Published online Sep 10, 2015. doi: 10.4239/wjd.v6.i11.1179
Peer-review started: June 16, 2015
First decision: August 4, 2015
Revised: August 11, 2015
Accepted: September 1, 2015
Article in press: September 2, 2015
Published online: September 10, 2015
Processing time: 94 Days and 17.6 Hours
Despite tremendous strides in modern medicine stringent control over insulin resistance or restoration of normoglycemia has not yet been achieved. With the growth of molecular biology, omics technologies, docking studies, and in silico pharmacology, modulators of enzymes and receptors affecting the molecular pathogenesis of the disease are being considered as the latest targets for anti-diabetic therapy. Therapeutic molecular targets are now being developed basing on the up or down regulation of different signaling pathways affecting the disease. Phytosynergistic anti-diabetic therapy is in vogue both with classical and non-classical medicinal systems. However its chemo-profiling, structural and pharmacokinetic validation awaits providing recognition to such formulations for international acceptance. Translational health research with its focus on benchside product development and its sequential transition to patient bedside puts the pharma RDs to a challenge to develop bio-waiver protocols. Pharmacokinetic simulation models and establishment of in vitro-in vivo correlation can help to replace in vivo bioavailability studies and provide means of quality control for scale up and post approval modification. This review attempts to bring different shades highlighting phyto-synergy, molecular targeting of antidiabetic agents via different signaling pathways and bio-waiver studies under a single umbrella.
Core tip: The current research scenario on anti-diabetic drug development pipeline focuses on pharmacological targets influencing the molecular pathogenesis of the disease. It encompasses receptors and enzymes that will increase insulin sensitivity, intracellular insulin signaling, enhance peripheral glucose utilizations, suppress hepatic glucose production and reduce circulating triglycerides levels. Combination therapy has gained significance either with herbal or synthetic drugs, though “phytosynergy” awaits proper validation to give rise to new generations of “phytopharmaceuticals”. Pharmacokinetic simulation models and established in vitro-in vivo correlation that may be extrapolated to humans can serve the purpose of bio-waiver in product transition from lab bench to patient bedside.
- Citation: De B, Bhandari K, Chakravorty N, Mukherjee R, Gundamaraju R, Singla RK, Katakam P, Adiki SK, Ghosh B, Mitra A. Computational pharmacokinetics and in vitro-in vivo correlation of anti-diabetic synergistic phyto-composite blend. World J Diabetes 2015; 6(11): 1179-1185
- URL: https://www.wjgnet.com/1948-9358/full/v6/i11/1179.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i11.1179
The constant escalations in the number of diabetics worldwide has given an alarming signal and fueled intensified research for the development of new therapeutic entities and latest effective therapeutic regimen. The statistics of the global diabetic population is expected to show a steady growth to 366 million by 2030 of which 90% will be type 2 diabetics. The international diabetes federation has estimated the number of diabetics in India to be 40.9 million, which is expected to grow to 60.9 million by 2025[1-3]. Diabetes is a common metabolic disorder with abnormal elevations in the blood-gluco-lipid profile, leading to major complications like diabetic neuropathy, nephropathy leading to end stage renal disease, retinopathy leading to blindness and diabetic foot ulcers necessitating limb amputations[1,2]. Type 2 diabetes is characterized by the hallmark of insulin resistance and β-cell dysfunction and ultimate destruction of pancreatic insulin secreting cells. Combating insulin resistance with the existing pharmacological approaches are unsatisfactory primarily because although they may compensate for the defects in insulin secretion and action, but are ineffective in counteracting β-cell dysfunction and handling the secondary complications of type 2 diabetes[1-3]. While developing a novel anti-diabetic chemical entity, latest drug design approaches focuses on activation-inhibition of enzymes in insulin-sensitive cells, minimization of associated side effects like obesity, substitution or antagonizing of physiological hormones and their pathways. With the advancements in high throughput screening, proteomics, genomics, molecular docking, and combinatorial chemistry, new therapeutic entities are being developed that influence enzyme activities, signaling receptors and pathophysiological pathways[1,2]. Modern day quantitative structural activity relationship and docking studies are enabling development of bio-active molecules that can achieve structural modifications and thereby alter their pharmacological actions and pharmacokinetic profile so as to maximize bioavailability and minimize the side effects[4-10].
Latest anti-diabetic drug development pipeline focuses on pharmacological targets which include receptors and enzymes that will increase insulin sensitivity, intracellular insulin signaling, enhance peripheral utilizations of glucose, suppress hepatic glucose production and reduce the levels of circulating tri glycerides[4-10].
Medicine in recent times, whether western classical or phyto-therapy, advocates for combination therapy, instead of single approach. Synergy research in phyto-therapy, with the aid of “omics technologies” needs a rationale for establishing its pharmacological and therapeutic superiority to treat diseases which have hitherto been treated using synthetic drugs alone[11-15].
Along with the paradigm of translational health research with the perspectives of bench to bedside approach; all pharmaceutical RDs target to develop robust, cost effective, enhanced throughput in vitro assays which may be extrapolated to humans and serve the purpose of bio-waiver. The development of increased number of new chemical entities obviates the need of enhanced pharmacokinetic studies. Though human pharmacokinetic in vivo studies are often considered as the “gold standard” for assessment of bioequivalence but it is expensive, time consuming and difficult to handle enormous amount of pharmacokinetic data. Development of pharmacokinetic simulation models which are computational or mathematical tools help to interpret drug kinetics in living environment under specified conditions and can waive off bioequivalence requirements called bio-waiver studies. Establishment of in vitro-in vivo correlation (IVIVC) provides a justified explanation for bio-waiver during scale up or post approval changes[16-25].
Thus the editorial encompasses the broad areas highlighting phyto-synergy, targeting of different signaling pathways of type 2 diabetes and how computational pharmacokinetics and development of IVIVC serves the purpose of bio-waiver.
Treatment regimen of type 2 diabetes advocates two different approaches, one recommending the sequential use of anti-diabetics and another is a pathophysiologic approach which aims to control the disease conditions basing on pathogenesis with a comparative preference on combination therapy.
American Diabetes Association guidelines incorporated an individualized ABCDE anti-diabetic therapy approach where each alphabet refers to A-age, B-body weight, C-complications (micro and macro vascular), D-disease duration and E-life expectancy and expense. Progressive β-cell destruction coupled with the development of insulin resistance in liver, muscles and adipocytes, subsequent elevation in glycated hemoglobin level being the common pathogenic hallmark in all type 2 diabetes mellitus, though variations are reported amongst different ethnic groups[4-6].
Apart from insulin resistance, a host of cardiovascular co-morbidities like dyslipidemia, hypertension, and central visceral adiposity occur in type 2 diabetes. Evidence based contemporary research paradigms have shown that intra abdominal or visceral fat depots synergize defective insulin action and secretion. Moreover leptins, adiponectins, tumor necrosis factor-α, resistin which are secreted from the adipose tissues interfere with glucose metabolism and insulin sensitivity giving rise to the concept of lipotoxicity in type 2 diabetes. These adipokines greatly modify insulin signaling pathways and promote development of insulin resistance. A triadic relation is found to exist amongst β-cell destruction, insulin resistance and adiposity[4-6].
Sedentary lifestyle, westernized dietary pattern, stress, anxiety, depression, smoking and alcohol consumption are other contributing risk factors of type 2 diabetes. Obesity is also found to be associated with endothelial dysfunction, impairs muscle microcirculation, retards entry of insulin and blood glucose into skeletal muscle and decreases their availability to muscle cells. Lack of physical activity is an important risk factor in type 2 diabetes. Daily physical activities decreases visceral and body fat, increase glycogen synthase (GS) content of the muscle, promotes non-oxidative disposal of glucose as glycogen and activates glucose transporter subtype 4 (GLUT4) to enhance peripheral glucose utilization. Physical activity up regulates expression and activity of proteins involved in insulin signal transduction, improves oxidative capacity of the skeletal muscles, decreases free fatty acid concentrations and enhances the increased expression of downstream signaling components of insulin. Regular exercises also trigger the release of anti-inflammatory cytokines, a protective role against insulin resistance[1,4-6].
An insight into the genetics of type 2 diabetes showed that genes encoding proteins are involved in insulin signaling, glycogen synthesis and glucose transportation, fatty acid uptake and synthesis, adipocyte differentiation and thus suggests associations with diabetes. A clear understanding of human genome sequence is necessary which will help in rapid identification of the genes associated with diabetes. Mutations of five genes viz. glucose metabolizing enzyme glucokinase, transcription factors hepatocyte nuclear factor (HNF) 1α and β, HNF4α and insulin promoter factor 1 (IPF1) affect moderate to significant reductions in insulin secretions. Latest research reporting does have mentioned that genetic variation of newly encoded gene Calpain, called as CAPN10 gene can cause diabetes[5-9].
Amongst the Oral Hypoglycemic Agents (OHAs) mostly recommended for type 2 anti-diabetic therapy, sulfonylureas (e.g., tolbutamide, glibenclamide, acetohexamide) and biguanides (e.g., phenformin, metformin) are in wide use followed by thiazolidinediones (also known as glitazones, e.g., Rosiglitazone, Pioglitazone) and alpha glucosidase inhibitors (acarbose, miglitol, voglibose). Sulphonyl ureas work primarily by stimulating pancreatic insulin secretion and reduce the hepatic glucose output and enhance peripheral glucose utilizations. Biguanides are anti-hyperglycemic agents rather than hypoglycemics, suppress excessive hepatic glucose production, increases peripheral glucose utilizations to a lesser extent, reduce intestinal glucose absorption by reducing food intake. Alpha glucosidase inhibitors delay the breakdown of disaccharides and polysaccharides and hence glucose absorption is delayed. Thiazolidinediones enhance insulin sensitivity in peripheral tissues.
However, the available pharmacological approaches for anti-diabetic therapy are not successful enough to put a stringent control on insulin resistance. Instead of mono therapy now combination therapy and muti-drug formulations are in vogue. With the development of proteomics, genomics and a thorough understanding of the molecular pathways, the development of new molecular targets with anti-diabetic potentials focuses in modulating pharmacokinetics, cellular location, overall distribution etc. Modulators of enzymes and receptors are now becoming the molecular targets for any disease therapy[8-10].
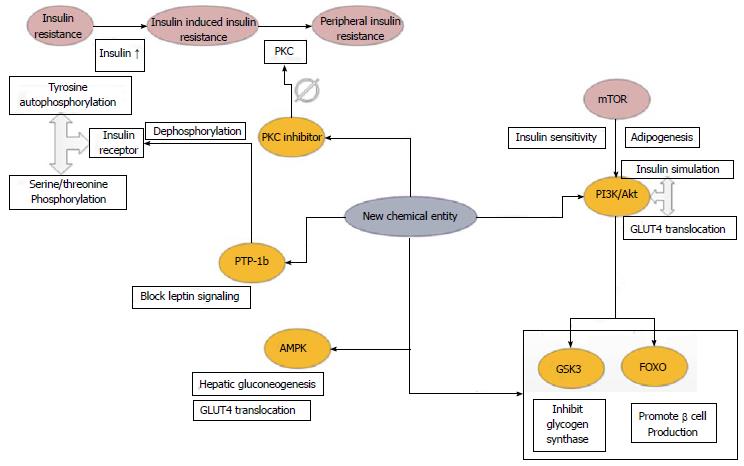
The three targeted tissues of insulin action include skeletal muscle, adipose tissue and liver. Insulin binds with the target cell surface receptor and activates the tyrosine kinase which is a constituent of the receptor molecule. Tyrosine residues of the insulin receptors undergo autophosphorylation and the serine/threonine residues become phosphorylated[7-10]. In type 2 diabetes elevated levels of insulin stimulates serine kinases via IGF-1 receptor (Insulin like growth factor 1) leading to insulin resistance[7-10]. Protein kinase C (PKC) is known to play a significant role in developing peripheral insulin resistance. Thus inhibition of PKC or its reduced expression may enhance insulin sensitivity and insulin receptor tyrosine kinase activity which can be an effective therapeutic strategy against type 2 diabetes. Protein tyrosine phosphatase-1b (PTP-1b) causes dephosphorylation of insulin receptor and is a negative regulator of the insulin signaling. It enhances insulin activity and is resistant to the development of obesity. PTP-1b down regulates or blocks leptin signaling by dephosphorylating Janus kinase (JAK). Thus PTP-1b serves as an essential therapeutic target. Phosphoinositide 3 kinase (PI3K) plays a significant role in the glucose uptake via insulin stimulation and GLUT4 translocation. PI3K is down regulated by two classes of serine/threonine kinases, Akt, also known as protein kinase B (PKB) and the isoforms of PKC[7-10]. Akt and isoforms of PKC are known to facilitate GLUT4 translocation. P70s6k directly phosphorylates IRS (insulin receptor substrate) which inhibits its activity and hinders Akt actively. Mammalian target of rapamycin (mTOR) has a significant role in obesity and IR and activates both Akt and p70s6k. The essential targets for Akt include the transcription factors, glycogen synthase kinase 3 (GSK3) and the forkhead box subgroup O (FOXO). GSK3 can phosphorylate and inhibit GS. Now phosphorylation of Akt inactivates GSK3 and leads to an increase in glycogen synthesis[7-10]. Akt phosphorylation also targets FOXO mediated transcription of target genes that promote the production of β-cells. To counteract IR and restore insulin sensitivity therapeutic agents should target to increase PI3K/Akt activity. Lipid phosphatase PTEN (phosphatase and tensin homolog) dephosphorylates phosphatidylinositol (3,4,5) trisphosphate (PIP3) making it less available to recruit Akt. Also downstream regulation of mTOR can regulate adipogenesis and insulin sensitivity[7-10].
AMPK (AMP activated protein kinase) regulates hepatic gluconeogenesis and increase muscle glucose uptake by translocation of GLUT4 which also serves the purpose of an essential therapeutic target[10]. A comprehensive scheme of the different receptor signaling pathways have been presented below in Figure 1.
Some of the novel molecular targets for anti-diabetic therapy have been mentioned in Table 1.
| Type | Target for action | Nature of action | Effect produced |
| Protein kinases | Protein kinase C | Inhibitory | Block receptor desensitization |
| AMP activated kinase | Activator | Enhance glucose transport | |
| GSK-3 | Inhibitory | Activate glycogen synthase | |
| MAP kinase | Inhibitory | Block receptor desensitization | |
| Protein phosphatases | PTP-1b | Inhibitory | Block receptor dephosphorylation |
| PP1 | Activator | Activate glycogen synthase | |
| LAR | Inhibitory | Block receptor dephosphorylation | |
| Lipid phosphatases | PTEN | Inhibitor | Increase PIP3-stimulated glucose transport |
| Cell surface receptors | Insulin receptor | Agonist | Insulin mimetic |
| Glucagon receptor | Antagonist | Low fasting glucose | |
| GLP receptor | Agonist | Increase insulin secretion | |
| β-3 adrenergic receptor | Agonist | Increase lipolysis | |
| Ion channels | Sulphonyl urea receptor | Inhibit K channel | Increase insulin secretion |
| Transcription factors | PPAR-γ | Selective modulator | Insulin sensitizer |
| HNF4 | Selective modulator | Increase insulin secretion |
Synergy refers to the increased effectiveness that results when two or more elements work together, though here we will refer to phytochemical constituents. Synergism is the total outcome of a cumulative effect which is greater than the sum of individual effects. From the dimensions of pharmacology, molecular biology or clinical research, synergism can be either in the form of multi target effect where different phyto-constituents of a single extract or a composite extract will affect more than one targets agonistically and exhibit synergism[11,12]. Synergy can give better outcomes in terms of pharmacokinetic profile or physicochemical effects based on enhanced solubility profile, improved absorption and ultimately better bioavailability. Use of synergistic combinations also helps to restrict the development of resistance due to single prolonged drug use. While synthesizing or processing a single entity, unwanted adverse effects may develop due to either the extraction procedure or synthetic scheme being followed, or development of any by products; such adverse effects can be minimized or eliminated by use of combo formulations. Moreover stability issues of one to several bio-actives on long storage are more protected in combined form than in isolated form[11,12].
Combination therapy has made its way in the treatment of type 2 diabetes whether it is western classical medicine or herbal formulations. Resveratrol, a phytoalexin found in grapes which acts on various molecular targets in adipocytes and osteoblasts decreases the number of adipocytes and acts synergistically with quercetin and genistein to reduce adipogenesis[12]. Evidence based clinical research results have shown that miglitol in combination with metformin provides a better glycemic control than metformin monotherapy which is an example of synergism in anti-diabetic therapy with western medicine. Oleanolic acid, a pentacyclic triterpene, a natural component of many medicinal herbs in combination with metformin, first line antidiabetic drug showed synergistic anti-diabetic potentials in animal studies[13]. Experimental results showed that the combination reduced hepatic gluconeogenesis by decreasing mRNA expressions of PGC-1α, G-6-Pase and PEPCK (Phosphoenol pyruvate carboxykinase 1). The combination is also found to stimulate the PI3K pathway that phosphorylates Akt and down regulates mTOR to improve insulin resistance. Sesame oil, an edible oil rich in mono and polyunsaturated fatty acids is found to show synergistic anti-diabetic potentials with sulphonyl ureas viz. glibenclamide[14]. In case of allopathy, results of clinical trials have shown that combination therapy with miglitol and metformin was found to be more effective than the use of single drug alone[15].
Establishment of standard quality control profile in global context to confirm the validity and reproducibility of phytochemical constituents in the form of processed extract rather than single isolated compound; proper analytical and spectroscopic method development for structural characterizations in combined forms; rigorous validation of safety profile and pharmacokinetic parameters is essential to find a scientific basic of phytosynergy which may give rise to a new generation of medicinal products - phyto-pharmaceuticals[11].
Drug development procedure is very tedious and expensive and in many cases due to lack of adequate pharmacokinetic data of the candidate drug, completion of further research becomes questionable. With the vast expansions in the research arenas undertaking the development of new chemical entities, bioequivalence studies are of vital concern in drug development especially when there are absolute new entities or having narrow therapeutic index. Though in vivo animal experimentation for establishing the pharmacokinetic profile is still the surrogate, yet it’s very tedious, expensive, and time consuming to handle enormous amount of data. Along with the development of in silico pharmacology, computational modeling now finds applications in pharmacokinetics and dynamics, as well as toxicokinetics and dynamics. Many multi-national pharma R&Ds are now focusing on bio-waiver where in many cases in vitro results were considered more acceptable in different dosage formulations especially immediate release solid dosage forms[16-18]. In that condition to proceed with a bio-waiver study there’s a need to establish dissolution profile and is to be characterized with both model dependent and independent approaches. In vivo performance of a dosage formulation or new chemical entity can be simulated from the in vitro dissolution data after establishing a definitive IVIVC[19-23].
The biopharmaceutics classification system (BCS) proceeds with a predictive approach for developing correlation between physicochemical criteria of drug formulations and its in vivo bioavailability. BCS is not the direct IVIVC; IVIVC develops a mathematical relation between in vitro and in vivo data by either linear or non- linear correlation[19-25]. As per FDA guidelines IVIV correlation ranges from A-D with multiple level C correlation, the details of which have been presented in Table 2.
| Level | In vitro parameters | In vivo parameters | Utility |
| Level A: direct relationship with in vivo data based on in vitro measurement alone | Dissolution curves | Absorption curves | Highest level of correlation depicting point to point relation between in vitro dissolution rate and in vivo input rate of drug from dosage form. Marks in vitro dissolution as the surrogate of in vivo performance |
| Level B: relation based on statistical moments analysis | MDT | MAT; MRT | Mean in vitro dissolution time of the product compared to mean in vivo residence time or mean in vivo dissolution time |
| Level C: relates one dissolution time point (t50%, t90%, etc.) to one mean pharmacokinetic parameter (AUC, Cmax, tmax) | Disintegration time, time to have 10%, 50%, 90% dissolved, dissolution rate, dissolution efficiency | Cmax, Tmax, Ka, time to have 10%, 50%, 90% absorbed, AUC (total or cumulative) | Single point weak correlation showing a partial relation between absorption and dissolution. Used in early stages of formulation development before pilot production |
Apart from these three types of correlation, level D correlation is a rank order and qualitative method which may be applicable in some steps of formulation development but not recommended for regulatory purposes. A multiple point level C correlation is really a justified bio-waiver where correlation is established over the entire dissolution profile with one or more pharmacokinetic parameters of interest. This correlation is based on three dissolution points (early, middle and end stages) and on achievement of this correlation level, the level A correlation is also likely to develop[19-21].
Even after the attainment of high level of correlation, till date no in vitro method can exactly simulate physiological conditions in vivo especially when it comes to replicate the exact gastro-intestinal (GI) conditions in vitro viz. appropriate amount, pH and exact physiological amounts of enzymes needed for digestion, physiological transits during digestion process, exact replication of peristalsis, food - drug interactions and its impact on dosage formulations. An artificial digestive system known as TIM1 have been developed by TNO Nutrition and Food Research mimicking the human stomach and three segments of small intestine where pH is monitored and computer controlled, constant generation of water pressure ensures mixing of enzymes by alternate compression and relaxation of flexible walls and removal of water and small molecules from lumen compartment by pumping dialysis fluid mimics the GI motility. Though such artificial models find applications in nutrition research but to be an effective quality control tool in drug development studies warrants further research[21].
Translational health research is the latest buzzword in the field of biomedicine which aims to bridge basic research with medical innovation with the perspectives of sequential development of products from lab bench to patient bedside. The landscape of drug discovery which is just the initiation of creating new chemical entities has undergone a drastic change after the emergence of computational biology, combinatorial chemistry and in silico docking studies. Now drug molecules are tailored as per requirements for maximizing bioavailability and stringent control over pharmacokinetics. Combination therapies with synergistic potentials are finding more prominence than monotherapy and even documentations are available in some anti-diabetic medications where combination of natural and synthetic medicine showed better results. However to capture the international pharma market and speed up the pilot scale production, there is an urgent need to boost bio-waivers which necessitates to develop robust and reproducible in vitro models simulating in vivo conditions.
P- Reviewer: Fang Y, Hssan M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Mitra A, Dewanjee D, Dey B. Mechanistic studies of lifestyle interventions in type 2 diabetes. World J Diabetes. 2012;3:201-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Dey B, Mitra A. Chemo-profiling of eucalyptus and study of its hypoglycemic potential. World J Diabetes. 2013;4:170-176. [PubMed] [DOI] [Full Text] |
| 3. | Dey B, Mitra A, Katakam P, Singla RK. Exploration of natural enzyme inhibitors with hypoglycemic potentials amongst Eucalyptus Spp. by in vitro assays. World J Diabetes. 2014;5:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 621] [Cited by in RCA: 616] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 5. | Scheen AJ. Pathophysiology of type 2 diabetes. Acta Clin Belg. 2003;58:335-341. [PubMed] |
| 6. | DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36 Suppl 2:S127-S138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 586] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 8. | Bhattacharya S, Dey D, Roy SS. Molecular mechanism of insulin resistance. J Biosci. 2007;32:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | May O. Diabetes and insulin signaling: A new strategy to promote pancreatic β cell survival. Available from: https://www.caymanchem.com/app/template/Article.vm/article/2108. |
| 10. | Bahare RS, Gupta G, Malik S, Sharma N. New emerging targets for type 2 diabetes. Int J Pharm Tech Res. 2011;3:809-818. |
| 11. | Wagner H, Merzenich GU. Synergy research: approaching a new generation of phytopharmaceuticals. J Nat Reme. 2009;9:121-141. [RCA] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 703] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 12. | Rayalam S, Della-Fera MA, Baile CA. Synergism between resveratrol and other phytochemicals: implications for obesity and osteoporosis. Mol Nutr Food Res. 2011;55:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Wang X, Chen Y, Abdelkader D, Hassan W, Sun H, Liu J. Combination therapy with oleanolic acid and metformin as a synergistic treatment for diabetes. J Diabetes Res. 2015;2015:973287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Sankar D, Ali A, Sambandam G, Rao R. Sesame oil exhibits synergistic effect with anti-diabetic medication in patients with type 2 diabetes mellitus. Clin Nutr. 2011;30:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Chiasson JL, Naditch L. The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care. 2001;24:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Mishra V, Gupta U, Jain NK. Biowaiver: an alternative to in vivo pharmacokinetic bioequivalence studies. Pharmazie. 2010;65:155-161. [PubMed] |
| 17. | Bois FY. Computational pharmacokinetics at a crossroads. In Silico Pharmacol. 2013;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Polli JE. In vitro studies are sometimes better than conventional human pharmacokinetic in vivo studies in assessing bioequivalence of immediate-release solid oral dosage forms. AAPS J. 2008;10:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Murtaza G, Azhar S, Khalid A, Nasir B, Ubaid M, Shahzad MK, Saqib F, Afzal I, Noreen S, Tariq M. Development of in vitro-in vivo correlation for pharmacokinetic simulation. Afri J Pharm Pharmcol. 2012;6:257-263. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Nainar S, Rajiah K, Angamuthu S, Prabakaran D, Kasibhatta R. Biopharmaceutical classification system in vitro/in vivo correlation: concept and development strategies in drug delivery. Trop J Pharm Res. 2012;11:319-329. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Cardot JM, Beyssac E, Alric M. In vitro-In vivo correlation: Importance of dissolution in IVIVC. Dissolu Technol. 2007;15-19. |
| 22. | Hardikar S, Bhosale AV, Budhawant RN. Establishment of In vitro-In vivo correlation: a cogent strategy in product development process. Ind J Pharm Edu Res. 2014;48:66-73. [DOI] [Full Text] |
| 23. | Chowdhury AK, Islam S. In vitro-In vivo correlation as a surrogate for bioequivalence testing: the current state of play. Asian J Pharm Sci. 2011;6:176-190. |
| 24. | Emami J. In vitro - in vivo correlation: from theory to applications. J Pharm Pharm Sci. 2006;9:169-189. [PubMed] |
| 25. | Shohin IE, Ramenskaya GV, Savchenko AY. Developing In vitro-In vivo correlation (IVIVC) for trimetazidine, indapamide and ciprofloxacin extended release solid oral dosage forms. Int J Pharma Biosci. 2011;2:573-580. |