Published online Dec 15, 2024. doi: 10.4239/wjd.v15.i12.2338
Revised: August 28, 2024
Accepted: October 10, 2024
Published online: December 15, 2024
Processing time: 246 Days and 1 Hours
Shikonin is a natural remedy that is effective at treating diabetic wounds. NFAT5 is a potential therapeutic target for diabetes, and mitochondrial function is essen
To assess the therapeutic mechanism of shikonin in diabetic wounds, its rela
Hypertonic cell and diabetic wound mouse models were established. NFAT5 expression was measured through western blotting and immunofluorescence, in vivo and in vitro. Mitochondrial function was evaluated using reactive oxygen species (ROS) detection and JC-1 and Calcein AM dyes. Mitochondrial structures were observed using transmission electron microscopy. The NFAT5/AMPK pathway was analyzed using a transfection vector and an inhibitor. The effect of shikonin on cells under hypertonic conditions via the NFAT5/AMPK pathway was assessed using western blotting.
Shikonin treatment preserved HaCaT cell viability, while significantly reducing cyclooxygenase-2 expression levels in a high-glucose environment (P < 0.05). Additionally, shikonin maintained mitochondrial morphology, enhanced membrane potential, reduced membrane permeability, and decreased ROS levels in HaCaT cells under hyperosmolar stress. Furthermore, shikonin promoted wound healing in diabetic mice (P < 0.05). Shikonin also inhibited NFAT5, in vivo and in vitro (P < 0.05). Shikonin treatment reduced NFAT5 expression levels, subsequently inhibiting AMPK expression in vitro (P < 0.05). Finally, shikonin inhibited several key downstream molecules of the NFAT5/AMPK pathway, including mammalian target of rapamycin, protein kinase B, nuclear factor kappa-light-chain-enhancer of activated B cells, and inducible nitric oxide synthase (P < 0.05).
Shikonin protects mitochondria via the NFAT5/AMPK-related pathway and enhances wound healing in diabetes.
Core Tip: NFAT5 is a potential therapeutic target for diabetes. Shikonin is a natural remedy that is ideal for treating diabetic wounds. In this study we confirmed that shikonin suppresses the pathological expression of NFAT5, thereby protecting mitochondrial function, inhibiting cellular inflammation, and reducing reactive oxygen species production. Furthermore, we found that shikonin protects mitochondria through the NFAT5/AMPK pathway. Our research provides novel insights into the contribution of shikonin to the treatment of diabetes.
- Citation: Cen LS, Cao Y, Zhou YM, Guo J, Xue JW. Shikonin protects mitochondria through the NFAT5/AMPK pathway for the treatment of diabetic wounds. World J Diabetes 2024; 15(12): 2338-2352
- URL: https://www.wjgnet.com/1948-9358/full/v15/i12/2338.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i12.2338
Diabetes affects 537 million people worldwide, and its prevalence is expected to increase to 783 million by 2045[1]. Approximately 10%-20% of patients with diabetes experience life-threatening chronic wounds[2]. Patients with diabetes who develop active ulcers within 5 years have a mortality rate of up to 40%. The mortality rate following amputation is even higher[3]. Diabetic wounds are characterized by persistent inflammation, high susceptibility to infection, micro
NFAT5, a Rel/NF-κB family member, is the only known genetic regulator of the mammalian adaptive response to osmotic stress[5,6]. NFAT5 expression levels and nuclear translocation and NFAT5-driven transcriptional activity are increased upon exposure to elevated glucose levels in mammals[7]. As diabetes progresses, NFAT5 may exacerbate the disease. The pathogenic response of NFAT5 can result in diabetes-related complications, such as atherosclerosis, diabetic retinopathy, and diabetic nephropathy[8]. Thus, NFAT5 is a potential therapeutic target for diabetes. In an inflammatory environment, ROS may be important molecular sensors that direct NFAT5 activity in a proinflammatory direction[9]. Mitochondria are the primary sites of ROS production. High glucose levels induce mitochondrial swelling, outer membrane rupture, and ROS overproduction, exacerbating the proinflammatory effects of NFAT5[10,11].
Shikonin is the primary active ingredient of extracts of the rhizome of the traditional Chinese medicinal herb known as “Zi Cao”. This herb is been used for external treatment of hypertonic stress-related skin diseases, including burns, dermatitis, and diabetic wounds[12,13]. Previous studies have demonstrated that shikonin can inhibit downstream NFAT5 targets, such as NF-κB, TNF-α, and IL-1β[14,15]. Furthermore, shikonin possesses antioxidant properties that reduce ROS production and mitigate cell damage under high-glucose conditions[16]. We propose that shikonin protects mitochondrial function under high osmotic stress conditions through the NFAT5 pathway.
We developed a hypertonic cell model to investigate the effects of shikonin on NFAT5 expression and mitochondrial function. Additionally, we established a diabetic mouse wound model to examine the impact of shikonin on wound healing and NFAT5 expression. Furthermore, we assessed proteins closely associated with mitochondrial function, including AMPK, mammalian target of rapamycin (mTOR), AKT, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2), as well as ROS production, to further explore the mechanisms by which shikonin alleviates hyper
The human epidermal keratinocyte cell line, HaCaT, was obtained from the Chinese Academy of Sciences (Shanghai, China). HaCaT cells were cultured in high-glucose Dulbecco’s modified Eagle medium (Aladdin, Shanghai, China) supplemented with RPMI 1640 (Corning Inc., Corning, NY, United States), 10% fetal bovine serum (TBD, Tianjin, China), and 200 U/mL gentamicin. The cells were maintained in standard incubators at 37 °C in a 5% CO2 atmosphere.
Cell viability was assessed using cell counting kit-8 (CCK-8; Solarbio-CA1210, China) according to the manufacturer’s instructions. HaCaT cells were plated at a density of 8 × 103 cells/well in 200 µL of complete medium in 96-well plates. The cells were cultured at 37 °C and incubated for 24 hours for viability assessment. HaCaT cells were treated with a normal glucose concentration (NG group, 25 mmol/L glucose, Aladdin), a high glucose concentration (HG group, 200 mmol/L glucose), a high mannitol concentration (175 mmol/L mannitol + 25 mmol/L glucose, Aladdin), and different concentrations (2, 5 μmol/L) of shikonin (Ruifensi Company, Chengdu, China). The SHG group underwent 2 μmol/L shikonin pretreatment for 4 hours and then treatment with a high glucose concentration. The NC + HG group was treated with 0.1 µg/µL NC oligos (sense, 5’-UUCUCCGAACGUGUCACGUTT-3’; antisense, 5’-ACGUGACACGUUCGGAGAATT-3’; GenePharma, Shanghai, China). The NFAT5 overexpression + HG group (0.1 µg/L, vector, pcDNA3.1 (+); cloning site, BamHI/EcoRI; GenePharma) and siNFAT5 + HG group (0.1 µg/µL, sense, 5’-GGCCAUGAAAGC
Seven-week-old SPF C57 mice were provided by Sippe-Bk (Shanghai, China; animal qualification certificate no. SCXK[Hu]2018-0006). The experimental protocol for this study was reviewed and approved by the Experimental Animal Ethics Committee of Zhejiang Chinese Medical University (No. IACUC-202312-03). All mice were given a standard rodent diet and free access to water. Streptozotocin (Boaigang, Beijing, Chia) was dissolved in 50 mmol/L sodium citrate buffer (pH 4.5) and stored in a refrigerator away from light. After fasting for 12 hours, the mice were intraperitoneally injected with 100 mg/kg streptozotocin once a day for 2 consecutive days. The blood glucose levels of the mice were measured using a blood glucose meter. After the final injection, blood glucose tests were conducted once a day for 1 week, and a random blood glucose level ≥ 16.8 mmol/L was used to indicate successful modeling. Blood glucose levels were measured every week until the end of the experiment, and mice whose levels returned to normal were removed. Diabetic mice that had been subjected to the modeling conditions for 4 weeks underwent anesthesia using a pentobarbital sodium, after which, a full-skin-thickness circular wound with a diameter of 30 mm was generated using a trephine at the back of the skull along the midline of the back. Diabetic mice with wounds were divided into a blank group and a shikonin group, with 12 mice per group.
Zicao (Huadong, Hangzhou, China) was baked at 80 °C for 15 minutes, cooled to room temperature and ground into a powder. The powder (100 g/L) was added to sesame oil after boiling, heated on low heat for 30 minutes, and then stored in a glass bottle for subsequent administration to the shikonin group. To prepare the treatment for the blank group, sesame oil was boiled, heated on low heat for 30 minutes, and then stored in a glass bottle. The concentration of shikonin in the Zicao oil (100 g/L) was 4 μmol/L. The shikonin external wound medication was applied once a day until wound healing.
The wounds were photographed every 3 days and the wound areas were measured using Image J (National Institutes of Health, Bethesda, MD, United States). NFAT5 expression was analyzed using immunofluorescence. Skin samples were taken from the edge of the wound and sent for examination on ice. After the specimen was fixed, the skin was washed with phosphate-buffered saline (PBS) three times, with each wash lasting 5 minutes. A monoclonal antibody (anti-NFAT5, Huabio, Hangzhou, China) was added and the samples were incubated at 37 °C for 1 hour or 4 °C overnight. After washing with PBS three times, with each wash lasting 5 minutes, the samples were incubated with the secondary antibody at 37 °C for 20-30 minutes. The samples were sealed with an anti-fluorescence-quencher containing 4′,6-diamidino-2-phenylindole.
COX-2 levels were determined using a commercial ELISA kit (Shimadzu, Shanghai, China), according to the manu
Intracellular ROS generation was measured using the oxidation-sensitive fluorescent probe DCFH-DA (Beyotime, Shanghai, China). Briefly, after the indicated treatments, the cells were washed twice with PBS and incubated with 5 μmol/L DCF-DA for 30 minutes at 37 °C in the dark. After incubation, the cells were washed and resuspended in culture medium for analysis using a microplate reader. The intensity of green fluorescence was positively correlated with ROS levels.
The fluorescent dye JC-1 (Beyotime, Shanghai, China) was used to assess mitochondrial membrane potential. Treated cell groups were placed in a 37 °C incubator for 24 hours. The washed cells were then incubated with the JC-1 dye for 20 minutes. After incubation, the cells were washed with PBS. They were then observed and photographed under a microscope, and red signal ratios were evaluated.
A Calcein AM dye solution (Beyotime, Shanghai, China) was used to assess mitochondrial membrane permeability. Treated cell groups were incubated with Calcein AM dye for 30-45 minutes at 37 °C. CoCl2 was added and the cells were incubated at 37 °C for a further 30-45 minutes. After incubation at 37 °C for 30 minutes in the dark, the culture medium was removed, the cells were washed 2-3 times with PBS, and detection buffer was added. A microscope was used to capture images.
After the cells were digested, 2.5% glutaraldehyde was added to 2 mL and the cells were fixed for 40 minutes. They were scraped off, transferred to 5 mL centrifuge tubes, and centrifuged. 1 mL of glutaraldehyde was absorbed, then the contents were blown into a 1.5 mL EP tube, centrifuged, the glutaraldehyde was absorbed, 50 µL of fetal bovine serum was added, the contents were blown again, centrifuged, and the excess fetal bovine serum was absorbed after clumps of cells had formed.
Glutaraldehyde was carefully added along the tube wall to secure. Samples were treated with double fixation, dehydration, and coating in the Medical Research Center, Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University. Sections were observed under a transmission electron microscope.
Total RNA was extracted from the cells and cDNA was synthesized using a reverse transcription kit (Yisheng, Shanghai, China). The PCR amplification system was prepared according to the instructions of the PCR kit manufacturer (Yisheng, Shanghai, China), in total volume of 50 µL. The PCR products were analyzed using agarose electrophoresis. The NFAT5 primer sequences were forward, 5′ GCTGGTGCTTTGAATGTAAATGTG 3′ and reverse, 5′ AGTCATCAGGGCATTAGGGATAATA 3′ and the h-GAPDH primer sequences were forward, 5' CATGAGAAGTATGACAACAGCCT 3′ and reverse 5′ AGTCCTTCCACGATACCAAAGT 3′. Amplification was performed using the preset reaction procedure. After 35 cycles, the amplified product was analyzed using on-board fluorescent quantification and were collected at the end of the PCR for agarose gel electrophoresis.
Western blotting assays were used to evaluate the levels of NFAT5, AMPK, mTOR, Akt, p-Akt, iNOS, VEGF, and NF-κB. The cells were washed three times with PBS and treated with 1 mL of radioimmunoprecipitation assay lysis buffer containing phenylmethylsulfonyl fluoride. The cells were centrifuged at 13500 × g for 15 minutes at 4 °C. The cells were mixed with loading buffer and boiled for 10 minutes. Equal amounts of proteins were loaded and separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were transferred from the gel to polyvinylidene fluoride membranes. The samples were blocked for 1 hour at room temperature with 5% nonfat dry milk in Tris-saline containing 0.1% Tween-20 (TBST). The following primary antibodies (1:1000; Huabio, Hangzhou, China) were used: anti-mTOR, anti-Akt, anti-p-Akt, anti-NF-κB, anti-VEGF, anti-iNOS, and anti-Glut-1. These were diluted in TBST and incubated with the blots overnight at 4 °C. The blots were then washed three times in TBST, treated with a peroxidase-conjugated secondary antibody (goat anti-rabbit, 1:1000) for 1 hour, and washed three more times with TBST. A Gel Doc XR + Molecular Imager (Bio-Rad, Hercules, CA, United States) was used to image the membranes. Gel EQ Quantity One software (Bio-Rad, United States) was used to quantify the grayscale intensity of the immunoreactive bands.
All data are presented as the mean ± SD of a minimum of three independent experiments. One-way analysis of variance was used to analyze differences between three or more groups. Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad, San Diego, CA, United States), and statistical significance was set at P < 0.05. The statistical methods of this study were reviewed by Professor Chen Ling from the Zhejiang University of Finance and Economics.
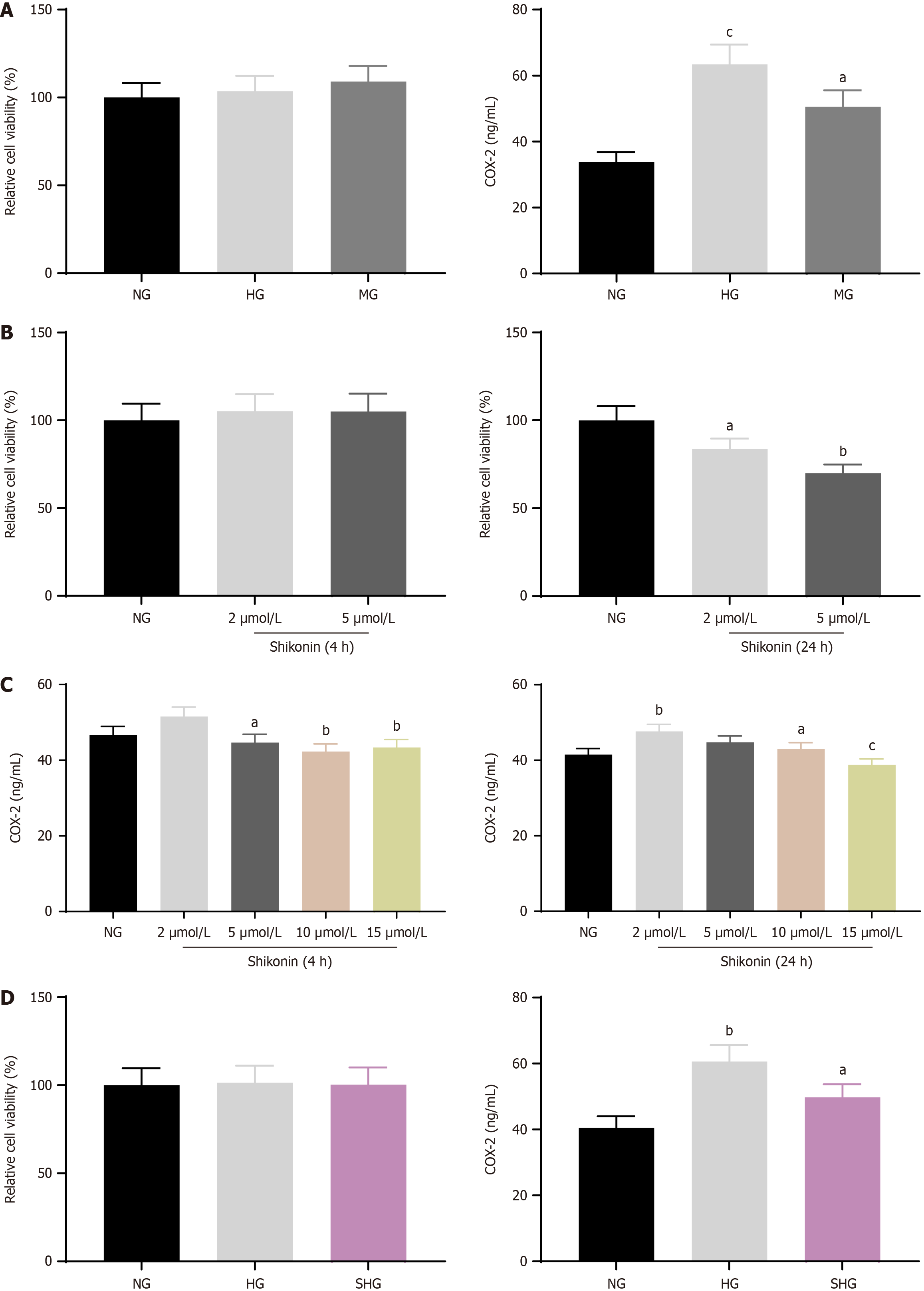
Cell viability of hypertonic cell model and COX-2 expression: Relative cell viability was 100% in the NG group (25 mmol/L glucose), 103.6% in the HG group (200 mmol/L glucose), and 109% in the MG group (175 mmol/L mannitol + 25 mmol/L glucose). The three groups exhibited no significant differences in cell viability. Thus, glucose concentrations used for the HG group (200 mmol/L glucose) and the MG group (175 mmol/L mannitol + 25 mmol/L glucose) were used for the hypertonic cell model. ELISA results showed COX-2 concentrations of 33.83 ng/mL in the NG group, 63.40 ng/mL in the HG group, and 50.55 ng/mL in MG group. This indicated that hypertonic concentrations of glucose or mannitol promoted the release of the pro-inflammatory factor COX-2 from HaCaT cells (Figure 1A).
Viability of shikonin-treated cells and COX-2 expression: The relative cell viability was 105.1%, and 105% after 4 hours treatment with 2 μmol/L or 5 μmol/L shikonin, respectively. These results showed that treatment with 2 μmol/L and 5 μmol/L for 4 hours had no effect on the viability of HaCaT cells. The relative cell viability was 83.6% and 69.9% after 24 hours of treatment with 2 μmol/L and 5 μmol/L shikonin, respectively. The results showed that HaCaT cell viability decreased gradually with increasing shikonin concentration, and prolonged treatment had an inhibitory effect on the viability of HaCaT cells (Figure 1B). The levels of COX-2 in HaCaT cells after 4 hours induction by different concentrations of shikonin were 46.62 ng/mL, 51.55 ng/mL, 44.67 ng/mL, 42.30 ng/mL, and 43.39 ng/mL. The expression level of COX-2 was highest after treatment with 2 μmol/L shikonin for 4 hours, and it decreased with increasing shikonin concentrations. The expression levels of COX-2 in HaCaT cells after 24 hours induced by different concentrations of comfrey were 41.5 ng/mL, 47.63 ng/mL, 44.74 ng/mL, 43.01 ng/mL, and 38.84 ng/mL. The expression level of COX-2 was highest after treatment with 2 μmol/L shikonin for 24 hours, and it then decreased significantly with increasing shikonin concentrations (Figure 1C). In summary, with increases in the shikonin concentration and treatment time, the expression levels of inflammatory factors can be effectively reduced.
Cell viability in shikonin-treated hypertonic cell models and COX-2 expression: The relative cell viability was 101.4% in the HG group. After 2 μmol/L shikonin pretreatment for 4 hours, cell viability was 100.3%. This indicated no significant effect of shikonin on cell viability. Thus, 2 μmol/L shikonin pretreatment for 4 hours (SHG group) was used for subsequent experiments. COX-2 levels were 40.50 ng/mL in the NG group, 60.60 ng/mL in HG group, and 49.72 ng/mL in the SHG group (Figure 1D).
Changes in mitochondria imaged by transmission electron microscopy: The mitochondrial ultrastructure was identified in the NG, MG, HG, and SHG groups. In the NG group, the structure of each layer of mitochondria (outer membrane, outer chamber, inner membrane, and inner chamber) was examined and the morphological boundaries were clear. The mitochondrial cristae were aligned. However, the MG and HG groups exhibited rounded and swollen mitochondria, nuclear shrinkage, and disorganized mitochondrial cristae. Compared to the MG and HG groups, the SHG group’s mitochondrial structure was clear, mitochondrial ridge swelling was not prominent, and the arrangement was organized., indicating that shikonin protected mitochondrial morphological integrity (Figure 2A).
ROS levels were lower in the MG and HG groups than the NG group. ROS levels in the SHG group were lower than those in the MG and HG groups, indicating that shikonin reduced ROS levels in cells in a hypertonic environment (Figure 2B).
The mitochondrial membrane potential was higher in the NG group than the MG and HG groups and higher in the SHG group than the MG and HG groups. The mitochondrial membrane potential increased after shikonin treatment (Figure 2C).
Compared with the NG group, the fluorescence expression of mitochondrial membrane potential was weakened in the HG and MG groups. The fluorescence intensity in the SHG group was higher than in the HG and MG groups. These results indicated that cells under hypertonic stress showed improved permeability of the mitochondrial membrane, but shikonin reduced the mitochondrial membrane permeability (Figure 2D).
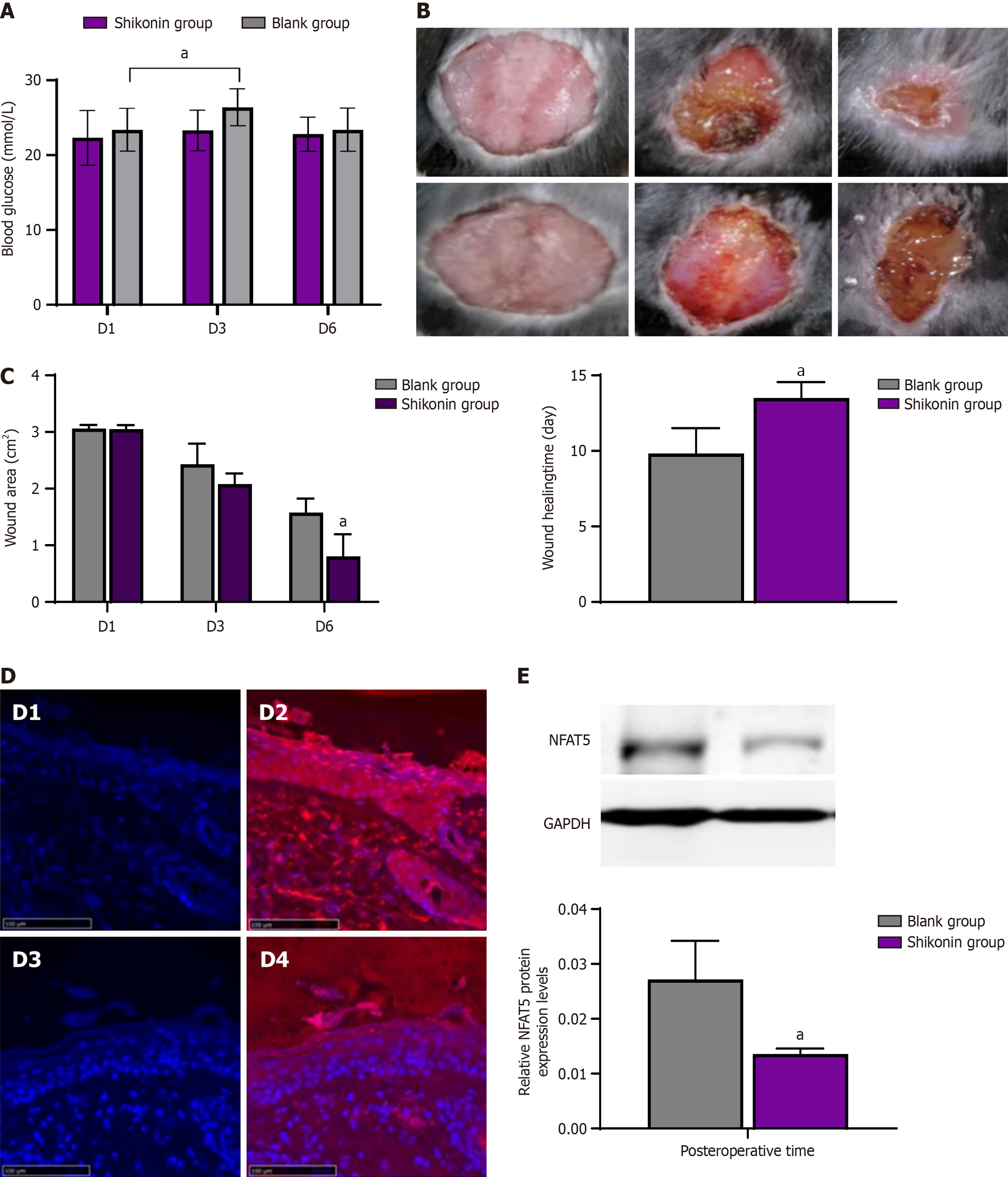
Changes of blood glucose in mice: On the first day after modeling, the blood glucose level of mice in the shikonin group was 22.31 ± 3.64 mmol/L, and that of mice in blank group was 23.40 ± 2.87 mmol/L, and there was no difference between the two groups. On the third day, the blood glucose level of mice in the shikonin group was 23.30 ± 2.72 mmol/L, and that in blank group was 26.40 ± 2.47 mmol/L, and there was no difference between the two groups. On the sixth day, the blood glucose level of mice in the shikonin group was 22.80 ± 2.28 mmol/L, and that in blank group was 23.40 ± 2.88 mmol/L, and there was no difference between the two groups. The blood glucose level of mice in the blank group was significantly higher on day 3 after modeling than on day 1 after modeling. There was no difference in the blood glucose level between the third day and the first day after molding in the shikonin group (Figure 3A).
Shikonin promoted wound healing: There was less exudation, a partial scab, and new granulation tissue at the wound margin in the shikonin group, while wound congestion with exudation was observed in the blank group on the third day after modeling (Figure 3B). On the sixth day after modeling, most of the wounds in the shikonin group had healed and formed a crust, and the wound area was significantly smaller than that in the blank group. The wound-healing time was 9.83 ± 1.17 days in the shikonin group, but 13.5 ± 1.05 days in the blank group. Thus, shikonin promoted wound healing in diabetic mice (Figure 3C).
Shikonin inhibited NFAT5 expression in diabetic mice: On the third day after modeling, the expression of NFAT5 protein at the wound margin was detected by immunofluorescence. NFAT5 expression levels were significantly reduced in the shikonin group compared with the blank group, as detected by western blotting (Figure 3D and E).
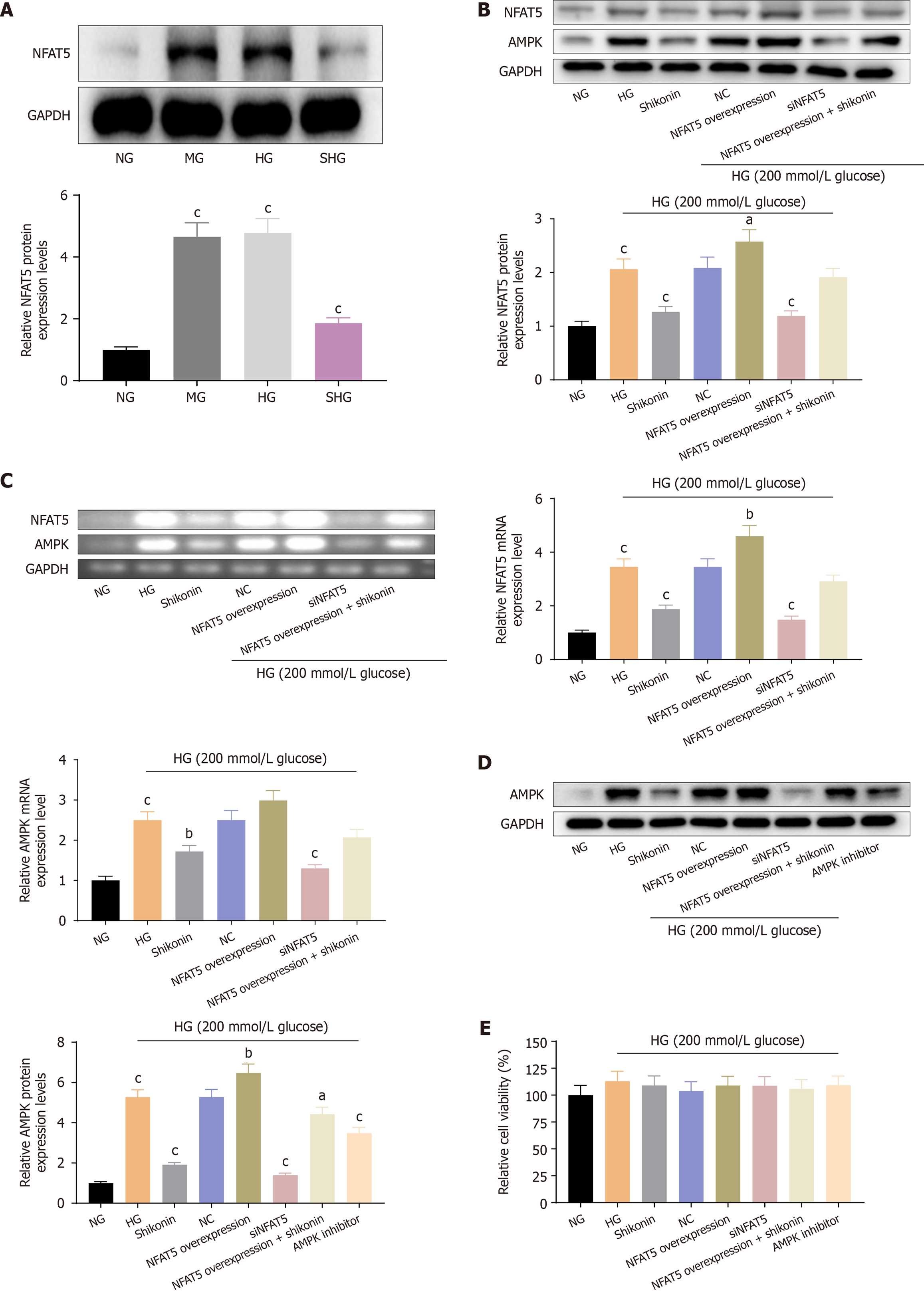
Shikonin inhibited NFAT5 expression under glucose-induced hyperosmotic stress: NFAT5 expression was detected by western blotting. Compared with the NG group, the HG and MG groups showed equally significant increase in NFAT5 expression levels. The NFAT5 expression level was reduced in the SHG group compared with HG and MG groups. These results indicated that shikonin inhibited NFAT5 expression under glucose-induced hyperosmotic stress (Figure 4A).
Shikonin inhibited AMPK expression via NFAT5 under glucose-induced hyperosmotic stress: We established NFAT5-overexpressing and NFAT5-silenced cell models. Compared with the NG group, the HG group showed significantly elevated expression levels of AMPK and NFAT5 protein and mRNA (Figure 4B and C). AMPK and NFAT5 expression levels were significantly reduced in the SHG group compared with the HG group. AMPK and NFAT5 expression levels were significantly reduced in the NFAT5-overexpression + HG group. The expression levels of AMPK and NFAT5 were decreased in the NFAT5-overexpression + HG group after shikonin treatment. The expression level of AMPK protein was significantly decreased in the AMPK inhibitor + HG group (Figure 4D). In addition, there were no differences in cell viability among all groups (Figure 4E). These results showed that the expression level of AMPK was elevated via NFAT5 in cells cultured in high-glucose medium. Moreover, shikonin inhibited AMPK expression via NFAT5 under conditions of glucose-induced hyperosmotic stress.
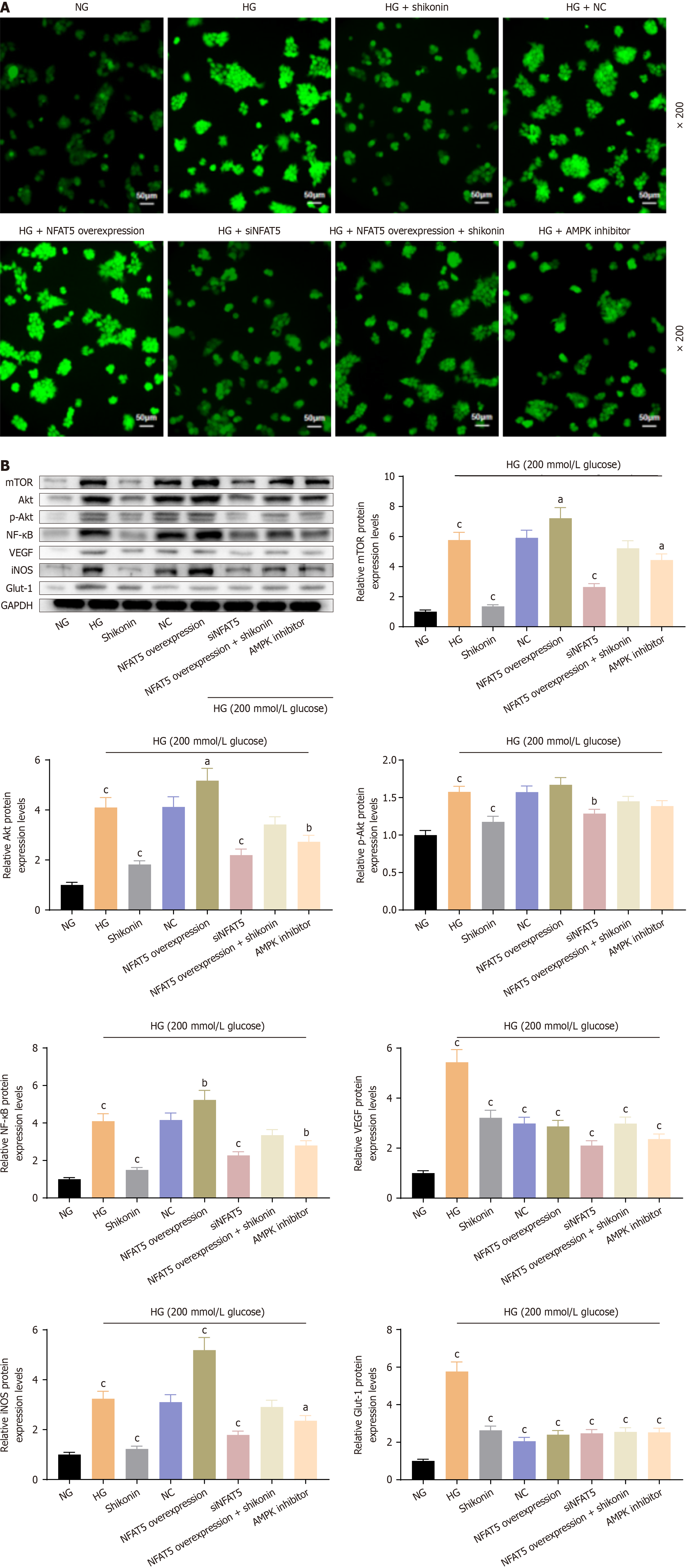
Shikonin inhibited ROS production via the NFAT5/AMPK pathway: ROS levels were lowest in the NG group. They were higher in the HG group than the NG group. ROS levels increased in the NFAT5-overexpression + HG group compared with the HG group and decreased in the SHG group, NFAT5-silenced + HG group, and AMPK inhibitor + HG group compared with the HG group. NFAT5 overexpression increased ROS levels and siNFAT5 and AMPK inhibitors reduced ROS levels in cells under hypertonic conditions. ROS levels were decreased in the NFAT5-overexpression + HG group with shikonin pretreatment compared with the NFAT5-overexpression + HG group. These results showed that shikonin inhibited ROS production under hypertonic stress via the NFAT5/AMPK pathway (Figure 5A).
Effects of shikonin on downstream molecules of the NFAT5/AMPK pathway under glucose-induced hyperosmotic stress: Compared with the NG group, the HG group showed significantly elevated expression levels of mTOR, Akt, p-Akt, NF-κB, VEGF, iNOS, and Glut-1. The expression levels of mTOR, Akt, p-Akt, NF-κB, and iNOS were reduced significantly in the HG group compared with the HG group. There were no significant differences in the expression levels of mTOR, Akt, p-Akt, NF-κB, or iNOS proteins in the NC + HG group, while the protein expression levels of VEGF and Glut-1 were significantly decreased. Compared with the HG group, the NFAT5-overexpression + hyperosmolar group showed significantly increased expression levels of mTOR, Akt, p-Akt, NF-κB, and iNOS proteins, and significantly decreased protein expression levels of VEGF and Glut-1. Compared with the HG group, the siNFAT5 + HG group and the NFAT5-overexpression + HG group with shikonin pretreatment showed significantly decreased protein expression levels of mTOR, Akt, p-Akt, NF-κB, VEGF, iNOS, and Glut-1. Moreover, compared with the HG group, the NFAT5-overexpression + HG group with shikonin pretreatment showed significantly decreased protein expression levels of VEGF and Glut-1. Compared with the HG group, the AMPK inhibitor + HG group showed significantly decreased protein expression levels of mTOR, Akt, p-Akt, NF-κB, iNOS, VEGF, and Glut-1 (Figure 5B). These results indicated that mTOR, Akt, NF-κB, and iNOS are closely associated with the NFAT5/AMPK pathway. Shikonin inhibited mTOR, Akt, NF-κB, and iNOS via the NFAT5/AMPK pathway. It also inhibited VEGF and Glut-1 without regulating NFAT5 ex
Zicao, a traditional Chinese medicine, has been well known as a topical medication for the treatment of various skin conditions since ancient times in China. Zicao is cold and bitter, which means it can make “heat blood” cold, according to the “Compendium of Materia Medica”. “Heat blood” means that hot blood increases circulation. “Heat blood” is associated with symptoms such as necrotic tissue, a high bacterial load, tissue damage, and other underlying causes of persistent inflammation in traditional Chinese medicine. Shikonin is the primary natural compound obtained from the root of Zicao, typically extracted through solvent extraction or ultrasonic extraction methods. For ultrasonic extraction, a solid-liquid ratio of 10.3 or 11:1 is recommended. According to Pei et al[17], Zicao oil is an effective solvent for shikonin extraction and is often utilized in clinical applications. Despite limited time for revisions, we analyzed the shikonin content in Zicao oil using HPLC and discovered that the concentration of shikonin in Zicao oil (100g/L) is 4μmol/L. Therefore, we opted for shikonin extraction using oil at a concentration of 100 g. Zicao oil is commonly applied to chronic wounds, such as burns, anal fistulas, pressure ulcers, and diabetic foot ulcers[12]. In addition, our previous research indicated that Zicao ointment is effective against persistent skin inflammatory conditions, such as verruca plana, urticaria, and neurodermatitis. Because shikonin is the main functional component of Zicao, the clinical dosage of shikonin should be refined in future studies. Shikonin should be stored at low temperatures and in the dark[18]. The traditional process of using high heat and hot oil for frying results in naphthoquinone pigment components in shikonin, which are unstable to heat, and the purple pigment is prone to modification[19]. Shikonin should be combined with polymeric materials or other novel materials to create more stable topical formulations in the future, ensuring greater dosage precision in clinical applications. The optimum dosage of shikonin can effectively exhibit benefits in the external treatment of dermatological conditions, decrease the overuse of glucocorticoids and antibiotics, enhance safety, and provide substantial social benefits at a minimal cost.
We developed a hypertonic cellular model. NFAT5 maintains normal physiological function only under normal osmotic pressure. A glucose concentration of 25 mmol/L is commonly used for cell culture[20]. Cells in different organs have different osmotic thresholds. Human keratinocytes exhibit a threshold of 450-600 mOsm for NFAT5 activation[21]. We found no difference in the viability of keratinocytes exposed to 25, 200, 175, or 25 mmol/L glucose for 24 hours. In addition, the expression of NFAT5 was upregulated in these hyperosmolar groups, and this upregulation of NFAT5 increased the expression level of COX-2, which is associated with activation of the downstream inflammatory response[22]. Inflammatory stimuli may induce an increase in blood glucose levels. Shikonin is a potential regulator of glucose tolerance[23]. We found that shikonin can relieve blood glucose fluctuations in diabetic mice with wounds. Our study showed that hyperosmolar stress is a potent inflammatory stimulus that promotes cytokine release. Shikonin suppresses inflammation via mediators such as NF-κB, TNF-α, and IL-1β[24]. We observed that shikonin also inhibited COX-2 expression, and that a small amount of inflammatory cytokine release improved the survival of cells under hyperosmolar stress, whereas excessive inflammatory cytokine release can damage cells. We observed that high concentrations or prolonged treatment with shikonin inhibited inflammation and cell viability, while appropriate times and concentrations did not affect cell viability and inhibited the release of inflammatory factors, indicating that moderate anti-inflammatory effects are beneficial for wound repair.
Shikonin may have a protective effect on cells under high-glucose conditions, particularly in terms of mitochondrial function. Our results are consistent with those of previous studies, showing that shikonin reduces ROS levels[25]. Generally, intracellular ROS levels are maintained within a low physiological range. Under pathological hypertonic conditions, the balance between the intracellular antioxidant system and oxygen free radicals is disturbed, and excess ROS can damage mitochondrial enzymes, lipids, and nucleic acids, causing oxidative stress and leading to structural and functional changes, such as swelling of the mitochondrial cristae and disruption of the mitochondrial membrane potential[26]. In our study, hypertonicity decreased mitochondrial membrane potential and increased mitochondrial membrane permeability, whereas shikonin had a protective effect on the mitochondrial membrane. Moreover, we observed that shikonin pretreatment maintained mitochondrial morphological integrity in that mitochondrial cristae swelling was relieved and the structure became orderly, as determined by transmission electron microscope imaging.
The physiological function of NFAT5 is to maintain normal cellular osmotic pressure and homeostasis. Here, NFAT5 expression increased after cells were exposed to hypertonic conditions. We observed that NFAT5 expression increased not only under high-glucose stress, but also under high-mannitol stress. As diabetes progresses, the NFAT5 hype
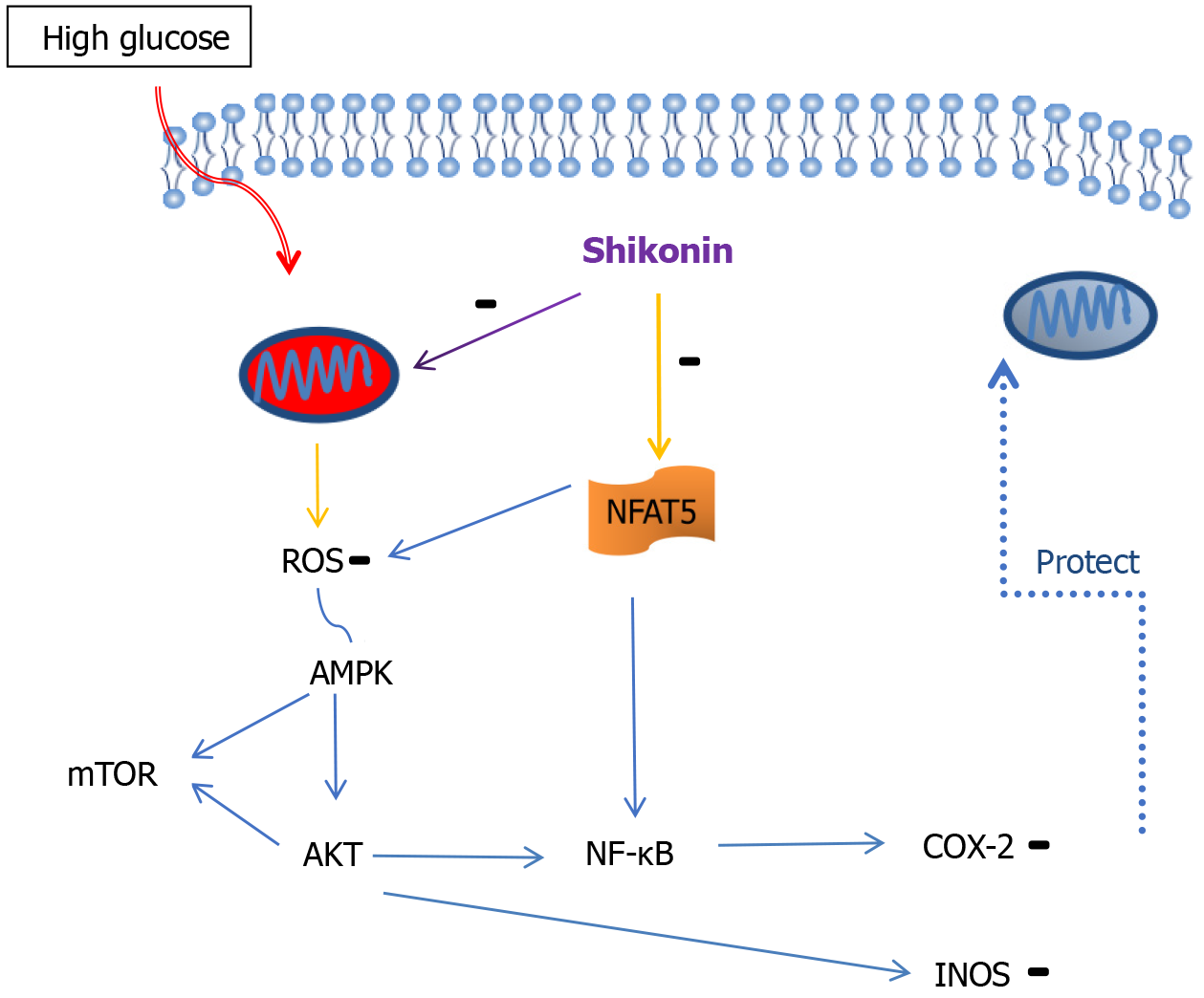
In conclusion, in this study we confirmed that shikonin can suppress the pathological expression of NFAT5, thereby protecting mitochondrial function, inhibiting cellular inflammation, and reducing ROS production. Futhermore, we found that shikonin protects mitochondria through the NFAT5/AMPK pathway (Figure 6). Our research provides novel insights into the contribution of shikonin in diabetes.
We appreciate the experimental support from the Medical Research Center, Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University and the technical support from the Laboratory Animal Research Center, Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University.
| 2. | Wu S, Zhou Z, Li Y, Jiang J. Advancements in diabetic foot ulcer research: Focus on mesenchymal stem cells and their exosomes. Heliyon. 2024;10:e37031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Edmonds M, Manu C, Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma. 2021;17:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 4. | Smith J, Rai V. Novel Factors Regulating Proliferation, Migration, and Differentiation of Fibroblasts, Keratinocytes, and Vascular Smooth Muscle Cells during Wound Healing. Biomedicines. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Ono M, Izumi Y, Maruyama K, Yasuoka Y, Hiramatsu A, Aramburu J, López-Rodríguez C, Nonoguchi H, Kakizoe Y, Adachi M, Kuwabara T, Mukoyama M. Characterization of gene expression in the kidney of renal tubular cell-specific NFAT5 knockout mice. Am J Physiol Renal Physiol. 2024;326:F394-F410. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Sumida TS. Hyperosmotic stress response regulates interstitial homeostasis and pathogenic inflammation. J Biochem. 2023;173:159-166. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Choi SY, Lee-Kwon W, Kwon HM. The evolving role of TonEBP as an immunometabolic stress protein. Nat Rev Nephrol. 2020;16:352-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Kim NH, Choi S, Han EJ, Hong BK, Choi SY, Kwon HM, Hwang SY, Cho CS, Kim WU. The xanthine oxidase-NFAT5 pathway regulates macrophage activation and TLR-induced inflammatory arthritis. Eur J Immunol. 2014;44:2721-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Lee N, Kim D, Kim WU. Role of NFAT5 in the Immune System and Pathogenesis of Autoimmune Diseases. Front Immunol. 2019;10:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Deng L, Du C, Song P, Chen T, Rui S, Armstrong DG, Deng W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid Med Cell Longev. 2021;2021:8852759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 305] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Li M, Wang Y, Han F, Shen K, Luo L, Li Y, Jia Y, Zhang J, Cai W, Wang K, Zhao M, Wang J, Gao X, Tian C, Guo B, Hu D. Exosome/metformin-loaded self-healing conductive hydrogel rescues microvascular dysfunction and promotes chronic diabetic wound healing by inhibiting mitochondrial fission. Bioact Mater. 2023;26:323-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 12. | Song Y, Ding Q, Hao Y, Cui B, Ding C, Gao F. Pharmacological Effects of Shikonin and Its Potential in Skin Repair: A Review. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 13. | Sun Q, Hu S, Lou Z, Gao J. The macrophage polarization in inflammatory dermatosis and its potential drug candidates. Biomed Pharmacother. 2023;161:114469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 14. | Yang C, Liu P, Wang S, Zhao G, Zhang T, Guo S, Jiang K, Wu H, Deng G. Shikonin exerts anti-inflammatory effects in LPS-induced mastitis by inhibiting NF-κB signaling pathway. Biochem Biophys Res Commun. 2018;505:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Sun Q, Gong T, Liu M, Ren S, Yang H, Zeng S, Zhao H, Chen L, Ming T, Meng X, Xu H. Shikonin, a naphthalene ingredient: Therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine. 2022;94:153805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 16. | Guo H, Sun J, Li D, Hu Y, Yu X, Hua H, Jing X, Chen F, Jia Z, Xu J. Shikonin attenuates acetaminophen-induced acute liver injury via inhibition of oxidative stress and inflammation. Biomed Pharmacother. 2019;112:108704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 17. | Pei XW, Wang KZ, Chen JC, Dang XQ, Shi ZB, Gao DF. [The effect of Lithospermum oil on wound tissue repair and bFGF mRNA expression]. Zhongguo Zhongxiyi Jiehe Zazhi. 2005;892-894. |
| 18. | Kaur K, Singh A, Sharma H, Punj S, Bedi N. Formulation Strategies and Therapeutic Applications of Shikonin and Related Derivatives. Recent Adv Drug Deliv Formul. 2022;16:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Yadav S, Sharma A, Nayik GA, Cooper R, Bhardwaj G, Sohal HS, Mutreja V, Kaur R, Areche FO, AlOudat M, Shaikh AM, Kovács B, Mohamed Ahmed AE. Review of Shikonin and Derivatives: Isolation, Chemistry, Biosynthesis, Pharmacology and Toxicology. Front Pharmacol. 2022;13:905755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Wang D, Jiang Y, Li Z, Xue L, Li X, Liu Y, Li C, Wang H. The Effect of Candida albicans on the Expression Levels of Toll-like Receptor 2 and Interleukin-8 in HaCaT Cells Under High- and Low-glucose Conditions. Indian J Dermatol. 2018;63:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Szél E, Danis J, Sőrés E, Tóth D, Korponyai C, Degovics D, Prorok J, Acsai K, Dikstein S, Kemény L, Erős G. Protective effects of glycerol and xylitol in keratinocytes exposed to hyperosmotic stress. Clin Cosmet Investig Dermatol. 2019;12:323-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Cen L, Xing F, Xu L, Cao Y. Potential Role of Gene Regulator NFAT5 in the Pathogenesis of Diabetes Mellitus. J Diabetes Res. 2020;2020:6927429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 23. | Guo Y, Zhou M, Mu Z, Guo J, Hou Y, Xu Y, Geng L. Recent advances in shikonin for the treatment of immune-related diseases: Anti-inflammatory and immunomodulatory mechanisms. Biomed Pharmacother. 2023;165:115138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 24. | Kim YG, Lee JH, Kim SH, Park SY, Kim YJ, Ryu CM, Seo HW, Lee JT. Inhibition of Biofilm Formation in Cutibacterium acnes, Staphylococcus aureus, and Candida albicans by the Phytopigment Shikonin. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 25. | Malik S, Brudzyńska P, Khan MR, Sytar O, Makhzoum A, Sionkowska A. Natural Plant-Derived Compounds in Food and Cosmetics: A Paradigm of Shikonin and Its Derivatives. Materials (Basel). 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | Nivedha J, Vennila L, Sindhu G, Kanimozhi K, Raj TC. Investigating the Anticancer Potential of Biochanin A in KB Oral Cancer Cells Through the NFκB Pathway. Cell Biochem Funct. 2024;42:e4130. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Chun Y, Kim J. AMPK-mTOR Signaling and Cellular Adaptations in Hypoxia. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 28. | Xie X, Shu R, Yu C, Fu Z, Li Z. Mammalian AKT, the Emerging Roles on Mitochondrial Function in Diseases. Aging Dis. 2022;13:157-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Yuan R, Adlimoghaddam A, Zhu Y, Han X, Bartke A. Early Life Interventions: Impact on Aging and Longevity. Aging Dis. 2024. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Zhang S, Xia B, Kalionis B, Li H, Zhang X, Zhang X, Xia S. The Role and Mechanism of Vascular Aging in Geriatric Vascular Diseases. Aging Dis. 2024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |