Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.107187
Revised: May 6, 2025
Accepted: June 5, 2025
Published online: July 15, 2025
Processing time: 111 Days and 0.5 Hours
Underdiagnosis of peripheral arterial disease results in inadequate treatment and more serious consequences. Hence, clinicians have focused on early diagnosis and treatment.
To investigate the effectiveness of the combination of doppler ultrasonography (DUS), three-dimensional dynamic contrast-enhanced magnetic resonance an
This study retrospectively analyzed the imaging and clinical data of 116 patients diagnosed with DM complicated with lower extremity vascular diseases from January 2021 to June 2023. All patients underwent unilateral or bilateral DUS, CTA, and CE-MRA as well as invasive digital subtraction angiography (DSA). The application values of DUS, CE-MRA, and CTA were compared.
A total of 152 lower extremity arteries in the 116 patients were graded following the classification of vascular branches. The Kappa values between DUS and DSA were 0.780, 0.755, and 0.806 for diagnosing moderate stenosis and 0.484, 0.699, and 0.449 for severe stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries, respectively. The Kappa values between CE-MRA and DSA were 0.784, 0.814, and 0.835 for diagnosing moderate stenosis and 0.694, 0.748, and 0.606 for severe stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries, respectively. The Kappa values between CTA and DSA were 0.900, 0.858, and 0.878 for diagnosing moderate stenosis and 0.882, 0.823, and 0.756 for severe stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries, respectively.
DUS, CE-MRA, and CTA demonstrated comparable accuracy in diagnosing lower extremity arterial disease in DM, and the consistency between CTA and DSA diagnoses was higher than the other two imaging methods.
Core Tip: Diabetes mellitus is a major risk factor for lower extremity peripheral arterial disease, and lower extremity complications are prevalent and increasing globally. However, peripheral arterial disease underdiagnosis results in undertreatment and subsequent complications with more serious consequences. Therefore, in this study we analyzed the diagnostic value of color doppler ultrasonography, magnetic resonance multimodal imaging, and CT angiography in diabetes mellitus complicated with lower limb arterial disease using digital subtraction angiography as the gold standard for diagnosing this disease.
- Citation: Bo Y, Xie J, Xu F, Yang G, Li DL, Yan XH. Comparison of three diagnostic imaging modalities for use in diabetic inferior arterial lesions. World J Diabetes 2025; 16(7): 107187
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/107187.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.107187
Peripheral arterial disease (PAD) of the lower limbs is a local lesion and is a local manifestation of systemic atherosclerosis[1]. Partial or complete occlusion of one or more arteries of the lower extremities primarily causes PAD and is associated with prevalent cardiovascular risk factors[2]. Individuals may be asymptomatic; however, symptoms typically involve intermittent claudication and chronic limb-threatening ischemia (with resting pain, ulceration, or gangrene)[3]. PAD of the lower extremities is endemic globally due to an aging population and an increase in cardiovascular risk factors such as hypertension, dyslipidemia, smoking, and type 2 diabetes mellitus (DM)[4].
DM is a major risk factor for PAD; thus, it is more prevalent in patients with PAD than in those with no PAD[5]. Further, PAD is one of the major complications of DM, and symptomatic PAD adversely affects the quality of life of patients[6]. Lower extremity complications are prevalent and are increasing globally, affecting approximately 131 million people with an estimated global prevalence of 1.8%[7]. They significantly increase morbidity and mortality in DM, resulting in leg ulcers and amputations in some cases, and are characterized by physical disability, decreased productivity, and mood disturbances. Neuropathy is a crucial cause of leg ulcers and amputations, and PAD is equally important[8]. However, PAD underdiagnosis results in inadequate treatment and more serious consequences. Therefore, clinicians have focused on early diagnosis and treatment.
Digital subtraction angiography (DSA) is the gold standard for PAD diagnosis[9]. However, DSA is an invasive test that causes complications in approximately 1.5% of patients, with a high cost of testing, which limits its clinical use. Further, non-invasive imaging examination is the most important assessment method for lower extremity vascular diseases. Considering the continuous development of medical technology, imaging modalities mainly include doppler ultrasonography (DUS)[10], three-dimensional (3D) dynamic contrast-enhanced magnetic resonance angiography (CE-MRA)[11], and CT angiography (CTA)[12]. Each modality demonstrates its own merits, with no comprehensive assessment criterion for selecting a particular approach. Hence, the novelty of the study was to comprehensively evaluate non-invasive diagnostic methods in selected patients with DM complicated with lower extremity arterial disease (LEAD) and assessed the efficacy of DUS, CE-MRA, and CTA to help in the early diagnosis and treatment of LEAD in patients with DM in the future.
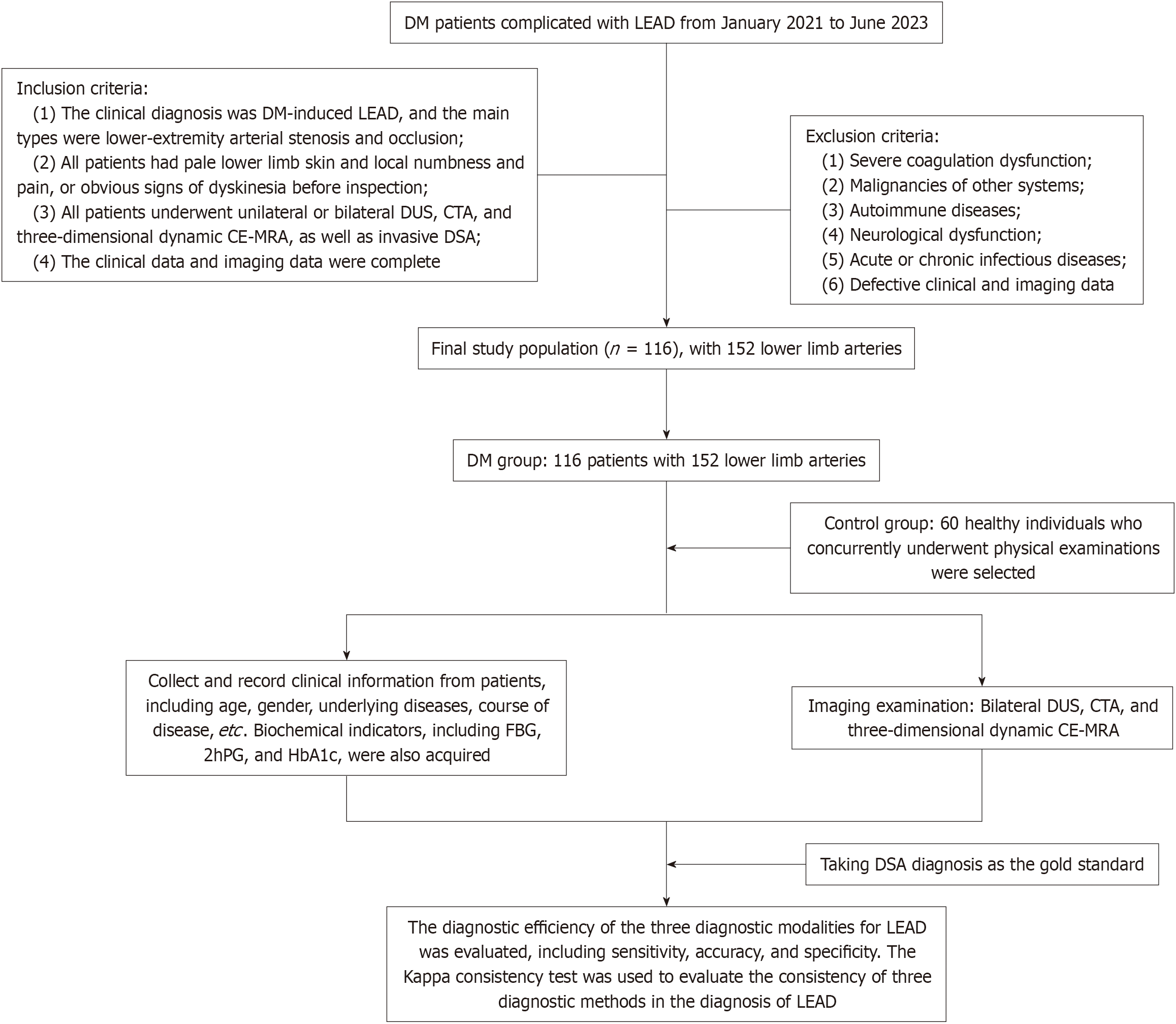
This study retrospectively analyzed the imaging and clinical data of patients with DM complicated with lower extremity vascular diseases diagnosed at Chongqing Kang Hua Zhong Lian Cardiovascular Hospital from January 2021 to June 2023. Inclusion criteria were: (1) The clinical diagnosis of DM-induced LEAD, with the main types of lower extremity arterial stenosis and occlusion; (2) All patients with pale lower limb skin and local numbness and pain or obvious signs of dyskinesia before inspection; (3) All patients who underwent unilateral or bilateral DUS, CTA, and CE-MRA as well as invasive DSA; and (4) Complete clinical and imaging data. Exclusion criteria were: (1) Severe coagulation dysfunction; (2) Malignancies of other systems; (3) Autoimmune diseases; (4) Neurological dysfunction; (5) Acute or chronic infectious diseases; and (6) Missing clinical and imaging data. The control group included another 60 healthy individuals who concurrently underwent physical examinations. A total of 116 eligible patients were finally included, with 152 lower limb arteries. Figure 1 illustrates the flow chart of the study.
General data (age, gender, underlying diseases, disease course, etc.) of patients and controls were obtained by reviewing the electronic medical records. Biochemical indicators, including fasting blood glucose, 2-h postprandial blood glucose, and glycosylated hemoglobin, were acquired.
DSA: The Philips Allura Xper FD20 angiography system was utilized. Seldinger’s technique was used to puncture the affected or contralateral radial artery or femoral artery, and retrograde or anterograde intubation was conducted until reaching the affected limb artery for routine DSA.
DUS: DUS was performed using the Philips IE22 ultrasound system. The lower limb arteries of the patients in a supine or prone position were observed through ultrasound images, including lumen stenosis, lumen occlusion, and plaques, and the smoothness of the vascular lumen–intima was analyzed.
CTA: Somatom Definition AS 128-slice 64-row CT scanner (Siemens, Germany) was employed. The patient was placed in a dorsal elevated and supine position for scanning from the abdomen to the dorsum pedis. The automatic bolus tracking technique was used for scanning after injecting 80 mL of iopromide (300 mg/mL), a contrast medium, into the anterior elbow vein of the patient with a high-pressure syringe at a flow rate of 4.0 mL/sec and 30 mL of normal saline administration at 4.0 mL/sec. After completing the scanning process, the images during the optimal diastolic and systolic phases were selected for automatic reconstruction.
CE-MRA: CE-MRA was performed with the German Siemens Skyra 3.0T magnetic resonance imaging machine. In the first step, with the patient in a supine or dorsal elevated position, 3D rapid gradient-echo imaging was performed. Gadobenate dimeglumine (20 mL) was pushed through the anterior cubital vein with a high-pressure syringe at a speed of 3.0 mL/sec. The second step was to push normal saline (30 mL) with the same injection rate. The area from the dorsal artery to the abdominal aorta was scanned using automatic table movement and segmentation techniques and time-resolved imaging of contrast kinetics. The image acquisition involved three segments, namely from the calf to the dorsal foot artery, from the abdominal aorta to the pelvic artery, and from the femoral artery to the popliteal artery.
To obtain the highest quality images, a team consisting of one senior and one junior physician in the Angiography Unit of the Department of Ultrasound, who had received specialized training in standardized examination of lower extremity arteries, performed all ultrasound or imaging assessments in this study. The examination procedures and points were consistent. Further, two radiologists with > 10 years of work experience conducted image assessments. Vascular branches involve three grades: Grade 1 indicates the superior geniculate artery, including the superficial femoral artery, external iliac artery, and common iliac artery; grade 2 denotes the inferior genicular artery, including the popliteal artery, posterior tibial artery, anterior tibial artery, and peroneal artery; grade 3 represents the dorsal aorta. Vascular lesions were classified as mild stenosis (< 50% stenosis), moderate stenosis (50%-75% stenosis), and severe stenosis or occlusion (> 75% stenosis).
Statistical Package for the Social Sciences version 25.0 statistical software was used to sort out and analyze data. Count data were presented as cases or percentages, and the χ2 test was conducted for between-group comparisons. Continuous data were all expressed as mean ± SD, and the comparison of means between groups was performed using the t-test. Considering DSA diagnosis as the gold standard, the diagnostic efficiency of the three diagnostic modalities for LEAD was assessed, including sensitivity, accuracy, and specificity. The Kappa consistency test was conducted to assess the consistency of three diagnostic methods in diagnosing LEAD.
The DM group consisted of 54 males and 62 females, with an average age of 53.62 ± 3.37 years and an average course of 7.35 ± 0.41 years. Patients with DM demonstrated statistically higher fasting blood glucose, 2-h postprandial blood glucose, and glycosylated hemoglobin levels than healthy individuals (P < 0.05; Table 1).
| Characteristics | DM group (n = 116) | Control group (n = 60) | χ2/t | P value |
| Age (year) | 53.62 ± 3.37 | 53.78 ± 3.09 | 0.307 | 0.759 |
| Gender | 2.158 | 0.142 | ||
| Male | 54 | 21 | ||
| Female | 62 | 39 | ||
| Hyperlipidemia | 41 | 19 | 0.238 | 0.626 |
| Hypertension | 54 | 23 | 1.085 | 0.297 |
| Coronary heart disease | 38 | 16 | 0.690 | 0.406 |
| Course of DM (year) | 7.35 ± 0.41 | |||
| Fasting blood glucose (mmol/L) | 8.17 ± 1.04 | 4.36 ± 0.59 | 26.253 | < 0.0001 |
| 2-h postprandial blood glucose (mmol/L) | 12.87 ± 1.48 | 7.01 ± 0.63 | 29.296 | < 0.0001 |
| Glycosylated hemoglobin (%) | 9.81 ± 0.67 | 5.41 ± 0.52 | 44.399 | < 0.0001 |
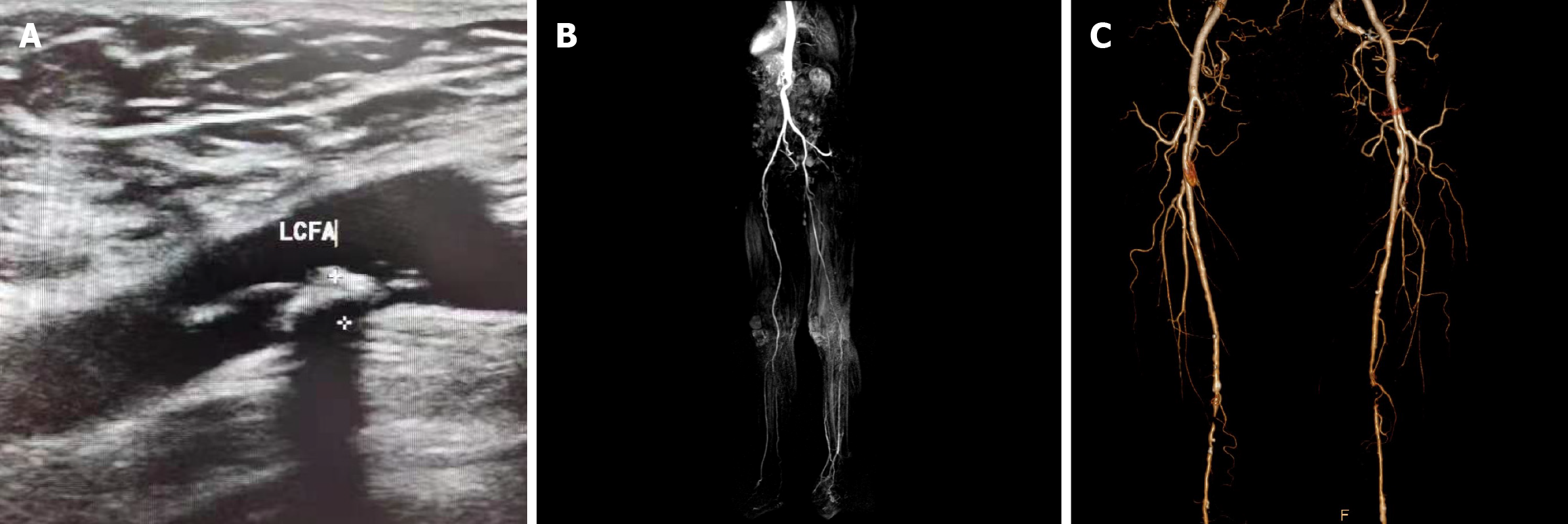
Table 2 shows the DSA diagnosis of 152 lower extremity arteries in 116 patients with DM based on arterial branch classification. A total of 78, 51, and 23 grade 1 arteries, 50, 53, and 49 grade 2 arteries, and 85, 47, and 20 grade 3 arteries demonstrated mild, moderate, and severe stenosis or occlusion, respectively. Figure 2 illustrates the imaging diagnosis results of different arterial branches in patients with DM complicated with LEAD.
| Arterial branch classification | Mild stenosis | Moderate stenosis | Severe stenosis or occlusion | Total |
| Grade 1 | 78 | 51 | 23 | 152 |
| Grade 2 | 50 | 53 | 49 | 152 |
| Grade 3 | 85 | 47 | 20 | 152 |
The sensitivity of DUS in diagnosing moderate stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries were 86.3%, 84.9%, and 91.5%, with specificity of 92.1%, 90.9%, and 91.4% and accuracy of 90.1%, 88.8%, and 91.4%, respectively. The Kappa values between DUS and DSA for diagnosing moderate stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries were 0.780, 0.755, and 0.806, respectively.
The sensitivity of DUS in diagnosing severe stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries was 82.6%, 79.6%, and 70.0%, with specificity of 82.2%, 90.3%, and 86.4% and accuracy of 82.2%, 86.8%, and 84.2%, respectively. The Kappa values of DUS vs DSA in diagnosing severe stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries were 0.484, 0.699, and 0.449, respectively (Table 3).
| Degree of stenosis | Arterial branch classification | DUS diagnosis | DSA diagnosis | Kappa | Sensitivity (%) | Specificity (%) | Accuracy (%) | |
| Moderate/severe | Non-moderate/non-severe | |||||||
| Moderate stenosis | Grade 1 | Moderate | 44 | 8 | 0.780 | 86.3 | 92.1 | 90.1 |
| Non-moderate | 7 | 93 | ||||||
| Grade 2 | Moderate | 45 | 9 | 0.755 | 84.9 | 90.9 | 88.8 | |
| Non-moderate | 8 | 90 | ||||||
| Grade 3 | Moderate | 43 | 9 | 0.806 | 91.5 | 91.4 | 91.4 | |
| Non-moderate | 4 | 96 | ||||||
| Severe stenosis or occlusion | Grade 1 | Severe | 18 | 23 | 0.484 | 82.6 | 82.2 | 82.2 |
| Non-severe | 5 | 106 | ||||||
| Grade 2 | Severe | 39 | 10 | 0.699 | 79.6 | 90.3 | 86.8 | |
| Non-severe | 10 | 93 | ||||||
| Grade 3 | Severe | 14 | 18 | 0.449 | 70.0 | 86.4 | 84.2 | |
| Non-severe | 6 | 114 | ||||||
The sensitivity of CE-MRA in diagnosing grade 1 arteries, grade 2 arteries, and grade 3 arteries with moderate stenosis were 90.2%, 90.6%, and 93.6%, with specificity of 90.1%, 91.9%, and 92.4% and accuracy of 90.1%, 91.4%, and 92.8%, respectively. The Kappa values between CE-MRA and DSA in diagnosing moderate stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries were 0.784, 0.814, and 0.835, respectively.
The sensitivity of CE-MRA in diagnosing severe stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries was 82.6%, 85.7%, and 80.0%, with specificity of 93.0%, 90.3%, and 90.9% and accuracy of 91.4%, 88.8%, and 89.5%, respectively. The Kappa values for CE-MRA diagnosis of severe stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries were 0.694, 0.748, and 0.606, respectively (Table 4).
| Degree of stenosis | Arterial branch classification | CE-MRA diagnosis | DSA diagnosis | Kappa | Sensitivity (%) | Specificity (%) | Accuracy (%) | |
| Moderate/severe | Non-moderate/non-severe | |||||||
| Moderate stenosis | Grade 1 | Moderate | 46 | 10 | 0.784 | 90.2 | 90.1 | 90.1 |
| Non-moderate | 5 | 91 | ||||||
| Grade 2 | Moderate | 48 | 8 | 0.814 | 90.6 | 91.9 | 91.4 | |
| Non-moderate | 5 | 91 | ||||||
| Grade 3 | Moderate | 44 | 8 | 0.835 | 93.6 | 92.4 | 92.8 | |
| Non-moderate | 3 | 97 | ||||||
| Severe stenosis or occlusion | Grade 1 | Severe | 19 | 9 | 0.694 | 82.6 | 93.0 | 91.4 |
| Non-severe | 4 | 120 | ||||||
| Grade 2 | Severe | 42 | 10 | 0.748 | 85.7 | 90.3 | 88.8 | |
| Non-severe | 7 | 93 | ||||||
| Grade 3 | Severe | 16 | 12 | 0.606 | 80.0 | 90.9 | 89.5 | |
| Non-severe | 4 | 120 | ||||||
The sensitivity of CTA in diagnosing moderate stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries was 100.0%, 94.3%, and 93.6%, with specificity of 93.1%, 92.9%, and 95.2% and accuracy of 95.4%, 93.4%, and 94.7%, respectively. The Kappa values between CTA and DSA for diagnosing moderate stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries were 0.900, 0.858, and 0.878, respectively.
The sensitivity of CTA in diagnosing severe stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries was 100.0%, 91.8%, and 85.0%, with specificity of 96.1%, 92.2%, and 95.5% and accuracy of 96.7%, 92.1%, and 94.1%, respectively. The Kappa values between CTA and DSA for diagnosing grade 1 arteries, grade 2 arteries, and grade 3 arteries with stenosis were 0.882, 0.823, and 0.756, respectively (Table 5).
| Degree of stenosis | Arterial branch classification | CTA diagnosis | DSA diagnosis | Kappa | Sensitivity (%) | Specificity (%) | Accuracy (%) | |
| Moderate/sever | Non-moderate/non-severe | |||||||
| Moderate stenosis | Grade 1 | Moderate | 51 | 7 | 0.900 | 100.0 | 93.1 | 95.4 |
| Non-moderate | 0 | 94 | ||||||
| Grade 2 | Moderate | 50 | 7 | 0.858 | 94.3 | 92.9 | 93.4 | |
| Non-moderate | 3 | 92 | ||||||
| Grade 3 | Moderate | 44 | 5 | 0.878 | 93.6 | 95.2 | 94.7 | |
| Non-moderate | 3 | 100 | ||||||
| Severe stenosis or occlusion | Grade 1 | Severe | 23 | 5 | 0.882 | 100.0 | 96.1 | 96.7 |
| Non-severe | 0 | 124 | ||||||
| Grade 2 | Severe | 45 | 8 | 0.823 | 91.8 | 92.2 | 92.1 | |
| Non-severe | 4 | 95 | ||||||
| Grade 3 | Severe | 17 | 6 | 0.756 | 85.0 | 95.5 | 94.1 | |
| Non-severe | 3 | 126 | ||||||
The incidence of DM is rapidly increasing. More than 700 million individuals globally are predicted to suffer from DM by 2045[13]. LEAD is a complication of DM and exhibits no obvious clinical symptoms in the early stage[14], making it an important public health problem. DSA is the gold standard for the clinical diagnosis of LEAD, but its invasiveness may cause vascular injury and other complications that are unacceptable to patients.
DUS, CE-MRA, and CTA, all non-invasive imaging modalities, have their merits and demerits in diagnosing LEAD comorbid with DM. In imaging arterial diseases are characterized by a lipid-rich necrotic core, intraplaque hemorrhage, calcification, etc.[15]. The proximal superficial femoral artery is the most predominant site of femoral artery stenosis (41.1%)[16], followed by the common femoral artery. The most common compression site of the popliteal artery is the gastrocnemius head[17]. The most predominantly seen site of anterior tibial artery damage or occlusion is the distal two-thirds, causing dorsalis pedis artery ischemia and blood redistribution through posterior tibial artery anastomosis[18].
In this study the sensitivity of DUS and CE-MRA for severe stenosis of grade 3 arteries was lower than that of grade 1 arteries and grade 2 arteries. CTA exhibited high diagnostic efficiency for both patients with moderate and severe stenosis. CE-MRA provides good visualization and grading technique for arterial stenosis. One of the advantages of MRA is its non-invasive nature and is less operator-dependent than DUS and develops a comprehensive image of the entire artery. Unlike DSA and CT, MRA does not involve radiation exposure. However, the contrast agent used in MRA causes kidney diseases. In addition, intravenous contrast utilization induces mild adverse effects in patients with renal failure.
DUS is a superior vascular mapping tool because it is cost-effective and eliminates complications associated with iodine contrast agents and ionizing radiation, especially for patients with renal diseases[19]. Further, DUS is an important tool for detecting lipid-rich plaques[20], lumen stenosis, and arterial wall thickness that is used to diagnose focal stent stenosis[21].
CTA is another excellent technique to provide high-resolution tissue contrast imaging in vascular diseases. It utilizes an iodinated contrast agent, like DSA, but is less invasive with a low risk of kidney disease caused by contrast agents and radiation[22]. Further, benefits that outweigh the risks can still be used in patients with kidney disease[23].
However, as previously noted, different approaches have their advantages and disadvantages. MRA is better at mapping calcified arteries and reducing artifacts compared with CTA[24]. In the popliteal artery, CTA can overcome the anatomical limitations of the popliteal fossa (gastrocnemius muscle) observed in DUS and MRA[25].
The results of this study indicated that the accuracy of CTA in diagnosing moderate and severe stenosis of grade 1 arteries, grade 2 arteries, and grade 3 arteries has a high consistency with the DSA diagnostic results. Thus, the results indicated that CTA has very high diagnostic efficiency, consistent with the research results of most scholars at home and abroad[26,27]. This is because CTA can display changes in lower limb varicose veins, especially in the venous equilibrium phase. Further, with higher resolution CTA effectively reflects the situation of vascular calcification. Therefore, to improve the diagnostic value of DM complicated with LEAD, appropriate examination methods should be selected in clinical practice. We believe that CTA should be utilized in the examination of patients with DM with severe stenosis of grade 3 lower extremity arteries.
Foreign studies indicated that DSA should only be utilized as a pretreatment diagnosis and non-invasive imaging as a routine assessment[28], indicating that non-invasive imaging examination has significant guiding value in clinical applications. Another study revealed that DUS demonstrated a diagnostic accuracy of up to 95% for moderate and severe anastomosed or occluded lower limb arteries[29]. In this study, the accuracy of DUS for moderate stenosis arteries and the accuracy for severe stenosis arteries were all < 95% reported in the aforementioned studies. This may be closely associated with the personal operation and experience of imaging physicians.
The cost-effectiveness of different imaging methods in clinical practice still needs to be considered. De Vries et al[30] conducted a randomized trial and revealed that the use of CE-MRA over standard DUS for the initial workup of PAD reduced the number of additional imaging procedures by 42%, with no difference in total cost. However, MRA is more expensive and less broadly available in contrast to carotid DUS and CTA[31]. Further, 3D approaches are more precise than 2-dimensional ultrasonography, and the costly expense of 3D imaging equipment and software to assess a 3D image may restrict its practical use[32]. In conclusion, in selecting a non-invasive diagnostic method that improves diagnostic accuracy, a combination of all three can be used, but only if the use of a single diagnostic method is not clearly identifiable and if economic conditions allow.
Limitations of this study should be acknowledged. The included participants were limited and exclusively inpatients; thus, the single-center small sample size may affect the generalizability of the results. Further, some important characteristics of patients may not be recorded due to the nature of the retrospective study. In the future, a large sample size prospective multicenter study is warranted to further investigate the application of DUS, CE-MRA, and CTA in DM complicated with LEAD.
DUS, CE-MRA, and CTA all have high accuracy in diagnosing LEAD, and the diagnostic efficacy of CTA was superior over the other two imaging methods.
| 1. | Soyoye DO, Abiodun OO, Ikem RT, Kolawole BA, Akintomide AO. Diabetes and peripheral artery disease: A review. World J Diabetes. 2021;12:827-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (8)] |
| 2. | Takahara M. Diabetes Mellitus and Lower Extremity Peripheral Artery Disease. JMA J. 2021;4:225-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Aboyans V, Ricco JB, Bartelink ML, Björck M, Brodmann M, Cohner T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor R, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I. [2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS)]. Kardiol Pol. 2017;75:1065-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Correction to: Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation. 2021;144:e193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Akalu Y, Birhan A. Peripheral Arterial Disease and Its Associated Factors among Type 2 Diabetes Mellitus Patients at Debre Tabor General Hospital, Northwest Ethiopia. J Diabetes Res. 2020;2020:9419413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Bourrier M, Ferguson TW, Embil JM, Rigatto C, Komenda P, Tangri N. Peripheral Artery Disease: Its Adverse Consequences With and Without CKD. Am J Kidney Dis. 2020;75:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global Disability Burdens of Diabetes-Related Lower-Extremity Complications in 1990 and 2016. Diabetes Care. 2020;43:964-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (2)] |
| 8. | Herman WH, Kalyani RR, Wexler DJ, Matthews DR, Inzucchi SE. Response to Comment on American Diabetes Association. Approaches to Glycemic Treatment. Sec. 7. In Standards of Medical Care in Diabetes-2016. Diabetes Care 2016;39(Suppl. 1):S52-S59. Diabetes Care. 2016;39:e88-e89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Lapeyre M, Kobeiter H, Desgranges P, Rahmouni A, Becquemin JP, Luciani A. Assessment of critical limb ischemia in patients with diabetes: comparison of MR angiography and digital subtraction angiography. AJR Am J Roentgenol. 2005;185:1641-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Donohue CM, Adler JV, Bolton LL. Peripheral arterial disease screening and diagnostic practice: A scoping review. Int Wound J. 2020;17:32-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Bisgaard M, Houlind KC, Blankholm AD, Ringgaard S, Christensen J, Precht H. Validation of MRI assessment of foot perfusion for improving treatment of patients with peripheral artery disease. Radiography (Lond). 2024;30:1116-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Shwaiki O, Rashwan B, Fink MA, Kirksey L, Gadani S, Karuppasamy K, Melzig C, Thompson D, D'Amico G, Rengier F, Partovi S. Lower extremity CT angiography in peripheral arterial disease: from the established approach to evolving technical developments. Int J Cardiovasc Imaging. 2021;37:3101-3114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4813] [Article Influence: 1604.3] [Reference Citation Analysis (36)] |
| 14. | Tan J, Lv H, Ma Y, Liu C, Li Q, Wang C. Analysis of angiographic characteristics and intervention of vitamin D in type 2 diabetes mellitus complicated with lower extremity arterial disease. Diabetes Res Clin Pract. 2020;169:108439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Liu Y, Han Y, Guan M, Cai Y, Wang W, Chen H, Zhao X. Added value of femoral artery atherosclerosis for determining severity of white matter lesion by carotid atherosclerosis: a magnetic resonance imaging study. Acta Radiol. 2021;62:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Gao M, Zhao X, Tao Y, Wang L, Xia M, Tong Z, Hou C, Hua Y. Incidence and Predictors of In-stent Re-Stenosis in the Superficial Femoral Artery: Evaluation of Long-Term Outcomes by Color Duplex Ultrasound. Ultrasound Med Biol. 2016;42:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Lovelock T, Claydon M, Dean A. Functional Popliteal Artery Entrapment Syndrome: An Approach to Diagnosis and Management. Int J Sports Med. 2021;42:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Cang ZQ, Xu Y, Wang M, Xu MN, Yuan SM. Anterior tibial artery injury is not the contraindication of medial plantar flap: digital subtraction angiography evidence and clinical application. J Plast Reconstr Aesthet Surg. 2021;74:2512-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Niu L, Zhang F, Luo X, Feng Y, Zhang C. Ultrasound-Guided Retrograde Infrapopliteal Artery Access for Recanalization of Complex Femoral-Popliteal Artery Occlusions. Ann Vasc Surg. 2021;76:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Fedak A, Ciuk K, Urbanik A. Ultrasonography of vulnerable atherosclerotic plaque in the carotid arteries: B-mode imaging. J Ultrason. 2020;20:e135-e145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Rteil A, Draxler M, Al Adas Z, Mohammad F, Kavousi Y, Kabbani L. Progressive stenosis of a popliteal artery stent graft by laminated thrombus. J Vasc Surg Cases Innov Tech. 2020;6:189-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Marko X, Peña CS. Computed Tomography of Acquired Aortic Diseases. Radiol Clin North Am. 2019;57:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Onaca N, Martinez E, Bayer J, Wall A, Fernandez H, Ruiz R, Ma TW, Gupta A, McKenna G, Testa G. Selective screening imaging of the aortoiliac arterial system in kidney transplant candidates with non-contrast pelvic computed tomography. Clin Transplant. 2021;35:e14331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Stoumpos S, Hennessy M, Vesey AT, Radjenovic A, Kasthuri R, Kingsmore DB, Mark PB, Roditi G. Ferumoxytol-enhanced magnetic resonance angiography for the assessment of potential kidney transplant recipients. Eur Radiol. 2018;28:115-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Altintas U, Helgstrand UV, Hansen MA, Stentzer KF, Schroeder TV, Eiberg JP. Popliteal artery entrapment syndrome: ultrasound imaging, intraoperative findings, and clinical outcome. Vasc Endovascular Surg. 2013;47:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Martinelli O, Alunno A, Drudi FM, Malaj A, Irace L. Duplex ultrasound versus CT angiography for the treatment planning of lower-limb arterial disease. J Ultrasound. 2021;24:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Zhang S, Wu Y, Guo Y, Jia X, Kang Y, Shen X, Song J, Yang A. Application opportunity of Doppler ultrasound combined with CT angiography in diabetic lower extremity arterial disease and the analysis of the risk factors. Front Endocrinol (Lausanne). 2023;14:1257241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Muhlestein JB, Lappé DL, Lima JA, Rosen BD, May HT, Knight S, Bluemke DA, Towner SR, Le V, Bair TL, Vavere AL, Anderson JL. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014;312:2234-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 29. | Jiang L, Zhao Y. The value of color Doppler ultrasound in the diagnosis of lower extremity vascular disease in type 2 diabetes and an analysis of related factors. Minerva Endocrinol. 2017;42:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | de Vries M, Ouwendijk R, Flobbe K, Nelemans PJ, Kessels AG, Schurink GH, van der Vliet JA, Heijstraten FM, Cuypers PW, Duijm LE, van Engelshoven JM, Hunink MG, de Haan MW. Peripheral arterial disease: clinical and cost comparisons between duplex US and contrast-enhanced MR angiography--a multicenter randomized trial. Radiology. 2006;240:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Bir SC, Kelley RE. Carotid atherosclerotic disease: A systematic review of pathogenesis and management. Brain Circ. 2022;8:127-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 32. | Laksono S, Kusharsamita H. Unravelling the role of carotid atherosclerosis in predicting cardiovascular disease risk: A review. ARYA Atheroscler. 2024;20:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |