Published online Oct 15, 2024. doi: 10.4239/wjd.v15.i10.2022
Revised: July 23, 2024
Accepted: July 26, 2024
Published online: October 15, 2024
Processing time: 109 Days and 16.8 Hours
Since the discovery of insulin over 100 years ago, the focus of research in the management of type 1 diabetes (T1D) has centered around glycemic control and management of complications rather than the prevention of autoimmune destruc
Core tip: There has been a paradigm shift in research on type 1 diabetes (T1D) in the last decade. From managing the consequences of β cell death to prevention of β cell destruction, immunotherapy is showing the path forward. Recent regulatory approval of teplizumab in stage 2 of T1D marks the first significant advance in research of immunotherapy. In this Editorial, we briefly explore the recent developments and prospects in the field of immunotherapy in T1D encompassing antibody-based therapy, antigen-based therapy, and stem-cell-based immunotherapy.
- Citation: Ray S, Palui R. Immunotherapy in type 1 diabetes: Novel pathway to the future ahead. World J Diabetes 2024; 15(10): 2022-2035
- URL: https://www.wjgnet.com/1948-9358/full/v15/i10/2022.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i10.2022
The basic pathophysiology behind the development of type 1 diabetes (T1D) is immune-mediated destruction of insulin-producing pancreatic β cells[1]. The insulin-producing capacity of the endocrine pancreas depends upon its functional β cell number and size, collectively known as β cell mass. The body’s aberrant immune system attacks and self-destroys the normal host tissue in autoimmune disease. Like any other autoimmune disease, progressive immune-mediated de
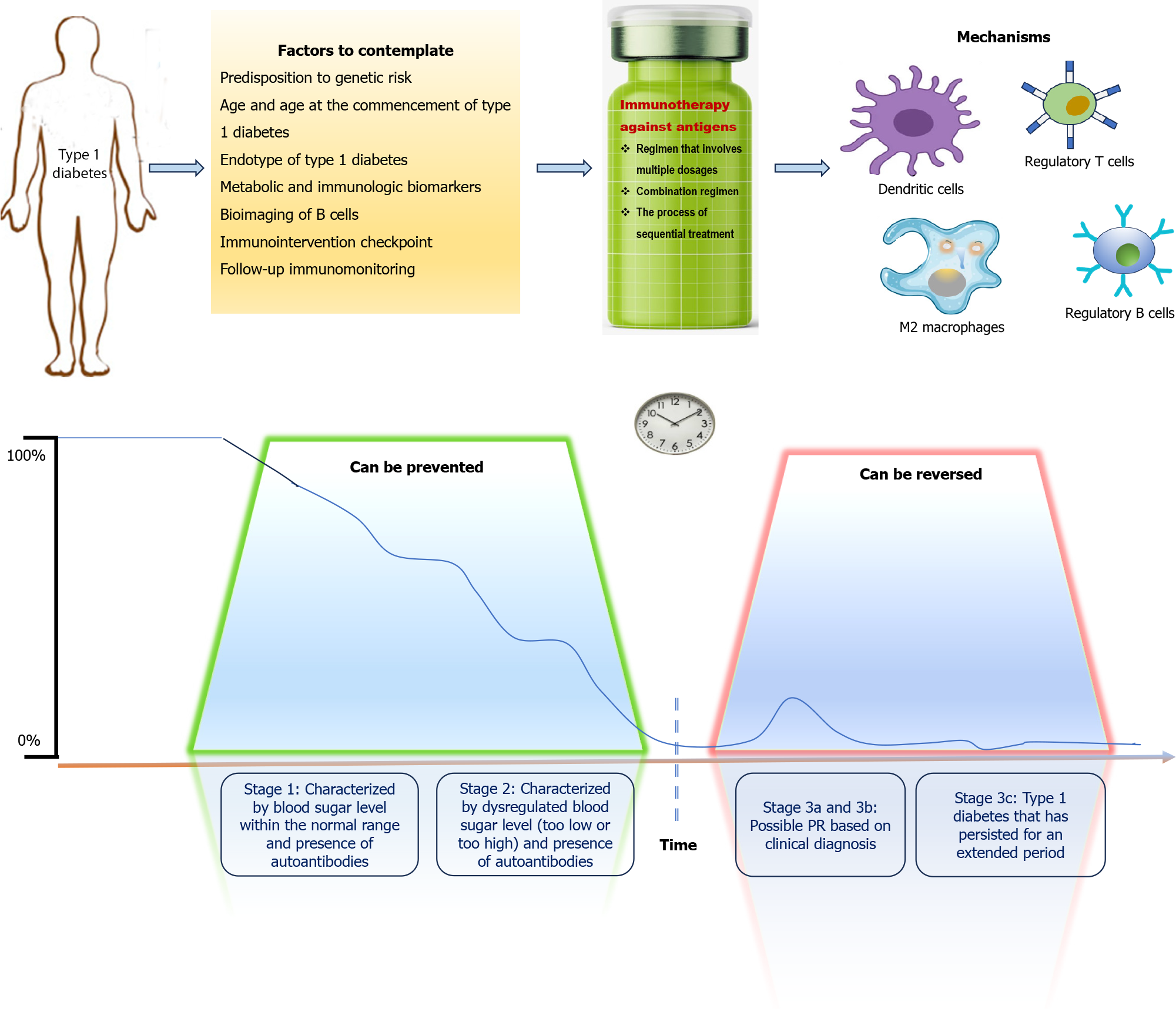
The pathophysiological stages of development of T1D are now being classified into three distinct stages: stage 1-positive antibody status with normoglycemia; stage 2-positive antibody status with dysglycemia or prediabetes; and stage 3-positive antibody status with frank diabetes or overt hyperglycemia[3]. Stage 3 of T1D development marks the onset of significant destruction of β cells. Stage 3 of disease development has been further subclassified into: stage 3a (not insulin requiring); stage 3b (insulin-requiring but with residual clinically relevant β cell mass); and stage 3c (insulin requiring without any clinically relevant β cell mass)[4]. The immune system plays a pivotal role in the de
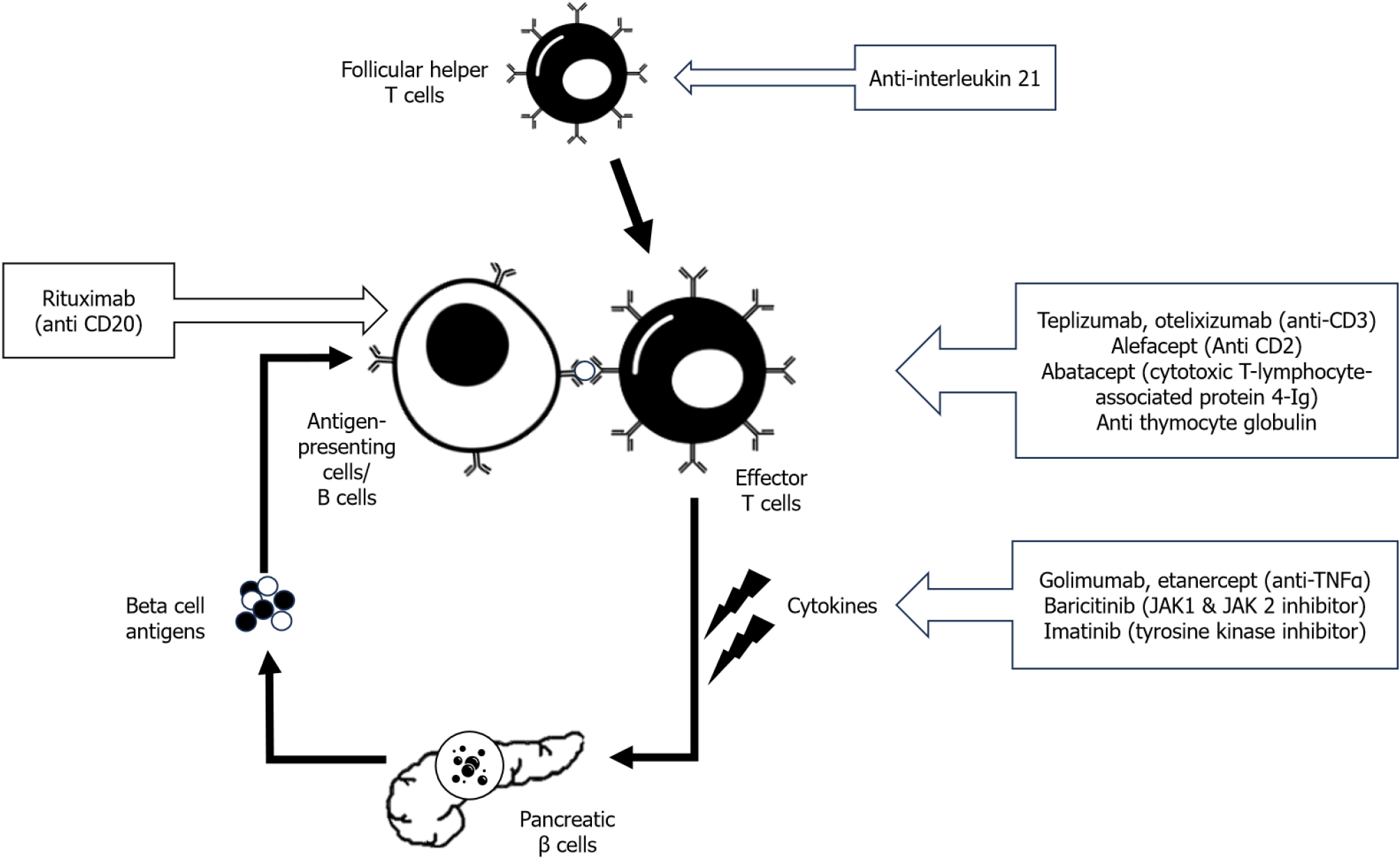
Antibody-based therapies against different target antigens have been tried to halt the autoimmune destruction of pancreatic β cells. These antibody-based therapies (monoclonal and polyclonal) are directed mainly against T cells or B cells or cytokine signaling (Figure 1). Most of these studies that evaluated antibody-based immunotherapies in T1D were done in stage 2 or early stage 3 of T1D. The details of the important antibody-based immunotherapies are given in the next section.
Teplizumab: Teplizumab is a humanized monoclonal antibody [immunoglobulin G (IgG) 1 kappa] directed against the CD3 portion of T-cell receptors[8]. This molecule is non-Fc binding, thus reducing the risk of cytokine release syndrome (CRS) compared to previous generations of anti-CD3 molecules. Teplizumab produces early suppression of cellular immune response by preventing the binding of CD4+ T helper cells to APCs by inhibiting CD3 of T-cell receptors. Prolonged and sustained binding of this molecule exerts a state of CD8+ T-cell exhaustion and thus induces chronic immunosuppression[9]. In 2022, teplizumab obtained US Food and Drug Administration (FDA) approval for delaying or prevention the onset of stage 3 of T1D in patients currently in stage 2 of T1D. It was approved for use in adults and children aged > 8 years[10].
The landmark trials that evaluated the safety and efficacy of teplizumab are summarized in Table 1[11-15]. All of these studies are done in stage 3 of the T1D except the TrialNet study, which was done in stage 2 of T1D[12]. These trials showed a more favorable c-peptide response in teplizumab than in the placebo arm. In the meta-analysis by Kamrul-Hasan et al[16], 834 subjects from six studies that evaluated the efficacy and safety of teplizumab as a disease-modifying therapy in T1D, were included. The authors reported greater preservation of area under the curve (AUC) of c-peptide in the teplizumab arm through 6-24 mo of follow-up [mean difference 0.07 nmol/L (95% confidence interval: 0.01-0.14, P = 0.03)]. Moreover, fewer patients reported reduced c-peptide response after 2 years of follow-up in the teplizumab arm (odds ratio 0.12). However, the authors also reported an increased risk of grade 3 or higher adverse events, nausea, rash, lymphopenia, and discontinuation of the study drug in the teplizumab arm than placebo. Adverse events are commonly associated with any monoclonal-antibody-based therapy. In the case of teplizumab, most adverse drug reactions occurred during the treatment period and were mild to moderate and manageable. Similarly, other recent meta-analyses also reported that patients in the teplizumab arm had higher AUC of c-peptide and lower exogenous insulin requirement but similar glycosylated hemoglobin (HbA1c) levels in comparison to placebo[17,18]. In another meta-analysis, Liu et al[19] included both the anti-CD3 monoclonal antibodies, teplizumab (seven studies) and otelixizumab (five studies). The authors also reported greater AUC of c-peptide and decreased exogenous insulin requirement in the anti-CD3 antibody arm. They found no significant difference in HbA1c and serious adverse events between the study and placebo arm[19]. Moreover, in the follow-up study of the AbATE trial, prolonged immunological response was reported even after 7 years of diagnosis of T1D in patients who initially responded to teplizumab[20].
| Ref. | Study design | Intervention | Population | Major outcome |
| Ramos et al[11], 2023 | PROTECT study | Phase 3, randomized, placebo-controlled trial with teplizumab or placebo for two 12-day courses | The 328 participants, stage 3 T1D, age 8-17 years, within 6 weeks of diagnosis | Higher stimulated c-peptide levels (teplizumab vs placebo) (least squares mean difference, 0.13 pmol per mL; 95%CI: 0.09-0.17; P < 0.001); no significant difference in HbA1c level, insulin requirement or hypoglycemia; ADR: headache, gastrointestinal symptoms, rash, lymphopenia, and mild cytokine release syndrome |
| Herold et al[12], 2019 | TrialNet study | Phase 2, randomized, placebo-controlled, double-blind trial of teplizumab (single 14-day course) | The 76 participants, relatives of T1D, stage 2, age > 8 years | Low-risk diagnosis of T1D (teplizumab vs placebo) (hazard ratio 0.41; 95%CI: 0.22-0.78; P = 0.006); longer median time to diagnose T1D (teplizumab vs placebo) (48.4 months vs 24.4 months); ADR of lymphopenia and rash |
| Herold et al[13], 2013 | AbATE Study | An open-label, randomized, controlled trial with teplizumab (two of 14-day course, one year apart) | The 83 participants, stage 3 T1D, age 8-30 years, within 8 weeks of diagnosis | Reduced decline in c-peptide at 2 years (-0.28 nmol/L; 95%CI: 0.36-0.20) vs control (-0.46 nmol/L; 95%CI: 0.57-0.35; P = 0.002); ADR: Rash, transient upper respiratory infections, headache, and nausea |
| Hagopian et al[14], 2013 | Protégé study | Phase 3, randomized, double-blind, parallel, placebo-controlled 2-years with teplizumab (3 dosing regimens, two of 14 days course, 26 weeks apart) | The 462 of 516 participants completed 2 years follow up, stage 3 T1D, age 8-35 years, within 12 weeks of diagnosis | Reduced the loss of area under curve mean c-peptide at 2 years (teplizumab vs placebo) (P = 0.027); ADR: lymphopenia; no differences in adverse events or serious adverse events among groups at 2 years |
| Herold et al[15], 2013 | Randomized placebo-controlled trial | The 63 participants, stage 3 T1D, within 4-12 months of diagnosis | The 21.7% higher c-peptide response (teplizumab vs placebo) [0.45 vs 0.371; difference, 0.059 nmol/L (95%CI: 0.006-0.115 nmol/L)] (P = 0.03); the teplizumab group required less exogenous insulin (P < 0.001) with no significant difference in HbA1c level; ADR: rash, lymphopenia and nausea |
Predictors of therapeutic response: The studies done with teplizumab also tried to find out possible predictors of response to therapy. If we can establish predictors for therapeutic response for this expensive therapy, it can be used cost-effectively in selective patients who are more likely to respond. The authors of the Protégé Trial reported that the recently diagnosed patients (< 6 wk) had the highest response. Moreover, patients living in the USA, patients with lower HbA1c, higher c-peptide, and lower insulin requirement at baseline were more likely to respond[14]. As younger patients exhibit stronger immune reactions than adult patients, they have a higher chance to respond[14,15]. Better glycemic control as measured by lower HbA1c level was also reported as a favorable predictor in trials done in stage 3 T1D[15,21]. This may be due to higher preserved β cell mass or increased insulin sensitivity in the treatment responders. The relationship of treatment outcome with baseline β cell reserve as measured by AUC of c-peptide is heterogeneous and may be related to the stage of T1D. Patients in stage 2 T1D are likely to respond when the baseline c-peptide level is lower, whereas a higher baseline c-peptide is a response predictor for patients in early stage 3 disease[12,14,21]. This paradoxical finding can be explained by the hypothesis that prior to significant immune mediated destruction in early stage of disease (stage 2), patients with stronger immune reaction are more likely to respond to immunomodulatory therapy. On the contrary, in patients with a later stage of disease (stage 3), when immune-mediated destruction is already significant, patients with higher residual functioning β cell mass respond better to therapy. The TrialNet study group also reported that patients who were anti-zinc transporter 8 (ZnT8) antibody negative, HLA-DR3 negative, and HLA-DR4 positive responded better to teplizumab than placebo[12]. Increased CD8+ T cell (cytotoxic and memory) and decreased CD4+ T cell (helper and memory) cells were observed in treatment responders in different trials[12,13,15,22]. However, none of these metabolic or immunologic predictors were consistently reported across all the studies and thus needed further validation in future studies.
Cost-effectiveness: One of the significant limitations of the broader use of this novel molecule is its premium cost. Teplizumab costs around $193000 for a single course of 14 d therapy. Mital et al[23] tried to analyze the cost-effectiveness of teplizumab depending on the HLA-DR3, HLA-DR4, and ZnT8 antibody status. They predicted if the cost of therapy is more than $100000, treating only a quarter of the patients at risk will be cost-effective. If we consider current annual cost of management of T1D patients and cost of teplizumab therapy, it may be cost-effective only if the prospective patient fulfills all the favorable criteria of therapeutic response- HLA-DR3 negative, HLA-DR4 positive and negative anti ZnT8 antibody status[23,24]. However, cost cannot be the sole deciding factor for restricting the benefit of this drug to whom it can be effective. We hope that in future this drug will be more affordable for the larger number of T1D patients. Guidelines for practical clinical use, screening, and proper patient selection for this molecule are now being formulated[25].
Otelixizumab: Otelixizumab is another anti-CD3 monoclonal chimeric and humanized antibody that had been evaluated in T1D. In a dose-finding, safety, and tolerability assessment randomized control trial (RCT), a 6-d course of otelixizumab in four different dosages was given to 30 T1D patients within 32 d of diagnosis[26]. A sustained metabolic response of preserved c-peptide level was found for up to 18 mo following a 9 mg dose of otelixizumab. However, not all the studies showed significant improvement in c-peptide level when compared to placebo, specifically when it is used in lower dosage[27,28]. The presence of a positive insulin autoantibody is reported to be a predictor of therapeutic response[29].
Alefacept: Alefacept is a fusion protein that antagonizes CD2 costimulatory receptors, inhibiting T cell proliferation and action. In T1DAL RCT, two 12-d courses of alefacept were given at 12 wk apart in 49 newly diagnosed (< 100 d) T1D patients[30]. After 24 mo, patients in the alefacept arm showed significantly higher AUC for c-peptide response for 2 and 4 h (P = 0.015 and P = 0.002, respectively). There was also a significant reduction of exogenous insulin requirement and hypoglycemia event in the alefacept arm. Alefacept also induced favorable immunological response by depleting CD4+ and CD8+ T cells[30]. However, at 12 mo follow-up, there was no significant difference in c-peptide AUC compared to the placebo[31].
Abatacept: Abatacept is a fusion protein of the Fc portion of IgG1 and cytotoxic T-lymphocyte-associated antigen 4, which blocks the costimulatory signal by blocking the CD28 T-cell receptor. In a multicenter RCT, 112 newly diagnosed T1D patients (between 6 and 45 years of age) were included and intravenous abatacept was given for 27 infusions over 2 years. The authors reported significant preservation of β cell function as measured by higher AUC of c-peptide at 2 years of follow-up in patients who received abatacept (P = 0.0029)[32]. In the follow-up study, the authors also reported persistent beneficial effects in the abatacept arm even after 1 year of cessation of treatment (P = 0.046)[33]. However, in a phase 2 RCT where 101 participants of stage 1 T1D patients were included, monthly infusion of abatacept for one year failed to significantly delay the progression of T1D (P = 0.11)[34].
Antithymocyte globulin: Anti-thymocyte globulin (ATG) is a rabbit polyclonal IgG antibody that acts against multiple T-cell antigens. It may act through various mechanisms like T-cell depletion, induction of anergy in T cells, and selective induction of regulatory T (Treg) cells[35]. In the START trial, high-dose ATG (6.5 mg/kg) failed to show any significant preservation of c-peptide response in recent onset T1D patients[36]. ATG showed acute T-cell depletion sparing effector memory T cells. Higher adverse events, including CRS, were reported in the ATG arm. To decrease the risk of these adverse events, later studies used a lower dosage of ATG. In the TrialNet ATG-granulocyte colony-stimulating factor (G-CSF) study, low-dose ATG (2.5 mg/kg) or low-dose ATG with pegylated G-CSF were studied in recent onset (< 100 d) T1D patients[37]. The authors reported significant HbA1c reduction and slowing of c-peptide decline after 1 year of follow-up in the low-dose ATG group without any extra benefit with the addition of GCSF. In the 2-year follow-up of the same trial, although reduction in HbA1c and T-cell depletion with preservation of Treg cells were reported in both ATG as well as ATG with G-CSF arm, higher AUC of c-peptide in comparison to placebo was seen only in the low-dose ATG arm but not in the ATG with G-CSF arm[38]. However, low-dose ATG with GSF had been reported to preserve the AUC of c-peptide following mixed meal tolerance tests in other studies[39,40]. In a cost-effectiveness analysis study, low-dose ATG was found to be more cost-effective than other immunotherapies (including teplizumab) or no therapy in patients with recent onset T1D[41].
Anti-interleukin 21 and liraglutide: Researchers have also explored the role of combination therapy in immunomodulatory therapy for T1D. Anti-interleukin (IL)-21 antibody antagonizes IL-21-mediated autoreactive T-cell trafficking to pancreatic islets as well as proliferation of effector and follicular helper T cells[42,43]. Glucagon-like peptide-1 agonists like liraglutide have been reported to improve β cell survival[44,45]. In the proof of concept animal study in the T1D mouse model, Rydén et al[46] reported that the combined anti-IL-21 and liraglutide therapy can reverse diabetes. In a phase 2, multicenter, parallel-group, placebo-controlled RCT, 308 T1D patients were randomized to four arms – anti-IL-21 only, liraglutide only, combined anti-IL-21 with liraglutide, or placebo. The decline of post-mixed meal tolerance test c-peptide level at 52 wk was significantly smaller (P = 0.0017) in the combined group in comparison to the placebo, but not in the anti-IL-21 only (P = 0.093) or liraglutide only (P = 0.38) groups[47]. Although the authors also reported a reasonable safety profile for this combination therapy, it should be further confirmed in future phase 3 trials.
Rituximab: Like various T-cell depletion therapies, depletion of B cells using anti-CD20 monoclonal antibody rituximab has also been studied in T1D. In a placebo-controlled RCT (TrialNet Anti-CD20), 87 recently diagnosed T1D patients were randomized to four different dosage infusions of rituximab or placebo[48]. The patients in the rituximab arm showed significantly higher AUC of c-peptide than placebo during the mixed meal tolerance test at 1-year follow-up. The patients in rituximab also had lower HbA1c and required less exogenous insulin. Patients who responded to rituximab showed greater T-cell proliferative response to islet cell antigens[49]. However, at 30 mo follow-up of the same study, there was no significant difference in the AUC of c-peptide between the rituximab and placebo arms[50]. The effect on B-cell depletion by rituximab also weaned off by 18 mo. The authors concluded that although rituximab can delay the decline of c-peptide in T1D, it cannot prevent the inevitable β cell loss[50]. In a recent RCT, combined therapy of autologous CD4+ CD25highCD127- Treg cells and rituximab was found to be superior in maintaining remission in recently diagnosed T1D patients in comparison to either the monotherapy or control[51].
Golimumab: The proinflammatory cytokine, tumor necrosis factor (TNF)-α plays an essential role in the pathogenesis of various autoimmune diseases[52]. In animal model studies, antagonizing TNF-α has been shown to prevent the development of autoimmune diabetes[53,54]. In the phase 2 T1GER study, the efficacy and safety of golimumab (anti TNF-α monoclonal antibody) were evaluated in recently diagnosed T1D patients (stage 3 of T1D)[55]. Patients were randomized to every fortnightly subcutaneous injection of golimumab (56 patients) or placebo (28 patients) for 52 wk. The authors reported higher 4-h mixed meal tolerance test AUC of c-peptide (0.64 pmol/mL vs 0.43 pmol/mL, P < 0.001) and lower exogenous insulin requirement (0.51 U/kg/day vs 0.69 U/kg/day) in golimumab arm than placebo. In the 2-year follow-up study (52 wk of therapy and 52 wk of off-therapy) of the same trial, patients in the golimumab arm showed persistently lower reductions in AUC of c- peptide at 78 and 104 wk compared to placebo[56]. The adverse events were reported to be similar in both arms of the study.
Etanercept: Etanercept, the recombinant soluble TNF-α receptor protein, blocks the activity of proinflammatory cytokine TNF-α and thus can be helpful in autoimmune diseases. In a pilot RCT, 18 patients with recently diagnosed T1D were given subcutaneous twice weekly etanercept or placebo for 24 wk[57]. The patients in the etanercept arm showed better HbA1C levels (5.9% vs 6.8%; P < 0.05) and AUC of c-peptide (+ 39% vs -20%; P < 0.05) than placebo, suggesting β cell preservation. In a recent study, etanercept was combined with glutamic acid decarboxylase (GAD-alum) and vitamin D (etanercept diamyd combination regimen) to evaluate the efficacy in newly diagnosed anti-GAD antibody-positive T1D patients[58]. However, this combination failed to show any significant beneficial effect in this trial.
Baricitinib: Baricitinib is a Janus kinase (JAK1 and JAK2) cytokine inhibitor which had been used successfully in au
Imatinib: The role of small tyrosine kinase inhibitors like imatinib also had been evaluated in T1D. In a nonobese diabetic mouse study, imatinib had been reported to induce durable remission[62]. Imatinib has been postulated to act through both immunological and metabolic pathways involving endoplasmic reticulum stress in β cells[63]. In a phase 2 mul
Apart from the pharmaceutical agents mentioned above, other molecules like verapamil, ladarixin, canakinumab and anakinra were also studied in recent onset T1D with variable success[65-67]. In a recent meta-analysis, which evaluated the effect of monoclonal antibody-based immunotherapy on c-peptide level in patients with recent onset T1D, 11 studies of four antibody-based immunotherapy (teplizumab, rituximab, otelixizumab and abatacept) were included[68]. The authors reported favorable c-peptide response in favor of β cell protection with all these four molecules. In a comparative study, the results (AUC of c-peptide response) from the primary studies of various immunotherapies (teplizumab, alefacept, abatacept, rituximab, high dose ATG, and low dose ATG ± G-CSF) in T1D were evaluated to rank them according to their effectiveness[69]. The authors reported that low-dose ATG and teplizumab showed maximum impact in preserving β cell function among the molecules studied. However, when these immunotherapies are used, we must be careful about the potential risk of adverse events like lymphopenia, viral infections, and CRS.
We should also remember that the sole FDA-approved immunotherapy, teplizumab, is indicated to be used only in stage 2 of T1D and thus practically has minimal application unless the disease is diagnosed early in the dysglycemia stage. More widespread screening programs like TrialNET, ASK, Sanford Population-Level Estimation of T1D Risk GEnes in Children, and T1 Detect JDRF are needed to find suitable candidates who are at the window of opportunity for this therapy[70]. The durability of the immunotherapies and frequency of dosage to maintain a state of disease remission should also be evaluated in longitudinal follow-up studies. Another concern is the safety of the long-term use of these immunomodulatory therapies due to the risk of CRS, reactivation of viral infections, etc. Thus, further well-designed RCTs with longer follow-up are needed to evaluate the efficacy, durability of therapeutic response, and safety of these immunotherapies in T1D. Moreover, the drugs should be affordable and cost-effective to ensure the access of newer therapies to the target populations.
Although several nonspecific approaches of immunoregulation have been trialed for T1D, antigen-based immunotherapy is thought to be a more favorable approach owing to its specificity and possible long-lived effects, without broad immunosuppression being needed[71]. The basis of antigen-specific immunotherapy is either inactivation of the pathogenic effector T (Teff) cells functionally, transforming them into Treg cells, or both. An ideal antigen-based immunotherapy could be administered multiple times (nonimmunogenic by itself) and would induce Treg cells that could repress islet-specific Teff cells of various specificities and lead to long-standing tolerance[72]. However, many questions are to be considered, along with the duration of the treatment and cost when designing antigen-specific immunotherapy.
For antigen-based immunotherapy to be fruitful, the subject getting treatment must have a T-cell population specific for the therapy-delivered antigen. Therefore, when developing an immunotherapy for individuals with T1D, the major specificities of circulating autoreactive T cells are essential factors to consider. In T1D individuals, the isolated CD4+ and CD8+ T-cell clones with specificity for a range of autoantigens have been identified. These include insulin, pre-proinsulin, GAD, islet antigen-2, islet glucose-6-phosphatase catalytic subunit related protein, and chromogranin A. Recently, proinsulin (c-peptide region) has been a hotspot for responsiveness of T cells in patients with T1D[73,74]. Furthermore, islet inflammation along with endoplasmic reticulum stress can cause further islet-derived antigen release, thereby inducing epitope spreading, post-translational modification of peptides, and fusion of peptide fragments originating from different proteins with subsequent generation of hybrid peptides[75]. Therefore, an in-depth knowledge of the critical antigenic targets and their kinetics during the progression of disease is crucial for choosing the most fitting targets for antigen-based therapy. Disease heterogeneity adds to the complexity of antigen-specific immunotherapy in T1D because of a subgroup of patients depending on their HLA alleles or disease endotypes[76]. Heterogeneity and HLA haplotype can influence the presentation of epitopes from islet antigens to the autoreactive T cells as well as the response of T cells[77,78]. Considerations and requirements for antigen-based immunotherapy in T1D are illustrated in Figure 2. Experts have used different delivery strategies for antigen-specific immunotherapy, for example, proteins and peptides, plasmid DNA, cell-based strategies, antigen-loaded nanoparticles, and liposome-based approaches (Table 2). The key question is which approach is more likely to restore β cell tolerance?
| Strategies | Immunological target | Advantages | Disadvantages |
| Autoantigenic peptides and proteins | APCs | Biocompatible; possibility to conjugate to a vehicle | Short half-life; adjuvant required |
| Autoantigen-encoding plasmid DNA | APCs | Long-lived effect | Gene therapy |
| Antigen-loaded cell-based strategies | Autoreactive T cells | Powerful immunoregulatory effect | Leukapheresis required; personalized medicine |
| Antigen-loaded nanoparticles and liposomes | APCs and T cells | Customizable; powerful immunoregulatory effect; might act by biomimicry | Synthetic; preclinical developmental phase |
The antigen-specific approach involves the delivery of β cell autoantigens through a route and regimen that induces immune tolerance. Several antigen-specific approaches have entered into trials in the last decade to explore their safety, feasibility, and efficacy using different delivery strategies[79]. Recently completed and ongoing immunotherapy trials using antigen-specific strategies in T1D are reviewed elsewhere[80]. Single-peptide immunotherapy using a proinsulin sequence showed hints of efficacy and immunomodulation and was well tolerated[81]. Gibson et al[82] used a preclinical, humanized model of peptide immunotherapy. They showed that combining numerous different β cell peptides into a single injectable may produce a significantly increased effect compared with a single peptide in generating immune regulation. A recent study has addressed whether therapies delivering several antigens concurrently are efficacious and any safety issues that can arise from administration of multiple antigens. A mixture of six β cell peptides from two islet autoantigens was administered to patients with recent-onset T1D[83]. Multiple-peptide immunotherapy showed the potential to rectify immune regulatory defects central to the pathobiology of T1D in this first-in-human study. No serious adverse effects were observed in groups that received drug treatment. Together with the observations in pre-clinical models that delivery of multiple peptides from more than one antigen may have more impact than a monopeptide[82], these recent findings justify future well-designed clinical trials.
The last decade has seen the knowledge translated into definite antigen-based immunotherapies, promising to restore the breach of immunological tolerance to β cell autoantigens selectively. However, in both prevention and reversion trials in T1D, suboptimal effects have been obtained so far. Consequently, there is still a need to optimize those immunotherapies and their associated factors, such as patient and disease heterogeneity; choice of antigen (peptide or whole molecule, conventional or unconventional, single antigen or cocktail); posology; administration patterns, route, timing and use of adjuvants; biomarkers for stratification and therapeutic outcome[80].
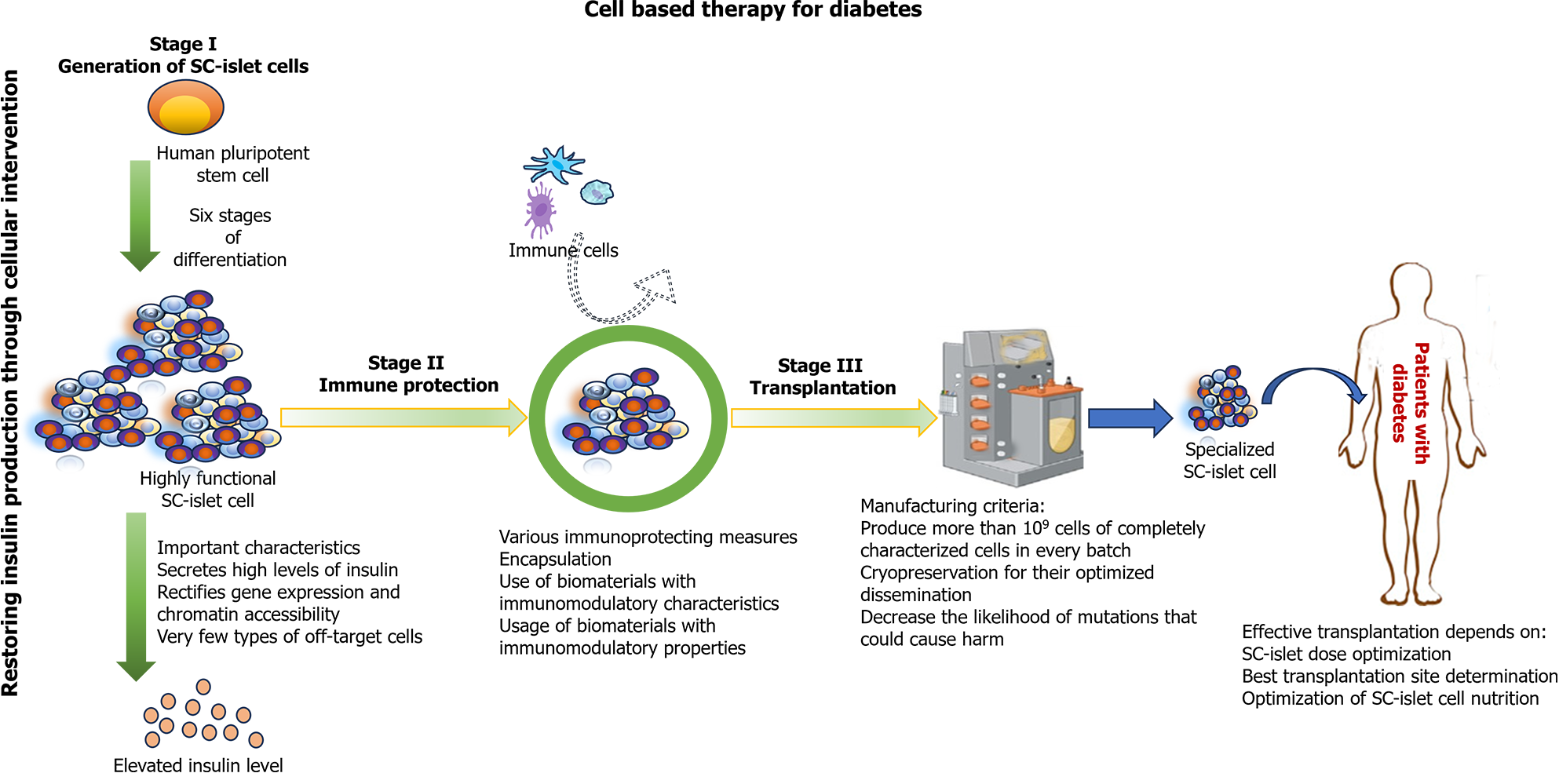
T1D can be reversed by transplantation of pancreas or islet cells, which serves as proof of principle for cell-based therapy[84]. However, several issues limit its widespread use, particularly the insufficient supply of highly functional β cells. Yet, the sourcing problem could be circumvented by differentiating stem cells (SCs) into insulin-producing cells, and it has garnered the most enthusiasm for creating functional β cells[85,86]. These SC-derived islets could be derived from a single-cell source using a standardized process. The resulting cell product could be well characterized, allowing for more predictable transplant outcomes.
SCs [embryonic SCs (ESCs), induced pluripotent SCs (iPSCs)], and adult SCs are being widely explored for T1D therapeutics[87]. In preclinical studies, ESC-derived β cells have shown favorable results by insulin production in response to glucose stimulation and restoration of normoglycemia[87]. iPSCs offer a practical alternative to ESCs. They can be derived from adult somatic cells, thus eliminating ethical concerns. In diabetic mouse models, iPSC-derived β cells have also exhibited the ability to secrete insulin and restore normoglycemia. Besides iPSCs, adult SCs, including mesenchymal SCs and hematopoietic SCs, have been investigated for T1D as well[88].
Recent technological advances have made human clinical trials utilizing SC-derived pancreatic endoderm cells (PECs) possible. An initial 2014 human clinical trial used the ViaCyte Inc. device (VC-01) to immunoprotect the cells using a cell-impermeable membrane entirely. Although some endocrine cells were found, fibrosis around the capsule led to graft loss, and insulin secretion was not detected from the device[89,90]. To circumvent this issue, a clinical trial (NCT03163511) was initiated in 2017 to evaluate the newer PEC-Direct device (PEC-01 cells implanted subcutaneously in VC-02 devices) that contained membrane openings allowing vascularization to develop nutrient exchange and promote survival of cells. Total immunosuppression was required after transplantation. In 63% of units, insulin expression within β cells was observed at 3-12 mo post-transplantation, with a preponderance of α cells reflecting the immature graft state. The recently published reports from this ongoing trial demonstrated detectable levels of c-peptide in peripheral blood by 6-9 mo post-transplantation[91,92]. Vertex pharmaceuticals embarked on a clinical trial with T1D patients in 2021, where an ESC-derived islet, VX-880, was transplanted without an immunoprotective device under immunosuppressive coverage. Initial findings seem promising[93]. An ongoing Vertex trial (NCT04786262) will determine whether the success can be rep
As things stand, the clinical trial results highlight the great promise SC-islets hold for treating T1D. The last few years have been notable for game-changing early progress in clinical trials with SC-based therapies for T1D[95]. Nevertheless, several remaining challenges need to be addressed before this SC therapy can be converted into a routine procedure. The central pillars of a successful SC-islet therapy for T1D are illustrated in Figure 3.
T1D involves the autoimmune destruction of insulin-producing β cells in the pancreas. Over 100 years since the discovery of insulin, there is still no cure for T1D. However, therapeutic options for T1D are again at a turning point. Years of effort to develop immune interventions are ultimately starting to pay off, with hints of progress in both new onset and preventative settings. We discussed the recently completed and ongoing clinical trials that have studied the efficacy and safety of several immunotherapeutic strategies targeting various mechanisms of autoimmunity, which are considered significant in disease pathogenesis. While more targeted immunotherapies with potentially fewer adverse effects get closer to the translation into clinical practice, new challenges may need to be faced. A better understanding of disease endotypes may facilitate the stratification of individuals to different treatment options. While moving forward, success lies in selecting which interventions are best suitable for which stage of the disease. Therefore, the timing and benefit/risk profile of candidate approaches should be considered carefully. It is also essential to conduct more clinical trials at T1D diagnosis to compare interventions. With the increasing interest in combination approaches, immunotherapeutic strategies targeting different aspects of the immune system are likely to be essential contributors to the future therapeutic landscape, together with the practice of individualized patient-tailored approaches, a change towards early intervention, and an emphasis on outcome measures.
We thank Dr. Somnath Mondal, PhD, Associate Director, IPD Analytics, Bhubaneswar for preparing the illustrations for this editorial.
| 1. | Paschou SA, Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. On type 1 diabetes mellitus pathogenesis. Endocr Connect. 2018;7:R38-R46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 2. | Subramanian S, Khan F, Hirsch IB. New advances in type 1 diabetes. BMJ. 2024;384:e075681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 723] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 4. | Tatovic D, Narendran P, Dayan CM. A perspective on treating type 1 diabetes mellitus before insulin is needed. Nat Rev Endocrinol. 2023;19:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Samassa F, Mallone R. Self-antigens, benign autoimmunity and type 1 diabetes: a beta-cell and T-cell perspective. Curr Opin Endocrinol Diabetes Obes. 2022;29:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Johnson MB, Hattersley AT, Flanagan SE. Monogenic autoimmune diseases of the endocrine system. Lancet Diabetes Endocrinol. 2016;4:862-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Lio CW, Hsieh CS. Becoming self-aware: the thymic education of regulatory T cells. Curr Opin Immunol. 2011;23:213-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Goldman JD, Choi H. Teplizumab: The First Treatment to Delay the Progression of Type 1 Diabetes. Clin Diabetes. 2023;41:474-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Herold KC, Delong T, Perdigoto AL, Biru N, Brusko TM, Walker LSK. The immunology of type 1 diabetes. Nat Rev Immunol. 2024;24:435-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 43] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 10. | Carvalho T. FDA approves first drug to delay type 1 diabetes. Nat Med. 2023;29:280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Ramos EL, Dayan CM, Chatenoud L, Sumnik Z, Simmons KM, Szypowska A, Gitelman SE, Knecht LA, Niemoeller E, Tian W, Herold KC; PROTECT Study Investigators. Teplizumab and β-Cell Function in Newly Diagnosed Type 1 Diabetes. N Engl J Med. 2023;389:2151-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 100] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 12. | Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler AG, Greenbaum CJ; Type 1 Diabetes TrialNet Study Group. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 2019;381:603-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 846] [Cited by in RCA: 713] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 13. | Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA; AbATE Study Team. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766-3774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 14. | Hagopian W, Ferry RJ Jr, Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, Ludvigsson J; Protégé Trial Investigators. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901-3908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, Sherr J, Rosenthal SM, Adi S, Jalaludin MY, Michels AW, Dziura J, Bluestone JA. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Kamrul-Hasan ABM, Mondal S, Nagendra L, Yadav A, Aalpona FTZ, Dutta D. Role of Teplizumab, a Humanized Anti-CD3 Monoclonal Antibody, in Managing Newly Diagnosed Type 1 Diabetes: An Updated Systematic Review and Meta-Analysis. Endocr Pract. 2024;30:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Reference Citation Analysis (0)] |
| 17. | Nourelden AZ, Elshanbary AA, El-Sherif L, Benmelouka AY, Rohim HI, Helmy SK, Sayed MK, Ismail A, Ali AS, Ragab KM, Zaazouee MS. Safety and Efficacy of Teplizumab for Treatment of Type One Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocr Metab Immune Disord Drug Targets. 2021;21:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Heidari E, Shafiee A, Noorian S, Rafiei MA, Abbasi M, Amini MJ, Safari O, Aghamahdi F, Bakhtiyari M. Efficacy of teplizumab for treatment of type 1 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2024;40:e3806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 19. | Liu Y, Li W, Chen Y, Wang X. Anti-CD3 monoclonal antibodies in treatment of type 1 diabetes: a systematic review and meta-analysis. Endocrine. 2024;83:322-329. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Perdigoto AL, Preston-Hurlburt P, Clark P, Long SA, Linsley PS, Harris KM, Gitelman SE, Greenbaum CJ, Gottlieb PA, Hagopian W, Woodwyk A, Dziura J, Herold KC; Immune Tolerance Network. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia. 2019;62:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG; Protégé Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 376] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 484] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 23. | Mital S, Nguyen HV. Cost Effectiveness of Teplizumab for Prevention of Type 1 Diabetes Among Different Target Patient Groups. Pharmacoeconomics. 2020;38:1359-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Novograd J, Frishman WH. Teplizumab Therapy to Delay the Onset of Type 1 Diabetes. Cardiol Rev. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 25. | Mehta S, Ryabets-Lienhard A, Patel N, Breidbart E, Libman I, Haller MJ, Simmons KM, Sims EK, DiMeglio LA, Gitelman SE, Griffin KJ, Tonyushkina KN. Pediatric Endocrine Society Statement on Considerations for Use of Teplizumab (Tzield™) in Clinical Practice. Horm Res Paediatr. 2024;1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Keymeulen B, van Maurik A, Inman D, Oliveira J, McLaughlin R, Gittelman RM, Roep BO, Gillard P, Hilbrands R, Gorus F, Mathieu C, Van de Velde U, Wisniacki N, Napolitano A. A randomised, single-blind, placebo-controlled, dose-finding safety and tolerability study of the anti-CD3 monoclonal antibody otelixizumab in new-onset type 1 diabetes. Diabetologia. 2021;64:313-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Aronson R, Gottlieb PA, Christiansen JS, Donner TW, Bosi E, Bode BW, Pozzilli P; DEFEND Investigator Group. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37:2746-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Ambery P, Donner TW, Biswas N, Donaldson J, Parkin J, Dayan CM. Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabet Med. 2014;31:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Demeester S, Keymeulen B, Kaufman L, Van Dalem A, Balti EV, Van de Velde U, Goubert P, Verhaeghen K, Davidson HW, Wenzlau JM, Weets I, Pipeleers DG, Gorus FK. Preexisting insulin autoantibodies predict efficacy of otelixizumab in preserving residual β-cell function in recent-onset type 1 diabetes. Diabetes Care. 2015;38:644-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Keyes-Elstein L, Long SA, Kanaparthi S, Lim N, Phippard D, Soppe CL, Fitzgibbon ML, McNamara J, Nepom GT, Ehlers MR. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125:3285-3296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 31. | Rigby MR, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Patel CM, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Moran A, Russell WE, Pinckney A, Keyes-Elstein L, Howell M, Aggarwal S, Lim N, Phippard D, Nepom GT, McNamara J, Ehlers MR; T1DAL Study Team. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 32. | Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Raskin P, Rodriguez H, Russell WE, Schatz D, Wherrett D, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 33. | Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Peakman M, Raskin P, Russell WE, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Abatacept Study Group. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 34. | Russell WE, Bundy BN, Anderson MS, Cooney LA, Gitelman SE, Goland RS, Gottlieb PA, Greenbaum CJ, Haller MJ, Krischer JP, Libman IM, Linsley PS, Long SA, Lord SM, Moore DJ, Moore WV, Moran AM, Muir AB, Raskin P, Skyler JS, Wentworth JM, Wherrett DK, Wilson DM, Ziegler AG, Herold KC; Type 1 Diabetes TrialNet Study Group. Abatacept for Delay of Type 1 Diabetes Progression in Stage 1 Relatives at Risk: A Randomized, Double-Masked, Controlled Trial. Diabetes Care. 2023;46:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 35. | Gaber AO, Monaco AP, Russell JA, Lebranchu Y, Mohty M. Rabbit antithymocyte globulin (thymoglobulin): 25 years and new frontiers in solid organ transplantation and haematology. Drugs. 2010;70:691-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Gitelman SE, Gottlieb PA, Rigby MR, Felner EI, Willi SM, Fisher LK, Moran A, Gottschalk M, Moore WV, Pinckney A, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, Ding L, Bluestone JA, Ehlers MR; START Study Team. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Haller MJ, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Miller JL, Atkinson MA, Becker DJ, Baidal D, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Herold KC, Marks JB, Moran A, Rodriguez H, Russell W, Wilson DM, Greenbaum CJ; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-Dose Anti-Thymocyte Globulin (ATG) Preserves β-Cell Function and Improves HbA(1c) in New-Onset Type 1 Diabetes. Diabetes Care. 2018;41:1917-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 38. | Haller MJ, Long SA, Blanchfield JL, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Geyer SM, Warnock MV, Miller JL, Atkinson MA, Becker DJ, Baidal DA, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Herold KC, Marks JB, Moran A, Rodriguez H, Russell WE, Wilson DM, Greenbaum CJ; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA(1c), and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 Diabetes: Two-Year Clinical Trial Data. Diabetes. 2019;68:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 39. | Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Rosenthal SM, Shuster JJ, Zou B, Brusko TM, Hulme MA, Wasserfall CH, Mathews CE, Atkinson MA, Schatz DA. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest. 2015;125:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Perry DJ, Schultz AR, Hulme MA, Shuster JJ, Zou B, Wasserfall CH, Posgai AL, Mathews CE, Brusko TM, Atkinson MA, Schatz DA. Antithymocyte Globulin Plus G-CSF Combination Therapy Leads to Sustained Immunomodulatory and Metabolic Effects in a Subset of Responders With Established Type 1 Diabetes. Diabetes. 2016;65:3765-3775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Nguyen HV, Schatz DA, Mital S, Jacobsen LM, Haller MJ. Cost-Effectiveness of Low-Dose Antithymocyte Globulin Versus Other Immunotherapies for Treatment of New-Onset Type 1 Diabetes. Diabetes Technol Ther. 2022;24:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Ferreira RC, Simons HZ, Thompson WS, Cutler AJ, Dopico XC, Smyth DJ, Mashar M, Schuilenburg H, Walker NM, Dunger DB, Wallace C, Todd JA, Wicker LS, Pekalski ML. IL-21 production by CD4+ effector T cells and frequency of circulating follicular helper T cells are increased in type 1 diabetes patients. Diabetologia. 2015;58:781-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 43. | Van Belle TL, Nierkens S, Arens R, von Herrath MG. Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity. 2012;36:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Rondas D, Bugliani M, D'Hertog W, Lage K, Masini M, Waelkens E, Marchetti P, Mathieu C, Overbergh L. Glucagon-like peptide-1 protects human islets against cytokine-mediated β-cell dysfunction and death: a proteomic study of the pathways involved. J Proteome Res. 2013;12:4193-4206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Wang W, Wu RD, Chen P, Xu XJ, Shi XZ, Huang LH, Shao ZL, Guo W. Liraglutide combined with human umbilical cord mesenchymal stem cell transplantation inhibits beta-cell apoptosis via mediating the ASK1/JNK/BAX pathway in rats with type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Rydén AK, Perdue NR, Pagni PP, Gibson CB, Ratliff SS, Kirk RK, Friesen TJ, Haase C, Coppieters K, von Herrath MG, Boursalian TE. Anti-IL-21 monoclonal antibody combined with liraglutide effectively reverses established hyperglycemia in mouse models of type 1 diabetes. J Autoimmun. 2017;84:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | von Herrath M, Bain SC, Bode B, Clausen JO, Coppieters K, Gaysina L, Gumprecht J, Hansen TK, Mathieu C, Morales C, Mosenzon O, Segel S, Tsoukas G, Pieber TR; Anti-IL-21–liraglutide Study Group investigators and contributors. Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9:212-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 48. | Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS; Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 802] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 49. | Herold KC, Pescovitz MD, McGee P, Krause-Steinrauf H, Spain LM, Bourcier K, Asare A, Liu Z, Lachin JM, Dosch HM; Type 1 Diabetes TrialNet Anti-CD20 Study Group. Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol. 2011;187:1998-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, Moran A, Raskin P, Rodriguez H, Schatz DA, Wherrett DK, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Anti-CD20 Study Group. B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014;37:453-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 51. | Zieliński M, Żalińska M, Iwaszkiewicz-Grześ D, Gliwiński M, Hennig M, Jaźwińska-Curyłło A, Kamińska H, Sakowska J, Wołoszyn-Durkiewicz A, Owczuk R, Młynarski W, Jarosz-Chobot P, Bossowski A, Szadkowska A, Siebert J, Myśliwiec M, Marek-Trzonkowska N, Trzonkowski P. Combined therapy with CD4(+) CD25highCD127(-) T regulatory cells and anti-CD20 antibody in recent-onset type 1 diabetes is superior to monotherapy: Randomized phase I/II trial. Diabetes Obes Metab. 2022;24:1534-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 52. | Kodama S, Davis M, Faustman DL. The therapeutic potential of tumor necrosis factor for autoimmune disease: a mechanistically based hypothesis. Cell Mol Life Sci. 2005;62:1850-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Koulmanda M, Bhasin M, Awdeh Z, Qipo A, Fan Z, Hanidziar D, Putheti P, Shi H, Csizuadia E, Libermann TA, Strom TB. The role of TNF-α in mice with type 1- and 2- diabetes. PLoS One. 2012;7:e33254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Grewal IS, Grewal KD, Wong FS, Picarella DE, Janeway CA Jr, Flavell RA. Local expression of transgene encoded TNF alpha in islets prevents autoimmune diabetes in nonobese diabetic (NOD) mice by preventing the development of auto-reactive islet-specific T cells. J Exp Med. 1996;184:1963-1974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, Leu JH, Zoka R, Hedrick JA, Rigby MR, Vercruysse F; T1GER Study Investigators. Golimumab and Beta-Cell Function in Youth with New-Onset Type 1 Diabetes. N Engl J Med. 2020;383:2007-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 56. | Rigby MR, Hayes B, Li Y, Vercruysse F, Hedrick JA, Quattrin T. Two-Year Follow-up From the T1GER Study: Continued Off-Therapy Metabolic Improvements in Children and Young Adults With New-Onset T1D Treated With Golimumab and Characterization of Responders. Diabetes Care. 2023;46:561-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 57. | Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 58. | Ludvigsson J, Routray I, Vigård T, Hanås R, Rathsman B, Carlsson A, Särnblad S, Albin AK, Arvidsson CG, Samuelsson U, Casas R. Combined Etanercept, GAD-alum and vitamin D treatment: an open pilot trial to preserve beta cell function in recent onset type 1 diabetes. Diabetes Metab Res Rev. 2021;37:e3440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Szekanecz Z, Buch MH, Charles-Schoeman C, Galloway J, Karpouzas GA, Kristensen LE, Ytterberg SR, Hamar A, Fleischmann R. Efficacy and safety of JAK inhibitors in rheumatoid arthritis: update for the practising clinician. Nat Rev Rheumatol. 2024;20:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 49] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 60. | Singh R, Driscoll MS. Review of Baricitinib in the Treatment of Alopecia Areata. J Drugs Dermatol. 2023;22:935-940. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Waibel M, Wentworth JM, So M, Couper JJ, Cameron FJ, MacIsaac RJ, Atlas G, Gorelik A, Litwak S, Sanz-Villanueva L, Trivedi P, Ahmed S, Martin FJ, Doyle ME, Harbison JE, Hall C, Krishnamurthy B, Colman PG, Harrison LC, Thomas HE, Kay TWH; BANDIT Study Group. Baricitinib and β-Cell Function in Patients with New-Onset Type 1 Diabetes. N Engl J Med. 2023;389:2140-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 62. | Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, Bluestone JA. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105:18895-18900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 63. | Welsh N. Are off-target effects of imatinib the key to improving beta-cell function in diabetes? Ups J Med Sci. 2022;127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 64. | Gitelman SE, Bundy BN, Ferrannini E, Lim N, Blanchfield JL, DiMeglio LA, Felner EI, Gaglia JL, Gottlieb PA, Long SA, Mari A, Mirmira RG, Raskin P, Sanda S, Tsalikian E, Wentworth JM, Willi SM, Krischer JP, Bluestone JA; Gleevec Trial Study Group. Imatinib therapy for patients with recent-onset type 1 diabetes: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9:502-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 65. | Ovalle F, Grimes T, Xu G, Patel AJ, Grayson TB, Thielen LA, Li P, Shalev A. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med. 2018;24:1108-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 66. | Piemonti L, Keymeulen B, Gillard P, Linn T, Bosi E, Rose L, Pozzilli P, Giorgino F, Cossu E, Daffonchio L, Goisis G, Ruffini PA, Maurizi AR, Mantelli F, Allegretti M. Ladarixin, an inhibitor of the interleukin-8 receptors CXCR1 and CXCR2, in new-onset type 1 diabetes: A multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2022;24:1840-1849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 67. | Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, Sanda S, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Canakinumab Study Group, Pickersgill L, de Koning E, Ziegler AG, Böehm B, Badenhoop K, Schloot N, Bak JF, Pozzilli P, Mauricio D, Donath MY, Castaño L, Wägner A, Lervang HH, Perrild H, Mandrup-Poulsen T; AIDA Study Group. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 68. | Roy SS, Keshri USP, Alam MS, Wasnik A. Effect of Immunotherapy on C-peptide Levels in Patients With Type I Diabetes Mellitus: A Systematic Review of Randomized Controlled Trials. Cureus. 2024;16:e58981. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Jacobsen LM, Bundy BN, Greco MN, Schatz DA, Atkinson MA, Brusko TM, Mathews CE, Herold KC, Gitelman SE, Krischer JP, Haller MJ. Comparing Beta Cell Preservation Across Clinical Trials in Recent-Onset Type 1 Diabetes. Diabetes Technol Ther. 2020;22:948-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 70. | Sims EK, Besser REJ, Dayan C, Geno Rasmussen C, Greenbaum C, Griffin KJ, Hagopian W, Knip M, Long AE, Martin F, Mathieu C, Rewers M, Steck AK, Wentworth JM, Rich SS, Kordonouri O, Ziegler AG, Herold KC; NIDDK Type 1 Diabetes TrialNet Study Group. Screening for Type 1 Diabetes in the General Population: A Status Report and Perspective. Diabetes. 2022;71:610-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 71. | Roep BO, Wheeler DCS, Peakman M. Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol. 2019;7:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 72. | Loaiza Naranjo JD, Bergot AS, Buckle I, Hamilton-Williams EE. A Question of Tolerance-Antigen-Specific Immunotherapy for Type 1 Diabetes. Curr Diab Rep. 2020;20:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 73. | Babon JA, DeNicola ME, Blodgett DM, Crèvecoeur I, Buttrick TS, Maehr R, Bottino R, Naji A, Kaddis J, Elyaman W, James EA, Haliyur R, Brissova M, Overbergh L, Mathieu C, Delong T, Haskins K, Pugliese A, Campbell-Thompson M, Mathews C, Atkinson MA, Powers AC, Harlan DM, Kent SC. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med. 2016;22:1482-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 74. | So M, Elso CM, Tresoldi E, Pakusch M, Pathiraja V, Wentworth JM, Harrison LC, Krishnamurthy B, Thomas HE, Rodda C, Cameron FJ, McMahon J, Kay TWH, Mannering SI. Proinsulin C-peptide is an autoantigen in people with type 1 diabetes. Proc Natl Acad Sci USA. 2018;115:10732-10737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 75. | Marre ML, McGinty JW, Chow IT, DeNicola ME, Beck NW, Kent SC, Powers AC, Bottino R, Harlan DM, Greenbaum CJ, Kwok WW, Piganelli JD, James EA. Modifying Enzymes Are Elicited by ER Stress, Generating Epitopes That Are Selectively Recognized by CD4(+) T Cells in Patients With Type 1 Diabetes. Diabetes. 2018;67:1356-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 76. | Battaglia M, Ahmed S, Anderson MS, Atkinson MA, Becker D, Bingley PJ, Bosi E, Brusko TM, DiMeglio LA, Evans-Molina C, Gitelman SE, Greenbaum CJ, Gottlieb PA, Herold KC, Hessner MJ, Knip M, Jacobsen L, Krischer JP, Long SA, Lundgren M, McKinney EF, Morgan NG, Oram RA, Pastinen T, Peters MC, Petrelli A, Qian X, Redondo MJ, Roep BO, Schatz D, Skibinski D, Peakman M. Introducing the Endotype Concept to Address the Challenge of Disease Heterogeneity in Type 1 Diabetes. Diabetes Care. 2020;43:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 77. | van Lummel M, Duinkerken G, van Veelen PA, de Ru A, Cordfunke R, Zaldumbide A, Gomez-Touriño I, Arif S, Peakman M, Drijfhout JW, Roep BO. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes. 2014;63:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 78. | van Lummel M, van Veelen PA, de Ru AH, Pool J, Nikolic T, Laban S, Joosten A, Drijfhout JW, Gómez-Touriño I, Arif S, Aanstoot HJ, Peakman M, Roep BO. Discovery of a Selective Islet Peptidome Presented by the Highest-Risk HLA-DQ8trans Molecule. Diabetes. 2016;65:732-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Rodriguez-Fernandez S, Almenara-Fuentes L, Perna-Barrull D, Barneda B, Vives-Pi M. A century later, still fighting back: antigen-specific immunotherapies for type 1 diabetes. Immunol Cell Biol. 2021;99:461-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M. The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol. 2019;7:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 81. | Alhadj Ali M, Liu YF, Arif S, Tatovic D, Shariff H, Gibson VB, Yusuf N, Baptista R, Eichmann M, Petrov N, Heck S, Yang JHM, Tree TIM, Pujol-Autonell I, Yeo L, Baumard L, Stenson R, Howell A, Clark A, Boult Z, Powrie J, Adams L, Wong FS, Luzio S, Dunseath G, Green K, O'Keefe A, Bayly G, Thorogood N, Andrews R, Leech N, Joseph F, Nair S, Seal S, Cheung H, Beam C, Hills R, Peakman M, Dayan CM. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med. 2017;9:eaaf7779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 82. | Gibson VB, Nikolic T, Pearce VQ, Demengeot J, Roep BO, Peakman M. Proinsulin multi-peptide immunotherapy induces antigen-specific regulatory T cells and limits autoimmunity in a humanized model. Clin Exp Immunol. 2015;182:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 83. | Liu YF, Powrie J, Arif S, Yang JHM, Williams E, Khatri L, Joshi M, Lhuillier L, Fountoulakis N, Smith E, Beam C, Lorenc A, Peakman M, Tree T. Immune and Metabolic Effects of Antigen-Specific Immunotherapy Using Multiple β-Cell Peptides in Type 1 Diabetes. Diabetes. 2022;71:722-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 496] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 85. | Migliorini A, Nostro MC, Sneddon JB. Human pluripotent stem cell-derived insulin-producing cells: A regenerative medicine perspective. Cell Metab. 2021;33:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 86. | Siehler J, Blöchinger AK, Meier M, Lickert H. Engineering islets from stem cells for advanced therapies of diabetes. Nat Rev Drug Discov. 2021;20:920-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 87. | Singh A, Afshan N, Singh A, Singh SK, Yadav S, Kumar M, Sarma DK, Verma V. Recent trends and advances in type 1 diabetes therapeutics: A comprehensive review. Eur J Cell Biol. 2023;102:151329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 88. | Park YM, Yang CM, Cho HY. Therapeutic Effects of Insulin-Producing Human Umbilical Cord-Derived Mesenchymal Stem Cells in a Type 1 Diabetes Mouse Model. Int J Mol Sci. 2022;23:6877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, Kerr J, Frazier MA, Kroon EJ, D'Amour KA. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl Med. 2015;4:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 90. | Hogrebe NJ, Ishahak M, Millman JR. Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell. 2023;30:530-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 91. | Ramzy A, Thompson DM, Ward-Hartstonge KA, Ivison S, Cook L, Garcia RV, Loyal J, Kim PTW, Warnock GL, Levings MK, Kieffer TJ. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell. 2021;28:2047-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 92. | Shapiro AMJ, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J, Wilensky J, Daniels M, Wang RM, Brandon EP, Jaiman MS, Kroon EJ, D'Amour KA, Foyt HL. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. 2021;2:100466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 93. | Kolata G. A Cure for Type 1 Diabetes? For One Man, It Seems to Have Worked. The New York Times: New York, 2021. Available from: https://www.nytimes.com/2021/11/27/health/diabetes-cure-stem-cells.html. |
| 94. | Sordi V, Monaco L, Piemonti L. Cell Therapy for Type 1 Diabetes: From Islet Transplantation to Stem Cells. Horm Res Paediatr. 2023;96:658-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 95. | Shapiro AMJ, Verhoeff K. A spectacular year for islet and stem cell transplantation. Nat Rev Endocrinol. 2023;19:68-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |