Published online Sep 10, 2015. doi: 10.4239/wjd.v6.i11.1186
Peer-review started: August 25, 2014
First decision: December 17, 2014
Revised: August 14, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 10, 2015
Processing time: 386 Days and 8.3 Hours
Hyperglycemia is associated with an increased risk of cardiovascular disease, and the consequences of intensive therapy may depend on the mechanism of the anti-diabetic agent(s) used to achieve a tight control. In animal models, stable analogues of glucagon-like peptide-1 (GLP-1) were able to reduce body weight and blood pressure and also had favorable effects on ischemia following coronary reperfusion. In a similar way, dipeptidyl peptidase IV (DPP-IV) showed to have favorable effects in animal models of ischemia/reperfusion. This could be due to the fact that DPP-IV inhibitors were able to prevent the breakdown of GLP-1 and glucose-dependent insulinotropic polypeptide, but they also decreased the degradation of several vasoactive peptides. Preclinical data for GLP-1, its derivatives and inhibitors of the DPP-IV enzyme degradation suggests that these agents may be able to, besides controlling glycaemia, induce cardio-protective and vasodilator effects. Notwithstanding the many favorable cardiovascular effects of GLP-1/incretins reported in different studies, many questions remain unanswered due the limited number of studies in human beings that aim to examine the effects of GLP-1 on cardiovascular endpoints. For this reason, long-term trials searching for positive cardiovascular effects are now in process, such as the CAROLINA and CARMELINA trials, which are supported by small pilot studies performed in humans (and many more animal studies) with incretin-based therapies. On the other hand, selective renal sodium-glucose co-transporter 2 inhibitors were also evaluated in the prevention of cardiovascular outcomes in type 2 diabetes. However, it is quite early to draw conclusions, since data on cardiovascular outcomes and cardiovascular death are limited and long-term studies are still ongoing. In this review, we will analyze the mechanisms underlying the cardiovascular effects of incretins and, at the same time, we will present a critical position about the real value of these compounds in the cardiovascular system and its protection.
Core tip: The dipeptidyl peptidase IV inhibitors prevent the breakdown of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide, but also decrease the degradation of several vasoactive peptides. Dipeptidyl peptidase IV inhibitors have shown to have favorable effects in animal models of ischemia/reperfusion and in hypertension. Clinical studies are most under way and final results could give reliable information on cardiovascular protection. Selective inhibitors of renal sodium glucose transport 2 have been also evaluated in the prevention of cardiovascular outcomes in type 2 diabetes. However, data on cardiovascular outcomes and cardiovascular death are limited and long term studies are on-going, therefore it is premature to draw conclusions.
- Citation: Sanchez RA, Sanabria H, Santos CL, Ramirez AJ. Incretins and selective renal sodium-glucose co-transporter 2 inhibitors in hypertension and coronary heart disease. World J Diabetes 2015; 6(11): 1186-1197
- URL: https://www.wjgnet.com/1948-9358/full/v6/i11/1186.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i11.1186
As a cardiovascular risk factor, hypertension together with disglycemia, hyperlipidemia and overweight, is one of the components of the so-called metabolic syndrome. From these four, hypertension takes the first position in mortality, particularly in middle- and low-income countries. Regarding disabilities, hypertension ranks in the third position after malnutrition and risky sex behavior[1-11]. As it is well known, diabetes mellitus is closely linked to cardiovascular diseases, and hypoglycemic agents may have either positive or negative effects on cardiovascular outcomes. Consequently, there is a growing interest in the evaluation of new compounds as therapeutic tools or with relation to side effects and interactions.
Incretins, either glucagon-like peptide-1 (GLP-1) analogues or dipeptidyl peptidase IV (DPP-IV) inhibitors, are just a new group of hypoglycemic drugs and their cardiovascular effects are being evaluated in different trials.
At the gastrointestinal level, incretins are able to increase insulin release after food intake in a glucose-dependent manner[12]. From these hormones, the most widely known ones are GLP-1 and the gastric inhibitory polypeptide.
The role of endogenous GLP-1 in the metabolic and cardiovascular systems has been intensively studied[13] with specific receptor antagonists (GLP-1R antagonists), with special attention to the cardiac effects of GLP-1 in different animal models. In conscious dogs with induced cardiomyopathy[14], GLP-1 infusion improved left ventricular contractility in 90%, stroke volume in 100% and cardiac output in 50%. Furthermore, an enhanced oxidative phosphorylation effect as a consequence of an increase in myocardial glucose uptake and oxygen consumption was also reported. Some authors suggested that the beneficial cardiovascular effects of GLP-1R stimulation are primarily due to the modulation of myocardial metabolism rather than direct mechanisms[14].
Other studies suggest that GLP-1 may induce vasodilation, possibly through the activation of specific endothelial and cardiovascular myocyte receptors[15].
In recent studies that used a mouse isolated heart preparation, both GLP-1 and its analog exenatide improved cardiac function following ischemia/reperfusion[16]. Moreover, data reported that GLP-1 cardio-protective effects result from additional mechanisms over the GLP receptor activation, affecting the GLP-1 degradation pathway[16-18]. Thus, the improvement of ischemic injury by coronary vasodilation induced by the metabolite GLP-1 seems to be mediated by a nitric oxide GLP-1 receptor-independent mechanism.
Studies in human beings seemed to have similar effects than those found in animal models. As an example of this, a significant improvement of left ventricular ejection fraction and wall motion scores were reported in a pilot study[19] in which 10 patients with acute myocardial infarction and coronary arterial graft surgery were perfused for three days with recombinant human GLP-1. These effects were independent from the infarction location or the diabetes history and, in some patients; they were detectable even months after cessation of the infusion. Similarly, the GLP-1 infusion improved left ventricular ejection fraction and exercise capacity in both diabetic and non-diabetic patients with congestive heart failure[15]. Finally, in diabetic patients with coronary heart disease that were pretreated with GLP-1 before cardiac surgery, an improvement of glycemic control and hemodynamic recovery indexes were reported[20] .
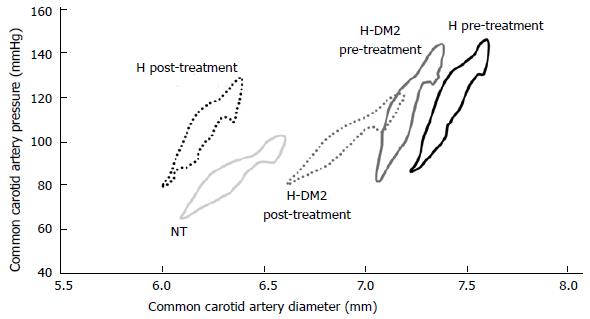
In type 2 diabetes, endothelial dysfunction is an early alteration of the consecutive vascular disease that is responsible for an increase in cardiovascular (CV) morbidity and mortality. Furthermore, endothelial dysfunction, as a cluster of the metabolic syndrome, together with postprandial hyperglycemia and postprandial hypertriglyceridemia are commonly associated with oxidative stress, decreased fibrinolysis, sympathetic activation, and increased atherosclerotic coronary plaque burden[21]. It is interesting that incretins play a role in reducing endothelial dysfunction in experimental studies. In accordance with this information, Basu et al[22] reported that administration of GLP-1 enhanced forearm vasodilator response to intra-arterial acetylcholine but not to nitroprusside, which was consistent with a nitric oxide synthase-dependent effect. However, whether the role of GLP-1 or the products of its degradation mediated these effects was not evaluated. In a review published by our group[23] that included patients with type 2 diabetes, we examined the endothelial function and the effects of treatment (Figure 1). The endothelial function was improved with ramipril, an angiotensin-converting enzyme inhibitor (ACEI), suggesting that GLP-1 may have endothelial effects that are similar to the ones of ACEI. In another study of Japanese diabetic patients with coronary artery disease, changes in endothelial function[24] were studied when patients were treated for 6 mo either with sitagliptin or conventional therapy. Patients receiving sitagliptin experienced a greater reduction in the C-reactive protein and systolic blood pressure (-7 mmHg), whereas hemoglobin A1c did not present any changes after treatment when compared to the control group. The authors concluded that sitagliptin, beyond its hypoglycemic action and blood pressure reduction, significantly improved the endothelial function and inflammatory state.
In conclusion, incretins as a family of anti-diabetic drugs may have additional protective effects on the cardiovascular system not only by improvement of glycemic control. In this regard, the mechanisms involved could be: the optimization of the endothelial function and the reduction of the inflammatory process with a subsequent improvement of the arterial and cardiac dynamics.
In addition to the well-demonstrated metabolic actions, incretins can reduce blood pressure as shown in different animal models of arterial hypertension. In Dahl salt-sensitive (DSS) rats, infusion of recombinant GLP-1 induces a reduction in blood pressure with concomitant attenuation of the development of hypertension[25]. This effect was related to higher levels of urine flow and sodium excretion, known as the natriuretic effect. In addition, a decrease in LV hypertrophy was observed. Similarly, in another study with DSS[26], a blunting effect of development of hypertension and cardiac left ventricular hypertrophy was described when the animals were pretreated with an exenatide-related GLP-1 receptor agonist. This was further confirmed during the pre-hypertensive period in spontaneously hypertensive rats[27] in which the administration of sitagliptin increased the levels of biologically active intact GLP-1 and significantly reduced the increase of blood pressure. These effects do not seem to be the only mechanisms involved in blood pressure reduction since, by using a mouse transgenic model, cardiac GLP-1R activation was able to induce the plasma levels of atrial natriuretic peptide (ANP) together with a decrease in blood pressure. Conversely, in GLP-1r-deficient mice, the GLP-1R agonist liraglutide failed to induce ANP secretion, vasodilation and blood pressure reduction. This supports the idea that different mechanisms of action like a gut-heart GLP-1R and an ANP-dependent axis are involved in blood pressure regulation with these compounds.
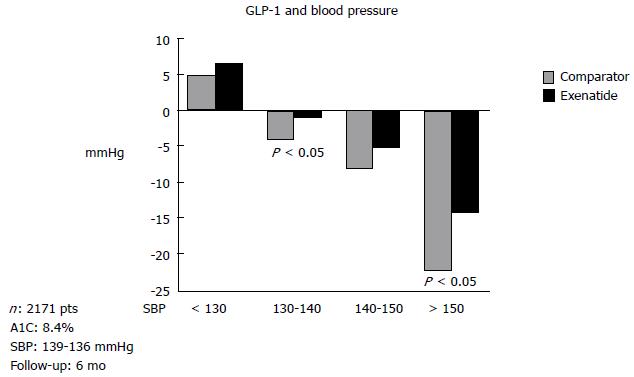
Studies of the stable GLP analogues on blood pressure were also performed in human beings[28]. Data obtained in six studies involving type 2 diabetic patients[29] showed that 6 mo of treatment with exenatide significantly reduces systolic blood pressure. Similarly, liraglutide in combination with other anti-diabetic drugs like metformin[30] also demonstrated the ability to reduce systolic blood pressure in diabetic hypertensive patients. In the LEAD-3 Mono trial[31], treatment with liraglutide vs glimepiride significantly decreased blood pressure. In a different study, Okerson et al[29] reported that six-month treatment with exenatide reduced systolic blood pressure when patients are pretreated with either insulin or placebo. The authors of these studies postulated that the exenatide antihypertensive effect seems to be partly independent from its metabolic activity. However, the weight loss effect cannot be ruled out[29] (Figure 2), raising one important point of discussion: How weight loss may contribute to lowering blood pressure and whether this reduction is linked to the antihypertensive effect. In fact, in the Okerson study[29] the decrease observed in systolic blood pressure was significantly related to weight loss. Likewise, in the LEAD-3 trial[32], liraglutide treatment significantly reduced weight, whereas glimepiride did not. However, in another study[33], a decrease in blood pressure was observed prior to a decrease in body weight. Thus, the real association between weight reduction and blood pressure reduction is not yet clear.
Different studies re-analyzed the effects of the pressure-natriuretic mechanism in lowering of blood pressure by both GLP-1 analogues[34] and DPP-IV inhibitors[35]. In addition, Crajoinas et al[35] recently suggested that the activation of the cAMP/PKA signaling pathway by incretins interferes with the normal Na+ transport in the proximal tubule that decreases sodium and water reabsorption, thus giving further support to the role of the natriuretic effect to the lowering of blood pressure through incretins.
Although a blood pressure decrease was reported in clinical studies with DPP-IV inhibitors in diabetes, these studies were not designed to evaluate the blood pressure effects and the conclusions were weak and failed to give support to the effect[36]. In this regard, patients with metabolic syndrome either under placebo or incomplete ACE inhibition were evaluated in one study carried out by Marney et al[37], who examined the interactive effect on blood pressure of the acute inhibition of both ACE and DPP-IV. The administration of sitagliptin was effective in lowering blood pressure. Yet, during maximal ACE inhibition sitagliptin had the opposite effect: It increased blood pressure with a concomitant increase in heart rate and circulating norepinephrine concentrations. These findings were similar to data previously reported in rats[38], where a dose-dependent decrease in blood pressure was observed with DPP-IV inhibition but later, when animals were pretreated with the ACE inhibitor captopril, the DPP-IV inhibition caused an increase in blood pressure. This effect was prevented with the blockade of the Neuropeptide Y (NPY1) receptors, thus suggesting that the combined inhibition of ACE and DPP-IV could raise blood pressure through their synergistic effects on substance P degradation. Moreover, Shah et al[39] showed that the inhibition of DPP-IV, similarly to GLP-1, is able to induce vasodilation (nitric oxide effect) with a consequent decrease in peripheral vascular resistance. Despite these controversial results, many investigators still favor the use of GLP-1 analogues and DPP-IV inhibitors for a better control of blood pressure in patients with diabetes and arterial hypertension[40,41]. In different studies performed in non-diabetic patients, sitagliptin[42] was associated with a 2-3 mmHg reduction in mean systolic blood pressure, assessed by 24-h ambulatory blood pressure monitoring and, in diabetic patients with inadequate glycemic control[43] that were receiving metformin, the addition of vildagliptin induced a dose-dependent decrease in both systolic and diastolic blood pressure.
Despite the data presented above, the ability of incretins to reduce blood pressure is still limited. Further studies must be performed in order to elucidate the real efficacy of GLP-1 analogues and DPP-IV inhibition on hypertension. Consequently, randomized trials in patients with either hypertension or diabetes and also with both hypertension and diabetes must be performed in order to elucidate this important question.
Although the CV protective effects of DPP-IV inhibitors seem to be a result of an improvement of type 2 diabetes, the accumulating evidence that was mentioned earlier also suggests a possible direct myocardial effect of GLP-1 on the improvement of the endothelial function, lowering blood pressure and preventing myocardial injury[44,45].
Another important mechanism of cardiovascular protection is associated with the immune modulatory role of DPP-IV on cardiovascular inflammation. Even though this concept has been minimally investigated, this seems to be an area of emerging importance to evaluate the role of DPP-IV inhibitors in the modulation of innate and adaptive immunity[46-50]. In this regard, the decreased accumulation of specific inflammatory macrophages present in adipose tissue or atherosclerotic lesions related to the DPP-IV inhibitor treatment was studied[51,52]. The data provided raises the possibility of a DPP-IV facilitatory interaction with inflammatory related macrophages, resulting in an impairment of inflammation. On the other hand, since DPP-IV activity in serum and tissues is markedly increased in obesity in both animal models and human beings[53-55], the inhibition of DPP-IV might offer a novel strategy for suppression of low-grade inflammation present in diabetes and associated tissue insulin resistance with favorable effects that improve heart and coronary artery function. Thus, it is possible that the common effects of DPP-IV inhibition/GLP-1 signaling, in opposition to angiotensin II/aldosterone effects, contribute to the beneficial modulation of immune responses in the cardio-renal system[56-58].
On the other hand, the vasodilator effect of both GLP-1 and DPP-IV inhibitors correlate with an increase in cGMP release, which is attenuated by the pre-incubation with nitric oxide synthase inhibitors, suggesting that at least part of their vasodilator mechanism is nitric oxide/cGMP-dependent. In addition, it seems that the anti-inflammatory effect precedes the blood pressure effect and mediates early improvements in endothelial function and atherosclerosis. Important in vitro studies with linagliptin performed in a mouse model of diabetic nephropathy[59] showed anti-inflammatory[49,60] and antioxidant[61] properties, improved re-epithelialization and healing of diabetes-related wounds[60] and, in a chronic renal failure rat model[62], renoprotective effects that were not linked to the worsening of glomerular and tubular pathological markers. In addition, in a uremic cardiomyopathy rat model, linagliptin significantly reduced the RNA messenger (mRNA) levels of several cardiac fibrosis markers and of a marker of left ventricular dysfunction. These results would demonstrate an important anti-fibrotic property of linagliptin[62].
In clinical studies, incretins seemed to reduce cardiovascular outcomes when compared to other hypoglycemic drugs as shown in a meta-analysis[63] in which the treatment with DPP-IV was associated with reduced CV events. The overall use of DPP-IV inhibitors compared to placebo or other oral hypoglycemic agents, apart from decreasing adverse CV effects, it was also able to reduce the risk of non-fatal myocardial infarction (MI) and acute coronary syndrome (ACS). Moreover, with DPP-IV inhibitor therapy the risk of adverse CV events was not significantly different compared to placebo, but was significantly lower compared to metformin and other oral hypoglycemic agents, including sulfonylureas and thiazolidinediones. In another small study[64] comparing sitagliptin vs placebo in patients with coronary artery disease and preserved left ventricular function awaiting revascularization, increased ejection fraction from 64.0% ± 8.0% to 73.0% ± 7.0% and increased plasma GLP-1 levels at peak stress (from 10.0 ± 9.0 pg/mL to 17.0 ± 11.0 pg/mL; P≤ 0.003) and at rest (from 9.0 ± 6.0 pg/mL to 12.0 ± 6.0 pg/mL) were reported.
In a large meta-analysis[65] of 25 phase III studies, vildagliptin was administered either as monotherapy or in combination therapy for a period of 12 wk to 2 years and the drug safety was compared to a pool of placebo and active comparators. Relative to all comparators, the RRs for the composite endpoint were < 1 for both vildagliptin 50mgqd and vildagliptin 50mgbid, and the results were consistent across subgroups defined by age, gender and CV risk status, including the higher CV risk subgroups of elderly patients, males, or patients with a high CV risk status. The exposure-adjusted incidences of each component of the composite endpoint for vildagliptin 50mgbid were also lower than or similar to those of all comparators. Based in these results, it was concluded that vildagliptin is a safe drug in the broad population of type 2 diabetes mellitus (T2DM), including patients at a higher risk of CCV events.
The incidence of major side effects (MACEs) was also evaluated in different studies with DPP-IV inhibitors. A meta-analysis[66] conducted to assess the effect of DPP-IV inhibitors on the incidence of MACE, cancer and pancreatitis compared to placebo or other treatment, determined that they were associated with a similar risk of cancer and pancreatitis and with a reduced risk of MACE. Frederich et al[67] analyzed eight randomized double-blind, phase II and III trials of patients with T2DM treated with saxagliptin, placebo, metformin, or glyburide. Cox proportional regression hazard model showed a 41% RR reduction of CV events with saxagliptin vs the comparators. The composite endpoint of CV death, MI or stroke was confirmed in 40 patients from whom 0.7% received saxagliptin and 1.4% received other comparator. The Cox RR estimate was 0.43 translating to a 57% risk reduction in patients assigned to saxagliptin. Thus, no CV harm and a potential for an actual reduction in CV events with saxagliptin was suggested[67].
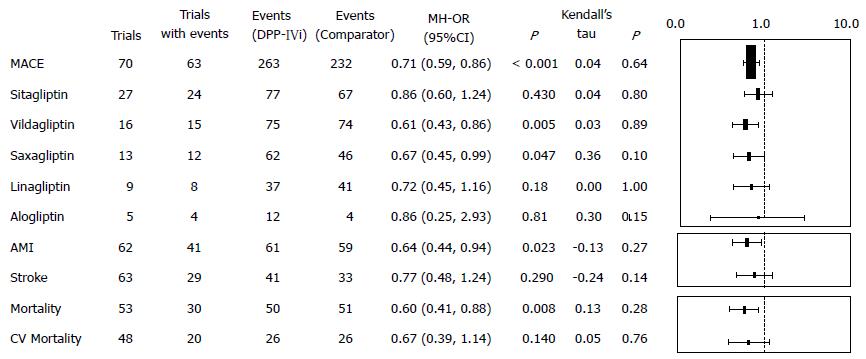
Pooled information of MACEs[68-70] from different DPP-IV inhibitors is shown in Figure 3.
More recently, in a pre-specified meta-analysis assessing cardiovascular safety[71], cardiovascular risk did not increase with linagliptin 5 or 10 mg once daily (as monotherapy). Additional data suggested that linagliptin was not associated with a significantly greater risk of the primary composite endpoints, regardless of age, gender, and race, use of rescue therapy, hypoglycemia or cardiovascular risk. In an extension of one clinical trial[72], after receiving linagliptin monotherapy, the rate of patients reporting cardiovascular/cerebrovascular events was 4.1% and the rate of those with ischemic events amounted up to only 1.9%.
Finally, in a study[73] of 52 wk of follow-up in which 2.9% of the patients had severe renal impairment (a population with high cardiovascular risk), linagliptin was added to their hypoglycemic therapy, and the rate of death from cardiovascular causes was significantly lower and did not differ from the one observed with placebo.
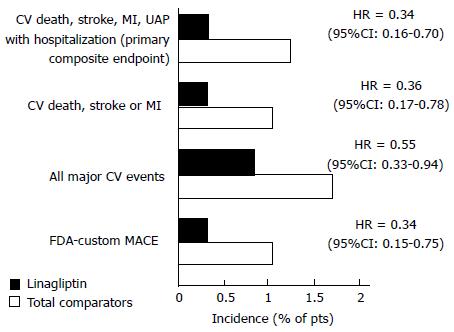
Figure 4 shows safety indicators in other studies with linagliptin compared to other hypoglycemic drugs[74].
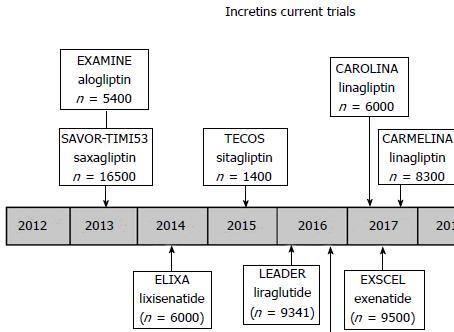
Trials specifically designed to evaluate the cardiovascular impact of DPP-IV inhibitors
In the SAVOR-TIMI 53 study[75], 16492 patients with type 2 diabetes and established atherosclerotic disease or high cardiovascular risk were randomized to receive saxagliptin or placebo. The primary endpoint was a composite of cardiovascular death, myocardial infarction or ischemic stroke, with a follow up of 2.1 years. No difference was observed for the primary endpoint when comparing saxagliptin to placebo (7.3% vs 7.2%, HR = 1.00, 95%CI: 0.89-1.12, P = 0.99 for superiority, P < 0.001 for non-inferiority). Surprisingly, a higher amount of hospitalizations due to heart failure were reported under saxagliptin compared to placebo (3.5% vs 2.8%; HR = 1.27, 95%CI: 1.07-1.51, P = 0.007). However, mortality secondary to heart failure did not increase (Figure 5).
The EXAMINE study[76] evaluated cardiovascular endpoints using alogliptin in patients with diabetes at very high cardiovascular risk. It randomized 5380 patients with diabetes and history of acute coronary syndrome. At a mean follow-up of 18 mo and compared to placebo, there was no difference in a composite of death from cardiovascular causes, non-fatal myocardial infarction, non-fatal stroke (11.3% vs 11.8%, HR = 0.96; P < 0.001 for non-inferiority), in the different components of the primary endpoint nor in the incidence of heart failure.
TECOS: In this randomized, double-blind study recent published, 14671 patients were assigned to add either sitagliptin or placebo to their existing therapy. The primary cardiovascular outcome was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina. Sitagliptin was noninferior to placebo for the primary composite cardiovascular outcome (HR = 0.98; 95%CI: 0.88-1.09; P < 0.001). Rates of hospitalization for heart failure did not differ between the two groups (HR = 1.00; 95%CI: 0.83-1.20; P = 0.98)[77].
The interim analysis of results of SITAGRAMI: Safety and Efficacy of Sitagliptin plus Granulocyte Colony-Stimulating Factor in Patients Suffering from Acute Myocardial Infarction[78]. It is a phase III multicenter trial testing the myocardial regenerating effects of Sitagliptin combined with G-CSF after an acute MI. The results are encouraging, but they still need to be confirmed once the long-term study has been analyzed.
CAROLINA: Cardiovascular Outcome Study of Linagliptin vs Glimepiride in Patients with T2DM[79]. It is a long-term multicenter study planning to enroll 6000 patients with an expected completion date in September 2018.
CARMELINA: Cardiovascular safety and Renal Microvascular outcome with linagliptin patients with T2DM at high vascular risk. It is a long-term study investigating the efficacy and safety of linagliptin vs placebo on cardiovascular and renal micro-vascular outcomes in patients with type 2 diabetes and risk of cardiovascular events. The study will randomize patients with type 2 diabetes and previous CV complications and albuminuria [urinary albumin creatinine ratio (UACR) ≥ 30 mg/g] with or without evidence of micro-vascular related end-organ damage and an estimated glomerular filtration rate (eGFR) between 15 and 45 mL/min and an UACR > 200 mg/g or eGFR ≥ 45-75. The study will include more than 8000 adults with type 2 diabetes. The primary endpoint will be the time to the first occurrence of either CV death (including fatal stroke and fatal MI); non-fatal MI; non-fatal stroke; or hospitalization for unstable angina pectoris. The renal outcome is measured as a composite of renal death, sustained end-stage renal disease and sustained decrease of ≥ 50% eGFR. The study will be completed in 2018. This kind of study could provide us with answers regarding the CV and renal outcomes for this type of drugs.
The glucose reabsorption regulation is mainly performed in the kidneys where more than 99% of the plasma glucose that filters through the kidneys is reabsorbed. There are two transporters of glucose across cell membranes, the GLUTs, facilitative glucose transporters and an active sodium-dependent transport process mediated by the sodium/glucose co-transporters (SGLTs). These are a large family of intestinal epithelium and of the proximal renal tubules membrane proteins involved in the transportation of glucose, amino acids, vitamins, osmolytes, and some ions[80].
The high-capacity, low-affinity transporter sodium-glucose co-transporter 2 (SGLT2) is expressed primarily in the kidney, while SGLT1 plays an important function in the absorption of glucose in the intestine. The issue of gene expression and the possibility of SGLT adaptation to chronic hyperglycemia is an area for further investigation. A small amount of adaptation and a near two-fold increase in the SGLT2 mRNA expression in diabetes animal models was shown. The induction of diabetes in rats increased mRNA expression of both SGLT2 and hepatocyte nuclear factor-1a in the renal cortex. Glycemic control was improved after 6 d of treatment with insulin or phlorizin accompanied by a reduced expression of SGLT2 and hepatocyte nuclear factor-1a to near-normal levels[81].
SGLT2 inhibitors are a new class of anti-diabetic drugs that reduce renal glucose reabsorption selectively in the proximal convoluted tubule leading to an increased urinary glucose excretion without potential gastrointestinal side effects. The SGLT2 inhibitors that are currently under investigation are dapagliflozin, a C-Aryl glucoside, empagliflozin and sergliflozin, an O-glucoside and canagliflozin[82,83] and represent an interesting and important tool to be added for the treatment of hyperglycemia. Additionally, SGLT2 inhibitors were associated with a reduction in systolic blood pressure compared to placebo (mean difference: -3.77 mmHg) and active comparators (mean difference: -4.45 mmHg). Diastolic blood pressure was also reduced with SGLT2 inhibitors compared to placebo (mean difference: -1.75 mmHg) and other anti-diabetic agents (mean difference: -2.01 mmHg). Risk of bias was high for both systolic and diastolic blood pressure analyses[84,85].
To be taken into account is the fact that SGLT2 inhibitors, like metformin, are associated with weight loss and also act as osmotic diuretics, resulting in a lowering of BP. While not approved for BP lowering, they may potentially aid BP goal achievement in people with a target reduction within 7-10 mmHg[86,87]. However, more studies are needed in order to determine a positive antihypertensive action of these compounds.
Regarding potential cardiovascular effects of SLGT2, different meta-analysis were performed: for dapagliflozin, the meta-analysis was based on 14 trials including 6300 patients. An OR of 0.73 (95%CI: 0.46-1.16) compared with the control group was reported, supporting the idea of an absence of cardiovascular risk. In a pooled analysis of two dapagliflozin trials[87] involving patients with established cardiovascular disease, the hazard ratio (HR) for the composite cardiovascular endpoint (cardiovascular death, myocardial infarction, stroke, and hospitalization for unstable angina) was 1.07 (95%CI: 0.64-1.72) compared to placebo. In another study that included data from 10 trials (10474 patients, OR = 0.95), of canagliflozin compared with placebo, no association of an increased risk for the composite cardiovascular outcome compared to placebo or an active comparator was found. Similarly, in the United States Food and Drug Administration report[87], the HR for non-fatal stroke was higher in patients receiving canagliflozin (6876 patient-years) than in the control groups (3470 patient-years; HR = 1.46; 95%CI: 0.83-2.58). On the other hand, an imbalance in the incidence of cardiovascular events was observed during the first 30 d[88] for canagliflozin (13 of 2886 patients) or placebo (1 of 1441 patients), which resulted in an HR = 6.50 (95%CI: 0.85-49.66). It was explained that this high risk of events resulted from volume depletion after the initiation of canagliflozin treatment, which failed to be observed after 30 d of treatment. In another recent study, systolic and diastolic blood pressure analyses were performed in response to empagliflozin during the euglycemic clamp in hypertensive patients. A reduction in systolic blood pressure was reported, as well as a decreased augmentation index at the radial, carotid and aortic arteries. Similar effects on arterial stiffness were observed, without changes in blood pressure. Carotid-radial pulse wave velocity decreased significantly under both glycemic conditions (P≤ 0.0001), whereas declines in carotid-femoral pulse wave velocity were only significant during clamped hyperglycemia. Finally, HRV, plasma noradrenalin and adrenaline remained unchanged under both euglycemic and hyperglycemic clamp conditions[89].
These new anti-diabetic compounds have shown additive CV protective effects in T2DM. Additional benefits include lowering of blood pressure, improvement of lipid profile and endothelial dysfunction, decrease in the macrophage-mediated inflammatory response, and reduction of myocardial injury. All these effects were mainly evaluated in animal models, since human clinical studies that include a high number of participants are still missing.
On the other hand, there are ongoing studies that aim to evaluate the CV effect and the safety of DPP-IV inhibitors. From the last studies that were published in which DPP-IV inhibitors were used, SAVOR TIMI, TECOS and EXAMINE, it seems that a neutral cardiovascular effect rather than a benefit is expected for these compounds. There are other studies with DPP-IV, which are still being developed, such as CAROLINA and CARMELINA, so additional effects could still be assessed.
As it was previously mentioned, further investigations in large cohorts of diabetic patients are needed in order to assess the exact mechanisms of CV protective effects held by renal glucose transport inhibitors. The reason supporting this need is based on the fact that these compounds have shown interesting natriuretic effect resulting in blood pressure decrease and loss of weight. Further trials may endorse these clinical features.
P- Reviewer: Parvizi N S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 1650] [Article Influence: 97.1] [Reference Citation Analysis (4)] |
| 2. | Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6631] [Cited by in RCA: 6786] [Article Influence: 295.0] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Prevention of Cardiovascular Disease. Guidelines for assessment and management of Cardiovascular Risk. [Accessed. 2007;Aug 24] Available from: http://www.who.int/cardiovascular_diseases/publications/Prevention_of_Cardiovascular_Disease/en/. |
| 4. | Lanas F, Avezum A, Bautista LE, Diaz R, Luna M, Islam S, Yusuf S. Risk factors for acute myocardial infarction in Latin America: the INTERHEART Latin American study. Circulation. 2007;115:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Albala C, Vio F, Kain J, Uauy R. Nutrition transition in Latin America: the case of Chile. Nutr Rev. 2001;59:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Murray CJL, Lopez AD, editors . The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Boston, Massachusetts: Harvard School of Public Health 1996; . |
| 7. | MacMahon S, Alderman MH, Lindholm LH, Liu L, Sanchez RA, Seedat YK. Blood-pressure-related disease is a global health priority. Lancet. 2008;371:1480-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 556] [Reference Citation Analysis (1)] |
| 8. | Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 610] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 9. | Jamieson DT, Breman JG, MeashamAR , Alleyne G, Claeson M, Evans D, Jha P, Mills A, Musgrove P. Disease control priorities in developing countries. New York: Oxford University Press 2006; . |
| 10. | López-Jaramillo P, Sánchez RA, Diaz M, Cobos L, Bryce A, Parra Carrillo JZ, Lizcano F, Lanas F, Sinay I, Sierra ID. Latin American consensus on hypertension in patients with diabetes type 2 and metabolic syndrome. J Hypertens. 2013;31:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 444] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 12. | Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 817] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 13. | Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 405] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 14. | Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 502] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 15. | Olsson J, Lindberg G, Gottsäter M, Lindwall K, Sjöstrand A, Tisell A, Melander A. Increased mortality in Type II diabetic patients using sulphonylurea and metformin in combination: a population-based observational study. Diabetologia. 2000;43:558-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Quast U, Stephan D, Bieger S, Russ U. The impact of ATP-sensitive K+ channel subtype selectivity of insulin secretagogues for the coronary vasculature and the myocardium. Diabetes. 2004;53 Suppl 3:S156-S164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Sack MN, Yellon DM. Insulin therapy as an adjunct to reperfusion after acute coronary ischemia: a proposed direct myocardial cell survival effect independent of metabolic modulation. J Am Coll Cardiol. 2003;41:1404-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 676] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 19. | Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ, Maher TD, Dean DA, Bailey SH, Marrone G, Benckart DH. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 339] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 21. | Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293:E1289-E1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Christen AI, Armentano RL, Miranda A, Graf S, Santana DB, Zócalo Y, Baglivo HP, Sánchez RA. Arterial wall structure and dynamics in type 2 diabetes mellitus methodological aspects and pathophysiological findings. Curr Diabetes Rev. 2010;6:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, Maeda H, Fujisue K, Yamamoto E, Kaikita K. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | Liu Q, Adams L, Broyde A, Fernandez R, Baron AD, Parkes DG. The exenatide analogue AC3174 attenuates hypertension, insulin resistance, and renal dysfunction in Dahl salt-sensitive rats. Cardiovasc Diabetol. 2010;9:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Pacheco BP, Crajoinas RO, Couto GK, Davel AP, Lessa LM, Rossoni LV, Girardi AC. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens. 2011;29:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Gustavson SM, Chen D, Somayaji V, Hudson K, Baltrukonis DJ, Singh J, Boyden TL, Calle RA. Effects of a long-acting GLP-1 mimetic (PF-04603629) on pulse rate and diastolic blood pressure in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2011;13:1056-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010;23:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complications. 2014;28:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab. 2009;11 suppl 3:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 791] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 32. | Lovshin JA, Zinman B. Blood pressure-lowering effects of incretin-based diabetes therapies. Can J Diabetes. 2014;38:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 402] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 34. | Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol. 2011;301:F355-F363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 35. | Marso SP, Poulter NR, Nissen SE, Nauck MA, Zinman B, Daniels GH, Pocock S, Steinberg WM, Bergenstal RM, Mann JF. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166:823-30.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 36. | Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension. 2010;56:728-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Jackson EK, Dubinion JH, Mi Z. Effects of dipeptidyl peptidase iv inhibition on arterial blood pressure. Clin Exp Pharmacol Physiol. 2008;35:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Shah Z, Pineda C, Kampfrath T, Maiseyeu A, Ying Z, Racoma I, Deiuliis J, Xu X, Sun Q, Moffatt-Bruce S. Acute DPP IV inhibition modulates vascular tone through GLP-1 independent pathways. Vascul Pharmacol. 2011;55:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Ogawa S, Ishiki M, Nako K, Okamura M, Senda M, Mori T, Ito S. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J Exp Med. 2011;223:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Anagnostis P, Athyros VG, Adamidou F, Panagiotou A, Kita M, Karagiannis A, Mikhailidis DP. Glucagon-like peptide-1-based therapies and cardiovascular disease: looking beyond glycaemic control. Diabetes Obes Metab. 2011;13:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, Herman GA. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 42. | Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 43. | Chrysant SG, Chrysant GS. Clinical implications of cardiovascular preventing pleiotropic effects of dipeptidyl peptidase-4 inhibitors. Am J Cardiol. 2012;109:1681-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Davey KA, Garlick PB, Warley A, Southworth R. Immunogold labeling study of the distribution of GLUT-1 and GLUT-4 in cardiac tissue following stimulation by insulin or ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H2009-H2019. [PubMed] |
| 45. | Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci. 2009;30:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 46. | Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP IV inhibition in diabetic mice. Diabetes. 2011;60:1246-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Satoh-Asahara N, Sasaki Y, Wada H, Tochiya M, Iguchi A, Nakagawachi R, Odori S, Kono S, Hasegawa K, Shimatsu A. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism. 2013;62:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 48. | Schürmann C, Linke A, Engelmann-Pilger K, Steinmetz C, Mark M, Pfeilschifter J, Klein T, Frank S. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J Pharmacol Exp Ther. 2012;342:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Koren S, Shemesh-Bar L, Tirosh A, Peleg RK, Berman S, Hamad RA, Vinker S, Golik A, Efrati S. The effect of sitagliptin versus glibenclamide on arterial stiffness, blood pressure, lipids, and inflammation in type 2 diabetes mellitus patients. Diabetes Technol Ther. 2012;14:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 51. | Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, Huang Y. DPP IV (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2011;58:157-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 52. | Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP4 inhibitors--from preclinical development to clinical research. Kidney Blood Press Res. 2012;36:65-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 53. | Yang J, Campitelli J, Hu G, Lin Y, Luo J, Xue C. Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin. Life Sci. 2007;81:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Li K, Xu W, Guo Q, Jiang Z, Wang P, Yue Y, Xiong S. Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ Res. 2009;105:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia. 2010;53:730-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 56. | Rodriguez R, Viscarra JA, Minas JN, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade increases pancreatic insulin secretion and decreases glucose intolerance during glucose supplementation in a model of metabolic syndrome. Endocrinology. 2012;153:1684-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 58. | Sharkovska Y, Reichetzeder C, Alter M, Tsuprykov O, Bachmann S, Secher T, Klein T, Hocher B. Blood pressure and glucose independent renoprotective effects of dipeptidyl peptidase-4 inhibition in a mouse model of type-2 diabetic nephropathy. J Hypertens. 2014;32:2211-2223; discussion 2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 59. | Klein T, Batra A, Mark M, Siegmund B. The DPP IV inhibitor linagliptin increases active GLP-2 and decreases colonic cytokines in a mouse inflammatory bowel disease model. Diabetes. 2011;60 Suppl 1:A309. |
| 60. | Schuff A, Steven S, Schell R. Comparison of the direct and indirect antioxidant effects of DPP IV inhibitors: the anti-inflammatory and vasodilatory potential of linagliptin. Diabetes. 2011;60 Suppl 1:A269. |
| 61. | Chaykovska L, von Websky K, Rahnenführer J, Alter M, Heiden S, Fuchs H, Runge F, Klein T, Hocher B. Effects of DPP IV inhibitors on the heart in a rat model of uremic cardiomyopathy. PLoS One. 2011;6:e27861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Patil HR, Al Badarin FJ, Al Shami HA, Bhatti SK, Lavie CJ, Bell DS, O’Keefe JH. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol. 2012;110:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 63. | Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP IV inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3:195-201. [PubMed] |
| 64. | Schweizer A, Dejager S, Foley JE, Couturier A, Ligueros-Saylan M, Kothny W. Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large Phase III type 2 diabetes population. Diabetes Obes Metab. 2010;12:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 65. | Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27 Suppl 3:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 66. | Frederich R, Alexander JH, Fiedorek FT, Donovan M, Berglind N, Harris S, Chen R, Wolf R, Mahaffey KW. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med. 2010;122:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 67. | Schernthaner G, Barnett AH, Emser A, Patel S, Troost J, Woerle HJ, von Eynatten M. Safety and tolerability of linagliptin: a pooled analysis of data from randomized controlled trials in 3572 patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Doucet J, Chacra A, Maheux P, Lu J, Harris S, Rosenstock J. Efficacy and safety of saxagliptin in older patients with type 2 diabetes mellitus. Curr Med Res Opin. 2011;27:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Neumiller JJ, Wood L, Campbell RK. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2010;30:463-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | Johansen OE, Neubacher D, von Eynatten M, Patel S, Woerle HJ. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol. 2012;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 71. | Araki E, Kawamori R, Inagaki N, Watada H, Hayashi N, Horie Y, Sarashina A, Thiemann S, von Eynatten M, Dugi K. Long-term safety of linagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | McGill JB, Sloan L, Newman J, Patel S, Sauce C, von Eynatten M, Woerle HJ. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36:237-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 73. | Deeks ED. Linagliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2012;72:1793-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2804] [Cited by in RCA: 2573] [Article Influence: 214.4] [Reference Citation Analysis (0)] |
| 75. | White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1896] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 76. | Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1831] [Cited by in RCA: 1873] [Article Influence: 187.3] [Reference Citation Analysis (0)] |
| 77. | Theiss HD, Brenner C, Engelmann MG, Zaruba MM, Huber B, Henschel V, Mansmann U, Wintersperger B, Reiser M, Steinbeck G. Safety and efficacy of SITAgliptin plus GRanulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction (SITAGRAMI-Trial)--rationale, design and first interim analysis. Int J Cardiol. 2010;145:282-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 78. | Rosenstock J, Marx N, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, Bluhmki E, Patel S, Johansen OE, Woerle HJ. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res. 2013;10:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 79. | Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004;447:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 333] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 80. | Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994;74:993-1026. [PubMed] |
| 81. | Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 82. | Meng W, Ellsworth BA, Nirschl AA, McCann PJ, Patel M, Girotra RN, Wu G, Sher PM, Morrison EP, Biller SA. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem. 2008;51:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 470] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 83. | Katsuno K, Fujimori Y, Takemura Y, Hiratochi M, Itoh F, Komatsu Y, Fujikura H, Isaji M. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J Pharmacol Exp Ther. 2007;320:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 84. | Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 662] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 85. | Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med. 2012;44:375-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 86. | United States Food and Drug Administration. FDA Briefing Document. NDA 204042. Invokana (Canagliflozin) Tablets. [Accessed. New York: Oxford University Press 2013; United States Food and Drug Administration, 2013 Available from: http//www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Endocrinologic and Metabolic Drugs Advisory Committee/UCM334550.pdf. |
| 87. | Matthews DR, Fulcher G, Perkovic V, DeZeeuw D, Mahaffey KW, Rosenstock J, Davies M, Capuano G, Desai M, Shaw W. Efficacy and safety of canagliflozin (CANA), an inhibitor of sodium glucose co-transporter 2 (SGLT2), added-on to insulin therapy/oral agents in type 2 diabetes. Diabetologia. 2012;55 Suppl 1:S314-S315. |
| 88. | Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, Woerle HJ, von Eynatten M, Broedl UC. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 89. | Monami M, Ahrén B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |