Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.104706
Revised: March 4, 2025
Accepted: March 28, 2025
Published online: June 15, 2025
Processing time: 166 Days and 14.2 Hours
The global prevalence of diabetes has surged in recent years, with diabetic kidney disease (DKD) emerging as a major complication. Traditional therapies have had limited success in slowing progression to end-stage kidney disease. However, novel therapies, particularly sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, which were initially developed for hyperglycemia management, have transformed the treatment of obesity, heart failure, cardiovascular disease, and more recently, DKD. SGLT2 inhibitors have consistently and significantly reduced cardiovascular events, albuminuria, and glomerular filtration rate, highlighting their efficacy across diverse clinical presentations for patients with kidney impairment. Although fewer studies have specifically investigated GLP-1 receptor agonists in patients with kidney disease, existing evidence underscores their potential to slow renal disease progression, reduce albuminuria, and improve clinically relevant outcomes. However, further research is needed to better identify patients most likely to benefit from treatment. Together, these therapies represent valuable advancements for DKD, offering significant reductions in morbidity and mortality and shifting the management of the disease by becoming essential pillars for the treatment of these patients.
Core Tip: Diabetes is the leading cause of chronic kidney disease. Until recently, treatment options for diabetes-associated renal impairment were limited until recently. Over the past decade, research in this field has expanded considerably, incorporating several new therapies with proven benefits in slowing kidney disease progression, especially sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists. Understanding the state of research with these therapies and clinical recommendations for their use in the context of diabetic kidney disease is a crucial step in providing optimized treatment options for patients with this condition.
- Citation: Santos GL, dos Santos CF, Rocha GR, Calmon MS, Lemos FF, Silva LG, Luz MS, Pinheiro SL, Botelho AC, de Melo FF. Beyond glycemic control: Roles for sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists in diabetic kidney disease. World J Diabetes 2025; 16(6): 104706
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/104706.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.104706
The global prevalence of diabetes has surged in recent years, now affecting over 10% of the adult population, with projections indicating it may exceed 1.3 billion cases by 2050[1,2]. Diabetic kidney disease (DKD), one of the greatest complications related to diabetes, currently affects 20% to 40% of patients with diabetes and ranks as the leading cause of chronic kidney disease (CKD) in the world, while accounting for more than 40% of the cases of end-stage kidney disease (ESKD) in the United States[1,3,4].
DKD is defined as a state of persistent elevation in albuminuria, reduction in estimated glomerular filtration rates (eGFR), or both, that is clinically attributed to diabetes mellitus without signs or symptoms of other causes of kidney disease[5].
Until recent years, the standard approach to CKD primarily consisted of dietary adjustments, controlling the underlying condition, and using renin-angiotensin system (RAS) inhibitors. All of these combined can slow the progression to ESKD and its related complications[6]. However, this approach has limited efficacy, particularly in specific patient subgroups where the benefits of intervention remained uncertain. For many years, these limitations sparked research for complementary therapies that could increase the likelihood of better prognosis for patients with DKD and better control disease progression.
In recent years, the emergence of novel therapies in this context has marked a paradigm shift in medicine. Particular emphasis is given to antidiabetic agents such as sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1 RAs). In addition to their effects on glucose control, these medications have been shown to be useful tools in the setting of heart failure (HF), cardiovascular disease, and, particularly kidney disease[7], as their use has been linked to slower progression to renal failure and prevention of several clinical outcomes of interest for all of these conditions.
This review aims to highlight the pivotal trials that established SGLT2i and GLP-1 RAs as essential pillars in CKD management, particularly within the context of diabetes, and to provide insights on current recommendations for the use of these drugs and management of DKD.
A robust body of evidence supports the efficacy of RAS inhibitors, namely angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARBs) in decelerating the rate of glomerular filtration rate (GFR) decline and attenuating albuminuria, thereby conferring long-term renal protection in both diabetic and non-diabetic setups[8-11]. Consequently, ACEi/ARBs have remained a cornerstone in CKD management for years[12,13].
In their 2024 guidelines, The Kidney Disease: Improving Global Outcomes (KDIGO) organization, recommends the use of these medications at the highest tolerated doses in patients with severe urinary albumin to creatinine ratios (uACR) ≥ 300 mg/g at baseline, irrespective of the presence of diabetes [14]. The American Diabetes Association (ADA) and KDIGO organization also highlight the significant role that these medications have in patients with concomitant hypertension and diabetes, which account for the most studied population in initial trials of RAS inhibitors[15].
Among patients with DKD, the use of ACEi or ARBs and their effects in reducing albumin excretion have been well-documented and serve as important assets in the preservation of GFR in patients that present severe albuminuria at baseline[16-19].
Prolonged aldosterone exposure and/or mineralocorticoid receptor (MR) hyperactivation have long been linked to increased inflammation, fibrosis, and tissue remodeling, contributing to cardiac and renal damage[20,21]. Steroidal MR antagonists (MRAs), such as spironolactone and eplerenone, are valuable in treating conditions like HF and resistant hypertension[22]. However, their use in CKD is limited by the heightened risk of hyperkalemia in this subset of patients, especially when diabetes is also present.
Non-steroidal MRAs (nsMRAs) differ significantly from steroidal MRAs and from each other, as they were developed based on distinct properties such as affinity, specificity, and tissue distribution[23]. Despite employment of different nsMRAs in a multitude of scenarios, we will discuss finerenone in depth for its better studied effects in the CKD context.
Finerenone offers a more selective action on MRs compared to eplerenone, but it is still able to maintain high potency and affinity for these receptors like spironolactone[24]. Overall, finerenone significantly reduces tissue remodeling and albuminuria while presenting less side effects, including a smaller risk of hyperkalemia compared to other steroidal MRAs, making it a safer option for CKD patients[23,25].
The definition of clinical aspects of finerenone was primarily conducted by the ARTS trials, which evaluated its safety, tolerability and non-inferiority compared to other MRAs or to placebo[26-28]. However, decisive clinical evidence regarding finerenone effects on DKD patients was only obtained following publication of the FIGARO-DKD and FIDELIO-DKD trials[29,30]. A pre-specified pooled analysis from these trials was conducted within the FIDELITY study[25], aimed at obtaining a more comprehensive estimate of both benefits and adverse events. Overall, finerenone treatment resulted in a 23% reduction for a composite outcome of renal events and a 14% reduction for a composite cardiovascular outcome. The risk of discontinuation due to hyperkalemia was low, at 1.7% for patients treated with finerenone compared to 0.6% for patients treated with placebo.
Currently, the use of finerenone is highly valuable for patients with diabetes, when elevated albuminuria persists after optimized therapy with SGLT2i and RAS blockade drugs[14].
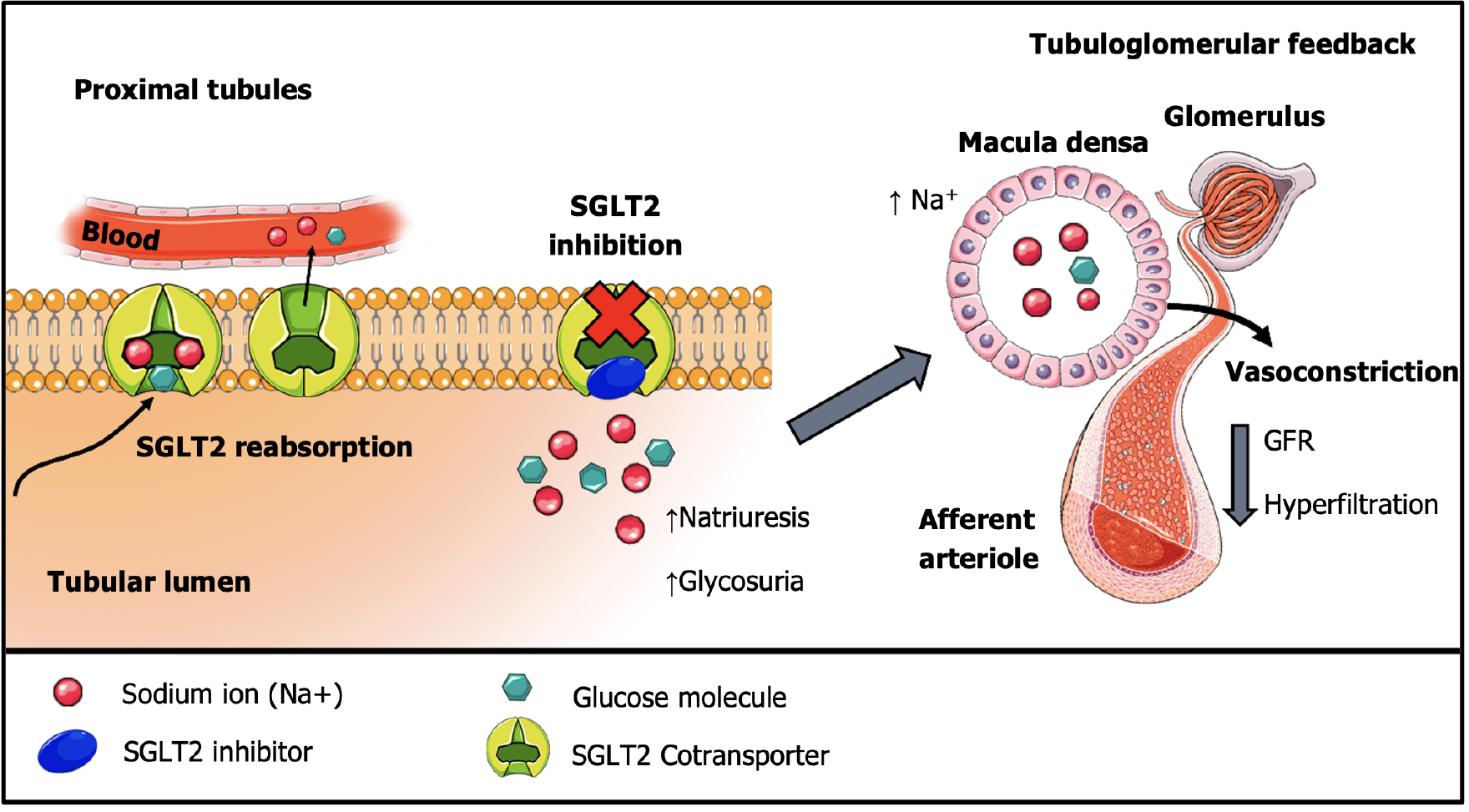
Sodium-glucose cotransporter-2 is a protein responsible for facilitating the reabsorption of the filtered sodium and glucose load at the renal proximal tubules[31,32]. SGLT2i interact with the active site of SGLT2 proteins, which includes the binding areas for glucose and sodium, blocking them from attaching and effectively impeding their reabsorption[32,33]. This process contributes to a decrease in blood glucose levels, which is important in a diabetes context[34,35].
Beyond natriuresis and glycosuria, SGLT2i are believed to exert significant cardioprotective and renoprotective effects through tubuloglomerular feedback modulation. SGLT2 inhibition results in an increased sodium concentration within the proximal tubule lumen, which is detected by macula densa cells[36]. As these cells take up sodium, they create an osmotic gradient that eventually leads to vasoconstriction in afferent arterioles and lower intraglomerular pressures by restrictive blood flow, as shown in Figure 1.
Clinically, these mechanisms initially lower GFR and likely contribute to the albuminuria reduction observed in SGLT2i-treated patients[37-39], similarly to what happens during treatment with RAS blockade. Therefore, SGLT2i are thought to act by reducing the strain caused by ultrafiltration of proteins and glomerular hypertension, ultimately preserving glomerular viability and kidney function.
The initial proposed use of SGLT2i was the management of hyperglycemia and consequently, diabetes control[40,41]. A 2024 Cochrane meta-analysis showed that the use of SGLT2i led to a 0.31% decrease in HbA1C levels compared to placebo[42]. However, these effects are not as significant as other antidiabetics in direct comparisons[43], indicating that while the glucose-lowering properties are important, they may not be the most critical effects of SGLT2i in type 2 diabetes (T2D) patients.
The 2024 ADA guidelines emphasize the use of SGLT2i for diabetic patients with concurrent renal impairment, atherosclerotic cardiovascular disease and/or HF for the prevention of disease progression and negative outcomes related to these conditions regardless of HbA1C levels[44].
After the primary studies in humans for T2D, the cardiovascular disease (CVD) safety establishment of SGLT2i became a priority. Several large-scale cardiovascular outcome trials (CVOT) were conducted to evaluate the cardiovascular effects of these medications. These studies were prompted by a 2008 Food and Drug Administration (FDA) guidance[45] for the pharmaceutical industry that recommended the safety assessments of anti-diabetes drugs due to concerns after rosiglitazone's association with increased myocardial infarction and cardiovascular mortality[46]. The publication of the EMPA-REG OUTCOME trial[47], followed by the CANVAS[48] and DECLARE-TIMI 58 trials[49], displayed the supe
In the context of HF, SGLT2i were initially assessed for patients with reduced ejection fraction through the DAPA-HF[50] and EMPEROR-Reduced trials[51] and later, for their effects in patients with preserved ejection fraction in the SOLOIST-WHF[52], EMPEROR-Preserved[53] and DELIVER trials[54]. Overall, SGLT2i significantly impacted the prevention of hospitalizations due to HF and the reduction of cardiovascular mortality, along with symptomatic relief of the condition[50-54].
Furthermore, in acute scenarios, the 2024 EMPACT-MI trial[55], which evaluated patients hospitalized for acute myocardial infarction, and the EMPULSE trial[56], which evaluated patients with acute HF or decompensated chronic HF, both demonstrated benefits for the use of SGLT2i compared to placebo.
Currently, both the 2022 guidelines by the American College of Cardiology/American Heart Association and the 2023 update to the 2021 guidelines by the European Society of Cardiology (ESC) recommends dapagliflozin and empagliflozin as key therapeutic options in the management of patients affected by chronic HF across the spectrum of ejection fractions[57-59].
Moreover, the ESC 2023 guidelines recommend that SGLT2i should be implemented in all patients with T2D and CVD, regardless of other parameters, in addition to the standard care with antiplatelet and lipid lowering drugs[60].
In summary, while current guidelines suggest that SGLT2i are not a first-line therapeutic option for patients diagnosed solely with T2D, their use is particularly relevant for patients with high cardiovascular risk and HF, even among patients without diabetes.
The use of SGLT2i in patients with CKD was initially limited due to concerns regarding their reliance on adequate kidney function to exert glucose-lowering effects, and to the absence of sufficient safety data in this population. Early small studies in patients with significantly reduced GFR confirmed the persistence of SGLT2i effects, even though glycemic control was diminished in those with moderate to severe renal impairment[61-63]. In EMPA-REG Renal, for instance, there was significant control of HbA1C levels for most stages of kidney impairment compared to placebo, with the exception of patients with stage 4 disease, in which differences were not significant. Stage 4 patients also experienced more urinary tract infections after empagliflozin use when compared to other stages[64]. Nevertheless, intervention with SGLT2i was deemed relatively safe for CKD patients with T2D[65].
Currently, around 50% of patients with CKD stages G4 and G5 present with some form of CVD[66] and an estimated 44% of patients on hemodialysis have concomitant HF[67]. The high degree of correlation between cardiovascular and renal diseases, along with shared mechanisms of pathogenesis, likely explains how several benefits of SGLT2i for CKD outcomes of interest were first proposed in subgroup analysis of renal impaired patients included in CVD and HF trials[67].
A subgroup analysis of EMPA-REG OUTCOME reported a 39% reduction in the composite outcome of worsening nephropathy in diabetic CKD patients with established CVD receiving SGLT2i[68]. Moreover, in patients with pre-existing CKD, after an initial drop in eGFR during short-term empagliflozin use - very similar from what is observed in RAS inhibitors - the filtration rate remained stable and discontinuation of the drug led to a recovery in the initially lost eGFR, while the placebo group exhibited a continuous decline in eGFR values over time, leading to faster CKD progression[68,69]. Initial decreases in eGFR are commonly observed in trials that include CKD patients. However, the use of SGLT2i has not been associated with an increased risk of acute kidney injury in recent meta-analyses; on the contrary, it has been shown to reduce this risk[70,71].
Additional work on the EMPA-REG OUTCOME trial demonstrated that empagliflozin promoted cardiovascular clinical benefits regardless of baseline uACR and GFR[72]. Altogether, these findings suggested a plausible benefit of SGLT2i therapy in slowing CKD progression and reducing clinical outcomes of interest, laying the foundation for further research into this topic.
In the CANVAS trials, albuminuria progression and regression, defined as changes in albuminuria status or a greater than 30% change in uACR levels, were key secondary outcomes in the original study[48]. A subsequent analysis focused on a composite kidney outcome, comprised of doubling in serum creatinine, evolution to ESKD, or death from renal causes, was published in detail in 2018[73]. The results demonstrated a significant 27% reduction in albuminuria progression and a 70% increase in albuminuria regression among patients who received canagliflozin compared to placebo. This finding was obtained in a population of both CKD and non-CKD T2D patients[22]. Furthermore, the prespecified analysis reported a 47% lower incidence of the composite kidney outcomes that was similar in CKD and non-CKD patients[48]. Although this did not provide definitive evidence, as more severe kidney outcomes (progression to ESKD and renal death) were reported in only a small number of participants and the studied population was not selected based on their propensity for kidney disease progression, these findings nonetheless encouraged further investigation into SGLT2i effects on CKD.
In 2019, the DECLARE-TIMI 58 trial[49] presented a subgroup analysis for renal events, which offered valuable insights into the population most likely to benefit of SGLT2i use[74]. The original trial enrolled T2D patients with little to no renal impairment on baseline. The eGFR slope analysis over time revealed the anticipated initial decline in GFR with dapagliflozin use. It took approximately two years for the mean change in eGFR to equalize with the placebo arm, after which a small increase was observed in dapagliflozin group through the end of the study. Despite the very small difference in eGFR progression, the renal-specific composite outcome, ESKD, sustained 40% decrease in eGFR and/or renal death, was clearly demonstrated, especially in patients with higher albuminuria levels[74], which showed that these patients could benefit more from SGLT2i therapy.
The evaluation of SGLT2i for their benefits in the reduction of albuminuria is well documented throughout literature. Despite being a surrogate end-point, albuminuria is one of the biomarkers more tightly linked to a faster decrease in eGFR levels, and consequently, CKD progression[75].
A 2019 meta-analysis demonstrated that sufficiently large trials of intervention reductions in albuminuria are highly confident to benefit clinical end-points, such as progression to ESKD[76]. In a pre-specified secondary analysis from the EMPA-KIDNEY trial, for instance, approximately 40% of the overall treatment effect of empagliflozin in slowing CKD progression or reducing CV death could be linked to reductions in uACR[77]. Analysis of surrogate end-points may be used as the initial indications of plausible drug effects and ways of encouraging the completion of event-driven studies, such as those evaluating eGFR slopes and clinical outcomes of interest, which are generally less economically feasible, require a higher number of participants, and a longer follow-up period.
Albuminuria control in the DKD context after SGLT2i use has been shown in several trials[78] and seems to be even greater when combined with other drugs, such as DPP-4 inhibitors[79], eplerenone[80] and endothelin receptor antagonists[81]. These effects could lead to a slower progression of both CKD and DKD among affected patients, especially when the baseline uACR levels are significantly high. Further ongoing studies to better determine these effects with finerenone, such as the CONFIDENCE trial, are still in progress[82]. These reductions in albuminuria, however, are usually not observed among populations with low baseline uACR and/or low risk of kidney disease development and progression, as shown in the DAPPER and RECEDE-CHF studies[83,84].
Determining which patients are at higher risk of kidney disease progression is an important step, as the first trials that evaluated SGLT2i effects for the CKD population were in populations at a higher probability of benefiting from the use of these medications. For this reason, patients with lower uACR levels were initially excluded from the analysis in these studies[85,86] and the absence of T2D was an exclusion criterion in the CREDENCE trial, for instance[86].
To date, four major randomized controlled trials have evaluated the benefits of SGLT2i therapy in renal disease progression with event-driven outcomes in patients with CKD. These are displayed in Table 1.
| Trial | SGLT2i | Population | Results (primary outcome) |
| CREDENCE[86] | Canagliflozin (100 mg) | Patients with T2D; eGFR 30-90 mL/minute and uACR > 300 to 5000 mg/g | ↓Evolution to ESKD; ↓death from cardiovascular causes |
| DAPA-CKD[85] | Dapagliflozin (10 mg) | Regardless of T2D status; eGFR 25-75 mL/minute and uACR > 200 to 5000 mg/g | ↓Sustained decline of eGFR of 50% or more; ↓evolution to ESKD |
| SCORED[88] | Sotagliflozin (200-400 mg) | Patients with T2D; eGFR 25-50 mL/minute (regardless of albuminuria); an additional CVD risk factor | ↓Hospitalization and urgent visits due to heart failure |
| EMPA-KIDNEY[89] | Empagliflozin (10 mg) | Patients with and without T1D or T2D; eGFR 20-45 mL/minute (regardless of albuminuria); or eGFR 45-90 mL/minute (with uACR > 200 mg/g) | ↓Progression of kidney disease |
In 2019, the CREDENCE trial[86] was the first of these studies and delved into the use of Canagliflozin in the selection of patients with T2D and an eGFR of 30-90 mL/minute/1.73 m² of body-surface area and uACR levels between 300 to 5000 mg/g. The results showed a decreased primary composite outcome of cardiovascular and renal death, as well as a smaller risk of progression to ESKD and doubling in serum creatinine levels.
Further data were obtained through DAPA-CKD[85], which enrolled patients with similar eGFR and uACR status to CREDENCE trial, but included those with and without T2D in the analysis. After randomization into groups of either dapagliflozin or placebo, the study demonstrated a reduction in the composite outcomes of sustained GFR decline, ESKD and renal death, as well as death from cardiovascular events and hospitalization due to HF. This demonstrated that the effects of SGLT2i use could be extrapolated to patients with severely elevated albuminuria at baseline, as later subgroup analysis showed that this benefit was irrespective of T2D status[87]. Furthermore, DAPA-CKD also provided evidence of benefit for all-cause mortality, an outcome in which the CREDENCE trial could not show strong significance for SGLT2i therapy[86].
Later, the SCORED trial[88] evaluated the use of sotagliflozin in T2D patients with cardiovascular risk factors and an eGFR of 25-60 mL/minute/1.73 m², regardless of albuminuria levels. It presented a significant reduction in time-to-event analysis for the first MACE among treated patients but was unable to provide conclusive evidence regarding renal disease progression, one of its secondary end-points of interest, due to funding issues that led to its early termination.
Finally, in 2022, with the publication of EMPA-KIDNEY[89], the analysis of SGLT2i effects extended to patients with and without T2D with eGFR of 20-45 mL/minute/1.73 m² irrespective of uACR status and patients with eGFR of 45-90 mL/minute/1.73 m² who had a urinary albumin-to-creatinine ratio of at least 200 mg/g. The results of this trial demonstrated a reduction in its primary composite outcome, especially related to a slower rate of kidney disease progression. This finding was observed even across patients with lower levels of albuminuria at baseline, although these patients were less impacted in absolute values compared to those with higher uACR levels and presented a small number of primary end-point events due to the slower progression of kidney disease. The impact on eGFR slope curves was also irrespective of the primary kidney diseases initially presented[77,90].
Overall, the combination of trials that included CKD-affected individuals showed that for a wide range of baseline conditions, patients benefitted from the use of SGLT2i. In a recent 2024 meta-analysis by Natale et al[42], a significant reduction was observed in several key clinical outcomes for patients with CKD, including progression to ESKD and composite kidney outcomes, hospitalizations for HF and MACE[42].
Likewise, a 2022 meta-analysis across 13 major randomized controlled trials (RCTs)[70] demonstrated that SGLT2i reduced the risk of kidney disease progression by 37% overall, and it was not related to the presence of T2D. This benefit also extended across different primary kidney disease diagnosis in all RCTs focused on CKD populations, except for conditions not included in the analysis of these trials, such as autosomal dominant polycystic kidney disease.
Most studies for SGLT2i focused on CKD populations were conducted in patients already receiving optimized treatment regimens, primarily with RAS blockade medications. Notably, the FIDELIO-DKD and FIGARO-DKD studies[29,30], which evaluated the effects of finerenone, were published after the recruitment phase in most of these trials and the use of MRAs was rare at baseline for these trials.
The current knowledge on the effects of MRAs combined with SGLT2i usually derives from HF trials, but are not detailed enough to provide information regarding the combined therapy effects for renal outcomes[91]. Also, head-to-head studies are still scarce to determine the isolated effects of SGLT2i in comparison to RAS inhibitor agents or MRAs.
In light of these factors, the United Kingdom Kidney Association (UKKA) recommends that patients receiving SGLT2i therapy should also be prescribed ACEi or angiotensin receptor blockers (ARB) whenever indicated and tolerated[92,93]. Additionally, ACEi or ARBs are independently recommended to SGLT2i by the KDIGO 2024 guidelines, based on their specific indications, while finerenone is recommended to be prescribed only after optimization with SGLT2i and ACEi/ARBs[14].
However, it is important to remember that these medications’ mechanisms of action in the context of preventing kidney disease progression are both by increasing sodium excretion. Therefore, their simultaneous implementation could lead to unpredicted effects, since this practice has not been specifically studied.
Currently, recommendations for the use of SGLT2i in the CKD population are well structured and drugs with established benefits in the CKD population, namely canagliflozin, dapagliflozin and empagliflozin, should be preferred[93]. The 2022 consensus guidelines by the ADA and the KDIGO organization recommend that, among patients with CKD and T2D, SGLT2i should be preferred for those that present atherosclerotic cardiovascular disease, regardless of HbA1C (%) at baseline or use of other antidiabetic medications[19].
Furthermore, the KDIGO 2024 guidelines recommend that SGLT2i should be started for patients with T2D and an eGFR ≥ 20 mL/minutes/1.73 m², regardless of albuminuria levels[14]. In contexts without diabetes, it recommends that patients should be eligible to SGLT2i therapy when they present eGFR levels ≥ 20 mL/minute/1.73 m² and uACR levels of at least 200 mg/g or HF, although it states that those with lower levels of albuminuria could also be treated, despite less certainty for this recommendation[14].
As previously mentioned, patients with smaller uACR levels from the EMPA-KIDNEY trial presented a small number of primary end-point events, which, combined with the trial’s early termination, resulted in compromised analysis of key clinical events, namely cardiovascular related ones[89].
Considering this, the use of SGLT2i in non-diabetic patients with low albuminuria status (< 200 mg/g) presents less clear benefits but is still suggested, as there is a large probability of benefits from the intervention by extrapolation of current data[14]. After treatment initiation with SGLT2i, it is recommended that the drug should be continued until progression to ESKD with need of kidney replacement therapy (KRT) unless it is not tolerated, such as with the development of diabetic ketoacidosis (DKA)[14,93].
SGLT2i are generally well-tolerated and have exhibited a favorable safety profile across the ranges of patients with CKD, CVD, T2D mellitus, and HF. However, clinicians must remain vigilant, and patients should be informed of potential adverse events to enable effective prevention and management strategies.
A systematic review and meta-analysis by Shi et al[94] established a correlation between the use of SGLT2i in T2D patients and an increase of 133 cases per 1000 patients-years of urinary tract infections, also shown in a 2024 study by Natale et al[42]. These infections are mostly related to mycotic genital infections, which accounted for an overall rate ratio of 2.97 for the use of SGLT2i[95]. In spite of these events, genital mycotic infections associated with SGLT2i usage are mostly mild to moderate and respond well to standard therapy[96-98].
As for DKA, this adverse effect seems to be almost completely dependent on the presence of diabetes at baseline, as a 2022 study pointed out only one event over a studied follow-up of 30.000 patient-years among individuals with no diabetes, in comparison to a 2.12 risk ratio among patients with diabetes, which also is observed in CKD patients with T2D[70,95]. Furthermore, risk of associated DKA can generally be mitigated through dose adjustments, patient education on carbohydrate intake and symptoms, proper medication adherence (especially insulin administration), and monitoring of blood ketone and glucose levels, especially during acute illness[99]. Furthermore, the UKKA guidelines recommend that SGLT2i therapy should be suspended whenever an individual develops DKA[92,93].
Moreover, a higher risk of amputation with canagliflozin use was specifically identified in the CANVAS trials regardless of patient’s baseline characteristics[48]. However, this association was not replicated in any other major trials involving SGLT2i. Recently, a Cochrane meta-analysis on the effects of SGLT2i in CKD patients found no significant difference in the amputation risk compared to placebo with the use of these medications, with the exception of patients with eGFR > 60 mL/minute/1.73 m², all of whom were included from the CANVAS studies due to lack of other trials that evaluated amputation events in this specific population as an adverse event of interest[42]. A 2022 meta-analysis showed that, when combined, studies other than the CANVAS did not present significant risks of this event[70].
In summary, the absolute benefits of SGLT2i in reducing CKD progression, acute kidney injury, hospitalization, and major cardiovascular events in patients with T2D mellitus have been consistently demonstrated in high-quality studies. It is also generally established that the significance of these benefits surpasses the potential risk of associated adverse events, which is why the treatment with SGLT2i for patients with T2D and CKD remains a recommendation with few restrictions[14].
In the context of type 1 diabetes (T1D) mellitus, the applicability and safety of SGLT2i remain understudied. In the EMPA-KIDNEY trial, the effect of empagliflozin on chronic eGFR slopes was consistent between T2D and T1D patients, but the very limited number of participants with T1D (68 total) hindered the ability of the study to identify any possible benefits for SGLT2i therapy, even after prespecified analysis[77]. Although minor RCTs have demonstrated that SGLT2i combined with insulin therapy can enhance glycemic control, reduce total daily insulin dose, and favor weight loss in T1D patients, these studies also observed an increased risk of DKA in those treated with SGLT2i[100-105].
In trials involving T1D patients, eGFR reduction was often an exclusion criterion[100,106]. A few studies enrolled individuals with eGFR values between 45 and 60 mL/minute/1.73 m², but subgroup analyses based on eGFR categories were not performed. Additionally, these trials did not assess changes in albuminuria or eGFR for these patients[103,104], leading to insufficient data on this specific population. The inTandem studies, particularly inTandem3, are the exception among these trials, as they provided uACR and eGFR measurements. However, sotagliflozin use did not yield statistically significant differences in this study[105], likely due to the low risk of kidney disease progression in the population.
In the EASE program trials[107], which evaluated empagliflozin use, less than 4% of T1D participants had an eGFR below 60 mL/minute/1.73 m², limiting the ability to conduct robust analyses of T1D in the CKD context. Nevertheless, an interesting finding from EASE was the comparable risk of DKA between low-dose empagliflozin (2.5 mg) and placebo (0.8% vs 1.2%, respectively)[107], showing that dose adjustments could be a possible future way of minimizing this adverse event in patients at high-risk of developing ketoacidosis.
Altogether, despite the scarce literature on the management of T1D patients for CKD, the current available evidence points to an increased number of DKA events as the major adverse effect of SGLT2i in this population. Although T1D patients may benefit from treatment, the risk-benefit profile of SGLT2i in this population remains unclear[108,109]. A 2023 cohort study, for instance, showed that patients with T1D treated with SGLT2i therapy were more likely to show preserved eGFR over a 5-year period compared to patients receiving GLP-1 RAs. Additionally, these patients presented a lessened risk of developing HF, CKD and being hospitalized for any cause, although they also presented with a higher rate of DKA and urinary tract infections[110].
Currently, larger trials assessing the specific effects of SGLT2i for T1D patients with CKD and HF are needed to better support the employment of appropriate therapeutic plans for these conditions, which could lead to significant im
Few guidelines make specific recommendations for the management of T1D patients with SGLT2i. For instance, the UKKA 2023 Clinical Practice Guideline Update directs that SGLT2i could be considered in patients with T1D who have an eGFR of at least 20 mL/minute/1.73 m² and a uACR ≥ 25 mg/mmol, despite the use of maximum tolerated ACEi or ARB and/or after a thorough evaluation and recommendation by a diabetes team[93]. Such patients should be educated on recognizing signs and symptoms of ketoacidosis and be closely monitored with ketone level measurements, which should not exceed 0.6 mmol/L. Also, medical attention should be sought if needed to prevent DKA. These recommendations, however, are based mostly on weak and conflicting evidence or data from general CKD patients and highlight the high demand for better quality studies in this field.
Other than patients presenting T1D, several populations and conditions have been analyzed regarding the effects and safety of SGLT2i in the context of CKD. Current epidemiology shows that older age is a major factor in the occurrence of kidney disease and T2D[114], which led to a necessity of defining the effects of SGLT2i in frail patients and possible risks associated with their prescription.
The 2023 EMPA-ELDERLY RCT showed that empagliflozin use was not a factor related to loss of muscle mass and strength in old patients without previous sarcopenia, despite their weight loss[115]. Comparably, sub analysis from data of the DAPA-CKD trial showed that patients classified as highly frail presented similar to higher benefits in the primary outcomes of kidney and cardiovascular disease events and all-cause mortality compared to patients with low to no frailty[116]. Similar results were obtained using age as a factor to distinguish the patient groups[117]. In general, presence of frailty or older age is not a factor related to important adverse effects and alone should not discourage SGLT2i pre
With regards to kidney transplant recipients (KTRs), the existing body of evidence is mostly composed of cohort studies and is insufficient to properly determine the benefits of SGLT2i therapy among these patients[118]. Current therapeutic decisions rely primarily upon a 2019 RCT conducted by Halden et al[119], which analyzed 44 KTRs with post-transplant diabetes mellitus and found no significant differences in adverse events in the use of empagliflozin compared to placebo, but showed limited benefits beyond HbA1C and weight reduction in these patients, which is expected considering the low number of patients included. Currently, the use of SGLT2i is recommended by the ADA/KDIGO consensus report from 2022 for management of hyperglycemia among transplant recipients[19]. However, recommendations for their use in preserving transplanted kidney function and preventing major negative clinical outcomes are based on extrapolations from better-studied populations and should be carefully considered by an experienced team.
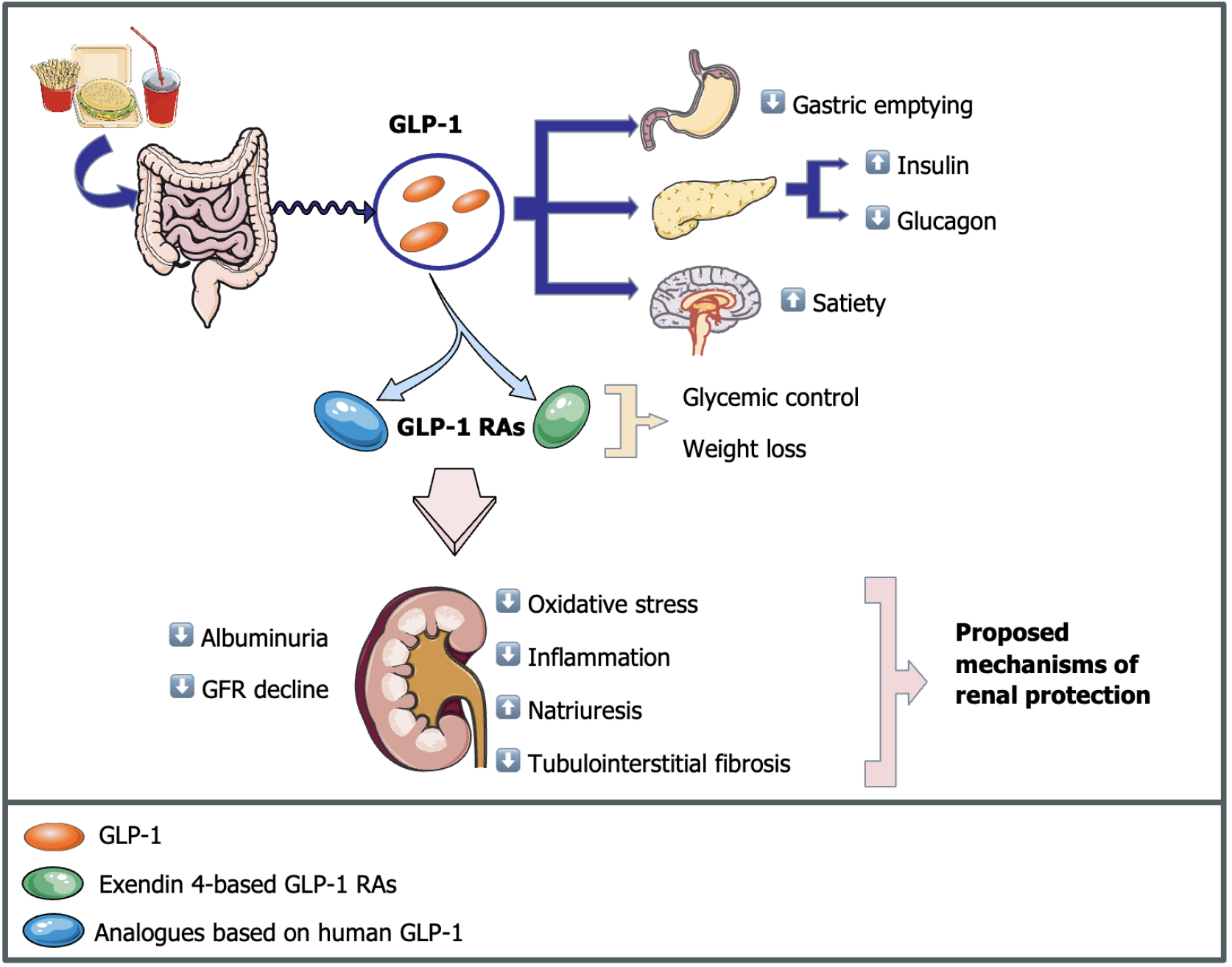
GLP-1 is a hormone that plays a crucial role in glycemic regulation by enhancing glucose-dependent insulin secretion, suppressing glucagon release, and decelerating gastric emptying[120,121]. Additionally, it modulates central pathways governing satiety, thereby regulating energy balance and postprandial glycemia[122]. GLP-1 is degraded through the actions of dipeptidyl peptidase-4 (DPP-4) enzymes[123].
GLP-1 RAs mimic endogenous GLP-1, eliciting comparable physiological effects[120]. GLP-1 RAs are divided into two main categories: Analogs based on human GLP-1[124] and exendin 4-based compounds. GLP-1 analogs are subject to DPP-4 actions and do not require dose adjustment in patients with compromised renal function[125]. Exendin 4-based compounds have shorter half-lives and weaker effects on hyperglycemia[126,127], significantly inhibiting gastric emptying in comparison to human-based compounds[128]. They are also resistant to degradation by DPP-4 enzymes, which do not metabolize them before being eliminated by the kidneys, requiring dose adjustments in patients with lower GFR[123].
Several studies have investigated the effects of GLP-1 and GLP-1RAs on renal metabolism, thus providing insights into the primary mechanisms behind the observed changes in eGFR and reductions in albuminuria, and other renal outcomes[129-131]. However, while these studies have advanced our understanding, the precise mechanisms by which GLP-1 RAs slow the decline in eGFR and reduce albuminuria remain unclear. Nevertheless, some pathways are proposed, including enhanced glycemic control, weight loss, increased natriuresis, and a reduction in inflammation and oxidative stress, as shown in Figure 2.
In recent years, the indications and uses of GLP-1 RAs have expanded significantly. Despite originally being destined for the management of diabetes, growing evidence has also supported their concomitant effectiveness in managing obesity, cardiovascular diseases and other metabolic disorders, making them a valuable option in the treatment of many conditions, even in the absence of T2D[132-134].
GLP-1 RAs are primarily recommended for treating patients with T2D who fail to achieve adequate glycemic control with other medications, such as metformin[135]. They are also approved for the treatment of obesity in patients with a body mass index (BMI) ≥ 30 kg/m² or ≥ 27 kg/m² with associated comorbidities[136]. Among the most commonly used GLP-1 RAs are liraglutide, exenatide, dulaglutide, and semaglutide, all of which have been shown to significantly reduce HbA1c levels and body weight, thereby improving overall metabolic outcomes.
A recent meta-analysis[137] revealed that GLP-1 RAs also promote weight loss, lower blood pressure, and improve lipid profiles in individuals without diabetes. Additionally, emerging evidence suggests that they have therapeutic potential in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), conditions common among patients with metabolic syndrome[138].
Currently, the 2022 ADA and the European Association for the Study of Diabetes guidelines for management of hyperglycemia in T2D have highlighted the roles of GLP-1 RA in reducing HbA1c, MACE, and in chronic overweight management[139]. Regarding NAFLD/NASH, the 2023 ADA Standards of Care in Diabetes recommends the use of GLP-1 RAs along with lifestyle interventions, especially for patients with associated T2D or obesity[140].
Despite the benefits, use of GLP-1 RAs is also accompanied by a range of adverse effects, especially gastrointestinal disorders, including nausea, vomiting, diarrhea, and constipation[141]. These symptoms are dose-dependent and tend to decrease over time as patients adjust to the medication[142].
Another possible adverse effect is the modestly increased risk of gallbladder issues, such as cholelithiasis and cholecystitis, particularly in those with rapid weight loss[143,144]. There is also controversy regarding whether GLP-1 RAs may increase the risk of pancreatitis, but the literature is inconsistent and studies with greater methodological robustness are still required[145,146].
In summary, GLP-1 RAs have become a key therapy in the treatment of T2D, obesity, and other metabolic conditions, offering various benefits such as glycemic control, weight reduction, and cardiovascular protection. Their expanding indications, including promising roles in NAFLD/NASH, highlight their therapeutic versatility. While generally well-tolerated, GLP-1 RAs are associated with some adverse effects, primarily gastrointestinal symptoms, which should be carefully managed to optimize patient outcomes. With growing evidence and ongoing research, these agents are likely to continue shaping the landscape of metabolic disease management.
Over the years, several trials have evaluated the benefits of GLP-1 RAs, most of which targeted populations at a high-risk of cardiovascular disease and its negative outcomes. Despite the lack of major trials focusing on CKD populations, secondary analysis of studies focused on other populations provided initial data on the possible impact of GLP-1 RA on the progression of kidney disease[147]. Similar to what happened with SGLT2i, these beginning results were essential for developing interest and future studies targeted at the CKD population, such as the recently published FLOW trial[148].
To date, a total of three exenatide-4 based GLP-1 RAs have been evaluated in major trials for their cardiorenal benefits: lixisenatide in the ELIXA trial[149], exenatide by the EXSCEL study[150], and more recently, efpeglenatide in the AMPLITUDE-O trial[151].
In the ELIXA trial, the use of lixisenatide significantly reduced uACR compared to control, especially for patients with severely increased albuminuria[149]. In the EXSCEL trial, exenatide was not superior to placebo for either cardiovascular benefits or albuminuria reduction[150,152].
In contrast to exenatide and lixisenatide trials, which did not provide substantial results, AMPLITUDE-O showed that efpeglenatide reduced the risk of cardiovascular events compared to placebo and reduced the occurrence of a composite kidney outcome for the incidence of albuminuria, decline in renal function and need for KRT[151]. Despite its effects, efpeglenatide is not currently available for clinical use and therefore is not a treatment option.
Regarding human GLP-1 analogs, four drugs were evaluated in CVOTs: Liraglutide, in the LEADER trial[153], semaglutide in subcutaneous and oral presentations in SUSTAIN 6[154] and PIONEER 6[155] studies, respectively; and dulaglutide in both the REWIND[156] and AWARD 7[157] trials. Furthermore, albiglutide was evaluated in the HARMONY trial, showing cardiovascular benefits[158], but renal outcomes of interest to our analysis were not reported.
The original LEADER[153] trial study reported important effects of liraglutide on cardiovascular protection. Later subgroup analysis showed that patients with eGFR of less than 60 mL/minute/1.73 m² at baseline presented a 31% lower risk of cardiovascular events with the use of liraglutide compared to placebo, suggesting possible benefits of GLP-1 RAs to this group of individuals[153].
Moreover, a prespecified secondary analysis focused on renal outcomes indicated that liraglutide reduced the risk of new-onset persistent macroalbuminuria by 22% and slightly slowed the decline in estimated GFR[159]. Post-hoc analyses that evaluated subgroups with CKD support the benefits of liraglutide in the prevention of death and MACE, even in stage 4 kidney disease. Finally, adverse events leading to discontinuation were similarly frequent for groups of patients with or without CKD[160,161].
In 2016, the SUSTAIN-6 trial evaluated the effects of subcutaneous semaglutide and showed a 24% reduced risk in the primary composite outcome of MACE. As for renal outcomes, new or worsening nephropathy was 46% lower. The main causes of discontinuation were gastrointestinal disorders[154].
A post-hoc analysis of data from the SUSTAIN 1-7 trials indicated a slower decline of eGFR for patients with pre-existing CKD and improvements in uACR, without significant increases in the occurrence of adverse events[162]. Similarly, in analyses from the LEADER trial, higher uACR levels were associated with greater risk of MACE. Additionally, patients who received semaglutide had a lower chance of progressing to a higher KDIGO risk category and a greater chance of moving to a lower category, primarily related to changes in uACR[162,163].
Analyses incorporating data from LEADER and SUSTAIN 6 trials reinforced the kidney-protective effects of semaglutide and liraglutide, demonstrating a consistent reduction in albuminuria and slowing of eGFR decline that was especially relevant in patients with preexisting kidney disease[164]. In a mediation analysis, the reductions in HbA1c, body weight, and systolic BP observed in these trials appeared to be responsible for only part of the benefits on kidney outcomes[164,165].
In the PIONEER 6 trial, oral semaglutide was only non-inferior compared to placebo in the prevention of cardio
In the context of dulaglutide, two major randomized studies were performed, AWARD-7[157] and REWIND[156] had different outlines but similar outcomes, pointing to a slight improvement in eGFR decline. In the AWARD-7 trial, dulaglutide significantly slowed the decrease in eGFR compared to insulin glargine, especially for participants with baseline uACR > 300 mg/g[157,166].
The REWIND trial showed that dulaglutide was associated with a reduced incidence of sustained eGFR decline of 40% or more. Furthermore, uACR levels did not increase in the dulaglutide group compared to placebo[167].
A summary of data from these trials was conducted by several meta-analyses. Despite a lack of significant effects in MACE[168,169], a meta-analysis from Sattar et al[170] showed a 21% reduction in composite kidney outcomes. A similar study conducted by Li et al[171] showed a 17% reduction in the composite renal outcome and the occurrence of new-onset macroalbuminuria by 25%. The human GLP-1 analogs and exendin-4-based GLP-1 RAs were analyzed separately for the main outcomes but achieved similar results[171].
Overall, the data suggest that albuminuria-lowering effects could be a class attribute of GLP-1 RAs, but the same cannot be inferred about other renal outcomes. Importantly, none of these studies was specifically targeted at the CKD population, which could hinder the evaluation of benefits from this therapy for non-surrogate end-points.
Considering these findings, even before the FLOW trial[148], GLP-1 RAs showed promising results for the control of diabetes-related kidney disease progression[147], especially the long-acting GLP-1 RAs, which is related to cardiovascular benefits. However, it was only after the publication of a definitive study analyzing a population of patients with CKD that clinical recommendations directly related to renal function could be published. A summary of all mentioned trials of GLP-1 RAs is shown in Table 2.
| Trial | GLP-1 RA | Population | Cardiovascular outcomes | Renal outcomes |
| ELIXA[149] | Lixisenatide (10-20 μg) | Patients with T2D and past MI or unstable angina; eGFR ≥ 30 mL/minute | No significant effect in MACE | ↓uACR |
| EXSCEL[150] | Exenatide (2 mg) | Patients with T2D; with and without CKD or CVD; eGFR ≥ 30 mL/minute | No significant effect in MACE | No significant effects in renal outcomes |
| AMPLITUDE-O[151] | Efpeglenatide (4-6 mg) | Patients with T2D and CVD or eGFR 25-59.9 mL/minute with CVD risk factors | ↓MACE | ↓uACR |
| LEADER[153] | Liraglutide (1.8 mg) | Patients with T2D; with and without CKD | ↓MACE | ↓uACR |
| SUSTAIN 6[154] | Subcutaneous semaglutide (0.5-1 mg) | Patients with T2D; With and without CKD; eGFR ≥ 30 mL/minute | ↓MACE | ↓uACR |
| PIONEER 6[155] | Oral semaglutide (14 mg) | Patients with T2D; With and without CKD; eGFR ≥ 30 mL/minute | ↓MACE | Not measured |
| REWIND[156] | Dulaglutide (1.5 mg) | Patients with T2D and CVD or CVD risk factors; eGFR ≥ 15 mL/minute | ↓MACE | ↓uACR |
| FLOW[148] | Subcutaneous semaglutide (1 mg) | Patients with T2D and CKD; eGFR of 25-75 mL/minute (uACR 300-5000 if eGFR ≥ 50 mL/minute) | ↓MACE | ↓uACR; ↓eGFR; ↓major kidney disease events |
The FLOW study was the first trial aimed at directly evaluating semaglutide in patients with T2D and CKD. In this study, a 1.0 mg once weekly dose of semaglutide was compared to placebo for composite primary outcome of kidney failure, substantial loss of kidney function, and death from kidney-related or cardiovascular causes in 3533 patients who met the inclusion criteria[148].
In this context, the semaglutide group showed a 24% reduced risk of major kidney disease events. Additionally, patients treated with semaglutide presented a 40% reduction in urinary albumin-to-creatinine ratio, compared to 12% in the placebo group. The loss of kidney function, measured by the creatinine-based eGFR from baseline to week 104, was 3.30 mL/minute/1.73 m² lower in the semaglutide group. The eGFR decline was also calculated using cystatin-C and showed equivalent results compared to the creatinine-based eGFR, which indicates that the variation in creatinine values was probably not related to patient’s weight loss. Other secondary outcomes showed additional benefits, such as greater body weight reduction, better control of glycated hemoglobin, and a reduction in systolic blood pressure. The main cause of discontinuation was gastrointestinal disorders, occurring in 4.5% of the semaglutide group[148].
A prespecified analysis of the FLOW trial showed that in general, the benefits of semaglutide were not influenced by the concomitant use of an SGLT2i. Outcomes including MACE and mortality presented similar results for patients using SGLT2i or not. However, the number of participants using SGLT2i at baseline was small, so this information should be interpreted with caution[172]. The benefits of semaglutide extend to the population with HF at baseline concomitant to CKD, which is expected considering the cardiovascular benefits of GLP-1 RAs[173]. In summary, this trial revealed that subcutaneous semaglutide can reduce kidney outcomes, slow the worsening of kidney function, and prevent car
While long-acting GLP-1 RAs were already established as an additional therapy for adults with T2D and CKD to manage hyperglycemia and promote intentional weight loss in obese patients by the KDIGO 2024 guidelines[14], they may also become part of the recommended treatments to prevent renal outcomes and progression of CKD, especially subcutaneous semaglutide, which shows stronger evidence. Consistent with this trend, guidelines by the Brazilian Diabetes Society already recommend that semaglutide should be considered in patients with T2D and CKD with eGFR > 25 mL/minute/1.73 m² and uACR > 100 mg/g to reduce renal outcomes in association with SGLT2i[174]. Moreover, guidelines by other organizations recommend that, in order to minimize the risk of hypoglycemia in patients previously using sulfonylureas or insulin, the doses of these drugs must be reduced in patients starting treatment with GLP-1 RAs, and combination with dipeptidyl peptidase-4 inhibitors should be avoided[19].
In addition to RCTs, several retrospective studies have been conducted on the long-term effects of GLP-1 RAs at markers and clinical outcomes related to renal impairment.
A Korean study evaluated the use of dulaglutide in patients with T2D and mild to severe CKD and showed that the eGFR decline was significantly slower after the use of dulaglutide[175]. A register-based cohort study compared the use of DPP-4 inhibitors vs GLP-1 RAs (mainly liraglutide) over 5 years and found that treatment with GLP-1 RAs led to a 24% lower risk of serious renal events[176].
A 2024 retrospective study showed that the treatment with GLP-1 RAs for 6 months significantly decreased uACR in patients with mild to moderate renal impairment[177], consistent with other studies by Osonoi et al[178] and Aviles Bueno et al[179]. Another study comparing GLP-1 RAs with long action insulin in T2D patients who required intensive glycemic control also showed a lower risk of renal outcomes among patients using GLP-1 RAs[180].
Overall, although the findings of these studies should be taken with caution, additional designs are valuable for complementing the present knowledge and exploring possibilities for clinical practice.
Drugs with a dual effect have recently been developed and can represent a new horizon for incretin-based therapies, among them tirzepatide. It is the first dual-action incretin and has been approved by the FDA since 2022. In contrast to other GLP-1 RAs, this drug activates both glucose-dependent insulinotropic polypeptide and GLP-1 receptors, resulting in greater glucose-lowering and weight loss effects with good tolerability among patients and similar adverse effects to other incretins[181,182].
The only RCT that evaluated the effects of tirzepatide on kidney markers was the SURPASS-4 trial, but it was focused on a population with low renal impairment risk[183]. A post-hoc analysis evaluating kidney outcomes showed that the decrease in eGFR was 1.4 mL/minute/1.73 m² per year in the tirzepatide group and 3.6 mL/minute/1.73 m² per year in the insulin group. The difference was more notable in patients with eGFR < 60 mL/minute/1.73 m². In addition, 40% of the patients who received tirzepatide had at least a 30% reduction in uACR, compared to 29% in the insulin glargine group.
Furthermore, patients who received tirzepatide had a higher chance of regressing to a less severe stage of albuminuria. Regarding clinical outcomes, tirzepatide reduced the risk of ESKD or death due to kidney failure by 42% compared to insulin glargine[184]. Despite significant weight loss in the patients who received tirzepatide, the equivalent results for cystatin C-derived eGFR confirmed the findings from creatinine-derived eGFR, supporting the plausibility of benefits on kidney function[185].
Tirzepatide seems to be a promising drug in the treatment of T2D-related kidney disease. However, despite the initial results, it is still not possible to infer from the current evidence whether there is a real benefit that translates into clinical practice for patients with CKD. Therefore, clinical recommendations should be taken with caution and studies focused on this specific population are necessary.
An important population to consider is patients with advanced kidney disease and in need of KRT. The research regarding the effects of the GLP-1 RAs in the context of complete renal failure is still incipient, as all major RCTs excluded patients with severe kidney function loss, including the FLOW trial, limiting available data for this population. Currently, most data for this population come from smaller studies that have evaluated GLP-1 RAs in this context.
Overall, the use of GLP-1 RAs seems to be beneficial for weight loss and glycemic control without significant adverse events[186,187]. In terms of clinical major outcomes, an observational study comparing GLP-1 RAs with long-acting insulin for diabetic patients undergoing acute dialysis found MACE benefits for those who discontinued dialysis, but not among those who remained dialysis-dependent[188].
Despite their benefits, the ADA/KDIGO 2022 consensus clearly states that caution is necessary in patients with or at risk for malnutrition due to the weight loss effect of GLP-1 RAs. However, among obese patients with T2D and advanced renal impairment who exceed the BMI required for kidney transplant listing, GLP-1 RAs can promote weight loss to facilitate qualification for transplant[19]. Among patients with eGFR < 15 mL/minute/1.73 m², only liraglutide and semaglutide are recommended and no dosage adjustment is required[15].
Altogether, data on ESKD patients are scarce and the clinical benefits of GLP-1 RAs are not as well defined for this population, but they could be used as tools for glycemic control and obesity management after due assessment.
Considering the benefits of GLP-1 RAs for patients with CKD and their imminent incorporation as recommended treatments for disease progression, the relevance of evaluating the synergy with other drugs is significant. In the FLOW trial, only 548 (15.5%) patients were using SGLT2i at baseline and no patients were using finerenone[189]. Further research is still necessary to measure the effects in this population and optimize recommendations.
A study analyzing data from several studies of SGLT2i, GLP-1 RAs and nsMRA estimated a gain of several years of survival free from CKD progression and MACE for patients with significant baseline albuminuria and T2D when adding these drugs to the base regimen with RAS blockade. Despite the limitations, this study supports the idea that combined treatment can significantly reduce mortality and promote health quality[190].
Furthermore, data from retrospective studies found that GLP-1 RAs and SGLT2i combined were related to a decrease in overall mortality for diabetes patients[191] and could reduce adverse cardiovascular and serious renal events[192], despite neither being targeted at patients with high risk of renal disease.
Beyond these benefits directly related to DKD progression, GLP-1 RAs and SGLT2i are attractive options for patients with multiple comorbidities and treatment with multiple drugs due to their multisystemic effects and the possibility of replacing other drugs that provide less cardiorenal benefits. However, their high cost is an important barrier to implementation[193].
Despite the current evidence, several gaps in knowledge regarding SGLT2i and GLP-1 RA treatment of patients with T2D and CKD still exist. Firstly, the exact mechanisms for the renal protection effects are still unknown. In this context, two trials are currently being conducted, the REMODEL study (NCT04865770)[194] - aimed at evaluating specific actions of semaglutide in kidneys of patients with T2D and CKD through the use of biopsies and magnetic resonance imaging and measurements of kidney oxygenation, perfusion and inflammatory markers - and the PROCEED trial (jRCTs071190054)[195] - aimed at evaluating endothelial dysfunction markers in DKD patients treated with ipragliflozin - could fill these blanks and lead to a better understanding of how these drugs act to impede kidney impairment.
The role of oral semaglutide in CKD remains uncertain, as highlighted by the PIONEER 6 findings[155]. The SOUL trial[196] aims to evaluate the effects of oral semaglutide vs placebo in patients with T2D, atherosclerotic CVD and/or CKD, focusing on time to first occurrence of CV death, non-fatal MI, or non-fatal stroke, but important kidney outcomes will be analyzed as confirmatory secondary outcomes. Importantly, the study will not exclude patients with ESKD, and more information on this population could be acquired, which may fill in gaps in knowledge for these patients.
Another scenario that should be explored by future studies is populations excluded from most analysis of SGLT2i and GLP-1 RAs trials, as patients who received kidney transplants and patients with T1D. Only small trials have been conducted in T1D patients and even when considering future ongoing research, such as the ATTEMPT trial (NCT
Moreover, the research regarding ESKD needs to be expanded. As previously mentioned, the current evidence is insufficient, and more information about the role of GLP-1 RAs in mortality and morbidity is necessary. Regarding SGLT2i, little to no inclusion of ESKD patients is conducted in most studies, which do not provide enough data to set recommendations among these patients, leading to their recommended suspension after initiation of KRT.
Finally, several ongoing trials comparing CKD therapies or evaluating combination strategies may refine prescribing practices and clarify potential synergistic benefits or adverse interactions for these drugs.
Considering the significant impacts of DKD, it is always valuable to explore new therapies, particularly those that may offer multiple benefits to patients. In recent years, the addition of SGLT2i to established treatment regimens has transformed the management of CKD across multiple clinical scenarios. Now, with the proven potential of therapy with GLP-1 RAs for DKD patients, new horizons for the management of CKD have begun to be uncovered. SGLT2i have shown clear benefits in slowing CKD progression, stabilizing GFR, and reducing albuminuria, in addition to preventing negative outcomes in the context of HF and MACE. As a result, current guidelines strongly recommend this drug class. As for GLP-1 RAs, current evidence suggests that therapy with these drugs may become a new avenue for managing DKD, alongside the standard treatments already in place. In addition to the known benefits related to cardiovascular risk reduction, hyperglycemia, and obesity management, subcutaneous semaglutide may offer a better prognosis for patients by delaying the loss of kidney function, as demonstrated in the FLOW trial. Over the past decade, well-structured research has led to paradigm-shifting advancements in DKD management, introducing several new therapies that reduce morbidity and mortality, slow disease progression, and greatly improve both lifespan and quality of life for affected patients. Furthermore, years of research to come will help in establishing additional benefits and better defining the effects of these therapies in specific subsets of patients both for CKD and for the multiple other conditions that benefit from SGLT2i and GLP-1 RA therapy.
| 1. | Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1792] [Article Influence: 224.0] [Reference Citation Analysis (0)] |
| 2. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1683] [Cited by in RCA: 1780] [Article Influence: 890.0] [Reference Citation Analysis (18)] |
| 3. | Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, Rossing P, Groop PH, Cooper ME. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 689] [Article Influence: 68.9] [Reference Citation Analysis (1)] |
| 4. | Al-Ghamdi SMG, Bieber B, AlRukhaimi M, AlSahow A, Al Salmi I, Al Ali F, Al Aradi A, Pecoits-Filho R, Robinson BM, Pisoni RL; GCC-DOPPS Study Group. Diabetes Prevalence, Treatment, Control, and Outcomes Among Hemodialysis Patients in the Gulf Cooperation Council Countries. Kidney Int Rep. 2022;7:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S191-S202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 165] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 6. | Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864-2883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 753] [Cited by in RCA: 786] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 7. | Padda IS, Mahtani AU, Parmar M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. 2023 Jun 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 8. | Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 694] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 9. | Giatras I, Lau J, Levey AS. Effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: a meta-analysis of randomized trials. Angiotensin-Converting-Enzyme Inhibition and Progressive Renal Disease Study Group. Ann Intern Med. 1997;127:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 263] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 443] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 11. | Gansevoort RT, Sluiter WJ, Hemmelder MH, de Zeeuw D, de Jong PE. Antiproteinuric effect of blood-pressure-lowering agents: a meta-analysis of comparative trials. Nephrol Dial Transplant. 1995;10:1963-1974. [PubMed] |
| 12. | Leon SJ, Tangri N. The Use of Renin-Angiotensin System Inhibitors in Patients With Chronic Kidney Disease. Can J Cardiol. 2019;35:1220-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Mukoyama M, Kuwabara T. Role of renin-angiotensin system blockade in advanced CKD: to use or not to use? Hypertens Res. 2022;45:1072-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 14. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105:S117-S314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1076] [Article Influence: 1076.0] [Reference Citation Analysis (0)] |
| 15. | Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102:S1-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 575] [Article Influence: 191.7] [Reference Citation Analysis (0)] |
| 16. | Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4068] [Cited by in RCA: 3991] [Article Influence: 166.3] [Reference Citation Analysis (1)] |
| 17. | Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5059] [Cited by in RCA: 5072] [Article Influence: 211.3] [Reference Citation Analysis (0)] |
| 18. | Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3760] [Cited by in RCA: 3556] [Article Influence: 111.1] [Reference Citation Analysis (2)] |
| 19. | de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, Rosas SE, Rossing P, Bakris G. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2022;102:974-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 20. | Shah M, Awad AS, Abdel-Rahman EM. Nonsteroidal Mineralocorticoid Receptor Antagonist (Finerenone) in Cardiorenal Disease. J Clin Med. 2023;12:6285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Fujii W, Shibata S. Mineralocorticoid Receptor Antagonists for Preventing Chronic Kidney Disease Progression: Current Evidence and Future Challenges. Int J Mol Sci. 2023;24:7719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Tsujimoto T, Kajio H. Spironolactone Use and Improved Outcomes in Patients With Heart Failure With Preserved Ejection Fraction With Resistant Hypertension. J Am Heart Assoc. 2020;9:e018827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Pandey AK, Bhatt DL, Cosentino F, Marx N, Rotstein O, Pitt B, Pandey A, Butler J, Verma S. Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Eur Heart J. 2022;43:2931-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Shaikh A, Ray J, Campbell KN. Role of Finerenone in the Treatment of Diabetic Kidney Disease: Patient Selection and Clinical Perspectives. Ther Clin Risk Manag. 2022;18:753-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, Kolkhof P, Nowack C, Gebel M, Ruilope LM, Bakris GL; FIDELIO-DKD and FIGARO-DKD investigators. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43:474-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 573] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 26. | Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM; Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) Study Group. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA. 2015;314:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 500] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 27. | Filippatos G, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Kim SY, Nowack C, Palombo G, Kolkhof P, Kimmeskamp-Kirschbaum N, Pieper A, Pitt B. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 303] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 28. | Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453-2463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 447] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 29. | Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020;383:2219-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1696] [Cited by in RCA: 1502] [Article Influence: 300.4] [Reference Citation Analysis (1)] |
| 30. | Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM; FIGARO-DKD Investigators. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2021;385:2252-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 862] [Article Influence: 215.5] [Reference Citation Analysis (0)] |
| 31. | Bailey CJ, Day C, Bellary S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr Diab Rep. 2022;22:39-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 32. | Brown E, Wilding JPH, Alam U, Barber TM, Karalliedde J, Cuthbertson DJ. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2021;53:2072-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Salvatore T, Galiero R, Caturano A, Rinaldi L, Di Martino A, Albanese G, Di Salvo J, Epifani R, Marfella R, Docimo G, Lettieri M, Sardu C, Sasso FC. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int J Mol Sci. 2022;23:3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 34. | Vallon V, Verma S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu Rev Physiol. 2021;83:503-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 290] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 35. | Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context. 2014;3:212264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Cherney DZ, Kanbay M, Lovshin JA. Renal physiology of glucose handling and therapeutic implications. Nephrol Dial Transplant. 2020;35:i3-i12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 37. | Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 38. | Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care. 2016;39 Suppl 2:S165-S171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 247] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 39. | Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 40. | Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 41. | Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, Woerle HJ; EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 42. | Natale P, Tunnicliffe DJ, Toyama T, Palmer SC, Saglimbene VM, Ruospo M, Gargano L, Stallone G, Gesualdo L, Strippoli GF. Sodium-glucose co-transporter protein 2 (SGLT2) inhibitors for people with chronic kidney disease and diabetes. Cochrane Database Syst Rev. 2024;5:CD015588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Scott R, Morgan J, Zimmer Z, Lam RLH, O'Neill EA, Kaufman KD, Engel SS, Raji A. A randomized clinical trial of the efficacy and safety of sitagliptin compared with dapagliflozin in patients with type 2 diabetes mellitus and mild renal insufficiency: The CompoSIT-R study. Diabetes Obes Metab. 2018;20:2876-2884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S158-S178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 323] [Article Influence: 323.0] [Reference Citation Analysis (1)] |
| 45. | Food and Drug Administration. Guidance for Industry on Diabetes Mellitus-Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. Dec 19, 2008. [cited 13 March 2025]. Available from: https://www.federalregister.gov/documents/2008/12/19/E8-30086/guidance-for-industry-on-diabetes-mellitus-evaluating-cardiovascular-risk-in-new-antidiabetic. |
| 46. | Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3497] [Cited by in RCA: 3343] [Article Influence: 185.7] [Reference Citation Analysis (0)] |
| 47. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8294] [Article Influence: 829.4] [Reference Citation Analysis (1)] |
| 48. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5399] [Article Influence: 674.9] [Reference Citation Analysis (0)] |
| 49. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE-TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4252] [Article Influence: 708.7] [Reference Citation Analysis (0)] |
| 50. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4445] [Article Influence: 740.8] [Reference Citation Analysis (0)] |
| 51. | Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 3175] [Article Influence: 635.0] [Reference Citation Analysis (0)] |
| 52. | Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021;384:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 1269] [Article Influence: 317.3] [Reference Citation Analysis (0)] |
| 53. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 2775] [Article Influence: 693.8] [Reference Citation Analysis (0)] |
| 54. | Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM; DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022;387:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 1444] [Article Influence: 481.3] [Reference Citation Analysis (0)] |
| 55. | Butler J, Jones WS, Udell JA, Anker SD, Petrie MC, Harrington J, Mattheus M, Zwiener I, Amir O, Bahit MC, Bauersachs J, Bayes-Genis A, Chen Y, Chopra VK, Figtree G, Ge J, Goodman SG, Gotcheva N, Goto S, Gasior T, Jamal W, Januzzi JL, Jeong MH, Lopatin Y, Lopes RD, Merkely B, Parikh PB, Parkhomenko A, Ponikowski P, Rossello X, Schou M, Simic D, Steg PG, Szachniewicz J, van der Meer P, Vinereanu D, Zieroth S, Brueckmann M, Sumin M, Bhatt DL, Hernandez AF. Empagliflozin after Acute Myocardial Infarction. N Engl J Med. 2024;390:1455-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 134] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 56. | Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, Ferreira JP, Nassif ME, Psotka MA, Tromp J, Borleffs CJW, Ma C, Comin-Colet J, Fu M, Janssens SP, Kiss RG, Mentz RJ, Sakata Y, Schirmer H, Schou M, Schulze PC, Spinarova L, Volterrani M, Wranicz JK, Zeymer U, Zieroth S, Brueckmann M, Blatchford JP, Salsali A, Ponikowski P. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 516] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 57. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1201] [Article Influence: 400.3] [Reference Citation Analysis (0)] |
| 58. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 7275] [Article Influence: 1818.8] [Reference Citation Analysis (0)] |
| 59. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Skibelund AK; ESC Scientific Document Group. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 922] [Article Influence: 461.0] [Reference Citation Analysis (0)] |
| 60. | Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, Christodorescu RM, Crawford C, Di Angelantonio E, Eliasson B, Espinola-Klein C, Fauchier L, Halle M, Herrington WG, Kautzky-Willer A, Lambrinou E, Lesiak M, Lettino M, McGuire DK, Mullens W, Rocca B, Sattar N; ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44:4043-4140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 622] [Article Influence: 311.0] [Reference Citation Analysis (0)] |
| 61. | Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, Wajs E, Figueroa K, Jiang J, Law G, Usiskin K, Meininger G; DIA3004 Study Group. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 62. | Kashiwagi A, Takahashi H, Ishikawa H, Yoshida S, Kazuta K, Utsuno A, Ueyama E. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 63. | Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 500] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 64. | Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC; EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 443] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 65. | Fioretto P, Del Prato S, Buse JB, Goldenberg R, Giorgino F, Reyner D, Langkilde AM, Sjöström CD, Sartipy P; DERIVE Study Investigators. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): The DERIVE Study. Diabetes Obes Metab. 2018;20:2532-2540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 66. | Xanthopoulos A, Papamichail A, Briasoulis A, Loritis K, Bourazana A, Magouliotis DE, Sarafidis P, Stefanidis I, Skoularigis J, Triposkiadis F. Heart Failure in Patients with Chronic Kidney Disease. J Clin Med. 2023;12:6105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, Kasiske BL, Deswal A, deFilippi CR, Cleland JGF, Anker SD, Herzog CA, Cheung M, Wheeler DC, Winkelmayer WC, McCullough PA; Conference Participants. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:1304-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 68. | Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2303] [Cited by in RCA: 2536] [Article Influence: 281.8] [Reference Citation Analysis (0)] |
| 69. | Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, Hantel S, Woerle HJ, Broedl UC, von Eynatten M, Groop PH; EMPA-REG OUTCOME Investigators. Empagliflozin and Kidney Function Decline in Patients with Type 2 Diabetes: A Slope Analysis from the EMPA-REG OUTCOME Trial. J Am Soc Nephrol. 2018;29:2755-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 70. | Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788-1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 505] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 71. | Chung MC, Hung PH, Hsiao PJ, Wu LY, Chang CH, Hsiao KY, Wu MJ, Shieh JJ, Huang YC, Chung CJ. Sodium-Glucose Transport Protein 2 Inhibitor Use for Type 2 Diabetes and the Incidence of Acute Kidney Injury in Taiwan. JAMA Netw Open. 2023;6:e230453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 72. | Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and Clinical Outcomes in Patients With Type 2 Diabetes Mellitus, Established Cardiovascular Disease, and Chronic Kidney Disease. Circulation. 2018;137:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 330] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 73. | Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Matthews DR, Neal B. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 468] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 74. | Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Kato ET, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 487] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 75. | Barzilay JI, Farag YMK, Durthaler J. Albuminuria: An Underappreciated Risk Factor for Cardiovascular Disease. J Am Heart Assoc. 2024;13:e030131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 76. | Heerspink HJL, Greene T, Tighiouart H, Gansevoort RT, Coresh J, Simon AL, Chan TM, Hou FF, Lewis JB, Locatelli F, Praga M, Schena FP, Levey AS, Inker LA; Chronic Kidney Disease Epidemiology Collaboration. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 252] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 77. | EMPA-KIDNEY Collaborative Group. Effects of empagliflozin on progression of chronic kidney disease: a prespecified secondary analysis from the empa-kidney trial. Lancet Diabetes Endocrinol. 2024;12:39-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 78. | Allegretti AS, Zhang W, Zhou W, Thurber TK, Rigby SP, Bowman-Stroud C, Trescoli C, Serusclat P, Freeman MW, Halvorsen YC. Safety and Effectiveness of Bexagliflozin in Patients With Type 2 Diabetes Mellitus and Stage 3a/3b CKD. Am J Kidney Dis. 2019;74:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 79. | Pollock C, Stefánsson B, Reyner D, Rossing P, Sjöström CD, Wheeler DC, Langkilde AM, Heerspink HJL. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:429-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 80. | Provenzano M, Puchades MJ, Garofalo C, Jongs N, D'Marco L, Andreucci M, De Nicola L, Gorriz JL, Heerspink HJL; ROTATE-3 study group; ROTATE-3 study group members. Albuminuria-Lowering Effect of Dapagliflozin, Eplerenone, and Their Combination in Patients with Chronic Kidney Disease: A Randomized Crossover Clinical Trial. J Am Soc Nephrol. 2022;33:1569-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 81. | Heerspink HJL, Kiyosue A, Wheeler DC, Lin M, Wijkmark E, Carlson G, Mercier AK, Åstrand M, Ueckert S, Greasley PJ, Ambery P. Zibotentan in combination with dapagliflozin compared with dapagliflozin in patients with chronic kidney disease (ZENITH-CKD): a multicentre, randomised, active-controlled, phase 2b, clinical trial. Lancet. 2023;402:2004-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 82. | Green JB, Mottl AK, Bakris G, Heerspink HJL, Mann JFE, McGill JB, Nangaku M, Rossing P, Scott C, Gay A, Agarwal R. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE). Nephrol Dial Transplant. 2023;38:894-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 83. | Yoshihara F, Imazu M, Sakuma I, Hiroi Y, Hara H, Okazaki O, Ishiguro C, Izumi C, Noguchi T, Shiraiwa T, Nishioka N, Fujii K, Iwakura K, Tomonaga O, Kobayashi K, Takihata M, Yumoto K, Takase H, Himi T, Shimizu I, Murakami T, Wagatsuma K, Sato K, Hiramatsu T, Akabame S, Hata S, Asakura M, Kawabata T, Omae K, Ito S, Kitakaze M; DAPPER Investigators. DAPagliflozin for the attenuation of albuminuria in Patients with hEaRt failure and type 2 diabetes (DAPPER study): a multicentre, randomised, open-label, parallel-group, standard treatment-controlled trial. EClinicalMedicine. 2023;66:102334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 84. | Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and Cardiovascular Effects of SGLT2 Inhibition in Combination With Loop Diuretics in Patients With Type 2 Diabetes and Chronic Heart Failure: The RECEDE-CHF Trial. Circulation. 2020;142:1713-1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 85. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3132] [Article Influence: 626.4] [Reference Citation Analysis (1)] |
| 86. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3979] [Article Influence: 663.2] [Reference Citation Analysis (0)] |
| 87. | Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, McMurray JJV, Correa-Rotter R, Rossing P, Toto RD, Sjöström CD, Langkilde AM, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 342] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 88. | Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG; SCORED Investigators. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 2021;384:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 785] [Article Influence: 196.3] [Reference Citation Analysis (0)] |
| 89. | Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ, Ng SYA, Sammons E, Zhu D, Hill M, Stevens W, Wallendszus K, Brenner S, Cheung AK, Liu ZH, Li J, Hooi LS, Liu W, Kadowaki T, Nangaku M, Levin A, Cherney D, Maggioni AP, Pontremoli R, Deo R, Goto S, Rossello X, Tuttle KR, Steubl D, Petrini M, Massey D, Eilbracht J, Brueckmann M, Landray MJ, Baigent C, Haynes R; The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1116] [Cited by in RCA: 1229] [Article Influence: 614.5] [Reference Citation Analysis (2)] |
| 90. | EMPA-KIDNEY Collaborative Group. Impact of primary kidney disease on the effects of empagliflozin in patients with chronic kidney disease: secondary analyses of the EMPA-KIDNEY trial. Lancet Diabetes Endocrinol. 2024;12:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 91. | Ferreira JP, Zannad F, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Jamal W, Steubl D, Schueler E, Packer M. Interplay of Mineralocorticoid Receptor Antagonists and Empagliflozin in Heart Failure: EMPEROR-Reduced. J Am Coll Cardiol. 2021;77:1397-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 92. | Authors N. UK Kidney Association Clinical Practice Guideline: Sodium-Glucose Co-transporter-2 (SGLT-2) Inhibition in Adults with Kidney Disease. Kidneys, Ukr J Ren Med. 2022;11:30-36. [DOI] [Full Text] |
| 93. | Roddick AJ, Wonnacott A, Webb D, Watt A, Watson MA, Staplin N, Riding A, Lioudaki E, Kuverji A, Kossi ME, Holmes P, Holloway M, Fraser D, Carvalho C, Burton JO, Bhandari S, Herrington WG, Frankel AH. UK Kidney Association Clinical Practice Guideline: Sodium-Glucose Co-transporter-2 (SGLT-2) Inhibition in Adults with Kidney Disease 2023 UPDATE. BMC Nephrol. 2023;24:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 94. | Shi Q, Nong K, Vandvik PO, Guyatt GH, Schnell O, Rydén L, Marx N, Brosius FC 3rd, Mustafa RA, Agarwal A, Zou X, Mao Y, Asadollahifar A, Chowdhury SR, Zhai C, Gupta S, Gao Y, Lima JP, Numata K, Qiao Z, Fan Q, Yang Q, Jin Y, Ge L, Yang Q, Zhu H, Yang F, Chen Z, Lu X, He S, Chen X, Lyu X, An X, Chen Y, Hao Q, Standl E, Siemieniuk R, Agoritsas T, Tian H, Li S. Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2023;381:e074068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 95. | Shiau CH, Tsau LY, Kao CC, Peng YC, Bai CH, Wu JC, Hou WH. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol. 2024;56:1359-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 96. | Nyirjesy P, Sobel JD, Fung A, Mayer C, Capuano G, Ways K, Usiskin K. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin. 2014;30:1109-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 97. | Rosenstock J, Perl S, Johnsson E, García-Sánchez R, Jacob S. Triple therapy with low-dose dapagliflozin plus saxagliptin versus dual therapy with each monocomponent, all added to metformin, in uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21:2152-2162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, Parikh SJ. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 99. | Goldenberg RM, Berard LD, Cheng AYY, Gilbert JD, Verma S, Woo VC, Yale JF. SGLT2 Inhibitor-associated Diabetic Ketoacidosis: Clinical Review and Recommendations for Prevention and Diagnosis. Clin Ther. 2016;38:2654-2664.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 100. | Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and Safety of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, as Add-on to Insulin in Patients With Type 1 Diabetes. Diabetes Care. 2015;38:2258-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 101. | Mathieu C, Dandona P, Gillard P, Senior P, Hasslacher C, Araki E, Lind M, Bain SC, Jabbour S, Arya N, Hansen L, Thorén F, Langkilde AM; DEPICT-2 Investigators. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes (the DEPICT-2 Study): 24-Week Results From a Randomized Controlled Trial. Diabetes Care. 2018;41:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 102. | Dandona P, Mathieu C, Phillip M, Hansen L, Tschöpe D, Thorén F, Xu J, Langkilde AM; DEPICT-1 Investigators. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care. 2018;41:2552-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 103. | Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, Danne T, Kushner JA, Lane WS, Lapuerta P, McGuire DK, Peters AL, Reed J, Sawhney S, Strumph P. Sotagliflozin in Combination With Optimized Insulin Therapy in Adults With Type 1 Diabetes: The North American inTandem1 Study. Diabetes Care. 2018;41:1970-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 104. | Danne T, Cariou B, Banks P, Brandle M, Brath H, Franek E, Kushner JA, Lapuerta P, McGuire DK, Peters AL, Sawhney S, Strumph P. HbA(1c) and Hypoglycemia Reductions at 24 and 52 Weeks With Sotagliflozin in Combination With Insulin in Adults With Type 1 Diabetes: The European inTandem2 Study. Diabetes Care. 2018;41:1981-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 105. | Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, Pozzilli P, Gesty-Palmer D, Lapuerta P, Simó R, Danne T, McGuire DK, Kushner JA, Peters A, Strumph P. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N Engl J Med. 2017;377:2337-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 316] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 106. | Dandona P, Mathieu C, Phillip M, Hansen L, Griffen SC, Tschöpe D, Thorén F, Xu J, Langkilde AM; DEPICT-1 Investigators. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:864-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 107. | Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, Zinman B, Skyler JS, George J, Soleymanlou N, Perkins BA. Empagliflozin as Adjunctive to Insulin Therapy in Type 1 Diabetes: The EASE Trials. Diabetes Care. 2018;41:2560-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 108. | van Raalte DH, Bjornstad P, Persson F, Powell DR, de Cassia Castro R, Wang PS, Liu M, Heerspink HJL, Cherney D. The Impact of Sotagliflozin on Renal Function, Albuminuria, Blood Pressure, and Hematocrit in Adults With Type 1 Diabetes. Diabetes Care. 2019;42:1921-1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 109. | Stougaard EB, Rossing P, Vistisen D, Banks P, Girard M, Davies MJ, Persson F. Sotagliflozin, a dual sodium-glucose co-transporter-1 and sodium-glucose co-transporter-2 inhibitor, reduces the risk of cardiovascular and kidney disease, as assessed by the Steno T1 Risk Engine in adults with type 1 diabetes. Diabetes Obes Metab. 2023;25:1874-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 110. | Anson M, Zhao SS, Austin P, Ibarburu GH, Malik RA, Alam U. SGLT2i and GLP-1 RA therapy in type 1 diabetes and reno-vascular outcomes: a real-world study. Diabetologia. 2023;66:1869-1881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 111. | Sridhar VS, Limonte CP, Groop PH, Heerspink HJL, Pratley RE, Rossing P, Skyler JS, Cherney DZI. Chronic kidney disease in type 1 diabetes: translation of novel type 2 diabetes therapeutics to individuals with type 1 diabetes. Diabetologia. 2024;67:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 112. | Mahmud F. Adolescent Type 1 Diabetes Treatment With SGLT2i for hyperglycEMia & hyPerfilTration Trial. [accessed 2025 Mar 13]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT04333823 ClinicalTrials.gov Identifier: NCT04333823. |
| 113. | ADA Meeting News. ATTEMPT trial shows kidney, glycemic benefits for SGLT2 inhibitor in adolescents with type 1 diabetes. Jul 11, 2024. [cited 3 March 2025]. Available from: https://www.adameetingnews.org/attempt-trial-shows-kidney-glycemic-benefits-for-sglt2-inhibitor-in-adolescents-with-type-1-diabetes/. |
| 114. | Ravender R, Roumelioti ME, Schmidt DW, Unruh ML, Argyropoulos C. Chronic Kidney Disease in the Older Adult Patient with Diabetes. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 115. | Yabe D, Shiki K, Homma G, Meinicke T, Ogura Y, Seino Y; EMPA-ELDERLY Investigators. Efficacy and safety of the sodium-glucose co-transporter-2 inhibitor empagliflozin in elderly Japanese adults (≥65 years) with type 2 diabetes: A randomized, double-blind, placebo-controlled, 52-week clinical trial (EMPA-ELDERLY). Diabetes Obes Metab. 2023;25:3538-3548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 116. | Vart P, Butt JH, Jongs N, Schechter M, Chertow GM, Wheeler DC, Pecoits-Filho R, Langkilde AM, Correa-Rotter R, Rossing P, McMurray JJV, Heerspink HJL. Efficacy and Safety of Dapagliflozin in Patients With Chronic Kidney Disease Across the Spectrum of Frailty. J Gerontol A Biol Sci Med Sci. 2024;79:glad181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 117. | Yu MK, Vart P, Jongs N, Correa-Rotter R, Rossing P, McMurray JJV, Hou FF, Douthat W, Khullar D, Langkilde AM, Wheeler DC, Heerspink HJL, Chertow GM. Effects of Dapagliflozin in Chronic Kidney Disease Across the Spectrum of Age and by Sex. J Gen Intern Med. 2024;39:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 118. | Ujjawal A, Schreiber B, Verma A. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney transplant recipients: what is the evidence? Ther Adv Endocrinol Metab. 2022;13:20420188221090001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 119. | Halden TAS, Kvitne KE, Midtvedt K, Rajakumar L, Robertsen I, Brox J, Bollerslev J, Hartmann A, Åsberg A, Jenssen T. Efficacy and Safety of Empagliflozin in Renal Transplant Recipients With Posttransplant Diabetes Mellitus. Diabetes Care. 2019;42:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 120. | Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. 2023;20:463-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 227] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 121. | DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19:1353-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 122. | Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27:740-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 1105] [Article Influence: 157.9] [Reference Citation Analysis (1)] |
| 123. | Meier JJ, Nauck MA, Kranz D, Holst JJ, Deacon CF, Gaeckler D, Schmidt WE, Gallwitz B. Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes. 2004;53:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 124. | Alicic RZ, Cox EJ, Neumiller JJ, Tuttle KR. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021;17:227-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 125. | Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 959] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 126. | Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2787] [Cited by in RCA: 2844] [Article Influence: 149.7] [Reference Citation Analysis (1)] |
| 127. | Trujillo JM, Nuffer W. GLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agents. Pharmacotherapy. 2014;34:1174-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 128. | Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001;281:E155-E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 129. | Kawanami D, Takashi Y. GLP-1 Receptor Agonists in Diabetic Kidney Disease: From Clinical Outcomes to Mechanisms. Front Pharmacol. 2020;11:967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 130. | Michos ED, Bakris GL, Rodbard HW, Tuttle KR. Glucagon-like peptide-1 receptor agonists in diabetic kidney disease: A review of their kidney and heart protection. Am J Prev Cardiol. 2023;14:100502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 131. | Rojano Toimil A, Ciudin A. GLP-1 Receptor Agonists in Diabetic Kidney Disease: From Physiology to Clinical Outcomes. J Clin Med. 2021;10:jcm10173955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 132. | Andrikou E, Tsioufis C, Andrikou I, Leontsinis I, Tousoulis D, Papanas N. GLP-1 receptor agonists and cardiovascular outcome trials: An update. Hellenic J Cardiol. 2019;60:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 133. | Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan JY, Yuan CS. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ. 2024;384:e076410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 155] [Reference Citation Analysis (12)] |
| 134. | Guo X, Zhou Z, Lyu X, Xu H, Zhu H, Pan H, Wang L, Yang H, Gong F. The Antiobesity Effect and Safety of GLP-1 Receptor Agonist in Overweight/Obese Patients Without Diabetes: A Systematic Review and Meta-Analysis. Horm Metab Res. 2022;54:458-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 135. | Gourgari E, Wilhelm EE, Hassanzadeh H, Aroda VR, Shoulson I. A comprehensive review of the FDA-approved labels of diabetes drugs: Indications, safety, and emerging cardiovascular safety data. J Diabetes Complications. 2017;31:1719-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 136. | Burcelin R, Gourdy P. Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obes Rev. 2017;18:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 137. | Ansari HUH, Qazi SU, Sajid F, Altaf Z, Ghazanfar S, Naveed N, Ashfaq AS, Siddiqui AH, Iqbal H, Qazi S. Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals With Obesity and Without Diabetes: A Systematic Review and Meta-Analysis. Endocr Pract. 2024;30:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 138. | Petit JM, Cercueil JP, Loffroy R, Denimal D, Bouillet B, Fourmont C, Chevallier O, Duvillard L, Vergès B. Effect of Liraglutide Therapy on Liver Fat Content in Patients With Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J Clin Endocrinol Metab. 2017;102:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 139. | Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, Rossing P, Tankova T, Tsapas A, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45:2753-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 828] [Article Influence: 276.0] [Reference Citation Analysis (1)] |
| 140. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Cusi K, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S49-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 132] [Article Influence: 66.0] [Reference Citation Analysis (1)] |
| 141. | Shetty R, Basheer FT, Poojari PG, Thunga G, Chandran VP, Acharya LD. Adverse drug reactions of GLP-1 agonists: A systematic review of case reports. Diabetes Metab Syndr. 2022;16:102427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 142. | Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 2204] [Article Influence: 551.0] [Reference Citation Analysis (0)] |
| 143. | Nauck MA, Muus Ghorbani ML, Kreiner E, Saevereid HA, Buse JB; LEADER Publication Committee on behalf of the LEADER Trial Investigators. Effects of Liraglutide Compared With Placebo on Events of Acute Gallbladder or Biliary Disease in Patients With Type 2 Diabetes at High Risk for Cardiovascular Events in the LEADER Randomized Trial. Diabetes Care. 2019;42:1912-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 144. | Faillie JL, Yu OH, Yin H, Hillaire-Buys D, Barkun A, Azoulay L. Association of Bile Duct and Gallbladder Diseases With the Use of Incretin-Based Drugs in Patients With Type 2 Diabetes Mellitus. JAMA Intern Med. 2016;176:1474-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 145. | Sodhi M, Rezaeianzadeh R, Kezouh A, Etminan M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA. 2023;330:1795-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 209] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 146. | Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Glucagon-like peptide-1 receptor agonists and pancreatitis: a meta-analysis of randomized clinical trials. Diabetes Res Clin Pract. 2014;103:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 147. | Naaman SC, Bakris GL. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care. 2023;46:1574-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 110] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 148. | Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, Baeres FMM, Idorn T, Bosch-Traberg H, Lausvig NL, Pratley R; FLOW Trial Committees and Investigators. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N Engl J Med. 2024;391:109-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 560] [Article Influence: 560.0] [Reference Citation Analysis (1)] |
| 149. | Muskiet MHA, Tonneijck L, Huang Y, Liu M, Saremi A, Heerspink HJL, van Raalte DH. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:859-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 150. | Bethel MA, Mentz RJ, Merrill P, Buse JB, Chan JC, Goodman SG, Iqbal N, Jakuboniene N, Katona B, Lokhnygina Y, Lopes RD, Maggioni AP, Ohman P, Tankova T, Bakris GL, Hernandez AF, Holman RR. Microvascular and Cardiovascular Outcomes According to Renal Function in Patients Treated With Once-Weekly Exenatide: Insights From the EXSCEL Trial. Diabetes Care. 2020;43:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 151. | Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, Lam CSP, Khurmi NS, Heenan L, Del Prato S, Dyal L, Branch K; AMPLITUDE-O Trial Investigators. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N Engl J Med. 2021;385:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 478] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 152. | Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 1728] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 153. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 4909] [Article Influence: 545.4] [Reference Citation Analysis (0)] |
| 154. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3025] [Cited by in RCA: 4080] [Article Influence: 453.3] [Reference Citation Analysis (1)] |
| 155. | Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC; PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1179] [Article Influence: 196.5] [Reference Citation Analysis (0)] |
| 156. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1921] [Cited by in RCA: 1794] [Article Influence: 299.0] [Reference Citation Analysis (1)] |
| 157. | Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, Botros FT. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 431] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 158. | Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1216] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 159. | Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB; LEADER Steering Committee and Investigators. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 869] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 160. | Mann JFE, Fonseca V, Mosenzon O, Raz I, Goldman B, Idorn T, von Scholten BJ, Poulter NR. Effects of Liraglutide Versus Placebo on Cardiovascular Events in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease. Circulation. 2018;138:2908-2918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 161. | Mann JFE, Fonseca VA, Poulter NR, Raz I, Idorn T, Rasmussen S, von Scholten BJ, Mosenzon O; LEADER Trial Investigators. Safety of Liraglutide in Type 2 Diabetes and Chronic Kidney Disease. Clin J Am Soc Nephrol. 2020;15:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 162. | Mann JFE, Hansen T, Idorn T, Leiter LA, Marso SP, Rossing P, Seufert J, Tadayon S, Vilsbøll T. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8:880-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 163. | Tuttle KR, Bain SC, Bosch-Traberg H, Khunti K, Rasmussen S, Sokareva E, Cherney DZ. Effects of Once-Weekly Semaglutide on Kidney Disease Outcomes by KDIGO Risk Category in the SUSTAIN 6 Trial. Kidney Int Rep. 2024;9:2006-2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 164. | Shaman AM, Bain SC, Bakris GL, Buse JB, Idorn T, Mahaffey KW, Mann JFE, Nauck MA, Rasmussen S, Rossing P, Wolthers B, Zinman B, Perkovic V. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145:575-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 152] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 165. | Mann JFE, Buse JB, Idorn T, Leiter LA, Pratley RE, Rasmussen S, Vilsbøll T, Wolthers B, Perkovic V. Potential kidney protection with liraglutide and semaglutide: Exploratory mediation analysis. Diabetes Obes Metab. 2021;23:2058-2066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 166. | Tuttle KR, Rayner B, Lakshmanan MC, Kwan AYM, Konig M, Shurzinske L, Botros FT. Clinical Outcomes by Albuminuria Status with Dulaglutide versus Insulin Glargine in Participants with Diabetes and CKD: AWARD-7 Exploratory Analysis. Kidney360. 2021;2:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 167. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Botros FT, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 402] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 168. | Pulipati VP, Ravi V, Pulipati P. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Eur J Prev Cardiol. 2020;27:1922-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 169. | Kelly M, Lewis J, Rao H, Carter J, Portillo I, Beuttler R. Effects of GLP-1 receptor agonists on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease: A systematic review and meta-analysis. Pharmacotherapy. 2022;42:921-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 170. | Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, Lam CSP, Lopes RD, McMurray JJV, Pratley RE, Rosenstock J, Gerstein HC. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 852] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 171. | Li X, Song Y, Guo T, Xiao G, Li Q. Effect of glucagon-like peptide 1 receptor agonists on the renal protection in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2022;48:101366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 172. | Mann JFE, Rossing P, Bakris G, Belmar N, Bosch-Traberg H, Busch R, Charytan DM, Hadjadj S, Gillard P, Górriz JL, Idorn T, Ji L, Mahaffey KW, Perkovic V, Rasmussen S, Schmieder RE, Pratley RE, Tuttle KR. Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial. Nat Med. 2024;30:2849-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 64.0] [Reference Citation Analysis (1)] |
| 173. | Pratley RE, Tuttle KR, Rossing P, Rasmussen S, Perkovic V, Nielsen OW, Mann JFE, MacIsaac RJ, Kosiborod MN, Kamenov Z, Idorn T, Hansen MB, Hadjadj S, Bakris G, Baeres FMM, Mahaffey KW; FLOW Trial Committees and Investigators. Effects of Semaglutide on Heart Failure Outcomes in Diabetes and Chronic Kidney Disease in the FLOW Trial. J Am Coll Cardiol. 2024;84:1615-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 174. | Sá JR, Canani LH, Rangel ÉB, Bauer AC, Escott GM, Zelmanovitz T, Silveiro SP, Betônico CDCR, Lauria MW, Lamounier RN, Moraes TPD, Bertoluci M. Avaliação e tratamento da doença renal do diabetes. Dira Soc Bras Diabetes. 2024;. [DOI] [Full Text] |
| 175. | Kim S, An JN, Song YR, Kim SG, Lee HS, Cho A, Kim JK. Effect of once-weekly dulaglutide on renal function in patients with chronic kidney disease. PLoS One. 2022;17:e0273004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 176. | Pasternak B, Wintzell V, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, Jonasson C, Melbye M, Svanström H, Ueda P. Use of Glucagon-Like Peptide 1 Receptor Agonists and Risk of Serious Renal Events: Scandinavian Cohort Study. Diabetes Care. 2020;43:1326-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 177. | Cao Y, Zhao J, Ma Y, Cao S, Liu Y. Real-World Clinical Effectiveness of Glucagon-Like Peptide-1 Receptor Agonist on Mild-to-Moderate Diabetic Kidney Disease in Patients with Type 2 Diabetes: A Retrospective, Single-Arm Clinical Trial. Diabetes Metab Syndr Obes. 2024;17:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 178. | Osonoi T, Saito M, Osonoi Y, Douguchi S, Ofuchi K, Katoh M. Liraglutide Improves Estimated Glomerular Filtration Rate Slopes in Patients with Chronic Kidney Disease and Type 2 Diabetes: A 7-Year Retrospective Analysis. Diabetes Technol Ther. 2020;22:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 179. | Aviles Bueno B, Soler MJ, Perez-Belmonte L, Jimenez Millan A, Rivas Ruiz F, Garcia de Lucas MD. Semaglutide in type 2 diabetes with chronic kidney disease at high risk progression-real-world clinical practice. Clin Kidney J. 2022;15:1593-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 180. | Peng ZY, Yang CT, Lin WH, Yao WY, Ou HT, Kuo S. Chronic kidney outcomes associated with GLP-1 receptor agonists versus long-acting insulins among type 2 diabetes patients requiring intensive glycemic control: a nationwide cohort study. Cardiovasc Diabetol. 2023;22:272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 181. | Forzano I, Varzideh F, Avvisato R, Jankauskas SS, Mone P, Santulli G. Tirzepatide: A Systematic Update. Int J Mol Sci. 2022;23:ijms232314631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 182. | MacIsaac RJ, Deed G, D'Emden M, Ekinci EI, Hocking S, Sumithran P, Rasalam R. Challenging Clinical Perspectives in Type 2 Diabetes with Tirzepatide, a First-in-Class Twincretin. Diabetes Ther. 2023;14:1997-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 183. | Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, Aizenberg D, Wynne AG, Riesmeyer JS, Heine RJ, Wiese RJ; SURPASS-4 Investigators. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398:1811-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 369] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 184. | Heerspink HJL, Sattar N, Pavo I, Haupt A, Duffin KL, Yang Z, Wiese RJ, Tuttle KR, Cherney DZI. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10:774-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 143] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 185. | Heerspink HJL, Sattar N, Pavo I, Haupt A, Duffin KL, Yang Z, Wiese RJ, Wilson JM, Hemmingway A, Cherney DZI, Tuttle KR. Effects of Tirzepatide Versus Insulin Glargine on Cystatin C-Based Kidney Function: A SURPASS-4 Post Hoc Analysis. Diabetes Care. 2023;46:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 186. | Vanek L, Kurnikowski A, Krenn S, Mussnig S, Hecking M. Semaglutide in patients with kidney failure and obesity undergoing dialysis and wishing to be transplanted: A prospective, observational, open-label study. Diabetes Obes Metab. 2024;26:5931-5941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 187. | Krisanapan P, Sanpawithayakul K, Pattharanitima P, Thongprayoon C, Miao J, Mao MA, Suppadungsuk S, Tangpanithandee S, Craici IM, Cheungpasitporn W. Safety and Efficacy of GLP-1 Receptor Agonists in Type 2 Diabetes Mellitus with Advanced and End-Stage Kidney Disease: A Systematic Review and Meta-Analysis. Diseases. 2024;12:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 188. | Lai HW, See CY, Chen JY, Wu VC. Mortality and cardiovascular events in diabetes mellitus patients at dialysis initiation treated with glucagon-like peptide-1 receptor agonists. Cardiovasc Diabetol. 2024;23:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 189. | Rossing P, Baeres FMM, Bakris G, Bosch-Traberg H, Gislum M, Gough SCL, Idorn T, Lawson J, Mahaffey KW, Mann JFE, Mersebach H, Perkovic V, Tuttle K, Pratley R. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant. 2023;38:2041-2051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 143] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 190. | Neuen BL, Heerspink HJL, Vart P, Claggett BL, Fletcher RA, Arnott C, de Oliveira Costa J, Falster MO, Pearson SA, Mahaffey KW, Neal B, Agarwal R, Bakris G, Perkovic V, Solomon SD, Vaduganathan M. Estimated Lifetime Cardiovascular, Kidney, and Mortality Benefits of Combination Treatment With SGLT2 Inhibitors, GLP-1 Receptor Agonists, and Nonsteroidal MRA Compared With Conventional Care in Patients With Type 2 Diabetes and Albuminuria. Circulation. 2024;149:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 109] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 191. | Riley DR, Essa H, Austin P, Preston F, Kargbo I, Ibarburu GH, Ghuman R, Cuthbertson DJ, Lip GYH, Alam U. All-cause mortality and cardiovascular outcomes with sodium-glucose Co-transporter 2 inhibitors, glucagon-like peptide-1 receptor agonists and with combination therapy in people with type 2 diabetes. Diabetes Obes Metab. 2023;25:2897-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 192. | Simms-Williams N, Treves N, Yin H, Lu S, Yu O, Pradhan R, Renoux C, Suissa S, Azoulay L. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study. BMJ. 2024;385:e078242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 193. | Nee R, Yuan CM, Narva AS, Yan G, Norris KC. Overcoming barriers to implementing new guideline-directed therapies for chronic kidney disease. Nephrol Dial Transplant. 2023;38:532-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 194. | Bjornstad P, Cherney D, Lawson J, Møntegaard C, Pruijm M, Tuttle K, Vrhnjak B, Kretzler M. MO399: Remodel: A Mechanistic Trial Evaluating the Effects of Semaglutide on the Kidneys In People With Type 2 Diabetes and Chronic Kidney Disease. Nephrol Dial Transpl. 2022;37:gfac070.013. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 195. | Tanaka A, Shimabukuro M, Okada Y, Sugimoto K, Kurozumi A, Torimoto K, Hirai H, Node K; PROCEED trial investigators. Rationale and design of an investigator-initiated, multicenter, prospective open-label, randomized trial to evaluate the effect of ipragliflozin on endothelial dysfunction in type 2 diabetes and chronic kidney disease: the PROCEED trial. Cardiovasc Diabetol. 2020;19:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 196. | McGuire DK, Busui RP, Deanfield J, Inzucchi SE, Mann JFE, Marx N, Mulvagh SL, Poulter N, Engelmann MDM, Hovingh GK, Ripa MS, Gislum M, Brown-Frandsen K, Buse JB. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes Metab. 2023;25:1932-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 197. | Krisanapan P, Suppadungsuk S, Sanpawithayakul K, Thongprayoon C, Pattharanitima P, Tangpanithandee S, Mao MA, Miao J, Cheungpasitporn W. Safety and efficacy of glucagon-like peptide-1 receptor agonists among kidney transplant recipients: a systematic review and meta-analysis. Clin Kidney J. 2024;17:sfae018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |