Published online May 15, 2012. doi: 10.4239/wjd.v3.i5.80
Revised: April 6, 2012
Accepted: May 11, 2012
Published online: May 15, 2012
Gastric intestinal symptoms common among diabetic patients are often caused by intestinal motility abnormalities related to enteric neuropathy. It has recently been demonstrated that the nitrergic subpopulation of myenteric neurons are especially susceptible to the development of diabetic neuropathy. Additionally, different susceptibility of nitrergic neurons located in different intestinal segments to diabetic damage and their different levels of responsiveness to insulin treatment have been revealed. These findings indicate the importance of the neuronal microenvironment in the pathogenesis of diabetic nitrergic neuropathy. The main focus of this review therefore was to summarize recent advances related to the diabetes-related selective nitrergic neuropathy and associated motility disturbances. Special attention was given to the findings on capillary endothelium and enteric glial cells. Growing evidence indicates that capillary endothelium adjacent to the myenteric ganglia and enteric glial cells surrounding them are determinative in establishing the ganglionic microenvironment. Additionally, recent advances in the development of new strategies to improve glycemic control in type 1 and type 2 diabetes mellitus are also considered in this review. Finally, looking to the future, the recent and promising results of metagenomics for the characterization of the gut microbiome in health and disease such as diabetes are highlighted.
- Citation: Bagyánszki M, Bódi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes 2012; 3(5): 80-93
- URL: https://www.wjgnet.com/1948-9358/full/v3/i5/80.htm
- DOI: https://dx.doi.org/10.4239/wjd.v3.i5.80
The gastrointestinal (GI) tract accomplishes a remarkable variety of functions, such as transport of luminal content, secretion and absorption of ions, water and nutrients, defence against pathogens and elimination of waste and/or noxious substances. Digestive functions are regulated by a complex neural network, known as the enteric nervous system (ENS), endowed in the gut wall and extending throughout its length from the esophagus to the internal anal sphincter[1-11]. The ENS derives from the neural crest[4-11] and consists of neurons distributed in two ganglionated plexuses, myenteric (Auerbach’s) and submucosal (Meissner’s), located within the gut wall. Enteric neurons can be identified according to their function, location, neurochemistry, shape, projections, quantitative properties and connections. After intensive research from several laboratories over the past two decades, a full description of all functional classes of enteric neurons has been recently achieved in the guinea pig and the mouse intestine[11-17].
GI motility disorders, such as vomiting, constipation, diarrhea and fecal incontinence, often accompany type-1 diabetes, both in patients and in animal models[18-20]. During the past decade, a growing amount of evidence has indicated[21-22] that the nitrergic subpopulation of myenteric neurons are the main points of attack of diabetic insults in the gut. Additionally, different susceptibility of nitrergic neurons located in different intestinal segments to diabetic damage and their different levels of responsiveness to insulin treatment have been revealed[22]. These findings implied that the development of diabetic nitrergic neuropathy is more complicated than suggested earlier[21] and that it differs from segment to segment along the GI tract. These findings initiated the investigation of the capillary endothelium within the gut wall and the glial cells surrounding enteric ganglia. The most recent evidence accumulating from these studies[23-25] prove that these cells play a determinative role creating the proper microenvironment for the ENS. Therefore, knowing the diabetes-related changes of these cells is important, not only with respect to pathogenesis, but also to therapeutic points.
In functional terms, intrinsic primary afferent neurons are determinative in the generation of intrinsic GI reflexes and also participate in the reflexes between the gut tube and accessory glands like the pancreas and liver[26-28].
There are five main types of enteric motor neuron: excitatory and inhibitory muscle motor neurons, motor neurons innervating endocrine cells, secretomotor/vasodilator neurons and simple secretomotor neurons[12]. Excitatory muscle motor neurons release acetylcholine and tachykinins, while inhibitory muscle motor neurons release nitric oxide (NO), adenosine triphosphate and vasoactive intestinal peptide. Besides muscle motor neurons, one type of orally directed (ascending) and three types of anally directed (descending) interneurons have been identified in the small intestine of the guinea pig[12].
All classes of enteric neurons are integrated in a continuous overlapping network along the GI tract. Small rings of circular muscle can contract independently; these rings and the associated enteric neurons can be regarded as functional modules. The spatiotemporal coordination of these interconnected modules is the determining factor for the generation of the rich repertoire of motor patterns[15,17].
ENS is also referred to as the “second brain” because of its capability to function in the absence of nerve inputs from the central nervous system[29]. However, extrinsic nerve pathways contribute to the regulatory mechanisms underlying gut functions[2,30-32].
Enteric glial cells (EGCs) represent an extensive but relatively poorly described cell population within the GI tract. The EGCs network has trophic and protective functions toward enteric neurons and is fully implicated in the integration and the modulation of neuronal activities[33-35]. In addition, EGCs within the ENS have a significant role in forming a diffusion barrier around the capillaries surrounding ganglia similar to that of blood-brain barrier[3,15,36-38].
Interstitial cells of Cajal (ICCs) are also related to the ENS and are electrically coupled to the smooth muscle cells. These pacemaker cells generate spontaneous electrical slow waves and mediate inputs from motor neurons[3,39-42]. ICCs are associated with afferent innervation and peristalsis of the stomach, suggestive of a key role in the pathophysiology of gastroparesis[43-47].
Nerve cells where transmission is mediated by NO are called nitrergic neurons[48-50]. In many organs of the urogenital, GI and cardiovascular systems, nitrergic neurotransmission plays a significant role as a major non-adrenergic non-cholinergic (NANC) neurotransmitter[51]. Nitrergic neurons in the myenteric plexus (MP) are inhibitory muscle motor neurons and descending interneurons[12,52,53].
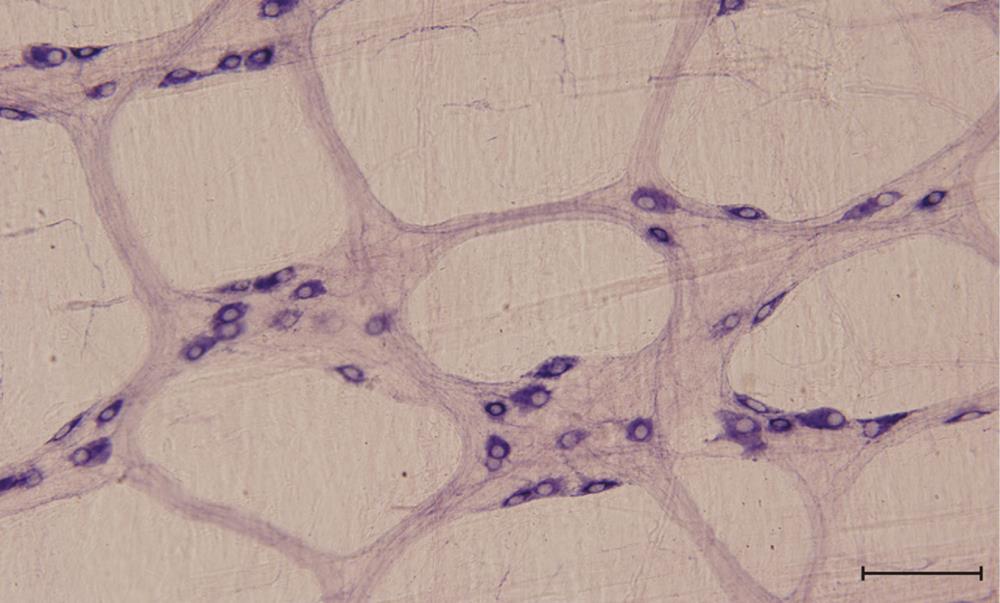
There have already been numerous investigations of the density and spatial distribution of nitrergic myenteric neurons[54-56]. In the MP of different mammalian species, nitric oxide synthase (NOS)-immunoreactive neurons constitutes approximately 25%-40% of the total myenteric neurons[14,56,57]. It is well established that within the ENS the neuronal NOS (nNOS) corresponds to nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d); therefore, NADPH-d histochemistry is used to label nitrergic enteric neurons (Figure 1).
Various reports have described the plastic remodeling of the nitrergic neurons during development[55,58,59], aging[60] and pathological conditions[22,61]. Several studies have suggested that nitrergic myenteric neurons are especially susceptible to the development of neuropathy in digestive tract diseases, like diabetes[22,62-65], chronic ethanol consumption[66-68] and inflammation[69].
