Published online Jun 15, 2014. doi: 10.4239/wjd.v5.i3.364
Revised: April 10, 2014
Accepted: May 8, 2014
Published online: June 15, 2014
Processing time: 264 Days and 0.7 Hours
Adrenomedullin (ADM) is a peptide hormone widely expressed in different tissues, especially in the vasculature. Apart from its vasodilatatory and hypotensive effect, it plays multiple roles in the regulation of hormonal secretion, glucose metabolism and inflammatory response. ADM regulates insulin balance and may participate in the development of diabetes. The plasma level of ADM is increased in people with diabetes, while in healthy individuals the plasma ADM concentration remains low. Plasma ADM levels are further increased in patients with diabetic complications. In type 1 diabetes, plasma ADM level is correlated with renal failure and retinopathy, while in type 2 diabetes its level is linked with a wider range of complications. The elevation of ADM level in diabetes may be due to hyperinsulinemia, oxidative stress and endothelial injury. At the same time, a rise in plasma ADM level can trigger the onset of diabetes. Strategies to reduce ADM level should be explored so as to reduce diabetic complications.
Core tip: Adrenomedullin (ADM) is a peptide hormone with vasorelaxing and hypotensive properties. It also plays multiple roles in the regulation of hormonal secretion, glucose metabolism and inflammatory response. A major observation is the elevation of plasma ADM level in diabetes, and is associated with diabetic complications in both type 1 and 2 diabetes. The increase could be resulted from oxidative stress, hyperinsulinemia and endothelial injury. This raises the potential application of ADM as a marker in diabetes, and strategies aimed at reducing ADM level could be explored so as to alleviate diabetic complications.
- Citation: Wong HK, Tang F, Cheung TT, Cheung BMY. Adrenomedullin and diabetes. World J Diabetes 2014; 5(3): 364-371
- URL: https://www.wjgnet.com/1948-9358/full/v5/i3/364.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i3.364
Adrenomedullin (ADM) is a peptide recently discovered with multiple functions. Its characteristic actions include vasorelaxing effect and hypotensive properties. Given its widespread expression and production in different organs, ADM can also act as an autocrine, endocrine or paracrine mediator in various biological systems. The prospects of ADM as a potential disease modulator comes from the observation of increased levels in plasma in various disease states. For instance, increased plasma ADM levels were observed in cardiovascular diseases and diabetes[1-3]. However, different from the observations in cardiovascular diseases, the explanation and significance for such an increase is not clear. Since then, research progress has been made in the association between ADM and diabetes. For instance, ADM plays a role in glucose metabolism and insulin balance[4]. These evidence may provide clue on the involvement of ADM in diabetes.
In this review, we summarized the current knowledge on ADM based on research progress in the recent decade and provided an account on the role of ADM played in the context of diabetes. This would help us understand better on the clinical application of ADM in diabetic patients.
ADM was initially discovered by Kitamura in 1993, extracted from pheochromocytoma in humans by monitoring the elevated 3’,5’ cyclic adenosine monophosphate (cAMP) production in human platelets[5]. It was later found that the peptide had a potent hypotensive and vasorelaxing effects. It forms a ring structure by 52 amino acid residues held by a disulfide bond. Since the peptide was abundantly found in the adrenal medulla, therefore this accounts for the name. The peptide is classified as a member of the calcitonin gene-related peptide (CGRP) superfamily. Although high level of ADM was identified in the adrenal medulla[6], circulating ADM was the most abundant in vascular wall[7].
ADM has a very high tissue distribution. Its biosynthesis has been studied by applying radioimmunoassays, and by detecting tissue ADM mRNA[8]. Immunoreactive ADM is detected in cardiovascular, respiratory, renal, endocrine, reproductive, neurological, intestinal and immune system[9,10]. Among these systems the highest ADM concentrations were detected at the adrenal glands. ADM mRNA is also detected in various peripheral tissues[11]. Such wide distributions indicate the multi-facet roles of ADM.
In the cardiovascular system, ADM is synthesized in both atria and ventricles in heart and blood vessels. Within the vasculature, ADM is actively manufactured and secreted by both the endothelial and the vascular smooth muscle cells[7,12]. It is also demonstrated that the vasculature had much higher ADM mRNA expression than the adrenal glands. This was further supported by the finding of a low ADM precursor ratio in the total ADM immunoreactivity in blood vessels[11].
Besides, ADM is synthesized in the lung[13], brain as well as in the pancreatic islets[14,15]. The widespread ADM expression suggests its diverse role in the regulations of cell functions. Since ADM is mainly produced by vascular endothelial and the smooth muscle cells, its regulatory function of vascular tone has become a major target for investigation.
ADM production is controlled by various humoral factors and physical factors. Inflammatory cytokines such as tumor necrosis factor (TNF)-α, TNF-β, interleukin (IL)-1α and IL-1β all are known to stimulate ADM production and secretion[16]. While mechanical factors like sheer stress and hypoxia are involved in the up-regulation of vascular ADM mRNA expression[17].
In healthy individuals, circulating plasma ADM level is as low as in the picomolar range, similar to the atrial natriuretic peptide, and its level changes in order to compensate for the vasoconstrictive effects. It is reported that in various pathological conditions, the increase in plasma ADM level correlates with severity of disease states. For instance, elevated plasma ADM level has been associated with heart failure, hypertension, artherosclerosis and diabetes mellitus[18].
Specific binding sites for ADM were identified in many different places in rat and in human models[19,20]. In humans, the binding sites are most abundant in the microvascular endothelium[20]. The biological actions of ADM are exerted mainly through CGRP receptors and the specific ADM receptors, which share a common molecular component of a G-protein coupled receptor called calcitonin receptor-like receptor (CRLR)[21]. The specificity of CRLR depends on different subtypes of another associated proteins, namely the receptor-activity-modifying proteins (RAMP1, 2 and 3)[22]. Co-expression of CRLR with different subtypes of RAMPs will form different ADM receptors. The specificity brought about by the RAMPs involves glycosylation and transport of the receptor-RAMP complex.
ADM can act as both a hormone and a cytokine to regulate the regional blood flow, vascular tone, leukocyte migration and differentiation, electrolyte balance, cardiac function, glucose uptake and hormone secretion[18]. It plays an important role in cardiovascular system[23]. ADM imposes a potent vasodilatory effect in humans and increases blood flow to various organs[24,25]. For instance, increased ADM expression could enhance hepatic and renal circulation[26]. In systemic circulation, vasodilation could be resulted from either endothelium-dependent[27], or endothelium-independent mechanisms[28], through ADM and CGRP receptors. In addition, the endothelium-derived vasodilation could be mediated by cAMP and nitric oxide[29,30].
Previous studies have identified the role of ADM in inflammation and immunity. ADM possesses anti-microbial properties against bacteria[31]. In vitro and in vivo study has demonstrated that ADM secretion and expression are up-regulated upon pathogenic exposure[32]. ADM expression also increases during local inflammation and sepsis[33] In particular, ADM levels in lung, heart and vasculature[34], liver and kidney[26], all increase upon endotoxin administration[35]. Macrophages could also augment ADM expression in inflammation[33].
The role of ADM in the inflammatory process varies after the onset of inflammation. ADM can activate and modulate cytokine production, while it can also inhibit overproduction of pro-inflammatory cytokines[36]. It plays a crucial role in initiating inflammatory response by stimulating the release of migratory inhibitory factor and IL-1β, while activate anti-inflammatory response by suppressing TNF-α production and up-regulating IL-6 production, as the latter is anti-inflammatory and inhibit lipopolysaccharide-induced TNF-α production[37-39]. Such co-ordinated functions of ADM suggest that it is associated with injury, infection and inflammation. Apart from inflammation, ADM expression in immune cells serves diverse functions. ADM can be detected in macrophages in the atherosclerotic plaques[40], where it may play a role in reducing inflammation and thereby exerting an anti-atherosclerotic effect.
While circulating ADM in plasma contributes to a large part of its physiological functions, ADM also serves as a local regulator of cellular functions. The paracrine effect of ADM can be demonstrated in the kidney, as it has been shown that ADM is histochemically localized in renal tubules, and recently mesangium was suggested to be one source of ADM in the kidney[41]. The local ADM modulates mesangial proliferation and is regulated by different growth factors and cytokines. This suggests that regulation of renal function by ADM may operate in an autocrine/paracrine manner. Another example of the localized effect of ADM is in the vascular smooth muscle cells, where its biosynthesis is regulated through a feedback loop. In one study, stimulation of ADM mRNA levels was observed together with a decrease in the immunoreactive ADM peptide secretion resulted from glycolytic inhibition[42]. As ADM could inhibit vascular smooth muscle cell migration and proliferation in response to growth factors[43], a decreased ADM secretion might stimulate its migration and growth locally, and lead to remodeling upon vascular injuries.
ADM is deeply involved in pancreatic endocrinology, mainly in insulin secretion[44]. It is known that ADM, CRLR and RAMPs are both expressed in the islets of the pancreas[45]. Previous findings demonstrated that exogenous ADM added to freshly isolated rat islets led to a dose-dependent inhibition of insulin secretion by 78% at 1 μmol/L ADM, and was accompanied by cAMP elevation[3]. Oral glucose tolerance tests have illustrated injection of ADM lowered insulin levels in blood by 2 folds 20 min after glucose administration, accompanied by an increase in circulating glucose[4]. This supports a role of ADM in insulin regulation in pancreas, and implies that ADM is associated with hyperglycemia[46].
Another function of ADM is inhibiting amylase secretion in pancreatic acini[47]. As ADM receptors were not identified in the acini, this suggest that such inhibition is mediated through other receptors[45].
As suggested above, ADM inhibits insulin release after an oral glucose load. Therefore, it can be expected that ADM contributes to diabetes and even leads to the development of diabetic complications[48].
Diabetes is characterized by hyperglycemia. It is resulted from dysregulation of insulin secretion or peripherial resistance. Diabetes mellitus causes retinopathy, neuropathy, nephropathy, and atherosclerosis. These complications are the results of prolonged hyperglycemia, altered metabolic pathways and non-enzymatic glycation of proteins[49].
There have been advances in the understanding of the relationship between ADM and diabetes. Plasma ADM level is elevated in patients with poorly controlled diabetes than in normal subjects, which suggests a direct effect of glucose on ADM release[1]. The effect of hyperglycemia on ADM expression is mediated through protein kinase C in vascular smooth muscle cells[50]. The observation that ADM expression in aorta, but not in adrenal gland, was raised in diabetic rats (plasma glucose = 567 ± 167 mg/dL) compared to control (plasma glucose = 94 ± 10 mg/dL), suggests that ADM expression in the vasculature could be the source of plasma ADM in diabetic patients[50]. In the streptozotocin-diabetic rat, there were increases in ADM synthesis in the ventricles and possible ADM secretion in the ventricles, atria and the thoracic aorta[51]. On the other hand, ADM may reduce the levels of inflammatory cytokines and endothelin in the adipose tissue and the skeletal muscle and hence increase glucose uptake[37].
However, another study examining the relationship between plasma ADM level and clinical parameters of diabetes demonstrated contradictory results. It showed no significant difference in plasma ADM level between diabetic patients without nephropathy and normal individuals, despite a significant higher level of HbA1c and plasma glucose in patients with diabetes[52]. Therefore, patients with renal impairment should be excluded when examining the relationship between plasma ADM level and blood glucose level, since patients with renal impairment might demonstrate an increase in the plasma ADM levels. Despite the direct effect of circulating glucose on plasma ADM level has not been well established, a positive association between plasma ADM level and the mean blood pressure has been demonstrated in the same study. Given the high plasma ADM levels in various disorders[53], the elevated ADM levels in diabetes might suggest that it has a protective role. Earlier research also showed an elevated plasma ADM level in patients with hypertension and chronic renal failure, particularly a 3-fold elevation in plasma ADM level associated with more severe renal failure. The elevation in ADM may help to prevent blood pressure increase and body fluid retention[54], and represent a compensatory mechanism for diabetic complications.
One characteristic of type 1 diabetes is the destruction of β-cells in the islets of Langerhans which produces insulin. Previously there was a report investigating the association of ADM and type 1 diabetes. ADM and cAMP levels were compared between type 1 diabetes patients with various complications and healthy individuals[55]. According to the data, increased plasma ADM level was identified only in patients having renal insufficiency, while patients with other complications had normal ADM level. A significant inverse correlation was also found between ADM levels and the creatinine clearance by multiple regression analysis. This suggested that when the kidney function was impaired, clearance of ADM was possibly decreased and resulted in an increase in the plasma level. Such hypothesis deserves further confirmation because most of the circulating ADM was shown to be cleared in the lungs instead of the kidneys[56]. In the same analysis, the relationship between the plasma ADM and the disease duration suggested the change in ADM level is resulted from the endothelial dysfunction.
Despite the uncertainty of the origin of plasma ADM, a recent study postulated that the selective dilation of glomerular capillaries in type 1 diabetes was attributed to the up-regulation of ADM and RAMP2 expression in the afferent arterioles and glomeruli, through the induced release of nitric oxide[57]. This may provide a hint that locally produced ADM can elicit vasodilatation action by paracrine control, independent of any changes in plasma ADM levels. ADM is also involved in the pathogenesis of retinopathy[58]. Since ADM is produced in the vasculature, endothelial activation caused by vessel damage may explain the increase in plasma ADM level. Another possibility is that ADM acts as a factor for survival of the endothelial cells[59], so plasma level of ADM increases upon endothelial injury. A significant positive association between ADM and cAMP in diabetic patients further supported the hypothesis that ADM plays a counter-regulatory role to prevent excessive vasoconstriction and vessel damage, and promotes natriuresis[54,60,61].
All these findings suggested an increase in plasma ADM level is the consequence rather than the cause of type 1 diabetes, since there are insufficient findings to demonstrate the direct link between ADM and the disease states. This can be further supported by the comparison of hypoglycemic- and hyperglycemic-patients in the same study in which no difference in the plasma ADM level was found.
Several studies have been carried out in an attempt to explain the rise in plasma ADM level and its implications in diabetic complications. One study showed that plasma ADM level was elevated in type 2 diabetes but did not correlate with glucose level in circulation[62]. Instead, increased ADM level was correlated with various diabetic complications, and the severity of diabetic nephropathy and retinopathy. Other parameters like serum creatinine level, systolic blood pressure, and urinary protein excretion were found to be related to ADM levels as well. ADM levels might therefore be related to the development of microangiopathy.
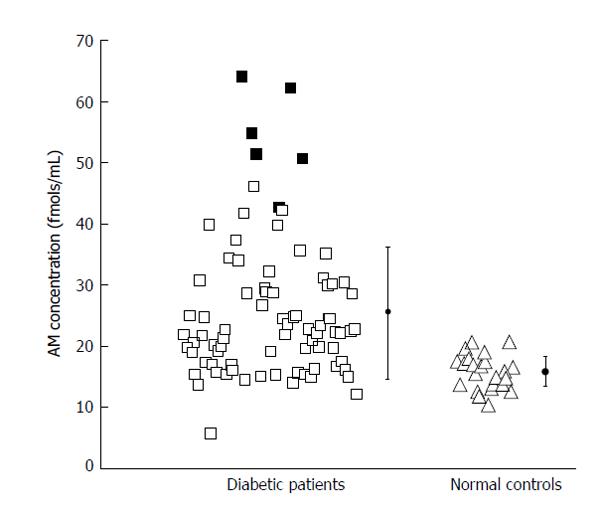
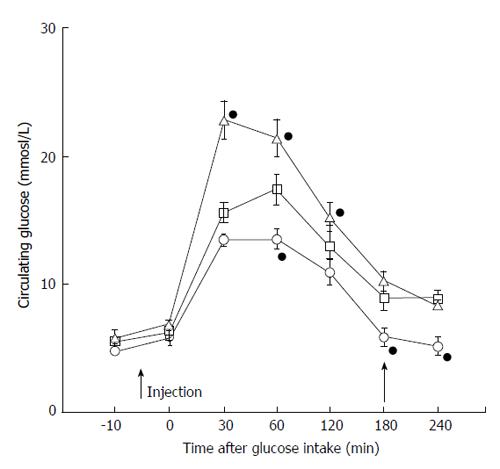
Another study examined a group of patients with a common feature of hyperglycemia development. The group had recent onset of diabetes induced by a drug treatment[63]. Results showed that the group can be characterized by a subset of patients with extremely high ADM levels (Figure 1). Even though the source of such excessive ADM is unknown, the results suggested that hyperglycemic patients are characterized by higher circulating ADM levels. In the same studies, the influence of ADM in blood glucose modulation was studied using an obese SHR rat model mimicking human type 2 diabetes. Synthetic ADM, blocking monoclonal antibody against ADM or saline were injected into the animals, and then glucose tolerance tests were carried out. In support to a previous study[4], ADM injection increased blood glucose level more significantly in diabetic rats, while application of antibody effectively reduced blood glucose level to even lower than saline control and improved postprandial recovery in diabetic rats (Figure 2). All these data raise the possibility that ADM is a causative factor in type 2 diabetes and has a negative impact on glycemic control.
To further explore the role of ADM incausing type 2 diabetes, the effect of ADM on insulin secretion has to be considered. There are studies addressing the association of ADM with insulin balance. There is a positive association between insulin resistance and plasma midregion pro-adrenomedullin levels[64]. The link between acute hyperinsulinemia and ADM has been proposed, in which plasma ADM levels increased in acute hyperinsulinemia[65]. There was a concomitant increase in plasma ADM levels with increasing insulin production, and a significant positive correlation between serum insulin levels and plasma ADM was seen in type 2 diabetic patients. The authors speculated that the increased insulin-stimulated ADM production from the pancreatic islets compensated for the diminished vasodilatory effect of insulin, hence this protects against arterial hypertension.
In the recent decade the effect of oxidative stress on ADM expression has been suggested. One study evaluated such relationship by measuring plasma levels of 8-epi-prostaglandin F2α (8-epi-PGF2α, a marker of oxidative stress) and ADM in normal and hypertensive subjects[66]. Both plasma levels were elevated in the hypertensive group (P < 0.05 for 8-epi-PGF2α and P < 0.02 for ADM respectively), and the data showed that 8-epi-PGF2α was associated with ADM in hypertensive patients with type 2 diabetes (r = 0.696, P < 0.01). It is known that oxidative stress could stimulate ADM mRNA expression and secretion from endothelial and vascular smooth muscle cells[67]. Sustained ADM deficiency increased oxidative stress and led to insulin resistance via impaired insulin signaling, which is supported by an angiotensin (Ang)-II treated mouse model[68]. Ang-II could induce oxidative stress and hypertensive conditions, and it was shown that Ang-II reduced insulin sensitivity in ADM-knockout heterozygous mice more than wild type mice. This suggests that endogenous ADM may act against insulin resistance induced by oxidative stress and offer protection from organ damage through its anti-oxidant action.
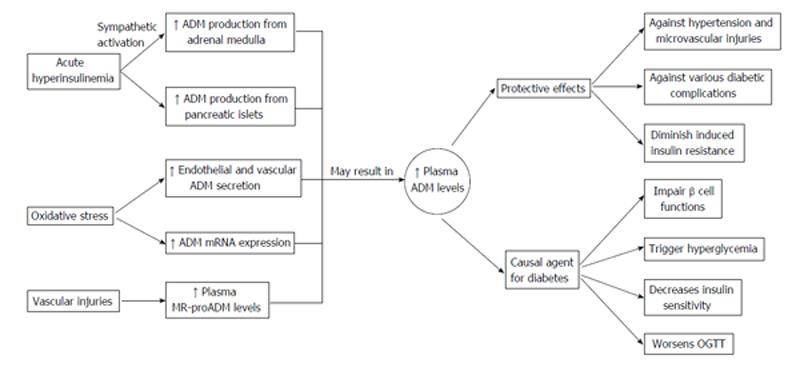
The interactions between ADM and diabetic complications are dynamic and complex. While conflicting arguments have been put forward to the link between poor metabolic control and increased ADM levels[64], it is generally accepted that plasma ADM levels are positively linked to oxidative stress[66], acute hyperinsulinemia[65], and other risk factors causing endothelial injury (Figure 3). This leaves much ground for further research about the causes and significance for the plasma ADM level increase.
There are two main questions that have to be answered in order to establish a link between ADM and diabetes: Firstly, what are the causes for the increase in plasma ADM levels in diabetic patients, and what are the sources for the elevated circulating ADM? What kind of stress or stimulation are involved? Secondly, what is the implication for the elevated level? Would it further worsen the glycemic condition and result in various diabetic complications?
Based on the above questions, numerous studies have been commenced. Research has demonstrated the association between diabetic complications and the increase in plasma ADM level. Plasma ADM levels were mainly associated with renal failure and retinopathy in type 1 diabetes. However, the correlation with hyperglycemia is still not clear and requires further investigation.
On the other hand, plasma ADM levels in type 2 diabetes patients are linked to a wider range of complications. The rise may be attributed to acute hyperinsulinemia, oxidative stress and endothelial damage. These stimuli increases ADM production from pancreatic islets and vascular endothelium. Such a rise may represent a causative factor triggering the onset of disease and insulin resistance. If this assumption holds, a controlled reduction in ADM levels may improve hyperglycemia. To understand the casual role of ADM in diabetes, genetic variants could be a potential variable to study using Mendalian randomization, since it is unlikely to be confounded by environmental factors. Our recent study has demonstrated a positive link between a single nucleotide polymorphism (SNP) of ADM gene and development of dysglycemia[69]. Our other studies also demonstrates that plasma ADM level is associated with one of its SNP, IL-6 and adiponectin SNPs[70-72]. In the future regulation of ADM level could be a key in controlling glycemia in people with diabetes and this warrants further investigation.
Permission was granted from the publisher to use the copyrighted figures in this manuscript.
P- Reviewers: Romani A, Swierczynski JT, Yin DP S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Hayashi M, Shimosawa T, Isaka M, Yamada S, Fujita R, Fujita T. Plasma adrenomedullin in diabetes. Lancet. 1997;350:1449-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Yu CM, Cheung BM, Leung R, Wang Q, Lai WH, Lau CP. Increase in plasma adrenomedullin in patients with heart failure characterised by diastolic dysfunction. Heart. 2001;86:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Wong HK, Cheung TT, Cheung BM. Adrenomedullin and Cardiovascular Diseases. J R Soc Med. 2012;1:14. [PubMed] |
| 4. | Martínez A, Weaver C, López J, Bhathena SJ, Elsasser TH, Miller MJ, Moody TW, Unsworth EJ, Cuttitta F. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology. 1996;137:2626-2632. [PubMed] |
| 5. | Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1585] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 6. | Ichiki Y, Kitamura K, Kangawa K, Kawamoto M, Matsuo H, Eto T. Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett. 1994;338:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 402] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, Eto T, Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 417] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Ohta H, Tsuji T, Asai S, Tanizaki S, Sasakura K, Teraoka H, Kitamura K, Kangawa K. A simple immunoradiometric assay for measuring the entire molecules of adrenomedullin in human plasma. Clin Chim Acta. 1999;287:131-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Marutsuka K, Hatakeyama K, Sato Y, Yamashita A, Sumiyoshi A, Asada Y. Immunohistological localization and possible functions of adrenomedullin. Hypertens Res. 2003;26 Suppl:S33-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Kitamura K, Sakata J, Kangawa K, Kojima M, Matsuo H, Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun. 1993;194:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 453] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Hwang IS, Tang F. Peripheral distribution and gene expression of adrenomedullin in the rat: possible source of blood adrenomedullin. Neuropeptides. 2000;34:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor-alpha. Biochem Biophys Res Commun. 1994;203:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 270] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Martinez A, Miller MJ, Unsworth EJ, Siegfried JM, Cuttitta F. Expression of adrenomedullin in normal human lung and in pulmonary tumors. Endocrinology. 1995;136:4099-4105. [PubMed] |
| 14. | Satoh F, Takahashi K, Murakami O, Totsune K, Sone M, Ohneda M, Abe K, Miura Y, Hayashi Y, Sasano H. Adrenomedullin in human brain, adrenal glands and tumor tissues of pheochromocytoma, ganglioneuroblastoma and neuroblastoma. J Clin Endocrinol Metab. 1995;80:1750-1752. [PubMed] |
| 15. | Washimine H, Asada Y, Kitamura K, Ichiki Y, Hara S, Yamamoto Y, Kangawa K, Sumiyoshi A, Eto T. Immunohistochemical identification of adrenomedullin in human, rat, and porcine tissue. Histochem Cell Biol. 1995;103:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Interleukin-1, tumor necrosis factor and lipopolysaccharide additively stimulate production of adrenomedullin in vascular smooth muscle cells. Biochem Biophys Res Commun. 1995;207:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 243] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Chun TH, Itoh H, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Doi K, Inoue M, Masatsugu K. Shear stress augments expression of C-type natriuretic peptide and adrenomedullin. Hypertension. 1997;29:1296-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Cheung BM, Tang F. Adrenomedullin: exciting new horizons. Recent Pat Endocr Metab Immune Drug Discov. 2012;6:4-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Owji AA, Smith DM, Coppock HA, Morgan DG, Bhogal R, Ghatei MA, Bloom SR. An abundant and specific binding site for the novel vasodilator adrenomedullin in the rat. Endocrinology. 1995;136:2127-2134. [PubMed] |
| 20. | Hagner S, Stahl U, Knoblauch B, McGregor GP, Lang RE. Calcitonin receptor-like receptor: identification and distribution in human peripheral tissues. Cell Tissue Res. 2002;310:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 600] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 22. | McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 1621] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 23. | Cheung BM, Li CY, Wong LY. Adrenomedullin: its role in the cardiovascular system. Semin Vasc Med. 2004;4:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Ishiyama Y, Kitamura K, Ichiki Y, Nakamura S, Kida O, Kangawa K, Eto T. Hemodynamic effects of a novel hypotensive peptide, human adrenomedullin, in rats. Eur J Pharmacol. 1993;241:271-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 219] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | He H, Bessho H, Fujisawa Y, Horiuchi K, Tomohiro A, Kita T, Aki Y, Kimura S, Tamaki T, Abe Y. Effects of a synthetic rat adrenomedullin on regional hemodynamics in rats. Eur J Pharmacol. 1995;273:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Li YY, Wong LY, Cheung BM, Hwang IS, Tang F. Differential induction of adrenomedullin, interleukins and tumour necrosis factor-alpha by lipopolysaccharide in rat tissues in vivo. Clin Exp Pharmacol Physiol. 2005;32:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Dettmann ES, Vysniauskiene I, Wu R, Flammer J, Haefliger IO. Adrenomedullin-induced endothelium-dependent relaxation in porcine ciliary arteries. Invest Ophthalmol Vis Sci. 2003;44:3961-3966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Barber DA, Park YS, Burnett JC, Miller VM. Adrenomedullin-mediated relaxations in veins are endothelium-dependent and distinct from arteries. J Cardiovasc Pharmacol. 1997;30:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Eguchi S, Hirata Y, Kano H, Sato K, Watanabe Y, Watanabe TX, Nakajima K, Sakakibara S, Marumo F. Specific receptors for adrenomedullin in cultured rat vascular smooth muscle cells. FEBS Lett. 1994;340:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 218] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Hayakawa H, Hirata Y, Kakoki M, Suzuki Y, Nishimatsu H, Nagata D, Suzuki E, Kikuchi K, Nagano T, Kangawa K. Role of nitric oxide-cGMP pathway in adrenomedullin-induced vasodilation in the rat. Hypertension. 1999;33:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Allaker RP, Grosvenor PW, McAnerney DC, Sheehan BE, Srikanta BH, Pell K, Kapas S. Mechanisms of adrenomedullin antimicrobial action. Peptides. 2006;27:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Allaker RP, Kapas S. Adrenomedullin expression by gastric epithelial cells in response to infection. Clin Diagn Lab Immunol. 2003;10:546-551. [PubMed] |
| 33. | Kubo A, Minamino N, Isumi Y, Katafuchi T, Kangawa K, Dohi K, Matsuo H. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J Biol Chem. 1998;273:16730-16738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Li YY, Cheung BM, Wong LY, Hwang IS, Kumana CR, Tang F. Adrenomedullin gene expression and levels in the cardiovascular system after treatment with lipopolysaccharide. Neuropeptides. 2005;39:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Cheung BM, Hwang IS, Li CY, O WS, Tsang KW, Leung RY, Kumana CR, Tang F. Increased adrenomedullin expression in lungs in endotoxaemia. J Endocrinol. 2004;181:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Elsasser TH, Kahl S. Adrenomedullin has multiple roles in disease stress: development and remission of the inflammatory response. Microsc Res Tech. 2002;57:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Liao SB, Wong PF, Cheung BM, Tang F. Effects of adrenomedullin on tumour necrosis factor alpha, interleukins, endothelin-1, leptin, and adiponectin in the epididymal fat and soleus muscle of the rat. Horm Metab Res. 2013;45:31-37. [PubMed] |
| 38. | Zudaire E, Portal-Núñez S, Cuttitta F. The central role of adrenomedullin in host defense. J Leukoc Biol. 2006;80:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Wong LY, Cheung BM, Li YY, Tang F. Adrenomedullin is both proinflammatory and antiinflammatory: its effects on gene expression and secretion of cytokines and macrophage migration inhibitory factor in NR8383 macrophage cell line. Endocrinology. 2005;146:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Nakayama M, Takahashi K, Murakami O, Murakami H, Sasano H, Shirato K, Shibahara S. Adrenomedullin in monocytes and macrophages: possible involvement of macrophage-derived adrenomedullin in atherogenesis. Clin Sci (Lond). 1999;97:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Michibata H, Mukoyama M, Tanaka I, Suga S, Nakagawa M, Ishibashi R, Goto M, Akaji K, Fujiwara Y, Kiso Y. Autocrine/paracrine role of adrenomedullin in cultured endothelial and mesangial cells. Kidney Int. 1998;53:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Autelitano DJ, Tang F, Little PJ. Rapid regulation of adrenomedullin in metabolically compromised vascular smooth muscle cells. J Hypertens. 1999;17:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Kano H, Kohno M, Yasunari K, Yokokawa K, Horio T, Ikeda M, Minami M, Hanehira T, Takeda T, Yoshikawa J. Adrenomedullin as a novel antiproliferative factor of vascular smooth muscle cells. J Hypertens. 1996;14:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | López J, Cuesta N. Adrenomedullin as a pancreatic hormone. Microsc Res Tech. 2002;57:61-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Zudaire E, Cuttitta F, Martínez A. Regulation of pancreatic physiology by adrenomedullin and its binding protein. Regul Pept. 2003;112:121-1.30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Martı’nez A, Cuttitta F. Adrenomedullin, pancreatic islets, and diabetes. Can J Diabetes Care. 2000;24:39-46. |
| 47. | Tsuchida T, Ohnishi H, Tanaka Y, Mine T, Fujita T. Inhibition of stimulated amylase secretion by adrenomedullin in rat pancreatic acini. Endocrinology. 1999;140:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Bunton DC, Petrie MC, Hillier C, Johnston F, McMurray JJ. The clinical relevance of adrenomedullin: a promising profile? Pharmacol Ther. 2004;103:179-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Wu JT. Review of diabetes: identification of markers for early detection, glycemic control, and monitoring clinical complications. J Clin Lab Anal. 1993;7:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Hayashi M, Shimosawa T, Fujita T. Hyperglycemia increases vascular adrenomedullin expression. Biochem Biophys Res Commun. 1999;258:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Tang F, Hwang IS, Wong MP, Li YY. Adrenomedullin gene expression and peptide levels in the heart and blood vessels of streptozotocin-diabetic rats. Horm Metab Res. 2007;39:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Kinoshita H, Kato K, Kuroki M, Nakamura S, Kitamura K, Hisanaga S, Fujimoto S, Eto T. Plasma adrenomedullin levels in patients with diabetes. Diabetes Care. 2000;23:253-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Cheung B, Leung R. Elevated plasma levels of human adrenomedullin in cardiovascular, respiratory, hepatic and renal disorders. Clin Sci (Lond). 1997;92:59-62. [PubMed] |
| 54. | Ishimitsu T, Nishikimi T, Saito Y, Kitamura K, Eto T, Kangawa K, Matsuo H, Omae T, Matsuoka H. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest. 1994;94:2158-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 341] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 55. | García-Unzueta MT, Montalbán C, Pesquera C, Berrazueta JR, Amado JA. Plasma adrenomedullin levels in type 1 diabetes. Relationship with clinical parameters. Diabetes Care. 1998;21:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Hirayama N, Kitamura K, Imamura T, Kato J, Koiwaya Y, Eto T. Secretion and clearance of the mature form of adrenomedullin in humans. Life Sci. 1999;64:2505-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Hiragushi K, Wada J, Eguchi J, Matsuoka T, Yasuhara A, Hashimoto I, Yamashita T, Hida K, Nakamura Y, Shikata K. The role of adrenomedullin and receptors in glomerular hyperfiltration in streptozotocin-induced diabetic rats. Kidney Int. 2004;65:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Ruzicska E, Toth M, Tulassay Z, Somogyi A. Adrenomedullin and diabetes mellitus. Diabetes Metab Res Rev. 2001;17:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Kato H, Shichiri M, Marumo F, Hirata Y. Adrenomedullin as an autocrine/paracrine apoptosis survival factor for rat endothelial cells. Endocrinology. 1997;138:2615-2620. [PubMed] |
| 60. | Shimosawa T, Shibagaki Y, Ishibashi K, Kitamura K, Kangawa K, Kato S, Ando K, Fujita T. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation. 2002;105:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 61. | Kato J, Tsuruda T, Kita T, Kitamura K, Eto T. Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:2480-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Nakamura T, Honda K, Ishikawa S, Kitamura K, Eto T, Saito T. Plasma adrenomedullin levels in patients with non-insulin dependent diabetes mellitus: close relationships with diabetic complications. Endocr J. 1998;45:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Martínez A, Elsasser TH, Bhathena SJ, Pío R, Buchanan TA, Macri CJ, Cuttitta F. Is adrenomedullin a causal agent in some cases of type 2 diabetes? Peptides. 1999;20:1471-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Lim SC, Morgenthaler NG, Subramaniam T, Wu YS, Goh SK, Sum CF. The relationship between adrenomedullin, metabolic factors, and vascular function in individuals with type 2 diabetes. Diabetes Care. 2007;30:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Katsuki A, Sumida Y, Gabazza EC, Murashima S, Urakawa H, Morioka K, Kitagawa N, Tanaka T, Araki-Sasaki R, Hori Y. Acute hyperinsulinemia is associated with increased circulating levels of adrenomedullin in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2002;147:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Maruyama N, Morioka K, Kitagawa N, Hori Y, Nakatani K, Yano Y. Increased oxidative stress is associated with elevated plasma levels of adrenomedullin in hypertensive patients with type 2 diabetes. Diabetes Care. 2003;26:1642-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Chun TH, Itoh H, Saito T, Yamahara K, Doi K, Mori Y, Ogawa Y, Yamashita J, Tanaka T, Inoue M. Oxidative stress augments secretion of endothelium-derived relaxing peptides, C-type natriuretic peptide and adrenomedullin. J Hypertens. 2000;18:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Xing G, Shimosawa T, Ogihara T, Matsui H, Itakura K, Qingyou X, Asano T, Ando K, Fujita T. Angiotensin II-induced insulin resistance is enhanced in adrenomedullin-deficient mice. Endocrinology. 2004;145:3647-3651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Ong KL, Tso AW, Leung RY, Cherny SS, Sham PC, Lam TH, Cheung BM, Lam KS. A genetic variant in the gene encoding adrenomedullin predicts the development of dysglycemia over 6.4 years in Chinese. Clin Chim Acta. 2011;412:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Cheung BM, Ong KL, Tso AW, Leung RY, Cherny SS, Sham PC, Lam TH, Lam KS. Plasma adrenomedullin level is related to a single nucleotide polymorphism in the adrenomedullin gene. Eur J Endocrinol. 2011;165:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Wong HK, Ong KL, Leung RY, Lam TH, Thomas GN, Lam KS, Cheung BM. A single nucleotide polymorphism of interleukin-6 gene is related to plasma adrenomedullin levels. Clin Endocrinol (Oxf). 2013;79:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Wong HK, Ong KL, Leung RY, Cheung TT, Xu A, Lam TH, Lam KS, Cheung BM. Plasma level of adrenomedullin is influenced by a single nucleotide polymorphism in the adiponectin gene. PLoS One. 2013;8:e70335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |