Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.105233
Revised: March 28, 2025
Accepted: May 14, 2025
Published online: July 15, 2025
Processing time: 181 Days and 14.8 Hours
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by hyperglycemia and insulin resistance, often leading to severe complications. Hemogram markers have attracted great attention from researchers for their established role in inflammatory conditions. In this respect, T2DM and its mi
Core Tip: Type 2 diabetes mellitus (T2DM) is a chronic condition marked by high blood glucose and insulin resistance, often accompanied by severe complications and systemic inflammation. Recent research highlights hemogram-derived markers, such as the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, red cell distribution width, and mean platelet volume, as valuable tools for predicting the onset, progression, and complications of T2DM. These markers, accessible through routine blood tests, provide insights into inflammation and vascular changes linked to T2DM including car
- Citation: Aktas G. Exploring the link: Hemogram-derived markers in type 2 diabetes mellitus and its complications. World J Diabetes 2025; 16(7): 105233
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/105233.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.105233
Type 2 diabetes mellitus (T2DM) is a prevalent chronic disease characterized by insulin resistance (IR) and chronic hyperglycemia. It significantly increases the risk of complications such as cardiovascular disease (CVD), diabetic retinopathy (DR), kidney disease, and diabetic neuropathy (DN). Inflammation has been identified as a key contributor to the development of T2DM and its associated complications, linking metabolic imbalance with vascular damage and tissue dysfunction[1]. A study indicated that of a total of half a billion people with diabetes, 9 of 10 of them have T2DM[2].
Markers derived from hemograms, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), mean platelet volume (MPV), and red cell distribution width (RDW), have gained attention for their ability to indicate systemic inflammation. The relationships between inflammatory conditions and the NLR[3], PLR[4], MPV[5], and RDW[6] are well established. These markers are readily available through routine blood tests, cost-effective, and non-invasive, making them valuable tools in the follow-up of disease progression and predicting complications in T2DM.
This review explored the potential role of these hemogram-derived markers in the context of T2DM, examining their relationship with disease severity, inflammation, and the development of diabetic complications. By integrating current research, the aim was to provide insights into their clinical utility and their potential to enhance patient management in T2DM.
T2DM is linked to a higher risk of various complications, such as CVD, diabetic kidney damage (DKD), DR, and DN, all of which substantially influence morbidity and mortality[7]. Growing evidence suggests that chronic low-grade inflammation plays a central role in the onset and progression of T2DM and its associated complications[8,9]. The NLR, a simple marker derived from routine blood tests, has attracted attention as a potential indicator of systemic inflammation. Elevated NLR has been reported in a range of inflammatory conditions including autoimmune disorders[10], inflammatory bowel disease[11], infectious diseases[12], metabolic syndrome[13], and obesity[14]. Like T2DM, these are characterized by a substantial inflammatory burden.
The NLR is calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. Neutrophils, key components of the innate immune system, typically increase in response to inflammation, infection, or tissue damage, whereas lymphocytes, part of the adaptive immune system, play a role in regulating inflammatory responses. In T2DM, chronic inflammation and immune system dysfunction can lead to alterations in both neutrophil and lymphocyte counts.
Inflammatory pathways, particularly those involving cytokines like tumor necrosis factor alpha (TNF-α) and in
The NLR has garnered attention as a potential biomarker for assessing the inflammatory burden in T2DM and its complications. Research indicates that elevated NLR is associated with poor glycemic control[18], increased levels of inflammatory markers[18], and common comorbidities such as hypertension[19] and dyslipidemia[19]. Moreover, elevated NLR has also been linked to DKD[3] and increased IR, underscoring its role as a marker of metabolic dysfunction. A Spanish study reported that higher NLR values were associated with IR in obese population[20]. Similarly, a study by Zhang and Liu[21] found a correlation between NLR and IR, as measured by the homeostasis model assessment (HOMA) of IR. Elevated NLR levels have also been associated with adverse outcomes in patients with T2DM, including increased risks of CV events, hospitalizations, and mortality. A study from China analyzing data from 1454 subjects found that elevated NLR was an independent risk factor for CV events and overall mortality in individuals with T2DM[22]. Subsequently, a meta-analysis, involving 407512 subjects, confirmed that the NLR serves as a prognostic marker for mortality in patients with T2DM[23]. These findings highlight the significant role of NLR in the management and prognosis of T2DM.
CVD is the leading cause of death in patients with T2DM. Chronic inflammation plays a central role in the deve
The inflammatory environment in T2DM, reflected by a high NLR, might contribute to endothelial dysfunction, plaque formation, and vascular remodeling, which are key factors in the pathogenesis of CVD[26]. Thus, NLR may serve as a simple, cost-effective tool to predict the risk of CV events in this high-risk population.
DKD, also known as diabetic nephropathy, is a common and serious complication of T2DM, characterized by progressive and eventual renal failure. Chronic inflammation plays a critical role in the development and progression of DKD, with immune cell infiltration and the release of inflammatory mediators driving glomerular injury and fibrosis[27]. Elevated NLR has been associated with an increased risk of developing DKD in patients with T2DM, and higher NLR levels correlate with worsening kidney function. A 2021 study demonstrated that increased NLR was linked to the presence of DKD in patients with T2DM[28]. Similarly, a cross-sectional study of 4813 adults with diabetesindicated that elevated NLR increased the risk of DKD by 2.5 times[29]. These findings were supported by a Japanese study, which showed that the NLR could be a useful marker for declining in renal function in subjects with diabetes[30]. Thus, the NLR is recommended as a predictor of early-stage , and may be valuable for monitoring renal function in patients with T2DM.
DR is an important cause of blindness in adults and is closely associated with the duration and severity of hy
DN is another common complication of T2DM, often resulting in pain, sensory loss, and functional impairment. The role of inflammation in the development of DN is well established, with immune-mediated damage to peripheral nerves contributing to the pathophysiology[35]. Studies have shown that elevated NLR is associated with the presence and severity of DN, suggesting that the NLR may reflect the extent of nerve injury and inflammatory involvement in patients with T2DM. One study revealed patients with diabeteswith DN had higher NLR levels than those without DN[36]. A recent meta-analysis confirmed these findings showing that patients with DN consistently exhibited higher NLR levels than those without the condition[37].
The PLR is a straightforward and easily calculated biomarker obtained from routine hemogram tests. In recent years, it has gained attention as a potential marker of systemic inflammation and a valuable tool for assessing the risk and severity of T2DM and its complications. The PLR represents the ratio of the absolute platelet count to the absolute lymphocyte count in the bloodstream. Platelets play a critical role in hemostasis and thrombosis and actively contribute to the inflammatory response by releasing various pro-inflammatory cytokines, growth factors, and chemokines. These substances promote endothelial dysfunction, IR, and atherosclerosis[15]. By contrast, lymphocytes are central to the adaptive immune response, and their depletion or dysfunction can result in a dysregulated immune system, contributing to chronic inflammation[38]. In T2DM, elevated platelet count and altered lymphocyte function are both observed as part of the inflammatory process, leading to an increased PLR in patients with this condition. Higher PLR levels have been reported in patients with T2DM compared to healthy controls[39]. Moreover, the PLR was correlated with glycated hemoglobin (HbA1c) level in that study[39]. In addition, Liu et al[40] reported that the PLR was an independent predictor of gestational diabetes in their study on 120 subjects. These data suggest its potential benefits in the follow-up of patients with gestational or T2DM.
Inflammatory pathways leading to IR and β-cell dysfunction are mediated by various pro-inflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and C-reactive protein (CRP). These cytokines activate platelets, promoting aggregation and driving vascular inflammation. Both processes play a key role in the progression of diabetic complications, particularly CVD[41]. The imbalance characterized by elevated platelet counts and relatively reduced lymphocyte counts is believed to indicate the extent of inflammation and oxidative stress in T2DM. This makes the PLR a convenient marker for evaluating the inflammatory burden in T2DM and related conditions.
PLR has been found to be significantly associated inflammation in T2DM. Elevated PLR is often seen in patients with poor glycemic control and advanced stages of the disease, suggesting its potential as a marker for disease severity. In 2019, authors compared the PLR levels in patients with T2DM and healthy subjects and revealed that subjects with diabeteshad increased PLR compared to the controls[39]. Higher PLR has been linked to IR, a hallmark of T2DM, and some studies have suggested that the PLR may serve as a predictor of metabolic syndrome, which frequently ac
Chronic inflammation and platelet activation are key factors in the development of atherosclerosis and thrombosis, both of which contribute to CV events. An elevated PLR has been consistently linked to a higher risk of CVD, including myocardial infarction, stroke, and peripheral artery disease, particularly in patients with T2DM. A 2021 meta-analysis reported that the PLR can predict prognosis in patients with myocardial infarction[42]. In another study, authors studied the role of the PLR in ischemic stroke subjects and found that it was an independent predictor of outcome in this population[43]. Additionally, Selvaggio et al[44] reported the PLR as a reliable marker of peripheral artery disease. A high PLR indicates increased platelet activation and inflammation, both of which play a role in endothelial dysfunction, plaque formation, and vascular damage[45]. Elevated PLR serves as an independent predictor of adverse CV events, making it a useful biomarker for evaluating CV risk in patients with diabetes.
The initiation and progression of DKD are closely linked to inflammation, oxidative stress, and vascular injury, all of which contribute to glomerular damage and fibrosis[46]. Studies have associated elevated PLR with both the presence and severity of DKD in patients with T2DM, suggesting its potential as a predictor of early and declining renal function. For example, Duan et al[47] studied 167 biopsy-proven subjects with DKD and revealed that the PLR was associated with proteinuria and the disease outcome. Considering the significant role of inflammation in the pathophysiology of DKD, PLR may serve as a valuable biomarker for identifying individuals at increased risk of renal complications and tracking the progression of kidney disease in patients with T2DM.
The development of DR is linked to vascular changes, inflammation, and oxidative stress in subjects with diabetes. Chronic low-grade inflammation plays a key role in the pathogenesis of DR[48], and platelet activation has been im
The interaction between DN and inflammation is well established in patients with T2DM. Serum levels of inflammation markers are reportedly increased in subjects with DN[51]. Accordingly, as an inflammation marker, the PLR has been associated with the presence and severity of DN, which suggests that increased platelet activation and reduced lymphocyte counts may reflect the underlying inflammatory processes involved in nerve damage. A study by Chen et al [36] confirmed that patients with diabetic polyneuropathy had higher PLR levels than the diabetic subjects without DN. Therefore, we conclude that high PLR may indicate diabetic peripheral neuropathy, which is helpful in identifying patients at risk of this debilitating complication.
As the prevalence of T2DM continues to rise globally, so too does the burden of its associated complications, including CVD, diabetic nephropathy, DR, and DN. These complications contribute significantly to morbidity and mortality among individuals with T2DM. MPV, a simple marker of platelet size, has emerged as a potential biomarker for assessing platelet activation and systemic inflammation[52]. A recent study suggested MPV in association with platelet adhesion in T2DM[53]. Moreover, MPV has been linked with inflammation and liver cirrhosis in patients with chronic hepatitis B[54]. Similar to T2DM, this chronic condition is also characterized with inflammatory burden.
MPV refers to the average size of platelets in circulation, with larger platelets generally considered more reactive and prothrombotic[55]. Platelets play an essential role in hemostasis, but are also involved in inflammation, contributing to the development of atherosclerosis, thrombosis, and vascular dysfunction. In T2DM, platelets are often hyperactivated due to the underlying inflammatory state and metabolic dysregulation, which leads to an increase in MPV levels[56].
The elevated MPV in T2DM is linked to several mechanisms, including oxidative stress, IR, and inflammatory cytokine release. Pro-inflammatory cytokines such as TNF-α and IL-6, which are elevated in T2DM, contribute to platelet activation and the release of pro-inflammatory mediators. Larger platelets have been shown to release more pro-inflammatory cytokines, which further exacerbate endothelial dysfunction and promote the development of diabetic complications[57]. The increase in MPV thus reflects both the degree of platelet activation and the overall inflammatory burden in T2DM.
It is obvious that MPV is a simple and inexpensive biomarker that can be easily measured through routine blood tests. Research has shown that higher MPV is associated with poor glycemic control and IR, both of which are hallmarks of T2DM[58]. Elevated MPV levels are also correlated with markers of systemic inflammation, such as CRP, suggesting that MPV may serve as a surrogate marker for inflammation in T2DM. Furthermore, elevated MPV has been associated with higher risks of adverse clinical outcomes, including an increased likelihood of developing diabetic complications. A study from Egypt reported that MPV was significantly higher in patients with T2DM with microvascular complications than the diabetics without those complications[59]. In addition, a Japanese study revealed that elevated MPV was also associated with macrovascular complications of T2DM[60].
In clinical practice, MPV can be used to assess the degree of platelet activation in patients with diabetes, offering insights into the patient's inflammatory status and potential risk for developing complications. Wu et al[61] found a correlation between MPV and HbA1c, supporting its role in glycemic monitoring. As a cost-effective and easily accessible marker, MPV holds promise as a useful tool for managing and monitoring T2DM, particularly in identifying individuals at higher risk of poor outcomes.
The association between MPV and CVD is well established as the association between MPV and T2DM. Platelet activation plays a central role in the pathogenesis of atherosclerosis and thrombosis[62]. In a recent work, Cassano et al[63] compared MPV levels among new-onset patients with T2DM, subjects with prediabetes, and controls with normal glycemia and reported that MPV was increased in subjects with diabetes and prediabetescompared to the controls. Moreover, the same study also revealed that MPV was correlated with arterial stiffness and subclinical myocardial damage[63]. Increased MPV has been consistently associated with elevated risk of CVD in patients with T2DM. Larger platelets are more thrombogenic, containing more pro-coagulant and pro-inflammatory substances, and they promote vascular injury, atherosclerotic plaque formation, and thrombus development. Elevated MPV is associated with poor endothelial function, a key factor in the development of atherosclerosis.
Several studies have identified high MPV as a predictor of adverse CV events, including myocardial infarction, stroke, and peripheral arterial disease in individuals with T2DM. A study from China showed that MPV was an independent risk factor of acute myocardial infarction[64]. Another study reported that MPV was associated with stroke risk[65]. Increased MPV has been recognized as a risk factor for peripheral artery disease according to a study by Li et al[66]. Elevated MPV levels have also been linked to the presence of coronary artery disease, suggesting that MPV can serve as a marker for subclinical CVD and a predictor of future CV events. Monitoring MPV levels may be useful in identifying patients with T2DM at high risk for CV complications and in guiding therapeutic interventions.
DKD is a common and severe complication of T2DM. It is characterized by progressive KD and eventual renal failure. Platelet activation and inflammation are key contributors to the development and progression of DKD. Elevated MPV has been shown to be associated with the presence and severity of diabetic nephropathy. One study demonstrated the utility of MPV in monitoring patients with DKD[67]. In patients with T2DM, larger platelets are more likely to promote microvascular injury in the kidneys, contributing to glomerular damage and fibrosis.
Studies have found that higher MPV levels are correlated with increased urinary albumin excretion, an early marker of KD in DKD. Sengupta et al[67] reported that MPV was increased in advanced stages of diabetic nephropathy compared to the early stages of the disease. Furthermore, increased MPV is associated with declining renal function, indicating its utility as an early marker of kidney dysfunction in patients with T2DM[68]. Monitoring MPV levels in patients with T2DM may help identify those at higher risk for developing nephropathy and could provide valuable insights into the progression of kidney disease.
DR is also associated with chronic inflammatory burden[69]. Platelet activation is thought to play a significant role in the development of DR, as activated platelets can contribute to retinal microvascular injury and thrombosis[70]. Elevated MPV has been associated with an increased risk of DR in patients with T2DM, with higher MPV values correlating with the severity of retinal damage. Authors have reported that elevated levels of MPV are associated with development and severity of DR in patients with T2DM[71].
As a reflection of systemic inflammation and endothelial dysfunction, two central processes in DR pathogenesis, elevated MPV may provide a valuable, non-invasive means of monitoring retinal health in patients with diabetes. Its ease of measurement makes MPV a practical tool for screening and tracking DR progression, potentially enabling earlier intervention to prevent vision loss.
DN is also characterized by a high burden of inflammation[35]. Inflammation and microvascular injury play a central role in the development of DN, and platelet activation is thought to contribute to these processes. Elevated MPV has been associated with the presence and severity of DN. A significant association between MPV and diabetic peripheral neuropathy was reported in a recent study[72]. Larger platelets, which are more reactive, may exacerbate nerve injury by promoting microvascular damage and inflammation.
Several studies have demonstrated that increased MPV correlates with the severity of neuropathic symptoms in patients with T2DM. Increased MPV was linked to the more serious neuropathic symptoms in patients with diabetesin a study by Nimmala et al[73]. Monitoring MPV levels may provide insights into the inflammatory processes that contribute to nerve damage and help identify individuals at a higher risk of neuropathy. By tracking MPV, clinicians may be able to identify those who require more intensive management to prevent the progression of neuropathy.
Among the biomarkers that reflect such systemic inflammation and vascular dysfunction, RDW measures the variation in the size of red blood cells (RBCs)[74]. Beyond its role as a marker of erythrocyte indices, it has been also linked to a variety of diseases, including T2DM, and serves as an indicator of underlying inflammatory and oxidative stress[75,76]. Elevated RDW levels are thought to reflect a dysregulated response to oxidative stress, inflammation, and changes in erythropoiesis. In T2DM, chronic hyperglycemia and IR lead to increased oxidative stress and inflammation, which in turn can alter RBC morphology and function, contributing to an increase in RDW[77]. The mechanisms linking RDW to T2DM involve various factors. The inflammatory environment in T2DM leads to the release of cytokines such as IL-6, TNF-α, and CRP, which can disrupt erythropoiesis and RBC turnover. Additionally, oxidative stress, which is prevalent in T2DM, can contribute to hemoglobin glycosylation and RBC damage, resulting in an increased RDW. Furthermore, the metabolic dysfunction in T2DM can lead to deficiencies in important nutrients such as iron, vitamin B12, and folic acid, all of which can further influence RBC production and lead to increased RDW.
An important feature of RDW as a disease marker that is inexpensive and readily available nature. It can be measured as part of a routine complete blood count test. In T2DM, elevated RDW has been linked to poor glycemic control. A 2021 study revealed that poorly controlled patients with diabeteshad elevated levels of RDW compared to the well-controlled subjects with diabetes,and RDW was correlated with HbA1c[78]. Conversely, another study reported higher RDW in patients with T2DM compared to healthy controls but found no significant difference between well-controlled and poorly controlled subgroups[79]. This discrepancy may be attributed to a relatively small sample size (n = 220) or the single-center design of the study. RDW has also been linked to IR in the literature. A study that analyzed data from more than 2000 subjects showed that RDW was correlated with an IR index, HOMA-beta[80]. In addition, an increased risk of developing diabetic complications is associated with RDW levels. Furthermore, increased RDW has been proposed as a risk factor for DR[81]. Moreover, DKDreportedly increases with elevated levels of RDW[82]. RDW may also reflect the degree of metabolic dysregulation in T2DM since literature suggested positive correlation between higher RDW levels and poor blood glucose control. Interestingly, a significant negative correlation between RDW and HbA1c was reported in subjects with diabetes; however, a positive correlation among them was notable in the non-diabetic population[83]. This finding was supported by a study by Zhang et al[84], which found a negative correlation between HbA1c levels and RDW in a diabetic cohort. Furthermore, RDW has also been shown to correlate with other inflammatory markers, such as CRP, suggesting that RDW may serve as a reflection of the systemic inflammatory state in T2DM. Zhao et al[81] reported a significant correlation between RDW and CRP in patients with diabeteswith DR. As an indicator of both inflammation and oxidative stress, RDW could be a valuable tool for monitoring disease progression and guiding treatment decisions in patients with T2DM.
