Published online Jan 15, 2024. doi: 10.4239/wjd.v15.i1.24
Peer-review started: September 25, 2023
First decision: October 10, 2023
Revised: October 22, 2023
Accepted: December 15, 2023
Article in press: December 15, 2023
Published online: January 15, 2024
Processing time: 108 Days and 14 Hours
Prediabetes is a well-established risk factor for major adverse cardiac and cerebrovascular events (MACCE). However, the relationship between prediabetes and MACCE in atrial fibrillation (AF) patients has not been extensively studied. Therefore, this study aimed to establish a link between prediabetes and MACCE in AF patients.
To investigate a link between prediabetes and MACCE in AF patients.
We used the National Inpatient Sample (2019) and relevant ICD-10 CM codes to identify hospitalizations with AF and categorized them into groups with and without prediabetes, excluding diabetics. The primary outcome was MACCE (all-cause inpatient mortality, cardiac arrest including ventricular fibrillation, and stroke) in AF-related hospitalizations.
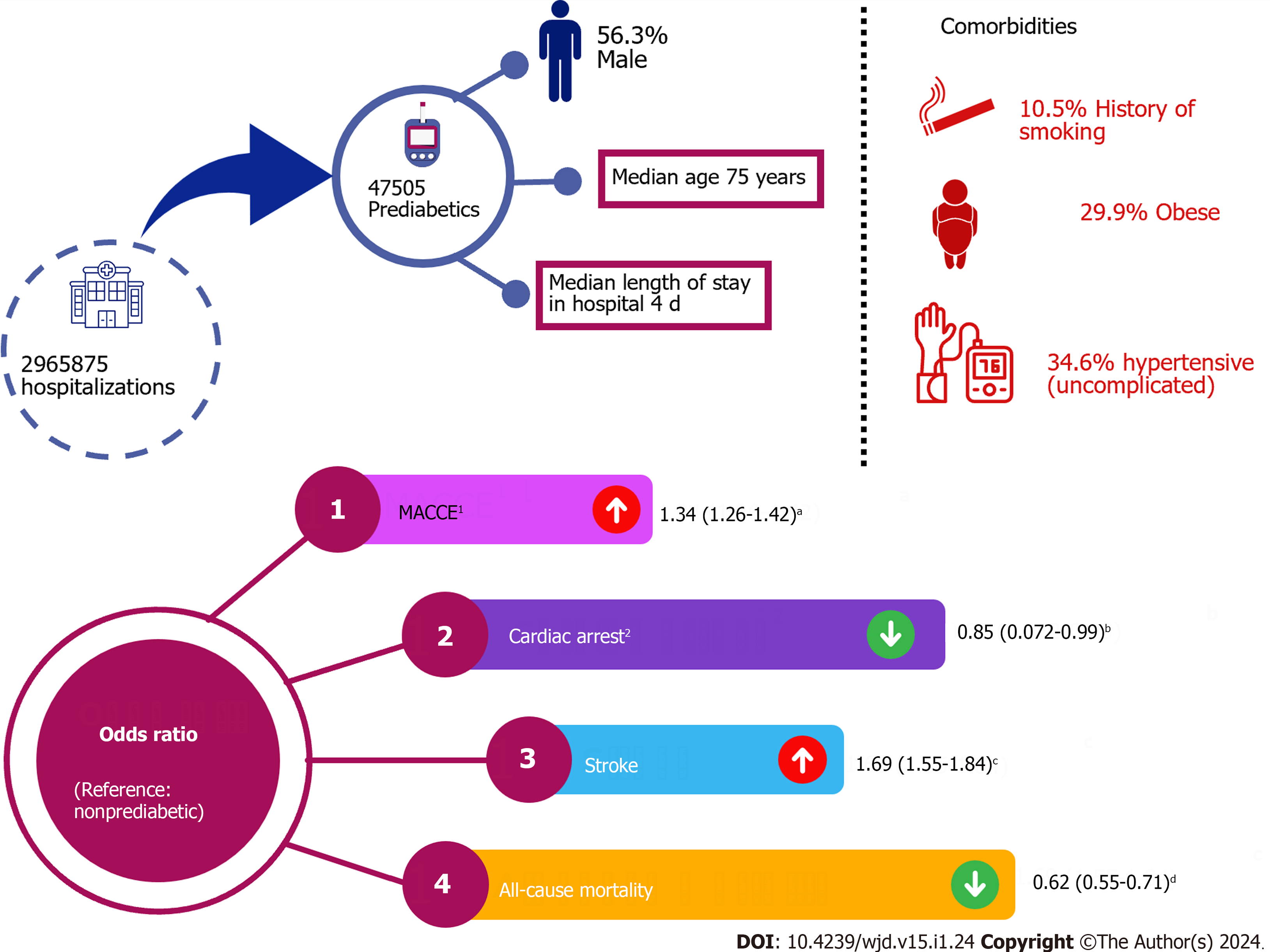
Of the 2965875 AF-related hospitalizations for MACCE, 47505 (1.6%) were among patients with prediabetes. The prediabetes cohort was relatively younger (median 75 vs 78 years), and often consisted of males (56.3% vs 51.4%), blacks (9.8% vs 7.9%), Hispanics (7.3% vs 4.3%), and Asians (4.7% vs 1.6%) than the non-prediabetic cohort (P < 0.001). The prediabetes group had significantly higher rates of hypertension, hyperlipidemia, smoking, obesity, drug abuse, prior myocardial infarction, peripheral vascular disease, and hyperthyroidism (all P < 0.05). The prediabetes cohort was often discharged routinely (51.1% vs 41.1%), but more frequently required home health care (23.6% vs 21.0%) and had higher costs. After adjusting for baseline characteristics or comorbidities, the prediabetes cohort with AF admissions showed a higher rate and significantly higher odds of MACCE compared to the non-prediabetic cohort [18.6% vs 14.7%, odds ratio (OR) 1.34, 95% confidence interval 1.26-1.42, P < 0.001]. On subgroup analyses, males had a stronger association (aOR 1.43) compared to females (aOR 1.22), whereas on the race-wise comparison, Hispanics (aOR 1.43) and Asians (aOR 1.36) had a stronger association with MACCE with prediabetes vs whites (aOR 1.33) and blacks (aOR 1.21).
This population-based study found a significant association between prediabetes and MACCE in AF patients. Therefore, there is a need for further research to actively screen and manage prediabetes in AF to prevent MACCE.
Core Tip: In our study, we've shed light on a critical yet often overlooked connection between prediabetes and major adverse cardiac and cerebrovascular events (MACCE) in atrial fibrillation (AF) patients. Our research revealed that AF patients with prediabetes are at significantly higher risk of experiencing MACCE, highlighting the importance of identifying and managing prediabetes in this population. This finding emphasizes the need for proactive screening and targeted interventions to reduce the burden of MACCE in AF patients with prediabetes. Further research and dedicated efforts are essential to enhance care and outcomes for these individuals.
- Citation: Desai R, Katukuri N, Goguri SR, Kothawala A, Alle NR, Bellamkonda MK, Dey D, Ganesan S, Biswas M, Sarkar K, Prattipati P, Chauhan S. Prediabetes: An overlooked risk factor for major adverse cardiac and cerebrovascular events in atrial fibrillation patients. World J Diabetes 2024; 15(1): 24-33
- URL: https://www.wjgnet.com/1948-9358/full/v15/i1/24.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i1.24
In 2019, more than one-third of the adult population, that is, 96 million adults aged 18 and above, received a diagnosis of prediabetes[1]. Prediabetes is a condition where people are associated with an increased risk of developing type 2 diabetes mellitus (DM), cardiovascular disease, and stroke due to elevated blood glucose levels that haven’t reached the threshold for diabetes. Impaired glucose tolerance (IGT) test was prevalent in 7.5% men and women in 2019 as per the International Diabetes Federation[2]. Among the arrhythmia's, atrial fibrillation (AF) was the most common arrhythmia and poses a significant healthcare burden due to its sequalae like stroke, heart failure, and increased mortality[3,4]. AF is often associated with other comorbid conditions like diabetes, hypertension, and obesity, which increases the major adverse cardiac and cerebrovascular events (MACCE)[4-6]. There is increasing evidence suggesting that prediabetes acts as a risk factor for MACCE in patients with AF; we have limited understanding regarding the underlying mechanisms[7,8].
The 2019 American Heart Association/American College of Cardiology/Heart Rhythm Society focused update for the management of patients with AF reported that increased risk of thromboembolic events may be due to the underlying vascular changes and prothrombotic state commonly seen with diabetes, however, the contemporary data and guidelines on the long-term impact of prediabetes on AF risk and outcomes remain largely unknown. Despite increasing evidence suggesting that prediabetes is one of the risk factors for MACCE in patients with AF, the understanding of the pathophysiology causing these adverse outcomes is still unclear. Previously proposed mechanisms of prediabetes leading to AF and associated cardiovascular complications include such as insulin resistance, oxidative stress, inflammation, autonomic dysfunction, fibrosis, and a prothrombotic state. Although these mechanisms are involved there is still a lack of understanding regarding the pathophysiology of how prediabetes contributes to AF. It entails an interaction, between factors that encompass the heart, metabolism and chronic inflammation. As it is crucial to understand the relationship between prediabetes and MACCE in patients with AF, which can help develop strategies for screening and managing this high-risk population, we analyzed the modern-day data from a nationally representative sample in the United States.
The study utilized the National Inpatient Sample (NIS) database for the year 2020, which is a part of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality. NIS is the largest all-payer inpatient healthcare dataset in the United States, representing about 20% of United States hospitals from 48 states, comprising average 7 million un-weighted discharges per year that approximate more than 35 million weighted nationwide discharges. The database provides one primary diagnosis and up to 24 secondary discharge diagnoses for each inpatient admission. As the NIS database contains deidentified data, Institutional review board approval was not necessary. Additional information about the database can be accessed from the HCUP website.
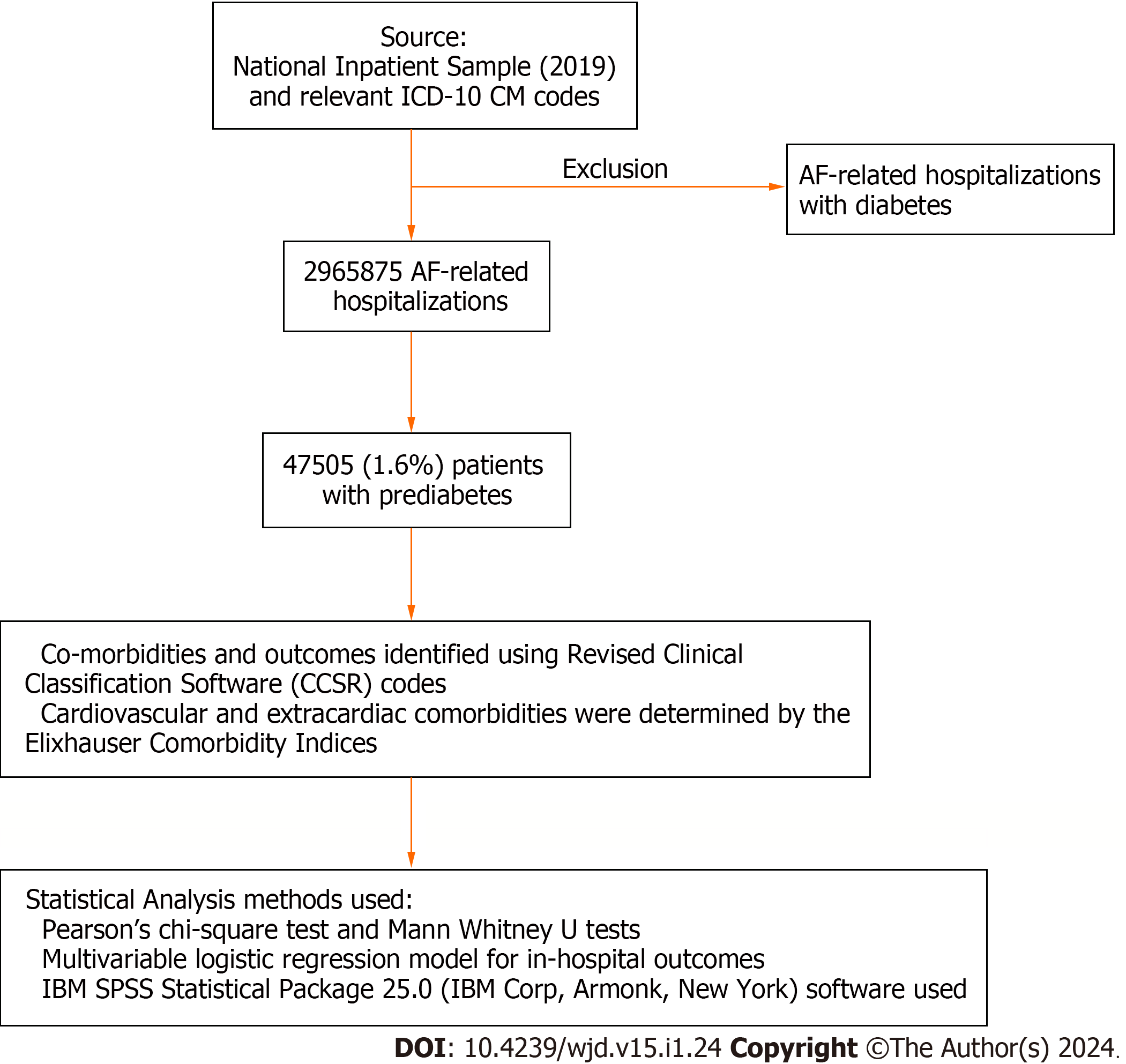
Patients with prediabetes (ICD code: R73. 03) and AF (ICD codes: I48.0, I48.1, I48.2, I48.3, I48.4, I48.9) were identified using relevant ICD-10 CM codes[9,10]. Patients AF are divided into two groups consisting of with and without prediabetes respectively excluding diabetic population. Patients with both prediabetes and AF are considered as exposure group and the other group is considered as control then the co-morbidities and outcomes are identified using Revised Clinical Classification Software codes. Cardiovascular and extracardiac comorbidities were determined by utilizing the Elixhauser comorbidity indices. Predefined criteria found in the NIS database, which are based on ICD-10 CM codes (Figures 1 and 2).
The primary objective of the study was to determine the relationship between prediabetes and in-hospital MACCE, including all-cause inpatient mortality, cardiac arrest including ventricular fibrillation and stroke. The secondary objective were length of hospital stays, hospital costs, and comorbidities related to AF hospitalizations.
Descriptive statistics were used to describe study population and initial characteristics were obtained. Categorical variables and continuous variables were reported as frequency and percentage, interquartile ranges respectively. Pearson's chi-square test and Mann Whitney U tests were utilized for categorical and continuous variables (non-normal distribution), respectively, to compare baseline demographics and hospital characteristics and other comorbidities between the two groups. Discharge weight provided in the database was used to generate national estimates. To evaluate the relationship between prediabetes (pDM) and MACCE in AF hospitalizations, multivariable logistic regression model was used to assess the risk of in-hospital outcomes. In conducting the regression analysis, we took into account factors including age, at admission, gender, race, income level, payment status, type of admission, hospital size teaching status of the facility geographical location and relevant medical conditions relevant cardiac and extra cardiac comorbidities and prior history of myocardial infarction or revascularization with percutaneous coronary intervention or coronary artery bypass grafting, stroke, venous thromboembolic events and cardiac arrest. Adjusted odds ratio (OR), 95% confidence interval (CI), and P-values were used to present logistic regression results. IBM SPSS Statistics 25.0 (IBM Corp, Armonk, New York) software was used for all statistical analyses using complex sample modules. A two-tailed P-value of less than 0.05 was used to determine statistical significance.
The study investigated the relationship between prediabetes and MACCE in individuals with AF in individuals hospitalized with AF using 2019 NIS, cohorts were divided into hospitalizations with AF into groups with and without prediabetes (pDM), excluding diabetics. MACCE defined as all-cause mortality, cardiac arrest including ventricular fibrillation, and stroke in AF- related hospitalizations, was the primary outcome. Of 2965875 total AF-related hospitalizations 47505 (1.6%) patients were identified with prediabetes.
The pDM cohort comprised a higher percentage of male population (56.3% vs 51.4%), were mostly younger (Median 75 vs 78 years) and had a greater proportion of black (9.8% vs 7.9%), Hispanic (7.3% vs 4.0%), and Asian or Pacific Islander patients (4.7% vs 1.6%) than the non-prediabetic cohort (P < 0.001) (Table 1).
| Variable | Prediabetes | No prediabetes | P value |
| N | 47505 | 2918370 | |
| Age (yr) | 75 (67-82) | 78 (68-85) | < 0.001 |
| Male sex | 56.3 | 51.4 | < 0.001 |
| Race/ethnicity | < 0.001 | ||
| White | 75.5 | 84 | |
| Black | 9.8 | 7.9 | |
| Hispanic | 7.3 | 4 | |
| API | 4.7 | 1.6 | |
| Comorbidities | |||
| Hypertension (uncomplicated) | 34.6 | 31.3 | < 0.001 |
| Hyperlipidemia | 64.7 | 48.7 | < 0.001 |
| Smoking | 10.5 | 10.2 | 0.033 |
| Obesity | 29.9 | 14.6 | < 0.001 |
| Drug abuse | 2.5 | 2.1 | < 0.001 |
| Prior myocardial infarction | 10.8 | 9.6 | < 0.001 |
| PVD | 20.2 | 12.7 | < 0.001 |
| Hypothyroidism | 18.1 | 20.4 | < 0.001 |
| Outcomes | |||
| Discharge disposition | |||
| Routine | 51.1 | 43.1 | < 0.001 |
| Home health care | 23.6 | 21 | < 0.001 |
| Total charges (USD), Median | 41155 | 41276 | < 0.001 |
Of the comorbid conditions hypertension, hyperlipidemia, smoking, obesity, drug misuse, prior myocardial infarction (MI), peripheral vascular disease (PVD), and hyperthyroidism were more prevalent in the pDM group (P < 0.05 for all the comorbidities).
Interestingly pDM were more often routinely discharged (23.6% vs 21.0%), however more often required home healthcare and incurred greater charges compared to patients without prediabetes.
After multivariable regression analysis and adjustment for baseline characteristics, the pDM cohort with AF hospitalizations had a substantially higher rate and chances of MACCE than the non-pDM cohort (18.6% vs. 14.7%, OR 1.34, 95%CI 1.25-1.45, P < 0.001). Figure 3 sub group analysis showed that males had a larger correlation (1.43 adjusted OR) than females (adjusted OR 1.22). Hispanics (1.43 adjusted OR) and Asians (1.36 adjusted OR) had a greater connection between MACCE and pDM than whites (1.33 adjusted OR) and blacks (1.21 adjusted OR) (Table 2).
| Outcome | Adjusted odds ratio (95%CI) | P value |
| MACCE | 1.34 (1.26-1.42) | < 0.001 |
| All-cause mortality | 0.62 (0.55-0.71) | < 0.001 |
| Cardiac arrest including ventricular fibrillation | 0.85 (0.072-0.99) | 0.043 |
| Stroke | 1.69 (1.55-1.84) | < 0.001 |
| Subgroup | Adjusted odds ratios of MACCE (95%CI) | P value |
| Sex | ||
| Male | 1.43 (1.33-1.54) | < 0.001 |
| Female | 1.22 (1.11-1.33) | < 0.001 |
| Race/ethnicity | ||
| White | 1.33 (1.24-1.42) | < 0.001 |
| Black | 1.21 (1.03-1.44) | < 0.001 |
| Hispanic | 1.43 (1.18-1.74) | < 0.001 |
| API | 1.36 (1.00-1.85) | 0.05 |
This study, based on the 2019 NIS, was conducted to understand and examine the effects of prediabetes in AF patients. According to the findings, 47505 (1.6%) of the 2965875 AF-related hospitalizations for MACCE were among patients with prediabetes. After correcting baseline characteristics or comorbidities, the prediabetes cohort had a greater frequency of MACCE, including all-cause inpatient mortality, cardiac arrest, including ventricular fibrillation, and stroke, in AF-related hospitalizations than the non-prediabetic cohort. This study points out that further research is needed to effectively manage prediabetes in AF patients to reduce MACCE (Figure 1).
Patients with prediabetes are thought to be responsible for endothelial dysfunction, chronic inflammation, and oxidative stress, which could lead to atherosclerosis, MI, stroke, and other cardiovascular disorders. The exact mechanisms underlying these changes are not well known[11,12]. Most of the patient population were younger males (56.3%); Hispanics or Asians had a stronger association with MACCE with prediabetes, followed by whites and blacks. As per the literature, certain ethnic groups and men were more predisposed to prediabetes and diabetes than others[13].
The prediabetic cohort was discharged from the hospital early, was associated with more home health care, and had greater overall cost. In addition, the prediabetes cohort with AF admissions showed a higher rate and significantly higher odds of MACCE compared to the non-prediabetic cohort. Despite having shorter hospital stays, they had higher healthcare charges, possible due to the requirement for frequent monitoring, more medications for comorbid conditions and management, of AF and subsequent in-hospital MACCE. Taking steps to tackle prediabetes and its potential progression towards diabetes can play a crucial role, in reducing these expenses and enhancing overall health outcomes in patients with multiple comorbidities and higher atherosclerotic cardiovascular disease risk.
Autonomic dysfunction associated with prediabetes can cause arrhythmias, which can increase the chances of developing AF[14]. In this study, involving 17943 patients with newly diagnosed AF, it was discovered that 20.7% of them had prediabetes while 56.4% had diabetes. Over a span of 4.7 years, heart failure occurred in 14, 15.7% and 17.7% in those with normal glucose levels, prediabetics and diabetics. This study concluded that prediabetes is associated with an elevated risk of HF in patients with AF. Moreover, individuals with a prediabetic state who develop diabetes within a span of two years are at a heightened risk of heart failure. On the hand those who revert to normal blood sugar levels experience a decreased risk of heart failure. It is essential for healthcare practitioners to diligently observe and control pre-diabetes in patients with AF in order to hinder the progression, towards diabetes and minimize the chances of developing heart failure. Additionally, interventions targeting glycemic control should be implemented early on to improve cardiovascular outcomes in this high-risk population.
Comorbid conditions such as hypertension, hyperlipidemia, smoking, obesity, substance abuse, prior MI, PVD, and hyperthyroidism are all prevalent among the exposure group, which is the prediabetic group with AF. These comorbid conditions are all identified as independent risk factors for MACCE[15], which brings to the point that the results could have been an effect of comorbidities rather than prediabetes itself. The presence of underlying health conditions in individuals with prediabetes as compared to those without prediabetes has significant implications for their overall outcomes. Among the comorbid conditions, hypertension is a well-recognized risk factor for MACCE, like stroke, MI, and cardiovascular mortality, irrespective of prediabetes status. An interesting correlation was found in recent literature in a retrospective analysis[16]. Hypertension and MACCE events are found in individuals with prediabetes. Other than hypertension, hyperlipidemia is another well-known risk factor for MACCE[17]. Hyperlipidemia is a well-established risk factor for coronary artery disease and can lead to premature cardiovascular mortality if not managed.
Smoking, alcohol, and obesity, major contributors to atherosclerosis, have been associated with MACCE in people with prediabetes[18-20]. PVD was also significantly higher in the AF cohort with prediabetes, PVD being a marker of systemic atherosclerosis and inflammation, could contribute to worsening AF prognosis and subsequent MACCE in patients with prediabetes.
The findings of this study shed light on the relationship between prediabetes and AF. Previous research has already established that prediabetes increases the risk of conditions like stroke and MI. The changes in insulin sensitivity and resistance observed in prediabetes may contribute to MACCE[21], which is concerning. Individuals with prediabetes have a 34% risk of MACCE compared to those without this condition in this study. However, it is important to note that correlation does not imply causation, and further research is needed to establish a direct link between prediabetes and AF. There is a link between prediabetes and complications such as retinopathy, nephropathy, and chronic kidney disease suggested in the literature. Furthermore, it has been observed that patients who have recently experienced stroke or transient ischemic attack are more likely to have prediabetes compared to the general population[21]. This indicates that prediabetes could potentially be a contributing factor to these events and warrant monitoring and interventions in order to prevent the progression to DM and these complications.
To effectively manage prediabetes, it's essential to understand the factors involved, such as IGT and impaired fasting glucose (IFG). Patients with IFG and IGT experience impaired beta cell function and insulin resistance, which is similar to type 2 diabetes. However, their pathophysiology differs slightly. IFG is mainly characterized by insulin resistance in the liver, while IGT involves muscle insulin resistance and mild hepatic resistance. Recognizing these differences is vital for tailoring treatment approaches that can prevent the progression of DM.
Patients who have AF face the risk of experiencing MACCE. These events can be attributed to both the pathological changes caused by AF itself well as the presence of other comorbid conditions associated with AF that directly or indirectly contribute to these adverse events. While research suggests that factors, like endothelial dysfunction, chronic inflammation, oxidative stress and autonomic dysfunction may be involved in the pathophysiology of MACCE, further investigation is necessary to confirm and expand upon these theories[22-24]. Moreover, future studies should prioritize developing strategies for reducing MACCE in patients, with prediabetes who also have AF. This is particularly important since diabetes is a known factor that leads to microvascular and macrovascular changes associated with disease.
Additionally, demographic differences revealed a greater risk of MACCE in men, Latinos, and Asians, which shows that understanding patient group diversity in terms of cardiovascular risk factors is critical for successful risk assessment and treatment. Additional studies quantifying the effects of multiple comorbidities in AF patients with prediabetes causing MACCE. Finally, it can be useful to find out potential advantages in lowering cardiovascular risk factors in prediabetes and AF patients with therapies and guidelines directed towards decreasing the inciting events. The findings of our study shed light on a topic that has not received much attention and could have important clinical implications. Our findings, in our opinion, should make physicians more aware of the prediabetes disease and any potential long term side effects. Considering the findings of this study, we would advocate for aggressive prediabetes treatment. This would entail behavioral modification, nutritional assessment and intervention, drug therapy, and optimization of risk factors.
To our knowledge, this is the largest study providing valuable insights into the relationship between prediabetes and AF-related MACCE in the United States adult population, however, it has a few drawbacks to consider while inferring results. Firstly, this study was mainly retrospective and was conducted on data provided by the administration, which could have coding errors and variable misclassification of different diseases and diagnoses. Furthermore, this study did not account for other potential confounding variables such as nutrition, physical exercise, the effects of other comorbid conditions. As a retrospective observational analysis, this study evaluated association and not causation. This study could not establish their causal relationship. Other study designs such as randomized controlled trials are needed to validate the association between prediabetes and MACCE in AF patients. Long-term follow up data were not available. Lastly, we could not evaluate whether any medications could have impacted AF outcomes in patients with prediabetes. Coding inaccuracies. Lack of lab value, residual confounding, absence of outpatient data.
This study suggests that AF patients with prediabetes are at increased risk of MACCE even after adjusting baseline characteristics and comorbidities compared to AF patients without prediabetes. These enlighten the importance of screening for prediabetes in AF patients and strict hyperglycemia control to prevent MACCE. Further study is required, as this study suggests that pre-diabetes may affect cardiovascular risk differently depending on the demographic group. Therefore, pre-diabetes screening should be done as a part of routine care for AF patients, especially if they are male, Hispanic, or Asian, to reduce the risk of MACCE.
Prediabetes is a well-established risk factor for major adverse cardiac and cerebrovascular events (MACCE). This observational retrospective cohort study examines relationship between prediabetes and MACCE in patients with atrial fibrillation (AF).
The study aims to fill a knowledge gap by studying the connection between prediabetes and major cardiac and cerebrovascular events in AF patients. The goal is to better understand the risks and implications for clinical practice in managing prediabetes in this population.
Our objective is to investigate and establish a link between prediabetes and MACCE in patients with AF.
Using National Inpatient Sample (2019) and relevant ICD-10 CM codes, hospitalizations with AF were categorized into groups with and without prediabetes, excluding diabetics. The primary outcome was MACCE (all-cause inpatient mortality, cardiac arrest including ventricular fibrillation, and stroke) in AF-related hospitalizations.
Key findings include: Prediabetes was present in 1.6% of AF-related hospitalizations. The prediabetes cohort was younger (median age 75 years) with a higher proportion of males, blacks, Hispanics, and Asians. Males had a stronger association between prediabetes and MACCE than females, and among different racial groups, Hispanics and Asians had a stronger association compared to whites and blacks. The prediabetes cohort with AF admissions had a higher rate of MACCE compared to the non-prediabetic cohort with an odds ratio of 1.34 and a 95% confidence interval of 1.26-1.42.
This study highlights the relationship between prediabetes and MACCE in AF patients, therefore emphasizing the importance of further research, awareness as well as the importance of screening and managing prediabetes in AF patients to prevent MACCE.
The research perspective is primarily epidemiological and clinical. It aims to understand the prevalence, risk factors, and clinical implications of prediabetes in the context of cardiovascular health.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar N, United Kingdom; Luo W, China S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | National Diabetes Statistics Report website. Centers for Disease Control and Prevention. April 9, 2023. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html. |
| 2. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5939] [Article Influence: 989.8] [Reference Citation Analysis (8)] |
| 3. | Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2658] [Cited by in RCA: 3341] [Article Influence: 278.4] [Reference Citation Analysis (0)] |
| 4. | Teh R, Kerse N, Pillai A, Lumley T, Rolleston A, Kyaw TA, Connolly M, Broad J, Monteiro E, Clair VW, Doughty RN. Atrial fibrillation incidence and outcomes in two cohorts of octogenarians: LiLACS NZ. BMC Geriatr. 2023;23:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Miao B, Hernandez AV, Roman YM, Alberts MJ, Coleman CI, Baker WL. Four-year incidence of major adverse cardiovascular events in patients with atherosclerosis and atrial fibrillation. Clin Cardiol. 2020;43:524-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Writing Group Members; January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66-e93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 7. | Huang PH, Huang CC, Lin SJ, Chen JW. Prediction of atrial fibrillation in patients with hypertension: A comprehensive comparison of office and ambulatory blood pressure measurements. J Clin Hypertens (Greenwich). 2022;24:838-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Schlesinger S, Neuenschwander M, Barbaresko J, Lang A, Maalmi H, Rathmann W, Roden M, Herder C. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65:275-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 169] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 9. | Dugan J, Shubrook J. International Classification of Diseases, 10th Revision, Coding for Diabetes. Clin Diabetes. 2017;35:232-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Sammani A, Bagheri A, van der Heijden PGM, Te Riele ASJM, Baas AF, Oosters CAJ, Oberski D, Asselbergs FW. Automatic multilabel detection of ICD10 codes in Dutch cardiology discharge letters using neural networks. NPJ Digit Med. 2021;4:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Wu JD, Liang DL, Xie Y, Chen MY, Chen HH, Sun D, Hu HQ. Association Between Hemoglobin Glycation Index and Risk of Cardiovascular Disease and All Cause Mortality in Type 2 Diabetic Patients: A Meta-Analysis. Front Cardiovasc Med. 2021;8:690689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 12. | Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 640] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 13. | Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 455] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 14. | Huang JY, Tse YK, Li HL, Chen C, Zhao CT, Liu MY, Wu MZ, Ren QW, Yu SY, Hung D, Li XL, Tse HF, Lip GYH, Yiu KH. Prediabetes Is Associated With Increased Risk of Heart Failure Among Patients With Atrial Fibrillation. Diabetes Care. 2023;46:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Lin FJ, Tseng WK, Yin WH, Yeh HI, Chen JW, Wu CC. Residual Risk Factors to Predict Major Adverse Cardiovascular Events in Atherosclerotic Cardiovascular Disease Patients with and without Diabetes Mellitus. Sci Rep. 2017;7:9179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Sun N, Chen Y, Xi Y, Wang H, Wang L. Association Between Heart Rate and Major Adverse Cardiovascular Events Among 9,991 Hypertentive Patients: A Multicenter Retrospective Follow-Up Study. Front Cardiovasc Med. 2021;8:741784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Neves JS, Newman C, Bostrom JA, Buysschaert M, Newman JD, Medina JL, Goldberg IJ, Bergman M. Management of dyslipidemia and atherosclerotic cardiovascular risk in prediabetes. Diabetes Res Clin Pract. 2022;190:109980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Yang Y, Peng N, Chen G, Wan Q, Yan L, Wang G, Qin Y, Luo Z, Tang X, Huo Y, Hu R, Ye Z, Qin G, Gao Z, Su Q, Mu Y, Zhao J, Chen L, Zeng T, Yu X, Li Q, Shen F, Zhang Y, Wang Y, Deng H, Liu C, Wu S, Yang T, Li M, Xu Y, Xu M, Zhao Z, Wang T, Lu J, Bi Y, Wang W, Ning G, Zhang Q, Shi L. Interaction between smoking and diabetes in relation to subsequent risk of cardiovascular events. Cardiovasc Diabetol. 2022;21:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Jiang H, Mei X, Jiang Y, Yao J, Shen J, Chen T, Zhou Y. Alcohol consumption and atrial fibrillation risk: An updated dose-response meta-analysis of over 10 million participants. Front Cardiovasc Med. 2022;9:979982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 20. | Piché ME, Tchernof A, Després JP. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ Res. 2020;126:1477-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 1091] [Article Influence: 218.2] [Reference Citation Analysis (0)] |
| 21. | Mijajlović MD, Aleksić VM, Šternić NM, Mirković MM, Bornstein NM. Role of prediabetes in stroke. Neuropsychiatr Dis Treat. 2017;13:259-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Samman Tahhan A, Sandesara PB, Hayek SS, Alkhoder A, Chivukula K, Hammadah M, Mohamed-Kelli H, O'Neal WT, Topel M, Ghasemzadeh N, Ko YA, Aida H, Gafeer M, Sperling L, Vaccarino V, Liang Y, Jones DP, Quyyumi AA. Association between oxidative stress and atrial fibrillation. Heart Rhythm. 2017;14:1849-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Chan YH, Chang GJ, Lai YJ, Chen WJ, Chang SH, Hung LM, Kuo CT, Yeh YH. Atrial fibrillation and its arrhythmogenesis associated with insulin resistance. Cardiovasc Diabetol. 2019;18:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Platonov PG. Atrial fibrosis: an obligatory component of arrhythmia mechanisms in atrial fibrillation? J Geriatr Cardiol. 2017;14:233-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |