Published online Apr 15, 2011. doi: 10.4239/wjd.v2.i4.54
Revised: March 28, 2011
Accepted: April 4, 2011
Published online: April 15, 2011
AIM: To investigate the differentiation and migration of endocrine cells to form the pancreatic islets of Langerhans in early human development.
METHODS: Embryonic pancreas of 6-14 wk gestation was observed using immunocytochemistry methods in early human development.
RESULTS: Insulin and glucagon are expressed in the same epithelium cells in the pancreas. In addition, insulin-producing cells also secrete somatostatin in early human embryonic development and these insulin-producing cells also express nestin.
CONCLUSION: Pancreatic duct epithelial cells that can produce insulin in early human development are precursors and still have the potential to differentiate other endocrine cells. These progenitors have differentiated before migration from primary ductal epithelium to form the pancreatic islets.
- Citation: Yang KM, Yong W, Li AD, Yang HJ. Insulin-producing cells are bi-potential and differentiatorsprior to proliferation in early human development. World J Diabetes 2011; 2(4): 54-58
- URL: https://www.wjgnet.com/1948-9358/full/v2/i4/54.htm
- DOI: https://dx.doi.org/10.4239/wjd.v2.i4.54
For decades, investigators have been studying pancreatic development in the hope of isolating a stem cell that could be induced to generate new β-cells. Recent clinical trials indicate that transplantation of isolated islets, combined with immune suppressive therapy, can cure type I diabetes[1]. This further raises the hopes of patients and researchers that a stem cell therapy for this disease is feasible. The problem is where to find such stem cells and how to control their differentiation. We know that the islets of Langerhans in the pancreas are specialized endocrine micro-organs composed of four distinctive cell types; insulin-producing β-cells, glucagon-producing α−cells, somatostatin-producing δ-cells and pancreatic polypeptide producing pp cells. During embryonic development, four endocrine cells of the pancreatic islet derive from a common set of epithelial cells that originate in the early gut endoderm[2]. The pancreas derives from two patches of epithelium that bud dorsally and ventrally from the gut epithelium, between the stomach and duodenum, beginning (in the mouse) at approximately embryonic day 9 (E9). Prior to and during budding, the organ primordium expresses the homeodomain protein Pdx1/Ipf1; all pancreatic cell types derive from Pdx1+progenitors[3,4]. After budding, the pancreatic primordia begin dramatic growth and branching while reorienting and fusing into a single bipolar organ. As we know, all adult pancreatic cells derive from Pdx1-expressing progenitors. During bud outgrowth, Pdx1 expression shifts from uniform to biphasic at high levels in β-cells and lower levels in undifferentiated precursors[5,6]. Inactivation of Pdx1 after bud formation, using the tTA system[7], prevents both islet and acinar differentiation; this general function in development may reflect a role in multipotent progenitors or stem cells. A key regulator of endocrine development is the bHLH protein Neurogenin3 (Ngn3), which is expressed exclusively in endocrine precursor cells and subsequently downregulated during differentiation[4,8]. Its absolute requirement for islet cell development[9] suggests that Ngn3 promotes endocrine fate in cells descended from Pdx1C progenitors. Moreover, misexpression of Ngn3 is sufficient to induce endocrine differentiation throughout the gut epithelium[10]. Ngn3 is ordinarily expressed in scattered cells of the epithelium; broader misexpression of Ngn3 in the early pancreas, using the Pdx1 promoter, results in complete diversion of the organ to an endocrine fate[10,11].
Lineage studies[12] illuminate additional aspects of endocrine development. Previously, cells co-expressing glucagon and insulin in the early pancreatic bud were suggested to represent bi-potential progenitor cells[13]. However, using the glucagon or insulin promoter to drive Cre-dependent lineage marking, it was found that adult β-cells derive from progenitors that had never expressed glucagon and vice-versa for α-cells. Surprisingly, it was also found that β-cells, but not α-cells, derive from progenitors that did express pancreatic polypeptide, although PP expression is not maintained in β-cells. Expression data, therefore, can be an unreliable guide to lineage.
During embryonic development, a cascade of transcriptional factors control β-cells formation in the pancreas. Different transcription factors control distinct checkpoints along the pathway to the differentiated β-cells. The first step of pancreatic epithelial cells towards an endocrine fate is controlled by neurogenin 3. Loss of neurogenin 3 function in mice results in a complete absence of endocrine cell differentiation[9]. β-cell competence factors likely include the NK-homeodomain genes Nkx2.2 and Nkx6.1[14,15]. Both of these factors act either downstream of or in parallel to Ngn3, as Ngn3 expression is normal in mice lacking Nkx2.2 or Nkx6.1[11]. Nkx2.2 mutants completely lack insulin expression; in place of normal β-cells, islets contain a large population of cells apparently arrested “just short” of β-cell fate. Nkx6.1 mutants have a phenotype that is both more and less dramatic: a small number of insulin-producing cells are generated during early pancreatic development but the normally exponential increase in β-cell generation that initiates during the secondary transition is completely absent and no immature β-like cells are formed.
There is considerable evidence suggesting that the differentiation of Pdx-1+ progenitor cells into pancreatic islet cells occurs by a multi-step process, involving successive changes in the antigenic profile of the stem cells. Pdx-1 cells co-expressing insulin and glucagon appear at E 9.5 in the pancreatic bud of mice before full morphogenesis of the pancreas[5]. From an embryonic development study, we found that at 6 wk gestation, the pancreatic primordium has branched and there is a lack of secreting role in the epithelial cells. At 10 wk gestation, the pancreatic ductal epithelial cells begin expressing insulin glucagon and somatostatin before migration from the duct. Interestingly, a few epithelial cells simultaneously express insulin and glucagon or insulin and somatostatin. Moreover, these insulin-producing cells also express nestin, which continues until the 14 wk when the process of islet formation begins. We conclude that endocrine cells differentiation prior to the migration and insulin-producing cells in pancreatic epithelium are endocrine progenitors, with bipotential in early human development.
Embryo samples of 6 cases (6 to 14 wk gestation, based on menolipsis and the size of the fetus) were obtained from donors at the department of Jiming Obstetrics hospital from first trimester spontaneous abortions. The investigation complies with the principles of the Declaration of Helsinki and has been approved by the Ethics Committee of SiChuan University where it was performed. The subjects gave informed consent to the work.
The embryonic tissues were fixed with 4% paraformaldehyde in phosphate buffer and 5 μm thick paraffin sections were mounted on silanized slides for immunocytochemical labeling.
For immunocytochemical labeling, we chose the streptavidin-biotin-peroxidase kits (Beijing Zhongshan Biotechnology Co. Ltd., Beijing, China). Mouse anti-insulin, glucagons, PCNA and rabbit anti-nestin and somatostatin were purchased from Santa Cruz Co. Ltd. (America). Immunocytochemistry sections were first blocked with 10% normal goat serum for 20 min at room temperature and incubated with primary antisera (Insulin: 1:300, Glucagon: 1:300, Somatostatin: 1:300, PCNA: 1:500, Nestin: 1:1000) overnight at 4°C. Then primary antisera were washed with phosphate-buffered saline (PBS) and incubated with the biotin labeled secondary mouse or rabbit antisera (1:100) for 2 h at 37°C, followed by a second PBS wash. Finally, slides were incubated with streptavidin for 1 h at 37°C, or streptavidin conjugated immunofluorescence FITC/Cy3, and washed extensively with PBS. After treatment with Streptavidin Affinity Complex (SABC), the tissues were oxidized by diaminobenzidine (Sigma, America) and counterstained with hematoxylin for microscopic observation. Fluorescence was visualized with a Nikon microscope. Double staining was according to the details of HistostainTM-DS kits (BeiJing Zhongshan Biotechnology Co. Ltd., Beijing, China). During the whole procedure, PBS was applied as a negative control in place of a primary antibody. Microscopy and imaging of the immunostained sections and the sections with haematoxylin and eosin (H&E) was done with an Olympus microscope (Model CX4IRF, Olympus Optical Co. Ltd.). At least 10 sections were observed from each pancreas. Images were recorded with a digital camera (C-5050zoom, Olympus Optical Co. Ltd.). The final images were assembled with Adobe Photoshop 7.0. The reaction results for the SABC method was yellow-brown adjacent to the top of cell or in cytoplasm of pancreatic tissues of human embryo. The immunofluorescence reaction is red or green but the cross-reaction was yellow. The reaction results of double staining (DS) were indigo and scarlet. The tissues of the control were all negative.
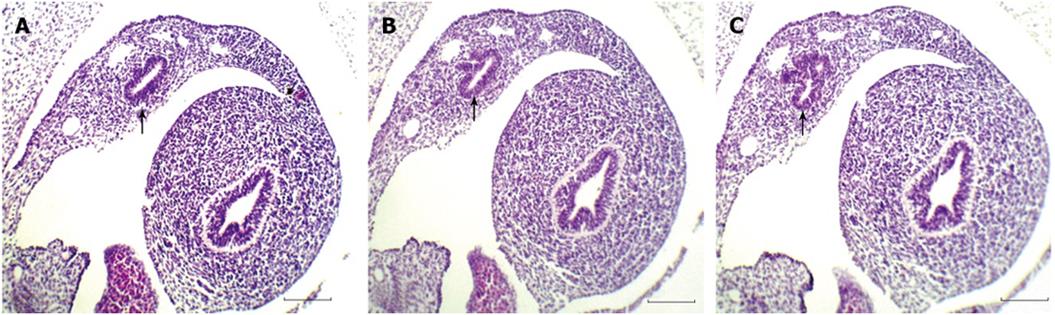
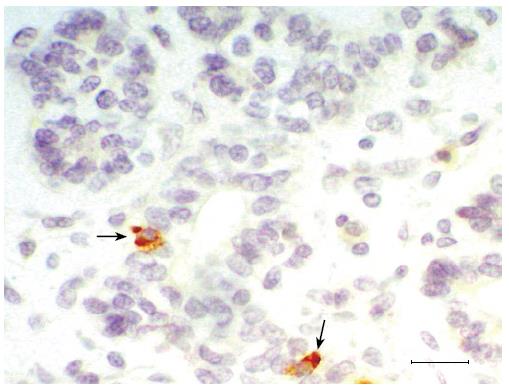
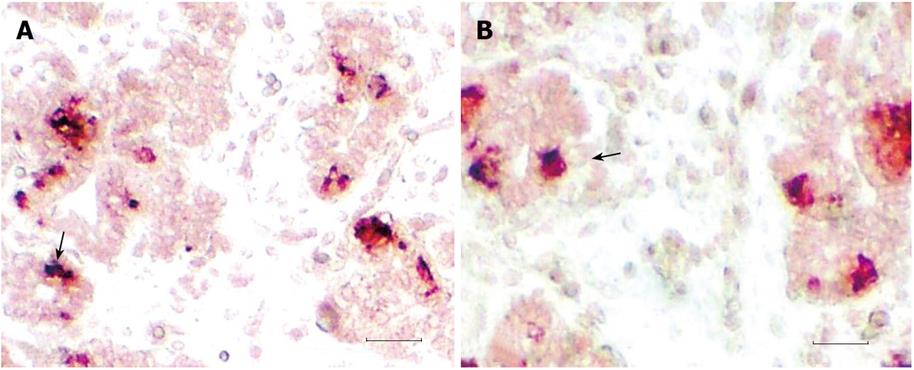
The pancreas derives from two patches of epithelium that bud dorsally and ventrally from the gut epithelium, between the stomach and duodenum. At 6 wk gestation, the pancreatic primordia begin to grow and branch. The growing epithelia cells concentrate and have polarity (Figure 1). The growing epithelium was surrounded by mesenchymal cells. At this stage, no insulin-positive cells and nestin+ cells were detected. Pancreatic epithelia cells begin to auto-secrete at 11 wk gestation. A large number of primary pancreatic duct and insulin (Figure 2), glucagon and somatostatin (data not shown) positive cells could be seen located the pancreatic epithelium. The positive reaction epithelia cells still remained in the duct and did not migrate. At this stage, the pancreatic premordia began dramatic growth with curvature and branching. With double staining, we detected some epithelial cells that co-express insulin and glucagon and somatostatin (Figure 3). The same insulin –producing epithelial cell co-expressed glucagon and somaotostaitin lasting up to 12 wk gestation. At this stage endocrine cells began to aggregate. To our surprise, we did not detect the proliferation of pancreatic epithelial cells to express antigen PCNA in this stage of epithelium differentiation but PCNA show the strong reaction in the liver (data not shown).
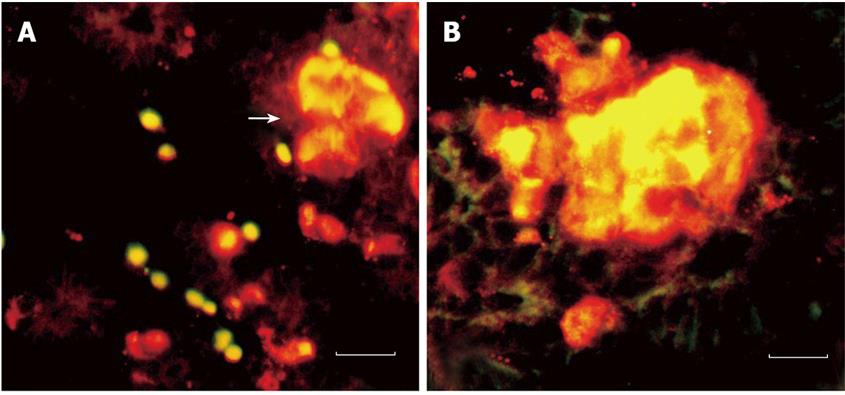
For the immunofluorescence, we observed that the insulin-producing epithelial cells not only co-express glucagon and somatostatin but also express nestin (Figure 4). The expression of nestin occurred in pancreatic epithelium (11 wk) and lasted until the formation of islets (14 wk). The insulin reaction is stronger than nestin. Meanwhile, a few insulin negative epithelia cells also express nestin. However, the endocrine cells began to differentiate and move from pancreatic ductal epithelium to form the islets. When the islet began to form, the insulin-positive cells still can be seen in ductal epithelial cells.
Pancreas development is a complex process that requires the timely expression of numerous factors; among them, the neurogenin3 and neuroD1/BETA2 drive endocrine differentiation. But the cell fate induced by ectopic Ngn3 expression is predominantly or exclusively α-cells. The ability to identify endocrine precursor cells was based on neurogenin3 expression. Previous studies suggested the cascade of transcription factors controlling islet cell differentiation[5,6-14]. It has been suggested that the early insulin/glucagon co-expression cells represent a transition state as cells differentiate from the early glucagon expressing cells into mature β-cells[11,12]. However, the investigation in mouse development does not support this view[14] because they do not detect significant replication of insulin-expressing cells. Maturation of the small early population of insulin/glucagon co-expressing cells can not explain the much larger number of mature β-cells that appear after E13. Furthermore, Herrera and colleagues have used lineage tracing in transgenic mouse model to show that the glucagon promoter is not active in the progenitor cells for mature β-cells[11,16].
In our study, we determined the expression of insulin, glucagon, somatostatin and nestin in early human embryonic pancreas development. We found that at 10 wk gestation, a few epithelial cells in pancreatic duct begin expressing insulin, glucagon and somotostaitin. It suggested that endocrine cells differentiated prior to migration. Double staining shows that a few epithelial cells co-express insulin/glucagon or insulin/somatostatin. But we could not detect the cells co-expressing glucagon/somatostatin. This suggested that insulin producing cells also have the potential to secrete glucagon or somatostatin. In addition, at the same gestational age, immunofluorescence staining showed that insulin-producing epithelium also expresses nestin, a marker that generally is considered as pancreas stem cell labeling[17]. All of this suggests that insulin producing epithelia cells are endocrine progenitors and bipotential cells. In contrast, we detected endocrine cells differentiation instead of proliferation in gestational age 6-14 wk, showing differentiation prior to proliferation. Therefore, we support that most of the β-cells in the late fetal pancreas must develop from non-hormone-expressing progenitor cells.
The above mentioned reminds us of mouse studies[17,18]. They identified that mature islets contain a stem cell population that can be induced to differentiate into insulin-producing cells following islet injury. Fernandes and his colleagues identified two subsets of PDX-1+β precursor cells in islet in mouse studies: the IN+/GLU+/PDX-1+ cell type which can lead to monospecific β-cells in embryo. Whereas IN+/SOM+/PDX-1+β cells, a cell type at a more advanced stage in the cellular hierarchy, are proposed to generate IN+ cells in regenerating islets of adults. It may be a hypothesis that progenitor from embryo set aside in islets lasts till the late postnatal life of adult. The transcription factor PDX-1 is expressed in pancreatic precursor cells and becomes restricted to β-cells in the mature islet where it controls insulin transcription [19].
A similar transient expression of nestin was proposed
to occur in the human insulin-producing β-cell precursors[17,20]. In our study we found the β-cells which secreted hormone in the duct prior to moving from duct, suggesting insulin may be a vital cytokine to promote the migration of β-cells and formation of islets. In addition, these insulin producing cells not only co-express glucagon or somatostatin but also express nestin and last up to the formation of islets. It indicated that insulin-producing cells are pancreatic stem cells and always remain in islets themselves. So we support the view that regeneration of β-cells after β-cells impaired showing the stem cells remaining of islets themselves[18]. Recently, to determine whether nestin can be used to identify β-cell progenitors in the developing human pancreas, nestin+ cells were purified by using an enhancer/promoter-driver selection plasmid to determine whether nestin+ cells differentiated into β-cells[21]. The experimental result suggested that nestin is not a specific marker of β-cell precursors in the developing human pancreas. According to our investigation, the expression of nestin may not be a specific marker of β-cell precursors but insulin-producing cells co-expressing glucagon or somatostatin in pancreas of early human development show that these cells have bi-potential. Simultaneously, these insulin-producing cells with nestin-positive reactions suggest that these cells are progenitors of islet. Nestin is localized specifically to the mesenchyme of the developing human pancreas but not to any epithelial cell population. Furthermore, after isolation, nestin+ cells do not differentiate into β-cells, nei-ther in vitro nor in vivo[21,22]. We hypothesize that expression of nestin shows the cells have the power of differentiation which can be differentiated under certain times and certain conditions, other than multipotential stem cells.
Differentiation of early gut endoderm cells into the endocrine cells forming the pancreatic islets of Langerhans in human embryonic development still needs to be clarified. To observe how gut endoderm cells differentiate into endocrine cells and migrate from epithelium was significant for the investigation of pancreatic stem cells.
In this article, embryonic pancreas of about 6-14 wk gestation was observed using immunohistochemistry methods in early human development. We found that insulin and glucagon are expressed in the same epithelium cells in the pancreas. In addition, insulin-producing cells also secrete somatostatin in early human embryonic development.
Insulin and glucagon are expressed in the same epithelium cells in the pancreas. In addition, insulin-producing cells also secrete somatostatin in early human embryonic development. Moreover, these insulin-producing cells also express nestin, generally considered the pancreatic stem cells marker.
These experiments revealed that pancreatic duct epithelial cells that can produce insulin in early human development are precursors and still have the potential to differentiate other endocrine cells. These progenitors have differentiated before migration from primary ductal epithelium to form the pancreatic islets.
This is an interesting descriptive study concerning an investigation about endocrine cells differentiation for pancreatic development in China.
Peer reviewers: Undurti Narasimha Das, MD, Professor, UND Life Sciences, 13800 Fairhill Road, Shaker Heights, OH 44120, United States
S- Editor Zhang HN L- Editor Roemmele A E- Editor Zhang L
| 1. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. |
| 2. | Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569-1580. |
| 3. | Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251-4259. |
| 4. | Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447-2457. |
| 5. | Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11-18. |
| 6. | Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163-176. |
| 7. | Holland AM, Hale MA, Kagami H, Hammer RE, MacDonald RJ. Experimental control of pancreatic development and maintenance. Proc Natl Acad Sci USA. 2002;99:12236-12241. |
| 8. | Alpert S, Hanahan D, Teitelman G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell. 1988;53:295-308. |
| 9. | Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. |
| 10. | Grapin-Botton A, Majithia AR, Melton DA. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 2001;15:444-454. |
| 11. | Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533-3542. |
| 12. | Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317-2322. |
| 13. | Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118:1031-1039. |
| 14. | Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533-5540. |
| 15. | Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213-2221. |
| 16. | Herrera PL, Orci L, Vassalli JD. Two transgenic approaches to define the cell lineages in endocrine pancreas development. Mol Cell Endocrinol. 1998;140:45-50. |
| 17. | Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521-533. |
| 18. | Fernandes A, King LC, Guz Y, Stein R, Wright CV, Teitelman G. Differentiation of new insulin-producing cells is induced by injury in adult pancreatic islets. Endocrinology. 1997;138:1750-1762. |
| 20. | Abraham EJ, Leech CA, Lin JC, Zulewski H, Habener JF. Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin-producing cells. Endocrinology. 2002;143:3152-3161. |