Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.106903
Revised: April 3, 2025
Accepted: June 18, 2025
Published online: July 15, 2025
Processing time: 125 Days and 0.8 Hours
Lung cancer (LC) is one of the most prevalent cancers globally, with a high incidence among the elderly population. Elderly patients, particularly those with diabetes mellitus, are at an increased risk of postoperative complications, in
To develop and validate a predictive model for PPI in elderly patients with dia
This retrospective study included 212 patients with LC who received treatment at our hospital from March 2015 to March 2022. General clinical information, sur
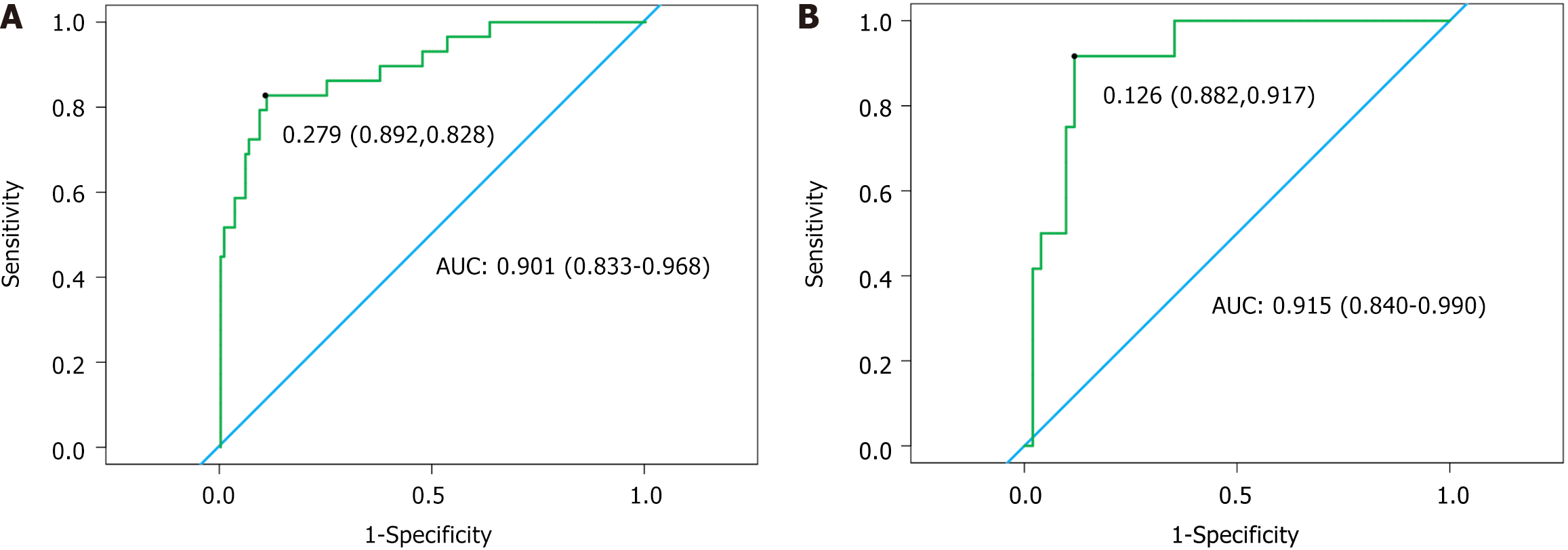
Among the 212 patients [median age: 72 years (interquartile range: 60-82 years)], 41 developed PPI (19.34%), with Gram-negative bacteria being the predominant pathogens (64.14%). Factors, such as age of ≥ 70 years, presence of respiratory diseases, maximum tumor diameter of ≥ 4 cm, stages II-III, receiving neoadjuvant chemotherapy of ≥ 2 times preoperatively, surgery duration of ≥ 3 hours, chest drainage tube placement duration of ≥ 3.5 days, preoperative fasting blood glucose levels, hemoglobin A1c (HbA1c) levels, and multi-leaf resection, were markedly higher in the infection group than in the non-infection group. Conversely, forced expiratory volume in 1 second (FEV1) of ≥ 80% and albumin (Alb) levels were lower in the infection group. Multivariate logistic regression analysis revealed that receiving neoadjuvant chemotherapy of ≥ 2 times [odds ratio (OR) = 2.987; P = 0.036], maximum tumor diameter of ≥ 4 cm (OR = 3.959; P = 0.013), multi-leaf resection (OR = 3.18; P = 0.036), preoperative FEV1 of ≤ 80% (OR = 3.305; P = 0.029), and high HbA1c levels (OR = 2.39; P = 0.003) as key risk factors for PPI, whereas high Alb levels (OR = 0.507; P < 0.001) was protective. The nomogram model demonstrated excellent diagnostic ability (area under the curve = 0.901, 0.915), and calibration curves and decision curve analysis revealed good predictive performance and clinical applicability of the model.
The primary pathogens of PPI in elderly patients with diabetes and LC undergoing thoracoscopic radical resection are Gram-negative bacteria. The nomogram model, based on preoperative neoadjuvant chemotherapy cycles, maximum tumor diameter, range of resection, and preoperative FEV1, Alb, and HbA1c levels, shows high clinical value in predicting the risk of PPI in this patient population.
Core Tip: This study analyzed the risk factors for postoperative pulmonary infection (PPI) in elderly patients with diabetes undergoing thoracoscopic radical resection of lung cancer and established a predictive model. The study revealed that the primary pathogens of PPI were Gram-negative bacteria (64.14%). Independent risk factors for PPI included receiving neoadjuvant chemotherapy of ≥ 2 times, maximum tumor diameter of ≥ 4 cm, multi-lobe resection, preoperative forced expiratory volume in 1 second of ≤ 80%, and high hemoglobin A1c levels, whereas high albumin levels were determined as a protective factor. The nomogram model established based on these factors demonstrated high predictive performance (area under the curve = 0.901, 0.915), effectively assessing the risk of postoperative infection and providing individualized prevention and management strategies for clinical practice.
- Citation: Chen ZY, Hong ZQ, Wang TQ, Fu GMZ, Su WM, Zhou CW. Risk factors for pulmonary infection after thoracoscopic radical resection of lung cancer in elderly patients with diabetes mellitus. World J Diabetes 2025; 16(7): 106903
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/106903.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.106903
Lung cancer (LC) is one of the most prevalent cancers globally and has consistently ranked as the second most prevalent, surpassed only by breast cancer. In 2020, approximately 2.2 million new LC cases were reported globally, accounting for 11.4% of all cancer diagnoses[1]. The accelerated pace of global industrialization, worsening environmental pollution, and increased number of smokers have all significantly raised the risk of LC[2]. Further, the growing issue of population aging has continuously increased LC incidence. The weakened immune function and diminished tissue repair capability in the elderly make them more vulnerable to these risk factors, causing a notably higher LC incidence among them, particularly in China.
Currently, the main treatment modalities for LC include radiotherapy, chemotherapy, and surgical intervention, with surgery frequently considered the most effective option for patients with early-stage LC[3]. However, surgery causes postoperative complications that affect patient outcomes, with pulmonary infections being among the most prevalent. Postoperative pulmonary infection (PPI) in patients with LC triggers systemic inflammation and causes severe complications, such as acute respiratory distress syndrome or sepsis, thereby exacerbating the patient’s condition and reducing survival rates[4]. Moreover, infections may change the tumor microenvironment, thereby increasing the risk of tumor recurrence and further worsening the patient’s long-term prognosis[5]. Further, the presence of infections slows down the recovery process, causing extended hospital stays, impaired physical recovery, and potential adjustments to subsequent treatment plans, such as delaying or reducing treatment intensity, which affects overall treatment outcomes. This issue is particularly pronounced in elderly patients with diabetes, where hyperglycemia causes immunosuppression and weakened leukocyte function, making them more susceptible to postoperative infections. In China, the prevalence of diabetes is increasing, with over 120 million people affected nationwide. Among them, elderly patients with diabetes represent a significant proportion, accounting for approximately 60% of the total diabetic population[6]. Therefore, determining and managing the risk of postoperative infections is crucial for improving the treatment outcomes of patients with diabetes and LC.
Current studies demonstrated considerable variation in assessing the risk factors for PPIs in patients with LC. In particular, some research indicates that gender, advanced age, prolonged surgery duration, diabetes, and forced expiratory volume in 1 second (FEV1)/forced vital capacity ratio are risk factors for postoperative infections in patients with LC[7]. Other studies indicate that surgical methods, postoperative pain management, and inspired oxygen concentration are crucial factors that induce pulmonary infections[8]. Further, existing studies have investigated the effect of diabetes on postoperative infections; however, the specific association between diabetes and PPIs in patients with LC remains unclear. The current literature lacks a systematic investigation into the interaction between diabetes and PPIs in elderly patients with LC, and no effective predictive model has been established to guide clinical practice. Hence, developing a predictive model for PPI in patients with diabetes and LC is of significant clinical importance. Accurate risk assessment helps clinicians devise more personalized management strategies, optimize postoperative care protocols, and reduce infection rates, thereby improving patient prognosis and efficiently allocating healthcare resources.
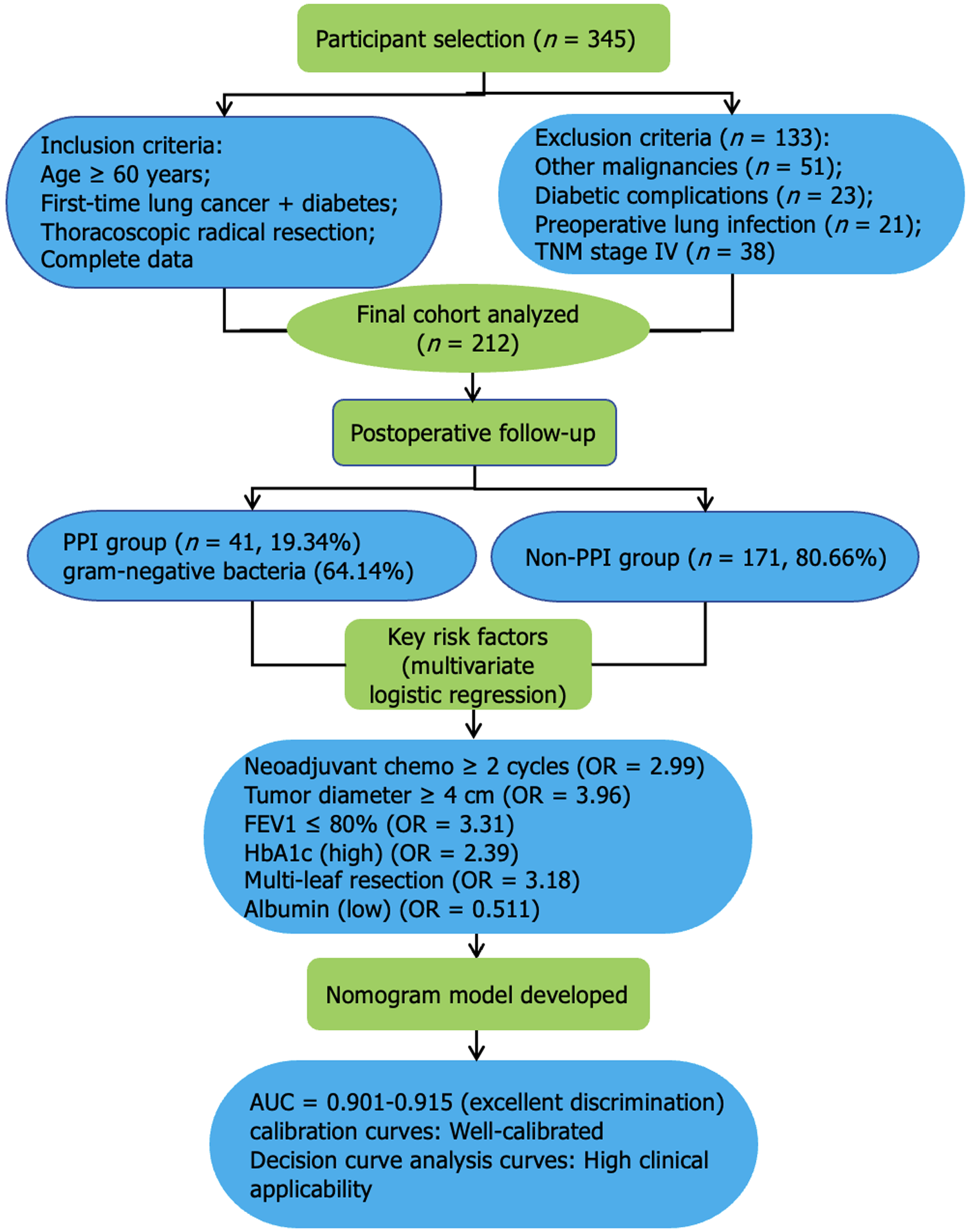
This study retrospectively analyzed patients with LC admitted to our hospital from March 2015 to March 2022 (Figure 1).
Inclusion criteria: (1) LC diagnosis based on the Guidelines for the Diagnosis and Treatment of Primary LC (2022 edition)[9]; (2) First-time LC diagnosis; (3) Age of ≥ 60 years; (4) Thoracoscopic radical resection for LC; (5) Diabetes diagnosis based on the Chinese Guidelines for the Diagnosis and Treatment of Elderly Diabetes (2024 edition)[10]; and (6) Complete clinical data availability.
Exclusion criteria: (1) Combined with other systemic malignancies; (2) Combined with diabetic complications, such as diabetic foot or diabetic nephropathy; (3) History of lung surgery; (4) Presence of preoperative pulmonary infection; (5) Underwent tracheostomy; and (6) Tumor node metastasis (TNM) classification stage IV.
Infection diagnostic criteria: (1) Symptomatic criteria: New onset of fever, purulent sputum, or worsening dyspnea; (2) Physical examination: New findings of wet rales or localized bronchial breath sounds on auscultation; (3) Imaging examination: New pulmonary infiltrates on chest X-ray or computed tomography scan; and (4) Laboratory findings: Significant serum inflammatory marker elevation, particularly white blood cell count (WBC) and C-reactive protein (CRP) levels.
Diabetes diagnostic criteria: Diagnostic criteria included fasting blood glucose (FBG) of ≥ 7.0 mmol/L or hemoglobin A1c (HbA1c) of ≥ 6.5%.
A total of 212 patients were selected and categorized into the infection (n = 41) and non-infection groups (n = 171) based on PPI occurrence.
Patients were placed in a lateral position under general anesthesia and single-lung ventilation. Three small incisions were established in the appropriate intercostal spaces on the patient’s chest wall after routine disinfection and draping: One for inserting the high-definition thoracoscope and the other two for the operation of surgical instruments. The thoracoscope provided an excellent view of the thoracic cavity. The lung hilum was carefully dissected to expose the pulmonary artery, pulmonary veins, and bronchi. Major vessels and bronchi were progressively dissected and divided with clamping and cutting devices to ensure clean incisions and prevent bleeding. Subsequently, the affected lung lobe was completely removed, ensuring that the resection margin was free of tumor tissue to achieve a safe surgical boundary. Hilar and mediastinal lymph nodes were thoroughly cleaned to reduce the risk of postoperative metastasis. Intraoperatively, bleeding points were promptly managed with electrocautery and titanium clips to maintain a clear surgical field.
The resected specimen was stored in a waterproof bag and removed through an auxiliary incision for pathological examination. The thoracic cavity was carefully rinsed after confirming the absence of active bleeding and significant residual lesions, and a chest drain was placed to drain postoperative effusion and gas. Incisions were closed in layers and covered with sterile dressings. The surgery went smoothly, and the patient’s vital signs remained stable upon return to the ward for observation.
Age, sex, body mass index (BMI), smoking history, drinking history, hypertension, hyperlipidemia, respiratory diseases (including mild chronic obstructive pulmonary disease, stable bronchial asthma, and mild pleural effusion), TNM classification stage, maximum tumor diameter, number of neoadjuvant chemotherapy cycles, and pathological type were collected and compared.
Surgical-related data included operative time, intraoperative blood loss, resection range, thoracic drainage tube time, and preoperative FEV1. Laboratory data included preoperative WBC, CRP, albumin (Alb), FBG, and HbA1c levels. These data were collected and compared.
Sample collection: Sputum samples for determining pathogens in patients with PPI were collected after waking up in the morning. To reduce bacterial interference from the oral cavity, the patient first rinsed the mouth with water and then with saline. The patient was instructed to deep breath and cough out deep sputum, discarding the first sputum to prevent oral cavity and upper respiratory tract contamination. The second sputum sample was collected and stored in a sterile container. Samples for patients who are unable to cough up sputum independently were collected directly from the lower respiratory tract using a fiberoptic bronchoscope.
Pathogen testing: A microscopic examination at low power was conducted before sending the sputum samples for testing to ensure the sample quality, with each field having > 25 WBC and < 10 epithelial cells to confirm that the sample primarily originated from the lower respiratory tract. The sputum sample was then inoculated onto a culture medium and incubated under proper conditions. Microbes were identified with a microbial identification instrument (Mettler Toledo 7000RMS) for colony counting and determination. Pathogens were confirmed if the colony count exceeded 105 CFU/mL and the same pathogen was determined in two consecutive cultures. This process helps in diagnosing and identifying specific pathogens in PPIs, providing a basis for subsequent clinical treatment.
Statistical Package for the Social Sciences version 25.0 software was used for data processing. Categorical variables were presented as n (%), and group differences were analyzed with the χ² test. Continuous variables were represented as mean ± SD and compared with the independent samples t-test. Logistic regression was used to identify risk factors for PPI in elderly patients with diabetes and LC, and receiver operating characteristic (ROC) curves were constructed. The dataset from the multivariate logistic regression was randomly divided into training and validation sets in a 7:3 ratio. R software (version 4.4.1) with the rms package was used to establish a nomogram prediction model for postoperative infections. The model’s predictive accuracy and clinical decision-making capabilities were assessed with ROC, calibration, and decision curve analysis (DCA) curves. A P value of < 0.05 indicates statistical significance.
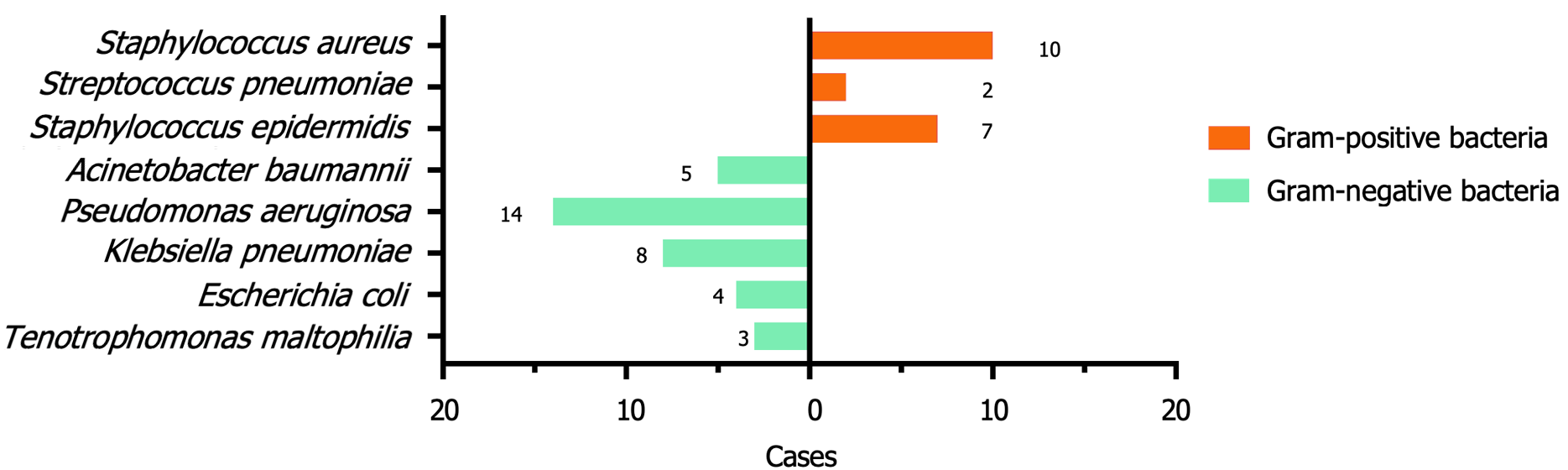
Among the 212 elderly patients with diabetes and LC, 41 developed PPIs, from which 53 strains of pathogens were isolated. The pathogens were predominantly Gram-negative bacteria, with 34 strains determined, among which Pseu
| Pathogenic bacteria | Number | Composition ratio (%) |
| Gram positive bacteria | 19 | 35.85 |
| Staphylococcus aureus | 10 | 18.87 |
| Streptococcus pneumoniae | 2 | 3.77 |
| Staphylococcus epidermidis | 7 | 13.21 |
| Gram negative bacteria | 34 | 64.15 |
| Acinetobacter baumannii | 5 | 9.43 |
| Pseudomonas aeruginosa | 14 | 26.42 |
| Klebsiella pneumoniae | 8 | 15.09 |
| Escherichia coli | 4 | 7.55 |
| Tenotrophomonas maltophilia | 3 | 5.66 |
No significant differences were observed between groups in terms of gender, BMI, smoking history, alcohol consumption history, hypertension, hyperlipidemia, or pathological types of LC. However, patients in the infection group were markedly more likely to be ≥ 70 years of age, have respiratory diseases, have tumors with a maximum diameter of ≥ 4 cm, be at stages II-III, and have received neoadjuvant chemotherapy of ≥ 2 times (Table 2).
| Items | Infected group (n = 41) | Non-infected group (n = 171) | t/χ2 value | P value | |
| Age (years old) | ≥ 70 | 27 (65.85) | 76 (44.44) | 4.767 | 0.029 |
| 60-69 | 14 (34.15) | 95 (55.55) | |||
| Sex (cases) | Male | 24 (58.54) | 112 (65.50) | 0.697 | 0.404 |
| Female | 17 (41.46) | 59 (34.50) | |||
| Body mass index (kg/m2) | 25.42 ± 2.13 | 24.79 ± 2.09 | 1.547 | 0.123 | |
| Smoking history | With | 23 (56.10) | 101 (59.06) | 0.120 | 0.729 |
| Without | 18 (43.90) | 70 (40.94) | |||
| Drinking history | With | 22 (53.66) | 79 (46.20) | 0.738 | 0.390 |
| Without | 19 (46.34) | 92 (53.80) | |||
| Hypertension | With | 27 (65.85) | 94 (54.97) | 1.599 | 0.206 |
| Without | 14 (34.15) | 77 (45.03) | |||
| Hyperlipidemia | With | 14 (34.15) | 82 (47.95) | 2.544 | 0.111 |
| Without | 27 (65.85) | 89 (52.05) | |||
| Respiratory diseases | With | 25 (60.98) | 71 (41.52) | 5.052 | 0.026 |
| Without | 16 (39.02) | 100 (58.48) | |||
| TNM stage | I stage | 16 (39.02) | 98 (57.31) | 4.448 | 0.035 |
| II-III stage | 25 (60.98) | 73 (42.69) | |||
| Maximum diameter of tumor | ≥ 4 cm | 22 (53.66) | 60 (35.09) | 4.809 | 0.028 |
| < 4 cm | 19 (46.34) | 111 (64.91) | |||
| Neoadjuvant chemotherapy | ≥ 2 times | 27 (65.85) | 64 (37.43) | 10.908 | < 0.001 |
| < 2 times | 14 (34.15) | 107 (62.57) | |||
| Pathological type | Squamous carcinoma | 22 (53.66) | 111 (64.91) | 1.792 | 0.181 |
| Adenocarcinoma | 19 (46.34) | 60 (35.09) | |||
No significant differences in intraoperative blood loss, preoperative WBC count, or preoperative CRP levels were found between groups. However, the infection group demonstrated significantly longer surgery times (≥ 3 hours), longer chest drain placement times (≥ 3.5 days), higher FBG and HbA1c levels, and a higher number of patients undergoing multi-lobe resection. Conversely, FEV1 and Alb levels were lower in the infection group (Table 3).
| Items | Infected group (n = 41) | Non-infected group (n = 171) | t/χ2 value | P value | |
| Operation time (hour) | ≥ 3 | 25 (60.98) | 72 (42.11) | 4.745 | 0.029 |
| < 3 | 16 (39.02) | 99 (57.89) | |||
| Intraoperative bleeding (mL) | ≥ 800 | 11 (26.83) | 38 (22.22) | 0.395 | 0.530 |
| < 800 | 30 (73.17) | 133 (77.77) | |||
| Extent of excision | Multi leaf | 28 (68.29) | 75 (43.86) | 7.903 | 0.005 |
| Single leaf | 13 (31.71) | 96 (56.14) | |||
| Thoracic drainage tube time (day) | ≥ 3.5 | 26 (63.41) | 61 (35.67) | 10.519 | < 0.001 |
| < 3.5 | 15 (36.59) | 110 (64.33) | |||
| Forced expiratory volume in one second (%) | < 80 | 28 (68.28) | 68 (39.77) | 10.861 | < 0.001 |
| ≥ 80 | 13 (31.71) | 103 (60.23) | |||
| White blood cell count (× 109/L) | 8.42 ± 1.39 | 8.79 ± 1.15 | -1.774 | 0.078 | |
| C-reactive protein (mg/L) | 3.42 ± 1.51 | 3.73 ± 1.45 | -1.220 | 0.224 | |
| Albumin (g/L) | 39.74 ± 1.72 | 41.87 ± 1.62 | -7.471 | < 0.001 | |
| Fasting blood glucose (mmol/L) | 7.02 ± 1.25 | 6.48 ± 1.21 | 2.55 | 0.011 | |
| Hemoglobin A1c (%) | 8.12 ± 1.04 | 7.12 ± 1.01 | 5.661 | < 0.001 | |
The following variables exhibited significant differences in univariate analysis and were assigned values: Age, TNM stage, respiratory diseases, neoadjuvant chemotherapy, maximum diameter of tumor, operative time, extent of resection, thoracic drainage tube time, FEV1, Alb, FBG, and HbA1c (Table 4).
| Factor | Variable | Assignment |
| Age | X1 | 60-69 years old, 0; ≥ 70 years old, 1 |
| TNM stage | X2 | I stage, 0; II-III stage, 1 |
| Respiratory diseases | X3 | No, 0; Yes, 1 |
| Neoadjuvant chemotherapy | X4 | < 2 times, 0; ≥ 2 times, 1 |
| Maximum diameter of tumor | X5 | < 4 cm, 0; ≥ 4 cm, 1 |
| Operation time | X6 | < 3 hours, 0; ≥ 3 hours, 1 |
| Range of excision | X7 | Single leaf, 0; multi leaf, 1 |
| Thoracic drainage tube time | X8 | < 3.5 days, 0; ≥ 3.5 days, 1 |
| Forced expiratory volume in one second | X9 | ≤ 80%, 0; > 80%, 1 |
| Albumin | X10 | Actual measurement value |
| Fasting blood glucose | X11 | Actual measurement value |
| Hemoglobin A1c | X12 | Actual measurement value |
Neoadjuvant chemotherapy of ≥ 2 times [odds ratio (OR) = 2.987; P = 0.036] and a maximum tumor diameter of ≥ 4 cm (OR = 3.959; P = 0.013) were significant risk factors for PPI. Further, multi-lobe resection, preoperative FEV1 of ≤ 80% (OR = 3.305; P = 0.029), and HbA1c (OR = 2.39; P = 0.003) were determined as risk factors for PPI, whereas high Alb levels (OR = 0.507; P < 0.001) were protective against infection (Table 5).
| Factor | P value | OR | 95%CI |
| Age | 0.063 | 2.735 | 0.948-7.89 |
| TNM stage | 0.084 | 2.486 | 0.886-6.973 |
| Respiratory diseases | 0.849 | 1.105 | 0.397-3.071 |
| Neoadjuvant chemotherapy | 0.036 | 2.987 | 1.072-8.318 |
| Maximum diameter of tumor | 0.013 | 3.959 | 1.341-11.69 |
| Operation time | 0.079 | 2.518 | 0.899-7.051 |
| Range of excision | 0.036 | 3.18 | 1.08-9.361 |
| Thoracic drainage tube time | 0.224 | 1.878 | 0.68-5.186 |
| Forced expiratory volume in one second | 0.029 | 3.305 | 1.129-9.673 |
| Albumin | 0.000 | 0.507 | 0.362-0.709 |
| Fasting blood glucose | 0.562 | 1.132 | 0.745-1.719 |
| Hemoglobin A1c | 0.003 | 2.39 | 1.346-4.241 |
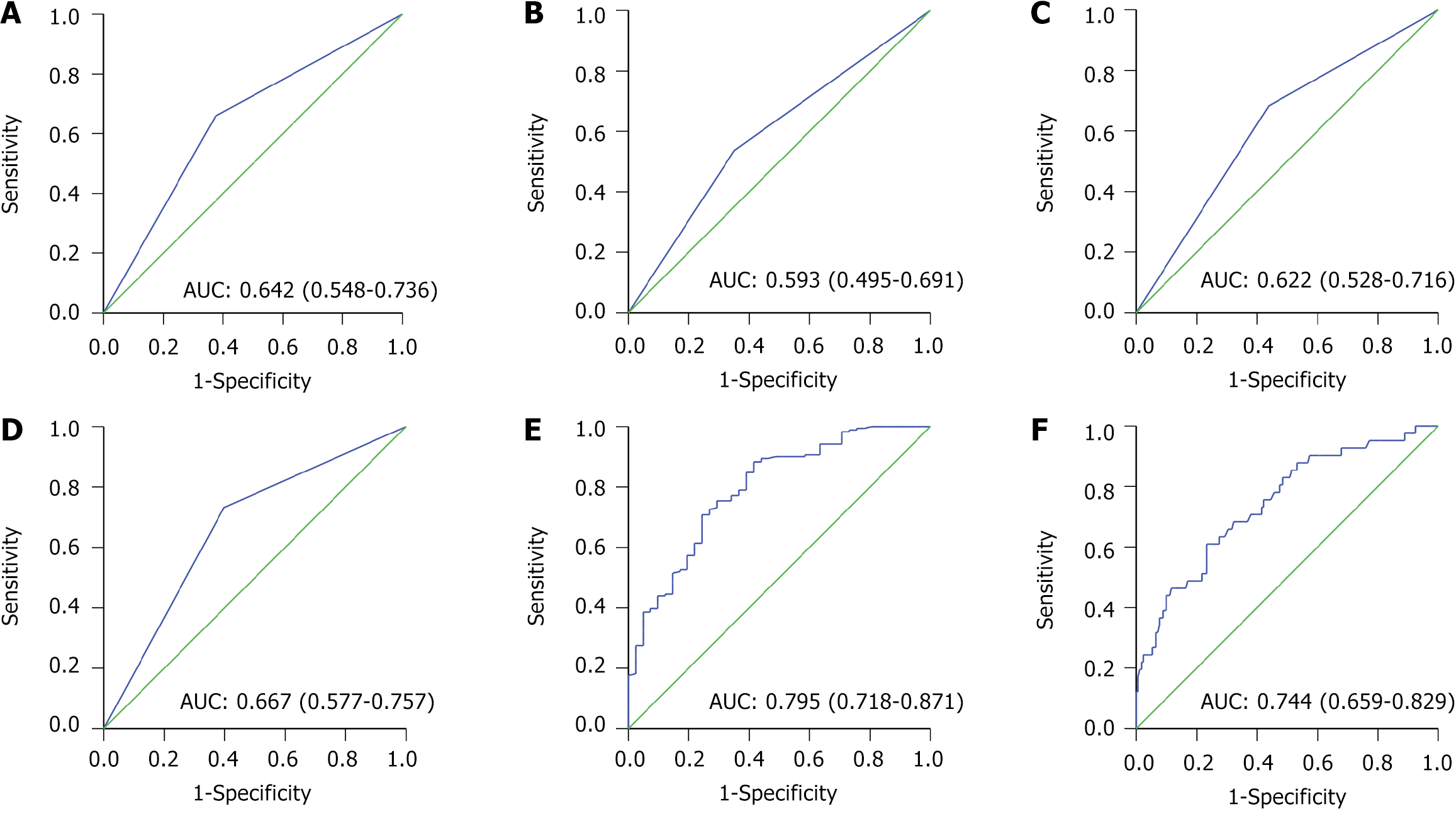
ROC curve analysis revealed that neoadjuvant chemotherapy, maximum tumor diameter, extent of resection, FEV1, Alb, and HbA1c demonstrated high predictive efficacy for PPI (Table 6 and Figure 3).
| Factor | AUC | SE | 95%CI |
| Neoadjuvant chemotherapy | 0.642 | 0.048 | 0.548-0.736 |
| Maximum diameter of tumor | 0.593 | 0.05 | 0.495-0.691 |
| Range of excision | 0.622 | 0.048 | 0.528-0.716 |
| Forced expiratory volume in one second | 0.667 | 0.046 | 0.577-0.757 |
| Albumin | 0.795 | 0.039 | 0.718-0.871 |
| Hemoglobin A1c | 0.744 | 0.044 | 0.659-0.829 |
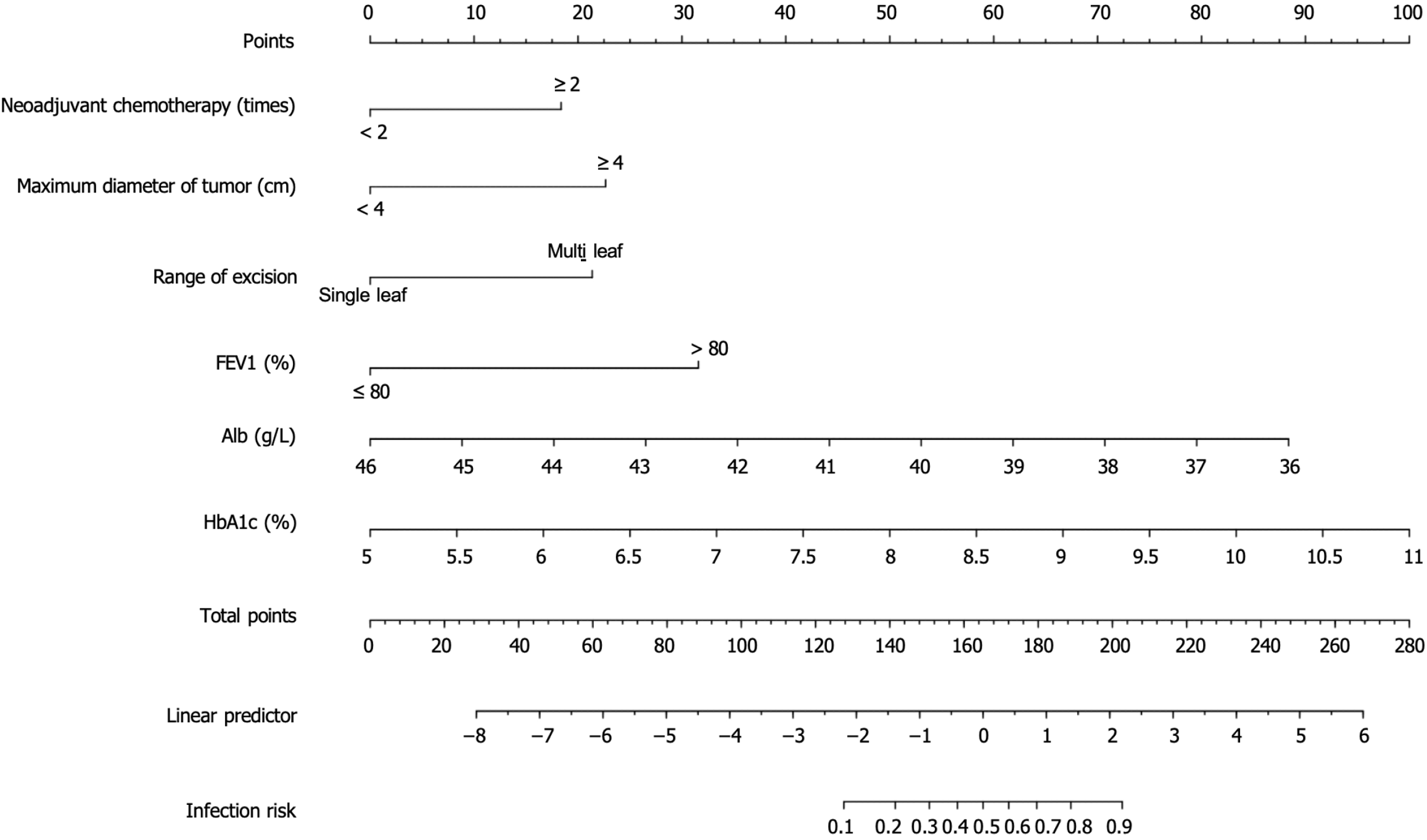
Nomogram development: The nomogram analysis revealed that the weights for various risk factors predicting PPI in elderly patients with diabetes and LC were as follows: Neoadjuvant chemotherapy of ≥ 2 times with 19 points, maximum tumor diameter of ≥ 4 cm with 21 points, multi-lobe resection with 20 points, FEV1 of ≤ 80% with 31 points, low Alb levels with 89 points, and high HbA1c levels with 100 points. These results indicate that high HbA1c levels have the greatest effect on the risk of PPI, followed by low Alb levels and FEV1 of ≤ 80% (Figure 4).
Validation of the nomogram prediction model: The ROC curve analysis of the model demonstrated an area under the curve (AUC) of 0.901 [95% confidence interval (CI): 0.833-0.968] for the training set and 0.915 (95%CI: 0.840-0.990) for the validation set, indicating excellent discriminatory ability (Figure 5).
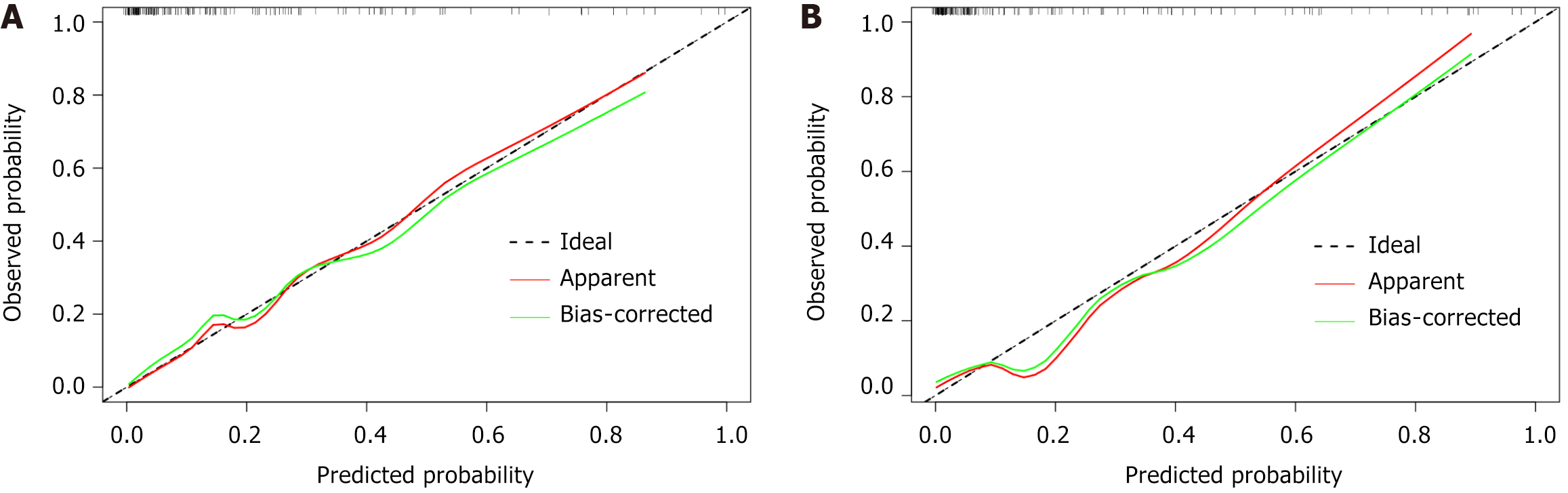
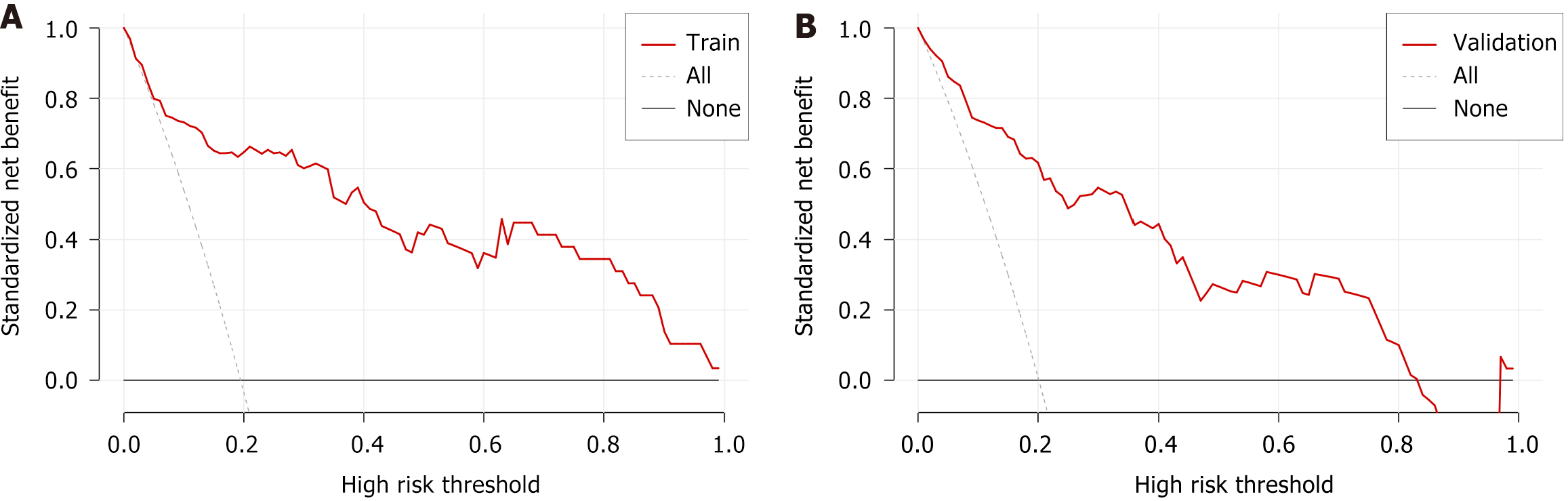
The calibration curve (Figure 6) and DCA (Figure 7) curve further confirmed that the nomogram prediction model exhibits strong predictive performance and clinical utility.
In recent years, thoracoscopic technology has rapidly advanced and become a crucial approach for LC radical resection. The incidence of perioperative complications remains relatively high despite the minimally invasive nature of thoracoscopic surgery and its quicker recovery times. Studies have revealed that the rate of perioperative complications reaches 15%-45%[11,12]. Among these complications, pulmonary infections are one of the most prevalent. Research has revealed that PPI incidence in patients with LC is approximately 7%-20%[13]. Elderly patients with diabetes and LC are at an even higher risk for PPIs due to their weakened immune function and metabolic abnormalities. Pulmonary infections not only affect the efficacy of thoracoscopic surgery but also increase the psychological stress and financial burden on patients, prolong hospital stays, and potentially elevate postoperative mortality rates. Therefore, screening for risk factors of PPIs in patients with LC needs to be strengthened, particularly in elderly patients with diabetes. Developing effective predictive models for early identification of infection risk and implementing individualized treatment strategies are crucial for improving patient outcomes and treatment effectiveness.
In this study, the incidence of PPIs in elderly patients with diabetes and LC undergoing thoracoscopic radical resection was 19.34%, emphasizing the urgent need for early preventive measures to improve infection control. Further analysis of pathogen distribution revealed that Gram-negative bacteria were predominant, accounting for 64.14% of the isolates, with Pseudomonas aeruginosa and Klebsiella pneumoniae being the most prevalent. These pathogens are widely seen in the respiratory tract and gastrointestinal tract, as well as in hospital environments, and they are often multidrug-resistant. They rapidly proliferate in patients with immunocompromise, especially elderly patients with diabetes[14]. Understanding the specific distribution of pathogens can help clinicians in selecting targeted antibiotic therapies early, improving infection control, and reducing the risk of antibiotic resistance.
Our retrospective analysis of clinical data from elderly patients with diabetes and LC undergoing thoracoscopic radical resection determined several risk factors for PPIs through univariate logistic regression analysis. The risk factors include preoperative neoadjuvant chemotherapy (≥ 2 times), maximum tumor diameter (≥ 4 cm), multi-lobar resection, preoperative FEV1 (≤ 80%), and high HbA1c levels. Conversely, low Alb level was identified as a protective factor against pulmonary infections.
Preoperative neoadjuvant chemotherapy is typically administered in patients with advanced LC to reduce tumor burden and improve resection rates[15]. However, our study revealed that receiving neoadjuvant chemotherapy of ≥ 2 times increased the risk of PPIs. This may be because of the indiscriminate damage to hematopoietic stem cells caused by chemotherapy drugs, causing leukopenia and bone marrow suppression, thereby impairing immune function and increasing susceptibility to infections[16]. Further, the cumulative toxic effects of chemotherapy drugs across multiple cycles weaken systemic immunity. Moreover, respiratory epithelial cell damage due to chemotherapy drugs may compromise mucosal barrier functions, establishing conditions conducive to pathogen invasion[17]. However, Bai et al[18] revealed that receiving neoadjuvant chemotherapy ≥ 2 times preoperatively was not a risk factor for PPI in patients with LC. This may be associated with differences in patients’ immune status and underlying health conditions. In particular, our study included elderly patients with diabetes, who tended to have weaker immune function and were more prone to infections.
An increased number in the maximum diameter of the lung tumor significantly raises the incidence of PPIs. Larger tumors are related to a higher probability of lymphatic metastasis, requiring an enlarged surgical resection range and increased surgical difficulty, which prolongs operative time. Further, our study revealed that a larger extent of resection is a risk factor for PPIs. Extended operative time increases patient exposure to external environments and prolongs mechanical ventilation, potentially damaging the integrity of the respiratory mucosa and diminishing natural lung defenses[19]. Previous studies have indicated a positive correlation between prolonged operative time and PPI risks[11]. Furthermore, a larger extent of resection and longer surgery duration may impair postoperative immune function and can complicate airway management, thereby increasing infection risk[13]. Ishikawa et al[20] revealed that extensive resection may increase postoperative complications; however, it was not directly related to pulmonary infection. This may be associated with differences in the surgery type, patient individual differences, and postoperative management measures compared to this study. Therefore, enhanced perioperative management and early interventions are crucial in reducing PPI incidence for elderly patients with diabetes and larger tumors that require extensive resections.
Patients with lower preoperative FEV1 are more at risk of PPIs, primarily due to poorer lung function and respiratory muscle strength, which reduces cough and expectoration efficiency and makes it difficult to clear airway secretions effectively. Further, patients with low FEV1 frequently experience inadequate alveolar ventilation and increased residual volume, causing insufficient oxygen supply to some lung areas and mucus retention, thereby establishing a favorable environment for pathogen growth[21]. Impaired lung function indicates decreased lung elasticity, further weakening the respiratory system’s ability to clear infectious pathogens[22]. Previous research has revealed impaired pulmonary ventilation as an independent risk factor for PPIs, consistent with our results[11]. Notably, patients with both low preoperative FEV1 and substantial intraoperative resections are at higher risk of infections, as both factors compound their effects on pulmonary ventilation. Therefore, improved lung monitoring and management during the perioperative period are required to minimize infection risk.
Low preoperative Alb levels significantly increase PPI incidence. This is likely because low Alb levels indicate possible malnutrition, which weakens immune function, and particularly reduces cellular and humoral immunity, thereby decreasing resistance to pathogens[23]. Further, Alb plays a crucial role in tissue repair and wound healing, whereas low Alb levels may delay wound healing, thereby increasing the risk of postoperative infections. Further, Alb helps maintain fluid balance and colloid osmotic pressure. Low Alb levels decrease plasma colloid osmotic pressure, thereby increasing interstitial fluid accumulation and pulmonary edema, making lung tissue more susceptible to infection[24]. Hence, preoperative low Alb levels are an important risk factor for postoperative infections, highlighting the need for improved nutritional support during the perioperative period.
Increased blood glucose significantly elevates the risk of PPIs in patients with LC. This is primarily because patients with diabetes often exhibit a “low-efficiency” immune system. The hyperglycemic environment adversely affects leukocytes, especially neutrophils and macrophages, which weaken their phagocytic and bactericidal abilities. Studies have revealed that in a hyperglycemic environment, neutrophils in patients with diabetes demonstrate a significant decrease in chemotaxis and phagocytic function, thereby delaying immune response to infections[25]. Lai et al[26] emphasized that when patients with diabetes are exposed to bacterial infections, cytokine secretion and antigen presentation functions of their WBCs are significantly impaired. This immunodeficiency makes patients undergoing thoracoscopic LC radical resection more prone to PPI. Further, diabetes is frequently accompanied by a chronic low-grade inflammatory state. Studies have revealed that cytokines, such as tumor necrosis factor-α, interleukin (IL)-6, and IL-1β, in patients with diabetes are persistently increased, which intensifies local and systemic inflammatory responses, thereby weakening the body’s defense against external pathogens. Research has revealed that patients with diabetes experience more intense postoperative inflammatory responses, causing slower recovery and increased pulmonary infection risks[27]. This persistent low-grade inflammation not only disrupts the lung’s barrier function but also facilitates pathogen invasion into lung tissue. Moreover, the microecological environment in patients with diabetes may also change, especially in the oral and respiratory tracts, where the number of pathogenic bacteria (e.g., Staphylococcus aureus, Streptococcus pneumoniae) may increase, playing a crucial role in postoperative infection occurrence[28]. Hyperglycemia and blood glucose fluctuations delay wound healing and impair immune defense. Hyperglycemia inhibits fibroblast function and reduces collagen synthesis, resulting in a slow tissue repair process. Studies have demonstrated that patients with diabetes have longer incision healing times postoperatively, reduced immune defenses, and a higher risk of bacterial infections[29]. Univariate analysis revealed obvious differences in FBG and HbA1c regarding postoperative infection risk; however, only HbA1c was determined as an independent risk factor on multivariate analysis. This may be because preoperative blood glucose is typically managed, and fasting glucose reflects short-term glucose status, whereas HbA1c more accurately evaluates long-term glucose control. Therefore, high HbA1c is an important risk factor for postoperative infections, emphasizing the importance of evaluating long-term glucose control preoperatively.
The nomogram constructed in this study illustrates that preoperative neoadjuvant chemotherapy of > 2 times, tumor diameter of > 4 cm, multi-lobar resection, FEV1 of < 80%, low Alb levels, and high HbA1c levels contribute 19, 21, 20, 31, 89, and 100 points respectively to the risk of PPIs in elderly patients with diabetes and LC undergoing thoracoscopic radical resection. This further confirmed the potential value of HbA1c in predicting PPIs. Patients with diabetes, particularly elderly ones, were more prone to infections due to long-term hyperglycemia, which may suppress immune function. Therefore, HbA1c should be considered an important reference indicator in clinical practice for assessing the risk of PPIs in elderly patients with diabetes and LC, and it should be used in conjunction with other clinical parameters for risk assessment and intervention strategy development. The internal and external validation of the nomogram prediction model revealed good predictive discrimination, with AUCs of 0.901 and 0.915 for the training and validation sets, respectively, indicating excellent model performance. Calibration curves and DCA curves for the training and validation sets indicate that the model has strong predictive performance and clinical value.
The innovation of this study is its focus on the elderly patient population with diabetes, considering the specific circumstances of postoperative LC, which fills the gap in research on the association between diabetes and PPIs in patients with LC. This provides clinical practice guidance with an individualized predictive tool to optimize treatment strategies. However, this study has some limitations. First, all data were obtained from a single institution and multicenter data were lacking, which may introduce bias and limit the generalizability of the results. Further, this is a retrospective analysis; thus, controlling for potential confounding factors is challenging, and causal relationship assessments are limited. Furthermore, the study focuses on PPI incidences, but it lacks follow-up data on long-term outcomes, recurrence rates, and overall survival, which are essential for comprehensively assessing the effect of PPI on long-term prognosis. Future prospective, multicenter studies are warranted to further validate the risk factors for PPIs in elderly patients with diabetes and LC, which enhance the generalizability and reliability of the results, and to conduct long-term follow-ups to evaluate prognosis.
In summary, the nomogram model constructed using preoperative neoadjuvant chemotherapy, maximum tumor diameter, extent of lung resection, preoperative FEV1 levels, Alb levels, and HbA1c levels exhibits high clinical value in predicting PPIs in elderly patients with diabetes and LC. This model effectively evaluates the risk of postoperative infections, providing valuable information for clinicians and helping in the early implementation of targeted strategies for preventions and interventions, ultimately improving postoperative outcomes and overall treatment effectiveness.
| 1. | Li C, Lei S, Ding L, Xu Y, Wu X, Wang H, Zhang Z, Gao T, Zhang Y, Li L. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl). 2023;136:1583-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 126] [Reference Citation Analysis (32)] |
| 2. | Marcotte LM, Khor S, Flum DR, Akinsoto N, Chaudhari V, Wood DE, Lavallee DC, Triplette M, Farjah F. Factors associated with lung cancer risk factor documentation. Am J Manag Care. 2023;29:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Torasawa M, Shukuya T, Uemura K, Hayashi T, Ueno T, Kohsaka S, Masui Y, Shirai Y, Okura M, Asao T, Mitsuishi Y, Shimada N, Takahashi F, Takamochi K, Suzuki K, Takahashi K, Seyama K. Lymphangioleiomyomatosis as a potent lung cancer risk factor: Insights from a Japanese large cohort study. Respirology. 2024;29:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Elshami M, Mansour A, Alser M, Al-Slaibi I, Abukmail H, Shurrab H, Qassem S, Usrof FD, Alruzayqat M, Aqel W, Nairoukh R, Kittaneh R, Sawafta N, Habes YMN, Ghanim O, Aabed WA, Omar O, Daraghmeh M, Aljbour J, Elian REM, Zhor A, Habes H, Al-Dadah M, Abu-El-Noor N, Bottcher B. Current situation and future directions of lung cancer risk factor awareness in Palestine: a cross-sectional study. BMJ Open. 2023;13:e061110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Bernatsky S, Ramsey-Goldman R, Petri M, Urowitz MB, Gladman DD, Fortin PR, Yelin EH, Ginzler E, Hanly JG, Peschken C, Gordon C, Nived O, Aranow C, Bae SC, Isenberg D, Rahman A, Hansen JE, Pierre YS, Clarke AE. Smoking Is the Most Significant Modifiable Lung Cancer Risk Factor in Systemic Lupus Erythematosus. J Rheumatol. 2018;45:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Ren W, Liu Y, Jiang H, Lv X, Zhang N. Epidemiology of potential drug- drug interactions in hospitalized patients with type 2 diabetes mellitus in China: a retrospective study. Front Endocrinol (Lausanne). 2024;15:1387242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Cheng Y, Chen Y, Hou X, Yu J, Wen H, Dai J, Zheng Y. Development of a Nomogram for Predicting Surgical Site Infection in Patients with Resected Lung Neoplasm Undergoing Minimally Invasive Surgery. Surg Infect (Larchmt). 2022;23:754-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Wang JY, Pang QY, Yang YJ, Feng YM, Xiang YY, An R, Liu HL. Development and Validation of a Nomogram for Predicting Postoperative Pulmonary Infection in Patients Undergoing Lung Surgery. J Cardiothorac Vasc Anesth. 2022;36:4393-4402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Office of the National Health Commission. [Guidelines for Diagnosis and Treatment of Primary Lung Cancer (2022 Edition)]. Xiehe Yixue Zazhi. 2022;13:549-570. [DOI] [Full Text] |
| 10. | National Geriatrics Center; Geriatrics Branch of Chinese Medical Association; diabetes Professional Committee of China Geriatrics Health Care Association. [Chinese Guidelines for Diagnosis and Treatment of Elderly diabetes (2024 Edition)]. Zhonghua Laonian Yixue Zazhi. 2024;43:105-147. [DOI] [Full Text] |
| 11. | Ma S, Li F, Li J, Wang L, Song H. Risk factor analysis and nomogram prediction model construction of postoperative complications of thoracoscopic non-small cell lung cancer. J Thorac Dis. 2024;16:3655-3667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Furák J, Németh T, Lantos J, Fabó C, Géczi T, Zombori-Tóth N, Paróczai D, Szántó Z, Szabó Z. Perioperative Systemic Inflammation in Lung Cancer Surgery. Front Surg. 2022;9:883322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 13. | Ding Z, Wang X, Jiang S, Liu J. Risk factors for postoperative pulmonary infection in patients with non-small cell lung cancer: analysis based on regression models and construction of a nomogram prediction model. Am J Transl Res. 2023;15:3375-3384. [PubMed] |
| 14. | Wang Y, Li J, Wu Q, Chang Q, Guo S. Pathogen distribution in pulmonary infection in chinese patients with lung cancer: a systematic review and meta-analysis. BMC Pulm Med. 2023;23:402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Cascone T, Leung CH, Weissferdt A, Pataer A, Carter BW, Godoy MCB, Feldman H, William WN Jr, Xi Y, Basu S, Sun JJ, Yadav SS, Rojas Alvarez FR, Lee Y, Mishra AK, Chen L, Pradhan M, Guo H, Sinjab A, Zhou N, Negrao MV, Le X, Gay CM, Tsao AS, Byers LA, Altan M, Glisson BS, Fossella FV, Elamin YY, Blumenschein G Jr, Zhang J, Skoulidis F, Wu J, Mehran RJ, Rice DC, Walsh GL, Hofstetter WL, Rajaram R, Antonoff MB, Fujimoto J, Solis LM, Parra ER, Haymaker C, Wistuba II, Swisher SG, Vaporciyan AA, Lin HY, Wang J, Gibbons DL, Jack Lee J, Ajami NJ, Wargo JA, Allison JP, Sharma P, Kadara H, Heymach JV, Sepesi B. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat Med. 2023;29:593-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 137] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 16. | Miao H, Xu S, Gao M, Chen Z. Benefits and Relevant Risk Factor Assessment of Neoadjuvant Chemotherapy in Combination with Surgery in Limited-Stage Small-Cell Lung Cancer. J Environ Pathol Toxicol Oncol. 2023;42:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Kumar S, Saikia J, Kumar V Jr, Malik PS, Madan K, Jain D, Bharati S. Neoadjuvant chemotherapy followed by surgery in lung cancer: Indian scenario. Curr Probl Cancer. 2020;44:100563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Bai G, Chen X, Peng Y, Ji Y, Bie F, Liu Y, Yang Z, Gao S. Surgery challenges and postoperative complications of lung cancer after neoadjuvant immunotherapy. Thorac Cancer. 2024;15:1138-1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Choi Y, Noh JM, Shin SH, Lee K, Um SW, Kim H, Pyo H, Ahn YC, Jeong BH. The Incidence and Risk Factors of Chronic Pulmonary Infection after Radiotherapy in Patients with Lung Cancer. Cancer Res Treat. 2023;55:804-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 20. | Ishikawa S, Yamamori I, Takamori S, Kitabatake K, Edamatsu K, Sugano A, Oizumi H, Kato H, Suzuki J, Sato K, Yusa K, Sadahiro M, Iino M. Evaluation of effects of perioperative oral care intervention on hospitalization stay and postoperative infection in patients undergoing lung cancer intervention. Support Care Cancer. 2021;29:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Chen J, Liu Y, Cai H, Zheng W. Risk factors for pulmonary infection in patients with non-small cell lung cancer: a Meta-analysis. BMC Pulm Med. 2024;24:353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | He R, Zhu Q, Wang Y, Chen G, Chen S, Wang Y. Influence of respiratory function training under the mode of mutual-assisted patients on postoperative pulmonary infection and immune function on lung cancer. Am J Transl Res. 2021;13:9260-9268. [PubMed] |
| 23. | Shimizu T, Okachi S, Imai N, Hase T, Morise M, Hashimoto N, Sato M, Hasegawa Y. Risk factors for pulmonary infection after diagnostic bronchoscopy in patients with lung cancer. Nagoya J Med Sci. 2020;82:69-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Alturaiki W. Immunomodulatory effects of BAFF and APRIL cytokines in post-pulmonary infection lung cancer: Implications for drug resistance and progression. Saudi Med J. 2024;45:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Herder C, Roden M, Venteclef N. Diabetes and pulmonary infection: how hyperglycaemia shapes the immune system. Signal Transduct Target Ther. 2024;9:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 26. | Lai W, Liu L, Wang S, Tang Q, Liu Y, Chai Y. The impact of diabetes on Sepsis-induced cardiomyopathy. Diabetes Res Clin Pract. 2025;220:112001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Zhang J, Zhao T, Long S, Liu X, Yu H. Risk factors for postoperative infection in Chinese lung cancer patients: A meta-analysis. J Evid Based Med. 2017;10:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Liu YX, Cao QM, Ma BC. Pathogens distribution and drug resistance in patients with acute cerebral infarction complicated with diabetes and nosocomial pulmonary infection. BMC Infect Dis. 2019;19:603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Zhang W, Wu Y. Refinement of Specialized Nursing Intervention in Elderly Patients with Diabetes Complicated by Pulmonary Infection and the Impact on the Patient's Condition and Prognosis. Altern Ther Health Med. 2024;30:511-517. [PubMed] |