ercan E, Asgun HF, Ickin M, Ercan F, Yavuz O, Bagla S, Kaplan A. Experimental acute myocardial infarction in rats: HIF-1α, caspase-3, erythropoietin and erythropoietin receptor expression and the cardioprotective effects of two different erythropoietin doses. Acta Histochem. 2013;115:658-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Nishimura K, Tokida M, Katsuyama H, Nakagawa H, Matsuo S. The effect of hemin-induced oxidative stress on erythropoietin production in HepG2 cells. Cell Biol Int. 2014;38:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Ali AA, Coulter JA, Ogle CH, Migaud MM, Hirst DG, Robson T, McCarthy HO. The contribution of N2O3 to the cytotoxicity of the nitric oxide donor DETA/NO: an emerging role for S-nitrosylation. Biosci Rep. 2013;33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Deng A, Arndt MA, Satriano J, Singh P, Rieg T, Thomson S, Tang T, Blantz RC. Renal protection in chronic kidney disease: hypoxia-inducible factor activation vs. angiotensin II blockade. Am J Physiol Renal Physiol. 2010;299:F1365-F1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Singh N, Sharma G, Mishra V. Hypoxia inducible factor-1: its potential role in cerebral ischemia. Cell Mol Neurobiol. 2012;32:491-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Korzeniewski SJ, Allred E, Logan JW, Fichorova RN, Engelke S, Kuban KC, O’Shea TM, Paneth N, Holm M, Dammann O. Elevated endogenous erythropoietin concentrations are associated with increased risk of brain damage in extremely preterm neonates. PLoS One. 2015;10:e0115083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Stoppe C, Coburn M, Fahlenkamp A, Ney J, Kraemer S, Rossaint R, Goetzenich A. Elevated serum concentrations of erythropoietin after xenon anaesthesia in cardiac surgery: secondary analysis of a randomized controlled trial. Br J Anaesth. 2015;114:701-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Tsai CF, Kuo YH, Yeh WL, Wu CY, Lin HY, Lai SW, Liu YS, Wu LH, Lu JK, Lu DY. Regulatory effects of caffeic acid phenethyl ester on neuroinflammation in microglial cells. Int J Mol Sci. 2015;16:5572-5589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Díez-Padrisa N, Aguilar R, Machevo S, Morais L, Nhampossa T, O’Callaghan-Gordo C, Nhalungo D, Menéndez C, Roca A, Alonso PL. Erythropoietin levels are not independently associated with malaria-attributable severe disease in Mozambican children. PLoS One. 2011;6:e24090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Symeonidis A, Kouraklis-Symeonidis A, Psiroyiannis A, Leotsinidis M, Kyriazopoulou V, Vassilakos P, Vagenakis A, Zoumbos N. Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus. Ann Hematol. 2006;85:79-85. [PubMed] |
| 43. | Teramo K, Kari MA, Eronen M, Markkanen H, Hiilesmaa V. High amniotic fluid erythropoietin levels are associated with an increased frequency of fetal and neonatal morbidity in type 1 diabetic pregnancies. Diabetologia. 2004;47:1695-1703. [PubMed] |
| 44. | Masuda S, Chikuma M, Sasaki R. Insulin-like growth factors and insulin stimulate erythropoietin production in primary cultured astrocytes. Brain Res. 1997;746:63-70. [PubMed] |
| 45. | Maiese K. mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015;6:217-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Rjiba-Touati K, Ayed-Boussema I, Guedri Y, Achour A, Bacha H, Abid-Essefi S. Effect of recombinant human erythropoietin on mitomycin C-induced oxidative stress and genotoxicity in rat kidney and heart tissues. Hum Exp Toxicol. 2015;pii:Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Maiese K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2015;2015:875961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 48. | Harish G, Mahadevan A, Pruthi N, Sreenivasamurthy SK, Puttamallesh VN, Keshava Prasad TS, Shankar SK, Srinivas Bharath MM. Characterization of traumatic brain injury in human brains reveals distinct cellular and molecular changes in contusion and pericontusion. J Neurochem. 2015;134:156-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Maiese K. SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015;7:235-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant stress and signal transduction in the nervous system with the PI 3-K, Akt, and mTOR cascade. Int J Mol Sci. 2012;13:13830-13866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Palma HE, Wolkmer P, Gallio M, Corrêa MM, Schmatz R, Thomé GR, Pereira LB, Castro VS, Pereira AB, Bueno A. Oxidative stress parameters in blood, liver, and kidney of diabetic rats treated with curcumin and/or insulin. Mol Cell Biochem. 2014;386:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, Harris DA, Abraham CR. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem. 2014;289:24700-24715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 53. | Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207-246. [PubMed] |
| 54. | Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 55. | Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr. 2014;6:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 56. | Haldar SR, Chakrabarty A, Chowdhury S, Haldar A, Sengupta S, Bhattacharyya M. Oxidative stress-related genes in type 2 diabetes: association analysis and their clinical impact. Biochem Genet. 2015;53:93-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217-234. [PubMed] |
| 58. | Mhillaj E, Morgese MG, Trabace L. Early life and oxidative stress in psychiatric disorders: what can we learn from animal models? Curr Pharm Des. 2015;21:1396-1403. [PubMed] |
| 59. | Nakka VP, Prakash-Babu P, Vemuganti R. Crosstalk Between Endoplasmic Reticulum Stress, Oxidative Stress, and Autophagy: Potential Therapeutic Targets for Acute CNS Injuries. Mol Neurobiol. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 60. | Patel SA, Velingkaar NS, Kondratov RV. Transcriptional control of antioxidant defense by the circadian clock. Antioxid Redox Signal. 2014;20:2997-3006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Vitale G, Salvioli S, Franceschi C. Oxidative stress and the ageing endocrine system. Nat Rev Endocrinol. 2013;9:228-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 62. | Zolotukhin P, Kozlova Y, Dovzhik A, Kovalenko K, Kutsyn K, Aleksandrova A, Shkurat T. Oxidative status interactome map: towards novel approaches in experiment planning, data analysis, diagnostics and therapy. Mol Biosyst. 2013;9:2085-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Overnutrition, mTOR signaling, and cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1198-R1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 64. | Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets. 2012;16:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 65. | Yamada E, Singh R. Mapping autophagy on to your metabolic radar. Diabetes. 2012;61:272-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Damasceno DC, Sinzato YK, Bueno A, Netto AO, Dallaqua B, Gallego FQ, Iessi IL, Corvino SB, Serrano RG, Marini G. Mild diabetes models and their maternal-fetal repercussions. J Diabetes Res. 2013;2013:473575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Xu YJ, Tappia PS, Neki NS, Dhalla NS. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart Fail Rev. 2014;19:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Bi L, Hou R, Yang D, Li S, Zhao D. Erythropoietin protects lipopolysaccharide-induced renal mesangial cells from autophagy. Exp Ther Med. 2015;9:559-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Yu Y, Shiou SR, Guo Y, Lu L, Westerhoff M, Sun J, Petrof EO, Claud EC. Erythropoietin protects epithelial cells from excessive autophagy and apoptosis in experimental neonatal necrotizing enterocolitis. PLoS One. 2013;8:e69620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Bendix I, Schulze C, Haefen Cv, Gellhaus A, Endesfelder S, Heumann R, Felderhoff-Mueser U, Sifringer M. Erythropoietin modulates autophagy signaling in the developing rat brain in an in vivo model of oxygen-toxicity. Int J Mol Sci. 2012;13:12939-12951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Bennis Y, Sarlon-Bartoli G, Guillet B, Lucas L, Pellegrini L, Velly L, Blot-Chabaud M, Dignat-Georges F, Sabatier F, Pisano P. Priming of late endothelial progenitor cells with erythropoietin before transplantation requires the CD131 receptor subunit and enhances their angiogenic potential. J Thromb Haemost. 2012;10:1914-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Bond WS, Rex TS. Evidence That Erythropoietin Modulates Neuroinflammation through Differential Action on Neurons, Astrocytes, and Microglia. Front Immunol. 2014;5:523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Chamorro ME, Wenker SD, Vota DM, Vittori DC, Nesse AB. Signaling pathways of cell proliferation are involved in the differential effect of erythropoietin and its carbamylated derivative. Biochim Biophys Acta. 2013;1833:1960-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Kaneko N, Kako E, Sawamoto K. Enhancement of ventricular-subventricular zone-derived neurogenesis and oligodendrogenesis by erythropoietin and its derivatives. Front Cell Neurosci. 2013;7:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Chattong S, Tanamai J, Kiatsomchai P, Nakatsu M, Sereemaspun A, Pimpha N, Praditpornsilpa K, Rojanathanes R, Sethpakadee A, Tungsanga K. Glutaraldehyde erythropoietin protects kidney in ischaemia/reperfusion injury without increasing red blood cell production. Br J Pharmacol. 2013;168:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Costa DC, Alva N, Trigueros L, Gamez A, Carbonell T, Rama R. Intermittent hypobaric hypoxia induces neuroprotection in kainate-induced oxidative stress in rats. J Mol Neurosci. 2013;50:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmoulière A. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen. 2014;22:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839-850. [PubMed] |
| 79. | Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 is an integral regulatory component of erythropoietin mTOR signaling and cytoprotection. PLoS One. 2012;7:e45456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr Neurovasc Res. 2011;8:220-235. [PubMed] |
| 81. | Jun JH, Shin EJ, Kim JH, Kim SO, Shim JK, Kwak YL. Erythropoietin prevents hypoxia-induced GATA-4 ubiquitination via phosphorylation of serine 105 of GATA-4. Biol Pharm Bull. 2013;36:1126-1133. [PubMed] |
| 82. | Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973-2979. [PubMed] |
| 83. | Sanchez PE, Fares RP, Risso JJ, Bonnet C, Bouvard S, Le-Cavorsin M, Georges B, Moulin C, Belmeguenai A, Bodennec J. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc Natl Acad Sci USA. 2009;106:9848-9853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Soliz J, Thomsen JJ, Soulage C, Lundby C, Gassmann M. Sex-dependent regulation of hypoxic ventilation in mice and humans is mediated by erythropoietin. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1837-R1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Lourhmati A, Buniatian GH, Paul C, Verleysdonk S, Buecheler R, Buadze M, Proksch B, Schwab M, Gleiter CH, Danielyan L. Age-dependent astroglial vulnerability to hypoxia and glutamate: the role for erythropoietin. PLoS One. 2013;8:e77182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2:387-399. [PubMed] |
| 87. | Esmaeili Tazangi P, Moosavi SM, Shabani M, Haghani M. Erythropoietin improves synaptic plasticity and memory deficits by decrease of the neurotransmitter release probability in the rat model of Alzheimer’s disease. Pharmacol Biochem Behav. 2015;130:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Lee ST, Chu K, Park JE, Jung KH, Jeon D, Lim JY, Lee SK, Kim M, Roh JK. Erythropoietin improves memory function with reducing endothelial dysfunction and amyloid-beta burden in Alzheimer’s disease models. J Neurochem. 2012;120:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Ma R, Hu J, Huang C, Wang M, Xiang J, Li G. JAK2/STAT5/Bcl-xL signalling is essential for erythropoietin-mediated protection against apoptosis induced in PC12 cells by the amyloid β-peptide Aβ25-35. Br J Pharmacol. 2014;171:3234-3245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of β-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY). 2012;4:187-201. [PubMed] |
| 91. | Nakazawa Y, Nishino T, Obata Y, Nakazawa M, Furusu A, Abe K, Miyazaki M, Koji T, Kohno S. Recombinant human erythropoietin attenuates renal tubulointerstitial injury in murine adriamycin-induced nephropathy. J Nephrol. 2013;26:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Nguyen AQ, Cherry BH, Scott GF, Ryou MG, Mallet RT. Erythropoietin: powerful protection of ischemic and post-ischemic brain. Exp Biol Med (Maywood). 2014;239:1461-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 93. | Rocha J, Eduardo-Figueira M, Barateiro A, Fernandes A, Brites D, Pinto R, Freitas M, Fernandes E, Mota-Filipe H, Sepodes B. Erythropoietin reduces acute lung injury and multiple organ failure/dysfunction associated to a scald-burn inflammatory injury in the rat. Inflammation. 2015;38:312-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Shen W, Chung SH, Irhimeh MR, Li S, Lee SR, Gillies MC. Systemic administration of erythropoietin inhibits retinopathy in RCS rats. PLoS One. 2014;9:e104759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Wang GB, Ni YL, Zhou XP, Zhang WF. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem. 2014;385:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 96. | Zhang X, Dong S, Qin Y, Bian X. Protective effect of erythropoietin against myocardial injury in rats with sepsis and its underlying mechanisms. Mol Med Rep. 2015;11:3317-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Yu T, Li L, Chen T, Liu Z, Liu H, Li Z. Erythropoietin attenuates advanced glycation endproducts-induced toxicity of Schwann cells in vitro. Neurochem Res. 2015;40:698-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Choi D, Schroer SA, Lu SY, Wang L, Wu X, Liu Y, Zhang Y, Gaisano HY, Wagner KU, Wu H. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med. 2010;207:2831-2842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 99. | Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3β, and β-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8:103-120. [PubMed] |
| 100. | Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194-204. [PubMed] |
| 101. | Wang L, Teng R, Di L, Rogers H, Wu H, Kopp JB, Noguchi CT. PPARα and Sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes. 2013;62:4122-4131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 102. | Yu T, Li L, Bi Y, Liu Z, Liu H, Li Z. Erythropoietin attenuates oxidative stress and apoptosis in Schwann cells isolated from streptozotocin-induced diabetic rats. J Pharm Pharmacol. 2014;66:1150-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J Cereb Blood Flow Metab. 2003;23:320-330. [PubMed] |
| 104. | Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107-1118. [PubMed] |
| 105. | Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003;71:659-669. [PubMed] |
| 106. | Hussein MH, Daoud GA, Kakita H, Kato S, Goto T, Kamei M, Goto K, Nobata M, Ozaki Y, Ito T. High cerebrospinal fluid antioxidants and interleukin 8 are protective of hypoxic brain damage in newborns. Free Radic Res. 2010;44:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Park KH, Choi NY, Koh SH, Park HH, Kim YS, Kim MJ, Lee SJ, Yu HJ, Lee KY, Lee YJ. L-DOPA neurotoxicity is prevented by neuroprotective effects of erythropoietin. Neurotoxicology. 2011;32:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Wang ZY, Shen LJ, Tu L, Hu DN, Liu GY, Zhou ZL, Lin Y, Chen LH, Qu J. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med. 2009;46:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 109. | Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res. 2011;8:270-285. [PubMed] |
| 110. | Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22:1251-1267. [PubMed] |
| 111. | Chong ZZ, Shang YC, Wang S, Maiese K. A Critical Kinase Cascade in Neurological Disorders: PI 3-K, Akt, and mTOR. Future Neurol. 2012;7:733-748. [PubMed] |
| 112. | Fong Y, Lin YC, Wu CY, Wang HM, Lin LL, Chou HL, Teng YN, Yuan SS, Chiu CC. The antiproliferative and apoptotic effects of sirtinol, a sirtuin inhibitor on human lung cancer cells by modulating Akt/β-catenin-Foxo3a axis. ScientificWorldJournal. 2014;2014:937051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 113. | Toba H, Kojima Y, Wang J, Noda K, Tian W, Kobara M, Nakata T. Erythropoietin attenuated vascular dysfunction and inflammation by inhibiting NADPH oxidase-derived superoxide production in nitric oxide synthase-inhibited hypertensive rat aorta. Eur J Pharmacol. 2012;691:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Ma R, Xiong N, Huang C, Tang Q, Hu B, Xiang J, Li G. Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology. 2009;56:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 115. | Maurice T, Mustafa MH, Desrumaux C, Keller E, Naert G, de la C García-Barceló M, Rodríguez Cruz Y, Garcia Rodríguez JC. Intranasal formulation of erythropoietin (EPO) showed potent protective activity against amyloid toxicity in the Aβ25-35 non-transgenic mouse model of Alzheimer’s disease. J Psychopharmacol. 2013;27:1044-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 116. | Sun ZK, Yang HQ, Pan J, Zhen H, Wang ZQ, Chen SD, Ding JQ. Protective effects of erythropoietin on tau phosphorylation induced by beta-amyloid. J Neurosci Res. 2008;86:3018-3027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 117. | Khan AI, Coldewey SM, Patel NS, Rogazzo M, Collino M, Yaqoob MM, Radermacher P, Kapoor A, Thiemermann C. Erythropoietin attenuates cardiac dysfunction in experimental sepsis in mice via activation of the β-common receptor. Dis Model Mech. 2013;6:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 118. | Parvin A, Pranap R, Shalini U, Devendran A, Baker JE, Dhanasekaran A. Erythropoietin protects cardiomyocytes from cell death during hypoxia/reperfusion injury through activation of survival signaling pathways. PLoS One. 2014;9:e107453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 119. | Busch S, Kannt A, Kolibabka M, Schlotterer A, Wang Q, Lin J, Feng Y, Hoffmann S, Gretz N, Hammes HP. Systemic treatment with erythropoietin protects the neurovascular unit in a rat model of retinal neurodegeneration. PLoS One. 2014;9:e102013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Chang ZY, Yeh MK, Chiang CH, Chen YH, Lu DW. Erythropoietin protects adult retinal ganglion cells against NMDA-, trophic factor withdrawal-, and TNF-α-induced damage. PLoS One. 2013;8:e55291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 121. | Fu W, Liao X, Ruan J, Li X, Chen L, Wang B, Wang K, Zhou J. Recombinant human erythropoietin preconditioning attenuates liver ischemia reperfusion injury through the phosphatidylinositol-3 kinase/AKT/endothelial nitric oxide synthase pathway. J Surg Res. 2013;183:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 122. | Kwon MS, Kim MH, Kim SH, Park KD, Yoo SH, Oh IU, Pak S, Seo YJ. Erythropoietin exerts cell protective effect by activating PI3K/Akt and MAPK pathways in C6 Cells. Neurol Res. 2014;36:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Kang J, Yun JY, Hur J, Kang JA, Choi JI, Ko SB, Lee J, Kim JY, Hwang IC, Park YB. Erythropoietin priming improves the vasculogenic potential of G-CSF mobilized human peripheral blood mononuclear cells. Cardiovasc Res. 2014;104:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 124. | Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 125. | Neasta J, Barak S, Hamida SB, Ron D. mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem. 2014;130:172-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 126. | Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99:128-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 127. | Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246-3256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 459] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 128. | Maiese K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014;46:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 129. | Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21-35. [PubMed] |
| 130. | Maiese K. Cutting through the complexities of mTOR for the treatment of stroke. Curr Neurovasc Res. 2014;11:177-186. [PubMed] |
| 131. | Marfia G, Madaschi L, Marra F, Menarini M, Bottai D, Formenti A, Bellardita C, Di Giulio AM, Carelli S, Gorio A. Adult neural precursors isolated from post mortem brain yield mostly neurons: an erythropoietin-dependent process. Neurobiol Dis. 2011;43:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, Wang J, Krebsbach PH, Taichman RS. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 133. | Sanghera KP, Mathalone N, Baigi R, Panov E, Wang D, Zhao X, Hsu H, Wang H, Tropepe V, Ward M. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol Cell Neurosci. 2011;47:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 134. | Andreucci M, Fuiano G, Presta P, Lucisano G, Leone F, Fuiano L, Bisesti V, Esposito P, Russo D, Memoli B. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009;42:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Maiese K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res. 2014;9:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 136. | Maiese K. Neuronal Activity, Mitogens, and mTOR: Overcoming the Hurdles for the Treatment of Glioblastoma Multiforme. J Transl Sci. 2015;1:2. [PubMed] |
| 137. | Tasioudi KE, Sakellariou S, Levidou G, Theodorou D, Michalopoulos NV, Patsouris E, Korkolopoulou P, Saetta AA. Immunohistochemical and molecular analysis of PI3K/AKT/mTOR pathway in esophageal carcinoma. APMIS. 2015;123:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 138. | Yang C, Zhang Y, Zhang Y, Zhang Z, Peng J, Li Z, Han L, You Q, Chen X, Rao X. Downregulation of cancer stem cell properties via mTOR signaling pathway inhibition by rapamycin in nasopharyngeal carcinoma. Int J Oncol. 2015;47:909-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 139. | Maiese K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr Neurovasc Res. 2014;11:378-389. [PubMed] |
| 140. | Aly H, Rohatgi N, Marshall CA, Grossenheider TC, Miyoshi H, Stappenbeck TS, Matkovich SJ, McDaniel ML. A novel strategy to increase the proliferative potential of adult human β-cells while maintaining their differentiated phenotype. PLoS One. 2013;8:e66131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 141. | Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19:1150-1162. [PubMed] |
| 142. | Shang YC, Chong ZZ, Wang S, Maiese K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013;10:29-38. [PubMed] |
| 143. | Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010;3:153-165. [PubMed] |
| 144. | Xing XS, Liu F, He ZY. Akt regulates β-catenin in a rat model of focal cerebral ischemia-reperfusion injury. Mol Med Rep. 2015;11:3122-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 145. | Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869-81, 881.e1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 146. | Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr Neurovasc Res. 2013;10:54-69. [PubMed] |
| 147. | Wei L, Sun C, Lei M, Li G, Yi L, Luo F, Li Y, Ding L, Liu Z, Li S. Activation of Wnt/β-catenin pathway by exogenous Wnt1 protects SH-SY5Y cells against 6-hydroxydopamine toxicity. J Mol Neurosci. 2013;49:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 148. | Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22:1317-1329. [PubMed] |
| 149. | Wang S, Sun Z, Zhang X, Li Z, Wu M, Zhao W, Wang H, Chen T, Yan H, Zhu J. Wnt1 positively regulates CD36 expression via TCF4 and PPAR-γ in macrophages. Cell Physiol Biochem. 2015;35:1289-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 150. | Yang M, Zhao X, Liu Y, Tian Y, Ran X, Jiang Y. A role for WNT1-inducible signaling protein-1 in airway remodeling in a rat asthma model. Int Immunopharmacol. 2013;17:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 151. | Chen X, Wang CC, Song SM, Wei SY, Li JS, Zhao SL, Li B. The administration of erythropoietin attenuates kidney injury induced by ischemia/reperfusion with increased activation of Wnt/β-catenin signaling. J Formos Med Assoc. 2015;114:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 152. | Maiese K. MicroRNAs and Stem Cells to the Rescue. Curr Neurovasc Res. 2015;12:211-213. [PubMed] |
| 153. | Maiese K, Chong ZZ, Hou J, Shang YC. The “O” class: crafting clinical care with FoxO transcription factors. Adv Exp Med Biol. 2009;665:242-260. [PubMed] |
| 154. | Danielyan L, Schäfer R, Schulz A, Ladewig T, Lourhmati A, Buadze M, Schmitt AL, Verleysdonk S, Kabisch D, Koeppen K. Survival, neuron-like differentiation and functionality of mesenchymal stem cells in neurotoxic environment: the critical role of erythropoietin. Cell Death Differ. 2009;16:1599-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 155. | Ogunshola OO, Moransard M, Gassmann M. Constitutive excessive erythrocytosis causes inflammation and increased vascular permeability in aged mouse brain. Brain Res. 2013;1531:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 156. | Hedley BD, Allan AL, Xenocostas A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clin Cancer Res. 2011;17:6373-6380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 158. | Zhang C, Li Z, Cao Q, Qin C, Cai H, Zhou H, Qian J, Tao L, Ju X, Yin C. Association of erythropoietin gene rs576236 polymorphism and risk of adrenal tumors in a Chinese population. J Biomed Res. 2014;28:456-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 159. | Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14:219-227. [PubMed] |
| 160. | Maiese K. FoxO Transcription Factors and Regenerative Pathways in Diabetes Mellitus. Curr Neurovasc Res. 2015;12:404-413. [PubMed] |
| 161. | Maiese K. FoxO proteins in the nervous system. Anal Cell Pathol (Amst). 2015;2015:569392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 162. | Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29:395-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 163. | Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond). 2009;116:191-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 164. | Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319-1322. [PubMed] |
| 165. | Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1465] [Cited by in RCA: 1498] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 166. | Yoo KY, Kwon SH, Lee CH, Yan B, Park JH, Ahn JH, Choi JH, Ohk TG, Cho JH, Won MH. FoxO3a changes in pyramidal neurons and expresses in non-pyramidal neurons and astrocytes in the gerbil hippocampal CA1 region after transient cerebral ischemia. Neurochem Res. 2012;37:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 167. | Peng S, Zhao S, Yan F, Cheng J, Huang L, Chen H, Liu Q, Ji X, Yuan Z. HDAC2 selectively regulates FOXO3a-mediated gene transcription during oxidative stress-induced neuronal cell death. J Neurosci. 2015;35:1250-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 168. | Hou J, Chong ZZ, Shang YC, Maiese K. FOXO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010;321:194-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 169. | Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7:95-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 170. | Wilk A, Urbanska K, Yang S, Wang JY, Amini S, Del Valle L, Peruzzi F, Meggs L, Reiss K. Insulin-like growth factor-I-forkhead box O transcription factor 3a counteracts high glucose/tumor necrosis factor-α-mediated neuronal damage: implications for human immunodeficiency virus encephalitis. J Neurosci Res. 2011;89:183-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 171. | Bakker WJ, van Dijk TB, Parren-van Amelsvoort M, Kolbus A, Yamamoto K, Steinlein P, Verhaak RG, Mak TW, Beug H, Löwenberg B. Differential regulation of Foxo3a target genes in erythropoiesis. Mol Cell Biol. 2007;27:3839-3854. [PubMed] |
| 172. | Kaushal N, Hegde S, Lumadue J, Paulson RF, Prabhu KS. The regulation of erythropoiesis by selenium in mice. Antioxid Redox Signal. 2011;14:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 173. | Paraíso AF, Mendes KL, Santos SH. Brain activation of SIRT1: role in neuropathology. Mol Neurobiol. 2013;48:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 174. | Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470-1482. [PubMed] |
| 175. | Iyer S, Han L, Bartell SM, Kim HN, Gubrij I, de Cabo R, O’Brien CA, Manolagas SC, Almeida M. Sirtuin1 (Sirt1) promotes cortical bone formation by preventing β-catenin sequestration by FoxO transcription factors in osteoblast progenitors. J Biol Chem. 2014;289:24069-24078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 176. | Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139-150. [PubMed] |
| 177. | Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 881] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 178. | Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J Biol Chem. 2011;286:5289-5299. [PubMed] |
| 179. | Akasaki Y, Alvarez-Garcia O, Saito M, Caramés B, Iwamoto Y, Lotz MK. FoxO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis Rheumatol. 2014;66:3349-3358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 180. | Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, Kaplun A, VanBerkum MF, Arking R, Freeman DC. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810-27819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 181. | Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52:1173-1185. [PubMed] |
| 182. | Maiese K. Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Curr Neurovasc Res. 2015;12:173-188. [PubMed] |
| 183. | World Health Organization. Global status report on noncommunicable diseases 2010. Geneva, 2011. Available from: http://www.who.int/nmh/publications/ncd_report_full_en.pdf. |
| 184. | Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000;16:230-236. [PubMed] |
| 185. | Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14:1729-1738. [PubMed] |
| 186. | da Rosa LC, Chiuso-Minicucci F, Zorzella-Pezavento SF, França TG, Ishikawa LL, Colavite PM, Balbino B, Tavares LC, Silva CL, Marques C. Bacille Calmette-Guérin/DNAhsp65 prime-boost is protective against diabetes in non-obese diabetic mice but not in the streptozotocin model of type 1 diabetes. Clin Exp Immunol. 2013;173:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 187. | Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446-3485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 188. | Portbury AL, Ronnebaum SM, Zungu M, Patterson C, Willis MS. Back to your heart: ubiquitin proteasome system-regulated signal transduction. J Mol Cell Cardiol. 2012;52:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 189. | Puthanveetil P, Wan A, Rodrigues B. FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell survival. Cardiovasc Res. 2013;97:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 190. | Zhao Y, Scott NA, Fynch S, Elkerbout L, Wong WW, Mason KD, Strasser A, Huang DC, Kay TW, Thomas HE. Autoreactive T cells induce necrosis and not BCL-2-regulated or death receptor-mediated apoptosis or RIPK3-dependent necroptosis of transplanted islets in a mouse model of type 1 diabetes. Diabetologia. 2015;58:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 191. | Caron AZ, He X, Mottawea W, Seifert EL, Jardine K, Dewar-Darch D, Cron GO, Harper ME, Stintzi A, McBurney MW. The SIRT1 deacetylase protects mice against the symptoms of metabolic syndrome. FASEB J. 2014;28:1306-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 192. | Castaño D, Larequi E, Belza I, Astudillo AM, Martínez-Ansó E, Balsinde J, Argemi J, Aragon T, Moreno-Aliaga MJ, Muntane J. Cardiotrophin-1 eliminates hepatic steatosis in obese mice by mechanisms involving AMPK activation. J Hepatol. 2014;60:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 193. | Gong H, Pang J, Han Y, Dai Y, Dai D, Cai J, Zhang TM. Age-dependent tissue expression patterns of Sirt1 in senescence-accelerated mice. Mol Med Rep. 2014;10:3296-3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 194. | Lee JH, Lee JH, Jin M, Han SD, Chon GR, Kim IH, Kim S, Kim SY, Choi SB, Noh YH. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med. 2014;46:e111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 195. | Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert Opin Drug Discov. 2013;8:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 196. | Malla R, Wang Y, Chan WK, Tiwari AK, Faridi JS. Genetic ablation of PRAS40 improves glucose homeostasis via linking the AKT and mTOR pathways. Biochem Pharmacol. 2015;96:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 197. | Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, Maeder C, Fournier M, Montet X, Rohner-Jeanrenaud F. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165:2325-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 198. | Gao J, Li J, An Y, Liu X, Qian Q, Wu Y, Zhang Y, Wang T. Increasing effect of Tangzhiqing formula on IRS-1-dependent PI3K/AKT signaling in muscle. BMC Complement Altern Med. 2014;14:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 199. | Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med. 2012;29:613-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 200. | Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, Xu A, Sweeney G. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes. 2015;64:36-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 201. | Zhang T, Fang M, Fu ZM, Du HC, Yuan H, Xia GY, Feng J, Yin GY. Expression of PI3-K, PKB and GSK-3 β in the skeletal muscle tissue of gestational diabetes mellitus. Asian Pac J Trop Med. 2014;7:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 202. | Hao J, Li F, Liu W, Liu Q, Liu S, Li H, Duan H. Phosphorylation of PRAS40-Thr246 involved in renal lipid accumulation of diabetes. J Cell Physiol. 2014;229:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 203. | Hao J, Zhu L, Li F, Liu Q, Zhao X, Liu S, Xing L, Feng X, Duan H. Phospho-mTOR: a novel target in regulation of renal lipid metabolism abnormality of diabetes. Exp Cell Res. 2013;319:2296-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 204. | Nakazawa J, Isshiki K, Sugimoto T, Araki S, Kume S, Yokomaku Y, Chin-Kanasaki M, Sakaguchi M, Koya D, Haneda M. Renoprotective effects of asialoerythropoietin in diabetic mice against ischaemia-reperfusion-induced acute kidney injury. Nephrology (Carlton). 2010;15:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 205. | Pandya KG, Budhram R, Clark GJ, Lau-Cam CA. Taurine can enhance the protective actions of metformin against diabetes-induced alterations adversely affecting renal function. Adv Exp Med Biol. 2015;803:227-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 206. | Pérez-Gallardo RV, Noriega-Cisneros R, Esquivel-Gutiérrez E, Calderón-Cortés E, Cortés-Rojo C, Manzo-Avalos S, Campos-García J, Salgado-Garciglia R, Montoya-Pérez R, Boldogh I. Effects of diabetes on oxidative and nitrosative stress in kidney mitochondria from aged rats. J Bioenerg Biomembr. 2014;46:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 207. | Aragno M, Mastrocola R, Ghé C, Arnoletti E, Bassino E, Alloatti G, Muccioli G. Obestatin induced recovery of myocardial dysfunction in type 1 diabetic rats: underlying mechanisms. Cardiovasc Diabetol. 2012;11:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 208. | Chong ZZ, Maiese K. Mammalian target of rapamycin signaling in diabetic cardiovascular disease. Cardiovasc Diabetol. 2012;11:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 209. | Das A, Durrant D, Koka S, Salloum FN, Xi L, Kukreja RC. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem. 2014;289:4145-4160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 210. | He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62:1270-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 302] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 211. | Ling S, Birnbaum Y, Nanhwan MK, Thomas B, Bajaj M, Li Y, Li Y, Ye Y. Dickkopf-1 (DKK1) phosphatase and tensin homolog on chromosome 10 (PTEN) crosstalk via microRNA interference in the diabetic heart. Basic Res Cardiol. 2013;108:352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 212. | Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: Developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev. 2009;2:119-129. [PubMed] |
| 213. | Zhang C, Zhang L, Chen S, Feng B, Lu X, Bai Y, Liang G, Tan Y, Shao M, Skibba M. The prevention of diabetic cardiomyopathy by non-mitogenic acidic fibroblast growth factor is probably mediated by the suppression of oxidative stress and damage. PLoS One. 2013;8:e82287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 214. | Liu Q, Li J, Cheng R, Chen Y, Lee K, Hu Y, Yi J, Liu Z, Ma JX. Nitrosative stress plays an important role in Wnt pathway activation in diabetic retinopathy. Antioxid Redox Signal. 2013;18:1141-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 215. | Schaffer SW, Jong CJ, Mozaffari M. Role of oxidative stress in diabetes-mediated vascular dysfunction: unifying hypothesis of diabetes revisited. Vascul Pharmacol. 2012;57:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 216. | Wang F, Ma X, Zhou M, Pan X, Ni J, Gao M, Lu Z, Hang J, Bao Y, Jia W. Serum pigment epithelium-derived factor levels are independently correlated with the presence of coronary artery disease. Cardiovasc Diabetol. 2013;12:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 217. | Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, Liu LB, Wu K, Liu R, Wang JZ. AMPK activation ameliorates Alzheimer’s disease-like pathology and spatial memory impairment in a streptozotocin-induced Alzheimer’s disease model in rats. J Alzheimers Dis. 2015;43:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 218. | Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 219. | White MF. IRS2 integrates insulin/IGF1 signalling with metabolism, neurodegeneration and longevity. Diabetes Obes Metab. 2014;16 Suppl 1:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 220. | Fu D, Wu M, Zhang J, Du M, Yang S, Hammad SM, Wilson K, Chen J, Lyons TJ. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 2012;55:3128-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 221. | Lee K, Hu Y, Ding L, Chen Y, Takahashi Y, Mott R, Ma JX. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes. 2012;61:2948-2957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 222. | Alexandru N, Popov D, Georgescu A. Platelet dysfunction in vascular pathologies and how can it be treated. Thromb Res. 2012;129:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 223. | Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171:3146-3157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 224. | Xiao FH, He YH, Li QG, Wu H, Luo LH, Kong QP. A genome-wide scan reveals important roles of DNA methylation in human longevity by regulating age-related disease genes. PLoS One. 2015;10:e0120388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 225. | Silverberg DS, Wexler D, Iaina A, Schwartz D. The interaction between heart failure and other heart diseases, renal failure, and anemia. Semin Nephrol. 2006;26:296-306. [PubMed] |
| 226. | Kristensen PL, Pedersen-Bjergaard U, Kjær TW, Olsen NV, Dela F, Holst JJ, Faber J, Tarnow L, Thorsteinsson B. Influence of erythropoietin on cognitive performance during experimental hypoglycemia in patients with type 1 diabetes mellitus: a randomized cross-over trial. PLoS One. 2013;8:e59672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 227. | Lewis EF, Pfeffer MA, Feng A, Uno H, McMurray JJ, Toto R, Gandra SR, Solomon SD, Moustafa M, Macdougall IC. Darbepoetin alfa impact on health status in diabetes patients with kidney disease: a randomized trial. Clin J Am Soc Nephrol. 2011;6:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 228. | Katz O, Stuible M, Golishevski N, Lifshitz L, Tremblay ML, Gassmann M, Mittelman M, Neumann D. Erythropoietin treatment leads to reduced blood glucose levels and body mass: insights from murine models. J Endocrinol. 2010;205:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 229. | Chattopadhyay M, Walter C, Mata M, Fink DJ. Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons. Brain. 2009;132:879-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 230. | Dang J, Jia R, Tu Y, Xiao S, Ding G. Erythropoietin prevents reactive oxygen species generation and renal tubular cell apoptosis at high glucose level. Biomed Pharmacother. 2010;64:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 231. | Chu Q, Zhang J, Wu Y, Zhang Y, Xu G, Li W, Xu GT. Differential gene expression pattern of diabetic rat retinas after intravitreal injection of erythropoietin. Clin Experiment Ophthalmol. 2011;39:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 232. | Ghaboura N, Tamareille S, Ducluzeau PH, Grimaud L, Loufrani L, Croué A, Tourmen Y, Henrion D, Furber A, Prunier F. Diabetes mellitus abrogates erythropoietin-induced cardioprotection against ischemic-reperfusion injury by alteration of the RISK/GSK-3β signaling. Basic Res Cardiol. 2011;106:147-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 233. | Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 234. | Hamed S, Ullmann Y, Egozi D, Daod E, Hellou E, Ashkar M, Gilhar A, Teot L. Fibronectin potentiates topical erythropoietin-induced wound repair in diabetic mice. J Invest Dermatol. 2011;131:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 235. | Hralová M, Angerová Y, Gueye T, Bortelová J, Svestková O, Zima T, Lippertová-Grünerová M. Long-term results of enriched environment and erythropoietin after hypobaric hypoxia in rats. Physiol Res. 2013;62:463-470. [PubMed] |
| 236. | Skali H, Parving HH, Parfrey PS, Burdmann EA, Lewis EF, Ivanovich P, Keithi-Reddy SR, McGill JB, McMurray JJ, Singh AK. Stroke in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia treated with Darbepoetin Alfa: the trial to reduce cardiovascular events with Aranesp therapy (TREAT) experience. Circulation. 2011;124:2903-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 237. | Frietsch T, Maurer MH, Vogel J, Gassmann M, Kuschinsky W, Waschke KF. Reduced cerebral blood flow but elevated cerebral glucose metabolic rate in erythropoietin overexpressing transgenic mice with excessive erythrocytosis. J Cereb Blood Flow Metab. 2007;27:469-476. [PubMed] |
| 238. | Semeraro F, Cancarini A, Morescalchi F, Romano MR, dell’Omo R, Ruggeri G, Agnifili L, Costagliola C. Serum and intraocular concentrations of erythropoietin and vascular endothelial growth factor in patients with type 2 diabetes and proliferative retinopathy. Diabetes Metab. 2014;40:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 239. | Bode-Böger SM, Böger RH, Kuhn M, Radermacher J, Frölich JC. Recombinant human erythropoietin enhances vasoconstrictor tone via endothelin-1 and constrictor prostanoids. Kidney Int. 1996;50:1255-1261. [PubMed] |
| 240. | De Palo T, Giordano M, Palumbo F, Bellantuono R, Messina G, Colella V, Caringella AD. Clinical experience with darbepoietin alfa (NESP) in children undergoing hemodialysis. Pediatr Nephrol. 2004;19:337-340. [PubMed] |
| 241. | Lombardero M, Kovacs K, Scheithauer BW. Erythropoietin: a hormone with multiple functions. Pathobiology. 2011;78:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 242. | Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 409] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 243. | Wang L, Di L, Noguchi CT. AMPK is involved in mediation of erythropoietin influence on metabolic activity and reactive oxygen species production in white adipocytes. Int J Biochem Cell Biol. 2014;54:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 244. | Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661-673. [PubMed] |
| 245. | Maiese K, Chong ZZ, Shang YC, Hou J. Novel avenues of drug discovery and biomarkers for diabetes mellitus. J Clin Pharmacol. 2011;51:128-152. [PubMed] |
| 246. | Jin X, Chen M, Yi L, Chang H, Zhang T, Wang L, Ma W, Peng X, Zhou Y, Mi M. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Mol Nutr Food Res. 2014;58:1941-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 247. | Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374-391. [PubMed] |
| 248. | Kopp C, Hosseini A, Singh SP, Regenhard P, Khalilvandi-Behroozyar H, Sauerwein H, Mielenz M. Nicotinic acid increases adiponectin secretion from differentiated bovine preadipocytes through G-protein coupled receptor signaling. Int J Mol Sci. 2014;15:21401-21418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 249. | Martínez de Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, Dieguez C, Lopez M. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med. 2014;14:3-21. [PubMed] |
| 250. | Paiva MA, Rutter-Locher Z, Gonçalves LM, Providência LA, Davidson SM, Yellon DM, Mocanu MM. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300:H2123-H2134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 251. | Jessen N, Koh HJ, Folmes CD, Wagg C, Fujii N, Løfgren B, Wolf CM, Berul CI, Hirshman MF, Lopaschuk GD. Ablation of LKB1 in the heart leads to energy deprivation and impaired cardiac function. Biochim Biophys Acta. 2010;1802:593-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 252. | Lai CS, Tsai ML, Badmaev V, Jimenez M, Ho CT, Pan MH. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARγ and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J Agric Food Chem. 2012;60:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 253. | Guan FY, Gu J, Li W, Zhang M, Ji Y, Li J, Chen L, Hatch GM. Compound K protects pancreatic islet cells against apoptosis through inhibition of the AMPK/JNK pathway in type 2 diabetic mice and in MIN6 β-cells. Life Sci. 2014;107:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 254. | Elliott S, Lorenzini T, Asher S, Aoki K, Brankow D, Buck L, Busse L, Chang D, Fuller J, Grant J. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol. 2003;21:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 366] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 255. | Macdougall IC, Padhi D, Jang G. Pharmacology of darbepoetin alfa. Nephrol Dial Transplant. 2007;22 Suppl 4:iv2-iv9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 256. | Sasu BJ, Hartley C, Schultz H, McElroy P, Khaja R, Elliott S, Egrie JC, Browne JK, Begley CG, Molineux G. Comparison of epoetin alfa and darbepoetin alfa biological activity under different administration schedules in normal mice. Acta Haematol. 2005;113:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 257. | Kent SB. Bringing the science of proteins into the realm of organic chemistry: total chemical synthesis of SEP (synthetic erythropoiesis protein). Angew Chem Int Ed Engl. 2013;52:11988-11996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 258. | Sinclair AM. Erythropoiesis stimulating agents: approaches to modulate activity. Biologics. 2013;7:161-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 259. | Makropoulos DA, Achuthanandam R, Avery J, Wilson K, Brosnan K, Miller A, Nesspor T, Chroscinski D, Walker M, Egenolf D. CNTO 530 increases expression of HbA and HbF in murine models of β-thalassemia and sickle cell anemia. Curr Pharm Biotechnol. 2013;14:242-248. [PubMed] |