Elevated RDW levels have been associated with a higher risk of adverse CV outcomes in individuals with T2DM. These include including myocardial infarction, stroke, and peripheral artery disease. Higher RDW values reflect increased erythrocyte turnover and a pro-inflammatory state, which may contribute to endothelial injury and the progression of atherosclerosis[85]. RDW has been linked with an increased risk of CVDs in patients with diabetes[86]. The association between RDW and CVDs in T2DM is further supported by its correlation with B-type natriuretic factor[87]. Therefore, RDW may serve as a valuable biomarker for identifying patients with T2DM at high risk of CV events and for assessing the efficacy of therapeutic interventions.
Several studies have highlighted the relationship between RDW and DKD, a common and serious microvascular complication of T2DM. The pathogenesis of DKD involves chronic hyperglycemia, increased oxidative stress, inflammation, and microvascular damage[88]. RDW is also associated with inflammation, oxidative stress and hyperglycemia. A study by Tonelli et al[89] revealed that elevation in RDW was associated with end-stage renal disease in the general population. In addition, higher levels of RDW in subjects with diabetes have been reported, particularly in those with poorly controlled disease[90]. RDW has been shown to be elevated in patients with DKD and correlates with markers of kidney dysfunction, such as albuminuria and glomerular filtration rate (GFR). RDW is correlated with GFR and albuminuria in patients with DKD[91]. Moreover, RDW has been proposed as an independent risk factor of diabetic chronic kidney disease[92]. In addition to CVD, elevated RDW has also been linked to risk of kidney dysfunction in subjects with diabetes[93]. High RDW values are associated with both diabetic chronic kidney disease[94] and acute kidney injury[95]. By contrast, a prospective cohort study by Engström et al[96], which followed 26709 non-diabetic individuals over 14 years, found that higher baseline RDW was associated with a lower incidence of DM[96]. Elevated RDW levels may reflect the ongoing inflammatory process in the kidneys and the increased oxidative stress that accompanies diabetic nephropathy. Given the association between RDW and inflammation, it is thought that RDW may serve as an early indicator of in patients with T2DM, providing valuable information about the need for early inter
The RDW has been studied in DR, another microvascular complication of diabetes mellitus. Chronic hyperglycemia and associated inflammatory processes play a significant role in the development of DR. As mentioned before, RDW is also associated with inflammation and correlated with plasma glucose levels. Elevated RDW has been reported in subjects with DR compared to those without[97]. Moreover, RDW has been identified as a potential marker for the presence and severity of DR[81]. Studies have shown that elevated RDW levels are associated with more severe stages of DR, and RDW may reflect the inflammatory and microvascular changes occurring in the retina[98]. Another study reported that RDW was associated with the presence of DR and positively correlated with HbA1c in patients with diabetes[99]. Increased RDW could serve as a valuable tool for identifying patients at risk of developing DR or for monitoring disease progression.
While the association between RDW and DN is well documented, no significant relationship has been observed between RDW and DN in studies[100,101]. DN is associated with inflammatory burden[35]. RDW is also associated with inflammation; however, no association was reported between RDW and neuropathy in patients with T2DM. Yet, diabetic foot ulcer, a complication of DN has been reported to be related with elevated levels of RDW[76]. This association is thought to reflect the underlying inflammatory and microvascular damage that contributes to nerve injury in T2DM.
Elevated RDW levels have been linked to an increased risk of CV events, kidney disease, and retinopathy. RDW is a simple, cost-effective, and widely available biomarker that can provide valuable insights into the inflammatory and oxidative stress burden in patients with T2DM. Regular monitoring of RDW levels may help clinicians identify individuals at higher risk for complications and allow for earlier intervention to prevent or mitigate the effects of these complications. Moreover, RDW may be useful for assessing the effectiveness of treatment regimens aimed at controlling inflammation, improving glycemic control, and reducing the risk of complications in patients with T2DM. As a tool for monitoring disease progression and guiding therapeutic decisions, RDW could play a significant role in improving the long-term outcomes for individuals with T2DM.
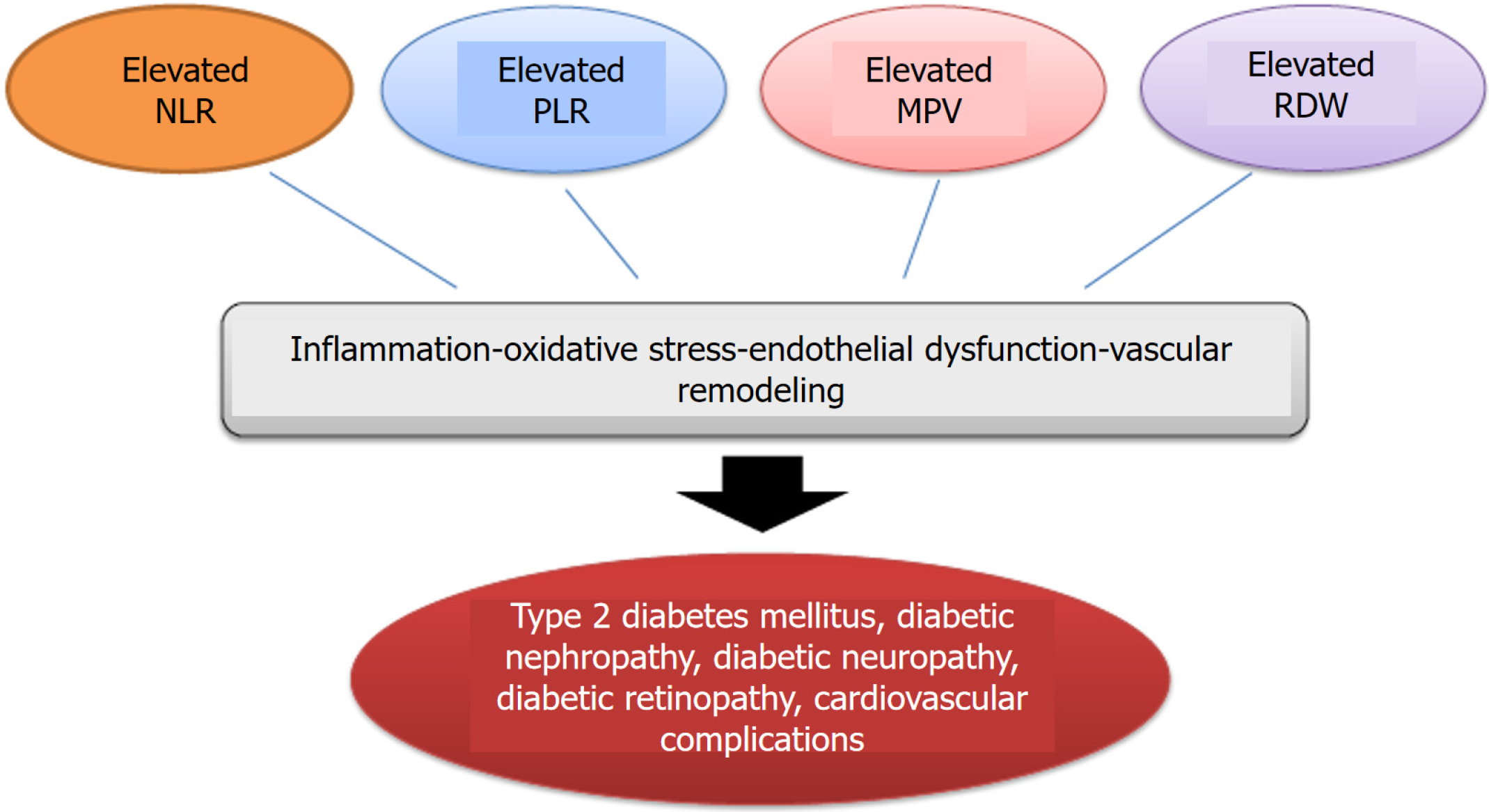
In light of the available data, it is appropriate to state that the NLR, PLR, MPV, and RDW are associated with both T2DM and its chronic complications. Figure 1 illustrates the associations between these markers and T2DM, as well as chronic diabetescomplications. It is also important to consider the implications of these associations in clinical practice. As inflammatory markers, the NLR, PLR, MPV, and RDW have been linked to T2DM and various diabetes-related complications, making them potentially useful clinical tools for risk stratification, prognosis, and disease monitoring. Chronic low-grade inflammation plays a central role in the pathogenesis of T2DM, contributing to IR, β-cell dysfunction, and vascular complications. Neutrophils, lymphocytes, erythrocytes, and platelets contribute to oxidative stress and tissue damage, and elevated levels of these markers reflect the inflammatory burden in T2DM. The predictive value of these markers for CVD, nephropathy, DR, and DN underscores their potential role in clinical settings. However, although they are simple and cost-effective biomarkers, they should be used in conjunction with other diagnostic and prognostic tools to ensure comprehensive patient management. Furthermore, the clinical application of these markers remains largely investigational. Most available evidence regarding their association with T2DM is derived from retrospective cohort studies. Prospective clinical trials are necessary to provide more robust evidence supporting their use in routine clinical practice.
Hemogram-derived inflammatory markers could be useful in diagnosis and follow up of the patients with T2DM and its complications. Their cost effectiveness and accessibility make them valuable supplementary diagnostic and prognostic tools in the diabetic population. However, most studies that have reported their association with DM and diabetic complications are cross-sectional, which limit causal interference.
| 1. | Aktas G, Atak Tel BM, Tel R, Balci B. Treatment of type 2 diabetes patients with heart conditions. Expert Rev Endocrinol Metab. 2023;18:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400:1803-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 516] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 3. | Li X, Wang L, Liu M, Zhou H, Xu H. Association between neutrophil-to-lymphocyte ratio and diabetic kidney disease in type 2 diabetes mellitus patients: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1285509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 4. | Li J, Wang X, Jia W, Wang K, Wang W, Diao W, Ou F, Ma J, Yang Y. Association of the systemic immuno-inflammation index, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio with diabetic microvascular complications. Front Endocrinol (Lausanne). 2024;15:1367376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 5. | Li HY, Liu TM. Platelet indices and inflammatory bowel disease: a Mendelian randomization study. Front Immunol. 2024;15:1377915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | García-Escobar A, Lázaro-García R, Goicolea-Ruigómez J, González-Casal D, Fontenla-Cerezuela A, Soto N, González-Panizo J, Datino T, Pizarro G, Moreno R, Cabrera JÁ. Red Blood Cell Distribution Width is a Biomarker of Red Cell Dysfunction Associated with High Systemic Inflammation and a Prognostic Marker in Heart Failure and Cardiovascular Disease: A Potential Predictor of Atrial Fibrillation Recurrence. High Blood Press Cardiovasc Prev. 2024;31:437-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 7. | Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, Di Palo KE, Golden SH, Sperling LS; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; and Council on Hypertension. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation. 2022;145:e722-e759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 323] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 8. | Pellegrini V, La Grotta R, Carreras F, Giuliani A, Sabbatinelli J, Olivieri F, Berra CC, Ceriello A, Prattichizzo F. Inflammatory Trajectory of Type 2 Diabetes: Novel Opportunities for Early and Late Treatment. Cells. 2024;13:1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 9. | Varra FN, Varras M, Varra VK, Theodosis-Nobelos P. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammationmediating treatment options (Review). Mol Med Rep. 2024;29:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 10. | Aktas G, Sit M, Dikbas O, Erkol H, Altinordu R, Erkus E, Savli H. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of Hashimoto's thyroiditis. Rev Assoc Med Bras (1992). 2017;63:1065-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Kurimoto N, Nishida Y, Hosomi S, Itani S, Kobayashi Y, Nakata R, Ominami M, Nadatani Y, Fukunaga S, Otani K, Tanaka F, Nagami Y, Taira K, Kamata N, Fujiwara Y. Neutrophil-to-lymphocyte ratio may predict clinical relapse in ulcerative colitis patients with mucosal healing. PLoS One. 2023;18:e0280252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Aktas G. Hematological predictors of novel Coronavirus infection. Rev Assoc Med Bras (1992). 2021;67 Suppl 1:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 13. | Hashemi Moghanjoughi P, Neshat S, Rezaei A, Heshmat-Ghahdarijani K. Is the Neutrophil-to-Lymphocyte Ratio an Exceptional Indicator for Metabolic Syndrome Disease and Outcomes? Endocr Pract. 2022;28:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Bagyura Z, Kiss L, Lux Á, Csobay-Novák C, Jermendy ÁL, Polgár L, Tabák ÁG, Soós P, Szelid Z, Merkely B, Kőhidai L, Pállinger É. Neutrophil-to-Lymphocyte Ratio Is an Independent Risk Factor for Coronary Artery Disease in Central Obesity. Int J Mol Sci. 2023;24:7397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 15. | Al-Mansoori L, Al-Jaber H, Prince MS, Elrayess MA. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation. 2022;45:31-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 16. | Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, Basson AK, Pheiffer C, Kengne AP. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes. 2023;14:130-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 102] [Article Influence: 51.0] [Reference Citation Analysis (12)] |
| 17. | Ge T, Yu Y, Cui J, Cai L. The adaptive immune role of metallothioneins in the pathogenesis of diabetic cardiomyopathy: good or bad. Am J Physiol Heart Circ Physiol. 2019;317:H264-H275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Chen HL, Wu C, Cao L, Wang R, Zhang TY, He Z. The association between the neutrophil-to-lymphocyte ratio and type 2 diabetes mellitus: a cross-sectional study. BMC Endocr Disord. 2024;24:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | He J, Bian X, Song C, Zhang R, Yuan S, Yin D, Dou K. High neutrophil to lymphocyte ratio with type 2 diabetes mellitus predicts poor prognosis in patients undergoing percutaneous coronary intervention: a large-scale cohort study. Cardiovasc Diabetol. 2022;21:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 20. | Rodríguez-Rodríguez E, Salas-González MD, Ortega RM, López-Sobaler AM. Leukocytes and Neutrophil-Lymphocyte Ratio as Indicators of Insulin Resistance in Overweight/Obese School-Children. Front Nutr. 2021;8:811081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Liu H. Correlation between insulin resistance and the rate of neutrophils-lymphocytes, monocytes-lymphocytes, platelets-lymphocytes in type 2 diabetic patients. BMC Endocr Disord. 2024;24:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 22. | Qiao S, Gao W, Guo S. Neutrophil-Lymphocyte Ratio (NLR) for Predicting Clinical Outcomes in Patients with Coronary Artery Disease and Type 2 Diabetes Mellitus: A Propensity Score Matching Analysis. Ther Clin Risk Manag. 2020;16:437-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Ghasempour Dabaghi G, Rabiee Rad M, Mortaheb M, Darouei B, Amani-Beni R, Mazaheri-Tehrani S, Izadan M, Touhidi A. The Neutrophil-to-Lymphocyte Ratio Predicts Cardiovascular Outcomes in Patients With Diabetes: A Systematic Review and Meta-Analysis. Cardiol Rev. 2025;33:202-211. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 489] [Cited by in RCA: 586] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 25. | Zhu B, Liu Y, Liu W, Cao C, Chen Y, Yi Y, Guo X, Luo Y, Weng S, Peng D. Association of neutrophil-to-lymphocyte ratio with all-cause and cardiovascular mortality in CVD patients with diabetes or pre-diabetes. Sci Rep. 2024;14:24324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Trtica Majnarić L, Guljaš S, Bosnić Z, Šerić V, Wittlinger T. Neutrophil-to-Lymphocyte Ratio as a Cardiovascular Risk Marker May Be Less Efficient in Women Than in Men. Biomolecules. 2021;11:528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Aktas G, Yilmaz S, Kantarci DB, Duman TT, Bilgin S, Balci SB, Atak Tel BM. Is serum uric acid-to-HDL cholesterol ratio elevation associated with diabetic kidney injury? Postgrad Med. 2023;135:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 28. | Jaaban M, Zetoune AB, Hesenow S, Hessenow R. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as novel risk markers for diabetic nephropathy in patients with type 2 diabetes. Heliyon. 2021;7:e07564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Wan H, Wang Y, Fang S, Chen Y, Zhang W, Xia F, Wang N, Lu Y. Associations between the Neutrophil-to-Lymphocyte Ratio and Diabetic Complications in Adults with Diabetes: A Cross-Sectional Study. J Diabetes Res. 2020;2020:6219545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 30. | Akase T, Kawamoto R, Ninomiya D, Kikuchi A, Kumagi T. Neutrophil-to-lymphocyte ratio is a predictor of renal dysfunction in Japanese patients with type 2 diabetes. Diabetes Metab Syndr. 2020;14:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Kang Q, Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 573] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 32. | Tang Y, Li L, Li J. Association between neutrophil-to-lymphocyte ratio and diabetic retinopathy in patients with type 2 diabetes: a cohort study. Front Endocrinol (Lausanne). 2024;15:1396161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Dascalu AM, Georgescu A, Costea AC, Tribus L, El Youssoufi A, Serban D, Arsene AL, Stana D, Alexandrescu C, Cristea BM, Tanasescu D, Bobirca A, Serboiu C, Alius C, Bratu DG. Association Between Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) With Diabetic Retinopathy in Type 2 Diabetic Patients. Cureus. 2023;15:e48581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Rajendrakumar AL, Hapca SM, Nair ATN, Huang Y, Chourasia MK, Kwan RS, Nangia C, Siddiqui MK, Vijayaraghavan P, Matthew SZ, Leese GP, Mohan V, Pearson ER, Doney ASF, Palmer CNA. Competing risks analysis for neutrophil to lymphocyte ratio as a predictor of diabetic retinopathy incidence in the Scottish population. BMC Med. 2023;21:304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Aktas G. Serum C-reactive protein to albumin ratio as a reliable marker of diabetic neuropathy in type 2 diabetes mellitus. Biomol Biomed. 2024;24:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Reference Citation Analysis (0)] |
| 36. | Chen M, Zhu Y, Wang J, Wang G, Wu Y. The Predictive Value of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Levels of Diabetic Peripheral Neuropathy. J Pain Res. 2021;14:2049-2058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Rezaei Shahrabi A, Arsenault G, Nabipoorashrafi SA, Lucke-Wold B, Yaghoobpoor S, Meidani FZ, Rahmati R, Ghaedi A, Khanzadeh S. Relationship between neutrophil to lymphocyte ratio and diabetic peripheral neuropathy: a systematic review and meta-analysis. Eur J Med Res. 2023;28:523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly : scientific basis and clinical implications. Drugs Aging. 2005;22:589-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Atak B, Aktas G, Duman TT, Erkus E, Kocak MZ, Savli H. Diabetes control could through platelet-to-lymphocyte ratio in hemograms. Rev Assoc Med Bras (1992). 2019;65:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Liu W, Lou X, Zhang Z, Chai Y, Yu Q. Association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume with the risk of gestational diabetes mellitus. Gynecol Endocrinol. 2021;37:105-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Sobol AB, Watala C. The role of platelets in diabetes-related vascular complications. Diabetes Res Clin Pract. 2000;50:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Dong G, Huang A, Liu L. Platelet-to-lymphocyte ratio and prognosis in STEMI: A meta-analysis. Eur J Clin Invest. 2021;51:e13386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Chen C, Gu L, Chen L, Hu W, Feng X, Qiu F, Fan Z, Chen Q, Qiu J, Shao B. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Potential Predictors of Prognosis in Acute Ischemic Stroke. Front Neurol. 2020;11:525621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Selvaggio S, Abate A, Brugaletta G, Musso C, Di Guardo M, Di Guardo C, Vicari ESD, Romano M, Luca S, Signorelli SS. Platelettolymphocyte ratio, neutrophiltolymphocyte ratio and monocytetoHDL cholesterol ratio as markers of peripheral artery disease in elderly patients. Int J Mol Med. 2020;46:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Wang RH, Wen WX, Jiang ZP, Du ZP, Ma ZH, Lu AL, Li HP, Yuan F, Wu SB, Guo JW, Cai YF, Huang Y, Wang LX, Lu HJ. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. 2023;14:1115031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 155] [Reference Citation Analysis (0)] |
| 46. | Jha JC, Ho F, Dan C, Jandeleit-Dahm K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin Sci (Lond). 2018;132:1811-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 47. | Duan S, Sun L, Zhang C, Wu L, Nie G, Huang Z, Xing C, Zhang B, Yuan Y. Association of platelet-to-lymphocyte ratio with kidney clinicopathologic features and renal outcomes in patients with diabetic kidney disease. Int Immunopharmacol. 2021;93:107413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Tang L, Xu GT, Zhang JF. Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural Regen Res. 2023;18:976-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 49. | Chen J, Tan W. Platelet activation and immune response in diabetic microangiopathy. Clin Chim Acta. 2020;507:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Wang JR, Chen Z, Yang K, Yang HJ, Tao WY, Li YP, Jiang ZJ, Bai CF, Yin YC, Duan JM, Zhou YY, Geng XQ, Yang Y. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol Metab Syndr. 2020;12:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 51. | Herder C, Kannenberg JM, Huth C, Carstensen-Kirberg M, Rathmann W, Koenig W, Heier M, Püttgen S, Thorand B, Peters A, Roden M, Meisinger C, Ziegler D. Proinflammatory Cytokines Predict the Incidence and Progression of Distal Sensorimotor Polyneuropathy: KORA F4/FF4 Study. Diabetes Care. 2017;40:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 52. | Zhang H, Lin F, Wang Z. Mean platelet volume/platelet count ratio in combination with tumor markers in colorectal cancer: a retrospective clinical study. BMC Cancer. 2023;23:124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 53. | Johansson M, Eriksson AC, Östgren CJ, Whiss PA. Platelet adhesion in type 2 diabetes: impact of plasma albumin and mean platelet volume. Thromb J. 2021;19:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Kosekli MA. Mean platelet volume and platelet to lymphocyte count ratio are associated with hepatitis B-related liver fibrosis. Eur J Gastroenterol Hepatol. 2022;34:324-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 55. | Llobet D, Vallvé C, Tirado I, Vilalta N, Carrasco M, Oliver A, Mateo J, Fontcuberta J, Souto JC. Platelet hyperaggregability and venous thrombosis risk: results from the RETROVE project. Blood Coagul Fibrinolysis. 2021;32:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Kelem A, Adane T, Shiferaw E. Insulin Resistance-Induced Platelet Hyperactivity and a Potential Biomarker Role of Platelet Parameters: A Narrative Review. Diabetes Metab Syndr Obes. 2023;16:2843-2853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 57. | Das D, Shruthi NR, Banerjee A, Jothimani G, Duttaroy AK, Pathak S. Endothelial dysfunction, platelet hyperactivity, hypertension, and the metabolic syndrome: molecular insights and combating strategies. Front Nutr. 2023;10:1221438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 58. | Ding Q, Wang F, Guo X, Liang M. The relationship between mean platelet volume and metabolic syndrome in patients with type 2 diabetes mellitus: A retrospective study. Medicine (Baltimore). 2021;100:e25303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Mi AE, Abdallah N, Eldars W. Mean Platelet Volume and Platelet Distribution Width Correlate with Microvascular Complications in Egyptian People with Type 2 Diabetes Mellitus. Curr Diabetes Rev. 2021;17:e080621193947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 60. | Inoue H, Saito M, Kouchi K, Asahara SI, Nakamura F, Kido Y. Association between mean platelet volume in the pathogenesis of type 2 diabetes mellitus and diabetic macrovascular complications in Japanese patients. J Diabetes Investig. 2020;11:938-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Wu M, Xiao L, Yang X. Positive Relationship of Platelet Volume Indices with HbA1c in Unselected Type-2 Diabetes Mellitus Patients. Clin Lab. 2019;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Lordan R, Tsoupras A, Zabetakis I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 2021;45:100694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 63. | Cassano V, Armentaro G, Iembo D, Miceli S, Fiorentino TV, Succurro E, Perticone M, Arturi F, Hribal ML, Montalcini T, Andreozzi F, Sesti G, Pujia A, Sciacqua A. Mean platelet volume (MPV) as new marker of diabetic macrovascular complications in patients with different glucose homeostasis : Platelets in cardiovascular risk. Cardiovasc Diabetol. 2024;23:89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 64. | Tian C, Song J, He D, Wu J, Sun Z, Sun Z. Predictive Value of Mean Platelet Volume/Platelet Count for Prognosis in Acute Myocardial Infarction. Int Heart J. 2018;59:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Ot S, Zafar L, Beg M, Siddiqui OA. Association of Mean Platelet Volume with Risk Factors and Functional Outcome in Acute Ischemic Stroke. J Neurosci Rural Pract. 2021;12:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Li S, Wang C, Zhong XW, Li HQ, Fu XQ, Ran XW. [Variance of mean platelet volume in subjects with normal glucose tolerance, impaired glucose regulation and type 2 diabetic mellitus and its relationship with diabetic peripheral artery disease]. Zhonghua Yi Xue Za Zhi. 2012;92:232-235. [PubMed] |
| 67. | Sengupta P, Priyadarshini A, Kumar Behera P, Padarabinda Tripathy K. Exploring Platelet Indices as Predictors of Nephropathy Severity in Type 2 Diabetes Mellitus: A Hospital-Based Cross-Sectional Analysis. Cureus. 2024;16:e71796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Xu B, Zhang Y, Chen G, Feng J, Gan L. Association of mean platelet volume/lymphocyte ratio with inflammation in non-dialysis patients with chronic kidney disease stages 1-4: A retrospective study. Front Immunol. 2022;13:1041356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Aktas G. Association between the Prognostic Nutritional Index and Chronic Microvascular Complications in Patients with Type 2 Diabetes Mellitus. J Clin Med. 2023;12:5952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 70. | Karagöz IK, Karagöz A, Özkalaycı F, Doğan C, Kocabay G, Elbay A. Relation Between Platelet Reactivity Levels and Diabetic Retinopathy Stage in Patient with Type 2 Diabetes Mellitus by Using Multiplate Whole Blood Aggregometry. Semin Ophthalmol. 2021;36:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Rasoulinejad SA. Is there an association between mean platelet volume and diabetic retinopathy? A case-control study. Caspian J Intern Med. 2021;12:129-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 72. | Wang Y, Miao Y, Wan Q. Association of white blood cell count to mean platelet volume ratio with type 2 diabetic peripheral neuropathy in a Chinese population: a cross-sectional study. BMC Endocr Disord. 2024;24:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 73. | Nimmala SG, Gokhale VS, Yadav P, Mangudkar S, Malik S. A Study of the Mean Platelet Volume and Plasma Fibrinogen in Type Two Diabetes Mellitus Patients Versus Healthy Controls and Their Role as Early Markers of Diabetic Microvascular Complications. Cureus. 2024;16:e65458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 74. | Ananthaseshan S, Bojakowski K, Sacharczuk M, Poznanski P, Skiba DS, Prahl Wittberg L, McKenzie J, Szkulmowska A, Berg N, Andziak P, Menkens H, Wojtkowski M, Religa D, Lundell F, Guzik T, Gaciong Z, Religa P. Red blood cell distribution width is associated with increased interactions of blood cells with vascular wall. Sci Rep. 2022;12:13676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 75. | Namazi G, Heidar Beygi S, Vahidi MH, Asa P, Bahmani F, Mafi A, Raygan F. Relationship Between Red Cell Distribution Width and Oxidative Stress Indexes in Patients with Coronary Artery Disease. Rep Biochem Mol Biol. 2023;12:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 76. | Hong J, Hu X, Liu W, Qian X, Jiang F, Xu Z, Shen F, Zhu H. Impact of red cell distribution width and red cell distribution width/albumin ratio on all-cause mortality in patients with type 2 diabetes and foot ulcers: a retrospective cohort study. Cardiovasc Diabetol. 2022;21:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 77. | Williams A, Bissinger R, Shamaa H, Patel S, Bourne L, Artunc F, Qadri SM. Pathophysiology of Red Blood Cell Dysfunction in Diabetes and Its Complications. Pathophysiology. 2023;30:327-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 78. | Asmamaw M, Sime T, Kene K, Fekadie Baye M, Teshome M, Zawdie B. Evaluation of Red Blood Cell Parameters as a Biomarker for Long-Term Glycemic Control Monitoring Among Type 2 Diabetic Patients in Southwest Ethiopia: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2021;14:4993-5000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Arkew M, Asmerom H, Tesfa T, Tsegaye S, Gemechu K, Bete T, Haile K. Red Blood Cell Parameters and Their Correlation with Glycemic Control Among Type 2 Diabetic Adult Patients in Eastern Ethiopia: A Comparative Cross-Sectional Study. Diabetes Metab Syndr Obes. 2022;15:3499-3507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 80. | Nah EH, Cho S, Park H, Kim S, Cho HI. Associations of complete blood count parameters with pancreatic beta-cell function and insulin resistance in prediabetes and type 2 diabetes mellitus. J Clin Lab Anal. 2022;36:e24454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Zhao F, Liu M, Kong L. Association between red blood cell distribution width-to-albumin ratio and diabetic retinopathy. J Clin Lab Anal. 2022;36:e24351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 82. | Chen J, Zhang D, Zhou D, Dai Z, Wang J. Association between red cell distribution width/serum albumin ratio and diabetic kidney disease. J Diabetes. 2024;16:e13575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 83. | Tsilingiris D, Makrilakis K, Barmpagianni A, Dalamaga M, Tentolouris A, Kosta O, Eleftheriadou I, Liatis S. The glycemic status determines the direction of the relationship between red cell distribution width and HbA1c. J Diabetes Complications. 2021;35:108012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Zhang D, Zhang S, Wang L, Pan T, Zhong X. The relationship between red blood cell distribution and islet β-cell function indexes in patients with type 2 diabetes. BMC Endocr Disord. 2021;21:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Gusev E, Sarapultsev A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int J Mol Sci. 2023;24:7910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 86. | Ainiwaer A, Kadier K, Abulizi A, Hou WQ, Rehemuding R, Maimaiti H, Yakufu M, Ma X, Ma YT. Association of red cell distribution width (RDW) and the RDW to platelet count ratio with cardiovascular disease among US adults: a cross-sectional study based on the National Health and Nutrition Examination Survey 1999-2020. BMJ Open. 2023;13:e068148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 87. | Subhashree AR. Red Cell Distribution Width and Serum BNP Level Correlation in Diabetic Patients with Cardiac Failure: A Cross - Sectional Study. J Clin Diagn Res. 2014;8:FC01-FC03. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 88. | Bhutto AR, Abbasi A, Abro AH. Correlation of Hemoglobin A1c with Red Cell Width Distribution and Other Parameters of Red Blood Cells in Type II Diabetes Mellitus. Cureus. 2019;11:e5533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Tonelli M, Wiebe N, James MT, Naugler C, Manns BJ, Klarenbach SW, Hemmelgarn BR. Red cell distribution width associations with clinical outcomes: A population-based cohort study. PLoS One. 2019;14:e0212374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Nada AM. Red cell distribution width in type 2 diabetic patients. Diabetes Metab Syndr Obes. 2015;8:525-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 91. | Afonso L, Zalawadiya SK, Veeranna V, Panaich SS, Niraj A, Jacob S. Relationship between red cell distribution width and microalbuminuria: a population-based study of multiethnic representative US adults. Nephron Clin Pract. 2011;119:c277-c282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Gu L, Xue S. The Association Between Red Blood Cell Distribution Width and the Severity of Diabetic Chronic Kidney Disease. Int J Gen Med. 2021;14:8355-8363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Xiong XF, Yang Y, Chen X, Zhu X, Hu C, Han Y, Zhao L, Liu F, Sun L. Red cell distribution width as a significant indicator of medication and prognosis in type 2 diabetic patients. Sci Rep. 2017;7:2709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Zhang J, Zhang R, Wang Y, Li H, Han Q, Wu Y, Wang S, Guo R, Wang T, Li L, Liu F. The association between the red cell distribution width and diabetic nephropathy in patients with type-2 diabetes mellitus. Ren Fail. 2018;40:590-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Wang B, Lu H, Gong Y, Ying B, Cheng B. The Association between Red Blood Cell Distribution Width and Mortality in Critically Ill Patients with Acute Kidney Injury. Biomed Res Int. 2018;2018:9658216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Engström G, Smith JG, Persson M, Nilsson PM, Melander O, Hedblad B. Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J Intern Med. 2014;276:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 97. | Arif MA, Syed F, Niazi R, Arif SA, Javed MU, Bashir A, Mansoor S. Assessment of red cell distribution width, glycaemic control and diabetes related complications - the ARDENT Study. J Pak Med Assoc. 2019;69:483-488. [PubMed] |
| 98. | Malandrino N, Wu WC, Taveira TH, Whitlatch HB, Smith RJ. Association between red blood cell distribution width and macrovascular and microvascular complications in diabetes. Diabetologia. 2012;55:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 99. | Blaslov K, Kruljac I, Mirošević G, Gaćina P, Kolonić SO, Vrkljan M. The prognostic value of red blood cell characteristics on diabetic retinopathy development and progression in type 2 diabetes mellitus. Clin Hemorheol Microcirc. 2019;71:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Magri CJ, Fava S. Red blood cell distribution width and diabetes-associated complications. Diabetes Metab Syndr. 2014;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | AlShareef AA, Alrawaili MS, Almutairi SA, Ayyad MM, Alshora W. Association of Hematological Parameters and Diabetic Neuropathy: A Retrospective Study. Diabetes Metab Syndr Obes. 2024;17:779-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |