INTRODUCTION
Thromboxane A2 (TxA2) is a clinically important prostaglandin metabolite derived from arachidonic acid through the cyclo-oxygenase (COX) pathway with roles in hemostasis and cardiovascular disease (CVD)[1,2]. Platelet enzyme COX-1 converts arachidonic acid into prostaglandin G2 (PGG2), followed by the action of peroxidases into PGH2 and into the biologically active TxA2 by thromboxane synthases[3]. Mainly produced by stimulated platelets, TxA2 behaves as a vasoactive agent that affects blood flow and pressure[4] as well as a pro-thrombotic agent capable of promoting the activation and subsequent aggregation of nearby platelets. The latter function is accomplished by TxA2 binding to thromboxane platelet receptors (TPR), a typical G protein-coupled receptor system with trans-membrane segments. Once bound to TPR receptors, phospholipase C is activated to stimulate cytoplasmic Ca2+-dependent Rho Kinases that activate phospholipase A2 and the up-regulation and expression of glycoprotein complex GPIIb/IIIa on the surface of platelets[5,6].
Because TxA2 is the bioactive and clinically relevant pro-thrombotic thromboxane metabolite, it would be the logical choice for testing in the clinical laboratory. However, its high instability and very short half-life (20-30 s) makes the routine measurement technically difficult and impractical. Indeed TxA2 is quickly hydrolyzed into a biologically inactive but more stable thromboxane B2 (TxB2) metabolite[7]. Serum TxB2 may be measured in the laboratory but its concentration can be overestimated due to ex vivo platelet activation during blood collection and processing. Other serum factors may also interfere with TxB2 measurements. TxB2 is further metabolized by the liver primarily into an 11-dehydro-thromboxane B2 (11dhTxB2) form. This and other minor stable metabolites like 11dehydro-2,3-dinorTxB2 and 2,3-dinorTxB2 are excreted in the urine (Figure 1). Urinary 11dhTxB2 directly reflects the platelet production of TxA2[8,9], and represents a good and reliable biomarker for the laboratory assessment of platelet activity.
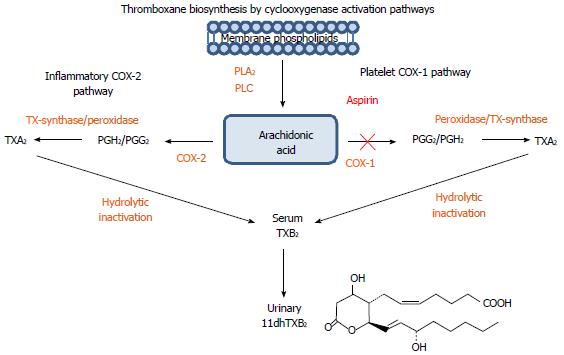
Figure 1 A schematic representation of the arachidonic/thromboxane metabolic pathway: Arachidonic acid generated from membrane phospholipids by phospholipase A2 and phospholipase C undergoes additional enzymatic transformation by cyclooxygenases (COX-1 and COX-2) into prostaglandin and thromboxane metabolites.
In platelets, Arachidonic acid (AA) is metabolized by COX-1 into prostaglandins PGG2, PGH2 and by thromboxane synthase into the bioactive thromboxane A2 (TXA2), which is a potent activator of platelet aggregation with a short half-life. TXA2 is quickly inactivated into a more stable thromboxane B2 (TXB2) and converted in the liver into an 11-dehydro-thromboxane B2 (11dhTXB2) metabolite excreted in the urine. Aspirin (ASA) irreversibly inhibits platelet COX-1 leading to decreased thromboxane-mediated platelet activation. TXA2 and 11dhTXB2 can be generated by COX-2 present in various inflammatory cells, pathway not affected by ASA.
Aspirin (Acetylsalicylic acid, ASA) irreversibly acetylates platelet COX-1 for the entire life cycle of the platelet. Ingestion of low doses of ASA blocks over 95% of platelet COX-1 activity resulting in the inhibition of TxA2 production. For these reasons, ASA is widely prescribed as an aid in the primary and secondary prevention of CVD. Despite its widespread use, not all individuals respond to ASA in the same way[10,11]. In addition, ASA effectiveness is limited because over 15%-25% of patients with arterial thrombosis may develop recurrent vascular events while on ASA treatment. This incomplete ASA response (or poor-responsiveness) to therapeutic doses has been referred to as “aspirin (ASA) resistance”, a phenomenon described in healthy populations as well as in patients with diabetes (DM) and CVD. The exact mechanisms responsible for this clinical unresponsiveness remain unclear[12,13].
Currently, ASA is largely prescribed for the primary prevention of cardiovascular events in DM but the evidence supporting its efficacy is surprisingly scarce and controversial[14-16]. Recent observations demonstrate that healthy subjects and DM patients with poor ASA response not only seem to manifest an incomplete inhibition of COX-1, but also display a pro-inflammatory milieu and enhanced oxidative stress[17-19]. On the other hand, diet-induced weight loss in subjects with central obesity reduced platelet reactivity and restored platelet sensitivity to nitric oxide, prostacyclin, and physiologic anti-aggregating agents. High on-ASA Platelet Reactivity (HAPR) has been proposed as a more appropriate term than “ASA resistance” to describe a high platelet reactivity status despite ASA therapy in an individual patient. Further, HAPR has been associated with atherothrombotic events following major vascular procedures and may identify patients at high risk for re-occlusion following percutaneous intervention (PCI) with stenting[20].
11DHTXB2 DETERMINATION AND ASA RESPONSE
There are two distinct groups of tests commonly used to measure platelet activity and response to ASA. The first group is blood-based and relies on platelet aggregation response to exogenous agonists or inhibitors by various means[21]. Because platelet activation or inhibition can be mediated by different receptors and pathways, it is not surprising to see a lack of correlation between the assays[22-24]. The second group of tests consists of serologic or urine-based immunoassays that measure both platelet (COX-1) and non-platelet (COX-2) production of thromboxanes. This discussion will focus on the measurement of urinary 11dhTxB2 as a direct indicator of TxA2 activity and platelet activation. One advantage of this type of assays is that thromboxane production is the primary target of ASA through an effective and irreversible COX-1 inhibition.
11dhTxB2 is a biologically inactive down-stream metabolite of TxA2 with a long (stable) circulating half-life that is readily excreted in the urine and relatively unaffected by ex vivo platelet activation and other pre-analytical variables[25,26], hence 11dhTxB2 usefulness as a reliable biomarker to assess platelet activation. Due to its relative small size and low concentrations, urinary 11dhTxB2 levels are measured by a competitive enzyme-linked immunsorbent assay (ELISA) that uses a spot urine sample without time constraints. Spot urine 11dhTxB2 levels are normalized against urine creatinine concentration making the 24 h collection unnecessary. It is important to point out that this ELISA measures the systemic production of thromboxanes (COX-1 and COX-2-derived), and directly reflects COX-1 inhibition by ASA. 11dhTxB2 results are first calculated against a reference curve prepared from a reference solution and the final results are reported as pg/mg (pg 11dhTxB2 per mg creatinine) to normalize results for urine concentration.
To assess the demographic and clinical variables influencing urinary excretion of 11dhTxB2 we first studied apparently healthy adults before and after receiving controlled doses of ASA. Based on the resulting frequency of 11dhTxB2 levels, we established a cut-off value to assess an adequate ASA response at 1500 pg/mg of 11dhTxB2. This cut-off has been re-confirmed in subsequent studies using both healthy and diseased populations before and after ASA ingestion[27]. Those individuals with urinary 11dhTxB2 levels after ASA ingestion below the cut-off of 1500 pg/mg are considered good ASA responders while those with levels above 1500 pg/mg are poor ASA responders (“ASA resistance”). It is important to assess high platelet reactivity in spite of ASA ingestion because a series of actions may be undertaken to manage and reverse the incomplete effect of ASA. The rest of this discussion will focus on a series of clinical studies performed on DM and coronary artery disease (CAD) patients measuring 11dhTxB2 and using the quoted 1500 pg/mg cut-off to assess the significance of the ASA response in the development of CVD complications.
ASA poor response or “resistance”: definition and clinical implications
ASA “resistance” has been referred to as the lack of a clinical and/or laboratory beneficial effect from ASA ingestion[28-30]. A true or complete ASA “resistance”, defined as a lack of response to ASA ingestion due to pharmacologic and/or genetic deficiencies, has not been described to date. The great majority of individuals respond to ASA ingestion as defined by ex vivo measurements of platelet aggregation or thromboxane production. However, in most individuals the response seems to be only partial or incomplete. From the clinical point of view, the term ASA “resistance” has neither been fully described nor properly standardized, thus it lacks a nosological clinical definition. Furthermore, consensus guidelines for treatment or management of ASA resistance have not been put forward[31]. Most experts prefer the term ASA “poor or incomplete response” or ASA “insensitivity” to the term ASA “resistance”. Throughout this discussion, we will occasionally use the term ASA “resistance” but with the clear understanding that we definitely prefer “poor or incomplete” ASA response as a more appropriate term.
Increased platelet turnover, platelet activation by alternative pathways, alternative/additional sources of TxA2 production such as macrophage/monocyte COX-2, drug bioavailability, and genetic polymorphisms, have been implicated in ASA poor responsiveness[13]. Recent reports suggested that CAD patients with high serum concentrations of cholesterol, triglyceride and C-reactive protein had reduced response to ASA measured by platelet aggregation and urinary 11dhTxB2[29]. Compared to asymptomatic patients, those with full blown CAD had significantly higher levels of urinary 11dhTxB2 following ASA ingestion. The HOPE[32] and CHARISMA[33] studies showed that urinary 11dhTxB2 levels in ASA-treated patients predicted the future risk of stroke, myocardial infarction and cardiovascular death. These findings raised the possibility that elevated urinary 11dhTxB2 excretion identifies patients on ASA treatment that are at elevated risk of adverse events and may benefit from additional anti-platelet agents or treatment modification.
Patients who experience a vascular ischemic event while taking ASA have been referred to as having a clinical ASA “resistance”. Patients who show a limited inhibition of thromboxane levels, platelet activation, or aggregation after ASA ingestion assessed by biochemical or laboratory tests are referred to as having a laboratory ASA “resistance”[30]. This discussion focuses on the biochemical or laboratory ASA resistance, and more specifically on the clinical impact of the reduced inhibition of COX-1 thromboxane levels. As with any other drug, the dose, drug interference and poor patient compliance should be kept in mind when evaluating ASA responsiveness. The prevalence of laboratory ASA “resistance” ranges from 10% to 25% with occasional peaks up to 60%. However, this wide variability depends on the methods used to measure the ASA response and the patient population under study rather than on the individual response. Nonetheless, other important causes of a poor response to ASA are emerging amongst which is stress-induced inflammation/oxidation[34,35].
An overall poor response to ASA has been associated with up to 13-fold increase risk of atherothrombotic complications in patients with CVD[13,36,37]. A recent meta-analysis of over 20 clinical studies performed on a total of 2930 CVD patients taking ASA (75-325 mg) demonstrated a 4-fold increased risk for any cardiovascular (CV) event including CV death in those patients with poor ASA response[38]. About twenty-eight percent (28%) patients were classified as ASA poor responders (“resistance”) suggesting an association with CVD risk. CV-related events were observed in 41%, death in 5.7%, and acute coronary syndrome (ACS) in 39.4% of patients with poor ASA response. It must be pointed out that the clinical studies included in the meta-analysis used different methods to measure platelet ASA inhibition and their own criteria to classify the response to ASA. An interesting observation of the meta-analysis is that ASA poor responders did not benefit from other anti-platelet therapy. The HOPE[32] study screened 5529 patients and measured urinary 11dhTxB2 in 488 ASA-treated CVD patients. Age and sex matched controls also received ASA. CV outcomes including CV death were recorded during a 5-year follow-up. Poor ASA responders with urinary 11dhTxB2 levels in the upper quartile had a 2-fold increasing risk of heart attacks and 3.5-fold risk of CV death. A sub-study of CHARISMA[33] that included 3261 ASA treated CVD patients confirmed the increased CVD risk in patients with 11dhTxB2 in the upper quartile as previously reported by the HOPE study.
11DHTXB2 AND ASA RESPONSE IN DM
CVD has been long recognized as a leading cause of morbidity and mortality in patients with type 1 and type 2 DM mainly by ischemic heart disease[39,40]. The use of ASA is known to reduce future secondary events in DM[41], however, a meta-analysis of randomized controlled trials failed to demonstrate a clear benefit of aspirin in the primary prevention of major cardiovascular events in patients with DM[42]. To further assess thromboxane levels and aspirin response in DM patients, two clinical studies were conducted on consecutive type 2 DM patients attending the endocrinology and diabetes outpatient clinics in Mexico. The diagnosis of DM was made by the attending physician following internationally accepted diagnostic criteria (World Health Organization DM criteria, 1985) that relied on the presence of abnormal fasting glucose (normal range 70-110 mg/dL), abnormal glucose tolerance test, chronic hyperglycemia and metabolic disturbances of lipid, carbohydrate and protein metabolism due to defects in insulin production or activity. Males and females between 18 and 79 years of age who had not taken ASA or other non-steroidal anti-inflammatory drugs for the previous 2 wk were included. Subjects with liver and kidney disease, symptomatic cardiovascular disease requiring ASA therapy (myocardial infarction, angina, stroke, peripheral artery disease), concomitant acute or chronic inflammatory diseases (bacterial or viral infections), autoimmune disorders, pregnancy, allergy or intolerance to ASA, and bleeding disorders were excluded. The use of ASA in Mexican patients with DM for primary prevention of CVD was significantly less common compared to the US, ensuring a good recruitment of DM patients not taking ASA while avoiding possible unethical discontinuation of the medication.
Baseline 11dhTxB2 levels in DM
Baseline (ASA-free) urinary 11dhTxB2 levels were measured in 100 subjects, 53 with DM and 47 healthy volunteers. None of the patients or controls in this group had received ASA for at least 2 wk prior to testing. The main objective of this study was to establish an average baseline urinary 11dhTxB2 level in DM. The hypothesis was that patients with DM had increased baseline urinary 11dhTxB2 levels hence a higher risk of developing cardiovascular atherothrombotic complication and would receive ASA therapy compared to healthy controls. The mean age of the population studied was 53.9 ± 12.6 years (54 females, 46 males) with mean disease duration of 9.1 ± 7.7 years.
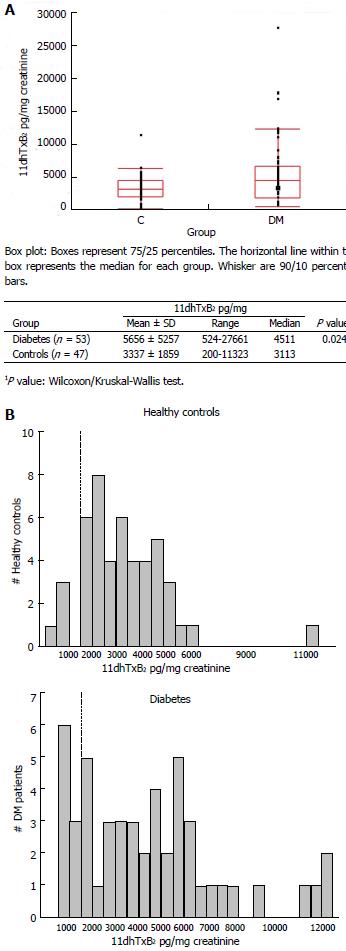
The distribution of baseline (ASA-free) 11dhTxB2 levels of DM patients and healthy volunteers is shown in Figure 2A. DM patients presented with a baseline mean urinary 11dhTxB2 excretion of 5656 pg/mg, a value 69.5% higher than the mean baseline 11dhTxB2 excretion of healthy controls at 3337 pg/mg, (P = 0.024). The highest 11dhTxB2 value seen in the DM group reached 27661 pg/mg while the highest value in healthy controls was 11323 pg/mg. Figure 2B shows the cumulative baseline frequency of urinary 11dhTxB2 excretion of healthy controls (up) and DM patients (down). The frequency of healthy controls followed a normal (Gaussian-like) distribution while DM patients had a distinctive flat distribution.
Figure 2 Distribution of baseline (aspirin-free) urinary 11-dehydro-thromboxane B2 levels (pg/mg) measured in healthy individuals and diabetes patients (A, top), and frequency distribution (histogram) of baseline urinary 11-dehydro-thromboxane B2 levels in the two groups studied (B, bottom).
A: Comparison of baseline 11-dehydro-thromboxane B2 levels of diabetes and controls; B: Frequency (Histogram) of baseline 11-dehydro-thromboxane B2 levels in diabetes and controls.
Influence of gender, age and disease duration on 11dhTxB2 levels
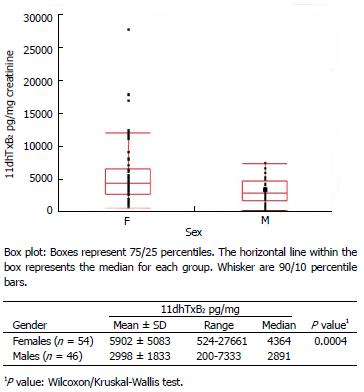
There were 34 females plus 19 males with DM, and 20 females plus 27 males in the healthy control group. Figure 3 depicts the urinary baseline (ASA-free) 11dhTxB2 levels according to gender. When evaluating all 100 subjects (DM patients and healthy controls), females exhibited a mean baseline urinary 11dhTxB2 excretion 50.9% higher than that of males (5902 vs 2998 pg/mg, P = 0.0004). When evaluating the influence of gender separately in DM patients and healthy controls, females consistently display significantly higher baseline 11dhTxB2 levels than males (DM P = 0.01, controls P = 0.02).
Figure 3 Distribution of baseline (aspirin-free) urinary 111-dehydro-thromboxane B2 levels (pg/mg) measured in healthy individuals and diabetes patients according to gender.
F: Females; M: Males.
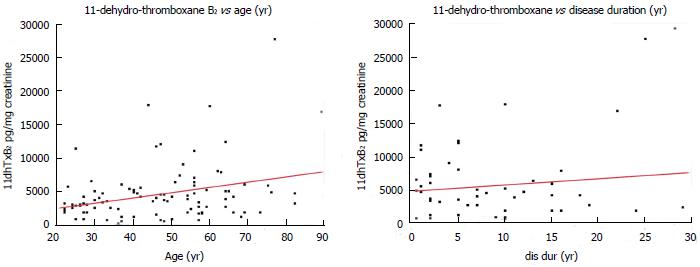
The mean age of DM patients was 56 years (range 29-80 years) and that of healthy controls 35 years (range 22-82 years). Figure 4 depicts the association of urinary baseline (ASA-free) 11dhTxB2 levels with the subject’s age (in years). In this analysis all 100 subjects (DM patients and healthy controls) were included. The mean disease duration for the DM patients was 9.6 years (range 1-29 years). There was weakly positive correlation (r = 0.322, P = 0.001) between age (in years) and baseline urinary 11dhTxB2 levels (left), and a weak (but not statistically significant) positive correlation (r = 0.124, P = 0.3) between disease duration (in years) and baseline urinary 11dhTxB2 levels (right).
Figure 4 Correlation of baseline (aspirin-free) urinary 11-dehydro-thromboxane B2 levels (pg/mg) measured in healthy individuals and diabetes patients with age (left), and disease duration of diabetes patients (right).
Red lines: Linear regression fit.
These variables were entered into a linear regression model to predict 11dhTxB2 levels (as a dependent variable). Only female gender remained as a significant (P = 0.02) predictor of 11dhTxB2 levels. Sex-related differences in platelet function and aspirin pharmacokinetics in rabbits and man have been previously described[43]. These results support previous findings that ASA reduces the risk of first heart attack in men but not in women suggesting that the ASA effect in women is different[42,44,45]. The results also support the relevance of measuring urinary 11dhTxB2 levels in DM patients to assist health care providers in assessing the risk for CVD and implementing an ASA preventive regimen.
Effect of ASA on 11dhTxB2 levels in DM
The effect of ASA in DM was studied in 137 subjects, 54 patients with DM and 83 healthy volunteers. Each DM patient or control subject contributed two urine samples: one before receiving ASA (baseline) and a second sample after receiving 100 or 325 mg of ASA for 7 d. The main objective of the study was to corroborate that ASA ingestion reduces 11dhTxB2 levels in DM patients. The hypothesis was that ASA would inhibit urinary 11dhTxB2 excretion in DM patients but with more ASA non-responders (11dhTxB2 levels > 1500 pg/mg) compared to healthy controls. The mean age of the final population under study was 54.3 ± 13.1 years with 90 females and 47 males. The mean disease duration was 9.6 ± 7.6 years.
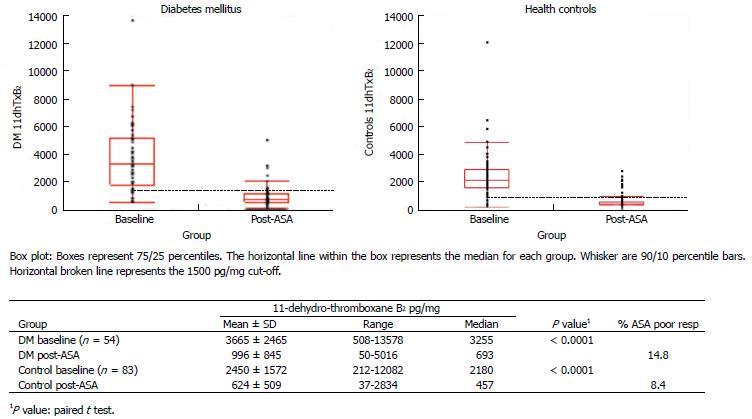
The baseline (ASA-free) and post-ASA ingestion values of DM patients and healthy controls are shown in Figure 5. ASA ingestion suppressed the mean baseline 11dhTxB2 excretion of DM patients by 71.5% (P < 0.0001) as well as the mean baseline of healthy controls (75.1%, P < 0.0001). The baseline 11dhTxB2 excretion of DM patients was greater than that of controls (3664 vs 2450 pg/mg, P = 0.001). Similarly, post-ASA 11dhTxB2 excretion of DM patients was greater than that of healthy controls (995 vs 624 pg/mg, P < 0.0001). Regarding the effect of the dose of ASA, the mean 11dhTxB2 excretion of subjects taking 100 mg of ASA was 708 pg/mg ± 507, whereas the mean of subjects taking 325 mg was 827 pg/mg ± 811 (P = 0.8). In this study, ASA dose used in DM and healthy controls had no significant influence of post-ASA 11dhTxB2 levels. Furthermore, a regression model to predict 11dhTxB2 levels (as a dependent variable) showed ASA (P = 0.0293) and obesity (P = 0.0467) as statistically significant predictors of 11dhTxB2 levels.
Figure 5 Distribution of baseline (aspirin-free) and post-aspirin urinary 11-dehydro-thromboxane B2 levels (pg/mg) measured in healthy individuals (right) and diabetes patients (left).
14.8% of diabetes patients were classified as aspirin (ASA) poor responders compared to 8.4% of healthy controls (post-ASA 11-dehydro-thromboxane B2 over the cutoff 1500 pg/mg).
11dhTxB2 excretion shifted below the cut-off (1500 pg/mg) after ASA treatment in the majority of healthy controls, leaving 8.4% (7/83) of subjects classified as non-responders. In DM patients, 11dhTxB2 excretion shifted below the cut-off (1500 pg/mg) after ASA ingestion in the majority of patients, except for 14.8% (8/46) of subjects subsequently classified as ASA non-responders. These results confirm that ASA treatment significantly inhibits baseline urinary 11dhTxB2 levels in both healthy individuals and DM patients, However, there were twice as many ASA poor responders among the DM patients possibly implicating a high platelet reactive phenotype associated with DM [40,46,47].
Having established that DM patients express elevated baseline levels of 11dhTxB2 and twice as many ASA non-responders, we investigated the effect of oxidative stress and anti-oxidant biomarkers on 11dhTxB2 excretion in DM[35]. Urinary 8-iso-prostaglandin-F2α (8-isoPGF2α) and sP-Selectin, nitrite (NO2-), nitrate (NO3-) and paraoxonase 1 (PON1) activity were measured in baseline (ASA free) and post-ASA samples from these DM patients and controls. Compared to controls, DM expressed increased levels of 8-isoPGF2α (1457 vs 1009 pg/mg, P < 0.0001), NO2- (11.8 vs 4.8 μmol/L, P < 0.0001), NO3- (50.4 vs 20.9 μmol/L, P < 0.0001) and sP-Selectin (120.8 vs 93.0 ng/mL, P = 0.02). ASA demonstrated no effect on 8-isoPGF2α, NO2-, NO3-, sP-Selectin or PON1 activity in either DM or controls. Again, higher urinary 11dhTxB2 levels in DM suggest a state of heightened platelet activation. In addition to platelet hyperactivity, DM patients presented with an inflammatory/oxidative background not affected by ASA. In fact, among the biomarkers measured, only urinary 8-isoPGF2α was significantly higher (P < 0.009) in DM patients with poor ASA response. These findings are in agreement with the hypothesis that an oxidative and inflammatory stress may maintain platelet activation irrespective of COX-1 pathway inhibition and/or increase the systemic generation of thromboxane from non-platelet sources via COX-2 pathway[34,48-50].
ASA treatment for CVD prevention is a widely accepted practice according to recommended guidelines, but evidence supporting its efficacy is somewhat conflictive and scarce, particularly for patients with DM[51]. The JPAD study (Japanese Primary Prevention of Atherosclerosis with ASA for Diabetes) involved 2539 type 2 DM patients between 40-85 years with no history of atherosclerosis randomized into ASA (81 or 100 mg/d) or non-ASA groups. ASA did not demonstrate a significant reduction in risk for any of the CVD-related endpoints. The POPADAD study (Prevention of Progression of Arterial Disease and Diabetes) included 1276 adults (> 40 years) with type 1 or 2 DM asymptomatic for CVD (ankle-brachial index less than 0.99). ASA (100 mg/d) also failed to demonstrate a significant reduction in risk for any CVD endpoint. Finally, the AAA study (Aspirin for Asymptomatic Atherosclerosis) included 3350 adults (50-75 years) asymptomatic for CVD (ankle-brachial index less than 0.95). ASA (100 mg/d) again did not demonstrate a significant reduction in risk for any endpoint. These studies suggest that DM somehow blunts the beneficial effect of ASA in CVD prevention. Additional mechanisms to explain these clinical findings are forthcoming and likely will help clarify the controversy surrounding the concept of clinical ASA “resistance”.
11DHTXB2 AND ASA RESPONSE IN ACS
Two clinical studies of ACS patients will be discussed. One study measured urinary 11dhTxB2 levels after ASA ingestion on 77 consecutive patients attending acute care facilities. ACS patients over 18 years of age undergoing elective PCI at the participating institutions were enrolled. All patients were treated with 325 mg of ASA for at least one week. Each patient provided one urine sample while on ASA. The main objective of the study was to assess urinary 11dhTxB2 excretion in response to 325 mg of ASA in relation to the manufacture’s cut-off value of 1500 pg/mg established in apparently healthy individuals. The mean levels of urinary 11dhTxB2 after 325 mg of ASA ingestion was 1550 pg/mg. The majority of ACS patients responded to ASA with 11dhTxB2 levels below the cut-off. However, the percent of ASA non-responders in this ACS population was 28.6%. One common question ponders the dose of daily ASA necessary to inhibit COX-1 and overcome ASA poor response. Urinary 11dhTxB2 levels were measured in 71 consecutive patients with stable CAD and randomized to receive 81 mg, 162 mg and 325 mg per day of ASA for 4 wk. The mean 11dhTxB2 decreased from 931 to 763 pg/mg (P = 0.046) with increasing doses of ASA. In this study, the rate of ASA poor responders decreased with increasing ASA dosage. This ASA dose-dependent response is in agreement with previous reports by Gurbel et al[22]. Thus, ASA dose should be considered when evaluating ASA poor responses.
A second study included 287 consecutive aspirin-free ACS patients admitted to a hospital in Japan for PCI to evaluate a possible association between urinary 11dhTxB2 levels before and after aspirin ingestion with adverse events (AE)[52]. Inclusion criteria included ST elevation myocardial infarction (STEMI), non-STEMI or early onset (within 24 h) invasive revascularization procedure. Upon enrollment and prior to PCI, a baseline (ASA-free) urine sample was obtained, followed by a daily regimen of 100 mg of ASA. Urine samples from ASA-treated patients were collected at hospital discharge (7-14 d) and upon follow up at 6 and 12 mo. Adverse cardiovascular events (AE) were recorded during a 12 mo patient follow-up. Primary end-points included stent thrombosis, Q wave myocardial infarction (QMI), non-QMI, and death (cardiac and non-cardiac). Secondary end-points included stroke, transient ischemic attack (TIA), target lesion revascularization of PCI or CABG, or other vascular event.
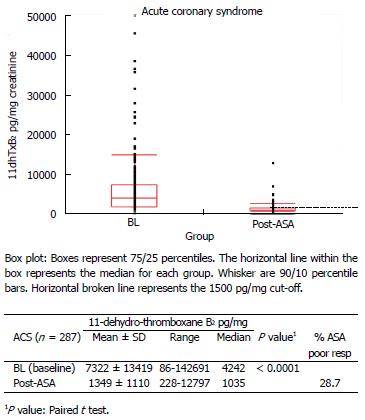
The mean age of these ACS patients was 68.9 years. Age did not influence baseline 11dhTxB2 levels (r = 0.060, P = 0.310), but females had significantly higher mean baseline 11dhTxB2 (7675 pg/mg) compared to males (6949 pg/mg, P = 0.0171). The mean baseline ASA-free 11dhTxB2 was 7322 pg/mg for this cohort of ACS patients and was 2-3 times higher than healthy individuals (range 2450-3337 pg/mg). ASA significantly suppressed (81%, P < 0.0001) of baseline 11dhTxB2 levels to 1349 pg/mg at discharge and subsequent time points. The distribution of baseline (before ASA) 11dhTxB2 levels of the ACS patients is shown in Figure 6. In spite of a significant inhibition of 11dhTxB2 by ASA, 28.7% of ACS patients were classified as poor responders by failing to achieve levels below the 1500 pg/mg cut-off. The overall rate of AEs was 17.1%. The rate of AEs according to baseline (ASA-free) 11dhTxB2 levels decreased slightly from 19.4% in quartile 1 to 15.5% in quartile 4. In contrast, the rate of AEs in ASA treatment quartiles increased from 9.1% in quartile 1 to 24.2% in quartile 3 and 20% in quartile 4. The relative risk for AEs of quartile 3 was 2.7 (P = 0.019). When upper quartiles (3 and 4) were compared to lower quartiles (1 and 2), the relative risk was 2.1 (P = 0.011).
Figure 6 Distribution of baseline (aspirin-free) and post-aspirin urinary 11-dehydro-thromboxane B2 levels (pg/mg) measured in acute coronary syndrome patients.
28.7% of acute coronary syndrome patients were classified as ASA poor responders (post-ASA 11-dehydro-thromboxane B2 over the cutoff 1500 pg/mg). ACS: Acute coronary syndrome; ASA: Aspirin.
High baseline 11dhTxB2 levels were consistent with an underlying platelet hyperactivity that may contribute to the development of atherothrombosis. However, baseline ASA-free 11dhTxB2 levels did not predict 1-year AEs. High levels (> 1500 pg/mg) of 11dhTxB2 after ASA ingestion likely represent extra-platelet (i.e., monocyte/macrophage-derived) COX-2 production of thromboxane. The increased relative risk (2.7) for AEs associated with high post-ASA 11dhTxB2 levels (upper quartiles) suggest that COX-2 production of thromboxane may be a factor associated with a cardiovascular inflammatory process. It is important to point out that ASA insensitive thromboxane generation has been associated with a pro-inflammatory milieu and enhanced oxidative stress in diabetes. Among several biomarkers tested, only baseline urinary 8-isoPGF2α discriminated between normal and poor thromboxane responders, suggesting that oxidative stress may maintain platelet function irrespective of COX-1 inhibition and/or increased systemic generation of thromboxane from non-platelet sources. Thromboxane alone may not be directly implicated in atherothrombosis. Nonetheless, these results confirm previous reports that post-ASA urinary 11dhTxB2 may be useful in predicting adverse outcomes in ACS patients.
Oxidative inflammation (stress) refers to prevailing levels of reactive oxygen species (ROS) in biological systems that overcome their removal by cellular or plasma repair (anti-oxidant) mechanisms[53]. The excess of superoxide anion (O2•-) produced by inflammatory cells may exert a free radical attack on cell membranes and/or lipoproteins in a process called lipid peroxidation. While the arachidonic acid metabolism mediated by enzymatic (COX) pathways has received most attention, a non-enzymatic free radical pathway is demonstrating relevance. The free radical oxidation of arachidonic acid generates biologically active F2-isoprostanes reflecting the oxidative status of the organism; is considered a reliable marker of oxidative stress in vivo; and has been shown to be an independent risk factor for CAD[54,55]. Some in vitro studies have demonstrated that 8-isoPGF2α is capable of stimulating platelet activation while other studies described pro-atherogenic properties through its interaction with the thromboxane platelet receptor (TPR). If 8-isoPGF2α binds to TPR, it may also be capable of competing with TxA2 and activating the Ca2+/Rho kinase pathway[56,57]. This may be particularly important because while TxA2 enzymatic synthesis is inhibited by ASA, the non-enzymatic 8-isoPGF2α production increases, perhaps as an alternative mechanism to maintain physiologic platelet activity.
Low dose ASA ingestion blocks COX-1 but has no effect on COX-2 or 8-isoPGF2α. During an oxidative inflammatory response, increased platelet hyperactivity would come from the combined COX-1, COX 2 and isoprostane (8-isoPGF2α) pathways. If ingesting ASA, platelet hyperactivity would be induced by COX-2 and 8-isoPGF2α alone. Limited or no COX-1 TxA2 production after ASA ingestion would leave unoccupied TPR available to bind 8-isoPGF2α that has a longer half-life (1-10 min vs 20-30 s) and higher plasma concentration (351-1831 vs 1-66 pg/mL) than TxA2[6]. Thus, blocking F2-isoprostane derived from oxidative inflammatory pathways not affected by ASA may be considered in CVD management especially in those individuals with poor ASA response.
CLINICAL SIGNIFICANCE OF 11DHTXB2 MEASUREMENTS
The irreversible inhibition of platelet COX-1 and subsequent reduction of TxA2 production by ASA has been recognized long ago, making ASA a cost-effective prevention regimen for atherothrombotic CVD. Low doses of ASA have been claimed to prevent over 150000 heart attacks annually. Furthermore, ASA ingestion has accounted for an overall 25% risk reduction of CV events, including a 34% reduction of non-fatal heart attacks, 25% of non-fatal strokes and 18% of all-cause mortality. However, between 25% to 50% of the patients with CAD and ACS did not fully benefit from ASA ingestion[12,13,58]. Thus, TxB2 measurements to detect those individuals with poor ASA response and higher CVD risk is clinically relevant.
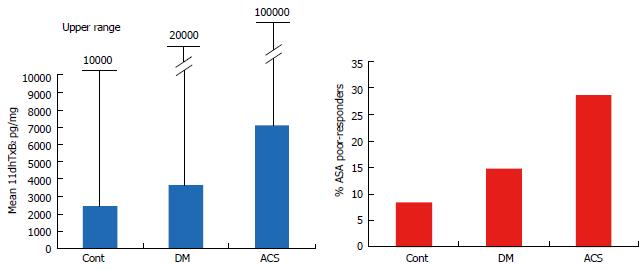
Our studies demonstrated that baseline (ASA-free) urinary 11dhTxB2 excretion showed an upward trend across healthy controls, DM and ACS (Figure 7). The mean 11dhTxB2 of the two control groups studied was 2893.5 pg/mg with an upper range up to 11702 pg/mg. The mean for DM groups was 4660.5 pg/mg with an upper range up to 20619 pg/mg and for ACS patients the mean was 7322 pg/mg with an upper range over 100000 pg/mg. The rate of ASA poor responders had a similar upward trend: controls with 8.4%, DM 14.8% and ACS over 28%.
Figure 7 Mean and upper range of baseline (aspirin-free) urinary 11-dehydro-thromboxane B2 levels (pg/mg) measured in healthy controls, diabetes and acute coronary patients (left), and percent (%) of aspirin poor responders (post-aspirin 11-dehydro-thromboxane B2 over the cutoff 1500 pg/mg) in the populations studied (right).
DM: Diabetes; ACS: Acute coronary syndromes.
Baseline 11dhTxB2 levels in both the healthy and diseased populations clearly indicated a wide range of platelet reactivity with a considerable overlap among the groups. This wide range observed likely prevented the establishment of an ASA-free 11dhTxB2 cut-off or even a normal range for clinical use. One relevant observation from the ACS study was that over 40% of ACS patients with high baseline (ASA-free) 11dhTxB2 showed a poor response after ASA ingestion. This observation is in agreement with the concept that higher baseline levels in DM and ACS patients may predict higher rates of ASA poor responders. An explanation for these findings comes from reports that patients with metabolic syndrome (obesity, dyslipidemia, insulin resistance) have increased oxidative stress (oxLDL), higher CVD risk[59], platelet hyperactivity[60] and suboptimal inhibition of platelet COX-1 by aspirin[61], suggesting that higher TxB2 places these patients at higher risk for thromboembolic events.
Russo et al[18] described that diet-induced weight loss in subject with central obesity reduces platelet activation restoring the sensitivity to anti-platelets agents. The Health Aging and Body Composition Study reported that the inflammatory marker interleukin-6 was a robust predictor for new negative health-related events and high urinary 8-isoPGF2α and 11dhTxB2 were associated with higher mortality risk[62]. More recently, Santilli et al[63] reported that high intensity physical exercise has broad beneficial effect on platelet activation biomarkers; urinary 11dhTxB2 and 8-isoPGF2α decreased 26% and 21% respectively and esRAGE increased 61% compared to the sedentary control group and multiple regression analysis demonstrated that 8-isoPGF2α and esRAGE were the only significant predictors of 11dhTxB2 levels.
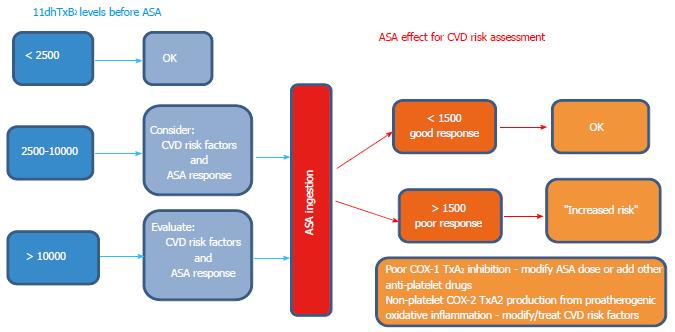
The suggestive algorithm discussed below (Figure 8) was developed taking into account the clinical studies discussed above and is proposed to interpret urinary 11dhTxB2 results for CVD risk management.
Figure 8 Proposed schematic representation (algorithm) to guide the clinical interpretation and decision making process for assessing CVD risk using baseline (aspirin-free) urinary 11-dehydro-thromboxane B2 (left), and post-aspirin 11-dehydro-thromboxane B2 levels (right).
Baseline 11-dehydro-thromboxane B2 levels suggested were taken from the mean and upper range of healthy controls. The cut-off of 1500 pg/mg applies only for subjects on aspirin (ASA) therapy to be classified as good or poor responders.
If the subject is not taking aspirin and the 11dhTxB2 level is
Below 2500 pg/mg: no action is necessary; Between 2500 and 10000 pg/mg: consider giving ASA to assess ASA response and/or consider other underlying CVD risk factors; Over 10000 pg/mg: give ASA to assess ASA response and/or look for other CVD risk factors.
If the subject is taking ASPIRIN and the 11dhTxB2 level is
Below 1500 pg/mg: no action is necessary (good ASA response), continue monitoring CVD risk; Above 1500 pg/mg: the subject is a poor ASA responder (“resistance”). Consider patient compliance, adjusting ASA dosage, additional anti-platelet therapy, etc. And more importantly investigate and modify underlying CVD risk factors such as dyslipidemia and inflammatory/oxidative pro-atherogenic background likely responsible for the incomplete inhibition of thromboxanes.
The major impact of this algorithm is that consistently high baseline 11dhTxB2 levels in subjects not taking ASA may justify further investigations for underlying CVD risks. However, only the presence of post-ASA high 11dhTxB2 levels predicts increased risk of atherothrombotic disease. This highlights the need of a comprehensive (multimodal-approach) management that includes both anti-platelet as well as anti-atherogenic treatments.
CONCLUSION
Poor response to ASA frequently indicates an underlying incomplete COX-1 inhibition and increased CVD risk. Among several assays used to measure ASA effect on platelets, urinary 11dhTxB2 reflects systemic production of thromboxanes and platelet reactivity directly affected by ASA. The incidence of ASA poor responders increases in DM and ACS patients, suggesting an active oxidative/inflammatory background likely responsible for both a continued platelet hyperactivity and a pro-atherogenic phenotype not affected by ASA.
Our studies of urinary 11dhTxB2 levels in response to ASA ingestion in diseased populations indicate the following: (1) patients with DM and CAD have significantly higher mean baseline levels of urinary 11dhTxB2 than healthy controls likely indicating a higher platelet activation and risk for CVD. Female gender seems to have a weak positive influence on 11dhTxB2 and platelet reactivity; (2) ASA ingestion significantly inhibited urinary 11dhTxB2 in DM, ACS and controls. However, the rate of DM ASA poor responders (14.8%) was about 2 times higher than controls (8.4%). This may also be a reflection of an increased platelet activation status in DM patients; (3) the rate of ACS ASA poor responders (28.7%) was about 3 times higher than controls; and (4) The results of the studies provide additional support to the laboratory measurement of urinary 11dhTxB2 levels not only in apparently healthy individuals but also in patients with DM and CAD to assess their response to ASA ingestion.
ACKNOWLEDGMENTS
Authors thank Beth L Hurley and Ivana J Muncy for their technical expertise and assistance in testing the samples and organizing the data.
P- Reviewers: Espino J, Papazoglou S- Editor: Song XX L- Editor: A E- Editor: Wu HL