Published online Jun 15, 2014. doi: 10.4239/wjd.v5.i3.244
Revised: January 29, 2014
Accepted: May 8, 2014
Published online: June 15, 2014
Processing time: 185 Days and 18.5 Hours
In healthy people, balance between glucose production and its utilization is precisely controlled. When circulating glucose reaches a critical threshold level, pancreatic β cells secrete insulin that has two major actions: to lower circulating glucose levels by facilitating its uptake mainly into skeletal muscle while inhibiting its production by the liver. Interestingly, dietary triglycerides are the main source of fatty acids to fulfill energy needs of oxidative tissues. Normally, the unconsumed fraction of excess of fatty acids is stored in lipid droplets that are localized in adipocytes to provide energy during fasting periods. Thus, adipose tissue acts as a trap for fatty acid excess liberated from plasma triglycerides. When the buffering action of adipose tissue to store fatty acids is impaired, fatty acids that build up in other tissues are metabolized as sphingolipid derivatives such as ceramides. Several studies suggest that ceramides are among the most active lipid second messengers to inhibit the insulin signaling pathway and this review describes the major role played by ceramide accumulation in the development of insulin resistance of peripherals tissues through the targeting of specific proteins of the insulin signaling pathway.
Core tip: Muscle and liver represent major sites for insulin-mediated glucose metabolism. The ability of insulin to promote its peripheral action is reduced significantly by excess of saturated fat that stimulates intracellular production of second-messenger lipids such as ceramide. Studies suggest that ceramide could be important contributors to lipotoxicity, as the inhibition of early steps its biosynthesis pathway has large beneficial effects in rodent models of obesity and diabetes. In this review, we describe mechanisms by which ceramide acts on insulin-sensitive tissues and we propose that targeting this lipid family could be an interesting approach to fight diabetes.
- Citation: Hage Hassan R, Bourron O, Hajduch E. Defect of insulin signal in peripheral tissues: Important role of ceramide. World J Diabetes 2014; 5(3): 244-257
- URL: https://www.wjgnet.com/1948-9358/full/v5/i3/244.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i3.244
Diabetes has become a serious public health problem in both developed and developing countries. Indeed, there is a dramatic increasing incidence of diabetes in most of these countries. In 2005, 217 million people worldwide had diabetes, and the World Health Organisation predicts that it will increase to 366 million in 2030[1]. In 2050, 33% of the population of the United States will suffer from diabetes[2]. One consequence is that over the years, diabetes has become life-threatening, with increased risk of cardiovascular diseases, retinopathy, kidney failure, and nerve and artery damages[3]. Diabetes is one of the first causes of haemodialysis, of blindness and of non-traumatic amputation of the legs. Another consequence is the increasing of health spending due to diabetes. For example, in the United States, diabetes costing is actually evaluated to more than $174 billion per year and it’s expected to increase in subsequent years[2].
There are different types of diabetes: (1) type 1 diabetes or maturity onset diabetes of the young associated to impairment of insulin production; and (2) type 2 diabetes, corresponding to 85%-90% of all diabetes, with both insulin secretion defects and peripheral insulin resistance. Type 2 diabetes is associated with obesity and although genetic factors play a role in the pathophysiology of this disease, other environmental factors such as diet and physical activity both play large roles. Several mechanisms have been proposed to explain both insulin resistance and insulin secretion defects observed in type 2 diabetes. Lipotoxicity, glucotoxicity, low grad systemic inflammation, oxidative stress and endoplasmic reticulum stress[4-6] correspond to different mechanisms that converge on a common pathway to induce insulin resistance. In this review we will focus on cellular lipid toxicity, i.e., lipotoxicity.
Systemic lipid imbalances are common in metabolic syndrome, in pre-diabetes and in type 2 diabetes and it is now clear that lipotoxicity can induce glucose dysregulation and participate to the pathophysiology of type 2 diabetes[7-9]. For example, prospective epidemiological studies performed in population with low or high risk to develop type 2 diabetes have shown that high free fatty acid (FFA) concentrations in plasma are associated with the risk of incident type 2 diabetes[10-12].
A major characteristic of type 2 diabetes is the loss of the ability of pancreatic β cells to increase insulin secretion to maintain normoglycemia in the face of insulin resistance[13]. Because of genetic predisposition, β cells could be unable to compensate the insulin resistance induced by FFA, but chronic exposition of β cells to high levels of FFA could equally explain defects in β cell function and decreased mass observed in type 2 diabetes. Indeed, in vitro studies have shown that FFA are associated with a decrease of insulin expression, synthesis and processing[14-16]. Another mechanism that can explain insulin secretion dysfunction in type 2 diabetes is that high FFA levels in islets induce β cell death[17]. In this review, we will not deal with this topic but we will rather focus our message on lipid-induced peripheral insulin resistance. To more information on lipotoxicity in pancreatic beta cells, confer to the excellent review of Boslem et al[18].
Since skeletal muscle constitutes 40% of human body mass and is quantitatively the most important tissue in regard to insulin-stimulated glucose disposal, it is considered the main cellular target in the development of insulin resistance. Thus, most of the studies investigating mechanisms of lipotoxicity induced insulin resistance were mostly performed in muscle tissue.
In 1963, Randle et al[19] have postulated that a competition between glucose and fatty acids for their oxidation and uptake is responsible for the onset of insulin resistance in muscle and adipose tissue. In vivo studies performed in both rodents and humans confirmed such insulin resistance obtained after lipid infusion but they also demonstrated that, in opposite to Randle’s hypothesis, insulin resistance induced by lipids was not secondary to decreased glycolysis[20]. Indeed, lipids act directly on insulin signaling, resulting in an inhibition of the translocation of the insulin sensitive glucose transporter GLUT4 to the plasma membrane in response to the hormone, with subsequent reduced glucose uptake[21-25]. In human, data clearly show a strong correlation between lipid intramuscular content and insulin resistance[26-28] and a cross-sectional analysis performed in young, normal weight and non-diabetic adults reveals that a better correlation exists between muscle insulin sensitivity, assessed by the hyperinsulinaemic-euglycaemic clamp technique, and intra-myocellular lipid content rather than with circulating lipid levels, body mass index, fasting blood glucose and age[29].
Liver is another important organ implicated in insulin resistance and, like in muscle indirect data also suggest an inverse relationship between lipid liver content and insulin sensibility. Indeed, ectopic lipid accumulation in the liver, termed nonalcoholic fatty liver disease (NAFLD), is associated with insulin resistance. Interestingly, in an animal model of lipodystrophy with steatosis, but without increased visceral fat, lipid liver content is associated with insulin resistance. Insulin resistance is reversed after reduction of steatosis with liver transplantation or recombinant leptin treatment[30]. Such association between steatosis and insulin resistance has also been observed in patients with severe lipodystrophy with equally a good response to recombinant leptin therapy[31]. Similarly, hepatic specific overexpression of lipoprotein lipase leads specifically to hepatic steatosis and hepatic insulin resistance[32,33]. During type 2 diabetes, reduction of steatosis by caloric restriction, or gastric bypass, is associated with increased insulin sensibility independently of visceral fat mass reduction[34,35].
Strong evidence exists between ectopic lipid accumulation and insulin resistance. However, in some cases, like in the “athlete’s paradox”, there is a lack of correlation between ectopic lipid accumulation and peripheral insulin resistance. Indeed, athletes display high insulin sensitivity but also present increased levels of intramuscular fatty acids[36]. Thus, it seems that ectopic accumulation of fatty acids in non-adipose tissues can only be used as markers for the onset of insulin resistance but cannot be considered as a direct cause. Even if they do not seem to be directly involved, fatty acids contribute to insulin resistance as they lead to the synthesis of many lipid derivative intermediates such as diacylglycerol (DAG) and ceramide.
Over the years, studies have provided conclusive proof that ceramide plays a key role in the progression of insulin resistance in insulin sensitive tissues, targeting and inhibiting specific actors of the insulin signaling pathway.
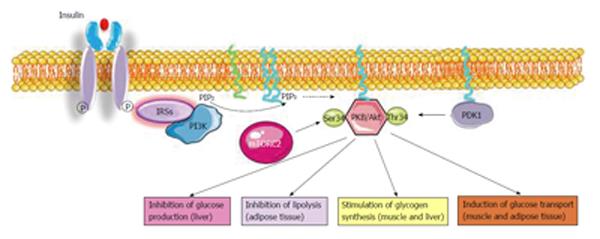
Insulin is a polypeptide hormone whose major physiological role is to control glucose homeostasis by stimulating glucose uptake into insulin sensitive tissues (skeletal muscle and adipose tissue) and by inhibiting glucose output from the liver[37]. Insulin consists of two polypeptide chains, a α chain of 21 amino acid residues linked by two disulfide bonds to a β chain of 30 amino acid residues. Insulin is produced in the β cells of the Islets of Langerhans found in the pancreas. It is initially synthesized as an immature single polypeptide chain of 110 amino acids called pre-proinsulin. Pre-proinsulin contains an N-terminal domain of 24 amino acids that acts to direct the polypeptide to the endoplasmic reticulum during translation. This domain is later cleaved to yield proinsulin. Proinsulin is transported to the secretory vesicles of the pancreatic β cells, where a proteolytic enzyme removes the central 35 residues of the peptide (termed the C-peptide) that connect α and β chains to produce insulin. Insulin is then released into the blood stream by exocytosis. Secretion of the hormone is regulated by the glucose abundance in the plasma.
In skeletal muscle, insulin promotes the uptake of glucose and its conversion into glycogen. This tissue is an important target of the hormone, representing the major site of glucose disposal in vivo[37] and is reported to mediate 70%-80% of whole body insulin-stimulated glucose transport[38]. In the liver, insulin stimulates the synthesis of glycogen while inhibiting gluconeogenesis and glycogenolysis, halting hepatic glucose output. In adipocytes, insulin promotes the uptake of glucose and its conversion into a glycerophosphate of which can be esterified by 3 fatty acids, allowing to form triglycerides for long term storage, whereas simultaneously inhibiting the lipolytic pathway[39]. In addition to glucose metabolism, insulin also regulates many other cellular processes including amino acid transport, lipogenesis, protein synthesis and mitogenesis.
The first step in the activation of the insulin signaling pathway is the binding of insulin with its membrane receptor, the insulin receptor (IR). IR is a heterotetrameric complex of two subunits: α-subunit, and β-subunit that possess a transmembrane domain and an intracellular part. Binding of insulin to α subunits of IR induces a rapid conformational change in the receptor. This in turn stimulates the intrinsic tyrosine kinase activity of the β subunit resulting in trans-autophosphorylation of tyrosine residues in the intracellular region of the β subunits[40]. As a result of this autophosphorylation, the IR becomes catalytically active and promotes the tyrosine phosphorylation of a number of cellular proteins including the IR Substrate (IRS) proteins.
IRS proteins are major physiological targets of the activated insulin receptor kinase. Six different IRS isoforms have been identified so far[41]. In skeletal muscle and adipose tissue, IRS1 is the isoform that mediate insulin signaling. In the liver, however, IRS2 is the one that drives insulin metabolic functions. In the pancreas, IRS2 is an important regulator of cell growth and regeneration[41]. Studies have also shown that both IRS3 and IRS4 can be activated in response to insulin and insulin-like growth factor 1 (IGF1)[42] and that IRS3 can mediate insulin signaling in adipocytes[42]. Mice lacking either IRS3 or IRS4, however, display no major phenotype, suggesting that neither isoform plays a direct role in controlling glucose metabolism[43,44] but may rather act as negative regulators of the IGF1 signaling pathway by suppressing the function of other IRS isoforms[45].
One key molecule that is activated by the IRSs in response to insulin is phosphoinositide-3-kinase (PI3K). PI3K is a lipid kinase, which phosphorylates the D3 position of the inositol ring within inositol lipids resulting in the generation of 3-phosphoinositides (e.g., PI-3P, PI-3,4P2, and PI-3,4,5P3). Eight mammalian isoforms of PI3K exist and they are grouped into three classes on the basis of their substrate specificity and structure: class I, class II, and class III. Only class I can phosphorylate phosphatidylinositol, 4, 5-bisphosphate (PIP2)[46]. Following PI3K activation, PIP3 is generated from the substrate PIP2. PIP3 binds a protein displaying a PH domain and called the 3-phosphoinositide-dependent protein Kinase 1 (PDK1). Activated-PDK1 triggers downstream targets such as protein kinase B (PKB/Akt)[47].
PKB/Akt also called Akt is the third central node activated by insulin. It plays a crucial role in mediating signaling effects on metabolism, cell growth and cell cycle[48,49]. PKB/Akt has three isoforms: PKBα/Akt1, ubiquitously expressed, PKBβ/Akt2 mostly present in insulin responsive tissues (liver, adipose tissue and muscle), and PKBγ/Akt3 predominant in the brain. PKBβ/Akt2 is the isoform implicated in the regulation of glucose metabolism since neither PKBα Akt1 nor PKBγ/Akt3 ablation affects glucose metabolism[50].
PKB/Akt is activated through PI3K-produced PIP3 which binds its PH domain. Then, PKB/Akt is recruited to the plasma membrane where it is activated by phosphorylation on two critical sites: threonine 308 (T308) in the activation loop and serine 473 (S473) in the hydrophobic motif[51]. PDK1 phosphorylates PKB/Akt on T308. The kinase that phosphorylates the S473 site is the complex mammalian target of rapamycin complex 2, a regulator of cell growth and proliferation[52].
PKB/Akt is highly activated within minutes following cell exposure to insulin to mediate the metabolic effects of the hormone[49,53].
Indeed, principle roles of PKB/Akt in insulin sensitive tissues are to: (1) Stimulate glucose uptake in muscle and adipose tissue; (2) Trigger glucose storage as glycogen in muscle and in the liver; (3) Stimulate the conversion of glucose excess into lipids in the liver; (4) Induce protein synthesis in muscle; (5) Inhibit glycogen breakdown in both muscle and liver; (6) Suppress liberation of free fatty acids from adipose tissue; (7) Inhibit de novo production of glucose in the liver; and (8) Impede protein breakdown in muscle (Figure 1).
Considering the crucial role PKB/Akt plays in mediating insulin metabolic actions in cells, impairing PKB/Akt activity represents the best way to compromise the whole system.
In pathological situations such as obesity and type 2 diabetes that are characterized by insulin resistance, ectopic fatty acid accumulation is increased due to reduced mitochondrial fatty acid oxidation and to enhanced fatty acid uptake[54-57]. This increased fat content inversely correlates with insulin sensitivity in skeletal muscle, liver and adipocytes[58-61].
Interestingly and depending on the degree of saturation, free fatty acid may exert different effects on insulin signaling. Studies have demonstrated that saturated fatty acids such as palmitate (16:0) and stearate (18:0) impair insulin sensitivity in muscle[62,63], whereas mono-unsaturated fatty acids or poly-unsaturated fatty acids have no effect or even enhance insulin action[64-66]. Although the exact reasons behind these differences are unclear, studies have suggested that unsaturated fatty acids may be preferentially targeted for triglyceride synthesis and storage, whilst saturated fatty acids may be used for synthesis of critical lipid intermediates such as DAG and ceramide. These two lipid second messengers have been demonstrated to mediate deleterious actions of saturated fatty acids on insulin signaling.
DAG is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule. DAG, intermediate of both triglyceride and phospholipid metabolism, is an important second messenger involved in intracellular signaling[67].
DAG has been shown to accumulate in insulin resistant liver[68,69] and studies have shown that intra-hepatic DAG is an important mediator of hepatic insulin resistance in obese people with nonalcoholic fatty liver disease[70,71]. Elevated DAG content and activation of protein kinase C (PKC)ε has been associated with hepatic insulin resistance and the involvement of this “lipid-activated pathway” has been validated through the use of antisense oligonucleotide against PKCε in rats. Knocking down PKCε expression in liver protected rats from lipid-induced hepatic insulin resistance, despite increase in hepatic lipid content[72].
Several studies have decrypted the mechanism by which DAG-activated PKCs inhibit insulin signaling in liver. They show that IRS proteins are likely to be PKC’s preferential targets. DAG-activated PKCs inhibit IRSs activity through their phosphorylation on several serine residues, preventing consequently insulin activation of IRSs through their phosphorylation on tyrosine residues[73-75].
In muscle, however, data are contradictory. Itani et al[76] were the first to point out the positive association between DAG content and muscle insulin resistance by comparing a group of subject receiving a lipid infusion to a control group. Lipid infusion resulted in a 3-fold increase in total DAG content in muscle, and reduced insulin sensitivity. Straczkowski et al[77] observed that total muscle DAG concentrations were higher in obese compared to lean controls and lean offspring type 2 diabetics, and this increased DAG content was inversely related to insulin sensitivity. Other studies have also confirmed this correlation[78,79].
However, the association between DAG and muscle insulin resistance is still questioned. Indeed, Vistisen et al[80] performed muscle biopsies during glucose clamps and they observed a reduction in insulin sensitivity after lipid infusion, without any changes in muscle DAG content. These results were confirmed by Anastasiou et al[81] that compared obese type 2 diabetic patients to non-diabetics subjects and found no difference in muscle DAG content between the groups. Similarly, Perreault et al[82] compared insulin resistant obese patients to glucose tolerant obese patients and again found no difference in DAG content between the groups. Even more intriguing, Amati el al[83] observed a two-fold increase in DAG content in insulin sensitive human muscle biopsies compared to insulin resistant human muscle biopsies. More recently, the same group showed no difference in muscle DAG content between lean subjects compared to obese insulin resistance patients[84].
Altogether, and in opposite to liver, it seems that DAG does not appear to be a crucial player in the onset of insulin resistance in muscle, and maybe more investigations are needed to really be able to conclude.
One of the main sphingolipid that has been demonstrated to play a crucial role in insulin resistance is ceramide. During obesity, ceramide is mainly generated from long chain fatty acyl-CoAs[85,86], and has been shown to be toxic lipid when it accumulates in tissues during obesity[87-89].
Ceramide is a bioactive sphingolipid that has been implicated in mediating or regulating many cellular processes, including cell cycle arrest, proliferation, apoptosis, senescence, and stress responses. Ceramide plays also an important role in cell membrane structure[90].
Formation of ceramide can be induced by different stimuli such as tumor necrosis factor-α, heat stress, oxidative stress, ionizing radiation, and chemotherapeutics[91].
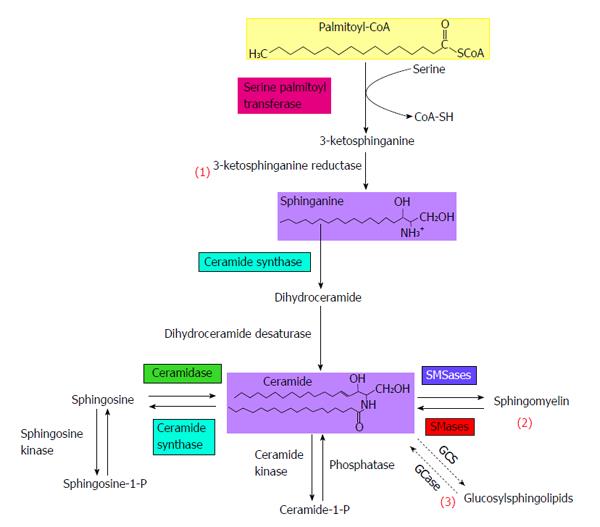
Multiple metabolic pathways converge to ceramide (Figure 2): (1) The de novo synthesis pathway from saturated fatty acids that takes place in the endoplasmic reticulum; (2) The sphingomyelinase pathway that uses sphingomyelinase to break down sphingomyelin in the cell membrane to release ceramide; and (3) The salvage pathway in lysosomes that occurs through breakdown of complex sphingolipids to give sphingosine, which is then rescued by reacylation to form ceramide.
In time of fatty acid plethora, the de novo ceramide biosynthesis pathway is the pathway that is likely to be most harnessed to synthesize ceramide. It occurs in the leaflet membrane of the endoplasmic reticulum where ceramide is synthesized through a series of reactions[92,93]. De novo synthesis of ceramide begins with the condensation of palmitate and serine to form 3-keto-dihydrosphingosine (Figure 2). This reaction is catalyzed by serine palmitoyl transferase (SPT) and is the rate-limiting step of the pathway. In turn, 3-keto-dihydrosphingosine is reduced to dihydrosphingosine, which is then followed by acylation by ceramide synthases (CerS) to produce dihydroceramide. In mammals, six CerS isoforms are expressed and are called CerS 1 to 6. They carry out the same chemical reaction, but display distinct specificities for the acyl-CoA chain length they use for N-acylation[94]. Thus, CerS isoforms are responsible for the fatty acid composition of ceramide. Interestingly, several studies have shown distinct cellular functions for ceramides with different N-acyl chain length[95,96]. The final reaction to produce ceramide is catalysed by dihydroceramide desaturase.
Studies in animal and models: One of the early studies that analyzed ceramide content in obese Zucker fa/fa rats (rats homozygous for truncated, non-functional leptin receptor) was Turinsky et al[97] in 1990. The authors found that these rats present an increase in ceramide content in both muscle and liver. Increased ceramide content was also detected in insulin resistant models of rodents, as in ob/ob mice, mice fed on high fat diet, and in intra-lipid infused mice[85,98,99]. Altogether these reports illustrate the inverse relationship between ceramide and insulin sensitivity in rodent muscle. This association was also confirmed in vitro in cultured C2C12 and L6 myotubes, as well as in adipocytes[99-101]. Exposing cultured muscle cells to saturated fatty acids (like palmitate) attenuates insulin activation of glycogen synthesis and glucose transport concomitantly with increasing intracellular ceramide amounts[63,99]. Additionally, incubation of muscle cells and adipocytes with analogues of ceramide mimics the inhibitory effects of FFAs on insulin signaling and suppresses insulin-stimulated glycogen synthesis and glucose transport[100,101].
Studies in human subjects: In accordance with data obtained in rodents, studies in human subjects also support the inverse relationship between ceramide accumulation and insulin sensitivity. It has been shown that under basal conditions, total amount of ceramide in skeletal muscle is increased in obese subjects compared to lean ones[83,84,87]. Another study performed in human skeletal muscle of lean normoglycemic subjects revealed again an inverse relationship between muscle ceramide accumulation and insulin sensitivity[102]. The same authors show in another study a ceramide accumulation in muscle of type 2 diabetic patient offsprings compared to muscle of control subjects[77]. Furthermore, the group of Goodpaster demonstrated that physical exercise reduces ceramide content in obese and insulin resistant subjects, and this was correlated with improved insulin sensitivity[83,103]. Like in muscle, accumulation of ceramide content in human adipocytes has also been demonstrated to be related to insulin resistance[104,105].
Altogether, these studies prove a solid association between insulin resistance and an increase in ceramide content in both muscle and adipocytes.
Unlike in muscle and adipose cells, a role of ceramide in the onset of hepatic insulin resistance is more debated. Indeed, some studies see no ceramide accumulation in fatty liver[68,70,71], making improbable these lipids as mediators hepatic insulin resistance. This is in contradiction with another study showing increases in hamster hepatic ceramide levels in response to lipopolysaccharide administration[106]. In addition, Longato et al[107] saw a dysregulated ceramide metabolism in high fat diet-induced hepatic steatosis.
Interestingly, and in opposite to muscle and adipose tissue, ceramide cannot accumulate in the liver. Indeed, very recently, Watt et al[108] have shown that lipid infusion in healthy subjects resulted in a rapid hepatic secretion of ceramide in the circulation, primarily within very low-density lipoprotein[109,110], thereby protecting the liver from the deleterious effects of their intracellular accumulation. It would be interesting, however, to assess whether lipid-induced ceramide secretion is affected in fatty liver (steatosis).
Altogether, if ceramide does not seem to accumulate in liver during lipotoxic conditions, its secretion into the circulation could be deleterious for other peripheral tissues such as pancreatic β cells and muscle cells.
Two methods were used to validate the implication of ceramide in impaired insulin sensibility: the first one was to inhibit ceramide production, and the second was to enhance ceramide metabolism towards less harmful sphingolipid species.
Inhibition of ceramide production improves insulin sensitivity: One method used to demonstrate the role of ceramide in the onset of insulin resistance was to inhibit ceramide biosynthesis. The most commonly studied molecular target involved in suppressing ceramide production is the enzyme SPT, enzyme that catalyzes the initial rate-limiting step in de novo ceramide synthesis (Figure 3)[90]. Several potent inhibitors of SPT have been documented, although the most widely used is myriocin, a naturally occurring fungal metabolite isolated from Myriococcum albomyces[111]. In studies carried out in vivo, administration of myriocin was found to attenuate PKB/Akt inhibition in response to lipid infusion or high-fat feeding, as well as improving glucose tolerance and peripheral insulin sensitivity in obese ob/ob mice and Zucker Diabetic Fatty rats[112-114]. As expected, these beneficial effects of myriocin were associated with reduced levels of ceramide and were reproduced when alternative inhibitors of de novo ceramide synthesis such as L-cycloserine (which also inhibits SPT) and Fenretinide (dihydroceramide synthase inhibitor) were used[63,115].
Studies performed in vitro in myotubes confirmed what was observed in vivo. They demonstrated that acute inhibition of SPT using myriocin ameliorates the loss in insulin-stimulated PKB/Akt activation in cultured L6 or C2C12 myotubes caused by palmitate-driven ceramide synthesis[62,63].
Interestingly, a very recent study shows that inhibition of the de novo synthesis of ceramide using myriocin reduces hepatic lipid accumulation in liver of rats with NAFLD[116]. This inhibition of ceramide biosynthesis is accompanied with decreased in both DAG and triglyceride contents, resulting in amelioration of hepatic insulin resistance and improvement of glucose homeostasis[116].
Stimulation of ceramide conversion into less harmful sphingolipids improves insulin sensibility: The degradation of ceramide is initiated by the action of ceramidase that produces sphingosine, which is then phosphorylated to sphingosine-1-phosphate (S1P) by sphingosine kinase[117]. S1P is the final metabolic product of sphingolipid degradation and can function as an intracellular second messenger or in an autocrine and/or paracrine manner to activate and signal through S1P receptors[118]. Interestingly, S1P itself opposes the effects of ceramide on intracellular signaling. S1P has been shown to ameliorate insulin-stimulated glucose uptake, possibly through the activation of PKB/Akt[118-121]. Therefore, studies have aimed at finding ways to enhance ceramide metabolism into S1P in muscle in order to restore their insulin sensitivity. Bruce et al[122] used transgenic mice overexpressing sphingosine kinase. They show that high fat fed transgenic mice display improved insulin sensitivity compared to control mice. In addition, they used a drug called FTY720 which inhibits ceramide synthase activity and decrease ceramide accumulation in skeletal muscle[123]. As expected, they saw an improvement of insulin sensitivity. FTY720 prevented muscle ceramide accumulation in high fat fed mice and subsequently improved glucose homeostasis[124]. Other studies show that overexpression of ceramidase (converting ceramide to sphingosine) protects from lipid-induced muscle insulin resistance in C2C12 myotubes[125].
Altogether, these results demonstrate that preventing the aberrant accumulation of ceramide by promoting its metabolism into sphingosine and sphingosine-derivatives might restore normal insulin sensitivity and glucose metabolism in models of insulin resistance.
Several studies have reported that ceramide may attenuate insulin-stimulated glucose transport and glycogen synthesis by antagonizing early events in insulin signaling such as activation of IRS-1[126] and possibly PI3K[127]. However, these results are controversial, as several groups reported no defects in the activation of these molecules upon challenging cells with ceramide[100,101]. In contrast, a number of groups suggested that PKB/Akt is the target of ceramide, and that inhibition of this kinase may account for reduced glucose transport and apoptosis observed in ceramide treated cells[99-101,128]. Consistent with this, defects in PKB/Akt activation have been noted in a variety of ceramide-treated cell types, including 3T3-L1 adipocytes[101], foetal brown adipocytes[129], L6 rat and C2C12 mouse skeletal muscle[99,100], A75R5 smooth muscle cells[130], and MCF7 breast cancer cells[131].
Furthermore, the inhibition of PKB/Akt by ceramide is not limited to experiments using exogenously supplied lipids. The hormonal activation of PKB/Akt is also blunted in muscle cells treated with free fatty acids in a manner which is dependent on the intracellular conversion of palmitate to ceramide[62,63,99]. Taken together these results suggest that ability of ceramide to impair PKB/Akt activity may be an important determinant of insulin sensitivity.
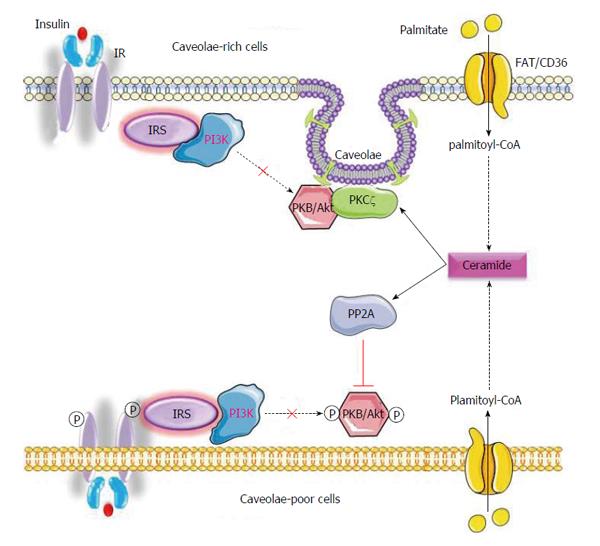
A key issue is the mechanism by which ceramide inhibits PKB/Akt activity. Depending on the cell enrichment in caveolin-enriched domain[132], ceramide inhibits the insulin-stimulated PKB/Akt either through the protein phosphatase 2A (PP2A), or via the atypical PKC (aPKC) pathway (Figure 3).
PP2A depended inhibition of insulin-induced activation of PKB/Akt: PP2A is a cytoplasmic serine/threonine phosphatase ubiquitously expressed that plays an important role in the regulation of diverse cellular processes, including metabolic enzymes, hormone receptors, kinase cascades, and cell growth[133]. It has been shown that insulin inhibits PP2A in physiologic conditions[134]. In contrast, several groups demonstrated that ceramide activates PP2A to promote the de-phosphorylation of PKB/Akt[62,135,136]. Two different inhibitors of PP2A activity, okadaic acid or SV40 small T antigen that binds with PP2A[137] were used to demonstrate the role of ceramide-induced PP2A inactivation of PKB/Akt. The presence of either inhibitor in cells treated with palmitate or short chain ceramide analogue (C2-ceramide), alleviated inhibition on PKB/Akt and re-established a normal, insulin signaling[62,128]. Therefore, one way for ceramide to inhibit PKB/Akt activity is by promoting its dephosphorylation at Thr308 and Ser473 through activation of PP2A.
Atypical PKCs another ceramide-stimulated protein altering PKB/Akt activation: The second mechanism of inactivation of PKB/Akt by ceramide requires the activation of aPKCs (PKCζ/λ). There is mounting evidence in the literature suggesting that aPKC may regulate PKB/Akt signaling and that the relationship between the two kinases may be subject to modulation by ceramide. It is 20 years since investigators first demonstrated that PKCζ/λ could associate with PKB/Akt in COS-7 fibroblasts[138]. It has also been demonstrated that PKCζ interacts directly with PKB/Akt in other cells types such as Chinese hamster ovary cells and COS-1 cells[139], as well as the BT-549 human breast cancer cell line[140].
In pathological conditions, ceramide-activated aPKCs impair insulin signaling. aPKCs phosphorylate PKB/Akt on its Thr34/Ser34 residue (Thr34 in PKBα and PKBβ, Ser34 in PKBγ), thus preventing PIP3 to bind the kinase on its PH domain, and to translocate to the plasma membrane and its subsequent activation in response to insulin[132,141,142]. Based on these observations, it was proposed that an increase in intracellular ceramide leading to the activation of aPKCs promotes the stabilization of the aPKC-PKB/Akt complex and attenuates the recruitment of PKB/Akt to the plasma membrane as a result of disrupted PIP3 binding (Figure 3).
Mechanisms by which saturated fatty acids act on insulin signaling are now getting clearer. They involve several lipid and protein intermediates that play an essential role to mediate the deleterious effects of accumulated saturated lipids in insulin sensitive tissues. Thus, two main options exist to counteract the action of these fatty acids on insulin signaling: (1) acting on ceramide downstream signaling targets (aPKCs or PP2A); or (2) modulating directly ceramide content[143]. Considering the large involvement of both aPKCs and PP2A in numerous paths[144,145], it would be more logical to try to directly inhibit the accumulation of ceramides in tissues. Several problems would arise with a complete inhibition of ceramide biosynthesis since these bioactive sphingolipids are in the center of sphingolipid metabolism. Indeed, ceramide signaling has been directly or indirectly involved in the diverse functions such as regulation of cell growth, differentiation, senescence, necrosis, proliferation, and apoptosis[90]. Therefore, inhibiting completely ceramide biosynthesis would be likely to be very harmful to the cells. Targeting specific ceramides species would be more appropriate since it has been shown that specific ceramide species could be associated with different functions, depending upon the cell type[94].
Concretely, it will be important to determine which ceramide species accumulate under lipotoxic conditions and then to evaluate whether these identified ceramide species enhance or reduce the deleterious effects of lipotoxicity in insulin sensitive tissues.
Interestingly, data existing already suggest that ceramide with distinct acyl chain-length are associated with different cell dysfunction in lipotoxic conditions. The enzyme responsible of generating different ceramide acyl chain-length is the CerS. Six mammalian CerS have been described, with each utilizing fatty acyl CoAs of relatively defined chain lengths for ceramide synthesis[94]. In pancreatic β-cells, C18:0, C22:0 and C24:1 ceramides induce apoptosis, and inhibition of the CerS (CerS4) responsible for their synthesis blocks this phenomenon[146]. In the liver, CerS1 and CerS6, producing mainly C16:0 and C18:0 ceramides are associated with insulin resistance[147], whereas C22:0 and C24:0 ceramides produced through CerS2 are rather protective[148].
In muscle cells, however, no definitive and conclusive investigation has been carried out to date. The expression of C16:0, C18:0 and C24:0 ceramide species are increased in myotubes of type 2 diabetic patients compared to lean donors[149]. However, one recent paper shows that overexpression of each CerS isoform in L6 muscle cells does not point out any ceramide species in the generation of insulin resistance[150]. Since the implication of ceramide in the onset of insulin resistance in muscle has been convincingly demonstrated both in vivo and in vitro (see previous chapters), more investigations are needed before to make any conclusion in this tissue.
In summary, deciphering the mechanisms by which ceramides act negatively on insulin signaling has already been a step forward. However, the identification of the putative ceramide species that mediates lipotoxicity in cells or pushing ceramides to be converted into less toxic lipids remains the priority in order to find a way to counteract ceramide negative actions.
P- Reviewer: Sasaoka T S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
| 1. | Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 389] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 868] [Cited by in RCA: 922] [Article Influence: 61.5] [Reference Citation Analysis (1)] |
| 3. | White JR. Economic considerations in treating patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2002;59 Suppl 9:S14-S17. [PubMed] |
| 4. | Flamment M, Hajduch E, Ferré P, Foufelle F. New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab. 2012;23:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1393] [Cited by in RCA: 1594] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 6. | Mlinar B, Marc J. New insights into adipose tissue dysfunction in insulin resistance. Clin Chem Lab Med. 2011;49:1925-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Reaven GM. The fourth musketeer--from Alexandre Dumas to Claude Bernard. Diabetologia. 1995;38:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 118] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Golay A, Felber JP, Jequier E, DeFronzo RA, Ferrannini E. Metabolic basis of obesity and noninsulin-dependent diabetes mellitus. Diabetes Metab Rev. 1988;4:727-747. [PubMed] |
| 9. | Abbasi F, Carantoni M, Chen YD, Reaven GM. Further evidence for a central role of adipose tissue in the antihyperglycemic effect of metformin. Diabetes Care. 1998;21:1301-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Charles MA, Eschwège E, Thibult N, Claude JR, Warnet JM, Rosselin GE, Girard J, Balkau B. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Pankow JS, Jacobs DR, Steinberger J, Moran A, Sinaiko AR. Insulin resistance and cardiovascular disease risk factors in children of parents with the insulin resistance (metabolic) syndrome. Diabetes Care. 2004;27:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Paolisso G, Gambardella A, Amato L, Tortoriello R, D’Amore A, Varricchio M, D’Onofrio F. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia. 1995;38:1295-1299. [PubMed] |
| 13. | Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110-125. [PubMed] |
| 14. | Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. 1995;80:1584-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Lewis GF. Lipid metabolism. Curr Opin Lipidol. 2002;13:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Chen NG, Romsos DR. Enhanced sensitivity of pancreatic islets from preobese 2-week-old ob/ob mice to neurohormonal stimulation of insulin secretion. Endocrinology. 1995;136:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patané G, Boggi U, Piro S, Anello M. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 457] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 18. | Boslem E, Meikle PJ, Biden TJ. Roles of ceramide and sphingolipids in pancreatic β-cell function and dysfunction. Islets. 2012;4:177-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785-789. [PubMed] |
| 20. | Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578-E591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 534] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 21. | Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 424] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 856] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 23. | Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 843] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 24. | Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1024] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 25. | Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest. 1998;101:2377-2386. [PubMed] |
| 26. | Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim Biophys Acta. 2010;1801:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Muoio DM, Newgard CB. Fatty acid oxidation and insulin action: when less is more. Diabetes. 2008;57:1455-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7-18. [PubMed] |
| 29. | Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113-116. [PubMed] |
| 30. | Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology. 2000;141:2328-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Merkel M, Weinstock PH, Chajek-Shaul T, Radner H, Yin B, Breslow JL, Goldberg IJ. Lipoprotein lipase expression exclusively in liver. A mouse model for metabolism in the neonatal period and during cachexia. J Clin Invest. 1998;102:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001;98:7522-7527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 550] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 34. | Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430-15435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 750] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 35. | Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, Klein S, Abumrad NN. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755-5761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2168] [Cited by in RCA: 2264] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 38. | Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand. 2003;178:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 39. | Ahmadian M, Duncan RE, Sul HS. The skinny on fat: lipolysis and fatty acid utilization in adipocytes. Trends Endocrinol Metab. 2009;20:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40 Suppl 2:S2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 384] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 41. | Lee YH, White MF. Insulin receptor substrate proteins and diabetes. Arch Pharm Res. 2004;27:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Giovannone B, Scaldaferri ML, Federici M, Porzio O, Lauro D, Fusco A, Sbraccia P, Borboni P, Lauro R, Sesti G. Insulin receptor substrate (IRS) transduction system: distinct and overlapping signaling potential. Diabetes Metab Res Rev. 2000;16:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Fantin VR, Wang Q, Lienhard GE, Keller SR. Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, and glucose homeostasis. Am J Physiol Endocrinol Metab. 2000;278:E127-E133. [PubMed] |
| 44. | Liu SC, Wang Q, Lienhard GE, Keller SR. Insulin receptor substrate 3 is not essential for growth or glucose homeostasis. J Biol Chem. 1999;274:18093-18099. [PubMed] |
| 45. | Tsuruzoe K, Emkey R, Kriauciunas KM, Ueki K, Kahn CR. Insulin receptor substrate 3 (IRS-3) and IRS-4 impair IRS-1- and IRS-2-mediated signaling. Mol Cell Biol. 2001;21:26-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346 Pt 3:561-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 372] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 47. | Cohen P, Alessi DR, Cross DA. PDK1, one of the missing links in insulin signal transduction? FEBS Lett. 1997;410:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 194] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Litherland GJ, Hajduch E, Hundal HS. Intracellular signalling mechanisms regulating glucose transport in insulin-sensitive tissues (review). Mol Membr Biol. 2001;18:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 198] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 49. | Hajduch E, Litherland GJ, Hundal HS. Protein kinase B (PKB/Akt)--a key regulator of glucose transport? FEBS Lett. 2001;492:199-203. [PubMed] |
| 50. | Schultze SM, Jensen J, Hemmings BA, Tschopp O, Niessen M. Promiscuous affairs of PKB/AKT isoforms in metabolism. Arch Physiol Biochem. 2011;117:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1943] [Cited by in RCA: 2021] [Article Influence: 106.4] [Reference Citation Analysis (3)] |
| 52. | Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4837] [Cited by in RCA: 5250] [Article Influence: 262.5] [Reference Citation Analysis (0)] |
| 53. | Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA. 2003;100:7569-7574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 295] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 54. | Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039-E1044. [PubMed] |
| 55. | Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1699] [Cited by in RCA: 1737] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 56. | Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 653] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 57. | Heilbronn LK, Gan SK, Turner N, Campbell LV, Chisholm DJ. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab. 2007;92:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Koyama K, Chen G, Lee Y, Unger RH. Tissue triglycerides, insulin resistance, and insulin production: implications for hyperinsulinemia of obesity. Am J Physiol. 1997;273:E708-E713. [PubMed] |
| 59. | Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45:947-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 282] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 60. | Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol. 2009;20:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 61. | Thomas EL, Fitzpatrick JA, Malik SJ, Taylor-Robinson SD, Bell JD. Whole body fat: content and distribution. Prog Nucl Magn Reson Spectrosc. 2013;73:56-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 62. | Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 382] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 63. | Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 64. | Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J. 2006;399:473-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 65. | Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 932] [Cited by in RCA: 861] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 66. | Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 465] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 67. | Brose N, Betz A, Wegmeyer H. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Curr Opin Neurobiol. 2004;14:328-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Kotronen A, Seppänen-Laakso T, Westerbacka J, Kiviluoto T, Arola J, Ruskeepää AL, Oresic M, Yki-Järvinen H. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. 2009;58:203-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 69. | Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 1042] [Article Influence: 57.9] [Reference Citation Analysis (1)] |
| 70. | Magkos F, Su X, Bradley D, Fabbrini E, Conte C, Eagon JC, Varela JE, Brunt EM, Patterson BW, Klein S. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology. 2012;142:1444-1446.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 71. | Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. 2011;108:16381-16385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 437] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 72. | Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Turban S, Hajduch E. Protein kinase C isoforms: mediators of reactive lipid metabolites in the development of insulin resistance. FEBS Lett. 2011;585:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 74. | Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metab. 2012;15:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 75. | van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 374] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 76. | Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1033] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 77. | Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 78. | Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 79. | Chibalin AV, Leng Y, Vieira E, Krook A, Björnholm M, Long YC, Kotova O, Zhong Z, Sakane F, Steiler T. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 80. | Vistisen B, Hellgren LI, Vadset T, Scheede-Bergdahl C, Helge JW, Dela F, Stallknecht B. Effect of gender on lipid-induced insulin resistance in obese subjects. Eur J Endocrinol. 2008;158:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Anastasiou CA, Kavouras SA, Lentzas Y, Gova A, Sidossis LS, Melidonis A. Diabetes mellitus is associated with increased intramyocellular triglyceride, but not diglyceride, content in obese humans. Metabolism. 2009;58:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Perreault L, Bergman BC, Hunerdosse DM, Eckel RH. Altered intramuscular lipid metabolism relates to diminished insulin action in men, but not women, in progression to diabetes. Obesity (Silver Spring). 2010;18:2093-2100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Amati F, Dubé JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic-Racic M, Toledo FG. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588-2597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 84. | Coen PM, Hames KC, Leachman EM, DeLany JP, Ritov VB, Menshikova EV, Dubé JJ, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring). 2013;21:2362-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 85. | Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 86. | Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125-3128. [PubMed] |
| 87. | Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25-31. [PubMed] |
| 88. | Coen PM, Dubé JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FG, Goodpaster BH. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes. 2010;59:80-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 89. | Lipina C, Hundal HS. Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance. Diabetologia. 2011;54:1596-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50 Suppl:S91-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 537] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 91. | Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855-1859. [PubMed] |
| 92. | Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 93. | Futerman AH. Intracellular trafficking of sphingolipids: relationship to biosynthesis. Biochim Biophys Acta. 2006;1758:1885-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J. 2012;441:789-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 407] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 95. | Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 397] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 96. | Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855-27862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 478] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 97. | Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880-16885. [PubMed] |
| 98. | Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55:2579-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 241] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 99. | Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202-24210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 471] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 100. | Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, Hundal HS. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001;44:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 101. | Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457-5464. [PubMed] |
| 102. | Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, Zendzian-Piotrowska M, Brzezinska Z, Gorski J. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes. 2004;53:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 103. | Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882-E888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 283] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 104. | Blachnio-Zabielska AU, Koutsari C, Tchkonia T, Jensen MD. Sphingolipid content of human adipose tissue: relationship to adiponectin and insulin resistance. Obesity (Silver Spring). 2012;20:2341-2347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 105. | Błachnio-Zabielska AU, Pułka M, Baranowski M, Nikołajuk A, Zabielski P, Górska M, Górski J. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. J Cell Physiol. 2012;227:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 106. | Memon RA, Holleran WM, Moser AH, Seki T, Uchida Y, Fuller J, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler Thromb Vasc Biol. 1998;18:1257-1265. [PubMed] |
| 107. | Longato L, Tong M, Wands JR, de la Monte SM. High fat diet induced hepatic steatosis and insulin resistance: Role of dysregulated ceramide metabolism. Hepatol Res. 2012;42:412-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 108. | Watt MJ, Barnett AC, Bruce CR, Schenk S, Horowitz JF, Hoy AJ. Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia. 2012;55:2741-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 109. | Lightle S, Tosheva R, Lee A, Queen-Baker J, Boyanovsky B, Shedlofsky S, Nikolova-Karakashian M. Elevation of ceramide in serum lipoproteins during acute phase response in humans and mice: role of serine-palmitoyl transferase. Arch Biochem Biophys. 2003;419:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 110. | Merrill AH, Lingrell S, Wang E, Nikolova-Karakashian M, Vales TR, Vance DE. Sphingolipid biosynthesis de novo by rat hepatocytes in culture. Ceramide and sphingomyelin are associated with, but not required for, very low density lipoprotein secretion. J Biol Chem. 1995;270:13834-13841. [PubMed] |
| 111. | Kluepfel D, Bagli J, Baker H, Charest MP, Kudelski A. Myriocin, a new antifungal antibiotic from Myriococcum albomyces. J Antibiot (Tokyo). 1972;25:109-115. [PubMed] |
| 112. | Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59:2453-2464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 113. | Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 966] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 114. | Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211-E224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 115. | Bikman BT, Guan Y, Shui G, Siddique MM, Holland WL, Kim JY, Fabriàs G, Wenk MR, Summers SA. Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J Biol Chem. 2012;287:17426-17437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 116. | Kurek K, Piotrowska DM, Wiesiołek-Kurek P, Lukaszuk B, Chabowski A, Górski J, Zendzian-Piotrowska M. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 117. | Le Stunff H, Milstien S, Spiegel S. Generation and metabolism of bioactive sphingosine-1-phosphate. J Cell Biochem. 2004;92:882-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 118. | Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 831] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 119. | Ma MM, Chen JL, Wang GG, Wang H, Lu Y, Li JF, Yi J, Yuan YJ, Zhang QW, Mi J. Sphingosine kinase 1 participates in insulin signalling and regulates glucose metabolism and homeostasis in KK/Ay diabetic mice. Diabetologia. 2007;50:891-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 120. | Rapizzi E, Taddei ML, Fiaschi T, Donati C, Bruni P, Chiarugi P. Sphingosine 1-phosphate increases glucose uptake through trans-activation of insulin receptor. Cell Mol Life Sci. 2009;66:3207-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 121. | Morales-Ruiz M, Lee MJ, Zöllner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672-19677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 222] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 122. | Bruce CR, Risis S, Babb JR, Yang C, Kowalski GM, Selathurai A, Lee-Young RS, Weir JM, Yoshioka K, Takuwa Y. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes. 2012;61:3148-3155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 123. | Lahiri S, Park H, Laviad EL, Lu X, Bittman R, Futerman AH. Ceramide synthesis is modulated by the sphingosine analog FTY720 via a mixture of uncompetitive and noncompetitive inhibition in an Acyl-CoA chain length-dependent manner. J Biol Chem. 2009;284:16090-16098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 124. | Bruce CR, Risis S, Babb JR, Yang C, Lee-Young RS, Henstridge DC, Febbraio MA. The sphingosine-1-phosphate analog FTY720 reduces muscle ceramide content and improves glucose tolerance in high fat-fed male mice. Endocrinology. 2013;154:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 125. | Chavez JA, Holland WL, Bär J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005;280:20148-20153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 126. | Kanety H, Hemi R, Papa MZ, Karasik A. Sphingomyelinase and ceramide suppress insulin-induced tyrosine phosphorylation of the insulin receptor substrate-1. J Biol Chem. 1996;271:9895-9897. [PubMed] |
| 127. | Zundel W, Giaccia A. Inhibition of the anti-apoptotic PI(3)K/Akt/Bad pathway by stress. Genes Dev. 1998;12:1941-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 128. | Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608-36615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 129. | Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 130. | Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem. 2002;277:3286-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 131. | Martin D, Salinas M, Fujita N, Tsuruo T, Cuadrado A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J Biol Chem. 2002;277:42943-42952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 132. | Blouin CM, Prado C, Takane KK, Lasnier F, Garcia-Ocana A, Ferré P, Dugail I, Hajduch E. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes. 2010;59:600-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 133. | Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 943] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 134. | Srinivasan M, Begum N. Regulation of protein phosphatase 1 and 2A activities by insulin during myogenesis in rat skeletal muscle cells in culture. J Biol Chem. 1994;269:12514-12520. [PubMed] |
| 135. | Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes. 2001;50:2210-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 136. | Salinas M, López-Valdaliso R, Martín D, Alvarez A, Cuadrado A. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol Cell Neurosci. 2000;15:156-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 137. | Cho US, Morrone S, Sablina AA, Arroyo JD, Hahn WC, Xu W. Structural basis of PP2A inhibition by small t antigen. PLoS Biol. 2007;5:e202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 138. | Konishi H, Kuroda S, Kikkawa U. The pleckstrin homology domain of RAC protein kinase associates with the regulatory domain of protein kinase C zeta. Biochem Biophys Res Commun. 1994;205:1770-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 139. | Doornbos RP, Theelen M, van der Hoeven PC, van Blitterswijk WJ, Verkleij AJ, van Bergen en Henegouwen PM. Protein kinase Czeta is a negative regulator of protein kinase B activity. J Biol Chem. 1999;274:8589-8596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 140. | Mao M, Fang X, Lu Y, Lapushin R, Bast RC, Mills GB. Inhibition of growth-factor-induced phosphorylation and activation of protein kinase B/Akt by atypical protein kinase C in breast cancer cells. Biochem J. 2000;352 Pt 2:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 141. | Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003;23:7794-7808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 142. | Fox TE, Houck KL, O’Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem. 2007;282:12450-12457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 143. | Hajduch E, Bourron O. Type 2 diabetes: ceramides as a therapeutic rarget? Clin Lipidol. 2013;8:607-609. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 144. | Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci (Landmark Ed). 2009;14:2386-2399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 145. | Lambrecht C, Haesen D, Sents W, Ivanova E, Janssens V. Structure, regulation, and pharmacological modulation of PP2A phosphatases. Methods Mol Biol. 2013;1053:283-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 146. | Véret J, Coant N, Berdyshev EV, Skobeleva A, Therville N, Bailbé D, Gorshkova I, Natarajan V, Portha B, Le Stunff H. Ceramide synthase 4 and de novo production of ceramides with specific N-acyl chain lengths are involved in glucolipotoxicity-induced apoptosis of INS-1 β-cells. Biochem J. 2011;438:177-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 147. | Cinar R, Godlewski G, Liu J, Tam J, Jourdan T, Mukhopadhyay B, Harvey-White J, Kunos G. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology. 2014;59:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 148. | Park JW, Park WJ, Kuperman Y, Boura-Halfon S, Pewzner-Jung Y, Futerman AH. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology. 2013;57:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 149. | Bajpeyi S, Myrland CK, Covington JD, Obanda D, Cefalu WT, Smith SR, Rustan AC, Ravussin E. Lipid in skeletal muscle myotubes is associated to the donors’ insulin sensitivity and physical activity phenotypes. Obesity (Silver Spring). 2014;22:426-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 150. | Frangioudakis G, Diakanastasis B, Liao BQ, Saville JT, Hoffman NJ, Mitchell TW, Schmitz-Peiffer C. Ceramide accumulation in L6 skeletal muscle cells due to increased activity of ceramide synthase isoforms has opposing effects on insulin action to those caused by palmitate treatment. Diabetologia. 2013;56:2697-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |