Published online Dec 15, 2013. doi: 10.4239/wjd.v4.i6.372
Revised: October 30, 2013
Accepted: November 15, 2013
Published online: December 15, 2013
Processing time: 200 Days and 14.9 Hours
AIM: To examine skin perfusion in dependency on insulinemia in healthy subjects.
METHODS: All volunteers were informed in detail about the procedures and signed informed consent. The protocol of this study was approved by the ethical committee. In our study, a two stage hyperinsulinemic euglycemic clamp was performed, with insulinemia 100 and 250 mIU/mL and glycemia 5.0 mmol/L (3% standard deviation). Before the clamp and in steady states, microcirculation was measured by laser Doppler flowmetry and transcutaneous oximetry and energy expenditure was measured by indirect calorimetry. Results (average and standard deviation) were evaluated with paired t-test.
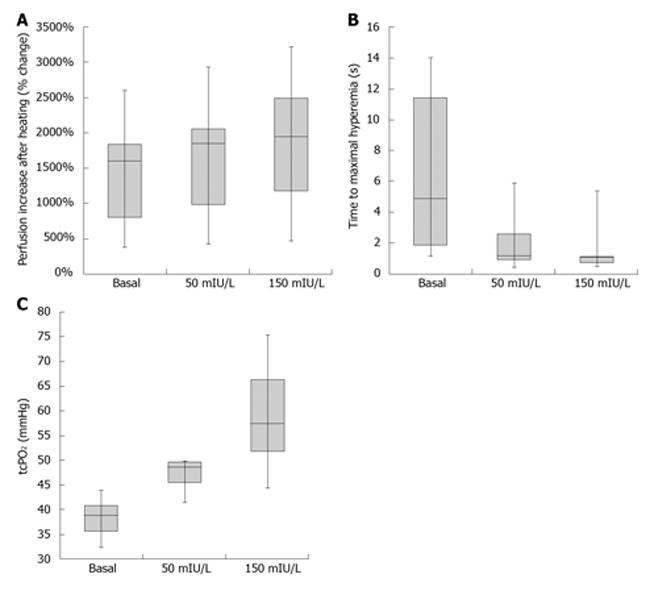
RESULTS: Physiological (50 mIU/L) insulinemia led to higher perfusion in both tests; hyperemia after heating to 44%-1848% (984-2046) vs 1599% (801-1836), P < 0.05, half time of reaching peak perfusion after occlusion release 1.2 s (0.9-2.6) vs 4.9 s (1.8-11.4), P < 0.05. Supraphysiological (150 mIU/L) insulinemia led to even higher perfusion in both tests; hyperemia after heating to 44%-1937% (1177-2488) vs 1599% (801-1836), P < 0.005, half time to reach peak perfusion after occlusion release 1.0 s (0.7-1.1) vs 4.9 s (1.8-11.4), P < 0.005. A statistically significant increase occurred in tissue oxygenation in both insulinemia. The difference in perfusion and oxygenation between physiological and supraphysiological hyperinsulinemia was not statistically significant.
CONCLUSION: The post occlusive hyperemia test in accordance with heating test showed significantly increasing skin perfusion in the course of artificial hyperinsulinemia. This effect rises non-linearly with increasing insulinemia. Dependency on the dose was not statistically significant.
Core tip: Insulin mediated small vessel vasodilatation is a hot topic in microcirculation research. Our work describes skin microcirculation response to increasing insulinemia in the physiological and supraphysiological level. Simultaneous use of two laser Doppler flowmetry and transcutaneous oximetry was used for discriminating between total blood flow and perfusion of the nutritive bed. Both methods reveal increasing skin microcirculation perfusion due to insulin infusion with this effect rising non-linearly with increasing insulinemia.
- Citation: Krčma M, Čechurová D, Brožová J, Jankovec Z, Lacigová S, Žourek M, Rušavý Z. Influence of physiological and supraphysiological hyperinsulinemia on skin microcirculation in healthy volunteers. World J Diabetes 2013; 4(6): 372-377
- URL: https://www.wjgnet.com/1948-9358/full/v4/i6/372.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i6.372
Since the mid 1980s, a lot of attention has been dedicated to the importance of microcirculation, a part of the arterial bed including arterioles, precapillary sphincters, capillaries, venules and arteriovenous shunts. It is a structure of decisive importance for an organism; an exchange of blood gases and metabolic products takes place in its domain and it contributes to thermoregulation. Mediation of the vasomotor reaction and vaso-arterial reflex maintaining a stable hydrostatic pressure is also an important function. Microcirculation is relatively difficult to access for more detailed examination due to its dimensions (capillary diameter approx. 5 × 10-5 mm2, blood flow velocity around 0.4 mm/s), yet its impairments are very severe and dominate in many metabolic disorders. Microcirculation impairment is crucial in diabetes mellitus, where arteriovenous shunts open at the expense of the nutritive bed due to a loss of sympathetic tone in peripheral circulation in diabetic neuropathy[1,2]. Blood flow is therefore seemingly sufficient, but the affected tissue undergoes ischemia (warm ischemia). To what extent hyperinsulinemia contributes to this effect is not yet clearly known; one of the possible explanations may be a stimulation of sympathetic activity. Several studies are dealing with insulin’s vasodilatory effect with inconsistent findings regarding the extent of microcirculatory response at various insulin levels upon acute and chronic insulin administration.
Experimental work in rats showed an improvement in blood perfusion of sciatic nerve perineurium after a one month insulin treatment and concurrently, amelioration in nerve conduction was proven electromyographically[3]. A short-term continuous subcutaneous insulin infusion (insulin pump treatment) led to an increase in capillary perfusion[4] and to a decrease in venous oxygen tension in diabetic patients after 9 d of treatment. This result suggests an existence of redistribution of skin perfusion favoring the nutritive capillary bed.
Acute hemodynamic effects of insulin were tested in an experiment where healthy young men were treated with a short insulin, locally administered into brachial artery with a rate of 1 and 5 mU/min for a period of 90 min in a double blinded study design. Blood flow was measured using body plethysmography. A higher insulin dose led to a statistically significant vasodilatation (20%) compared to placebo. Administration of insulin + L-glucose (metabolically inactive stereoisomer) did not produce a further increase in vasodilatation in comparison with pure insulin administration. Administration of insulin + D-glucose led to an increase in perfusion in comparison with pure insulin administration (47% compared to placebo). Glucose infusion itself did not cause any significant changes in blood flow[5]. In diabetes type 1 patients, when using different insulin infusion rates (1.5 IU/h, 15 IU/h), an increase in blood flow measured by laser Doppler flowmetry (LDF) at the low dose of insulin occurred, while a decrease occurred at the higher dose[6]. These measurements, however, were not performed in steady state conditions during clamp examination. Conversely, no statistically significant changes in perfusion of skin microcirculation were found in an experiment in healthy volunteers, where skin perfusion was monitored during a three step insulin clamp with gradually increasing insulinemia levels 60-500 IU/mL. Arteriovenous difference of glucose already attained a maximum value at the lowest insulinemia and subsequently remained constant[7].
An increase in perfusion measured using LDF was noted at supraphysiological hyperinsulinemia in anesthetized rats under clamp conditions, concurrently associated with an increase in femoral artery flow[8].
Some studies dealing with physiological hyperinsulinemia (approximately to 50 mIU/L) proved an increased blood flow through muscle and skin microvascular beds and increased density of opened capillaries under this condition[9,10]. They used LDF and video capillary microscopy for measurements. In addition, local skin administration of insulin using iontophoresis has a vasodilatory effect in healthy volunteers, which diminishes with age[11], was not proven in diabetes type 2 patients and is decreased in obese non-diabetic women[12]. An improvement in glycemic control leads to an increase in microvascular reactivity in diabetes type 2 patients[13].
To summarize the abovementioned findings, it is relatively well proven that physiological and even supraphysiological hyperinsulinemia leads to an increase in total limb blood perfusion. Study results regarding insulin influence on microcirculation vary. Some authors[7] suggest an insignificant role of physiological as well as supraphysiological insulinemia, others[6,9] observed an increase in perfusion only in physiological hyperinsulinemia. Furthermore, it is not completely clear what part of the vascular bed contributes to the increase in perfusion, whether it is an increase in blood flow through nutritive capillaries or arteriovenous shunts.
This study examined the vasodilatory effect of insulin on perfusion of skin microcirculation in healthy volunteers and assessed whether this effect follows a linear trend with insulinemia.
Microcirculation was examined at rest and after stimulation by physiologically (50 mIU/L) and supraphysiologically (150 mIU/L) increased level of insulin. Examinations was performed in 12 non-obese healthy volunteers with no history of diabetes in parents and siblings, with no chronic disease or chronic medication except hormonal contraception in women, and matched in age as well as in basic anthropometric and biochemical parameters (Table 1). The study protocol was approved by the ethical committee of Medical Faculty in Pilsen, Charles University in Prague. All volunteers were fully acquainted in advance with the experiment and methods used, which they confirmed by signing an informed consent. The day before study commencement, the volunteers maintained an ordinary daily routine with the exception of heavy physical exercise, excessive consumption of carbohydrates, fats and alcohol, and last meal until 9 pm. The following morning, a two step hyperinsulinemic clamp with target insulinemia 50 and 150 mIU/L was performed according to a well-established method[14], i.e., rate of insulin infusion 2.4 and 6.0 IU/m2 per hour. The order of insulinemias was inverted in one half of the sujects (i.e., first 6.0 then 2.4 IU/m2 per hour) and its sequence was random. We measured skin perfusion using LDF and transcutaneous oxymetry and respiratory quotient and energy expenditure by indirect calorimetry (V-max Sensormedics, Yorba Linda, CA, United States) according to a standard method[15] at basal conditions and in both steady states. M-value of each clamp was calculated to assess the change in insulin resistance. Results in the form of median and interquartile range were evaluated by the Wilcoxon test.
| Characteristics | Median (interquartile range) |
| Number (male/female) | 12 (6/6) |
| Age (yr) | 24 (23-25) |
| BMI (kg/m2) | 21.6 (20.7-23.7) |
| Waist (cm) | 74.5 (66.3-80.0) |
| Blood pressure (mmHg) | 113/75 (107/66-117/80) |
| Fasting plasma glucose (mmol/L) | 4.7 (4.6-5.3) |
| Plasma triglycerides (mmol/L) | 0.8 (0.7-0.9) |
| HDL cholesterol (mmol/L) | 1.4 (1.1-1.6) |
| LDL cholesterol (mmol/L) | 2.5 (2.3-3.1) |
| Fibrinogen (mmol/L) | 2.3 (2.2-2.5) |
Skin perfusion was examined at basal conditions before the clamp and in steady state at both insulin levels. System Periflux 5000 (Perimed, Sweden) with PF 5010 probe emitting laser with a wavelength of 780 nm and power output 1 mW was used for the measurement. The probe was placed to the dorsum of the non-dominant foot and measurement was performed in all subjects at a stable temperature of 33 °C. Subsequently, stimulation tests[16] were employed: heating (probe heating to 44 °Cinducing maximal vasodilatation) and occlusion (3 min occlusion of a limb using a sphygmomanometer cuff inflated to a pressure of 30 mmHg higher than systolic blood pressure), where time necessary for attaining maximal perfusion after cuff release was measured. These stimulation tests are a standard in examination of tissue perfusion[17-20] owing to a considerable time and spatial variability of plain basal perfusion measurement. Sampling rate was 31 ms and firmware Perisoft (Perimed, Sweden) was used for data evaluation.
Partial pressure of oxygen was measured using tcpO2 probe PF 5040 of Periflux 5000 system (Perimed, Sweden), based on the principle of polarography[21]. A heated Clark electrode (45 °C) was attached to the skin of the foot dorsum at a standard location (between the 1st and 2nd metatarsus) using an adhesive ring and the space between the electrode and skin was filled with contact solution supplied by the producer. The probe was applied at least 10 min prior to measurement commencement. Sampling rate was 31 ms and firmware Perisoft (Perimed, Sweden) was used for data evaluation.
Data are clearly summarized in Table 2 and Figure 1. The group in which the clamp with lower target insulinemia was performed first did not statistically differ in baseline characteristics from the group with the initial higher insulinemic clamp. Statistically significant higher perfusion in skin microcirculation was achieved at physiological hyperinsulinemia in both tests [hyperemia after heating to 44 °C: 1848% (984-2046) vs 1599% (801-1836), P < 0.05; half time of reaching peak perfusion after occlusion release 1.2 s (0.9-2.6) vs 4.9 s (1.8-11.4), P < 0.05]. A statistically significant increase occurred in tissue oxygenation [tcpO2: 48.6 mmHg (45.5-49.7) vs 38.9 mmHg (35.5-40.8), P < 0.05].
| Basal | 50 mIU/L | 150 mIU/L | |
| Measured insulin level (mIU/L) | 3.5 | 47.5b | 144.5b |
| (1.8-4.1) | (36.0-53.3) | (115.9-170.5) | |
| LDF baseline (PU) | 7.5 | 12.3 | 12.9 |
| (6.8-10.2) | (9.2-21.8) | (8.6-29.9) | |
| LDF heating test (%) | 1599 | 1848a | 1937b |
| (801-1836) | (984-2046) | (1177-2488) | |
| LDF post-occlusion | 4.9 | 1.2a | 1.0b |
| Hyperemia test (s) | (1.8-11.4) | (0.9-2.6) | (0.7-1.1) |
| tcpO2 (mmHg) | 38.9 | 48.6a | 57.4a |
| (35.5-40.8) | (45.5-49.7) | (51.7-66.2) |
The perfusion of skin microcirculation was even higher at supraphysiological hyperinsulinemia in both tests [hyperemia after heating to 44 °C: 1937% (1177-2488) vs 1599% (801-1836), P < 0.005, half time to reach peak perfusion after occlusion release 1.0 s (0.7-1.1) vs 4.9 s (1.8-11.4), P < 0.005]. A statistically significant increase occurred in tissue oxygenation [tcpO2: 57.4 mmHg (51.7-66.2) vs 38.9 mmHg (35.5-40.8), P < 0.005]. The difference in perfusion and oxygenation between physiological and supraphysiological hyperinsulinemia was not statistically significant. M-value measured during the clamp for insulin resistance evaluation did not change.
Studies that monitored an influence of insulin on microcirculation used either local skin administration using iontophoresis[9,12] or systemic delivery[5-7]. The advantage of local administration is limited local hyperinsulinemia, which does not require a clamp examination associated with fluid infusion and change in hepatic production of glucose and pancreatic production of insulin. In our study, we chose the systemic administration with the advantage of physiological insulin distribution and elimination of influence of passage of electrical current, which can induce vasoconstriction via voltage-dependent sodium and calcium channels[22].
Simultaneous use of LDF and transcutaneous oxymetry was performed to distinguish perfusion of the nutritive bed (assessed through O2 release) from total blood flow through microcirculation (including arteriovenous shunts) in the region of interest of the LDF probe, to which microvascular reactivity corresponds. However, measuring transcutaneous partial oxygen pressure can only be considered a rough indicator of nutritive bed perfusion. The exchange of oxygen between the vascular bed and tissues also takes place on other levels (larger vessels via interstitial fluid) and a discrepancy was found between capillary density assessed through video capillary microscopy and transcutaneous oxymetry values[23].
In some older studies[6,7], the authors describe no increase (or non significant increase) in microcirculation perfusion as a result of insulin infusion. On the other hand, more recent studies[9,10] demonstrate an increase in perfusion at physiological hyperinsulinemia. The explanation may be due to different methodology being used. In older studies, perfusion was measured by a probe only at basal conditions and no stimulation test was employed. According to our findings, the value of basal LDF perfusion showed only an insignificant incremental trend (Table 2), which corresponds to data measured earlier.
Transcutaneous oxygen pressure monitoring is important for estimation of amputation wound healing in diabetic foot syndrome[24] as well as for angioplasty effect monitoring in patients with critical limb ischemia[25] with tcpO2 values at rest below 30 mmHg as an independent predictor of ischemia[26]. The increase in transcutaneous oxygen pressure observed in our study in consistent with previous study data[4], where arteriovenous difference of oxygen increased with continuous subcutaneous insulin infusion, suggesting a flow redistribution favoring a functional vascular bed. On the contrary, in patients with diabetes mellitus type 2 with insulin resistance and hyperinsulinemia present, transcutaneous oxygen pressure is inversely proportional to insulinemia and it falls with its increase[27]. In obese patients with metabolic syndrome but without diabetes, decreased vasomotion and reduced response to locally administered insulin was described[10]. These findings suggest a different behavior of microcirculatory vascular bed in hyperinsulinemic insulin-resistant patients, where the response of microcirculation to exogenous insulin administration is altered and there is no improvement of nutritive perfusion (possibly even deterioration), while in insulin-sensitive patients, insulin administration causes more nutritive capillaries to open. Pathogenesis mechanism of reactivity changes is put in connection with oxidative stress induced by hyperlipidemia and insulin resistance, which causes vasoconstriction through augmentation of endothelin receptor activity (for thromboxane A2) in smooth muscle tissue[28]. In this regard, it will be interesting to observe insulin mediated microcirculation redistribution in patients with chronic heart failure treated with newly developed endothelin receptor antagonists; such a study has not yet been done according to the available literary data. There was no true control group with only fluid intake (without glucose and insulin) in our study. This can be considered a limitation of the study because the most important question is whether the fluid load associated with clamp examination itself does not lead to sympathetic activation and microcirculation reactivity increase.
In the literature, we can find a mention about a slight microcirculatory reactivity increase associated with fast infusion of saline[13]. In our experiment, healthy volunteers always had a total of 1-1.5l of glucose solution administered during the 2 h clamp and no increase in heart rate was observed. This rate of fluid infusion cannot be considered sufficient to trigger a sympathetic response. In addition, no significant difference in measured parameters was observed between the two subgroups (initially lower and higher insulinemia) despite the fact that the administered fluid volume at the first and the second steady state varied. Therefore, we presume the potential effect of fluid intake as insignificant. Hyperinsulinemia causes an increase in reactivity of microcirculation as well as an increase in transcutaneous oxygen pressure in healthy volunteers upon systemic administration of insulin. This effect rises non-linearly with increasing insulinemia.
Insulin, as a most potent antidiabetic drug, is currently studied from many points of view. One of them is the influence on microcirculation and whether (and how much) insulin blood concentration contributes to vasodilatation.
There are new technologies for measuring microvasculatory perfusion, laser Doppler flowmetry and transcutaneous oximetry. Both of them contribute to distinguishing between nutrition and thermoregulation vessels.
This work describes the microcirculation response to increasing insulinemia; perfusion is increased in both physiological and supraphysiological levels.
In contrast to large vessel examination, microcirculation examination is quite complicated. The mentioned techniques can help to measure drug mediated microcirculation perfusion in acute and chronic states.
The authors investigated the vasodilatory potential of insulin on skin microcirculation in healthy volunteers. This is an interesting study but the authors need to include a description of statistical methods and specific statistical tests.
P- Reviewers: Raghow R, Sourij H, Traub M S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Houben AJ, Schaper NC, Slaaf DW, Tangelder GJ, Nieuwenhuijzen Kruseman AC. Skin blood cell flux in insulin-dependent diabetic subjects in relation to retinopathy or incipient nephropathy. Eur J Clin Invest. 1992;22:67-72. |
| 2. | Netten PM, Wollersheim H, Thien T, Lutterman JA. Skin microcirculation of the foot in diabetic neuropathy. Clin Sci (Lond). 1996;91:559-565. |
| 3. | Biessels GJ, Stevens EJ, Mahmood SJ, Gispen WH, Tomlinson DR. Insulin partially reverses deficits in peripheral nerve blood flow and conduction in experimental diabetes. J Neurol Sci. 1996;140:12-20. |
| 4. | Tymms DJ, Tooke JE. The effect of continuous subcutaneous insulin infusion (CSII) on microvascular blood flow in diabetes mellitus. Int J Microcirc Clin Exp. 1988;7:347-356. |
| 5. | Ueda S, Petrie JR, Cleland SJ, Elliott HL, Connell JM. The vasodilating effect of insulin is dependent on local glucose uptake: a double blind, placebo-controlled study. J Clin Endocrinol Metab. 1998;83:2126-2131. |
| 6. | Tooke JE, Lins PE, Ostergren J, Adamson U, Fagrell B. The effects of intravenous insulin infusion on skin microcirculatory flow in Type 1 diabetes. Int J Microcirc Clin Exp. 1985;4:69-83. |
| 7. | Utriainen T, Malmström R, Mäkimattila S, Yki-Järvinen H. Methodological aspects, dose-response characteristics and causes of interindividual variation in insulin stimulation of limb blood flow in normal subjects. Diabetologia. 1995;38:555-564. |
| 8. | Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418-1423. |
| 9. | Serné EH, IJzerman RG, Gans RO, Nijveldt R, De Vries G, Evertz R, Donker AJ, Stehouwer CD. Direct evidence for insulin-induced capillary recruitment in skin of healthy subjects during physiological hyperinsulinemia. Diabetes. 2002;51:1515-1522. |
| 10. | de Jongh RT, Clark AD, IJzerman RG, Serné EH, de Vries G, Stehouwer CD. Physiological hyperinsulinaemia increases intramuscular microvascular reactive hyperaemia and vasomotion in healthy volunteers. Diabetologia. 2004;47:978-986. |
| 11. | Rossi M, Cupisti A, Ricco R, Santoro G, Pentimone F, Carpi A. Skin vasoreactivity to insulin iontophoresis is reduced in elderly subjects and is absent in treated non-insulin-dependent diabetes patients. Biomed Pharmacother. 2004;58:560-565. |
| 12. | de Jongh RT, Serné EH, IJzerman RG, Jørstad HT, Stehouwer CD. Impaired local microvascular vasodilatory effects of insulin and reduced skin microvascular vasomotion in obese women. Microvasc Res. 2008;75:256-262. |
| 13. | Forst T, Lübben G, Hohberg C, Kann P, Sachara C, Gottschall V, Friedrich C, Rosskopf R, Pfützner A. Influence of glucose control and improvement of insulin resistance on microvascular blood flow and endothelial function in patients with diabetes mellitus type 2. Microcirculation. 2005;12:543-550. |
| 14. | DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214-E223. |
| 15. | WEIR JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1-9. |
| 16. | Müller P, Keller R, Imhof P. Laser Doppler flowmetry, a reliable technique for measuring pharmacologically induced changes in cutaneous blood flow? Methods Find Exp Clin Pharmacol. 1987;9:409-420. |
| 17. | Albrecht HP, Hiller D, Mück-Weymann M, Bühler-Singer S, Boateng B, Hornstein OP. [Dynamic function tests for detection of physiologic and pathophysiologic reactions in cutaneous microcirculation]. Hautarzt. 1995;46:455-461. |
| 18. | Leahy MJ, de Mul FF, Nilsson GE, Maniewski R. Principles and practice of the laser-Doppler perfusion technique. Technol Health Care. 1999;7:143-162. |
| 19. | Walmsley D, Wiles PG. Reactive hyperaemia in skin of the human foot measured by laser Doppler flowmetry: effects of duration of ischaemia and local heating. Int J Microcirc Clin Exp. 1990;9:345-355. |
| 20. | Wohlrab J, Körting R, Helmbold P, Marsch WC. The NO release test as a functional reference standard for laser Doppler fluxmetry in cutaneous microangiology. Skin Res Technol. 2001;7:172-175. |
| 21. | Lawall H, Amann B, Rottmann M, Angelkort B. The role of microcirculatory techniques in patients with diabetic foot syndrome. Vasa. 2000;29:191-197. |
| 22. | Figueroa XF, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, Duling BR. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol. 2007;293:H1371-H1383. |
| 23. | Ubbink DT, Jacobs MJ, Slaaf DW. Can transcutaneous oximetry detect nutritive perfusion disturbances in patients with lower limb ischemia? Microvasc Res. 1995;49:315-324. |
| 24. | Faglia E, Clerici G, Caminiti M, Quarantiello A, Curci V, Morabito A. Predictive values of transcutaneous oxygen tension for above-the-ankle amputation in diabetic patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2007;33:731-736. |
| 25. | Caselli A, Latini V, Lapenna A, Di Carlo S, Pirozzi F, Benvenuto A, Uccioli L. Transcutaneous oxygen tension monitoring after successful revascularization in diabetic patients with ischaemic foot ulcers. Diabet Med. 2005;22:460-465. |
| 26. | Cechurová D, Rusavý Z, Lacigová S, Růzicka J, Novák M, Jankovec Z. [Transcutaneous oxygen tension in hyperbaric condition as a predictor of ischaemia in non-healing diabetic foot ulcers]. Vnitr Lek. 2002;48:971-975. |
| 27. | Kizu A, Koyama H, Tanaka S, Maeno T, Komatsu M, Fukumoto S, Emoto M, Shoji T, Inaba M, Shioi A. Arterial wall stiffness is associated with peripheral circulation in patients with type 2 diabetes. Atherosclerosis. 2003;170:87-91. |
| 28. | Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294:H1658-H1666. |