Diabetes-related abnormalities in the ENS were reviewed in 2007[70]. Recent studies on the ENS in diabetes are summarized in Table 1.
| Location of change | Type of change | References | Species |
| Stomach | Gastroparesis, oxidative stress | Choi et al[50] | Mouse |
| Duodenum, jejunum, ileum, colon | Region specific nitrergic neuronal loss, gastrointestinal motility disorders | Izbéki et al[22] | Rat |
| Esophagus, stomach, intestine | Loss of ICCs | Ördög[46] | Human, mouse, rat |
| Ileum | Loss of enteric neurons | Pereira et al[78] | Rat |
| Jejunum | Decreased NO responsiveness, decreased nNOS protein expression | Zandecki et al[18] | Rat |
| Duodenum | Loss of enteric neurons | De Mello et al[88] | Rat |
| Esophagus, stomach, intestine | Diabetic gastroenteropathy | Ördög et al[47] | Human, mouse, rat |
| Stomach | Gastroparesis, regional injury of ICCs | Wang et al[43] | Rat |
| Colon | Reduction in GFAP and neurotrophins | Liu et al[82] | Rat |
| Small intestine | Loss of enteric neurons, gastrointestinal motility disorders | Nezami and Srinivasan[38] | Human, mouse, rat |
| Colon | Gastrointestinal motility disorders, loss of enteric neurons, increased oxidative stress | Chandrasekharan et al[72] | Human |
| Stomach | Gastroparesis | Hasler et al[106] | Human |
| Stomach, intestine | Oxidative stress | Kashyap et al[90] | Human, mouse, rat |
| Stomach | Gastroparesis | Tang et al[97] | Human |
| Duodenum, cecum | Loss of enteric neurons | Zanoni et al[156] | Rat |
The combination of intracellular signaling disorders with quantitative and neurochemical changes of the enteric neurons can be related to the neuronal loss and relevant clinical problems of the neurological manifestations of diabetes mellitus, such as dilatation of the stomach, small and large intestines, constipation and diabetic diarrhea[71,72]. A recent study found a significant decrease in ganglion size in diabetic patients compared with normal individuals and enhanced apoptosis of the enteric neurons[72]. There is also evidence of damage to the enteric neurons in animal models of diabetes[73-78].
Different subpopulations of myenteric neurons are differentially susceptible to the development of neuropathy in diabetes. Zandecki et al[18] characterized the myenteric neuropathy in the jejunum of spontaneously diabetic BioBreeding rats. Their results provide evidence for a selective nitrergic motor dysfunction in the jejunum of these diabetic rats. The underlying mechanism involved decreased nNOS protein expression, while the purinergic NANC transmission was not affected.
In animal models of type-1 diabetes, damage of the vagus nerve also contributes to changes in the ENS[79,80]. As nNOS expression is not controlled by the vagus nerve in the jejunum of rat, the nitrergic neuropathy is believed to result from a primary dysfunction in the ENS rather than from vagal dysfunction[18].
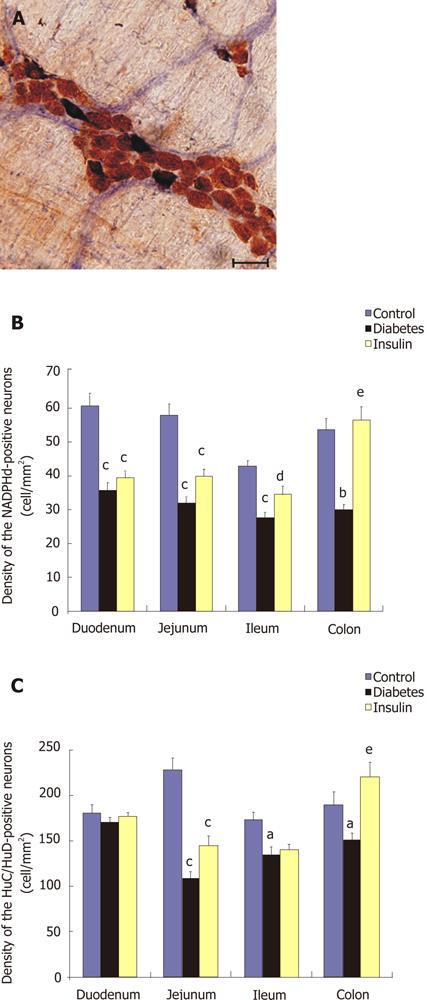
The available reports focusing only on single segments of the GI tract are somewhat contradictory. In our earlier study[22], the streptozotocin- (STZ) induced diabetic rat model was used to investigate the relationship between the deranged gut motility and the segment-specific quantitative changes in the nitrergic myenteric neurons. Additionally, we studied the effectiveness of early insulin replacement to prevent the development of diabetes-induced changes. The NADPH-d-stained cells were considered to be nitrergic neurons when they were double-labeled with HuC/HuD used as a pan-neuronal marker (Figure 2A). The duodenum of the diabetic rats was the only gut segment where the number of nitrergic neurons was decreased, while the total neuronal number was not altered. In the jejunum, ileum and colon, both the total and the nitrergic neuronal cell number decreased significantly (Figure 2B and C). Immediate insulin replacement did not prevent the nitrergic cell loss significantly in the duodenum and jejunum, but it did prevent it significantly in the ileum and colon. These findings comprise the first evidence that the nitrergic neurons located in different intestinal segments exhibit different susceptibilities to a diabetic state and different responsiveness to insulin treatment[22].
Other results also showed that nitrergic neuropathy appears to be more pronounced in the colon compared with the proximal gut[75,81-82]. The strict regionality of pathological processes called attention to the importance of the molecular differences in the neuronal microenvironment along the GI tract. Since myenteric ganglia are not vascularized capillaries, adjacent to them must be responsible to provide the ganglionic microenvironment, including the proper oxidative circumstances.
Literary data report about sex-dependent sensibility of enteric neurons to the diabetic state[83-84]. Apoptosis of enteric neurons was characteristic in diabetic males, but not in female rats[83]. Another study provides evidence that females may have a greater dependency on the nitrergic mechanisms in health. After induction of diabetes, gastric emptying was delayed in both male and female rats, but females exhibited significantly delayed gastric emptying compared to males. Furthermore, diabetes seems to affect the nitrergic system to a greater extent in females than in males. Together, these changes may account for the greater vulnerability of females to diabetic gastric dysfunction. These data are consistent with clinical observation that diabetic gastroparesis predominantly affects women[84].
Some studies have mentioned an increase in number and size of NOS neurons as well as fiber thickness[85-88]. Cellek’s biphasic model of nitrergic neuropathy can offer a good explanation for these contradictory results[21,65]. According to this model, nitrergic neurons innervating the urogenital and GI organs undergo a degenerative process in two phases in diabetes. The first phase is characterized by an insulin-reversible decrease in nNOS expression in the axons, while in the second phase, apoptotic cell death occurs in the nitrergic neurons which is not reversible by insulin treatment.
The nitrergic neurons are not a homogeneous cell population. Some of the nNOS-containing neurons also contain heme oxygenase-2 (HO-2). Double-labeling studies revealed that approximately 50% of nNOS-containing neurons also contained HO-2 and that the diabetes-induced change in size was confined to nNOS-immunoreactive neurons that did not contain HO-2. No change in the size and the distribution occurred in neurons in which nNOS and HO-2 were colocalized. This indicates that the antioxidant HO-2 protects those NOS-containing neurons in which it is colocalized against oxidative stress, pointing to the importance of oxidative stress in the development of diabetes-related neuropathies[89].
There is convincing evidence that the generation of reactive oxygen species (ROS) is increased in both type 1 and type 2 diabetes; that the onset of diabetes and its complications are closely associated with oxidative stress; and treatment with antioxidants minimizes or prevents development of these complications in diabetic patients[77-78]. In the non-obese diabetic model of type 1 diabetes, increased oxidative stress has been shown to lead to development of gastroparesis and colonic motor dysfunction[72]. Induction of HO-1, the inducible isoform of HO, at the same time has been identified as an important cellular defence mechanism against oxidative stress[90]. The HO-1 pathway prevents and reverses cellular changes that lead to development of GI complications of diabetes. Induction of HO-1 by hemin decreased ROS, rapidly restored nNOS expression, and completely normalized gastric emptying in mice. Inhibition of HO-1 activity with normal gastric emptying caused development of diabetic gastroparesis[50].
It was earlier found that in the GI tract the colon was more susceptible to damage by oxidative stress[91], and in the colon the apoptosis of the enteric neurons was increased. Detailed analysis of neuronal subtypes from the diabetic and normal colon revealed a selective susceptibility of the inhibitory neuronal sub-populations like nNOS in diabetic patients[90]. The sensitivity of colonic tissue to oxidative stress may arise due to antioxidant or reductant deficiencies. It was observed that colons from diabetic patients had decreased amounts of the non-enzymatic antioxidant reduced glutathione that correlated well with the duration of diabetes. There was also an increased expression of superoxide dismutase, possibly as a compensatory mechanism to match the increase in levels of various free radical scavengers or reductants[72].
ROS can be generated as a result of auto-oxidation of glucose and formation of advanced glycosylation end products (AGEs). AGEs are a group of heterogeneous compounds formed by the non-enzymatic reactions between aldehydic group of reducing sugars with proteins, lipids or nucleic acids. Formation and accumulation of AGEs are related to the aging process and accelerated in diabetes. The pathogenic role of AGEs in vascular diabetic complications is widely recognised[92]. AGEs elicit oxidative stress generation and subsequently cause inflammatory and thrombogenic reactions in various types of cells via interaction with a receptor for AGEs. In addition, mitochondrial superoxide generation has been shown to play an important role in the formation and accumulation of AGEs under diabetic conditions[80].
AGEs in the serum and tissues of diabetic rats increases gradually throughout the two phases of diabetes, but the AGEs accumulation in the tissues seems to begin at the time point when the nNOS depletion becomes irreversible. This model suggests that irreversible rise in the serum and accumulation of AGEs in the tissues is the trigger for nitrergic apoptosis. The time point where the two phases are separated was called “the point of no return”[65]. The selective nitrergic neuropathy[63] is most probably due to the fact that endogenous NO and accumulated AGEs synergistically cause oxidative stress within the nitrergic neuron, which leads to apoptosis[65].
GI motility disorders, such as gastroparesis, constipation and diarrhea, often accompany diabetes, both in patients and in animal models. Population-based studies have shown that 2%-19% of diabetic patients report upper GI symptoms and 48%-65% of those with abdominal symptoms have delayed gastric emptying[93-97].
Motility disorders in diabetes are traditionally considered to originate from visceral autonomic neuropathy, especially changes in vagal innervation. However, increasing evidence from animal models points towards changes in the ENS as the underlying mechanism for these motility disturbances[18,79,98-100]. Changes in adrenergic and cholinergic neurotransmission have been reported[101-102] but recent studies have focused on altered NANC innervation[18].
Nitrergic control was impaired in diabetic rats as a consequence of both decreased smooth muscle responsiveness to NO and decreased nNOS protein expression. Nitrergic enteric neuropathy in diabetes may be a primary dysfunction, occurring independently from vagal dysfunction[18]. Results indicate that the colonic peristaltic reflex is enhanced by impairment of enteric nitrergic inhibitory neurons in spontaneously diabetic rats[75].
Loss of nitrergic neurons in diabetes can result in delayed gastric emptying due to the loss of neurons in the pylorus and accelerated intestinal transit due to the loss of influence of these neurons in the small and large intestine[103].
Diabetic gastroparesis was initially described by Kassander in 1958 as “gastroparesis diabeticorum” in a patient with type 1 diabetes, but it is increasingly being recognized in patients with type 2 diabetes. Gastroparesis is defined as a syndrome characterized by abnormal gastric function resulting in delayed gastric emptying in the absence of mechanical obstruction[44,104]. The pathogenesis of diabetic gastroparesis is multifactorial and results in a neuromyopathy[97]. Most data from recent animal and human studies suggest that the two main findings in diabetic gastroparesis are the loss of ICCs and reduced expression of nNOS[44,50,73,105,106]. Experimental data indicate that in diabetes, increased oxidative stress due to the low HO-1 level in addition to reduced insulin and insulin-like growth factor-1 signaling, not hyperglycemia, is responsible for the loss of the ICCs. The depletion of ICCs causes abnormalities in gastric slow waves, absence of peristalsis and atrophy of gastric smooth muscle[50,97,107].
Our results[22] showed that the STZ-induced diabetic rats displayed faster small intestinal and colonic transit, as observed by others in different rat models of diabetes[108-110]. We therefore infer that our observations in this model with regard to the changes in the total myenteric neurons and the nitrergic subpopulation furnish data on the pathogenesis of diabetic diarrhea, which is a serious complication of diabetes in approximately 10% of diabetic patients.
Colorectal dysfunction is also common in diabetes. Of patients attending specialized diabetes clinics, up to 60% reported constipation, 22% had diarrhea and 20% had fecal incontinence[111]. In diabetic rodents, constipation was accompanied by reduced neuromuscular neurotransmission in the distal colon, whereas a paradoxical increase in contractile and underlying spike complex activity was noted in the proximal colon. The latter occurred in the absence of reduced inhibitory control and may have reflected functional compensation or a response to small intestinal bacterial overgrowth. ICCs were reduced in the colon of mice with both type 1- and type 2-like diabetes, as well as in type 2 diabetic patients[46,112-115].
Both type 1 and type 2 diabetes mellitus have long been recognized as an independent risk factor for cardiovascular disease (CVD), including coronary artery disease, stroke, peripheral arterial disease, cardiomyopathy and congestive heart failure. CVD is the leading cause of comorbidity and death in patients with diabetes[116-118]. Vascular complications of diabetes also extend to microvascular disease, manifested as diabetic nephropathy, neuropathy and retinopathy. Chronic hyperglycemia plays a major role in the initiation of diabetic vascular complications[119-120]; however, the mechanisms through which hyperglycemia promotes the development of vascular diseases remain incompletely understood.
Multiple mechanisms for this relationship between glucose and atherosclerosis have been proposed. Hyperglycemia may activate nuclear factor-κB, a key mediator that regulates proinflammatory and proatherosclerotic target genes in endothelial cells, vascular smooth muscle cells and macrophages[121]. Hyperglycemia can also foster the non-enzymatic formation of AGEs, protein cross-linking and ROS formation[122]. Hyperglycemia stimulates oxidative stress, which appears to be a driving force in atherosclerosis[123]. Common final pathways among most, if not all, of these various mechanisms are stimulation of inflammation, arterial remodeling and tissue damage[124,125]. In addition to systemic factors, organ-specific factors also appear to be important in the development of vascular disease. For example, in the kidney, stimulation of mesangial matrix production by hyperglycemia, activation of protein kinase C and an increasing degree of intraglomerular hypertension may contribute to glomerular injury[126]. Other factors associated with the development of vascular disease in type 2 diabetes include impaired endothelial-dependent relaxation, increased proliferation of vascular smooth muscle cells and increased non-enzymatic collagen glycation[127]. Hyperglycemia may also activate matrix-degrading metalloproteinases, enzymes implicated in plaque rupture and arterial remodeling, inducing similar responses in vascular smooth muscles[128].
Although intensive glycemic control has reduced the risks of micro- and macrovascular complications, this strategy is not successful in all patients; therefore, cardiovascular events remain the leading risk factor for mortality of diabetic patients worldwide[129-130]. Glycemic control in the context of type 2 diabetes, as well as pre-diabetes, is also intertwined with cardiovascular risk factors such as obesity, hypertriglyceridemia and blood pressure control[131-133]. Similarly, major issues and concerns have arisen around the cardiovascular safety of antidiabetic therapy[134-136]. Together, these issues have focused attention on the need to understand the cardiovascular effects of current treatments for diabetes and the optimal strategies for care of patients with this disease.
Since endothelium is the primary physiological source of endothelial NOS (eNOS) which then produce NO to regulate cardio- and cerebrovascular homeostasis, loss of the modulatory role of the endothelium may be a critical and initiating factor in the development of diabetic vascular disease. Impaired function of the vascular system then leads to ischemia, stroke and consequently hypoxia and neuropathy[137-139]. Because of their dominant clinical incidence, the diabetes-induced alterations in the capillary endothelium of retina[140-143] and renal glomerulus[144,145] have been the focus of a vast number of studies, while except for an early case report on microangiopathy in a bowel biopsy[146], the impact of diabetes on capillaries within the intestinal wall has been almost completely overlooked until now. The myenteric ganglia are not vascularized; accordingly, the capillaries adjacent to the MP have the role to supply them. Therefore, knowing the mechanisms by which diabetes inflicts structural, functional and molecular changes in these capillaries may open new directions in diabetes research and then offer alternative mechanisms to treat the complications associated with hyperglycemia. Due to the growing incidence of insulin resistance, it is becoming increasingly important for clinicians to introduce alternative therapies and be aware of diabetes-related vascular complications[130].
In our ongoing research, we provided evidence[25] that endothelial cells in capillaries adjacent to the MP are direct targets of diabetic damage. The microvessels in a particular gut segment were affected differentially by the pathophysiological conditions, allowing neurons in one intestinal region to survive, while causing them to die in another. Furthermore, we proved that structural and functional alterations which influence the permeability of these capillaries[25] coincide with the enteric neuropathy demonstrated in STZ-induced diabetic rats[22]. Investigations are currently in progress in our laboratory to explain the molecular background of the diabetes-related changes in capillaries supplying the MP.
Vascular permeability and the expression of cell adhesion molecules are regulated by many complex signaling pathways within endothelial cells[138,147,148]. The major negative regulatory protein for eNOS is caveolin-1 (Cav-1). The pathways which involve the regulation of eNOS by Cav-1 in different vascular beds[149] are the focus of research. Now, we want to know whether the diabetes-induced alterations in the microvasculature of the retina, renal glomerulus and nerves are accompanied by changes in the capillaries supplying the MP and whether such changes can result in an impairment of the strict control of capillary permeability, which then gives rise to the gut region-specific nitrergic neuropathy demonstrated in the MP of rats with STZ-induced diabetes[22].
Although the metabolic and cellular mechanisms leading to severe macro- and microvascular diseases may differ between type 1 and type 2 diabetes, both share a decreased NO bioavailability and altered vascular permeability[150]. A deficit in bioavailable NO could result from an impairment of the eNOS function or the inactivation of NO by oxidative stress. eNOS is a membrane-associated NOS isoform, and the proper localization of eNOS is therefore necessary for its interactions with other regulatory proteins (scaffolds, chaperones and kinases) that fine-tune the cycles of eNOS activation and inactivation[151,152]. Recent studies with Cav-1-deficient mouse models suggest that they may be profoundly important for postnatal cardiovascular functions, including the endothelial barrier function and the regulation of NO synthesis[151-153]. It has also been demonstrated that insulin regulates the distribution of Cav and stimulates the phosphorylation of Cav protein[115].
Enteric neurons are surrounded and outnumbered by EGCs. Recent data suggest that EGCs play an important role in the maintenance of tissue integrity in the GI tract[78,82,154]. Several lines of evidence implicate that the secretion of neurotrophic factors by EGCs may be a part of glial regulation of gut homeostasis. The secretion of glia cell-derived neurotrophic factor (GDNF), nerve growth factor (NGF) and transforming growth factor-beta contribute to the maintenance of endothelial integrity and vasodilatation[82]. Evidence is accumulating that EGCs share the ability of astrocytes to regulate tight-junction integrity and cellular interactions comparable with those maintaining the blood-brain barrier and creating the proper microenvironment for enteric neurons[36,38]. To know the exact mechanisms of how EGCs contribute to gut homeostasis under physiological and pathophysiological conditions is therefore important to work out new therapeutic strategies and to be aware of diabetes-related vascular complications.
Several authors have shown that autonomic neuropathy caused chronically by diabetes mellitus is related to quantitative and morphometric changes in the enteric neurons in various GI segments[22,77,155,156]. However, studies on the number and area of glial cells in diabetes mellitus are scarce. Recent data proves that, unlike the neurons, the diabetic condition in rats did not reduce the glial density per unit area of the intestine. This glial preservation may be attributable to the resistance of the glia cell population and a defense mechanism exerted by glia in an attempt to promote the maintenance of neurons that remain viable after the development of peripheral diabetic neuropathy[78]. Nerve cells cannot synthesize glutathione, the major endogenous cellular antioxidant, because they do not contain the enzyme gamma-glutamyl-cystein-synthetase which is responsible for formation of a peptide bond between cysteine and glutamate[157]. Thus, neurons depend directly on glial cells for glutathione synthesis. This dependence that neurons have on glial cells becomes even more important in diabetes, because changes in glutathione metabolism are common in diabetic patients and associated with reduced levels of these antioxidants[158]. Furthermore, glial cells directly promote neuronal protection by increasing the intracellular content of total glutathione in their own cells. Oxidative stress increases expression of the enzyme gamma-glutamyl-cystein-synthetase in glial cells, which promotes a neuroprotective mechanism by release of glutathione to neurons that survive diabetic neuropathy[159]. This increase in glutathione in glial cells is also a defence mechanism because it protects against the diabetes-induced death of glial cells by inhibition of lipid peroxidation reactions[78].
The area of the glial cells was decreased in the diabetic rats compared to the controls. This decrease may be related to a reduction in the expression of neurotrophic factors or neurotrophins responsible for promoting the survival and maintenance of neurons[78]. This hypothesis is consistent with other studies. The induction of diabetes is associated with a reduction in glial fibrillary acidic protein (GFAP) and neurotrophins expression in the colon, which may affect the role of EGCs and neurotrophins in the enteric plexuses. The changes of GFAP expression in glial cells could be the consequence of unviable extracellular conditions such as hyperosmolarity, low nutrient availability or increased oxidative stress. Immunostaining and western blot showed that diabetes induced a decrease in the intensity of staining of GFAP-positive EGCs and GFAP protein levels at 4 wk and attenuated GFAP expression were more evident at 12 wk[82].
Moreover, mRNA and protein analysis indicated that the levels of NGF were down-regulated in diabetic rats. These findings suggest that the induction of diabetes is associated with a reduction in GFAP and neurotrophins expression in the colon, which may affect the role of EGCs and neurotrophins in the enteric plexuses. This in turn may partly contribute to the physiopathological changes associated with the diabetic state in the GI tract[82]. The neurotrophic factor GDNF reverses hyperglycemia-induced neuronal apoptosis and loss of nitrergic neurons and also improves GI motility in diabetic mice. Therefore, GDNF may be a potential therapeutic target for GI motility disorders in diabetes[103].
New therapies using various drug treatments to improve glycemic control in diabetes have recently been developed or are under development. Current therapies for the treatment of type 1 diabetes include daily administration of exogenous insulin and less frequently, whole pancreas or islet transplantation. More recently, embryonic or induced pluripotent stem cells have also been examined for their ability to differentiate in vitro into pancreatic endocrine cells[160-162]. The first results of glucagonocentric reconstruction of diabetes at the same time open a new perspective over insulin monotherapy of type 1 diabetes. A recent publication[163] proposes that glucagon excess, rather than insulin deficiency, is the main cause of type 1 diabetes. Based on recently accumulated evidence[163,164], it was concluded that glucose-responsive β cells normally regulate juxtaposed α cells and that without intraislet insulin, unregulated α cells hypersecrete glucagon, which directly causes the symptoms of diabetes. Although patients with type 1 diabetes have an absolute deficiency of insulin, the pathogenesis of type 2 diabetes mellitus is associated with relative insulin deficiency and insulin resistance[165-167]. Therefore, in addition to insulin, a number of different classes of medication to treat patients with diabetes have been developed.
There is a growing body of evidence that the incretin hormone, glucagon-like peptide 1 (GLP-1), has profound effects on the GI motor system[168-170]. Moreover, the effects of GLP-1 on GI motility appear to be pivotal to its effect of reducing postprandial hyperglycemia[171-173]. In a recent study, exogenous GLP-1 was able to reduce mouse gastric motility by acting peripherally in the antral region, through neural NO release[174]. It has recently been demonstrated that GLP-1 receptors are expressed in the enteric neurons. Furthermore, 27% of GLP-1 receptor immunoreactive neurons in the duodenum and 79% of these neurons in the colon are co-expressed with nNOS[175].
Due to its promising potential in the treatment of type 2 diabetes and related intestinal motility disorders, the incretin-based therapies have been the focus of much interest during the last years[176-179]. Incretins, which are released by enteroendocrine cells in the intestine in response to a meal, have been implicated in contributing to the pathogenesis of type 2 diabetes mellitus. Injectable GLP-1 receptor agonists and orally administered dipeptidyl peptidase-4 (DPP-4) inhibitors have been developed[180,181] and introduced into clinical practice to specifically address the blunted incretin responses in patients with diabetes type 2. The GLP-1 receptor agonists potentiate insulin secretion, inhibit glucagon release, delay gastric emptying and reduce appetite. The DPP-4 inhibitors primarily improve insulin secretion and inhibit glucagon release.
In diabetic rats, a DPP-4 inhibitor improved the thickening of the glomerular basement membrane[182] which is the histological hallmark of diabetic microangiopathy. GLP-1 administration also decreases the damage of alveolar capillary basal lamina in rats with spontaneous type 2 diabetes mellitus[183]. The use of these drugs is also associated with improvements in blood pressure, diabetic dyslipidemia and myocardial function[184-186]. Therefore, they have a potential role to reduce the cardiovascular risk factors, a major cause of mortality in patients with diabetes.
Intestinal region-specific selective loss of enteric neurons in rat models of diabetes mellitus indicates the importance of the neuronal microenvironment in the pathogenesis of diabetic enteric neuropathy. Therefore, among the most important players of enteric microenvironments, capillary endothelium and EGCs have received much attention in recent years. Studies in humans and in animal models indicate that the mechanisms of endothelial dysfunction differ according to the diabetic model and the vascular bed under study. Therefore, different animal models and different vascular beds must be considered in future studies in order to be able to draw general conclusions on the anatomical, physiological and molecular mechanisms leading to the development of diabetic enteric neuropathies that generally appear as a consequence of vascular complications.
The gut region-specific neuronal and vascular damage demonstrated in STZ-induced diabetic rats[22] leads to the question of why the enteric neurons, glial cells and microvessels in the different intestinal segments are affected differentially by the diabetic condition. Since correlations have been suggested between the host’s health and the GI tract microbiota, numerous investigations in recent years have focused on the connection between the GI tract microbiota and metabolic diseases. Most recent findings[187-189] provide a sufficient basis for the speculation that the different degrees of susceptibility of enteric neurons and microvessels to a pathological stimulus such as hyperglycemia might be related to the prevalence of bacteria in the different parts of the GI tract. Accordingly, the differences in prevalence of bacteria in different gut segments[188,190] are influenced by the oxygen supply of the small and large intestine[191]. Knowledge of species and functional composition of the gut microbiome is rapidly increasing thanks to technological advances in culture independent methods[189,192-195]. The human GI tract is dominated by anerobic bacteria mainly in the distal part of the gut[190]. We presume that, due to the adequate oxidative environment in the proximal intestine, the enteric neurons or capillaries there can tolerate hyperglycemia-related oxidative stress better and for a longer time than they can in the colon, where the basal oxygen supply is far from optimal.
The intestinal microbiota have been shown to be different in composition and causally linked to metabolic diseases such as diabetes and obesity in humans and mice[187,196-199]. Furthermore, the divergences from the core microflora may define the status of disease[200-201]. The development of diabetes type 1 in rats was reported to be associated with higher amounts of Bacteroides ssp.[202]. It has been proposed that the gut microbiota directed increased monosaccharide uptake from the gut and instructed the host to increase hepatic production of triglycerides associated with the development of insulin resistance[203]. Larsen et al[187] demonstrated that type 2 diabetes is also associated with compositional changes in the intestinal bacteria. Accordingly, their results show that the relative abundance of Firmicutes was significantly lower, while the proportion of Bacteroidetes and Proteobacteria was somewhat higher in diabetic persons compared to non-diabetics.
The lactate- and butyrate-producing bacteria in a healthy gut induce a sufficient amount of mucin synthesis to maintain gut integrity. In contrast, non-butyrate-producing lactate-utilizing bacteria prevent optimal mucin synthesis, as identified in autoimmune subjects[204]. Obese and diabetic mice display enhanced intestinal permeability by reducing the expression of genes coding for two tight junction proteins, ZO-1 and occludin[198]. It was proved that prebiotic modulation of gut microbiota lowers intestinal permeability by increases in endogenous GLP-2 production, thereby improving gut barrier function, glucose-tolerance and low-grade inflammation[198-199].
In order to precisely determine the role of the gut microbiota in the development of metabolic diseases, among others, diabetes mellitus type 1 and type 2, and provide new therapeutic strategies, it is crucial to collect more detailed information on the host-microbial homeostasis.
We thank our colleague and teacher, Professor Éva Fekete for her valuable comments on the manuscript.
Peer reviewers: Dr. Adriana Georgescu, Department of Vascular Dysfunction in Diabetes and Obesity, Institute of Cellular Biology and Pathology Nicolae Simionescu, 8 BP Hasdeu Street, Bucharest 050568, Romania; David J Hill, Professor, Lawson Health Research Institute, St Joseph’s Health Care, St Joseph’s Health Care, 268 Grosvenor Street, London N6A4V2, Canada; Dr. Abd A Tahrani, Centre of Endocrinology, Diabetes and Metabol, University of Birmingham, The Biomedial Unit, Birmingham Heartlands Hospital, Birmingham, B9 5SS, United Kingdom
S- Editor Wu X L- Editor Roemmele A E- Editor Wu X
| 1. | Furness JB. The organisation of the autonomic nervous system: peripheral connections. Auton Neurosci. 2006;130:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Di Nardo G, Blandizzi C, Volta U, Colucci R, Stanghellini V, Barbara G, Del Tacca M, Tonini M, Corinaldesi R, De Giorgio R. Review article: molecular, pathological and therapeutic features of human enteric neuropathies. Aliment Pharmacol Ther. 2008;28:25-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Nezami BG, Srinivasan S. Enteric nervous system in the small intestine: pathophysiology and clinical implications. Curr Gastroenterol Rep. 2010;12:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Taraviras S, Pachnis V. Development of the mammalian enteric nervous system. Curr Opin Genet Dev. 1999;9:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 1. Pediatr Dev Pathol. 2002;5:224-247. [PubMed] |
| 6. | Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 2. Pediatr Dev Pathol. 2002;5:329-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Anderson RB, Newgreen DF, Young HM. Neural crest and the development of the enteric nervous system. Adv Exp Med Biol. 2006;589:181-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Burns AJ, Roberts RR, Bornstein JC, Young HM. Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin Pediatr Surg. 2009;18:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Gershon MD. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci. 2010;33:446-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Shepherd I, Eisen J. Development of the zebrafish enteric nervous system. Methods Cell Biol. 2011;101:143-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Wang X, Chan AK, Sham MH, Burns AJ, Chan WY. Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology. 2011;141:992-1002.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 573] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 13. | Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Qu ZD, Thacker M, Castelucci P, Bagyánszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 15. | Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut. 2000;47 Suppl 4:iv15-iv9; discussion iv26. [PubMed] |
| 16. | Furness JB. The enteric nervous system. Massachusetts: Blackwell Publishing Inc 2006; . |
| 17. | Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol. 2008;24:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Zandecki M, Vanden Berghe P, Depoortere I, Geboes K, Peeters T, Janssens J, Tack J. Characterization of myenteric neuropathy in the jejunum of spontaneously diabetic BB-rats. Neurogastroenterol Motil. 2008;20:818-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Adewoye EO, Ige AO, Latona CT. Effect of Methanolic extract of Musa sapientum leaves on Gastrointestinal Transit time in Normal and Alloxan induced Diabetic rats: Possible Mechanism of Action. Niger J Physiol Sci. 2011;26:83-88. [PubMed] |
| 20. | Ciobanu L, Dumitrascu DL. Gastrointestinal motility disorders in endocrine diseases. Pol Arch Med Wewn. 2011;121:129-136. [PubMed] |
| 21. | Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Izbéki F, Wittman T, Rosztóczy A, Linke N, Bódi N, Fekete E, Bagyánszki M. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res Clin Pract. 2008;80:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | von Boyen G, Steinkamp M. The role of enteric glia in gut inflammation. Neuron Glia Biol. 2010;6:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Xiao WD, Chen W, Sun LH, Wang WS, Zhou SW, Yang H. The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol Cell Neurosci. 2011;46:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Bódi N, Talapka P, Poles MZ, Hermesz E, Jancsó Z, Katarova Z, Izbéki F, Wittmann T, Fekete E, Bagyánszki M. Gut region-specific diabetic damage to the capillary endothelium adjacent to the myenteric plexus. Microcirculation. 2012;19:316-326. [PubMed] |
| 26. | Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277:G922-G928. [PubMed] |
| 27. | Weidmann S, Schrödl F, Neuhuber W, Brehmer A. Quantitative estimation of putative primary afferent neurons in the myenteric plexus of human small intestine. Histochem Cell Biol. 2007;128:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Mitsui R. Immunohistochemical characteristics of submucosal Dogiel type II neurons in rat colon. Cell Tissue Res. 2010;340:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Gershon MD. The enteric nervous system: a second brain. Hosp Pract (Minneap). 1999;34:31-2, 35-8, 41-2 passim. [PubMed] |
| 30. | Tan LL, Bornstein JC, Anderson CR. Neurochemical and morphological phenotypes of vagal afferent neurons innervating the adult mouse jejunum. Neurogastroenterol Motil. 2009;21:994-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Olsson C, Holmgren S. Autonomic control of gut motility: a comparative view. Auton Neurosci. 2011;165:80-101. [PubMed] |
| 32. | Ratcliffe EM. Molecular development of the extrinsic sensory innervation of the gastrointestinal tract. Auton Neurosci. 2011;161:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Wang H, Feng L, Hu JW, Xie CL, Wang F. Characterisation of the vitreous proteome in proliferative diabetic retinopathy. Proteome Sci. 2012;10:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Chalazonitis A, D'Autréaux F, Pham TD, Kessler JA, Gershon MD. Bone morphogenetic proteins regulate enteric gliogenesis by modulating ErbB3 signaling. Dev Biol. 2011;350:64-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Chalazonitis A, Gershon MD, Greene LA. Cell death and the developing enteric nervous system. Neurochem Int. 2012;[Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Rühl A. Glial cells in the gut. Neurogastroenterol Motil. 2005;17:777-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Rühl A. Glial regulation of neuronal plasticity in the gut: implications for clinicians. Gut. 2006;55:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | MacEachern SJ, Patel BA, McKay DM, Sharkey KA. Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus. J Physiol. 2011;589:3333-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Sanders KM, Ördög T, Koh SD, Ward SM. A Novel Pacemaker Mechanism Drives Gastrointestinal Rhythmicity. News Physiol Sci. 2000;15:291-298. [PubMed] |
| 40. | Ward SM. Interstitial cells of Cajal in enteric neurotransmission. Gut. 2000;47 Suppl 4:iv40-iv3; discussion iv52. [PubMed] |
| 41. | Bush TG. Enteric glial cells. An upstream target for induction of necrotizing enterocolitis and Crohn's disease? Bioessays. 2002;24:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Bassotti G, Villanacci V, Antonelli E, Morelli A, Salerni B. Enteric glial cells: new players in gastrointestinal motility? Lab Invest. 2007;87:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Wang XY, Huizinga JD, Diamond J, Liu LW. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil. 2009;21:1095-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 248] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 45. | Yamamoto T, Watabe K, Nakahara M, Ogiyama H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y, Hayashi N. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Ordög T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil. 2008;20:8-18. [PubMed] |
| 47. | Ordög T, Hayashi Y, Gibbons SJ. Cellular pathogenesis of diabetic gastroenteropathy. Minerva Gastroenterol Dietol. 2009;55:315-343. [PubMed] |
| 48. | Snyder SH, Bredt DS. Biological roles of nitric oxide. Sci Am. 1992;266:68-71, 74-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 402] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 49. | Smits GJ, Lefebvre RA. ATP and nitric oxide: inhibitory NANC neurotransmitters in the longitudinal muscle-myenteric plexus preparation of the rat ileum. Br J Pharmacol. 1996;118:695-703. [PubMed] |
| 50. | Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T, Szurszewski JH, Farrugia G. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055-264. [PubMed] |
| 51. | De Giorgio R, Parodi JE, Brecha NC, Brunicardi FC, Becker JM, Go VL, Sternini C. Nitric oxide producing neurons in the monkey and human digestive system. J Comp Neurol. 1994;342:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Brehmer A, Schrödl F, Neuhuber W. Morphology of VIP/nNOS-immunoreactive myenteric neurons in the human gut. Histochem Cell Biol. 2006;125:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Sung TS, La JH, Kim TW, Yang IS. Alteration of nitrergic neuromuscular transmission as a result of acute experimental colitis in rat. J Vet Sci. 2006;7:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Jarvinen MK, Wollmann WJ, Powrozek TA, Schultz JA, Powley TL. Nitric oxide synthase-containing neurons in the myenteric plexus of the rat gastrointestinal tract: distribution and regional density. Anat Embryol (Berl). 1999;199:99-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Román V, Bagyánszki M, Krecsmarik M, Horváth A, Resch BA, Fekete E. Spatial pattern analysis of nitrergic neurons in the developing myenteric plexus of the human fetal intestine. Cytometry A. 2004;57:108-112. [PubMed] |
| 56. | Bódi N, Battonyai I, Talapka P, Fekete E, Bagyánszki M. Spatial pattern analysis of nitrergic neurons in the myenteric plexus of the duodenum of different mammalian species. Acta Biol Hung. 2009;60:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Bagyánszki M, Román V, Fekete E. Quantitative distribution of NADPH-diaphorase-positive myenteric neurons in different segments of the developing chicken small intestine and colon. Histochem J. 2000;32:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Timmermans JP, Barbiers M, Scheuermann DW, Bogers JJ, Adriaensen D, Fekete E, Mayer B, Van Marck EA, De Groodt-Lasseel MH. Nitric oxide synthase immunoreactivity in the enteric nervous system of the developing human digestive tract. Cell Tissue Res. 1994;275:235-245. [PubMed] |
| 59. | Van Ginneken C, Van Meir F, Sommereyns G, Sys S, Weyns A. Nitric oxide synthase expression in enteric neurons during development in the pig duodenum. Anat Embryol (Berl). 1998;198:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 61. | Spångéus A, Suhr O, El-Salhy M. Diabetic state affects the innervation of gut in an animal model of human type 1 diabetes. Histol Histopathol. 2000;15:739-744. [PubMed] |
| 62. | Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Cellek S, Rodrigo J, Lobos E, Fernández P, Serrano J, Moncada S. Selective nitrergic neurodegeneration in diabetes mellitus - a nitric oxide-dependent phenomenon. Br J Pharmacol. 1999;128:1804-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 65. | Cellek S, Qu W, Schmidt AM, Moncada S. Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: a new insight into selective nitrergic neuropathy in diabetes. Diabetologia. 2004;47:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Krecsmarik M, Izbéki F, Bagyánszki M, Linke N, Bódi N, Kaszaki J, Katarova Z, Szabó A, Fekete E, Wittmann T. Chronic ethanol exposure impairs neuronal nitric oxide synthase in the rat intestine. Alcohol Clin Exp Res. 2006;30:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Bagyánszki M, Krecsmarik M, De Winter BY, De Man JG, Fekete E, Pelckmans PA, Adriaensen D, Kroese AB, Van Nassauw L, Timmermans JP. Chronic alcohol consumption affects gastrointestinal motility and reduces the proportion of neuronal NOS-immunoreactive myenteric neurons in the murine jejunum. Anat Rec (Hoboken). 2010;293:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Bagyánszki M, Torfs P, Krecsmarik M, Fekete E, Adriaensen D, Van Nassauw L, Timmermans JP, Kroese AB. Chronic alcohol consumption induces an overproduction of NO by nNOS- and iNOS-expressing myenteric neurons in the murine small intestine. Neurogastroenterol Motil. 2011;23:e237-e248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol. 2005;564:863-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951-960. [PubMed] |
| 71. | De Giorgio R, Camilleri M. Human enteric neuropathies: morphology and molecular pathology. Neurogastroenterol Motil. 2004;16:515-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 72. | Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131-18, e26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 73. | Watkins CC, Sawa A, Jaffrey S, Blackshaw S, Barrow RK, Snyder SH, Ferris CD. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 75. | Yoneda S, Kadowaki M, Kuramoto H, Fukui H, Takaki M. Enhanced colonic peristalsis by impairment of nitrergic enteric neurons in spontaneously diabetic rats. Auton Neurosci. 2001;92:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Furlan MM, Molinari SL, Miranda Neto MH. Morphoquantitative effects of acute diabetes on the myenteric neurons of the proximal colon of adult rats. Arq Neuropsiquiatr. 2002;60:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Pereira RV, de Miranda-Neto MH, da Silva Souza ID, Zanoni JN. Vitamin E supplementation in rats with experimental diabetes mellitus: analysis of myosin-V and nNOS immunoreactive myenteric neurons from terminal ileum. J Mol Histol. 2008;39:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Pereira RV, Tronchini EA, Tashima CM, Alves EP, Lima MM, Zanoni JN. L-glutamine supplementation prevents myenteric neuron loss and has gliatrophic effects in the ileum of diabetic rats. Dig Dis Sci. 2011;56:3507-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Itoh H, Yoneda M, Tamori K, Miyamoto Y, Morikawa A, Eto M, Makino I. Rapid gastric emptying and pathological changes of vagus nerve in the spontaneously diabetic Chinese hamster. Diabetes Res Clin Pract. 1995;28:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Yagihashi S, Sima AA. Diabetic autonomic neuropathy in BB rat. Ultrastructural and morphometric changes in parasympathetic nerves. Diabetes. 1986;35:733-743. [PubMed] [DOI] [Full Text] |
| 81. | Furlan MM, de Miranda Neto MH, Sant'ana Dde M, Molinari SL. Number and size of myenteric neurons of the duodenum of adult rats with acute diabetes. Arq Neuropsiquiatr. 1999;57:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Liu W, Yue W, Wu R. Effects of diabetes on expression of glial fibrillary acidic protein and neurotrophins in rat colon. Auton Neurosci. 2010;154:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 83. | Surendran S, Kondapaka SB. Altered expression of neuronal nitric oxide synthase in the duodenum longitudinal muscle-myenteric plexus of obesity induced diabetes mouse: implications on enteric neurodegeneration. Biochem Biophys Res Commun. 2005;338:919-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725-G733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 85. | Adeghate E, al-Ramadi B, Saleh AM, Vijayarasathy C, Ponery AS, Arafat K, Howarth FC, El-Sharkawy T. Increase in neuronal nitric oxide synthase content of the gastroduodenal tract of diabetic rats. Cell Mol Life Sci. 2003;60:1172-1179. [PubMed] |
| 86. | Shotton HR, Clarke S, Lincoln J. The effectiveness of treatments of diabetic autonomic neuropathy is not the same in autonomic nerves supplying different organs. Diabetes. 2003;52:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | LePard KJ. Choline acetyltransferase and inducible nitric oxide synthase are increased in myenteric plexus of diabetic guinea pig. Auton Neurosci. 2005;118:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | de Mello ST, de Miranda Neto MH, Zanoni JN, Furlan MM. Effects of insulin treatment on HuC/HuD, NADH diaphorase, and nNOS-positive myoenteric neurons of the duodenum of adult rats with acute diabetes. Dig Dis Sci. 2009;54:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Shotton HR, Lincoln J. Diabetes only affects nitric oxide synthase-containing myenteric neurons that do not contain heme oxygenase 2. Brain Res. 2006;1068:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Kashyap P, Farrugia G. Oxidative stress: key player in gastrointestinal complications of diabetes. Neurogastroenterol Motil. 2011;23:111-114. [PubMed] [DOI] [Full Text] |
| 91. | van der Vliet A, Tuinstra TJ, Bast A. Modulation of oxidative stress in the gastrointestinal tract and effect on rat intestinal motility. Biochem Pharmacol. 1989;38:2807-2818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Puddu A, Viviani GL. Advanced glycation endproducts and diabetes. Beyond vascular complications. Endocr Metab Immune Disord Drug Targets. 2011;11:132-140. [PubMed] |
| 93. | Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 419] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 94. | Maleki D, Locke GR, Camilleri M, Zinsmeister AR, Yawn BP, Leibson C, Melton LJ. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 218] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 96. | Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 97. | Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Camilleri M, Malagelada JR. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur J Clin Invest. 1984;14:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 219] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 99. | Keshavarzian A, Iber FL. Gastrointestinal involvement in insulin-requiring diabetes mellitus. J Clin Gastroenterol. 1987;9:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | Lincoln J, Bokor JT, Crowe R, Griffith SG, Haven AJ, Burnstock G. Myenteric plexus in streptozotocin-treated rats. Neurochemical and histochemical evidence for diabetic neuropathy in the gut. Gastroenterology. 1984;86:654-661. [PubMed] |
| 101. | Belai A, Burnstock G. Changes in adrenergic and peptidergic nerves in the submucous plexus of streptozocin-diabetic rat ileum. Gastroenterology. 1990;98:1427-1436. [PubMed] |
| 102. | Nowak TV, Harrington B, Kalbfleisch JH, Amatruda JM. Evidence for abnormal cholinergic neuromuscular transmission in diabetic rat small intestine. Gastroenterology. 1986;91:124-132. [PubMed] |
| 103. | Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 104. | Kashyap P, Farrugia G. Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut. 2010;59:1716-1726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 105. | Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis--a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 106. | Hasler WL. Gastroparesis: pathogenesis, diagnosis and management. Nat Rev Gastroenterol Hepatol. 2011;8:438-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 107. | Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 108. | Kaputlu I, Ozdem S, Sadan G, Gökalp O. Effects of diabetes on non-adrenergic, non-cholinergic relaxation induced by GABA and electrical stimulation in the rat isolated duodenum. Clin Exp Pharmacol Physiol. 1999;26:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 109. | Korenaga K, Micci MA, Taglialatela G, Pasricha PJ. Suppression of nNOS expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol Motil. 2006;18:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 110. | Yamada K, Hosokawa M, Fujimoto S, Nagashima K, Fukuda K, Fujiwara H, Ogawa E, Fujita Y, Ueda N, Matsuyama F. The spontaneously diabetic Torii rat with gastroenteropathy. Diabetes Res Clin Pract. 2007;75:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 111. | Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378-384. [PubMed] |
| 112. | Imaeda K, Takano H, Koshita M, Yamamoto Y, Joh T, Suzuki H. Electrical properties of colonic smooth muscle in spontaneously non-insulin-dependent diabetic rats. J Smooth Muscle Res. 1998;34:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 113. | Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, Miyagawa J, Chen H, Miyazaki Y, Kiyohara T. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Forrest A, Parsons M. The enhanced spontaneous activity of the diabetic colon is not the consequence of impaired inhibitory control mechanisms. Auton Autacoid Pharmacol. 2003;23:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 115. | Yamamoto M, Toya Y, Schwencke C, Lisanti MP, Myers MG, Ishikawa Y. Caveolin is an activator of insulin receptor signaling. J Biol Chem. 1998;273:26962-26968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 236] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 116. | Guasch-Ferré M, Bulló M, Costa B, Martínez-Gonzalez MÁ, Ibarrola-Jurado N, Estruch R, Barrio F, Salas-Salvadó J. A risk score to predict type 2 diabetes mellitus in an elderly Spanish Mediterranean population at high cardiovascular risk. PLoS One. 2012;7:e33437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 117. | Haidinger T, Zweimüller M, Stütz L, Demir D, Kaider A, Strametz-Juranek J. Effect of gender on awareness of cardiovascular risk factors, preventive action taken, and barriers to cardiovascular health in a group of austrian subjects. Gend Med. 2012;9:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 118. | Kaplan RC, Bùzková P, Cappola AR, Strickler HD, McGinn AP, Mercer LD, Arnold AM, Pollak MN, Newman AB. Decline in Circulating Insulin-Like Growth Factors and Mortality in Older Adults: Cardiovascular Health Study All-Stars Study. J Clin Endocrinol Metab. 2012;[Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Dailey G. Early and intensive therapy for management of hyperglycemia and cardiovascular risk factors in patients with type 2 diabetes. Clin Ther. 2011;33:665-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 120. | Shogbon AO, Levy SB. Intensive glucose control in the management of diabetes mellitus and inpatient hyperglycemia. Am J Health Syst Pharm. 2010;67:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 121. | Mazzone A, Strege PR, Tester DJ, Bernard CE, Faulkner G, De Giorgio R, Makielski JC, Stanghellini V, Gibbons SJ, Ackerman MJ. A mutation in telethonin alters Nav1.5 function. J Biol Chem. 2008;283:16537-16544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Averill MM, Bornfeldt KE. Lipids versus glucose in inflammation and the pathogenesis of macrovascular disease in diabetes. Curr Diab Rep. 2009;9:18-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Manna P, Sil PC. Impaired redox signaling and mitochondrial uncoupling contributes vascular inflammation and cardiac dysfunction in type 1 diabetes: Protective role of arjunolic acid. Biochimie. 2012;94:786-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 124. | Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 2010;151:4109-4115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 125. | Kampoli AM, Tousoulis D, Briasoulis A, Latsios G, Papageorgiou N, Stefanadis C. Potential pathogenic inflammatory mechanisms of endothelial dysfunction induced by type 2 diabetes mellitus. Curr Pharm Des. 2011;17:4147-4158. [PubMed] |
| 126. | Conway BR, Rennie J, Bailey MA, Dunbar DR, Manning JR, Bellamy CO, Hughes J, Mullins JJ. Hyperglycemia and renin-dependent hypertension synergize to model diabetic nephropathy. J Am Soc Nephrol. 2012;23:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 127. | Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53:S35-S42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 309] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 128. | Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD. Matrix metalloproteinases and diabetic vascular complications. Angiology. 2005;56:173-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 129. | Onat A. Metabolic syndrome: nature, therapeutic solutions and options. Expert Opin Pharmacother. 2011;12:1887-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 130. | Okamoto MM, Anhê GF, Sabino-Silva R, Marques MF, Freitas HS, Mori RC, Melo KF, Machado UF. Intensive insulin treatment induces insulin resistance in diabetic rats by impairing glucose metabolism-related mechanisms in muscle and liver. J Endocrinol. 2011;211:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 131. | Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, Emmons KM, Rosner BA, Colditz GA; for the Be Fit, Be Well Study Investigators. Obesity Treatment for Socioeconomically Disadvantaged Patients in Primary Care Practice. Arch Intern Med. 2012;172:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 132. | Crichton GE, Elias MF, Dore GA, Abhayaratna WP, Robbins MA. Relations between dairy food intake and arterial stiffness: pulse wave velocity and pulse pressure. Hypertension. 2012;59:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 133. | Masuyama H, Hiramatsu Y. Effects of a High-Fat Diet Exposure in Utero on the Metabolic Syndrome-Like Phenomenon in Mouse Offspring through Epigenetic Changes in Adipocytokine Gene Expression. Endocrinology. 2012;[Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 134. | Richard KR, Shelburne JS, Kirk JK. Tolerability of dipeptidyl peptidase-4 inhibitors: a review. Clin Ther. 2011;33:1609-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 135. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Price DL, Chen R, Udell J. The design and rationale of the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction (SAVOR-TIMI) 53 study. Am Heart J. 2011;162:818-825.e6. [PubMed] |
| 136. | White WB, Bakris GL, Bergenstal RM, Cannon CP, Cushman WC, Fleck P, Heller S, Mehta C, Nissen SE, Perez A. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.e1. [PubMed] |
| 137. | De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 842] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 138. | Simionescu M, Popov D, Sima A. Endothelial transcytosis in health and disease. Cell Tissue Res. 2009;335:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 139. | Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 140. | Barnett AH. Pathogenesis of diabetic microangiopathy: an overview. Am J Med. 1991;90:67S-73S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesburn AB, Kenney MC. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem. 1996;44:1469-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 142. | Roy S, Sato T, Paryani G, Kao R. Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes. 2003;52:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 143. | Lee SE, Ma W, Rattigan EM, Aleshin A, Chen L, Johnson LL, D'Agati VD, Schmidt AM, Barile GR. Ultrastructural features of retinal capillary basement membrane thickening in diabetic swine. Ultrastruct Pathol. 2010;34:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 144. | Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007;27:195-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 312] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 145. | Zhu WW, Chen HP, Ge YC, Xie HL, Zeng CH, Li LS, Liu ZH. Ultrastructural changes in the glomerular filtration barrier and occurrence of proteinuria in Chinese patients with type 2 diabetic nephropathy. Diabetes Res Clin Pract. 2009;86:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 146. | De Las Casas LE, Finley JL. Diabetic microangiopathy in the small bowel. Histopathology. 1999;35:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 147. | Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1321] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 148. | Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102:e120-e131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 149. | Li XA, Everson W, Smart EJ. Nitric oxide, caveolae, and vascular pathology. Cardiovasc Toxicol. 2006;6:1-13. [PubMed] [DOI] [Full Text] |
| 150. | Schmidt RE, Dorsey DA, Beaudet LN, Parvin CA, Zhang W, Sima AA. Experimental rat models of types 1 and 2 diabetes differ in sympathetic neuroaxonal dystrophy. J Neuropathol Exp Neurol. 2004;63:450-460. [PubMed] |
| 151. | Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv. 2003;3:445-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 152. | Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 672] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 153. | Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 278] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 154. | von Boyen GB, Steinkamp M, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut. 2004;53:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 155. | Tashima CM, Tronchini EA, Pereira RV, Bazotte RB, Zanoni JN. Diabetic rats supplemented with L-glutamine: a study of immunoreactive myosin-V myenteric neurons and the proximal colonic mucosa. Dig Dis Sci. 2007;52:1233-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 156. | Zanoni JN, Tronchini EA, Moure SA, Souza ID. Effects of L-glutamine supplementation on the myenteric neurons from the duodenum and cecum of diabetic rats. Arq Gastroenterol. 2011;48:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 157. | Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 619] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 158. | Tsai PH, Liu JJ, Chiu WC, Pai MH, Yeh SL. Effects of dietary glutamine on adhesion molecule expression and oxidative stress in mice with streptozotocin-induced type 1 diabetes. Clin Nutr. 2011;30:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 159. | Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J Neurochem. 1999;72:2334-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 160. | Voltarelli JC, Couri CE, Rodrigues MC, Moraes DA, Stracieri AB, Pieroni F, Navarro G, Leal AM, Simões BP. Stem cell therapies for type 1 diabetes mellitus. Indian J Exp Biol. 2011;49:395-400. [PubMed] |
| 161. | Zhu FF, Zhang PB, Zhang DH, Sui X, Yin M, Xiang TT, Shi Y, Ding MX, Deng H. Generation of pancreatic insulin-producing cells from rhesus monkey induced pluripotent stem cells. Diabetologia. 2011;54:2325-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 162. | Godfrey KJ, Mathew B, Bulman JC, Shah O, Clement S, Gallicano GI. Stem cell-based treatments for Type 1 diabetes mellitus: bone marrow, embryonic, hepatic, pancreatic and induced pluripotent stem cells. Diabet Med. 2012;29:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 163. | Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 539] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 164. | Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 165. | Donner T, Muñoz M. Update on insulin therapy for type 2 diabetes. J Clin Endocrinol Metab. 2012;97:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 166. | Ryan JP, Sheu LK, Critchley HD, Gianaros PJ. A Neural Circuitry Linking Insulin Resistance to Depressed Mood. Psychosom Med. 2012;[Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 167. | Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61:778-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 168. | Tolessa T, Gutniak M, Holst JJ, Efendic S, Hellström PM. Glucagon-like peptide-1 retards gastric emptying and small bowel transit in the rat: effect mediated through central or enteric nervous mechanisms. Dig Dis Sci. 1998;43:2284-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 169. | Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab. 2001;86:3853-3860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 170. | Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 626] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 171. | Schirra J, Kuwert P, Wank U, Leicht P, Arnold R, Göke B, Katschinski M. Differential effects of subcutaneous GLP-1 on gastric emptying, antroduodenal motility, and pancreatic function in men. Proc Assoc Am Physicians. 1997;109:84-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 172. | Schirra J, Leicht P, Hildebrand P, Beglinger C, Arnold R, Göke B, Katschinski M. Mechanisms of the antidiabetic action of subcutaneous glucagon-like peptide-1(7-36)amide in non-insulin dependent diabetes mellitus. J Endocrinol. 1998;156:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 173. | Schirra J, Göke B. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept. 2005;128:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 174. | Rotondo A, Amato A, Lentini L, Baldassano S, Mulè F. Glucagon-like peptide-1 relaxes gastric antrum through nitric oxide in mice. Peptides. 2011;32:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 175. | Amato A, Cinci L, Rotondo A, Serio R, Faussone-Pellegrini MS, Vannucchi MG, Mulè F. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol Motil. 2010;22:664-e203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 176. | Blonde L, Montanya E. Comparison of liraglutide versus other incretin-related anti-hyperglycaemic agents. Diabetes Obes Metab. 2012;14 Suppl 2:20-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 177. | Brown NJ. Cardiovascular effects of antidiabetic agents: focus on blood pressure effects of incretin-based therapies. J Am Soc Hypertens. 2012;6:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 178. | Cobble M. Differentiating among incretin-based therapies in the management of patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 179. | Cornell S. Differentiating among incretin therapies: a multiple-target approach to type 2 diabetes. J Clin Pharm Ther. 2012;[Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 180. | Rosenstock J, Zinman B. Dipeptidyl peptidase-4 inhibitors and the management of type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2007;14:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 181. | Cefalu WT. Evolving treatment strategies for the management of type 2 diabetes. Am J Med Sci. 2012;343:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 182. | Liu WJ, Xie SH, Liu YN, Kim W, Jin HY, Park SK, Shao YM, Park TS. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2012;340:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 183. | Tang X, Wang Y, Guo X, Wang S, Chai L, Zhang Y. [Effects of glucagon like peptide-1 treatment on the alveolar capillary basal lamina in Otsuka Long-Evans Tokushima Fatty rats]. Beijing Daxue Xuebao. 2008;40:178-180. [PubMed] |
| 184. | Davidson JA. Incorporating incretin-based therapies into clinical practice: differences between glucagon-like Peptide 1 receptor agonists and dipeptidyl peptidase 4 inhibitors. Mayo Clin Proc. 2010;85:S27-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 185. | Davies MJ, Kela R, Khunti K. Liraglutide - overview of the preclinical and clinical data and its role in the treatment of type 2 diabetes. Diabetes Obes Metab. 2011;13:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 186. | Dicembrini I, Pala L, Rotella CM. From theory to clinical practice in the use of GLP-1 receptor agonists and DPP-4 inhibitors therapy. Exp Diabetes Res. 2011;2011:898913. [PubMed] |
| 187. | Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1783] [Cited by in RCA: 2082] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 188. | Burcelin R, Serino M, Chabo C, Blasco-Baque V, Amar J. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol. 2011;48:257-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 189. | Maccaferri S, Biagi E, Brigidi P. Metagenomics: key to human gut microbiota. Dig Dis. 2011;29:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 190. | Greiner T, Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 191. | Chronopoulos A, Tang A, Beglova E, Trackman PC, Roy S. High glucose increases lysyl oxidase expression and activity in retinal endothelial cells: mechanism for compromised extracellular matrix barrier function. Diabetes. 2010;59:3159-3166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 192. | Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5700] [Cited by in RCA: 5592] [Article Influence: 279.6] [Reference Citation Analysis (2)] |
| 193. | Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57:1605-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 194. | Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J. Metagenomic pyrosequencing and microbial identification. Clin Chem. 2009;55:856-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 195. | Marchesi JR. Prokaryotic and eukaryotic diversity of the human gut. Adv Appl Microbiol. 2010;72:43-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 196. | Bäckhed F. Changes in intestinal microflora in obesity: cause or consequence? J Pediatr Gastroenterol Nutr. 2009;48 Suppl 2:S56-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 197. | Burcelin R, Luche E, Serino M, Amar J. The gut microbiota ecology: a new opportunity for the treatment of metabolic diseases? Front Biosci. 2009;14:5107-5117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 198. | Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3224] [Cited by in RCA: 3543] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 199. | Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1735] [Cited by in RCA: 1888] [Article Influence: 118.0] [Reference Citation Analysis (1)] |
| 200. | Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070-11075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4639] [Cited by in RCA: 4467] [Article Influence: 223.4] [Reference Citation Analysis (1)] |
| 201. | Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6397] [Cited by in RCA: 5669] [Article Influence: 354.3] [Reference Citation Analysis (1)] |
| 202. | Brugman S, Klatter FA, Visser JT, Wildeboer-Veloo AC, Harmsen HJ, Rozing J, Bos NA. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 203. | Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, Corthesy I, Macé K, Chou CJ. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 204. | Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6:e25792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 590] [Article Influence: 42.1] [Reference Citation Analysis (0)] |