Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.105069
Revised: March 12, 2025
Accepted: April 16, 2025
Published online: June 15, 2025
Processing time: 154 Days and 15.7 Hours
Post-transplant diabetes mellitus (PTDM) is a common metabolic adverse event following kidney transplantation, negatively impacting graft function and patient outcomes.
To evaluate the frequency of PTDM and to determine predictive factors in living donor individuals who have undergone kidney transplantation.
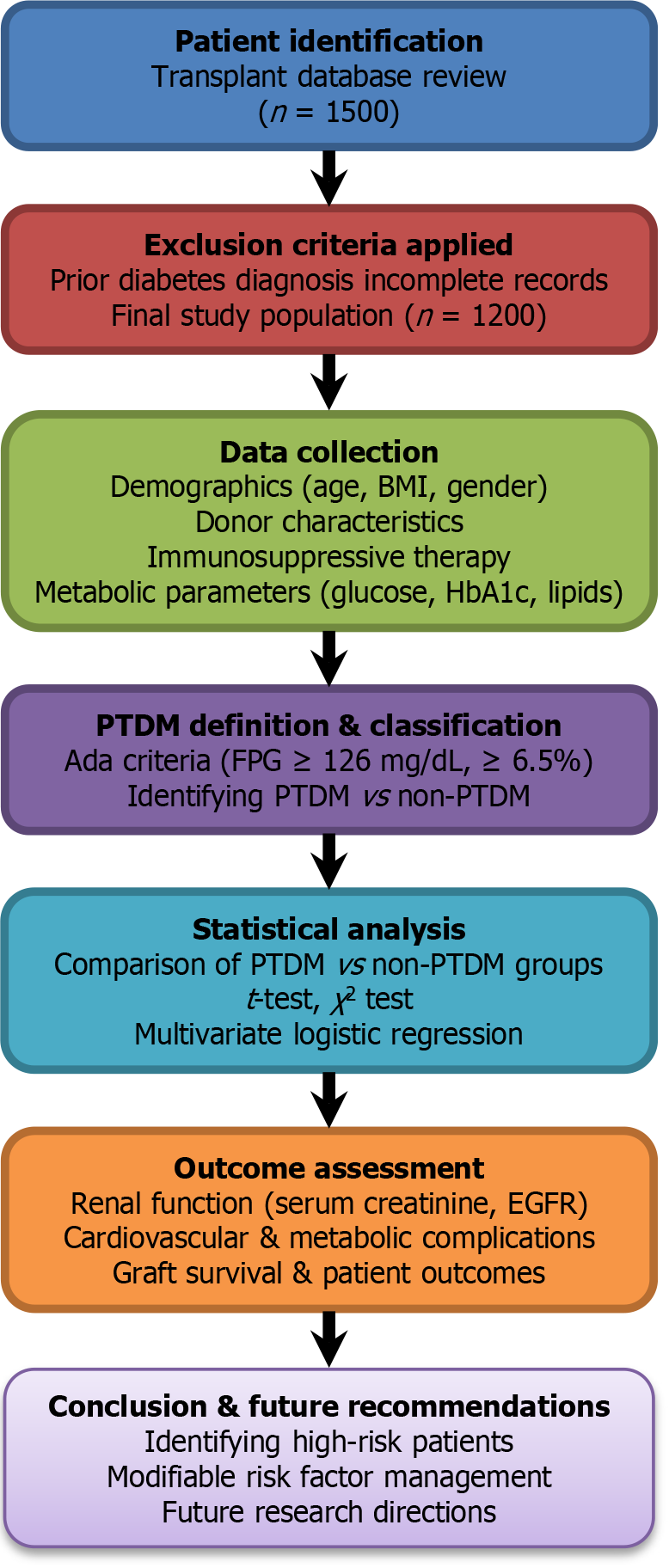
A retrospective analysis was conducted on 1200 living donor kidney transplant recipients treated between 2016 and 2023. Demographic, clinical, and treatment data were collected, and PTDM was identified based on American Diabetes Association criteria. Statistical analysis included logistic regression analysis to determine independent predictors of PTDM.
PTDM was diagnosed in 162 patients (13.5%). Risk factors included older age [odds ratio (OR) 1.03, P = 0.03], increased body mass index (OR 1.08, P = 0.02), a genetic predisposition to diabetes (OR 1.95, P = 0.001), and corticosteroid use (OR 1.30, P = 0.04). Most PTDM cases (61.7%) occurred during the initial 6 months after transplant. Tacrolimus-based regimens were more commonly associated with PTDM compared to other protocols. Renal function at 12 months was com
PTDM remains a significant concern in kidney transplantation, particularly among patients with modifiable risk factors. Optimizing immunosuppressive regimens, implementing early metabolic monitoring, and addressing modifiable risks such as BMI may help reduce PTDM incidence. Additional research is required to evaluate extended-term results and refine preventive strategies.
Core Tip: This study investigated the incidence and risk factors of post-transplant diabetes mellitus (PTDM) in a large cohort of living donor kidney transplant recipients. The findings highlight the significant role of modifiable risk factors such as body mass index and corticosteroid use, as well as non-modifiable factors like age and family history of diabetes. Early onset PTDM, predominantly within the first 6 months post-transplant, underscores the importance of vigilant metabolic monitoring and tailored immunosuppressive strategies to reduce PTDM risk and improve long-term patient and graft outcomes.
- Citation: Huseynov A, Kuşlu Çicek SN. Trends and predictors of diabetes mellitus after living kidney transplantation: A retrospective study. World J Diabetes 2025; 16(6): 105069
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/105069.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.105069
Kidney transplantation has become the recommended therapeutic choice for individuals with end-stage renal disease, substantially improving patient longevity and overall well-being[1,2]. However, a notable metabolic complication that arises in this population is post-transplant diabetes mellitus (PTDM), which can adversely affect both graft function and patient outcome[3]. PTDM is defined as new-onset hyperglycemia diagnosed after transplantation and is influenced by multiple factors including immunosuppressive regimens, recipient characteristics, and genetic predisposition[4]. Recent estimates suggest that the incidence of PTDM varies between 10% and 40%, contingent on the diagnostic standards and the population studied, highlighting the clinical importance of accurate identification and management strategies[5].
Living donor kidney transplantation offers several advantages over deceased donor transplantation, such as shorter waiting times and better graft survival. Despite these benefits, patients undergoing living donor transplantation remain at risk for PTDM, which can result in an increased frequency of cardiovascular adverse outcomes, reduced graft survival, and increased morbidity[1]. Nonetheless, it is important to acknowledge that the findings from living donor recipients may not be fully generalizable to deceased donor populations, necessitating future comparative research. Therefore, identifying key predictive factors and tracking incidence trends of PTDM in this specific population is critical for the occurrence of targeted interventions and improved prolonged-term outcomes[3,4,6].
Although this study provides valuable insights, the retrospective design may introduce potential biases, such as incomplete data and selection bias (e.g., the exclusion of prediabetic patients). Future work should consider sensitivity analysis to address missing or irregular follow-up data. By shedding light on modifiable and non-modifiable risk com
This retrospective analysis encompassed data from 1200 patients who received living donor kidney transplants at our center from January 2016 to December 2023. Individuals aged 18 years or older with sufficient clinical records and regular post-transplant follow-up were eligible for inclusion. Patients with a prior diagnosis of type 1 or type 2 diabetes, as well as those with incomplete medical records were excluded from the research. To visually summarize the study metho
Demographic variables: Age, gender, body mass index (BMI), family history of diabetes, and other comorbidities such as hypertension, dyslipidemia, and cardiovascular disease.
Donor characteristics: Donor age, gender, blood type compatibility, and whether the donor was a relative or non-relative.
Transplantation process and immunosuppressive therapy: Details on the specific immunosuppressive regimens used, including calcineurin inhibitors, antiproliferative agents, and corticosteroids. Although tacrolimus is the primary agent utilized, future analyses could include comparative data (e.g., cyclosporine, mTOR inhibitors) to provide odds ratios or risk stratification for different regimens.
Metabolic and clinical parameters: Fasting blood glucose, HbA1c levels, serum creatinine, blood urea nitrogen, lipid profile, blood pressure values, and other relevant laboratory test results recorded at various intervals following transplantation.
Definition of PTDM: PTDM was defined as new-onset hyperglycemia that met any of the following criteria at any time after transplantation: (1) Fasting plasma glucose ≥ 126 mg/dL; (2) Random plasma glucose ≥ 200 mg/dL; and (3) HbA1c ≥ 6.5%.
Based on these criteria, 13.5% of the study population (162 out of 1200) had PTDM.
All data were collected in accordance with ethical guidelines, with patient confidentiality maintained through anony
Data were analyzed using SPSS software. Continuous variables were presented as mean ± SD or median with minimum and maximum values, while categorical variables were summarized as frequency and percentage. Comparisons between patients who developed PTDM and those who did not were conducted using the independent samples t-test or Mann-Whitney U test for continuous variables, and the χ2 test for categorical variables. Where data were incomplete (e.g., missing laboratory values), bias was minimized through careful review and potential imputation strategies; however, incomplete follow-up remains a limitation. Multivariate logistic regression analysis was used to determine independent risk factors for PTDM. A P value < 0.05 was considered statistically significant.
A total of 1200 patients who underwent living donor kidney transplantation were included in the study. The mean age of the entire cohort was 46.2 ± 11.7 years, and 59.2% of the patients were male. PTDM was identified in 162 patients, resulting in an overall incidence rate of 13.5%.
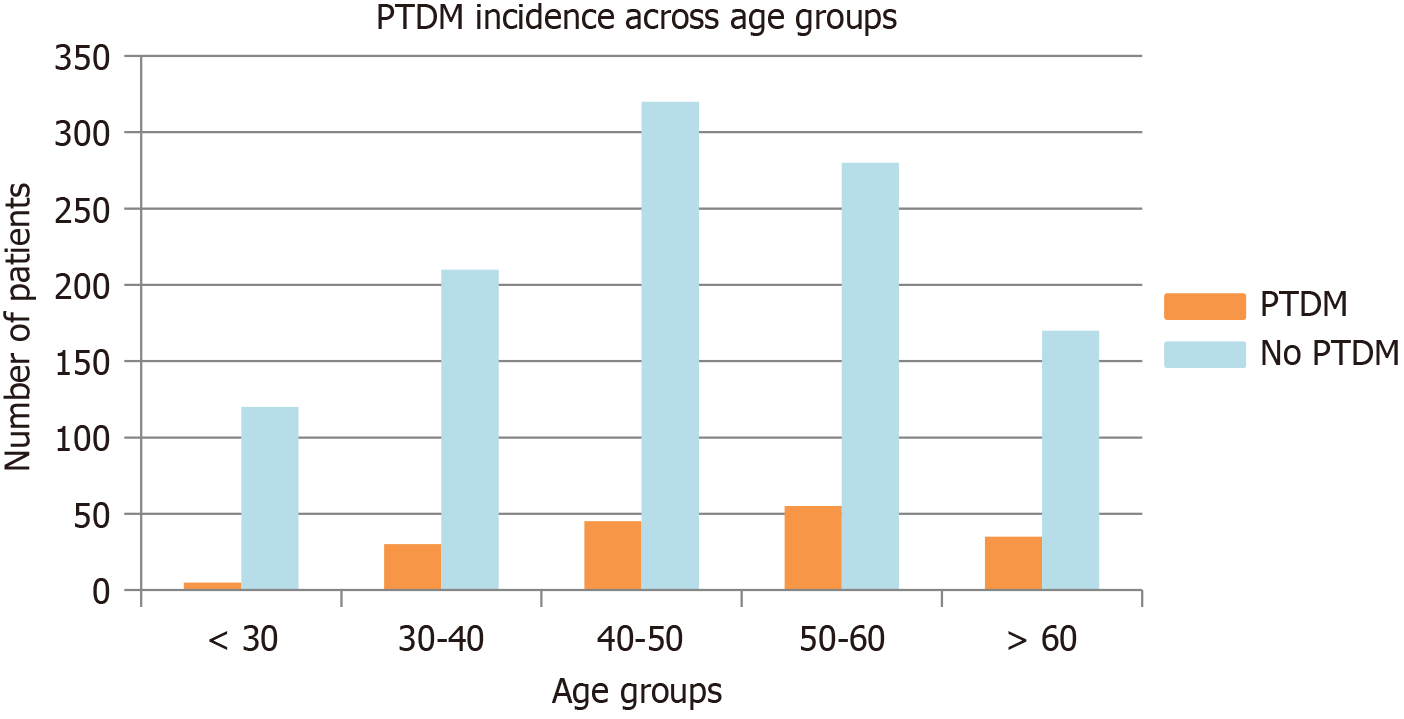
Table 1 provides a summary of the demographic and clinical characteristics of the PTDM and non-PTDM groups. Patients who developed PTDM were generally older (48.5 ± 12.3 years vs 45.8 ± 11.6 years, P = 0.02) and had a higher mean BMI (27.2 ± 3.5 kg/m² vs 25.5 ± 3.5 kg/m², P = 0.01) compared to those without PTDM (Figure 2). The bar chart in Figure 2 compares the number of patients with and without PTDM across different age groups (grouped in 10-year intervals), highlighting the higher prevalence of PTDM among older recipients. In addition, a significantly higher proportion of patients with PTDM reported a positive family history of diabetes (40.1% vs 24.6%, P = 0.003).
| Characteristic | All patients (n = 1200) | PTDM (n = 162) | No PTDM (n = 1038) | P value |
| Age (years), mean ± SD | 46.2 ± 11.7 | 48.5 ± 12.3 | 45.8 ± 11.6 | 0.02 |
| Male | 710 (59.2) | 100 (61.7) | 610 (58.8) | 0.45 |
| Female | 490 (40.8) | 62 (38.3) | 428 (41.2) | 0.41 |
| BMI (kg/m²), mean ± SD | 25.8 ± 3.6 | 27.2 ± 3.5 | 25.5 ± 3.5 | 0.01 |
| Family history of diabetes | 320 (26.7) | 65 (40.1) | 255 (24.6) | 0.003 |
| Corticosteroid use | 1160 (96.7) | 162 (100) | 998 (96.1) | 0.02 |
Despite a similar distribution of males and females between groups (P > 0.05), the PTDM group exhibited a statistically significant difference in corticosteroid use (100% vs 96.1%, P = 0.02). Other clinical parameters, including initial graft function, did not differ between the two groups.
A logistic regression model was used to identify independent predictors of PTDM (Table 2). Older age, higher BMI, family history of diabetes, and corticosteroid use were significantly associated with an increased risk of PTDM (P < 0.05 for all).
| Variable | OR | 95%CI | P value |
| Age (per 1 year increase) | 1.03 | 1.01-1.06 | 0.03 |
| BMI (per 1 kg/m² increase) | 1.08 | 1.02-1.13 | 0.02 |
| Family history of diabetes | 1.95 | 1.36-2.79 | 0.001 |
| Corticosteroid use | 1.30 | 1.01-1.60 | 0.04 |
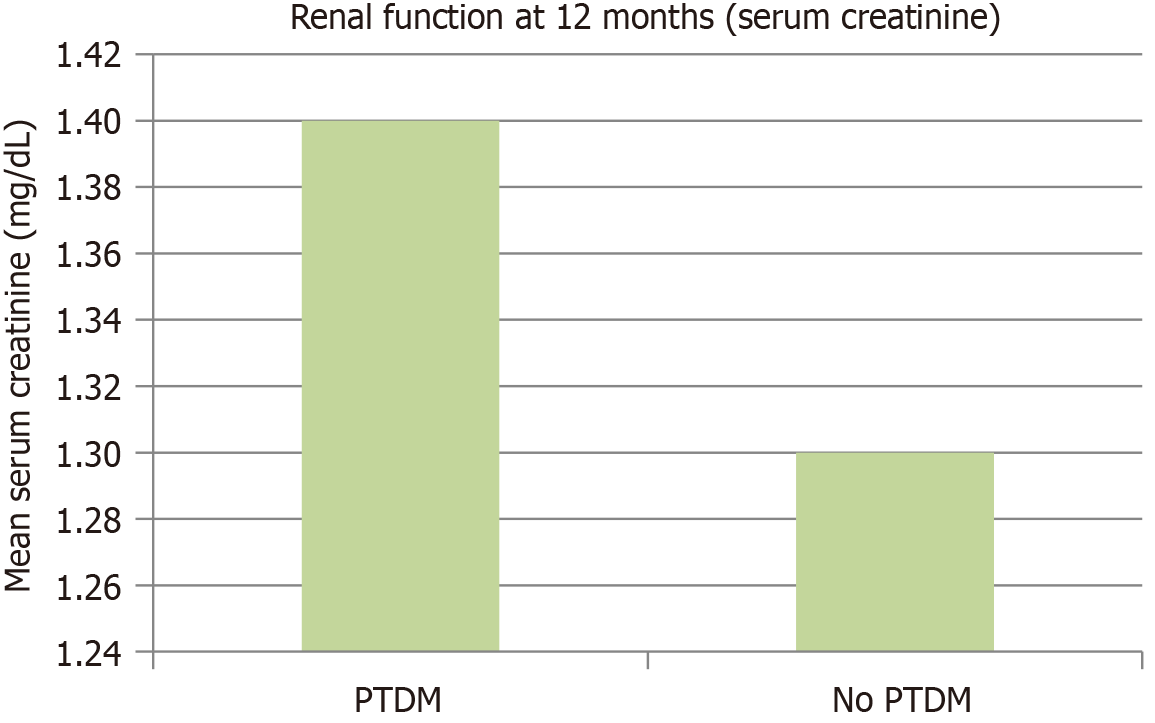
Renal function assessments (including serum creatinine and estimated glomerular filtration rate) at 12 months post-transplant did not differ significantly between the PTDM and non-PTDM groups (P > 0.05; Figure 3). Figure 3 demon
PTDM occurred in 13.5% (n = 162) of the study population. Advanced age and elevated BMI were strongly linked to PTDM. A genetic predisposition to diabetes emerged as a significant contributing factor. Corticosteroid use was also linked to increased PTDM incidence.
PTDM remains a significant complication following kidney transplantation, impacting both patient and graft outcomes. In this study, we observed a PTDM incidence rate of 13.5% among 1200 living donor kidney transplant recipients, con
A notable finding in this study was the absence of a statistically significant difference in renal function between PTDM and non-PTDM groups at 12 months post-transplant. Several factors may contribute to this result. First, the follow-up period of 12 months may not be sufficient to observe long-term renal function deterioration associated with PTDM. PTDM-related metabolic alterations, such as hyperglycemia-induced glomerular damage, often gradually develop over an extended period. Second, the relatively small number of PTDM cases (n = 162) compared to the non-PTDM group (n = 1038) may have limited the statistical power to detect subtle differences in renal outcomes. Third, aggressive post-transplant monitoring and optimized immunosuppressive regimens in our cohort may have mitigated early renal function decline, thereby minimizing short-term differences between the groups. Future studies with longer follow-up periods and larger, more diverse patient populations may be necessary to determine whether PTDM has a significant impact on long-term graft survival and renal function.
Nevertheless, it is crucial to note that the retrospective design introduces inherent limitations, including selection bias and potential missing data. We recommend sensitivity analyses or prospective cohort designs for future research to better handle these issues. Moreover, while our findings are specific to living donor recipients, additional research is needed to compare outcomes with deceased donor transplant populations, as these groups often have different risk profiles.
Furthermore, in terms of information collection, the scope of data gathered was relatively limited, potentially over
Several strategies can be employed to reduce the likelihood of PTDM among kidney transplant recipients. Modifiable risk factors, such as obesity and immunosuppressive regimens, present actionable targets. Weight management before and after transplantation can lower PTDM risk, with some studies advocating for structured preoperative weight loss programs[8]. Additionally, the careful selection and monitoring of immunosuppressive protocols, particularly mini
Although statistically significant, the difference in corticosteroid use between the PTDM group (100%) and the control group (96.1%) warrants further exploration regarding its clinical implications. Specifically, dosage and treatment duration were not comprehensively analyzed in this study, leaving open the question of whether varied corticosteroid regimens could differentially affect PTDM risk. Future investigations should focus on the precise dosing, cumulative exposure, and timing of corticosteroid administration to better elucidate its role in PTDM onset and severity.
Furthermore, regular post-transplant screening for hyperglycemia and preemptive management using lifestyle interventions or pharmacological agents, such as metformin, can help delay or prevent PTDM onset in susceptible individuals[11].
The association between PTDM and older age, higher BMI, and family history of diabetes observed in this study closely aligns with prior research[8,12]. For instance, a large multicenter study identified these variables as independent risk factors for PTDM, reinforcing the need for targeted interventions[9]. The predominance of early-onset PTDM during the initial 6 months following transplant in our cohort further mirrors findings in other studies, suggesting the influence of perioperative factors and initial immunosuppressive therapy[13].
A key strength of this research is the incorporation of a large, homogenous cohort of living donor kidney transplant recipients, enabling detailed analysis of PTDM risk factors in this specific population. The use of standardized diagnostic criteria for PTDM also enhances the reliability and comparability of the results with existing literature. Additionally, the retrospective design allowed for the analysis of long-term trends in PTDM incidence and associated factors.
However, certain limitations exist. The retrospective design of the study might introduce biases related to incomplete or missing data, particularly in cases with irregular follow-up. Moreover, the lack of data on insulin resistance markers
This research underscores the necessity for thorough strategies to reduce PTDM risk in living donor kidney transplant recipients. Addressing modifiable risk factors, optimizing immunosuppressive regimens, and implementing regular metabolic monitoring can significantly improve outcomes. Given the retrospective nature of this work, prospective studies with extended follow-up, inclusion of insulin resistance or genetic markers, and comparative analyses across various immunosuppressive protocols are needed to deepen our understanding of PTDM etiology and management. Future prospective studies involving diverse populations and exploring novel therapeutic approaches are essential to advancing our understanding and management of PTDM.
| 1. | Pham NT, Cruz D, Madera-Marin L, Ravender R, Garcia P. Diabetic Kidney Disease in Post-Kidney Transplant Patients. J Clin Med. 2024;13:793. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Tantisattamo E, Hanna RM, Reddy UG, Ichii H, Dafoe DC, Danovitch GM, Kalantar-Zadeh K. Novel options for failing allograft in kidney transplanted patients to avoid or defer dialysis therapy. Curr Opin Nephrol Hypertens. 2020;29:80-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Hecking M, Sharif A, Eller K, Jenssen T. Management of post-transplant diabetes: immunosuppression, early prevention, and novel antidiabetics. Transpl Int. 2021;34:27-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 4. | Addendum. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44(Suppl. 1):S15-S33. Diabetes Care. 2021;44:2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002;62:1440-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Lin H, Yan J, Yuan L, Qi B, Zhang Z, Zhang W, Ma A, Ding F. Impact of diabetes mellitus developing after kidney transplantation on patient mortality and graft survival: a meta-analysis of adjusted data. Diabetol Metab Syndr. 2021;13:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Palepu S, Prasad GV. New-onset diabetes mellitus after kidney transplantation: Current status and future directions. World J Diabetes. 2015;6:445-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 8. | Kawai T, Benedict Cosimi A. Induction of tolerance in clinical kidney transplantation. Clin Transplant. 2010;24 Suppl 22:2-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Axelrod DA, Cheungpasitporn W, Bunnapradist S, Schnitzler MA, Xiao H, McAdams-DeMarco M, Caliskan Y, Bae S, Ahn JB, Segev DL, Lam NN, Hess GP, Lentine KL. Posttransplant Diabetes Mellitus and Immunosuppression Selection in Older and Obese Kidney Recipients. Kidney Med. 2022;4:100377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Pilch NA, Bowman LJ, Taber DJ. Immunosuppression trends in solid organ transplantation: The future of individualization, monitoring, and management. Pharmacotherapy. 2021;41:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 11. | Yan J, Yang X, Wang J, Cai H, Che X, Ying L, Zhang T, Chen Q, Xia J, Gu L, Yuan X, Chen R, Li D, Liu Z, Dong K, He L, Zhang M, Mou S. Metabolic Risk Profile and Graft Function Deterioration 2 Years After Kidney Transplant. JAMA Netw Open. 2023;6:e2349538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Alajous S, Budhiraja P. New-Onset Diabetes Mellitus after Kidney Transplantation. J Clin Med. 2024;13:1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 13. | Rysz J, Franczyk B, Radek M, Ciałkowska-Rysz A, Gluba-Brzózka A. Diabetes and Cardiovascular Risk in Renal Transplant Patients. Int J Mol Sci. 2021;22:3422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Rudzki G, Knop-Chodyła K, Piasecka Z, Kochanowska-Mazurek A, Głaz A, Wesołek-Bielaska E, Woźniak M. Managing Post-Transplant Diabetes Mellitus after Kidney Transplantation: Challenges and Advances in Treatment. Pharmaceuticals (Basel). 2024;17:987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |