Published online Aug 15, 2013. doi: 10.4239/wjd.v4.i4.92
Revised: July 12, 2013
Accepted: July 18, 2013
Published online: August 15, 2013
Processing time: 69 Days and 15.8 Hours
There is a growing body of evidence that Diabetes Mellitus leads to a specific cardiomyopathy apart from vascular disease and bring about high morbidity and mortality throughout the world. Recent clinical and experimental studies have extensively demonstrated that this cardiomyopathy causes impaired cardiac performance manifested by early diastolic and late systolic dysfunction. This impaired cardiac performance most probably have emerged upon the expression and activity of regulatory proteins such as Na+/Ca2+ exchanger, sarcoplasmic reticulum Ca2+-ATPase, ryanodine receptor and phospholamban. Over years many therapeutic strategies have been recommended for treatment of diabetic cardiomyopathy. Lately, inorganic elements have been suggested to have anti-diabetic effects due to their suggested ability to regulate glucose homeostasis, reduce oxidative stress or suppress phosphatases. Recent findings have shown that trace elements exert many biological effects including insulin-mimetic or antioxidant activity and in this manner they have been recommended as potential candidates for treatment of diabetes-induced cardiac complications, an effect based on their modes of action. Some of these trace elements are known to play an essential role as component of enzymes and thus modulate the organ function in physiological and pathological conditions. Besides, they can also manipulate redox state of the channels via antioxidant properties and thus contribute to the regulation of [Ca2+]i homeostasis and cardiac ion channels. On account of little information about some trace elements, we discussed the effect of vanadium, selenium, zinc and tungstate on diabetic heart complications.
Core tip: Diabetic cardiomyopathy is one of the major causes of mortality in diabetic patients. Common cellular defects underlying the progressive cardiac complications of diabetes are reduction in the rate of contraction, low myosin ATPase activity, dysregulation of [Ca2+]i homeostasis and altered ionic currents. Accordingly, it is of critical importance to develop therapeutic strategies that will effectively inhibit diabetes induced fatal complications. In last decade, several trace elements have been suggested to improve performance of diabetic heart based due to their potential anti-diabetic and/or antioxidant activity. In this article the effects of trace elements on electrophysiological alterations of diabetic heart were discussed in detail.
- Citation: Ozturk N, Olgar Y, Ozdemir S. Trace elements in diabetic cardiomyopathy: An electrophysiological overview. World J Diabetes 2013; 4(4): 92-100
- URL: https://www.wjgnet.com/1948-9358/full/v4/i4/92.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i4.92
Cardiomyopathy, which develops independent of any major vascular disease, is one of the main complications of diabetes resulting in a high percentage of morbidity and mortality. Although atherosclerotic vascular diseases occur frequently in diabetic conditions, a specific type of cardiomyopathy that results in impaired cardiac performance has been widely described in clinical and experimental studies[1-7]. In clinical aspect, diabetic cardiomyopathy is a disease which manifests itself particularly by early diastolic and late systolic dysfunction. As a matter of fact, elevated end-diastolic left ventricular (LV) pressure, reduced end-diastolic LV volume, impaired LV function in response to physiological stress and reduced LV filling rates in diabetic humans and animals are well-characterized[7-9]. These functional abnormalities of diabetic heart are likely to stem from multiple cellular defects such as reduction in the rate of contraction and relaxation, low myosin ATPase activity, myosin isoforms’ shift from V1 (fast) to V3 (slow), deterioration of sarcoplasmic reticulum (SR) calcium uptake and reduction in glucose carrier (GLUT-4)[10,11]. Consistent with this, our reports and other studies have demonstrated prolonged periods of contraction and relaxation and in turn reduced tensile strength of rat papillary muscle in type 1 diabetes[12,13]. However, unchanged tensile strength despite slow left ventricular papillary muscle contraction and relaxation has been also suggested in experimental diabetes model in rats[3,4,14]. At cellular level prolongation of action potential (AP) duration has been consistently shown in diabetic hearts[3,4,15,16]. Significant alterations in the ionic currents that constitute AP configuration have been proposed as the main culprit of this prolongation, and indeed reduced transient outward (Ito) along with smaller steady-state K+ currents (or Iss) have been reported[3-6,15,16], despite unchanged Ca2+ currents[5,6]. Additionally, inward rectifier K+ current (IK1) has not been stated to have changed, but the delayed rectifier current (IK), thought to modulate late repolarization of AP, has decreased in diabetic ventricular cells[6,16].
On the other hand, regulation of intracellular Ca2+ concentration ([Ca2+]i) is very critical for myocardium and has overriding impact on the contraction of heart. Therefore, diabetes induced abnormalities in cardiac contractility have been correlated with the intracellular [Ca2+]i changes[5,6,12,14,17]. However, despite the plenty of data about dysregulated [Ca2+]i in diabetic myocardium, current findings are somehow controversial particularly in terms of the direction of change[14,18,19]. Nevertheless, amplitudes of Ca2+ transients recorded under electrical stimuli have been reported to be smaller, while their time to peak and decay were mostly longer[5,6,19-22]. Therefore, it is most likely that diabetic cardiac dysfunction arises due to changes in expression and/or activity of cellular mechanisms that regulate [Ca2+]i during cardiac cycle. This possibility has been widely studied over years and indeed a significant decrease has been found in expression of regulatory proteins such as Na+/Ca2+ exchanger NCX (NCX), sarcoplasmic reticulum Ca2+ ATPase (SERCA), ryanodine receptor (RyR) and phospholamban (PLB), along with reduced activity of NCX and SERCA[11,18,20,22-25].
However diabetes is characterized by complexity; it likely involves activation of different pathways leading to abnormal [Ca2+]i homeostasis and thus contractile dysfunction. For example, currently it is clearly evident that reactive oxygen species (ROS) and resultant oxidative stress is involved in the pathogenesis of diabetic cardiomyopathy. Hyperglycemia leads to generation of superoxide radicals from both mitochondrial (via oxidation of glucose) and non-mitochondrial sources (xanthine oxidase, nitric oxide synthase and NADPH-oxidase)[26].
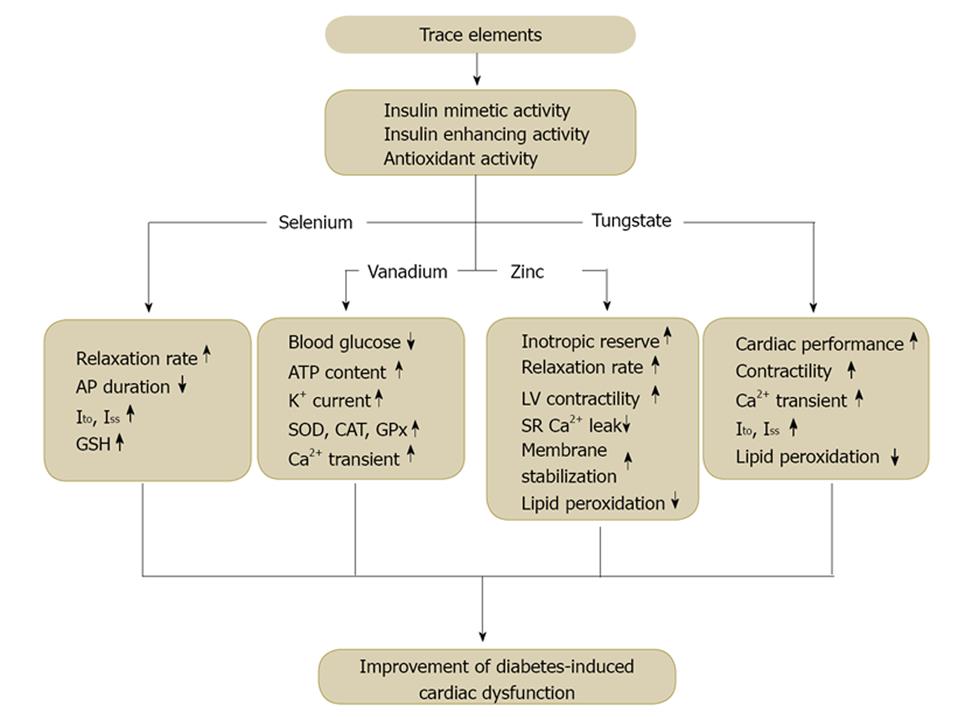
In recent years many inorganic elements have been recommended as dietary supplement to alleviate the impaired insulin metabolism in diabetic patients[5,26-30]. Being essential or not, trace elements have been identified for long time as potential candidates for treatment or to mitigate severity of complications of some metabolic disorders including diabetes (Figure 1). Activation of insulin receptor signaling, antioxidant properties or inhibition of phosphatases have been depicted as potential ways of action in modulating glucose homeostasis and preventing organ damage[26,29,31]. On the other hand, cardiac complications have been progressively becoming the main cause of death among diabetics due to the improvements in the treatment of diabetic complications with non-cardiac origin. Accordingly, it is of critical importance to develop therapeutic strategies that will effectively inhibit diabetes induced fatal cardiac disorders. Consistently, trace elements, some of which are involved in metabolism as essential components of enzymes, have also been suggested to improve the reduced cardiac performance in diabetic heart due to their presumed insulin-mimetic or antioxidant activity[3,5,28,32]. Furthermore, recent studies have demonstrated that the underlying mechanism of this improvement is due most probably to restoration of abnormal [Ca2+]i homeostasis and cardiac ion channels. Despite the limited number of studies, it is evident that either insulin-mimetic or antioxidant, trace elements are capable of modulating expression and/or redox status of ion channels and [Ca2+]i regulating proteins[34-36]. Of the inorganic or trace elements currently known; vanadium, selenium, zinc and tungstate were discussed in this review, since the effects of other inorganic elements on diabetic cardiac complications have not been well-documented yet.
Selenium was first discovered by Berzelius in 1818. This Swedish chemist named that new chemical element after Selene, the Greek goddess of the moon. Selenium is an essential trace element in man and animals, since it is an integral part of selenium dependent glutathione peroxidase[36]. In humans and experimental studies, selenium deficiency has been suggested to result in increased risk of various pathologies including cardiovascular diseases[37]. Particularly, selenium deficiency results in Keshan disease, which is a special type of cardiomyopathy caused by dietary inadequacy of selenium and responds to treatment with sodium selenite[38]. Furthermore, adequate selenium intake is required for optimal activity of some key antioxidant enzymes, including glutathione peroxidases and thioredoxin reductases, which act to prevent free radical damage to various cells[39,40]. As a result of its protective role against oxidative stress, selenium raised considerable expectations for the prevention of cardiovascular diseases including diabetic cardiomyopathy. It appears to have insulin-like effects when administered in vivo[41]. In fact, several reports have suggested that plasma glucose levels were significantly though not completely improved in the diabetic rats treated with selenite in different ways either orally or via injection[3,42-44]. Interestingly, similar to vanadium, selenite decreases plasma glucose levels in hypo-insulinemic rats without an accompanying correction of the insulin levels[3,42]. Nevertheless, the reduced cardiac performance characteristics of diabetic rats such as left ventricular developed pressure (LVDP), positive dP/dt (+ dP/dt) and negative dP/dt (- dP/dt) have been found to be reversed with selenite treatment[42,45].
The effects of sodium selenite treatment on mechanical and electrical properties of diabetic heart have been also studied in detail. Ayaz et al[3] demonstrated that selenium supplementation for 5 wk was capable of reducing the prolonged peak time and relaxation of electrically stimulated papillary muscle twitch in diabetic rats, although no change was reported between peak tension of the experimental groups. Additionally, the prolonged AP duration, which is a typical characteristic of diabetic heart, was shown to be restored after the treatment. In the same study, the major repolarizing currents of AP, Ito and Iss, were lower in untreated diabetic cardiomyocytes while selenium achieved an apparent increase in treated-diabetics. However, plasma insulin levels didn’t increase significantly despite the long-term administration of selenium[3]. Although the precise mechanism of this beneficial effect is not known currently, it is likely that oxidative stress, which has been suggested to involve in the etiology of diabetes-induced downregulation of ion channels, is balanced through selenite-mediated augmentation of glutathione levels, and resultant enhancement of endogenous antioxidant defense mechanisms[3,5,28,46]. Additionally, oxidative species have been recognized to modulate K+ channels and cellular Ca2+ regulation, notably via redox modifications of key amino acid residues involved in the function of ion channels and transporters[33-35,47]. Therefore, it is most likely that selenium may achieve recovery of impaired cardiac performance and altered K+ currents of diabetic cardiomyocytes via restoration of the oxidized groups of ion channel proteins.
Vanadium is a trace element that exists naturally in water and soil and found in different physiologically active oxidation states[29]. Although the exact physiological actions of vanadium are not known yet, it is supposed to be necessary for the body as a trace element since its deficiency has been suggested to result in a variety of side-effects[29,48]. In addition to reproductive problems and skeletal abnormalities observed in case of deficiency, vanadium is likely to have a significant role in thyroid, iron, glucose and lipid metabolism[29,49]. The total vanadium content of the body has been estimated to be approximately 200 μg[29,50]. The beneficial effects of vanadium have been widely studied in diabetic conditions and speculated to exert insulin-mimetic activity through a specific tyrosine kinase receptor or to annihilate free radicals due to its antioxidant activity[51-55]. Therefore, the potential use of vanadium in the treatment of diabetic complications including cardiomyopathy has been assessed and indeed its hypoglycemic effect along with reversal of functional abnormalities has been clearly demonstrated by several studies[28,56-59].
In the last decade, the effect of vanadate compounds on impaired performance of diabetic heart has been investigated in a large number of studies that have shown significant improvement in diabetes with vanadate treatment[28,56]. Ozcelikay et al[60] reported that vanadate treatment was capable of normalizing blood glucose and serum thyroid hormone levels, despite the fact that serum insulin level of diabetic animals was not corrected significantly. Moreover, vanadate treatment resulted in normalization of mechanical alterations and reversed the decreased responsiveness of diabetic atria to isoprenaline in spontaneously-beating preparations from diabetic rats. Similarly Heyliger et al[56] assessed the impact of vanadate on cardiac performance in diabetic female rats and found that vanadate was capable of restoring blood glucose but not insulin levels when administered for a 4-wk period to the diabetic rats. In the same study, vanadate treatment prevented the decline in cardiac performance due to diabetes. Organic vanadium complex, bis (maltolato) oxovanadium (IV) was also reported to correct working heart parameters such as LVDP and ± dP/dT values in streptozotocin-induced diabetic rats, which indicated the protective effect of vanadium derivatives against heart dysfunction associated with type 1 diabetes in rats[61]. Consistently, decreased peak ± dP/dt and reduced cardiac efficiency of diabetic hearts were fully restored while myocardial ATP content significantly increased by vanadate administration[62]. These results, thus, indicate that the normalizing effect of vanadate on diabetes can contribute to the prevention of cardiac changes observed at the early and late stages of diabetes.
Taking the central role that Ca2+ plays in cardiac electrical and mechanical activity, it is likely to suggest that the beneficial effects of vanadate entail modulation of Ca2+ regulation in diabetic cardiomyocyte. In fact Clark et al[28,29] demonstrated that tea-vanadate treatment had normalized the contractile response of diabetic cardiomyocytes and ameliorated the Ca2+ transients to an extent equal to or better than that of insulin treated diabetic animals. It is an effect that were attributed to the alleviated glycemic status because tea/vanadate decoction has been shown to restore glycemic status effectively in rodent models of both Type I and Type II diabetes mellitus[63,64]. Interestingly, tea/vanadate decoction exhibited vastly improved glycemic status that could persist beyond treatment period[63] and relieved diabetic animals from non-specific side-effects of vanadate or its analogues to other organs in the body[65,66]. Vanadate also mimics the enhancing effect of insulin on cardiac K+ currents (particularly Ito) in sucrose-fed rats with 3-4 wk treatment or 5-6 h incubation of myocytes, an effect suggested to arise due probably to synthesis of new channels[67]. Hence, although we don’t have such data, it is tempting to speculate that vanadate is likely to shorten AP duration in diabetic myocardium and thereby modulate ventricular repolarization and dispersion of repolarization that have been shown to be a major cause of cardiac arrhythmias in diabetes mellitus[68].
Vanadate is thought to act via insulin-mimetic and/or insulin-enhancing action[69] or through activation of lipid signaling mechanisms like the phosphatidylinositol pathway[54]. It can also scavenge free radicals[55], and accordingly, vanadate administration has been reported to decrease oxidative damage remarkably in the diabetic heart[70]. Therefore, the beneficial effect of vanadate on diabetes-induced cardiac dysfunction may stem from its ability to serve as a scavenger of free radicals[27,54,71]. With vanadium treatment, glutathione peroxidase, catalase and superoxide dismutase levels have been corrected to near normal values in diabetic rats[54,55]. However, one another study attributed some of these effects to vanadate’s ability to prevent diabetic hypothyroidism[60]. In conclusion, despite the plenty of findings that provide evidences for improving effect of vanadium on diabetic heart dysfunction due most probably to its insulin-mimetic and/or antioxidant action, further studies are needed to fully elucidate the molecular mechanism of these beneficial effects.
Zinc is an essential trace element that is critical in maintaining cellular functions since it is the cofactor of numerous enzymes and transcription factors[26,72]. In normal cellular physiology, much of the intracellular zinc is found in protein bound form and participates in phosphorylation/dephosphorylation cascades. Besides, it acts as a second messenger in the signaling system[73] and affects the redox status of the cell. Thus, in particular conditions zinc can either enhance the cell’s antioxidant capacity or trigger the production of reactive oxygen species[26,74]. Consistent with this, Zn deficiency has been suggested to result in increased oxidative damage in multiple organs including the heart[75-77] due to the decreased cardiac antioxidant capacity[76,78].
It has been demonstrated that Zn deficiency induced by low concentrations of Zn in drinking water[79] and by Zn chelators increases the likelihood of diabetes in humans and animals[80]. Therefore, it is likely that Zn deficiency can be a risk factor for the development of diabetes, and in reciprocal manner, diabetes itself can dysregulate Zn homeostasis. Indeed, systemic Zn deficiency has been associated with the high incidence of diabetic cardiovascular complications[72,79,81,82]. The potential role of zinc in the protection of diabetic patients from coronary heart disease has been investigated in a recent clinical trial in which serum zinc level was inversely proportional to cardiovascular complications[83]. Measurements of cardiac function have demonstrated that Zn is capable of improving left ventricular systolic and diastolic function. Moreover, inotropic reserve of left ventricle was enhanced in the heart of the diabetic mice treated with Zn compared to that without Zn, which implicates alleviated cardiac function with Zn supplementation[30]. Wang et al[84] observed lower ± dP/dtmax, suggesting reduced LV contractility along with slowing of relaxation in the diabetic mice, which both improved following Zn supplementation to near control levels. Furthermore, Zn ameliorated the diabetes-induced catecholamine desensitization markedly, which was quantified by measure of augmentation of dP/dtmax after β-adrenergic stimulation. Thus, they concluded that zinc is capable of improving both basal and stimulated LV function as well as inotropic reserve in diabetic hearts.
On the other hand, incomplete relaxation and reduced contractile function which were more prominent as pacing frequency increased has been reported in diabetic cardiomyocytes, but these changes were significantly restored by extracellular Zn exposure[8]. These findings provide evidences that suggest zinc administration could be a possible long term management regimen for incomplete relaxation and diastolic dysfunction associated with diabetic cardiomyopathy. In addition, extracellular zinc ion has been proposed to compete with Ca2+ for the cardiomyocyte L-type Ca2+ channel and, the release of SR Zn through the RyR also appears to be regulated similarly to that of SR Ca2+[85-87]. Moreover, extracellular Zn exposure could lower the open probability of RyR and presumably reduces SR Ca2+ leak through the RyR, which has been shown to be elevated in hyperglycemic conditions[88,89]. Given these results, it is likely that Zn exerts a competitive effect on Ca2+ regulatory mechanisms and modulates cardiomyocyte function.
Although the cellular and molecular mechanisms responsible for zinc-induced protection against diabetic cardiomyopathy has not been fully understood yet, zinc-binding protein metallothionein (MT) has been proposed to play a role in cellular defence against oxidative stress associated with diabetic cardiomyopathy[72,84,90]. Indeed, Zn supplementation provides significant protection of the heart from oxidative stress. Zn has been demonstrated to act as an antioxidant through participation in SOD and thioredoxin enzymatic and chelator activities, stabilizing cell membranes, and inhibiting lipid peroxidation[26,74,91]. Additionally, the relationship between Zn and diabetes appears to be complex. Several complications of diabetes have been supposed to be related to increased intracellular oxidants and free radicals associated with decreases in intracellular Zn and in Zn-dependent antioxidant enzymes[92]. Moreover, Zn is suggested to be important for the normal conformation, secretion and function of insulin[26,30].
All these observations strongly support the notion that Zn deficiency occurs in diabetic subjects[82] and Zn supplementation may improve cardiac dysfunction or damage in these patients due to its systemic antioxidant capacity or modulation of the cellular ionic mechanisms. However, the understanding of molecular mechanisms that involve in Zn related changes in diabetic heart deserves further investigation.
Over the past decade, sodium tungstate (Na2WO4), which chemically resembles vanadium has become a molecule of interest, since it has a relatively low toxicity and it has been suggested to have antidiabetic activity in experimental studies[93-96]. Although numerous studies have demonstrated the efficacy of tungstate as an antidiabetic agent in various models of experimental diabetes, only few of them have investigated whether it can improve cardiac performance of diabetic heart as well. One of these studies performed by Nagareddy et al[31] has assessed cardiac function by measuring left ventricular pressure, the rate of contraction and the rate of relaxation. An apparent cardiac dysfunction has been shown in untreated diabetic rat hearts, which exhibited an inability to respond to the increase in left atrial filling pressure. However, the treatment of diabetic rats with tungstate has improved LVP, + dP/dt, and - dP/dt, particularly at higher filling pressures.
On the other hand, recently we have studied the cellular mechanism of that beneficial effect of sodium tungstate on diabetic myocardium at cellular level. We demonstrated that long-term sodium tungstate treatment was capable of ameliorating the amplitude of shortening and associated Ca2+ transients of diabetic cardiomyocytes, although it didn’t improve the rate of relaxation in either traces. Moreover, we showed depressed Ito and Iss in diabetic cardiomyocytes which were recovered significantly by tungstate administration that might be accomplished due to its antioxidant property[5]. This finding is important because diminished potassium currents and thus prolonged action potential in ventricular cells have been suggested to increase the likelihood of arrhythmia in diabetic patients[4,33,67,97]. Hence, tungstate administration is likely to reduce this propensity in diabetic patients.
The underlying mechanism of these beneficial effects has been mostly attributed to antioxidant or insulin-like activity of tungstate. Because hyperglycemia leads to abnormal increase of ROS production[5,11,35] that have been recognized to be capable of modulating K+ channels and [Ca2+]i regulation due to redox modifications of key amino acid residues involved in the function of intracellular and plasma membrane ion channels and transporters[33,35]. In fact, tungstate treatment was associated with significant reduction of lipid and protein oxidation levels in treated-diabetic rats, a finding that further supports this hypothesis. Insulin-mimetic or insulin-enhancing activity of tungstate is less likely since we didn’t observe a remarkable change either in insulin or blood glucose levels after supplementation[5]. Contrary to this, some investigators have reported increased insulin and/or decreased glucose levels that might arise from very high level of tungstate they administered, which may cause side effects[98].
Diabetic cardiomyopathy, one of the major causes of mortality in diabetic patients, is associated with progressive contractile dysfunction. Therefore, it is crucial to develop therapeutic strategies that will effectively inhibit diabetes-induced fatal complications of the heart. Among the various therapeutic strategies, the restoration of glycemic status by insulin-enhancing or insulin-mimetic agents can be useful in the prevention of cardiomyopathy in diabetic patients.
In the last decade, several inorganic compounds such as selenium, vanadium, zinc and tungstate have been suggested to improve cardiac performance in diabetic heart based on its potential anti-diabetic and/or antioxidant activity. Some of these trace elements are known to play an essential role as components of enzymes and thus modulate the organ function in physiological and pathological conditions. Current findings clearly demonstrate that diabetic cardiomyopathy leads to ventricular dysfunction due to altered ionic homeostasis in myocytes which results in defective excitation-contraction coupling of myocardium and, trace element supplementation can prevent these changes and thus ameliorate the diminished cardiac function. Therefore, they may have a potential therapeutic use in preventing diabetic cardiomyopathy, although further investigations and substantial efforts are needed to elucidate the underlying mechanism of their beneficial effect. Furthermore, prior to clinical trials, the question whether they have side effects or not should be addressed unequivocally.
P- Reviewers Cai L, Kumar R S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Fein FS, Sonnenblick EH. Diabetic cardiomyopathy. Prog Cardiovasc Dis. 1985;27:255-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 191] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol. 1991;68:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 392] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Ayaz M, Ozdemir S, Ugur M, Vassort G, Turan B. Effects of selenium on altered mechanical and electrical cardiac activities of diabetic rat. Arch Biochem Biophys. 2004;426:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Ozdemir S, Ugur M, Gürdal H, Turan B. Treatment with AT(1) receptor blocker restores diabetes-induced alterations in intracellular Ca(2+) transients and contractile function of rat myocardium. Arch Biochem Biophys. 2005;435:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Aydemir M, Ozturk N, Dogan S, Aslan M, Olgar Y, Ozdemir S. Sodium tungstate administration ameliorated diabetes-induced electrical and contractile remodeling of rat heart without normalization of hyperglycemia. Biol Trace Elem Res. 2012;148:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Ozturk N, Yaras N, Ozmen A, Ozdemir S. Long-term administration of rosuvastatin prevents contractile and electrical remodelling of diabetic rat heart. J Bioenerg Biomembr. 2013;45:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 675] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 8. | Yi T, Cheema Y, Tremble SM, Bell SP, Chen Z, Subramanian M, LeWinter MM, VanBuren P, Palmer BM. Zinc-induced cardiomyocyte relaxation in a rat model of hyperglycemia is independent of myosin isoform. Cardiovasc Diabetol. 2012;11:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Scognamiglio R, Avogaro A, Negut C, Piccolotto R, Vigili de Kreutzenberg S, Tiengo A. Early myocardial dysfunction in the diabetic heart: current research and clinical applications. Am J Cardiol. 2004;93:17A-20A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Mahgoub MA, Abd-Elfattah AS. Diabetes mellitus and cardiac function. Mol Cell Biochem. 1998;180:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res. 1998;40:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH. Overexpression of the sarcoplasmic reticulum Ca(2+)-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes. 2002;51:1166-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Fein FS, Kornstein LB, Strobeck JE, Capasso JM, Sonnenblick EH. Altered myocardial mechanics in diabetic rats. Circ Res. 1980;47:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 215] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Ishikawa T, Kajiwara H, Kurihara S. Alterations in contractile properties and Ca2+ handling in streptozotocin-induced diabetic rat myocardium. Am J Physiol. 1999;277:H2185-H2194. [PubMed] |
| 15. | Shimoni Y, Firek L, Severson D, Giles W. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res. 1994;74:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Jourdon P, Feuvray D. Calcium and potassium currents in ventricular myocytes isolated from diabetic rats. J Physiol. 1993;470:411-429. [PubMed] |
| 17. | Norby FL, Aberle NS, Kajstura J, Anversa P, Ren J. Transgenic overexpression of insulin-like growth factor I prevents streptozotocin-induced cardiac contractile dysfunction and beta-adrenergic response in ventricular myocytes. J Endocrinol. 2004;180:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Yu JZ, Rodrigues B, McNeill JH. Intracellular calcium levels are unchanged in the diabetic heart. Cardiovasc Res. 1997;34:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Hayashi H, Noda N. Cytosolic Ca2+ concentration decreases in diabetic rat myocytes. Cardiovasc Res. 1997;34:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Lederer WJ, Matlib MA. Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1398-H1408. [PubMed] |
| 21. | Lagadic-Gossmann D, Buckler KJ, Le Prigent K, Feuvray D. Altered Ca2+ handling in ventricular myocytes isolated from diabetic rats. Am J Physiol. 1996;270:H1529-H1537. [PubMed] |
| 22. | Yaras N, Ugur M, Ozdemir S, Gurdal H, Purali N, Lacampagne A, Vassort G, Turan B. Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes. 2005;54:3082-3088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Hattori Y, Matsuda N, Kimura J, Ishitani T, Tamada A, Gando S, Kemmotsu O, Kanno M. Diminished function and expression of the cardiac Na+-Ca2+ exchanger in diabetic rats: implication in Ca2+ overload. J Physiol. 2000;527 Pt 1:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Norby FL, Wold LE, Duan J, Hintz KK, Ren J. IGF-I attenuates diabetes-induced cardiac contractile dysfunction in ventricular myocytes. Am J Physiol Endocrinol Metab. 2002;283:E658-E666. [PubMed] |
| 25. | Teshima Y, Takahashi N, Saikawa T, Hara M, Yasunaga S, Hidaka S, Sakata T. Diminished expression of sarcoplasmic reticulum Ca(2+)-ATPase and ryanodine sensitive Ca(2+)Channel mRNA in streptozotocin-induced diabetic rat heart. J Mol Cell Cardiol. 2000;32:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL. Zinc and cardiovascular disease. Nutrition. 2010;26:1050-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Bhuiyan MS, Fukunaga K. Cardioprotection by vanadium compounds targeting Akt-mediated signaling. J Pharmacol Sci. 2009;110:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Clark TA, Maddaford TG, Tappia PS, Heyliger CE, Ganguly PK, Pierce GN. Restoration of cardiomyocyte function in streptozotocin-induced diabetic rats after treatment with vanadate in a tea decoction. Curr Pharm Biotechnol. 2010;11:906-910. [PubMed] |
| 29. | Clark TA, Deniset JF, Heyliger CE, Pierce GN. Alternative therapies for diabetes and its cardiac complications: role of vanadium. Heart Fail Rev. 2013;Feb 21; [Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Li B, Tan Y, Sun W, Fu Y, Miao L, Cai L. The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol Mech Methods. 2013;23:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Nagareddy PR, Vasudevan H, McNeill JH. Oral administration of sodium tungstate improves cardiac performance in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2005;83:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Turan B, Ugur M, Ozdemir S, Yaras N. Altered mechanical and electrical activities of the diabetic heart: Possible use of new therapeutics? Exp Clin Cardiol. 2005;10:189-195. [PubMed] |
| 33. | Li X, Xu Z, Li S, Rozanski GJ. Redox regulation of Ito remodeling in diabetic rat heart. Am J Physiol Heart Circ Physiol. 2005;288:H1417-H1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Li X, Li S, Xu Z, Lou MF, Anding P, Liu D, Roy SK, Rozanski GJ. Redox control of K+ channel remodeling in rat ventricle. J Mol Cell Cardiol. 2006;40:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Bidasee KR, Nallani K, Besch HR, Dincer UD. Streptozotocin-induced diabetes increases disulfide bond formation on cardiac ryanodine receptor (RyR2). J Pharmacol Exp Ther. 2003;305:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590. [PubMed] |
| 37. | Tanguy S, Grauzam S, de Leiris J, Boucher F. Impact of dietary selenium intake on cardiac health: experimental approaches and human studies. Mol Nutr Food Res. 2012;56:1106-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Yang GQ, Chen JS, Wen ZM, Ge KY, Zhu LZ, Chen XC, Chen XS. The role of selenium in Keshan disease. Adv Nutr Res. 1984;6:203-231. [PubMed] |
| 39. | Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 893] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 40. | Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2798] [Cited by in RCA: 2533] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 41. | McNeill JH, Delgatty HL, Battell ML. Insulinlike effects of sodium selenate in streptozocin-induced diabetic rats. Diabetes. 1991;40:1675-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Battell ML, Delgatty HL, McNeill JH. Sodium selenate corrects glucose tolerance and heart function in STZ diabetic rats. Mol Cell Biochem. 1998;179:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Ayaz M, Can B, Ozdemir S, Turan B. Protective effect of selenium treatment on diabetes-induced myocardial structural alterations. Biol Trace Elem Res. 2002;89:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Becker DJ, Reul B, Ozcelikay AT, Buchet JP, Henquin JC, Brichard SM. Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia. 1996;39:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Aydemir-Koksoy A, Bilginoglu A, Sariahmetoglu M, Schulz R, Turan B. Antioxidant treatment protects diabetic rats from cardiac dysfunction by preserving contractile protein targets of oxidative stress. J Nutr Biochem. 2010;21:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Xu Z, Patel KP, Lou MF, Rozanski GJ. Up-regulation of K(+) channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc Res. 2002;53:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 48. | French RJ, Jones PJ. Role of vanadium in nutrition: metabolism, essentiality and dietary considerations. Life Sci. 1993;52:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Nielsen F. Vanadium and its role in life. New York: Taylor&Francis 1995; 543–573. [PubMed] |
| 50. | Byrne AR, Kosta L. Vanadium in foods and in human body fluids and tissues. Sci Total Environ. 1978;10:17-30. [PubMed] [DOI] [Full Text] |
| 51. | Fantus IG, Ahmad F, Deragon G. Vanadate augments insulin-stimulated insulin receptor kinase activity and prolongs insulin action in rat adipocytes. Evidence for transduction of amplitude of signaling into duration of response. Diabetes. 1994;43:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | D’Onofrio F, Le MQ, Chiasson JL, Srivastava AK. Activation of mitogen activated protein (MAP) kinases by vanadate is independent of insulin receptor autophosphorylation. FEBS Lett. 1994;340:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Pandey SK, Anand-Srivastava MB, Srivastava AK. Vanadyl sulfate-stimulated glycogen synthesis is associated with activation of phosphatidylinositol 3-kinase and is independent of insulin receptor tyrosine phosphorylation. Biochemistry. 1998;37:7006-7014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Matsubara T, Musat-Marcu S, Misra HP, Dhalla NS. Protective effect of vanadate on oxyradical-induced changes in isolated perfused heart. Mol Cell Biochem. 1995;153:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Sekar N, Kanthasamy A, William S, Balasubramaniyan N, Govindasamy S. Antioxidant effect of vanadate on experimental diabetic rats. Acta Diabetol Lat. 1990;27:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Heyliger CE, Tahiliani AG, McNeill JH. Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science. 1985;227:1474-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 496] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 57. | Clark TA, Heyliger CE, Kopilas M, Edel AL, Junaid A, Aguilar F, Smyth DD, Thliveris JA, Merchant M, Kim HK. A tea/vanadate decoction delivered orally over 14 months to diabetic rats induces long-term glycemic stability without organ toxicity. Metabolism. 2012;61:742-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Kopp SJ, Daar J, Paulson DJ, Romano FD, Laddaga R. Effects of oral vanadyl treatment on diabetes-induced alterations in the heart GLUT-4 transporter. J Mol Cell Cardiol. 1997;29:2355-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Li SH, McNeill JH. In vivo effects of vanadium on GLUT4 translocation in cardiac tissue of STZ-diabetic rats. Mol Cell Biochem. 2001;217:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Ozcelikay AT, Yidizoglu-Ari N, Ozuari A, Oztürk Y, Altan VM. The effect of vanadate on alloxan-diabetic rat atria. Diabetes Res Clin Pract. 1993;19:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Yuen VG, Orvig C, Thompson KH, McNeill JH. Improvement in cardiac dysfunction in streptozotocin-induced diabetic rats following chronic oral administration of bis(maltolato)oxovanadium(IV). Can J Physiol Pharmacol. 1993;71:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Noda C, Masuda T, Sato K, Ikeda K, Shimohama T, Matsuyama N, Izumi T. Vanadate improves cardiac function and myocardial energy metabolism in diabetic rat hearts. Jpn Heart J. 2003;44:745-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Clark TA, Heyliger CE, Edel AL, Goel DP, Pierce GN. Codelivery of a tea extract prevents morbidity and mortality associated with oral vanadate therapy in streptozotocin-induced diabetic rats. Metabolism. 2004;53:1145-1151. [PubMed] |
| 64. | Clark TA, Edel AL, Heyliger CE, Pierce GN. Effective control of glycemic status and toxicity in Zucker diabetic fatty rats with an orally administered vanadate compound. Can J Physiol Pharmacol. 2004;82:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Domingo JL. Vanadium and diabetes. What about vanadium toxicity? Mol Cell Biochem. 2000;203:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Srivastava AK. Anti-diabetic and toxic effects of vanadium compounds. Mol Cell Biochem. 2000;206:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Shimoni Y, Severson D, Ewart HS. Insulin resistance and the modulation of rat cardiac K(+) currents. Am J Physiol Heart Circ Physiol. 2000;279:H639-H649. [PubMed] |
| 68. | Lindström T, Jorfeldt L, Tegler L, Arnqvist HJ. Hypoglycaemia and cardiac arrhythmias in patients with type 2 diabetes mellitus. Diabet Med. 1992;9:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Cam MC, Brownsey RW, McNeill JH. Mechanisms of vanadium action: insulin-mimetic or insulin-enhancing agent? Can J Physiol Pharmacol. 2000;78:829-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Genet S, Kale RK, Baquer NZ. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: effect of vanadate and fenugreek (Trigonellafoenum graecum). Mol Cell Biochem. 2002;236:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 71. | Takeda S, Mochizuki S, Saini HK, Elimban V, Dhalla NS. Modification of alterations in cardiac function and sarcoplasmic reticulum by vanadate in ischemic-reperfused rat hearts. J Appl Physiol. 2005;99:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Song Y, Wang J, Li XK, Cai L. Zinc and the diabetic heart. Biometals. 2005;18:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U. Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem. 2002;277:44327-44331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37:1182-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 365] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 75. | Oteiza PI, Olin KL, Fraga CG, Keen CL. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J Nutr. 1995;125:823-829. [PubMed] |
| 76. | Noh SK, Koo SI. Feeding of a low-zinc diet lowers the tissue concentrations of alpha-tocopherol in adult rats. Biol Trace Elem Res. 2001;81:153-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc Natl Acad Sci USA. 2002;99:16770-16775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 293] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 78. | Kim SH, Keen CL. Influence of dietary carbohydrate on zinc-deficiency-induced changes in oxidative defense mechanisms and tissue oxidative damage in rats. Biol Trace Elem Res. 1999;70:81-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Haglund B, Ryckenberg K, Selinus O, Dahlquist G. Evidence of a relationship between childhood-onset type I diabetes and low groundwater concentration of zinc. Diabetes Care. 1996;19:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Goldberg ED, Eshchenko VA, Bovt VD. The diabetogenic and acidotropic effects of chelators. Exp Pathol. 1991;42:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 81. | Brandão-Neto J, Silva CA, Figueiredo NB, Shuhama T, Holanda MB, Diniz JM. Zinc kinetics in insulin-dependent diabetes mellitus patients. Biometals. 2000;13:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Viktorínová A, Toserová E, Krizko M, Duracková Z. Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metabolism. 2009;58:1477-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 83. | Soinio M, Marniemi J, Laakso M, Pyörälä K, Lehto S, Rönnemaa T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care. 2007;30:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, Cai L. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 85. | Atar D, Backx PH, Appel MM, Gao WD, Marban E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J Biol Chem. 1995;270:2473-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 171] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 86. | Tuncay E, Bilginoglu A, Sozmen NN, Zeydanli EN, Ugur M, Vassort G, Turan B. Intracellular free zinc during cardiac excitation-contraction cycle: calcium and redox dependencies. Cardiovasc Res. 2011;89:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Shao CH, Rozanski GJ, Patel KP, Bidasee KR. Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. J Mol Cell Cardiol. 2007;42:234-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 88. | Turan B. Zinc-induced changes in ionic currents of cardiomyocytes. Biol Trace Elem Res. 2003;94:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Tian C, Shao CH, Moore CJ, Kutty S, Walseth T, DeSouza C, Bidasee KR. Gain of function of cardiac ryanodine receptor in a rat model of type 1 diabetes. Cardiovasc Res. 2011;91:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 90. | Wold LE, Ceylan-Isik AF, Fang CX, Yang X, Li SY, Sreejayan N, Privratsky JR, Ren J. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med. 2006;40:1419-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Collet JF, D’Souza JC, Jakob U, Bardwell JC. Thioredoxin 2, an oxidative stress-induced protein, contains a high affinity zinc binding site. J Biol Chem. 2003;278:45325-45332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 92. | Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86:521-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 578] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 93. | Barberà A, Gomis RR, Prats N, Rodríguez-Gil JE, Domingo M, Gomis R, Guinovart JJ. Tungstate is an effective antidiabetic agent in streptozotocin-induced diabetic rats: a long-term study. Diabetologia. 2001;44:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 94. | Barberà A, Rodríguez-Gil JE, Guinovart JJ. Insulin-like actions of tungstate in diabetic rats. Normalization of hepatic glucose metabolism. J Biol Chem. 1994;269:20047-20053. [PubMed] |
| 95. | Barberà A, Fernàndez-Alvarez J, Truc A, Gomis R, Guinovart JJ. Effects of tungstate in neonatally streptozotocin-induced diabetic rats: mechanism leading to normalization of glycaemia. Diabetologia. 1997;40:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Fierabracci V, De Tata V, Pocai A, Novelli M, Barberà A, Masiello P. Oral tungstate treatment improves only transiently alteration of glucose metabolism in a new rat model of type 2 diabetes. Endocrine. 2002;19:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Lengyel C, Virág L, Kovács PP, Kristóf A, Pacher P, Kocsis E, Koltay ZM, Nánási PP, Tóth M, Kecskeméti V. Role of slow delayed rectifier K+-current in QT prolongation in the alloxan-induced diabetic rabbit heart. Acta Physiol (Oxf). 2008;192:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | McInturf SM, Bekkedal MY, Wilfong E, Arfsten D, Chapman G, Gunasekar PG. The potential reproductive, neurobehavioral and systemic effects of soluble sodium tungstate exposure in Sprague-Dawley rats. Toxicol Appl Pharmacol. 2011;254:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |